Abstract
Refinements in surgery, radiation therapy, and chemotherapy since the mid-20th century have resulted in a survival rate exceeding 90% for patients with Wilms tumor (WT). Although this figure is remarkable, a significant proportion of patients continue to have event-free survival (EFS) estimates of <75%, and nearly 25% of survivors experience severe chronic medical conditions. The first-generation Children’s Oncology Group (COG) renal tumor trials (AREN ‘0’), which opened to enrollment in 2006, focused on augmenting treatment regimens for WT subgroups with predicted EFS <75% to 80%, including those with the adverse prognostic marker of combined loss of heterozygosity (LOH) at chromosomes 1p/16q, pulmonary metastasis with incomplete lung nodule response after 6 weeks of chemotherapy, bilateral disease, and anaplastic histology. Conversely, therapy was reduced for patient subgroups with good outcomes and potential for long-term toxicity, such as those with lung metastasis with complete lung nodule response after 6 weeks of chemotherapy. This article summarizes the key findings of the first-generation COG renal tumor studies and their implications for clinical practice.
Background
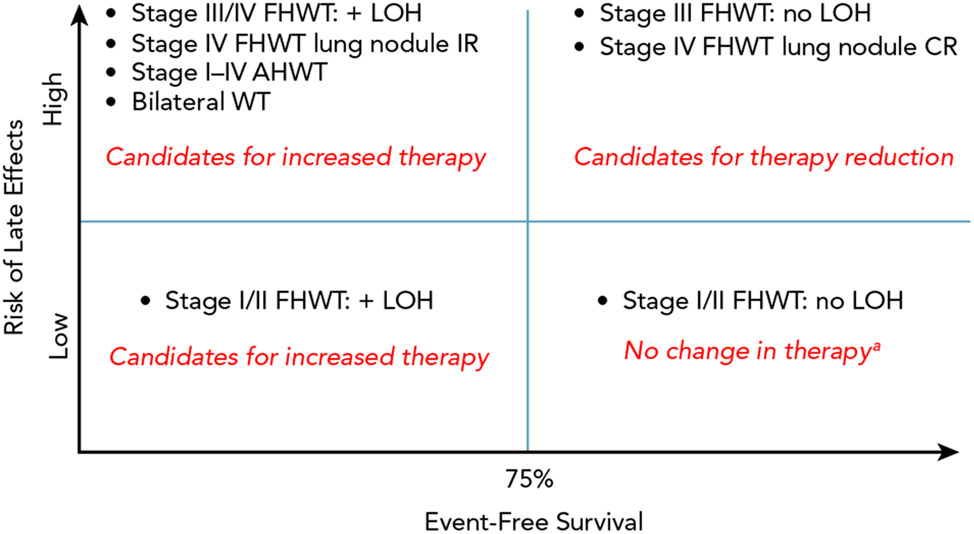
Clinical trials conducted over the past 50 years by the National Wilms Tumor Study Group (NWTSG), the International Society of Pediatric Oncology Renal Tumor Study Group (SIOP-RTSG), and others have resulted in survival rates exceeding 90% for patients with Wilms tumor (WT).1,2 Although the overall survival (OS) figure is remarkable, a significant proportion of patients have event-free survival (EFS) estimates of <75%, and nearly 25% of survivors experience severe chronic medical conditions.3,4 The mission of the Children’s Oncology Group (COG) Renal Tumor Committee, the successor to the NWTSG, is to improve cure rates for patients with pediatric renal tumors while minimizing treatment-related toxicities. To generate the scientific priorities for the first generation of COG studies (AREN ‘0’), which opened to enrollment in 2006, we classified WT subgroups according to expected EFS and potential for long-term treatment-related toxicities (Figure 1). The committee considered whether EFS or OS provides a more clinically meaningful endpoint for improvement. After careful deliberation, the committee determined that avoiding relapses should take top priority for subgroups with expected EFS <75% to 80%, because (1) the salvage rate for previously treated, relapsed, favorable-histology WT (FHWT) was only 50% to 80% depending on the initial therapy, and improving EFS would translate to improved OS; (2) patients who require salvage therapy are at greatest risk for long-term adverse effects, and avoiding relapses would avert the need for highly intensive treatment; and (3) relapse takes a significant psychosocial and economic toll on patients and families. The committee consensus was that a 20% to 25% event rate would justify therapy augmentation, recognizing that some patients would be exposed to unnecessary treatment.
Figure 1. Classification of WT subsets according to potential for late effects and event-free survival at the beginning of the AREN ‘0’ generation of studies.
Abbreviations: AHWT, anaplastic histology Wilms tumor; CR, complete response; FHWT, favorable histology Wilms tumor; IR, incomplete response; LOH, combined loss of heterozygosity at chromosomes 1p and 16q; WT, Wilms tumor.
aExcept for patients with very low risk WT, as described in the text, who were candidates to receive no adjuvant therapy.
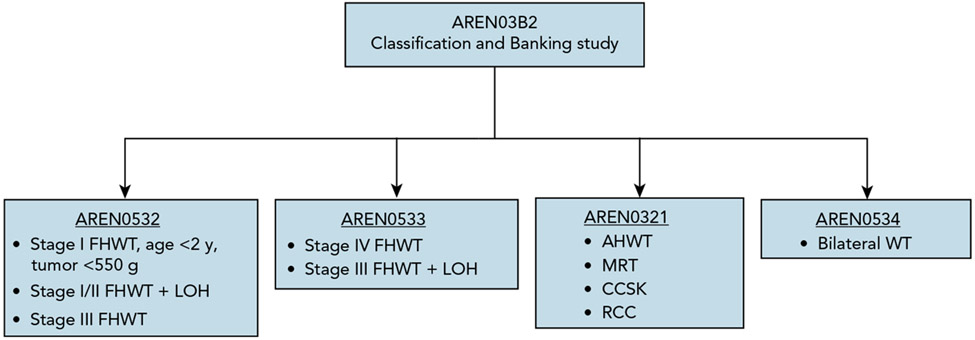
The structure of the AREN ‘0’ studies is depicted in Figure 2. An expanded menu of biologic and clinical prognostic factors, including loss of heterozygosity (LOH) at chromosomes 1p and 16q and completeness of lung nodule response after 6 weeks of chemotherapy, facilitated a more precise approach to therapy augmentation and reduction.
Figure 2. Organization of the first-generation COG AREN ‘0’ Renal Tumor Studies. The AREN03B2 Classification and Banking study served as a required gateway of entry to 1 of 4 frontline therapeutic studies.
Abbreviations: AHWT, anaplastic histology Wilms tumor; CCSK, clear cell sarcoma of the kidney; FHWT, favorable histology Wilms tumor; LOH, combined loss of heterozygosity at chromosomes 1p and 16q; MRT, malignant rhabdoid tumor; RCC, renal cell carcinoma; WT, Wilms tumor.
This article summarizes the key findings for WT and the implications for clinical practice in institutions that follow the NWTSG/COG treatment approach. We recognize that the complex risk classification schema and treatment regimens that were used are not achievable in all global settings and that clinical practice guidelines must be informed by the local context.
AREN03B2
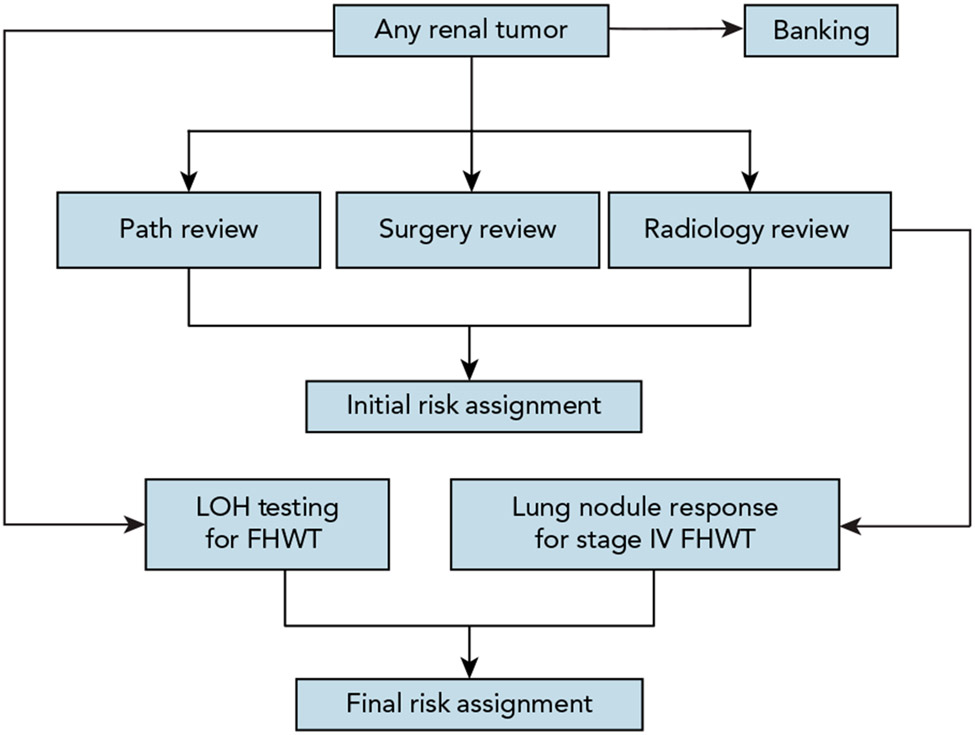
The AREN03B2 Renal Tumor Biology and Risk Stratification Protocol opened in 2006 with a primary goal to provide real-time risk stratification to enable appropriate enrollment of patients onto therapeutic trials, and to build a well-annotated tumor bank to enable basic and translational research. Submission of imaging studies, pathology slides, operative notes, and pathology reports was required within 14 days of initial surgical procedure for provision of a real-time initial risk assignment (IRA). Risk assignments were provided through rapid review by an expert panel of radiologists, pathologists, surgeons, and oncologists. Final risk assignments were issued for patients with FHWT based on presence or absence of combined LOH of 1p and 16q, and response of pulmonary nodules to 2 cycles of chemotherapy (Table 1, Figure 3).
Table 1.
Risk Stratification Schema for First-Generation COG Renal Tumor Studies
| Stage | Histology | Age | Tumor Weight | LOH 1p/16q |
Lung Nodule Response | Extrapulmonary Metastases | Final Study |
|---|---|---|---|---|---|---|---|
| I | FH | <2 y ≥2 y |
≥550 g Any |
No No |
N/A N/A |
N/A | None |
| II | FH | Any | Any | No | N/A | N/A | None |
| I | FH | <2 y | <550 g | Any | N/A | N/A | AREN0532 |
| I/II | FH | Any | Any | Yes | N/A | N/A | AREN0532 |
| III | FH | Any | Any | No | N/A | N/A | AREN0532 |
| IV | FH | Any | Any | No | CR | No | AREN0533 |
| III/IV | FH | Any | Any | Yes | N/A | Any | AREN0533 |
| IV | FH | Any | Any | Any | IR | Any | AREN0533 |
| IV | FH | Any | Any | Any | Any | Yes | AREN0533 |
| I | DA FA |
Any | Any | Any | N/A | N/A | AREN0321 |
| II–III | FA | Any | Any | Any | N/A | N/A | AREN0321 |
| II | DA | Any | Any | Any | Any | N/A | AREN0321 |
| III | DA | Any | Any | Any | Any | N/A | AREN0321 |
| IV | DA FA |
Any | Any | Any | Any | Any | AREN0321 |
| V | Any | Any | Any | Any | Any | Any | AREN0534 |
Abbreviations: CR, complete response; DA, diffuse anaplasia; FA, focal anaplasia; FH, favorable histology; IR, incomplete response; LOH, loss of heterozygosity at both 1p and 16q; N/A, not available.
Figure 3. Risk classification and banking flow diagram for AREN03B2.
Abbreviations: FHWT, favorable histology Wilms tumor; LOH, loss of heterozygosity testing for chromosomes 1p and 16q.
AREN03B2 remains open; 6,686 patients have enrolled as of February 2021, for an average enrollment of 485 patients per year during times of active accrual. Peak enrollment of 558 patients per year occurred from 2006 through 2014, when the first-generation therapeutic studies were open. The process of real-time review with timely assignment of IRA was feasible, with a median of 12 days from initial surgical procedure to IRA during the therapeutic study era. The study identified discrepancies in central review of imaging, histologic diagnosis, or stage that impacted IRA in 10% of patients. Among patients who received an IRA by September 2020, 91.3% were determined to have unilateral and 8.7% bilateral disease. Among those with unilateral disease, 82% had FHWT (21% stage I, 24% stage II, 33% stage III, and 22% stage IV), 5.8% had anaplastic WT (AWT), 5% diffuse AWT, and 0.8% focal AWT.
Impact on Clinical Practice
The rate of discrepancies between institutional and central reviews highlights the benefit for patients to receive expert review as part of this classification study.
AREN0532
Protocol AREN0532, open to enrollment from 2006 through 2013, included patients with very low risk (VLR) FHWT, stage I/II FHWT with LOH at chromosomes 1p and 16q, and stage III FHWT.
Very Low Risk FHWT
The previous North American standard of care for stage I FHWT was an 18-week regimen of vincristine/dactinomycin (EE4A).5 AREN0532 tested whether a defined group of VLR FHWT (age <2 years, tumor weight <550 g, stage I disease with a negative lymph node biopsy) could be managed with nephrectomy alone. The strategy of observation alone was previously tested in NWTS-5 but triggered a conservative stopping rule based on the assumption that only half of patients could be salvaged. In fact, 91% of patients were salvaged, and follow-up demonstrated no late events.6,7 Of note, 3 of the 11 recurrences were metachronous. AREN0532 therefore retested observation alone in VLR FHWT with the important exclusions of predisposition syndromes (with the intent to avoid metachronous recurrences) and lack of lymph node sampling. AREN0532 demonstrated an excellent outcome with a high salvage rate for patients with disease relapse using a standardized regimen (DD4A; doxorubicin/vincristine/dactinomycin) and radiation therapy (RT) (Table 2).8 A survey of reasons for an observed lower-than-expected accrual rate identified parental concern about the risk for relapse and increased-intensity salvage chemotherapy.9 Molecular analysis demonstrated the association of LOH at 11p15 and loss of imprinting at 11p15, which occurs much less commonly than LOH, with an increased risk of relapse.8,10,11
Table 2.
Treatment Regimens and Outcomes for First-Generation COG AREN ‘0’ Studies and Comparative Data From NWTS-5
| AREN ‘0’ |
NWTS-5 |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Stage/Histology | Study | Chemotherapy | Radiation | Number Treated |
4-y EFS (95% CI) |
4-y OS (95% CI) |
4-y EFS (95% CI) |
4-y OS (95% CI) |
References |
| Stage I FH (age <24 mo, tumor weight <550 g) | AREN0532 | Nephrectomy alone | None | 116 | 89.7% (84.1%–95.2%) | 100% | 84%a (73%–91%) | 98%a (87%–99%) | 7,8 |
| Stage I or II FH; + LOH | AREN0532 | DD4Ab | None | 32 | 87.3% (75.1%–99.5%) | 100% | 68.8% (55.2%–82.3%) | 91.6% (83.6%–99.6%) | 19 |
| Stage III FH; no LOH | AREN0532 | DD4Ab | Flankc | 533 | 88.0% (85.0%–91.0%) | 97.0% (95.0%–98.0%) | 86.5% (83.8%–89.1%) | 94.5% (92.8%–96.3%) | 13 |
| Stage III or IV FH; + LOH | AREN0533 | Md |
|
51 | 90.2% (81.7%–98.6%) | 96.1% (90.5%–100%) | 61.3% (44.9%–77.6%) | 86.0% (74.5%–97.5%) | 19 |
| Stage IV FH with lung metastasis only and CR at 6 wk; no LOH | AREN0533 | DD4Ab |
|
124 | 79.5% | 96.1% | NA | NA | 18 |
| Stage IV FH with lung metastasis only and incomplete response at 6 wk; no LOH | AREN0533 | Md |
|
131 | 88.5% | 95.4% | NA | NA | 18 |
| Stage V FH | AREN0534 | Per local stage | Per local stage | 189 | 82.1% | 94.9% | NA | NA | 23 |
| Stage I FA + DA | AREN0321 | DD4Ab | Flank | 18 | 100% | 100% | 70.0% (51.7%–88.2%) | 81.5% (66.1%–96.9%) | 26 |
| Stage II DA | AREN0321 | UH-1e | Flank | 15 | 86.7% (68.8%–100%) | 86.2% (68.0%–100%) | 79.2% (60.9%–97.5%) | 78.4% (60.0%–96.9%) | 28 |
| Stage III DA | AREN0321 | UH-1e | Flankc | 27 | 80.9% (65.8%–96.0%) | 88.6% (76.4%–100%) | 61.3% (47.8%–74.7%) | 64.7% (51.6%–77.8%) | 28 |
| Stage IV DA | AREN0321 | UH-1e /UH-2f |
|
24 | 41.7% (19.6%–63.7%) | 49.2% (27.5%–71.0%) | 32.1% (14.8%–49.4%) | 32.1% (14.8%–49.4%) | 28 |
| Stage V DA | AREN0534 | Per local stage |
|
17 | 58.2% (15.7%–100%) | 68.4% (24.9%–100%) | 25.1% (5.9%–51.0%) | 41.6% (19.7%–62.2%) | 23,25 |
| Unilateral WT with bilateral predisposition | AREN0534 | EE4Ag followed by treatment per stage/histology | Per local stage | 34 | 94% (85.2%–100%) | 100% | NA | NA | 24 |
Abbreviations: COG, Children’s Oncology Group; CR, complete response; DA, diffuse anaplasia; EFS, event-free survival; FA, focal anaplasia; FH, favorable histology; LOH, loss of heterozygosity at both 1p and 16q; NA, no applicable comparison group; OS, overall survival; WLI, whole lung irradiation; WT, Wilms tumor.
Reported as 5-year EFS and OS.
DD4A: vincristine/dactinomycin/doxorubicin × 25 wk; cumulative doxorubicin, 150 mg/m2.
Whole-abdomen radiation therapy indicated for diffuse intraperitoneal tumor rupture, peritoneal tumor seeding, and cytology-positive ascites.
M: vincristine/dactinomycin/doxorubicin/cyclophosphamide/etoposide × 31 wk; cumulative doxorubicin, 195 mg/m2; cumulative cyclophosphamide, 8.8 g/m2; cumulative etoposide, 2,000 mg/m2.
Revised UH-1: vincristine/doxorubicin/cyclophosphamide/carboplatin/etoposide × 30 wk; cumulative doxorubicin, 225 mg/m2; cumulative cyclophosphamide, 14.8 g/m2; cumulative etoposide, 2,000 mg/m2.
Revised UH-2: revised UH-1 with vincristine/irinotecan × 36 wk.
EE4A: vincristine/dactinomycin × 19 wk.
Impact on Clinical Practice
Observation alone in a defined group of patients with VLR FHWT, especially in the absence of adverse biomarkers, is a reasonable option that avoids the risks of central line placement and chemotherapy.
Stage III FHWT
AREN0532 collected clinicopathologic information, including biomarkers, but did not change standard therapy (regimen DD4A plus RT) for patients with stage III FHWT.12 Patients with combined LOH 1p/16q or anaplasia at delayed nephrectomy were excluded. The study demonstrated very good EFS and OS (Table 2).13 Positive or unknown lymph node status, positive LOH 1p or 16q, and blastemal predominant histology at delayed nephrectomy were identified as adverse prognostic factors. Patients with both positive lymph nodes and LOH at either 1p or 16q had 4-year EFS of only 73.8%.
Impact on Clinical Practice
Regimen DD4A plus RT remains the standard of care for patients with stage III FHWT except if they have combined LOH 1p and 16q (see “Augmentation of Therapy for Adverse Biomarkers”). Increasing chemotherapy for patients with positive lymph nodes and LOH of either 1p or 16q is not considered standard of care. Augmented chemotherapy with established treatment regimens may be considered after careful discussion of the risks and benefits.
AREN0533
Protocol AREN0533, open to accrual from 2007 through 2013, included patients with stage IV FHWT and stage III FHWT with LOH at chromosomes 1p and 16q.
Patients with stage IV FHWT have inferior outcomes compared with those with localized disease.14 Additionally, their treatment is complicated by risks for significant late effects, including cardiac dysfunction and pulmonary toxicity. The treatment of FHWT with lung metastases on NWTS-5 included regimen DD4A and lung RT, with a cumulative anthracycline exposure of 150 mg/m2. The SIOP 93-01 trial produced comparable results without lung RT for those with lung nodule complete response (CR) achieved with chemotherapy or surgical resection, although the cumulative anthracycline dose was 350 mg/m2.15 A subanalysis of the SIOP 93-01 and SIOP-2001 populations found that lung nodule incomplete response after 6 weeks of chemotherapy was an adverse prognostic indicator. Based on these observations, AREN0533 applied a new risk stratification and treatment strategy for patients with stage IV FHWT. Patients with isolated lung metastases—defined for the first time by the presence of nodules on centrally reviewed CT scans rather than chest radio-graph16,17—who achieved lung nodule CR after 6 weeks of DD4A continued the same chemotherapy without lung RT. Patients with lung nodule incomplete response or the presence of LOH 1p/16q received lung RT and switched to regimen M, which added 4 cycles of cyclophosphamide/etoposide in addition to vincristine/dactinomycin/doxorubicin. Patients with extrapulmonary metastases also received regimen M and RT to all metastatic sites, regardless of chemotherapy response.
The overall AREN0533 strategy was successful, with 4-year EFS and OS for patients with isolated lung metastases of 85.4% and 95.6%, respectively, compared with 72.5% and 84.0% for patients on NWTS-5.18 A total of 46% of assessable patients had lung nodule CR and 54% had incomplete response. For patients with incomplete response, EFS and OS were outstanding with regimen M (Table 2). For patients with CR, there were more events than expected without lung RT, but the OS was excellent (Table 2). A post hoc analysis revealed that 4-year EFS was only 57% in patients with 1q gain and lung nodule CR who were treated with DD4A without lung RT. Of 11 relapses in this patient group, 9 were pulmonary. Analysis is underway for patients with extrapulmonary metastases.
Impact on Clinical Practice
Omission of lung RT is an acceptable strategy for patients with isolated pulmonary metastasis with lung nodule CR after 6 weeks of chemotherapy, except for those with combined LOH 1p/16q or 1q gain. Regimen M and lung RT provide a good standard of care for patients with lung nodule incomplete response, unless residual nodules are proven benign or necrotic by biopsy. The risks for long-term toxicity associated with regimen M should be part of the risk/benefit discussion.
Augmentation of Therapy for Adverse Biomarkers
LOH at 1p/16q
AREN0532 and AREN0533 were the first studies to describe the effect of augmentation of therapy for patients with combined LOH at chromosomes 1p and 16q. The rationale for the increase in therapy was based on NWTS-5, which demonstrated that in unilateral FHWT, combined LOH at 1p and 16q was associated with significantly inferior relapse-free survival and OS in both stage I/II and stage III/IV groupings.5 LOH 1p and 16q did not have prognostic significance for AWT or bilateral WT (BWT). AREN0532 therefore added doxorubicin to the standard vincristine/dactinomycin for stages I/II FHWT, and AREN0533 added cyclophosphamide/etoposide to the standard vincristine/dactinomycin/doxorubicin for stage III/IV FHWT. The augmentation of therapy in both groups resulted in improved EFS and trends toward improved OS (Table 2).19
Impact on Clinical Practice
Augmentation of therapy for patients with FHWT and combined LOH 1p/16q reduces the risk for relapse. The increased risks of augmented chemotherapy should be part of the risk/benefit discussion.
1q Gain
Gain of 1q has been more recently investigated as a prognostic marker.20 In the NWTS-5 cohort, 1q gain was present in 28% of patients and was associated with inferior EFS within all disease stages, and significantly inferior OS in stages I (90% vs 98%; P=.0015) and IV (74% vs 92%; P=.0110).21 There was a marked correlation between 1q gain and LOH 1p/16q. When 1q gain was present, the independent prognostic significance of LOH 1p/16q disappeared, but when 1q gain was absent, LOH 1p/16q remained associated with inferior outcomes.21 The adverse prognostic significance of 1q gain was also demonstrated in the setting of preoperative chemotherapy.22
Although the first-generation COG protocols did not change therapy based on 1q gain status, 1q gain was retrospectively evaluated in patients with stage IV FHWT and lung nodules. As described earlier in “AREN0533,” patients with 1q gain and lung nodule CR who were treated without lung RT had 4-year EFS of only 57%, whereas those with 1q gain and lung nodule incomplete response who received regimen M and lung RT had 4-year EFS of 86%.18 Historically, patients with stage IV FHWT with 1q gain treated with DD4A and RT to metastatic sites had 4-year EFS of 64%,21 suggesting that regimen M may mitigate the effect of this negative biomarker. Analysis is underway for patients with extrapulmonary metastases.
Impact on Clinical Practice
Augmenting chemotherapy based solely on 1q gain is not considered standard of care, but may be considered for high-stage FHWT after careful discussion of the risks and benefits.
AREN0534
Protocol AREN0534, open to enrollment from 2009 through 2015, included patients with BWT, unilateral WT in the setting of a predisposition to BWT, and diffuse hyperplastic perilobar nephroblastomatosis (DHPLN).23 This was the first prospective clinical trial for BWT. The results for the bilateral and bilaterally predisposed arms have been reported and analysis of the DHPLN arm is underway.
Bilateral WT
The primary aims for BWT were to improve 4-year EFS to 73%, to prevent complete removal of at least one kidney in 50% of patients by using prenephrectomy chemotherapy with vincristine/dactinomycin/doxorubicin, and to have at least 75% of children undergo definitive surgical treatment by 12 weeks after initiation of chemotherapy. Postsurgical chemotherapy was based on histology and postsurgical stage. The 4-year EFS for 189 evaluable patients with BWT was 82.1%, indicating that the interventions were effective (Table 2).23 A total of 39% of patients were able to undergo bilateral nephron-sparing surgery and only 1% (n=2) became anephric; 84% of patients underwent definitive surgical treatment (partial or complete nephrectomy or wedge resection in at least one kidney) by 12 weeks after initiation of chemotherapy.
Impact on Clinical Practice
A treatment approach including standardized 3-drug preoperative chemotherapy, surgical resection within 12 weeks of diagnosis, and histology-based postoperative therapy provides a good standard of care for BWT.
Bilaterally Predisposed Unilateral WT
The aim of this stratum was to facilitate partial nephrectomy in lieu of nephrectomy in 25% of children with unilateral WT and bilateral predisposition (syndromic features, multicentric tumors) by using prenephrectomy chemotherapy with vincristine/dactinomycin. Postoperative therapy was based on stage and histology. Among the 34 patients evaluable in this stratum, 4-year EFS and OS were 94% and 100%, respectively; prenephrectomy chemotherapy facilitated renal preservation in 22 patients (65%).24
Impact on Clinical Practice
Preoperative chemotherapy to facilitate partial nephrectomy provides a reasonable standard of care for patients with unilateral WT with predisposition for metachronous bilateral disease.
AREN0321
Protocol AREN0321, open to accrual from 2006 through 2012, included patients with focal AWT, diffuse AWT, clear cell sarcoma of the kidney, malignant rhabdoid tumor, and renal cell carcinoma. This review focuses solely on outcomes for AWT.
AWT Stage I
In NWTS-5, the 4-year EFS and OS estimates for 29 patients with stage I AWT (10 focal and 19 diffuse) treated with vincristine/dactinomycin without flank radiation were 69.5% and 82.6%, respectively.25 Because disease relapses occurred in both the abdomen and distant sites, both doxorubicin (cumulative dose, 150 mg/m2) and flank radiation (10.8 Gy) were incorporated into the treatment regimen in AREN0321. The 4-year EFS and OS for 18 patients with stage I AWT (8 focal and 10 diffuse) treated on AREN0321 were 100%.26 A retrospective analysis of 112 patients with stage I AWT treated on NWTS 1–5 and AREN0321 protocols showed that the EFS was significantly improved with doxorubicin treatment, but not by flank radiation. The rate of local recurrence was low (3.6%) and occurred only in patients with diffuse AWT. Among those patients, rates of local recurrence were similar for patients who received (4.0%) or did not receive flank radiation (6.2%).26
Impact on Clinical Practice
The addition of doxorubicin to vincristine and dactinomycin is recommended for the treatment of stage I AWT. The use of flank radiation is not needed for focal AWT but warrants a discussion of risks versus benefits for diffuse AWT.
AWT Stages II–IV
In NWTS-3 and NWTS-4, the addition of cyclophosphamide to vincristine/dactinomycin/doxorubicin improved the 4-year relapse-free survival for patients with stages II–IV diffuse AWT.27 In NWTS-5, further augmentation of therapy using a regimen of vincristine/doxorubicin/cyclophosphamide alternating with cyclophosphamide/etoposide for 24 weeks plus RT (regimen I) resulted in additional improvement in patient outcomes.25 However, the survival outcomes were still unsatisfactory and 46% of the recurrences were local. AREN0321 was designed to achieve further gains in survival outcomes with continued augmentation of therapy.
AREN0321 showed that the combination of vincristine and irinotecan was well tolerated and highly active in patients with diffuse AWT. The response rate was 79% in 14 patients with measurable metastatic diffuse AWT who received the combination in an up-front window.28 In addition, AREN0321 evaluated an intensive regimen that used carboplatin in addition to the regimen I agents (regimen UH-1) for the treatment of stages II–IV diffuse AWT, with the incorporation of vincristine/irinotecan in patients with stage IV disease responding to treatment (regimen UH-2) and a higher radiation dose to the flank (20 Gy) for local stage III diffuse AWT. This treatment appeared to improve relapse-free survival for stage II–IV diffuse AWT compared with NWTS-5, but with increased toxicity, necessitating modification of the regimen part way through the study to reduce the doxorubicin, cyclophosphamide, and etoposide exposure (“revised” UH-1 and UH-2). Overall, 4 patients (6%) died of cardiac and pulmonary toxicity.28
Impact on Clinical Practice
Revised regimens UH-1 and UH-2 appear to reduce the risk of relapse for patients with stage II–IV diffuse AWT and provide a reasonable standard of care. If these regimens are used, measures should be taken to mitigate toxicity, such as using the cardioprotectant dexrazoxane and reducing the radiation dose (10 Gy) to the whole abdomen, especially in infants.
Future Directions
The COG Renal Tumor Committee plans to continue to refine the risk stratification schema by incorporating the new biomarkers of 1q gain for all stages of FHWT and 11p15 LOH for stage I FHWT to determine eligibility for treatment consisting of surgery only. Additionally, postchemotherapy histology for those who undergo delayed nephrectomy will be used to determine postoperative treatment. Patients with adverse biology will receive new treatment regimens that limit exposure to anthracyclines and alkylating agents. Patients with favorable prognostic factors will be candidates for therapy reduction, such as omission of doxorubicin for some with stage III FHWT. Radiation regimens will be refined, including use of cardiac-sparing intensity-modulated lung RT, to further improve the quality of life and overall survival of children with WT.
Acknowledgments
Children’s Oncology Group studies were sponsored by grants from the NIH (U10CA180886, U10CA180899, U10CA098543, U10CA098413, and U24CA114766). The authors are indebted to our fellow members of the COG Renal Tumor Committee, the many health professionals who supported the studies, and the parents and children who participated and helped advance the field.
Footnotes
Disclosures: The authors have disclosed that they have no financial interests, arrangements, or affiliations with the manufacturers of any products discussed in this article or their competitors.
Disclaimer: The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
References
- 1.Nakayama DK, Bonasso PC. The history of multimodal treatment of Wilms’ tumor. Am Surg 2016;82:487–492. [PubMed] [Google Scholar]
- 2.Dome JS, Graf N, Geller JI, et al. Advances in Wilms tumor treatment and biology: progress through international collaboration. J Clin Oncol 2015; 33:2999–3007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dome JS, Fernandez CV, Mullen EA, et al. Children’s Oncology Group’s 2013 blueprint for research: renal tumors. Pediatr Blood Cancer 2013;60: 994–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Termuhlen AM, Tersak JM, Liu Q, et al. Twenty-five year follow-up of childhood Wilms tumor: a report from the Childhood Cancer Survivor Study. Pediatr Blood Cancer 2011;57:1210–1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grundy PE, Breslow NE, Li S, et al. Loss of heterozygosity for chromosomes 1p and 16q is an adverse prognostic factor in favorable-histology Wilms tumor: a report from the National Wilms Tumor Study Group. J Clin Oncol 2005;23:7312–7321. [DOI] [PubMed] [Google Scholar]
- 6.Green DM, Breslow NE, Beckwith JB, et al. Treatment with nephrectomy only for small, stage I/favorable histology Wilms’ tumor: a report from the National Wilms’ Tumor Study Group. J Clin Oncol 2001;19:3719–3724. [DOI] [PubMed] [Google Scholar]
- 7.Shamberger RC, Anderson JR, Breslow NE, et al. Long-term outcomes for infants with very low risk Wilms tumor treated with surgery alone in National Wilms Tumor Study-5. Ann Surg 2010;251:555–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fernandez CV, Perlman EJ, Mullen EA, et al. Clinical outcome and biological predictors of relapse after nephrectomy only for very low-risk Wilms tumor: a report from Children’s Oncology Group AREN0532. Ann Surg 2017;265:835–840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fernandez CV, Li N, Mullen EA, et al. Barriers to the enrollment of children in the Children’s Oncology Group study of very low risk Wilms tumor: a report from the Children’s Oncology Group. J Pediatr Hematol Oncol 2011;33:521–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sredni ST, Gadd S, Huang CC, et al. Subsets of very low risk Wilms tumor show distinctive gene expression, histologic, and clinical features. Clin Cancer Res 2009;15:6800–6809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Perlman EJ, Grundy PE, Anderson JR, et al. WT1 mutation and 11P15 loss of heterozygosity predict relapse in very low-risk Wilms tumors treated with surgery alone: a Children’s Oncology Group study. J Clin Oncol 2011;29:698–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ehrlich PF, Anderson JR, Ritchey ML, et al. Clinicopathologic findings predictive of relapse in children with stage III favorable-histology Wilms tumor. J Clin Oncol 2013;31:1196–1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fernandez CV, Mullen EA, Chi YY, et al. Outcome and prognostic factors in stage III favorable-histology Wilms tumor: a report from the Children’s Oncology Group study AREN0532. J Clin Oncol 2018;36:254–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Green DM. The treatment of stages I-IV favorable histology Wilms’ tumor. J Clin Oncol 2004;22:1366–1372. [DOI] [PubMed] [Google Scholar]
- 15.Verschuur A, Van Tinteren H, Graf N, et al. Treatment of pulmonary metastases in children with stage IV nephroblastoma with risk-based use of pulmonary radiotherapy. J Clin Oncol 2012;30:3533–3539. [DOI] [PubMed] [Google Scholar]
- 16.Grundy PE, Green DM, Dirks AC, et al. Clinical significance of pulmonary nodules detected by CT and not CXR in patients treated for favorable histology Wilms tumor on national Wilms tumor studies-4 and -5: a report from the Children’s Oncology Group. Pediatr Blood Cancer 2012;59:631–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Smets AM, van Tinteren H, Bergeron C, et al. The contribution of chest CT-scan at diagnosis in children with unilateral Wilms’ tumour. Results of the SIOP 2001 study. Eur J Cancer 2012;48:1060–1065. [DOI] [PubMed] [Google Scholar]
- 18.Dix DB, Seibel NL, Chi YY, et al. Treatment of stage IV favorable histology Wilms tumor with lung metastases: a report from the Children’s Oncology Group AREN0533 study. J Clin Oncol 2018;36:1564–1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dix DB, Fernandez CV, Chi YY, et al. Augmentation of therapy for combined loss of heterozygosity 1p and 16q in favorable histology Wilms tumor: a Children’s Oncology Group AREN0532 and AREN0533 study report. J Clin Oncol 2019;37:2769–2777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gratias EJ, Jennings LJ, Anderson JR, et al. Gain of 1q is associated with inferior event-free and overall survival in patients with favorable histology Wilms tumor: a report from the Children’s Oncology Group. Cancer 2013;119:3887–3894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gratias EJ, Dome JS, Jennings LJ, et al. Association of chromosome 1q gain with inferior survival in favorable-histology Wilms tumor: a report from the Children’s Oncology Group. J Clin Oncol 2016;34:3189–3194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chagtai T, Zill C, Dainese L, et al. Gain of 1q As a prognostic biomarker in Wilms tumors (WTs) treated with preoperative chemotherapy in the International Society of Paediatric Oncology (SIOP) WT 2001 trial: a SIOP Renal Tumours Biology Consortium study. J Clin Oncol 2016;34:3195–3203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ehrlich P, Chi YY, Chintagumpala MM, et al. Results of the first prospective multi-institutional treatment study in children with bilateral Wilms tumor (AREN0534): a report from the Children’s Oncology Group. Ann Surg 2017;266:470–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ehrlich PF, Chi YY, Chintagumpala MM, et al. Results of treatment for patients with multicentric or bilaterally predisposed unilateral Wilms tumor (AREN0534): a report from the Children’s Oncology Group. Cancer 2020;126:3516–3525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dome JS, Cotton CA, Perlman EJ, et al. Treatment of anaplastic histology Wilms’ tumor: results from the fifth National Wilms’ Tumor Study. J Clin Oncol 2006;24:2352–2358. [DOI] [PubMed] [Google Scholar]
- 26.Daw NC, Chi YY, Kim Y, et al. Treatment of stage I anaplastic Wilms’ tumour: a report from the Children’s Oncology Group AREN0321 study. Eur J Cancer 2019;118:58–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Green DM, Beckwith JB, Breslow NE, et al. Treatment of children with stages II to IV anaplastic Wilms’ tumor: a report from the National Wilms’ Tumor Study Group. J Clin Oncol 1994;12:2126–2131. [DOI] [PubMed] [Google Scholar]
- 28.Daw NC, Chi YY, Kalapurakal JA, et al. Activity of vincristine and irinotecan in diffuse anaplastic Wilms tumor and therapy outcomes of stage II to IV disease: results of the Children’s Oncology Group AREN0321 study. J Clin Oncol 2020;38:1558–1568. [DOI] [PMC free article] [PubMed] [Google Scholar]