Abstract
Objective:
To evaluate the expression of an activator of nuclear factor-kappa (RANK), osteoprotegerin (OPG), osteopontin (OPN), and transforming growth factor ß1 (TGF-ß1) in gingival crevicular fluid (GCF) of teeth subjected to orthodontic forces.
Materials and Methods:
A randomized, pilot clinical trial including 10 healthy volunteers was conducted using a split-mouth design. Orthodontic elastic separators were placed between the second premolar and first molar, with the contralateral quadrant serving as a control. The GCF samples were collected from the tension and compression sites at baseline, 24 hours, and 7 days after the placement of separators. The GCF sample volumes were measured using a Periotron 8000, and total protein concentrations were determined. Levels of RANK, OPG, OPN, and TGF-ß1 were also analyzed using a multiplex enzyme-linked immunosorbent assay.
Results:
The control sites remained unchanged throughout the study. In contrast, the concentration of OPG significantly decreased at the compression site by 24 hours, and the amount and concentration of RANK differed significantly between the control, compression, and tension sites after 7 days. A significant increase in absolute TGF-ß1 levels was also detected at the compression site versus the control and tension sites after 7 days.
Conclusion:
Bone metabolism is affected by application of force to the teeth by elastic separators. Both increased expression of bone resorptive mediators (eg, RANK and TGF-ß1) and decreased expression of a bone-forming mediator (eg, OPG) on the compression side were detected.
Keywords: Bone metabolism marker, Orthodontic movement, Gingival crevicular fluid
INTRODUCTION
It has been hypothesized that periodontal ligament (PDL) cells that are stimulated by orthodontic forces produce local factors that participate in the maintenance and remodeling of the ligament and that affect the metabolism of the adjacent alveolar bone.1 Using both in vivo2–5 and in vitro6,7 studies, investigators have tried to elucidate the molecular patterns associated with orthodontic tooth movements in animals and humans. As a result, signaling pathways responsible for bone resorption and bone formation have recently been elucidated. Three novel members of the tumor necrosis factor (TNF) ligand and receptor superfamilies, including the receptor activator of nuclear factor-kappa B ligand (RANKL), the receptor activator of nuclear factor-kappa B (RANK), and osteoprotegerin (OPG), have been found to play key roles in the modulation of bone resorptive processes. These processes involve differentiation, activation, and function of osteoclasts, with interactions between RANKL and RANK (a receptor on the cell surface of osteoclasts and osteoclast precursors) leading to stimulation of proliferation and inhibition of apoptosis. Moreover, OPG is another receptor produced by osteoblasts that can inhibit RANKL/RANK interactions and modify the effects of RANKL.
Various cytokines, such as transforming growth factor-ß (TGF-ß) and interleukin-1 (IL-1), can also modulate bone metabolism by stimulating macrophage colony-stimulating factor (M-CSF) production. As a result, the number of pre-osteoclastic cells increases and RANKL expression is enhanced.8 TGF-ß has been shown to increase the production of OPG9 and to stimulate the expression of RANK through pre-osteoclastic cells, thus increasing osteoclastic sensibility to RANK-L.10 However, contributions by TGF-ß are controversial. Despite being characterized as an inhibitor of osteoclast precursor recruitment and a mediator of suppressed osteoclast activity,11–13 other authors have suggested that TGF-ß may contribute to the induction of bone resorption.14,15 Osteopontin (OPN) is another molecule that has been linked to bone resorption via promotion of osteoclast adhesion to the osseous matrix.16 Correspondingly, bone resorption is inhibited in OPN knockout mice.17
Gingival crevicular fluid (GCF) is an osmotically mediated inflammatory exudate present in the gingival sulcus. When inflamed, this region tends to increase in volume and exhibit increased capillary permeability. It is hypothesized that mechanisms of bone resorption are related to the release of inflammatory mediators present in GCF. Accordingly, various studies have attempted to clarify the molecular mechanisms affected by orthodontic tooth movement by performing biochemical analyses of GCF and markers related to bone resorption, including interleukin-1ß, interleukin-6, interleukin-8, prostaglandin E2, tumor necrosis factor-α, substance P, and ß-glucuronidase.18–20 However, these results have been inconclusive. In some studies, only one side of a tooth19–23 versus two sides (eg, compression and tension)18,24,25 were evaluated, or only one time point26 was analyzed, or consecutive sampling at only one site was used to obtain larger GCF samples.20 All of these factors have limited the overall understanding of the biology of bone resorption and apposition during orthodontic movements.
Therefore, the objective of this pilot study was to evaluate the expression of RANK, OPG, OPN, and TGF-ß1 in GCF volumes obtained from teeth subjected to early orthodontic forces and to compare the expression of these markers between tension and compression sides, as well as between buccal and lingual sides, at different time points.
MATERIALS AND METHODS
Study Sample
Subjects between 20 and 50 years old who were in overall good health and had contact points on both sides of test and control molars were eligible for this study. Subjects were excluded if they were smokers, had gingivitis, had probing pocket depths ≥4 mm, had loss of clinical attachment ≥2 mm in the selected or adjacent teeth, or had taken anti-inflammatory or antibiotic medications within the previous 6 months. A total of 10 healthy, adult volunteers (five men, five women) were selected to participate in this pilot study, and they ranged in age from 22 to 29 years. For each volunteer, one first molar was randomly selected as a test tooth, and the contralateral molar served as control.
Study Design
Two weeks before the beginning of the study, the selected volunteers were informed of the objectives of the study, and they indicated their agreement to participate by signing an informed consent form that had been approved by the Ethical Committee of our institution. The volunteers were then subjected to a supragingival prophylaxis and were given oral hygiene instructions to follow at home for procedures to eliminate inflammation. The study lasted for 7 days and involved three visits. At the first visit (baseline), the Silness and Löe Plaque (PI) Index, Lobene Modified Gingival Index (GI), and Bleeding on Probing (BOP) Index were recorded for the control molar and for adjacent teeth at six sites per tooth. The BOP Index was evaluated after GCF sampling to avoid possible variations. GCF samples were subsequently obtained at mesiobuccal and mesiolingual sites of control molars. An orthodontic elastic separator was then placed at the mesial site of test molars.
At 24 hours and 7 days later, same clinical measurements were recorded for the control molar, test molar, and adjacent teeth. GCF samples were obtained from mesiobuccal and distobuccal sites of test molars at each visit. At the latter visit, the orthodontic elastic separators were removed.
GCF Sampling
GCF samples were collected using periopaper strips (Harco, Tustin, Calif). Samples were collected at the mesiobuccal and mesiolingual sites of control molars at baseline and at 7 days, and the test molar samples were obtained 24 hours and 7 days after placement of elastic separators at the mesiobuccal (tension side) and distobuccal (compression side) sites. Teeth were isolated with cotton rolls, cleaned of plaque deposits, and dried gently with air before paper strips were applied 1 mm subgingivally for 30 seconds. The volume of the sample on the paper strips was measured using a calibrated Periotron 8000 (Harco). The readings from the Periotron were converted to an actual volume (microliters) by reference to the standard curve calibrated with human serum.27,28
Biochemical Analysis
Each GCF sample was diluted in 100 µL 1% Triton X-100/50 mM Tris-HCl (pH 7.5) (Sigma-Aldrich Chemie GmbH, Buchs, Switzerland) and centrifuged at 12,500 rpm for 5 minutes. This procedure was repeated after the addition of another 100 µL of buffer, resulting in a total volume of 200 µL. From these samples, 135 µL was used to analyze protein levels of RANK, OPG, OPN, and TGF-ß1 using the Searchlight System (Endogen, Pierce Biotechnology, Rockford, Ill); 5 µL was used to quantify the total amount of protein present using a spectrophotometer (NanoDrop ND-1000, NanoDrop Technology, Wilmington, Del); and 65 µL was stored at −80°C.
The amount of each biomarker was determined in picograms (pg). Cytokine concentration (pg/µL) was calculated from the volume of GCF according to the following formula: Cytokine concentration (pg/µL) = Total amount cytokine (pg)/Volume GCF (µL). Concentrations were also normalized for the protein content of the samples and given as pg/µg protein, according to the following formula: Cytokine concentration (pg/µg) = Total amount cytokine (pg)/Protein concentration in GCF (µg).
Data Analysis
The Friedman test (nonparametric repeated measures analysis of variance) and Dunn's multiple comparisons test as post hoc were used to evaluate the statistical significance (P < .05) between measurements for the control sites at the different time points as well as differences between and within measurements for the control and test sites (tension and compression) for the three time points.
RESULTS
The volume of GCF samples ranged from 0.1 to 0.9 µL. The mean value was 0.4 µL (SD = 0.3 µL).
Clinical gingival condition was evaluated at the baseline and during the experimental period. All patients maintained good oral hygiene throughout the study. No significant changes in the PI, GI, or BOP indexes were found.
GCF values recorded for control and test sites at various time points are listed in Tables 1 through 4. At the control sites, there were no differences between the values recorded for buccal and palatal/lingual sites, or between values recorded at different visits, for any of the four markers investigated. Therefore, although control sites were not assessed at 24 hours, control baseline values were used to test for differences between the control and test sites at 24 hours.
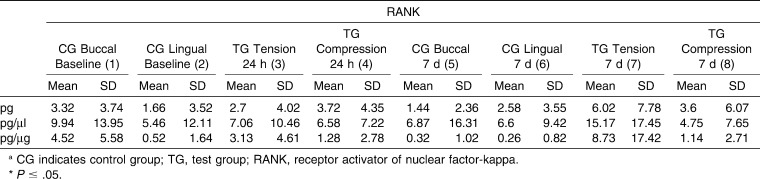
Table 1. .
Mean Values for Receptor Activator of Nuclear Factor-kB (RANK) of the Control Sites and Tension/Compression Sites at Different Time Points: Intragroup and Control versus Tension/Compression Site Comparisonsa
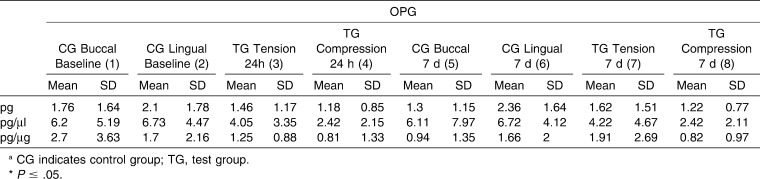
Table 2. .
Mean Values for Osteoprotegerin (OPG) of the Control Sites and Tension/Compression Sites at Different Time points: Intragroup and Control versus Tension/Compression Site Comparisonsa
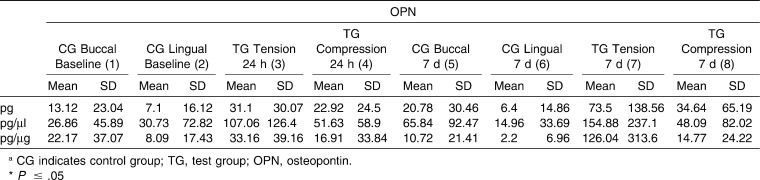
Table 3. .
Mean Values for Osteopontin (OPN) of the Control Sites and Tension/Compression Sites at Different Time points: Intragroup and Control versus Tension/Compression site Comparisons
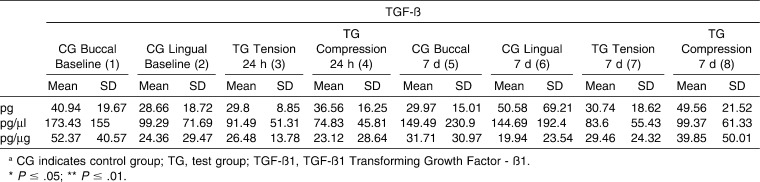
Table 4. .
Mean Values for Transforming Growth Factor-ß1 (TGF-ß1) of the Control Sites and Tension/Compression Sites at Different Time Points: Intragroup and Control versus Tension/Compression Site Comparisons
Table 1. .
Extended
Table 2. .
Extended
Table 3. .
Extended
Table 4. .
Extended
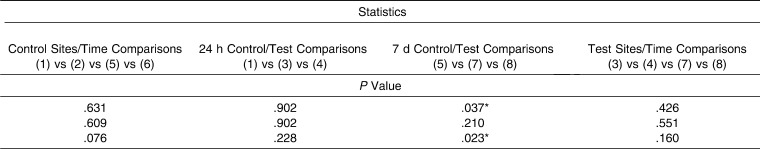
RANK levels (Table 1) at the control (baseline values) and test sites detected 24 hours after insertion of an orthodontic elastic separator were not statistically significant. However, after 7 days, statistically significant differences (P = .037) were identified between tension, compression, and control sites in regard to total cytokine levels (measured in picograms) and in regard to the ratio of RANK levels relative to total protein levels (pg/µg) (P = .023). However, post hoc tests did not identify significant two-by-two differences. No differences were found between compression and tension sites at 24 hours or 7 days.
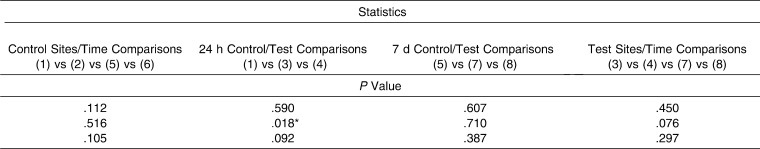
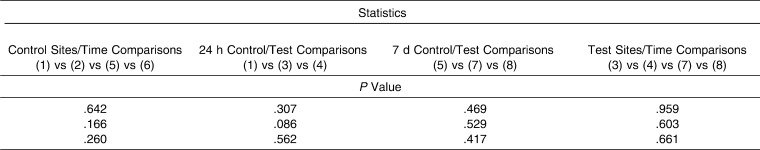
For OPG (Table 2), a statistically significant difference (P = .018) in GCF values recorded at 24 hours for tension, compression, and control (baseline values) sites was observed when results were expressed as pg/mL. Moreover, a post hoc test identified concentrations of OPG that were statistically significantly higher (P <.05) in control sites versus compression sites at the 24-hour point. Regarding the test molar, there were no differences between compression and tension sites at any time. In contrast, values recorded for OPN (Table 3) showed no significant differences between any of the values recorded at any of the time points evaluated.
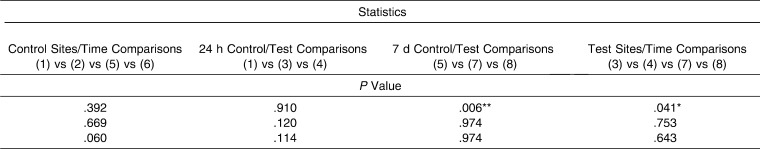
For TGF-ß1 (Table 4), no differences were found between the GCF values recorded at the baseline 24-hour time point. However, after 7 days, statistically significant differences (P = .006) between the tension, compression, and control sites were detected when the results were expressed as total amount of TGF-ß1 (pg). Moreover, protein levels were significantly higher at the compression test site than at the tension test site (P < .05) or the control site (P < .05) according to Dunn's multiple comparisons test.
In regard to the test sites, GCF TGFß1 values for tension and compression sites at the time points evaluated showed statistically significant (P = .04) differences in total cytokine levels (measured in picograms), although the post-test for multiple comparisons did not identify any statistically significant differences in the two-by-two analyses performed.
DISCUSSION
Recently, RANKL, RANK, OPG, and other signaling pathways have been shown to have important roles in bone metabolism. However, because these molecules are expressed at low levels, sensitive methods of detection are needed. In this study, multiplex sandwich enzyme-linked immunosorbent assay technology was used and provided a sensitivity of <1 pg/µL. Furthermore, to provide an accurate evaluation of the biochemical profile obtained from these assays, the total amount of a given cytokine in each sample (measured in picograms), the amount of marker relative to the total amount of protein (pg/µg), and the marker concentration relative to the volume of GCF (pg/µL) 29 were determined. A reason to show our results in these three ways is the lack of an internal method error analysis for the GCF sampling and quantification, which could make it unclear how much of these variations are due to biological processes or to random error. In an attempt to reduce this bias, the Periotron was always calibrated before GCF sampling.27,28
As a pilot experiment for detecting GCF components after the application of force to the teeth by means of elastic separators, this study included a small sample size, measurements of both tension and compression sides, one sampling per site, and a very simple initial tooth displacement (eg, insertion of an elastic separator). In addition, only two time intervals were evaluated—baseline and 7 days for the control sites, and 24 hours and 7 days for the test sites—because these time points were used in similar studies and provided the most significant changes for other biochemical factors.2,5,6 Although this is a limitation of the study, the fact that the control-site values remained stable during the study, considering both time and tooth aspects (buccal and lingual), could reduce this shortcoming.
Although no clinical differences in gingival inflammation could be detected during the study, the insertion of elastic separators may induce an undetectable subclinical inflammation.30 This fact points out the need for control sites to be used as reference, especially after the 7-day period. Another factor that could have prevented us from finding gingival changes could be the type of the patients that participated in the study (healthy young volunteers).
For the control sites, no significant changes were observed for any of the markers studied, including the comparison of buccal and lingual sites and the buccal and lingual sites at various time points. These results suggest that these markers are only expressed when significant alterations in bone metabolism occur.
Statistically higher concentrations of OPG were detected in control sites versus compression sites at 24 hours, yet these changes were not maintained at 7 days. These results, and those of other studies,6,22,23 suggest that a lower concentration of OPG, a marker associated with bone apposition, is present at sites where bone resorption appears (eg, compression sites). Similarly, RANK results also demonstrated significant differences between test and control sites at the 24-hour time point, which were not maintained through the 7-day time point. These results indicate that RANK may have a short-term role in bone changes that occur when orthodontic forces are applied.
The observation that TGF-ß1 was present at statistically higher concentrations in compression sites versus tension sites at the 7-day time point suggests that TGF-ß1 plays a role in bone destruction, a finding reported by other authors.14,15 However, these results are in contrast with those of Garlet and colleagues,26 who reported increased levels of this marker in compression and tension sites versus control sites, but not between tension and compression sites.
CONCLUSIONS
Changes in bone metabolism after insertion of an elastic separator included higher levels of bone resorptive mediators (eg, RANK and TGF-ß1) and lower levels of bone forming mediators (OPG). Moreover, the dynamics of these changes occurred over the short term (24 hours) and long term (7 day).
Further studies are needed to elucidate additional dynamics and signaling pathways of biological processes that occur during orthodontic movements.
Acknowledgments
This study was supported by Fundación Médica Mutua Madrileña.
REFERENCES
- 1.Yamaguchi M. RANK/RANKL/OPG during orthodontic tooth movement. Orthod Craniofac Res. 2009;12:113–119. doi: 10.1111/j.1601-6343.2009.01444.x. [DOI] [PubMed] [Google Scholar]
- 2.Kim T, Handa A, Iida J, Yoshida S. RANKL expression in rat periodontal ligament subjected to a continuous orthodontic force. Arch Oral Biol. 2007;52:244–250. doi: 10.1016/j.archoralbio.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 3.Dunn MD, Park CH, Kostenuik PJ, Kapila S, Giannobile WV. Local delivery of osteoprotegerin inhibits mechanically mediated bone modeling in orthodontic tooth movement. Bone. 2007;41:446–455. doi: 10.1016/j.bone.2007.04.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fujihara S, Yokozeki M, Oba Y, Higashibata Y, Nomura S, Moriyama K. Function and regulation of osteopontin in response to mechanical stress. J Bone Miner Res. 2006;21:956–964. doi: 10.1359/jbmr.060315. [DOI] [PubMed] [Google Scholar]
- 5.Kuroda S, Balam TA, Sakai Y, Tamamura N, Takano-Yamamoto T. Expression of osteopontin mRNA in odontoclasts revealed by in situ hybridization during experimental tooth movement in mice. J Bone Miner Metab. 2005;23:110–113. doi: 10.1007/s00774-004-0548-5. [DOI] [PubMed] [Google Scholar]
- 6.Nishijima Y, Yamaguchi M, Kojima T, Aihara N, Nakajima R, Kasai K. Levels of RANKL and OPG in gingival crevicular fluid during orthodontic tooth movement and effect of compression force on releases from periodontal ligament cells in vitro. Orthod Craniofac Res. 2006;9:63–70. doi: 10.1111/j.1601-6343.2006.00340.x. [DOI] [PubMed] [Google Scholar]
- 7.Kanzaki H, Chiba M, Sato A, et al. Cyclical tensile force on periodontal ligament cells inhibits osteoclastogenesis through OPG induction. J Dent Res. 2006;85:457–462. doi: 10.1177/154405910608500512. [DOI] [PubMed] [Google Scholar]
- 8.Hofbauer LC, Lacey DL, Dunstan CR, Spelsberg TC, Riggs BL, Khosla S. Interleukin-1beta and tumor necrosis factor-alpha, but not interleukin-6, stimulate osteoprotegerin ligand gene expression in human osteoblastic cells. Bone. 1999;25:255–259. doi: 10.1016/s8756-3282(99)00162-3. [DOI] [PubMed] [Google Scholar]
- 9.Takai H, Kanematsu M, Yano K, et al. Transforming growth factor-beta stimulates the production of osteoprotegerin/osteoclastogenesis inhibitory factor by bone marrow stromal cells. J Biol Chem. 1998;273:27091–27096. doi: 10.1074/jbc.273.42.27091. [DOI] [PubMed] [Google Scholar]
- 10.Yan T, Riggs BL, Boyle WJ, Khosla S. Regulation of osteoclastogenesis and RANK expression by TGF-beta1. J Cell Biochem. 2001;83:320–325. doi: 10.1002/jcb.1200. [DOI] [PubMed] [Google Scholar]
- 11.Janssens K, ten Dijke P, Janssens S, Van Hul W. Transforming growth factor-beta1 to the bone. Endocr Rev. 2005;26:743–774. doi: 10.1210/er.2004-0001. [DOI] [PubMed] [Google Scholar]
- 12.Kanaan RA, Kanaan LA. Transforming growth factor beta1, bone connection. Med Sci Monit. 2006;12:RA164–RA169. [PubMed] [Google Scholar]
- 13.Brady TA, Piesco NP, Buckley MJ, Langkamp HH, Bowen LL, Agarwal S. Autoregulation of periodontal ligament cell phenotype and functions by transforming growth factor-beta1. J Dent Res. 1998;77:1779–1790. doi: 10.1177/00220345980770100501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Itonaga I, Sabokbar A, Sun SG, et al. Transforming growth factor-beta induces osteoclast formation in the absence of RANKL. Bone. 2004;34:57–64. doi: 10.1016/j.bone.2003.08.008. [DOI] [PubMed] [Google Scholar]
- 15.Pilkington MF, Sims SM, Dixon SJ. Transforming growth factor-beta induces osteoclast ruffling and chemotaxis: potential role in osteoclast recruitment. J Bone Miner Res. 2001;16:1237–1247. doi: 10.1359/jbmr.2001.16.7.1237. [DOI] [PubMed] [Google Scholar]
- 16.Reinholt FP, Hultenby K, Oldberg A, Heinegard D. Osteopontin—a possible anchor of osteoclasts to bone. Proc Natl Acad Sci USA. 1990;87:4473–4475. doi: 10.1073/pnas.87.12.4473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ishijima M, Tsuji K, Rittling SR, et al. Resistance to unloading-induced three-dimensional bone loss in osteopontin-deficient mice. J Bone Miner Res. 2002;17:661–667. doi: 10.1359/jbmr.2002.17.4.661. [DOI] [PubMed] [Google Scholar]
- 18.Dudic A, Kiliaridis S, Mombelli A, Giannopoulou C. Composition changes in gingival crevicular fluid during orthodontic tooth movement: comparisons between tension and compression sides. Eur J Oral Sci. 2006;114:416–422. doi: 10.1111/j.1600-0722.2006.00387.x. [DOI] [PubMed] [Google Scholar]
- 19.Ren Y, Hazemeijer H, de Haan B, Qu N, de Vos P. Cytokine profiles in crevicular fluid during orthodontic tooth movement of short and long durations. J Periodontol. 2007;78:453–458. doi: 10.1902/jop.2007.060261. [DOI] [PubMed] [Google Scholar]
- 20.Uematsu S, Mogi M, Deguchi T. Interleukin (IL)-1 beta, IL-6, tumor necrosis factor-alpha, epidermal growth factor, and beta 2-microglobulin levels are elevated in gingival crevicular fluid during human orthodontic tooth movement. J Dent Res. 1996;75:562–567. doi: 10.1177/00220345960750010801. [DOI] [PubMed] [Google Scholar]
- 21.Griffiths GS, Moulson AM, Petrie A, James IT. Evaluation of osteocalcin and pyridinium crosslinks of bone collagen as markers of bone turnover in gingival crevicular fluid during different stages of orthodontic treatment. J Clin Periodontol. 1998;25:492–498. doi: 10.1111/j.1600-051x.1998.tb02478.x. [DOI] [PubMed] [Google Scholar]
- 22.Kawasaki K, Takahashi T, Yamaguchi M, Kasai K. Effects of aging on RANKL and OPG levels in gingival crevicular fluid during orthodontic tooth movement. Orthod Craniofac Res. 2006;9:137–142. doi: 10.1111/j.1601-6343.2006.00368.x. [DOI] [PubMed] [Google Scholar]
- 23.Toygar HU, Kircelli BH, Bulut S, Sezgin N, Tasdelen B. Osteoprotegerin in gingival crevicular fluid under long-term continuous orthodontic force application. Angle Orthod. 2008;78:988–993. doi: 10.2319/100507-483.1. [DOI] [PubMed] [Google Scholar]
- 24.Perinetti G, Paolantonio M, D'Attilio M, et al. Alkaline phosphatase activity in gingival crevicular fluid during human orthodontic tooth movement. Am J Orthod Dentofacial Orthop. 2002;122:548–556. doi: 10.1067/mod.2002.126154. [DOI] [PubMed] [Google Scholar]
- 25.Perinetti G, Serra E, Paolantonio M, et al. Lactate dehydrogenase activity in human gingival crevicular fluid during orthodontic treatment: a controlled, short-term longitudinal study. J Periodontol. 2005;76:411–417. doi: 10.1902/jop.2005.76.3.411. [DOI] [PubMed] [Google Scholar]
- 26.Garlet TP, Coelho U, Silva JS, Garlet GP. Cytokine expression pattern in compression and tension sides of the periodontal ligament during orthodontic tooth movement in humans. Eur J Oral Sci. 2007;115:355–362. doi: 10.1111/j.1600-0722.2007.00469.x. [DOI] [PubMed] [Google Scholar]
- 27.Chapple IL, Landini G, Griffiths GS, Patel NC, Ward RS. Calibration of the Periotron 8000 and 6000 by polynomial regression. J Periodontal Res. 1999;34:79–86. doi: 10.1111/j.1600-0765.1999.tb02226.x. [DOI] [PubMed] [Google Scholar]
- 28.Tozum TF, Hatipoglu H, Yamalik N, et al. Critical steps in electronic volume quantification of gingival crevicular fluid: the potential impact of evaporation, fluid retention, local conditions and repeated measurements. J Periodontal Res. 2004;39:344–357. doi: 10.1111/j.1600-0765.2004.00758.x. [DOI] [PubMed] [Google Scholar]
- 29.Vernal R, Dutzan N, Chaparro A, Puente J, Antonieta Valenzuela M, Gamonal J. Levels of interleukin-17 in gingival crevicular fluid and in supernatants of cellular cultures of gingival tissue from patients with chronic periodontitis. J Clin Periodontol. 2005;32:383–389. doi: 10.1111/j.1600-051X.2005.00684.x. [DOI] [PubMed] [Google Scholar]
- 30.Perinetti G, Paolantonio M, Serra E, et al. Longitudinal monitoring of subgingival colonization by Actinobacillus actinomycetemcomitans, and crevicular alkaline phosphatase and aspartate aminotransferase activities around orthodontically treated teeth. J Clin Periodontol. 2004;31:60–67. doi: 10.1111/j.0303-6979.2004.00450.x. [DOI] [PubMed] [Google Scholar]