Abstract
Objective:
To evaluate the color stability of six esthetic archwires at different time periods and their fluorescence.
Materials and Methods:
Samples were evaluated after 7, 14, and 21 days of immersion in staining solution. Color measurements were performed by means of a spectrophotometer according to the Commission Internationale de I'Eclairage L*a*b* system, and color changes (ΔE*) and National Bureau of Standards units were computed. The fluorescence of as-received samples was evaluated by two observers and compared with that of a bovine central incisor. Statistical differences were investigated using analysis of variance and Tukey's post hoc test.
Results:
All brands showed statistically significant color change after 21 days (ΔE* from 1.88 to 12.06). The Optis archwire (fiber-reinforced composite) presented the highest color alteration, although staining was observed only near its ends. The Trianeiro archwire (coated nickel-titanium) and the Ortho Organizers archwire (coated stainless steel) presented with less color change. The Optis archwire was the only one that presented with fluorescence similar to that of bovine teeth.
Conclusions:
All esthetic archwires assessed showed clinically noticeable color change after 21 days in staining solution. The optical properties of currently available esthetic archwires may not yet be ideal.
Keywords: Color stability, Fluorescence, Esthetic archwires
INTRODUCTION
Appearance is one of patients' main concerns during orthodontic treatment. There is a growing demand for esthetic appliances, but most fixed orthodontic appliance components are metallic and silver in color.1
This problem has been partially solved by the introduction of esthetic brackets made of ceramic or composite, which are becoming more popular.2 However, most archwires are still made of metal such as stainless steel and nickel-titanium. A number of alternatives have been explored to create an esthetic archwire that would allow efficient orthodontic treatment from the labial aspect.3
Metallic archwires coated with tooth-colored polymers or inorganic materials and fiber-reinforced composite wires are currently the existing solutions to this esthetic problem.4 Materials used in archwire coatings are plastic resin materials such as synthetic fluorine-containing resin or epoxy resin composed mainly of polytetrafluoroethylyene to simulate tooth color.5 However, some authors have suggested that the color of coated archwires tends to change over time and that the coating splits during use in the mouth, exposing the underlying metal.1,6,7
Like other esthetic orthodontic products, there are internal and external causes for the discoloration of esthetic archwires. External discoloration can be caused by food dyes and colored mouth rinses. The type of coating material and its surface roughness play decisive roles in the extent of the discoloration caused by diverse substances. The amount of color change can be influenced by a number of factors, including oral hygiene and water absorption.8
Fluorescence is an optical property inherent in the natural tooth when irradiated by ultraviolet light.9 Anterior tooth restorations that do not present this property become dark when submitted to ultraviolet light, whether from daylight or in rooms with black light, resulting in esthetic disharmony. An ideal esthetic archwire should present fluorescence similar to that of human teeth.
No studies have been conducted to examine the color stability of these esthetic archwires in different time periods and their fluorescence. Therefore, this in vitro study was undertaken to analyze the fluorescence of coated archwires and fiber-reinforced composite wires and their color stability after exposure to common staining solution for 7, 14, and 21 days.
MATERIALS AND METHODS
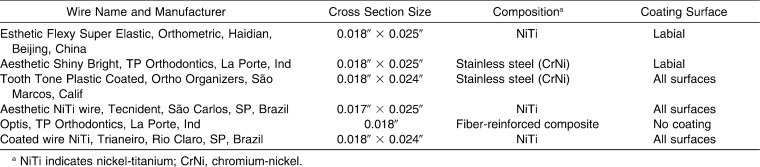
In this study, six brands of esthetic archwires were assessed. Based on the results of a pilot study, a sample size calculation showed that at least three specimens would be enough to detect a 10% difference with a significance level of 5% and a test power of 0.85. The brands, cross section size, composition, and coating surfaces are shown in Table 1, as described by the manufacturers.
Table 1. .
Characteristics of the Esthetic Archwires Used in the Study
Five samples of each brand were prepared. Each sample was made by placing 10-mm-long wire segments together and uniting their juxtaposed ends with ethyl cyanoacrylate (SuperBonder, Loctite Company, São Paulo, SP, Brazil). The esthetic coating surface of every wire segment was facing the same direction, and the total width of each sample had to be at least 7 mm so that its color could be properly measured.
Staining Solution Preparation
A staining coffee solution was prepared by pouring 500 mL of boiled distilled water over 15 g of coffee powder (Melitta Classic, Mellita, São Paulo, SP, Brazil). The solution was stirred every 30 minutes for 10 seconds until it cooled down to 37°C and then filtered through a paper filter (Melitta). This liquid mixture was then poured into a pot and kept in an incubator during the entire experiment. The solution was freshened once every 7 days. In addition, to reduce the precipitation of particles, the mixture was stirred once a day for 1 minute.
Color Measurements
Before the specimens were immersed into the solution, they were stored in distilled water at 37°C for 24 hours. After 24 hours of immersion (T0), the color of each sample was measured using the spectrophotometer VITA Easyshade Compact (VITA Zahnfabrik, Bad Säckingen, Germany, Model DEASYC220). After the first measurement (T0), the samples were placed in a container with the prepared staining coffee solution. Color measurements were repeated after 7 days (T1), 14 days (T2), and 21 days (T3) of immersion in the solution.
Before each measurement, samples were removed from the solution and rinsed with distilled water in an ultrasonic cleaning bath (Cristófoli Ltda, Campo Mourão, PR, Brazil) for 5 minutes. Excess water on the surfaces was removed with tissue papers, and the samples were allowed to dry.
Prior to performing the measurements, the spectrophotometer was calibrated according to the manufacturer's instructions. Five measurements of each of the five samples of each brand were made. The average value of these five readings of each sample was recorded by the examiner. Color changes were characterized using the Commission Internationale de I'Eclairage L*a*b* color space (CIE L*a*b*).10
Because visual color assessment is subjective, the color systems are quantitative systems with rectangular coordinates that allow an objective color measurement. These systems represent adequately the visual perception of color differences. Total color differences are expressed by the formula ΔE* = ([ΔL*]2 + [Δa*]2 + [Δb*]2)1/2, where ΔL*, Δa*, and Δb* are differences in L*, a*, and b* values before (T0) and after immersion at each time interval (T1, T2, T3).
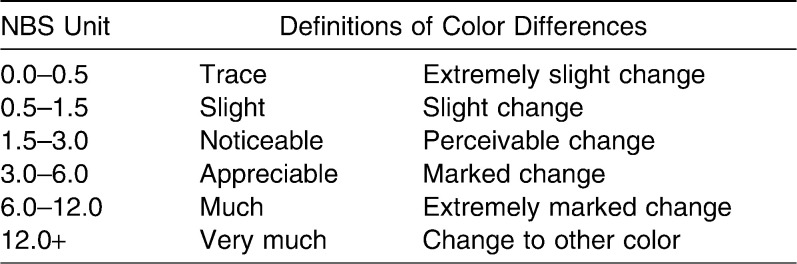
To relate the amount of color change (ΔE*) to a clinical environment, the data were converted to National Bureau of Standards (NBS) units11 as follows: NBS units = ΔE* × 0.92. The definitions of color changes quantified by NBS units were used. These values were suggested by Koksal and Dikbas12 and are shown in Table 2.
Table 2. .
Critical Marks of Color Change According to the National Bureau Standards
Because the Optis archwire is not coated and has a totally different composition than the other archwires tested, and because it showed the most staining, with a color change nearly twice as pronounced as that of the most stained coated archwire, it was investigated further. One archwire as received from the manufacturer and another archwire cut with a wire cutter 10 mm from its extremities were submitted to 21 days of immersion in the staining solution, as previously described. These samples were observed on a stereoscope, and one as-received sample and one cut tip were observed on a scanning electron microscope.
Fluorescence Assessment
The samples were randomly arranged in a dark atmosphere devoid of natural light. One fluorescent black lamp (G-Light, 40 W, 127 V, 60 Hz) was positioned 30 cm above the samples. To observe the fluorescence, each specimen was placed on the labial surface of a bovine incisor and surrounded by enamel for better visualization. A bovine central incisor was used because the specimens' dimensions were larger than those of a human incisor crown and because a bovine central incisor gives the same fluorescence as a human teeth.9 Two observers independently classified the samples according to fluorescence levels (high, medium, and low).
Statistics
After the ΔE* values were calculated with the described formula, descriptive statistical analyses were applied to each group at all times, and analysis of variance and Tukey's post hoc test were used to verify differences in color between groups and between times. Values were designated as statistically significant at P < .05. All statistical analyses were performed with SPSS software (16.0 version, SPSS Inc, Chicago, Ill).
RESULTS
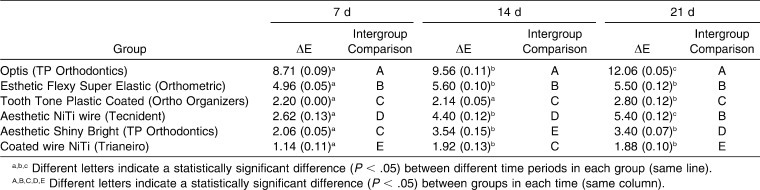
Table 3 shows the total color difference (ΔE*) of the esthetic archwires after 7 days, 14 days, and 21 days of immersion in the staining solution and intragroup and intergroup comparisons according to Tukey's post hoc test.
Table 3. .
Total Color Difference (ΔE*) and Intragroup (Between Time Periods) and Intergroup (in Each Time Period) Comparison Using Tukey Post Hoc Test
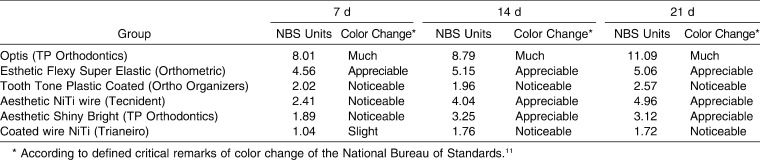
To relate the amount of color change (ΔE*) recorded by the spectrophotometer to the clinical environment, the data were converted into NBS units. The results of this conversion are shown in Table 4, and described in terms of the NBS-defined color differences.
Table 4. .
Color Change of Archwires Converted to NBS Units and Remark of Color Difference
All brands showed staining after 7 days, but at different intensities. Clinically apparent staining occurred in all brands after 21 days, except in Tooth Tone Plastic Coated (Ortho Organizers) and the Coated wire NiTi (Trianeiro).
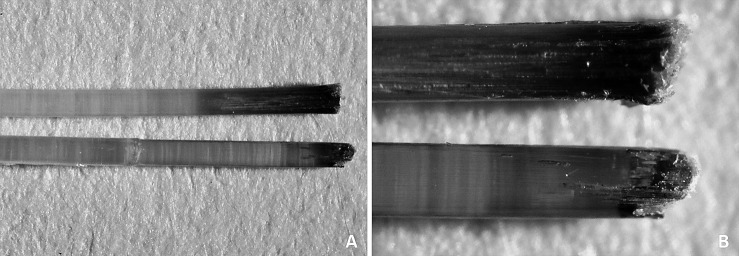
The Optis archwires (TP Orthodontics) that were immersed in the staining solution—both as received from the manufacturer and the one that was cut—both showed internal discoloration up to 3.8 mm and 1.9 mm from the wire ends, respectively. However, the intensity of staining of the archwire that had its extremities cut was greater (Figure 1).
Figure 1. .
Stereoscopic images of Optis archwire tips cut (upper) and as received (lower) after immersion in staining solution for 21 days. (A) At 12× magnification. (B) At 45× magnification.
The Optis archwire was the only one to present high fluorescence. The other archwires assessed were considered to present low fluorescence.
DISCUSSION
In addition to the color differences initially observed between the different existing esthetic archwires, the color stability of coated archwires during orthodontic treatment is also clinically important. In the current study, the color stability of these archwires could be reliably evaluated.
Ideally, the color of esthetic archwires should match that of natural teeth and esthetic brackets. However, the colors of natural teeth vary according to the color measurement protocols used and also by race, gender, and age.13,14
Generally, values in the range of one unit are considered exact color matches because they cannot be identified by independent observers.15 Since instrumental measurements eliminate the subjective interpretation of visual color comparison, spectrophotometers are used instead of visual evaluation.
Color changes were characterized using the CIE L*a*b* color space. The CIE L*a*b* color space is currently one of the most popular and widely used systems of color measurement, and it is well suited for the determination of small color differences.
Many authors12,16–18 have used ΔE* values to evaluate the “perceptibility” of color differences. However, it is noteworthy that the criteria for perceptibility adopted by each author were different. To counter such differences and disagreements in the criteria used, the NBS rating system is frequently used to determine the degree of color difference, since it offers absolute criteria by which ΔE* values can be converted to definitions with clinical significance.11,12
Some studies12,17,19 concluded that coffee was the most chromogenic agent in comparison with other staining substances, such as tea and cola drinks. For this reason, a coffee solution was used in this study to evaluate the effect of staining.
In the present study, the color changes of Optis (TP) (composite) and Tecnident (nickel-titanium) archwires intensified with a longer immersion period, while the Orthometric (nickel-titanium), Aesthetic Shiny Bright (TP) (stainless steel), and Trianeiro (nickel-titanium) wires showed a significant color change between 7 and 14 days and were stable thereafter (Table 3). When NBS values were evaluated after the 3-week immersion period, “extremely marked change” was observed in Optis archwires and “perceivable change” was seen in the Ortho Organizers (chromium-nickel) and Trianeiro archwires (Table 4).
Arthur et al.20 suggested that changes in the optical properties within a polymer could be responsible for the color changes seen clinically. They stated that chemical discoloration was caused by the oxidation of unreacted double bonds in the matrix of the polymer and the subsequent formation of degradation products from water diffusion or the oxidation of the polymer. This could explain the staining behavior of coated archwires over time.
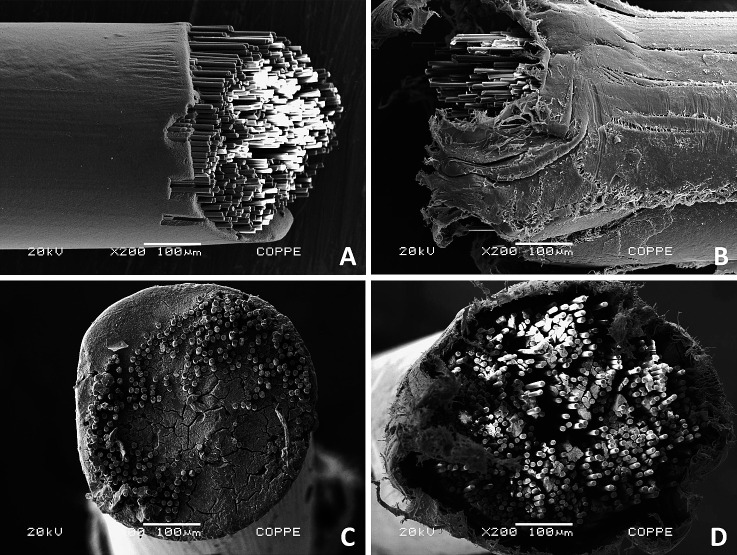
The fiber-reinforced composite wires (Optis) showed the lowest color stability at all evaluation periods. This could be explained by the difference in its composition vs the other wires evaluated. The cutting action performed on the Optis archwire for the setting up of the samples created cracks on the surface of the polymer and exposed the glass fibers, allowing the penetration of dyes into its internal matrix (Figure 2). In this study, both the Optis archwire immersed in the staining solution as received from the manufacturer and the one cut 10 mm from its extremities showed internal discoloration from the end of the archwire for less than 4 mm. This finding may suggest that the tendency to discoloration of Optis archwires occurs from their ends, even when they are not cut, which may be a result of a deficiency in the sealing of the internal fibers from the outside environment (Figure 2). According to these observations, although Optis archwires showed the lowest color stability of all the wires tested, clinically, this may not be relevant, as the staining is concentrated near the extremities of the archwire and will therefore be limited to the posterior region. Therefore, the color change in this archwire will not be visually detected in the smile as long as it is not used as a segmented archwire in the anterior region.
Figure 2. .
Scanning electron microscope photomicrographs of Optis archwire. (A,C) Longitudinal and cross-sectional views of as-received tip; (B,D) longitudinal and cross-sectional views of the tip of the archwire cut with wire cutter.
One aspect that must be considered is that the Optis archwire features a round cross section. Therefore, the specimens set out with Optis archwire segments did not present a flat surface, which might have altered the color measurements; this must be considered as a limitation of the study.
The bovine central incisor was used as a reference for high fluorescence, as bovine teeth give the same fluorescence as human teeth.9 The Optis archwire was the only one that presented a high fluorescence. The different composition and translucency of this archwire may be responsible for this difference, which gave it a unique esthetic characteristic and may add to its final appearance.
The optical properties of esthetic archwires available may not yet be ideal. More studies are needed to improve these characteristics. In the future, this aspect should be taken into account by the manufacturers.
CONCLUSIONS
All the esthetic archwires assessed showed noticeable color change after 21 days.
The Optis archwire showed the most pronounced color alteration, although staining was observed only near its ends. The Trianeiro and Ortho Organizers archwires presented less color change.
Only the Optis archwire presented fluorescence resembling that of bovine teeth. The coated archwires presented low fluorescence.
REFERENCES
- 1.Elayyan F, Silikas N, Bearn D. Ex vivo surface and mechanical properties of coated orthodontic archwires. Eur J Orthod. 2008;30:661–667. doi: 10.1093/ejo/cjn057. [DOI] [PubMed] [Google Scholar]
- 2.Russell JS. Esthetic orthodontic brackets. J Orthod. 2005;32:146–163. doi: 10.1179/146531205225021024. [DOI] [PubMed] [Google Scholar]
- 3.Burstone CJ, Liebler SAH, Goldberg AJ. Polyphenylene polymers as esthetic orthodontic archwires. Am J Orthod Dentofacial Orthop. 2011;139:e391–e398. doi: 10.1016/j.ajodo.2009.05.030. [DOI] [PubMed] [Google Scholar]
- 4.Elayyan F, Silikas N, Bearn D. Mechanical properties of coated superelastic archwires in conventional and self-ligating orthodontic brackets. Am J Orthod Dentofacial Orthop. 2010;137:213–217. doi: 10.1016/j.ajodo.2008.01.026. [DOI] [PubMed] [Google Scholar]
- 5.Ramadan AA. Removing hepatitis C virus from polytetrafluoroethylyene-coated orthodontics archwires and other dental instruments. East Mediterr Health J. 2009;9:274–278. [PubMed] [Google Scholar]
- 6.Postlethwaite KM. Advances in fixed appliance design and use: brackets and archwires. Dental Update. 1992;19:276–280. [PubMed] [Google Scholar]
- 7.Kusy RP. A review of contemporary archwires: their properties and characteristics. Angle Orthod. 1997;67:197–207. doi: 10.1043/0003-3219(1997)067<0197:AROCAT>2.3.CO;2. [DOI] [PubMed] [Google Scholar]
- 8.Faltermeier A, Rosentritt M, Reicheneder C, Behr M. Discolouration of orthodontic adhesives caused by food dyes and ultraviolet light. Eur J Orthod. 2008;30:89–93. doi: 10.1093/ejo/cjm058. [DOI] [PubMed] [Google Scholar]
- 9.Monsénégo G, Burdairon G, Clerjaud B. Fluorescence of dental porcelain. J Prosthet Dent. 1993;69:106–113. doi: 10.1016/0022-3913(93)90249-n. [DOI] [PubMed] [Google Scholar]
- 10.Commission Internationale de I'Ecleirage (CIE) Colorimetry Technical Report CIE publication n° 15 3rd ed. Vienna, Austria : Bureau Central de la CIE; 2004. [Google Scholar]
- 11.Nimeroff I. Colorimetry. Natl Bureau Stand Monogr. 1968;47:104. [Google Scholar]
- 12.Koksal T, Dikbas I. Color stability of different denture teeth materials against various staining agents. Dent Mater J. 2008;27:139–144. doi: 10.4012/dmj.27.139. [DOI] [PubMed] [Google Scholar]
- 13.Bolt RA, ten Bosch JJ, Coops JC. Influence of window size in small-window color measurements, particularly of teeth. Physics Med Biol. 1994;39:1133–1142. doi: 10.1088/0031-9155/39/7/006. [DOI] [PubMed] [Google Scholar]
- 14.Li Y. Tooth color measurement using Chroma Meter: techniques, advantages, and disadvantages. J Esthet Restor Dent. 2003;15:S33–S41. doi: 10.1111/j.1708-8240.2003.tb00316.x. [DOI] [PubMed] [Google Scholar]
- 15.Seghi RR, Hewlett ER, Kim J. Visual and instrumental colorimetric assessments of small color differences on translucent dental porcelain. J Dent Res. 1989;68:1760–1764. doi: 10.1177/00220345890680120801. [DOI] [PubMed] [Google Scholar]
- 16.Stober T, Gilde H, Lenz P. Color stability of highly filled composite resin materials for facings. Dent Mater. 2001;17:87–94. doi: 10.1016/s0109-5641(00)00065-8. [DOI] [PubMed] [Google Scholar]
- 17.Mutlu-Sagesen L, Ergün G, Ozkan Y, Semiz M. Color stability of dental composite after immersion in various media. Dent Mater J. 2005;24:382–390. doi: 10.4012/dmj.24.382. [DOI] [PubMed] [Google Scholar]
- 18.Eliades T, Gioka C, Heim M, Eliades G, Makou M. Color stability of orthodontic adhesive resins. Angle Orthod. 2004;74:391–393. doi: 10.1043/0003-3219(2004)074<0391:CSOOAR>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 19.Ertas E, Guler AU, Yucel AC, Koprulu H, Guler E. Color stability of resin composites after immersion in different drinks. Dent Mater J. 2006;25:371–376. [PubMed] [Google Scholar]
- 20.Arthur SK, Frederick CS, John C, Tak WC. Color stability of provisional prosthodontic materials. J Prosthet Dent. 2004;91:447–452. doi: 10.1016/S0022391304001283. [DOI] [PubMed] [Google Scholar]