Abstract
We report on a case of phaeohyphomycosis caused by Alternaria infectoria in a renal transplant recipient with pulmonary infiltrates and multiple skin lesions. Diagnosis was based on microscopy and culture of the skin lesions. Treatment consisted of a combination of surgical excision and systemic antifungal therapy, first with itraconazole and subsequently with liposomal amphotericin B, for 39 days. At a 20-month follow-up visit, no recurrence of the skin lesions or the pulmonary infiltrates had occurred.
Phaeohyphomycosis refers to a subcutaneous and systemic infection caused by dark-walled hyphae in culture and often in tissue (11). Agents that cause this infection are primarily recognized as soil saprophytes, plant pathogens, and contaminants living in the environment. With the growing number of patients who are immunocompromised, phaeohyphomycotic agents are increasingly being reported as the cause of human disease (14, 16). More than 100 species belonging to at least 57 genera are known to be agents of phaeohyphomycosis (17). Of these species, many have been reported only anecdotally, while others, such as Bipolaris, Curvularia, Exserohilum, and Alternaria species, have frequently been involved in human infections (20, 25). Among the members of the last genus, the most common species is Alternaria alternata. We report here on an immunocompromised patient with a documented phaeohyphomycosis caused by Alternaria infectoria, which has rarely been reported as a human pathogen before. The case presented here reflects yet an additional example of the growing number of fungi capable of eliciting phaeohyphomycosis in immunocompromised individuals.
Case report.
A 60-year-old male patient with end-stage renal disease due to diabetic nephropathy underwent a cadaveric kidney transplantation. The postoperative course was uneventful, and the patient was discharged 25 days after transplantation. Five months after transplantation he presented at the outpatient department with a painless skin lesion on the right thumb. The patient did not recall any local skin trauma since the transplantation. On the suspicion of Kaposi's sarcoma, a punch biopsy specimen was taken from the lesion for histological examination and the patient was sent home. Three days later he developed a painful red swelling on his right foot. He also suffered from a nonproductive cough. No dyspnea or fever was present. Routine laboratory tests revealed a leukocyte count of 9,300/mm3 and a hemoglobin level of 6.8 mmol/liter. His serum creatinine level was stable (133 μmol/liter). Chest X ray revealed in the right and left lower lobes pulmonary infiltrates that had been absent 5 months previously. The patient was admitted for further evaluation.
On admission, immunosuppression consisted of prednisolone at 10 mg once daily and tacrolimus, which inhibits interleukin-2 production and hence T-cell activation (3). The whole-blood tacrolimus level was 9.8 ng/ml (target level, 7 to 10 ng/ml). Mycophenolate mophetil (another antirejection drug, which blocks both B and T lymphocytes by inhibition of the de novo synthesis of guanosine nucleotides [2]) was instituted as an experimental medication at transplantation and had been stopped 2 months before. The patient was also treated with felodipine posttransplantation for hypertension.
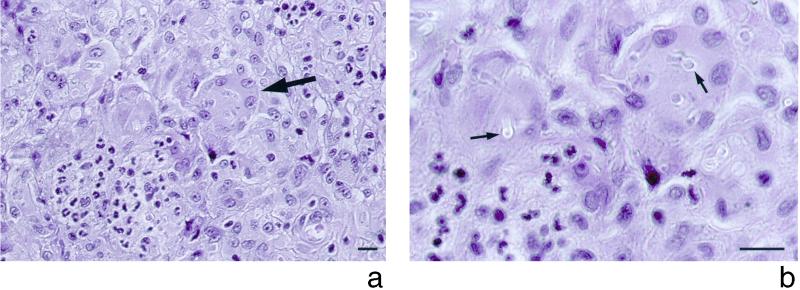
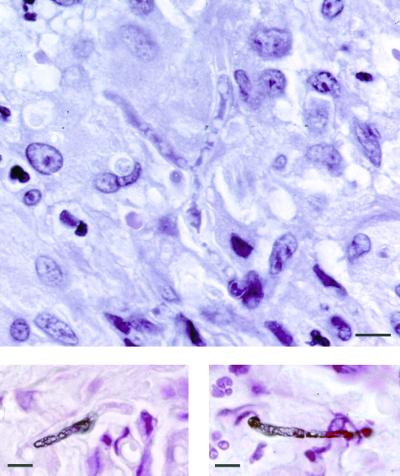
On physical examination, a nodular lesion (3 by 4 cm) on the medical right foot and a similar lesion (1 by 1 cm) on the left foot were found, in addition to the lesion (1 by 1.5 cm) on the right thumb. The lesions were painless, dark violaceous, elevated, and unattached to the underlying tissue; the overlying epidermis was unaffected. Further physical examination, including chest auscultation, was unremarkable. The large lesion on the medial right foot was completely resected. Histopathologic examination of this lesion revealed findings similar to those observed for the previous punch biopsy specimen from the right thumb. In both specimens, multiple intradermal microabscesses were seen, and these were surrounded by a cellular infiltrate consisting of epithelioid macrophages and giant cells engulfing many fungal forms (Fig. 1a). In the specimen stained with hematoxylin-eosin, many refringent hyaline capsules containing fungal elements were observed when the condensor diaphragm was narrowed (Fig. 1b) (15). Fungal elements appeared uncolored in tissue sections stained with hematoxylin-eosin (Fig. 2, top panel); however, in Fontana-Masson melanin-stained tissue sections, some of these fungal elements stained brown (Fig. 2, bottom panels), leading to the diagnosis of subcutaneous phaeohyphomycosis. Twelve days after the skin lesions were first noticed, itraconazole was started at 200 mg twice daily. Because of the pulmonary infiltrates found on admission, diagnostic bronchoscopy was performed 4 days after the initiation of itraconazole therapy. Raw, uncentrifuged bronchoalveolar lavage (BAL) fluid samples were quantitatively cultured for bacteria and yeast by inoculation of 0.010- and 0.001-ml volumes transferred with an adjustable air-displacement pipette (Pipetman P20; Gilson, Villiers-le-Bel, France) (8). Before inoculation, BAL fluid samples were gently mixed on a roller mixer (Coulter Electronics Ltd., Luton, England). The aliquots were plated onto blood agar base supplemented with 5% (vol/vol) sheep blood, chocolate agar, cystine lactose electrolyte-deficient agar, and Shaedler agar supplemented with 5% (vol/vol) sheep blood and vitamin K (Becton Dickinson Microbiology Europe, Meylan Cedex, France). After incubation for 48 h, the colonies were counted on the plates, and the number of CFU per milliliter was determined by multiplying the number of colonies by the dilution factor. In addition, cytocentrifuged preparations were made as described previously (9) and stained with May-Grünwald Giemsa, Gram, Grocott-Gomori methenamine silver, and auramine-rhodamine stains. BAL fluid samples were also cultured for mycobacteria with the MB/BacT process system (Organon Teknika, Durham, N.C.). Finally, the remaining BAL fluid samples (approximately 100 and 70 ml for the first and the second samples, respectively) were divided into 10-ml fractions, centrifuged at 1,500 × g for 10 min, and inoculated onto Sabouraud dextrose agar plates, which were incubated at 35°C for a 6-week period. None of the cultures of BAL fluid samples revealed any growth.
FIG. 1.
Hematoxylin-eosin stain of a biopsy specimen from the lesion of the right thumb. (a) Margin of an abscess with accumulation of polymorphonuclear leukocytes (left lower corner) and numerous multinucleated giant cells (arrow) engulfing fungal elements. (b) The fungal elements are surrounded by hyaline capsules (arrows) which become visible by lowering the condensor diaphragm. Bar, 10 μm.
FIG. 2.
Hematoxylin-eosin stain of a biopsy specimen form the lesion of the right foot. Note the uncolored branching hyphae in the center (top panel). Staining with the Fontana-Masson stain shows brown-pigmented hyphal fragments, disclosing the dematiaceous nature of the fungus (bottom panels). Bars, 10 μm.
After 14 days of treatment with itraconazole, the remainder of the right thumb lesion was completely resected and submitted for culture. Twenty-one days after the initiation of itraconazole, the patient gained 10 kg in weight due to fluid retention. He became progressively dyspneic, and his serum creatinine level increased to 207 μmol/liter. Repeat chest X rays showed progressive pulmonary infiltrations in the right lower lobe, with pulmonary congestion and a right-sided pleural effusion. Cultures of specimens of a repeat BAL grew 2 × 103 CFU of oral flora per ml but no respiratory pathogens. Cultures from the pleural fluid were negative for microorganisms. Treatment was subsequently changed to amphotericin B in a liposomal formulation. The patient improved gradually and regained his normal weight, the dyspnea resolved, and the serum creatinine level returned to the baseline level.
After 28 days of treatment with amphotericin B, the remaining lesion on the left foot was resected. Histological examination of this lesion showed no signs of mycosis. X ray of the chest showed no pulmonary infiltrate or pleural effusion. The patient was subsequently discharged from the hospital without antifungal therapy. At a 20-month follow-up visit, no recurrence of the skin lesions or the pulmonary infiltrates was observed.
Mycological findings.
Grocott-Gomori methenamine silver nitrate staining of touch preparations of a biopsy specimen from the right foot obtained on the day of admission showed smooth, branched hyphae. Biopsy specimens from the same lesion initially cultured in Sabouraud broth at 35°C yielded mycelial growth after 4 days of incubation and were subsequently cultured on Sabouraud agar at room temperature. After approximately 7 days, brown woolly colonies were seen. Examination of lactophenol preparations of these colonies showed conidia and septate hyphae. Conidia were avoid, smooth walled, or verrucose; some of them were muriform and often ended with apical beaks. The strain was initially identified as an Alternaria species, and a subculture was sent to the Scientific Institute of Public Health Louis Pasteur, Brussels, Belgium, for species identification.
Specimens from the subculture were inoculated onto malt extract agar for incubation at 25 and 35°C. After 7 days, scant growth was noted at 35°C. The samples incubated at 25°C showed readily sporulating olivaceous-black colonies with diameters of up to 5 cm. Conidiophores were dark, septate, simple, or geniculate. Conidia were ovoid or ellipsoidal, mostly 10 to 44 by 8 to 11 μm, and smooth walled or verrucose; some had one or more transverse and sometimes longitudinal or oblique septa. Some, mostly young, conidia showed apical growth of long, geniculate, secondary conidiophores (pseudorostrata) (Fig. 3a). Chains of a few conidia separated by secondary conidiophores were also seen (Fig. 3b). On the basis of the last two characteristics, the species was identified as A. infectoria (22, 23). The strain is preserved at the IHEM Culture Collection (IHEM 16110), and its identification was confirmed by the Institute of the Royal Netherlands Academy of Arts and Sciences (Centraalbureau voor Schimmelcultures), Baarn, The Netherlands.
FIG. 3.
Lactophenol cotton blue mount of A. infectoria. (a) Microscopic appearance of conidia (open arrow) with apical outgrowth of long, geniculate secondary conidiophore (pseudorostrata) (closed arrow). (b) Chain formation of conidia, separated by pseudorostrata. Bars, 10 μm.
The lesion on the right thumb was resected 2 weeks after itraconazole treatment had been started, Culture of this lesion yielded the same fungus.
Discussion.
Phaeohyphomycosis is a fungal infection caused by dematiaceous or darkly pigmented fungi (1). Among other mycoses, this fungal infection is increasingly seen in immunocompromised patients (25). Clinical manifestations may range from local skin lesions to invasive and disseminated infections (13).
Diagnosis is made by histopathological examination of tissue specimens. The early histopathologic picture is one of multiple stellate abscesses, which progress to a single circumscribed lesion with a central cavity filled with pus and surrounded by a fibrous wall (26). The margins of these abscesses and the granulomas are composed of giant cells, epithelioid cells, histiocytes, plasma cells, and lymphocytes; fungi are found in or adjacent to purulent areas (11). Despite the dematiaceous nature of the fungi, the brown pigment is not always apparent (13) and hyphae may appear hyaline in lesions when hematoxylin-eosin stain is used (15), such as was seen in our patient's specimen (Fig. 2, top panel); in contrast, the natural brown color of the hyphae is masked by the Grocott staining method. Therefore, confirmation of the presence of a dematiaceous mold can be achieved by using a melanin-specific stain, such as the Fontana-Masson stain (Fig. 2, bottom panels) (7).
More than 100 fungal species have been documented as agents of phaeohyphomycosis (17). The genera most frequently involved in human infections include Bipolaris, Curvularia, Exserohilum, and Alternaria (20, 25). Among the members of the last genus, the most commonly found species is A. alternata (4).
In this report we describe a case of phaeohyphomycosis caused by A. infectoria. The microscopic characteristics typical of A. infectoria include the presence of prominent pseudorostrata (secondary conidiophores) (Fig. 3a) and chains of conidia separated by secondary conidiophores (Fig. 3b) (5, 24).
A. infectoria has been found to cause cutaneous lesions in a cat (18) and, to our knowledge, has previously been reported as a human pathogen in only two cases. Laumaillé et al. (12) have described a cutaneous lesion caused by A. infectoria in a liver transplant recipient. Treatment consisted of a total resection without antifungal agents; however, recurrence of the lesion was noted 5 months later. In the second case, Alternaria infection presented as sinusitis and maxillary osteomyelitis in an otherwise healthy woman. (J. Garau, R. D. Diamond, L. B. Lagrotteria, and S. A. Kabins, Letter, Ann. Intern. Med. 86:747–748, 1977). Culture of a biopsy specimen grew a fungus that was identified as the Alternaria state of Pleospora infectoria (6). Surgical excision of the lesion was performed after a 7-week course of treatment with amphotericin B, but recurrence occurred 4 months later, and prolonged administration of amphotericin B was needed.
In our patient described here, the diagnosis of phaeohyphomycosis was confirmed by both microscopic and cultural evidence. The presence of microscopically confirmed lesions on three noncontiguous body sites (right thumb, right foot, left foot) strongly suggests that the disease was disseminated. Although the fungi could not be detected in the BAL fluid samples, we assume that a coexistent pulmonary phaeohyphomycosis was responsible for the pulmonary infiltrates, since the abnormalities noted on chest X ray normalized after the initiation of antifungal therapy. In addition, the pulmonary infiltrates could not be explained by fluid retention since no signs of heart failure were present at the time of presentation.
Treatment of phaeohyphomycosis should include complete surgical excision of accessible lesions combined with antifungal therapy, especially when invasive or systemic infection is present (17). The patient described here was successfully treated with a combination of surgery and systemic antifungal therapy. Itraconazole has been found to be as effective as amphotericin B in patients with phaeohyphomycosis (including those with skin and organ involvement), ac-counting for approximately 65% of successful outcomes (21). In our patient, however, the clinical effectiveness of itraconazole remained unclear. Itraconazole therapy was prematurely interrupted because of the development of fluid retention and dyspnea, which were considered adverse effects (19). Possible interaction with felodipine may also have contributed to the development of fluid retention (10). Since amphotericin B is nephrotoxic, the liposomal formulation of this agent was given. This treatment resulted in clinical and histological improvements.
In summary, this report presents the third case of human phaeohyphomycosis caused by A. infectoria. Diagnosis was based on cultural and histological examination of cutaneous lesions. The simultaneous presence of multiple skin lesions and pulmonary infiltrates which responded to effective antifungal therapy strongly suggest a disseminated infection. Therapy consisted of surgical excision of the lesions in combination with itraconazole and amphotericin B. We conclude that A. infectoria should be considered in the differential diagnosis of cutaneous and deep-seated phaeohyphomycosis.
REFERENCES
- 1.Ajello L. The gamut of human infections caused by dematiaceous fungi. Jpn J Med Mycol. 1981;22:1–5. [Google Scholar]
- 2.Allison A C, Eugui E M. Mycophenolate mofetil and its mechanisms of action. Immunopharmacology. 2000;47:85–118. doi: 10.1016/s0162-3109(00)00188-0. [DOI] [PubMed] [Google Scholar]
- 3.Almawi W S, Melemedjian O K. Clinical and mechanistic differences between FK506 (tacrolimus) and cyclosporin A. Nephrol Dial Transplant. 2000;15:1916–1918. doi: 10.1093/ndt/15.12.1916. [DOI] [PubMed] [Google Scholar]
- 4.Anaissie E J, Bodey G P, Rinaldi M G. Emerging fungal pathogens. Eur J Clin Microbiol Infect Dis. 1989;8:323–330. doi: 10.1007/BF01963467. [DOI] [PubMed] [Google Scholar]
- 5.Andersen B, Thrane U. Differentiation of Alternaria infectoria and Alternaria alternata based on morphology, metabolite profiles, and cultural characteristics. Can J Microbiol. 1996;42:685–689. [Google Scholar]
- 6.De Hoog R D, Guarro J. Atlas of clinical fungi. Baarn, The Netherlands: Centraalbureau voor Schimmelcultures; 1995. [Google Scholar]
- 7.Fothergill A W. Identification of dematiaceous fungi and their role in human disease. Clin Infect Dis. 1996;22:S179–S184. doi: 10.1093/clinids/22.supplement_2.s179. [DOI] [PubMed] [Google Scholar]
- 8.Jacobs J A, De Brauwer E I, Cornelissen E I, Drent M. Accuracy and precision of quantitative calibrated loops in transfer of bronchoalveolar lavage fluid. J Clin Microbiol. 2000;38:2117–2121. doi: 10.1128/jcm.38.6.2117-2121.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jacobs J A, De Brauwer E I, Ramsay G, Cobben N A, Wagenaar S S, van der Ven A J, Bruggeman C A, Drent M. Detection of non-infectious conditions mimicking pneumonia in the intensive care setting: usefulness of bronchoalveolar fluid cytology. Respir Med. 1999;93:571–578. doi: 10.1016/s0954-6111(99)90157-9. [DOI] [PubMed] [Google Scholar]
- 10.Jalava K M, Olkkola K T, Neuvonen P J. Itraconazole greatly increases plasma concentrations and effects of felodipine. Clin Pharmacol Ther. 1997;61:410–415. doi: 10.1016/S0009-9236(97)90191-0. [DOI] [PubMed] [Google Scholar]
- 11.Kwon-Chung K J, Bennett J E. Phaeohyphomycosis. In: Kwon-Chung K J, Bennett J E, editors. Medical mycology. Philadelphia, Pa: Lea & Febiger; 1992. pp. 620–677. [Google Scholar]
- 12.Laumaillé C, Le Gall F, Degeilh B, Gueho E, Huerre M. Cutaneous Alternaria infectoria infection after liver transplantation. Ann Pathol. 1998;18:192–194. [PubMed] [Google Scholar]
- 13.McGinnis M R. Chromoblastomycosis and phaeohyphomycosis: new concepts, diagnosis, and mycology. J Am Acad Dermatol. 1983;8:1–16. doi: 10.1016/s0190-9622(83)70001-0. [DOI] [PubMed] [Google Scholar]
- 14.Morrison V A, Haake R J, Weisdorf D J. The spectrum of non-Candida fungal infections following bone marrow transplantation. Medicine. 1993;72:78–89. doi: 10.1097/00005792-199303000-00002. [DOI] [PubMed] [Google Scholar]
- 15.Ramos A M, de O. Sales A, de Andrade M C, Bittencourt J F, Ramos C C. A simple method for detecting subcutaneous phaeohyphomycosis with light-colored fungi. A study of eight cases. Am J Surg Pathol. 1995;19:109–114. doi: 10.1097/00000478-199501000-00013. [DOI] [PubMed] [Google Scholar]
- 16.Rees J R, Pinner R W, Hajjeh R A, Brandt M E, Reingold A L. The epidemiological features of invasive mycotic infections in the San Francisco Bay area, 1992–1993: results of population-based laboratory active surveillance. Clin Infect Dis. 1998;27:1138–1147. [PubMed] [Google Scholar]
- 17.Rinaldi M G. Phaeohyphomycosis. Dermatol Clin. 1996;14:147–153. doi: 10.1016/s0733-8635(05)70335-1. [DOI] [PubMed] [Google Scholar]
- 18.Roosje P J, de Hoog G S, Koeman J P, Willemse T. Phaeohyphomycosis in a cat caused by Alternaria infectoria E. G Simmons Mycoses. 1993;36:451–454. doi: 10.1111/j.1439-0507.1993.tb00740.x. [DOI] [PubMed] [Google Scholar]
- 19.Rosen T. Debilitating edema associated with itraconazole therapy. Arch Dermatol. 1994;130:260–261. [PubMed] [Google Scholar]
- 20.Schell W A. New aspects of emerging fungal pathogens. Clin Lab Med. 1995;15:365–387. [PubMed] [Google Scholar]
- 21.Sharkey P K, Graybill J R, Rinaldi M G, Stevens D A, Tucker R M, Peterie J D, Hoeprich P D, Greer D L, Frenkel L, Counts G W, et al. Itraconazole treatment of phaeohyphomycosis. J Am Acad Dermatol. 1990;23:577–586. doi: 10.1016/0190-9622(90)70259-k. [DOI] [PubMed] [Google Scholar]
- 22.Simmons E G. Alternaria themes and variations (22–26) Mycotaxon. 1986;25:287–308. [Google Scholar]
- 23.Simmons E G. Alternaria themes and variations (73) Mycotaxon. 1993;48:109–140. [Google Scholar]
- 24.Simmons E G. Alternaria themes and variations (106–111) Mycotaxon. 1994;50:409–427. [Google Scholar]
- 25.Vartivarian S H, Anaissie E L, Bodey G P. Emerging fungal pathogens in immunocompromised patients: classification, diagnosis, and management. Clin Infect Dis. 1993;17:S487–S491. doi: 10.1093/clinids/17.supplement_2.s487. [DOI] [PubMed] [Google Scholar]
- 26.Ziefer A, Connor D H. Phaeomycotic cyst. A clinicopathologic study of twenty-five patients. Am J Trop Med Hyg. 1980;29:901–911. [PubMed] [Google Scholar]