INTRODUCTION
The outbreak of coronavirus disease 2019 (COVID-19), caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), was first identified in Wuhan, a city in Hubei province in China, in December 2019 which was declared as pandemic on March 11, 2020. Since identifying this novel virus, there has been a wide range of disease presentations [1–4]. Over the past year, the SARS-CoV-2 has undergone various mutations that resulted in a second wave. As of today, there were 91 vaccines under clinical development, 184 in the pre-clinical developmental stage, and three vaccines are approved for emergency use by the FDA [5]. According to clinical trial data, the vaccines have proven effective against novel SARS-CoV-2 and some of its variants. The Center for Disease Control and Prevention (CDC) started a vaccination drive and vaccinated 28.5% of the US population by April 25, 2021 [3]. In this paper, we present a case of positive COVID-19 infection after receiving the first dose of mRNA 1273 (Moderna) vaccine and discuss the questions and dilemmas that might arise in the management of patients infected with COVID-19 after they are vaccinated.
CASE REPORT
Patient is a 69-year-old Caucasian male patient, a nursing supervisor at a mental health facility, with a known history of alcoholic liver cirrhosis, essential hypertension, type 2 diabetes mellitus, obstructive sleep apnea, and hypothyroidism. He had a history of 10-year pack per day (PPD), quit smoking over 30 years ago, and no history of emphysema. He regularly uses continuous positive airway pressure (CPAP) at nighttime.
On January 9, 2021, he received the first dose of mRNA 1273 (Moderna) vaccine for COVID-19. A day after receiving the vaccine, he started having body aches and headaches. Later, his headache improved, but he progressively developed shortness of breath. He went to the urgent care with these symptoms and was tested positive for COVID-19 infection by PCR. His oxygen saturation on the pulse oximetry at home was 90% initially but later dropped to 85% over the next three days. He presented to the emergency room with worsening shortness of breath and denied cough, fever, chest pain or palpitations, trouble with urination, and bowel movements.
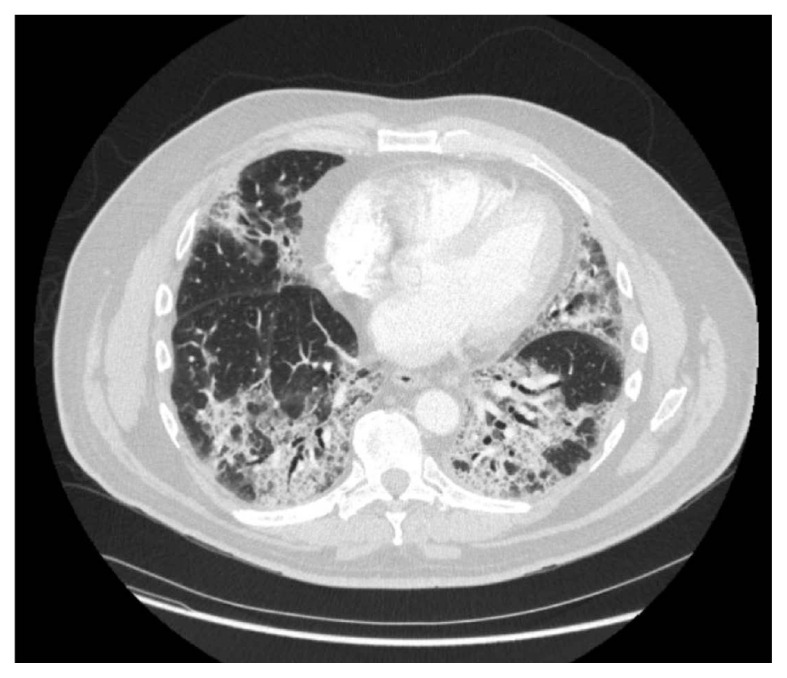
In the emergency room, he was hypoxic, re-tested for COVID-19 infection, which was positive. The CT scan of the chest in the emergency room showed ground-glass infiltrates in both lungs and associated interstitial infiltrates, consistent with COVID-19 multifocal pneumonia (Figure 1). His hepatic function panel and renal function panel were within normal limits. At the time of admission, laboratory parameters were normal for the most part other than a d-dimer level of 2.45 ug/mL (reference range <0.44 ug/mL), total bilirubin 1.8 (reference range 0.2–1.2 mg/dL), ALP 272 U/L (37–153 U/L), AST 44 U/L (6–29 U/L), LDH 220 U/L (98–192 U/L) and fibrinogen 730 mg/dL (190–480 mg/dL).
Figure 1.
COVID-19 induced multifocal ground glass infiltrates.
He was admitted to the medical floor for respiratory failure from COVID-19 multifocal pneumonia. He was initially treated with intravenous steroids, oxygen supplements, and intravenous remdesivir, which was the standard institutional protocol for managing mild to moderate COVID-19 infections at that period. He presented four days after the mRNA vaccine administration, and there were no substantial guidelines for the management of COVID-19 infections in vaccinated patients.
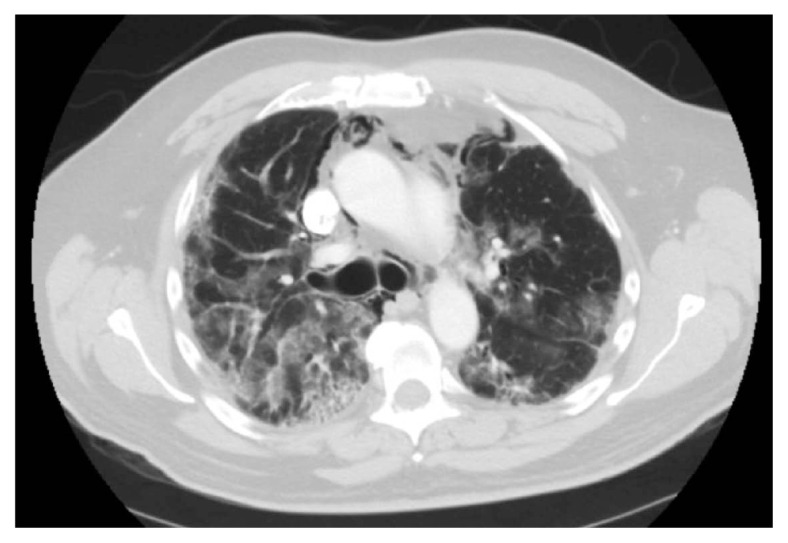
When there was no clinical improvement over the next 48 hours, he was started on combination of oral baricitinib and remdesivir as this combination was proven superior to remdesivir alone in the length of stay of hospitalized patients from COVID-19. There was still no improvement after 72 hours, and two doses of tocilizumab were administered. However, he continued to deteriorate with progressive hypoxia, requiring up to 15 L oxygen through a high-flow nasal cannula. Convalescent plasma was routinely administered to COVID-19 patients during that period. SARS-CoV-2 IgG antibody testing was done to see if he developed some neutralizing antibodies since he has received one vaccine dose. He was positive for IgG antibody and after much debate, it was decided not to administer convalescent plasma. Over the next 2 days, his hypoxia worsened, and the imaging showed a new pneumo-mediastinum (Figure 2). He was then transferred to the intensive care unit (ICU) for further management and completed the ten-day course of IV remdesivir therapy, and he received three doses of tocilizumab. He was also given intermittent diuretics, high-dose IV steroids; subsequently tapered to 20 mg daily dose of oral prednisone. Repeated imaging confirmed a pneumo-mediastinum with no pneumothorax (Figure 2). The lower extremity doppler study was negative for DVT; the CT angiogram of the chest was negative for pulmonary embolism. His liver enzymes, renal function panel, and inflammatory markers were within normal limits. He was eventually transferred back to the medical floor and discharged home with one-liter supplemental oxygen. He was advised to wait for three months before receiving the second dose of the vaccine.
Figure 2.
Pneumomediastinum in a severe case of COVID-19 infection
DISCUSSION
We present a case of a COVID-19 infection in a patient who received the first dose of the COVID-19 mRNA 1273 (Moderna) vaccine. Despite having a single dose of vaccine, the clinical course of the patient was quite typical for a severe COVID-19 infection. As of early 2021, there are established guidelines for the management of COVID-19 infection in the hospital or outpatient settings. Each and every hospital has established their own institutional guidelines in the management of the disease. Barring a few minor side effects, the vaccines currently in use are deemed extremely safe and have decreased the rate of infection and transmission quite significantly [6–8].
However, there have been a lot of dilemmas and debates in the management of the patients who are infected and hospitalized with the COVID-19 virus once they are vaccinated. We performed a literature review of the commonly encountered dilemmas in the management of such patients and illustrated them as follows.
Once infected with COVID-19, how long should one wait before receiving the first dose of COVID-19 mRNA vaccine?
According to the Center for Disease and Control (CDC), one should receive their vaccine even if previously infected with COVID-19. There is little data and research available to assess the safety after recovery from the virus. Patients who were asymptomatic or presented with mild to moderate symptoms and did not receive any medical therapy for COVID-19 should wait until the recovery from current illness and receive their vaccination once they are out of their recommended quarantine period. The isolation period is ten days from the beginning of the symptoms and being asymptomatic for at least 24 hrs without taking any medication [9]. If treated with monoclonal antibodies or the convalescent plasma during the COVID-19 infection, one must wait 90 days before getting the first dose as a precaution to avoid any potential interference between vaccine-induced immune response and passive antibody therapy [10].
If infected with COVID-19 after receiving the first vaccine dose, how long should one wait before receiving the second vaccine dose?
As per the CDC guidelines, the minimum interval between the two Pfizer-BioNTech vaccines is three weeks, and that of the Moderna vaccine is four weeks. However, because of any unavoidable reasons, if one cannot adhere to the recommended time interval, then the second dose of both vaccines may be administered a maximum of up to 6 weeks following the first dose. Currently, very little data is available on the efficacy of both Pfizer and Moderna vaccines if administered beyond this time frame [11].
Meanwhile, if a person becomes COVID-19 positive following the first dose of the vaccine, then the second dose of the vaccine can be scheduled following the isolation period in mild and moderate cases. If treated with monoclonal antibodies or convalescent plasma during the COVID-19 infection, one must wait 90 days before getting the second dose as a precaution to avoid any potential interference between vaccine-induced immune response and passive antibody therapy [10, 12].
Is the PCR test going to be positive after the vaccination?
The mRNA vaccines do not contain the entire viral genome. Instead, they are engineered using a small region of the viral genome, making a specific viral protein called spike protein after entering the host cells against which an immune response is generated. RNA molecules are unstable and are easily targeted by enzymes that disintegrate these molecules within hours. Therefore, mRNA-based vaccines will not cause a COVID-19 PCR test to be positive. Like PCR tests, these vaccines will not cause rapid antigen tests to be positive because the proteins produced following vaccination are not expressed in the respiratory tract. According to the available data, the current vaccines may not provide complete protection against COVID-19 but significantly reduce morbidity and mortality [13].
Would the COVID-19 antibody test be positive after receiving the mRNA vaccine?
Vaccines help in building immunity without getting ill. Different vaccines develop an immune response in different ways by producing neutralizing antibodies to battle future infections. The antibodies developed from vaccination are similar to that of the natural infection. During natural infection, the B-cells produce neutralizing antibodies that help to eliminate viruses, recover from illness, and prevent reinfection for a short duration. The median time for seroconversion was on day 10 for IgM and day 12 for IgG after the onset of symptoms and persists for varied periods [14]. Some studies demonstrated lower antibody titers and fast waning in mild and asymptomatic patients, while few studies reported 50% higher antibody responses in symptomatic patients [15]. The convalescent sera from the recovered patients strongly reacted to both receptor binding domain and spike proteins of the SARS-CoV-2 [16].
Similar to natural infection, the mRNA vaccines take at least 14 days to produce neutralizing antibodies. As the antibodies decrease after the first dose, it is essential to take a second dose to achieve the desired antibody response to provide lasting immunity. It is still not known how long the immunity lasts after the second dose, but a recent study reports the persistence of antibodies six months after the second dose of vaccine [17]. As COVID-19 vaccines are developed to encode spike protein, a positive test for IgM/IgG spike protein would indicate the prior infection or vaccination [10, 18].
Does the use of corticosteroids suppress the vaccine mechanism of action?
The main concern regarding COVID-19 vaccines in steroid use is the effects on the efficacy of the vaccine given the immunosuppressive actions of corticosteroids. Steroids could induce a temporary decline in lymphocyte groups with supraphysiologic dosing, T cell lymphocytic apoptosis and altered immunoglobulin secretion [19, 20]. Despite studies that reported corticosteroids influencing the development and function of B-cells in animals, the effects on vaccine responses are not apparent.
Further studies are required to know the impact of steroids on B and T-cell responses. There is some data available on functional vaccine outcomes in the setting of chronic steroid use [21]. The findings suggest that chronic higher doses of systemic steroids may impact the vaccine conferred immunity. Short-term and systemic bolus steroids did not show any impact on vaccine response in tetanus and influenza [22]. To date, there is no significant evidence suggesting that steroid therapy will impact the responsiveness and efficacy of the COVID-19 vaccine. However, it is helpful to consider the lack of data and evidence by physicians for individual patients who may require dosage modification of steroids in light of the COVID-19 vaccination.
What are the management guidelines for positive COVID-19 infection after COVID-19 vaccination?
For asymptomatic COVID-19 infection, providing supportive care, close monitoring with repeating the labs at regular intervals would suffice. As such, there is no specific treatment in such cases. Several clinical trials are going on to study the role of monoclonal antibodies in the prevention and treatment of high-risk outpatients and mild to moderate COVID-19 infection. Currently, the FDA has approved emergency use authorization to combine bamlanivimab and etesevimab or a combination of casirivimab and imdevimab for outpatients with mild to moderate symptoms [23].
In symptomatic COVID-19 infection patients, supplemental oxygen, dexamethasone, and remdesivir are initiated. All patients except pregnant women are placed on low molecular weight heparin prophylaxis to prevent thromboembolism. In patients who require supplemental oxygen but are not ventilated and corticosteroids are contraindicated, the combination of baricitinib and remdesivir is superior to that of the remdesivir alone by improving the clinical status and also reducing recovery time. A loading dose 200 mg of remdesivir on day 1 was given in the intravenously and followed by 100 mg daily until day 10. Addition of 4 mg of baricitinib or 2 mg if glomerular filtration rate is less than 30, daily either orally or through nasogastric tube for 14 days had shown significant improvement in clinical status [24]. Interleukin-6 receptor inhibitors, tocilizumab, and sarilumab have shown improved survival rates in critically ill patients [25]. Convalescent plasma is another therapeutic option in hospitalized patients [26].
In vaccinated patients who develop COVID-19 infection, the treatment options including antivirals, corticosteroids, monoclonal antibodies, or convalescent plasma or the timing of these treatments should not affect. If a person tests positive for SARS-CoV-2 after completing vaccination (≥2 weeks of two-dose mRNA vaccine), it is essential to report to the vaccine adverse event reporting system (VAERS) [10].
What are quarantine guidelines if exposed to suspected or confirmed COVID-19 individuals after completing a full dose of vaccination?
According to the CDC, after fully vaccinated against COVID-19 and exposed to positive or suspected patients with COVID-19 infection, he/she is not required to quarantine if asymptomatic, or the exposure to COVID-19 positive individual has occurred after three months of final dose of vaccine series. It involves monitoring for at least 14 days [27]. In symptomatic cases, the exposed person should be isolated, clinically evaluated, tested, and treated like a non-vaccinated person, as mentioned above [28].
After completing the full dose of COVID-19 vaccination, if a person is exposed to a COVID-19 positive individual and is asymptomatic, will the exposed person be a carrier/can transmit the infection to others? If so, for how long?
Based on the available data, a fully vaccinated individual is less likely to get an asymptomatic infection and less likely to transmit a COVID-19 infection. However, the risk of getting infected cannot be completely ruled out. Only limited data is available about the possible SARS-COV-2 infection in a fully vaccinated immunocompromised individual and immunosuppressive medications [29].
Is the person immune to variants after completing a full dose of the COVID-19 vaccine?
At the time of this paper, there were 91 COVID-19 vaccines in the clinical development stage and 184 COVID-19 vaccines in the pre-clinical developmental stage [8]. Several platforms have been utilized to develop these vaccines, including protein subunit, viral vector (replicating and non-replicating), DNA, inactivated virus, messenger RNA, virus-like particles and live attenuated viruses. As of now, the FDA has approved three vaccinations for emergency use in the USA [7].
Data suggests that the mRNA vaccines provide reasonable protection against the B.1.1.7 COVID-19 variant. The messenger RNA (mRNA) vaccines, Pfizer and Moderna, were authorized in the US before identifying the South African variant (B.1.351 or 20H/501Y.V2) strain. According to the latest studies, these two vaccines elicited lower neutralizing antibodies than that of the previous strains. Novavax, Janssen, and Astra-Zeneca conducted trials in South Africa that have dominant B.1.351 mutated strains. These studies demonstrated the lower vaccine efficacy than that of the other variants where this strain was not dominant [30].
B.1.351 and P.1 consist of similar receptor-binding mutations, and hence, the vaccine efficacy against P.1 strain is assumed to be identical to B.1.351. As the studies demonstrated reduced vaccine efficacy against B.1.351, the effectiveness against P. 1 strain is likely reduced [31]. Sinovac Biotech has initiated clinical trials, which revealed that the CoronoVac vaccine is 50% effective in preventing infection with the P.1 variant in Brazil [32].
Vaccine efficacy for another Brazilian variant, B.1.1.28, has not yet been reported. B.1.526 and B.1.525 have a reduction in vaccine efficacy [33]. According to the Indian Council of Medical Research Virology lab, Bharat Biotech’s COVAXIN vaccine has been found to neutralize effectively and is 78% effective against the double mutant variant, B.1.617 [34–36].
What precautions are required after the completion of a full dose of the COVID-19 vaccine?
On April 27, 2021, CDC revised the guidelines on preventive measures. If the person is not fully vaccinated, all precautions as previously mentioned are continued. After completing a full dose of the vaccine, the person can gather or conduct activities outdoors except in crowded places and gather indoors with fully vaccinated people without a mask or staying 6 feet apart. Domestic travel can be done without testing before or after travel but requires testing for international travel. As things are getting back to normal slowly, it is still vital to avoid large indoor gatherings and follow precautions to protect yourself [37].
CONCLUSION
Vaccines play a crucial role in teaching our immune system to recognize and fighting against the COVID-19 virus. It approximately takes two weeks after the second dose of mRNA vaccines to develop the neutralizing antibodies. This indicates a possibility of getting infected with SARS-CoV-2 just after the vaccination as there wasn’t sufficient time to provide protection. Studies show that vaccines are effective in reducing hospitalizations and deaths. But further studies are required to learn about how well the vaccines prevent from spreading the virus and how long the vaccines provide protection.
Footnotes
Conflict of interest
None.
Funding
No grants were obtained for this study.
REFERENCES
- 1.Malayala SV, Mohan G, Vasireddy D, Atluri P. A case series of vestibular symptoms in positive or suspected COVID-19 patients. Infez Med. 2021;29(1):117–122. [PubMed] [Google Scholar]
- 2.Malayala SV, Raza A. A Case of COVID-19-Induced Vestibular Neuritis. Cureus. 2020;12(6):e8918. doi: 10.7759/cureus.8918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Atluri P, Vasireddy D, Malayala S. COVID-19 Encephalopathy in adults. Cureus. 2021;13(2):e13052. doi: 10.7759/cureus.13052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vanaparthy R, Malayala SV, Balla M. COVID-19-Induced Vestibular Neuritis, Hemi-Facial Spasms and Raynaud’s Phenomenon: A Case Report. Cureus. 2020;12(11):e11752. doi: 10.7759/cureus.11752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vasireddy D, Atluri P, Malayala S, Vanaparthy R, Mohan G. Review of COVID-19 vaccines approved in the United States of America for emergency use. J Clin Med Res. 2021;3(4):204–13. doi: 10.14740/jocmr4490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kadali RAK, Janagama R, Peruru S, Malayala SV. Side effects of BNT162b2 mRNA COVID-19 vaccine: A randomized, cross-sectional study with detailed self-reported symptoms from healthcare workers. Int J Infect Dis. 2021;106:376–81. doi: 10.1016/j.ijid.2021.04.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kadali RAK, Janagama R, Peruru S, Gajula V, Madathala RR, Chennaiahgari N, Malayala SV. Non-life-threatening adverse effects with COVID-19 mRNA-1273 vaccine: A randomized, cross-sectional study on healthcare workers with detailed self-reported symptoms. J Med Virol. 2021 doi: 10.1002/jmv.26996. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Malayala SV, Mohan G, Vasireddy D, Atluri P. Purpuric rash and thrombocytopenia after the mRNA-1273 (Moderna) COVID-19 Vaccine. Cureus. 2021;13(3):e14099. doi: 10.7759/cureus.14099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.CDC. When to quarantine? [Accessed April 28, 2021]. Available at https://www.cdc.gov/coronavirus/2019-ncov/if-you-are-sick/quarantine.html.
- 10.Frequently Asked Questions about COVID-19 Vaccination. Centers for Disease Control and Prevention; [Accessed April 28, 2021]. Available at https://www.cdc.gov/coronavirus/2019-ncov/vaccines/faq.html. [Google Scholar]
- 11.Shannon AC. CDC recommends some coronavirus patients wait 90 days to get vaccine. abc10.com. [Accessed April 28, 2021]. Available at https://www.abc10.com/article/news/health/coronavirus/vaccine/vaccine-question-how-long-to-wait/103-b22ae306-8acd-4201-8258-695760277473.
- 12.Interim Clinical Considerations for Use of COVID-19 Vaccines. Centers for Disease Control and Prevention; [Accessed April 28, 2021]. Available at https://www.cdc.gov/vaccines/covid-19/info-by-product/clinical-considerations.html. [Google Scholar]
- 13.You asked, we answered: What if I get COVID-19 after my first vaccine shot?: Nebraska Medicine Omaha, NE. Nebraska Medicine; [Accessed April 28, 2021]. Available at https://www.nebraskamed.com/COVID/what-if-i-get-covid-19-after-my-first-vaccine-shot. [Google Scholar]
- 14.Binnicker M. Could Vaccination Cause Me To Test Positive For Covid-19 Forbes. [Accessed April 28, 2021]. Available at https://www.forbes.com/sites/coronavirusfrontlines/2021/02/26/could-vaccination-cause-me-to-test-positive-for-covid-19/?sh=171007de38b3.
- 15.Lou B, Li TD, Zheng SF, et al. Serology characteristics of SARS-CoV-2 infection after exposure and post-symptom onset. Eur Respir J. 2020;56(2):2000763. doi: 10.1183/13993003.00763-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Interim Guidance on Duration of Isolation and Precautions for Adults with COVID-19. Centers for Disease Control and Prevention; [Accessed April 28, 2021]. Available at https://www.cdc.gov/coronavirus/2019-ncov/hcp/duration-isolation.html. [Google Scholar]
- 17.Amanat F, Stadlbauer D, Strohmeier S, et al. A serological assay to detect SARS-CoV-2 seroconversion in humans. Nat Med. 2020;26(7):1033–6. doi: 10.1038/s41591-020-0913-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Doria-Rose N, Suthar MS, Makowski M, et al. Antibody Persistence through 6 Months after the Second Dose of mRNA-1273 Vaccine for Covid-19 [published online ahead of print, 2021 Apr 6] N Engl J Med. 2021 doi: 10.1056/NEJMc2103916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Burbelo PD, Riedo FX, Morishima C, et al. Detection of Nucleocapsid Antibody to SARS-CoV-2 is More Sensitive than Antibody to Spike Protein in COVID-19 Patients. Preprint. medRxiv. 2020 doi: 10.1101/2020.04.20.20071423. 2020.04.20.20071423. Published 2020 Apr 24. [DOI] [Google Scholar]
- 20.Fauci AS, Dale DC. The effect of in vivo hydrocortisone on subpopulations of human lymphocytes. J Clin Invest. 1974;53(1):240–6. doi: 10.1172/JCI107544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fischer L, Gerstel PF, Poncet A, et al. Pneumococcal polysaccharide vaccination in adults undergoing immunosuppressive treatment for inflammatory diseases - a longitudinal study. Arthritis Res Ther. 2015;17(1):151. doi: 10.1186/s13075-015-0663-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Johnson JR, Denis R, Lucas CE, et al. The effect of steroids for shock on the immune response to tetanus toxoid. Am Surg. 1987;53(7):389391. [PubMed] [Google Scholar]
- 23.Food and Drug Administration. Frequently asked questions on the emergency use authorization for bamlanivimab and etesevimab. 2021. [Accessed April 17, 2021]. Available at: https://www.fda.gov/media/145808/download.
- 24.Kalil AC, Patterson TF, Mehta AK, et al. Baricitinib plus Remdesivir for Hospitalized Adults with Covid-19. N Engl J Med. 2021;384(9):795–807. doi: 10.1056/NEJMoa2031994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.REMAP-CAP Investigators. Gordon AC, Mouncey PR, et al. Interleukin-6 Receptor Antagonists in Critically Ill Patients with Covid-19. N Engl J Med. 2021;384(16):1491–502. doi: 10.1056/NEJMoa2100433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.May B. Study: Convalescent Plasma Doesn’t Prevent Progression of COVID-19 in At-Risk People. Bio-Space. [Accessed May 4, 2021]. Available at https://www.biospace.com/article/study-convalescent-plasma-doesn-t-prevent-progression-of-covid-19-in-at-risk-people/
- 27.If you’re vaccinated and exposed to Covid-19 what do you do? Here’s CDC’s guidance. Advisory Board; [Accessed May 4, 2021]. Available at https://www.advisory.com/daily-briefing/2021/02/18/quarantine. [Google Scholar]
- 28.Interim Public Health Recommendations for Fully Vaccinated People. Centers for Disease Control and Prevention; [Accessed May 4, 2021]. Available at https://www.cdc.gov/coronavirus/2019-ncov/vaccines/fully-vaccinated-guidance.html. [Google Scholar]
- 29.Science Brief: Background Rationale and Evidence for Public Health Recommendations for Fully Vaccinated People. Centers for Disease Control and Prevention; [Accessed May 4, 2021]. Available at https://www.cdc.gov/coronavirus/2019-ncov/science/science-briefs/fully-vaccinated-people.html. [Google Scholar]
- 30.Vasireddy D, Vanaparthy R, Mohan G, Malayala SV, Atluri P. Review of COVID-19 Variants and COVID-19 Vaccine Efficacy: What the Clinician Should Know? [published corrections appears in J Clin Med Res. 2021 Jul; 13 (7): 412] J Clin Med Res. 2021;13(6):317–25. doi: 10.14740/jocmr4518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Existing vaccines may protect against the Brazilian coronavirus variant. University of Oxford; [Accessed May 4, 2021]]. Available at https://www.ox.ac.uk/news/2021-03-18-existing-vaccines-may-protect-against-brazilian-coronavirus-variant. [Google Scholar]
- 32.COVID-19 vaccine effective against new variant in Brazil Articles - COVID19 vaccine effective against new variant in Brazil - Emerging Pathogens Institute-University of Florida. [Accessed May 4, 2021]]. Available at https://epi.ufl.edu/articles/covid19-vaccine-effective-against-new-variant-in-brazil.html.
- 33.SARS-CoV-2 Variants of Concern. Centers for Disease Control and Prevention; [Accessed May 4, 2021]. Available at https://www.cdc.gov/coronavirus/2019-ncov/cases-updates/variant-surveillance/variant-info.html. [Google Scholar]
- 34.Coronavirus: ‘Double mutant’ Covid variant found in India. BBC News; [Accessed May 4, 2021]. Available at https://www.bbc.com/news/world-asia-india-56507988. [Google Scholar]
- 35.Choi J. First case of India’s ‘double mutant’ COVID-19 variant found in San Francisco Bay Area. TheHill. [Accessed May 4, 2021]. Available at https://thehill.com/homenews/state-watch/546491-first-case-of-indias-double-mutant-covid-19-variant-found-in-san.
- 36.COVAXIN works against double mutant, reduces hospitalisation, shows Phase 3 interim data. Tribuneindia News Service; [Accessed May 4, 2021]. Available at https://www.tribuneindia.com/news/nation/covaxin-works-against-double-mutant-shows-78-100-efficacy-against-severe-covidphase-3-interim-data-242191. [Google Scholar]
- 37.When You’ve Been Fully Vaccinated. Centers for Disease Control and Prevention; [Accessed May 4, 2021]. Available at https://www.cdc.gov/coronavirus/2019-ncov/vaccines/fully-vaccinated.html. [Google Scholar]