Abstract
Objective:
To determine the difference in the levels of Streptococcus mutans and S sobrinus in stimulated saliva in orthodontic patients with different bracket types (stainless steel and esthetic brackets) using polymerase chain reaction and cultivation method.
Materials and Methods:
Thirty-two patients, aged 13 to 30 years, were selected following these criteria: 1) orthodontic treatment indication, 2) systemic health, and 3) no tobacco and antibiotic consummation for three months prior to the commencement of the study. Patients were divided into two groups according to the bracket type; 16 patients formed the conventional bracket group (stainless steel brackets), and 16 patients formed the esthetic bracket group (plastic brackets). The levels of S mutans and S sobrinus in stimulated whole saliva samples were collected prior to fixed orthodontic appliance placement (T1) and 12 weeks after placement (T2), as were the Decayed, Missing, and Filled Surface Index (DMFS) and Oral Hygiene Index-Simplified (OHI-S). Mann-Whitney, Wilcoxon, and chi-square tests were used for statistical analysis.
Results:
Statistical analysis (chi-square test) showed no difference in S mutans and S sobrinus counts among patients with different brackets at either T1 or T2. There was no difference in total bacteria counts after fixed orthodontic appliance placement.
Conclusion:
The number of colony-forming units of S mutans and S sobrinus in stimulated saliva samples does not seem to be significantly different between patients with stainless steel brackets and patients with plastic brackets.
Keywords: S Mutans , S sobrinus , Polymerase chain reaction, Plastic brackets, Stainless steel brackets
INTRODUCTION
Complex design of fixed orthodontic appliances promotes development and retention of supragingival plaque due to reduced efficiency in self-cleaning and oral hygiene measures.1 Supragingival plaque presents a reservoir of complex cariogenic bacterial strains and a risk factor for enamel demineralization and dental caries.2 Microbial adhesion and plaque maturation in orthodontic patients depends on different variables, such as bracket design and material, proximity of the gingival sulcus to the bracket, surface area of the labial enamel relative to the bracket, position of the teeth in the dental arch, material of ligation and, mainly, individual oral hygiene habits.3 Numerous studies4,5 have proved that placement of fixed orthodontic appliance leads to an increase in the number of mutans Streptococci. Among them, Streptococcus mutans and S sobrinus have been recognized as prime causative organisms of dental caries.2 Since the popularity of plastic brackets has grown during the last few years due to increased demand for superior esthetics during orthodontic treatment, it is crucial to identify possible variations in the adhesion pattern of S mutans and S sobrinus on different bracket material in order to decrease the risk of possible side effects of such therapy.
In the literature there are diverse results regarding plaque and bacterial adhesion on different bracket materials that can be attributed to various methodologies used in published research. The aim of this clinical study was to detect the effect of two different types of brackets (esthetic and stainless steel) on caries-related factors such as a) Oral Hygiene Index-Simplified (OHI-S), b) paraffin-stimulated whole saliva, and c) levels of S mutans and S sobrinus in the paraffin-stimulated whole saliva in patients undergoing orthodontic treatment.
MATERIALS AND METHODS
Thirty-two patients of both sexes were included in this prospective clinical study. The Ethical Committee of the School of Dental Medicine approved the protocol of the research. Patients were recruited in a private orthodontic office in Zagreb, Croatia, and had to satisfy the following inclusion criteria: 1) indication for fixed orthodontic treatment, 2) healthy systemic condition, 3) healthy periodontium, 4) nonsmoker, and 5) without antibiotic therapy in the last three months. The aims and the methods of research were explained to all patients, and they or their parents/guardians were asked to sign informed consent.
Patients were divided into two groups according to bracket type: 16 patients formed the conventional bracket group (Discovery; Dentaurum, Ispringen, Germany), and 16 patients formed the esthetic (plastic) bracket group (Spirit MB; Ormco/A Company, Orange, Calif). All brackets were bonded with the same adhesive (Transbond XT; 3M Unitek, Monrovia, Calif) and ligated using elastomere ligature (Latex-free Unicyles α Chain; Masel, Carlsbad, Calif). All patients received oral hygiene instructions at baseline and at each regular check-up and were asked to refrain from any chlorhexidine use during the study.
From Pandis et al.,5 Forsberg et al.,6 and Attin et al.,7 it is known that a sample size of 16 patients per group, at α = .05, yields a statistical power close to 0.8 for this kind of study.5–6,7
All clinical measurements were performed by the same investigator at two time points: 1) prior to placement of fixed orthodontic appliance (T1) and 2) 12 weeks after placement of the appliance (T2). Time point T2 has been determined as a period with highest prevalence of oral microbiota according to Ristic et al.8
Further clinical measurements were performed at these two time points in the following sequence: 1) paraffin-stimulated whole saliva (SS), 2) OHI-S, 3) Decayed, Missing, and Filled Surface Index (DMFS).
Paraffin-Stimulated Whole Saliva Collection
All patients were asked to refrain from eating, drinking, and brushing 2 hours prior to all clinical examination. Collection of stimulated saliva and other clinical parameters took place in a dental chair between 0900 and 1100 hours. Patients were asked to chew 1 g of paraffin wax for 1 minute, followed by collection of saliva into measured, dry, and sterilized plastic tubes for 5 minutes. After sampling time, the tubes were measured and the whole amount of collected stimulated saliva was calculated by subtracting the weight of the empty tube. Total amount of saliva was measured in g/min, which is equal to ml/min.9
Decayed, Missing, and Filled Surface Index (DMFS)
Decayed, Missing, and Filled Surface Index was recorded using criteria of the World Health Organization for permanent dentition.10
Oral Hygiene Index Simplified
In order to quantify supragingival plaque, we used the OHI-S of Greene and Vermillion,11 which is divided into debris index and calculus index, the sum of which presents the value of OHI-S. Fluorescein dye (Plak-Check, Butler, Germany) was applied with cotton tips and illuminated with a halogen light (Blue phase G2; Ivoclar-Vivadent, Schaan, Liechtenstein) in order to detect yellow efflorescences. Supragingival plaque and calculus were recorded on the buccal aspects of the permanent right and left maxillary first molars, permanent right maxillary central incisor, and permanent left mandibular central incisor, as well as on the lingual aspects of the permanent right and left mandibular first molars. The OHI-S score was computed as the mean of the plaque scores for the observed and scored (index) teeth. The criteria for classification of the OHI-S are good (from 0.0 to 0.6), regular (from 0.7 to 1.8), and poor (from 1.9 to 3.0).
Bacterial Markers
Saliva samples were serially diluted 10-fold with potassium phosphate-buffered saline (pH 7), and 100 µL of diluted samples were plated, as previously described,12 on Mitis-Salivarius agar containing bacitracin and sucrose (MBS agar). Another 100 µL of diluted saliva was plated on nonselective agar (Columbia agar, BBL). Samples were incubated in a humified chamber at 37°C for 2 days. The number of colony-forming units (CFU) of S mutans and S sobrinus per milliliter of saliva was determined from typical colonial morphology. All colonies with typical macro- and micromorphology were further subcultivated overnight in thioglycollate broth in a shaker incubator (37°C). Pure culture strains were biochemically identified by API Strep test (BioMerieux Inc, Durham, NC).
Saliva (1 mL) was centrifuged for 15 minutes at 12,000 g, and the pellet was resuspended in 200 µL of cell lysis buffer (10 mM Tris-HCl, 1 mM Ethylenediaminetetraacetic acid, 1% Triton X-100; pH 8.0). The suspension was boiled for 10 minutes and centrifuged. Supernatant was used for DNA extraction.13,14
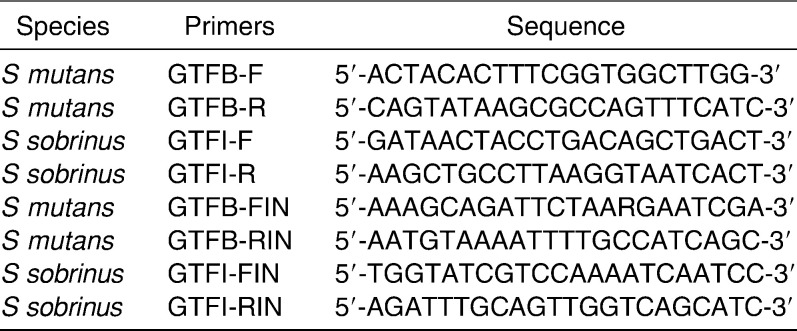
The sequences of the primers used for nested polymerase chain reaction (PCR) detection of S mutans and S sobrinus are shown in Table 1. Genomic DNA was analyzed by nested PCR for the glucosyltransferase genes (gtfB of S mutans and gtfI of S sobrinus). First sets of primers GTFB-F,-R and GTFI-F,-R were used for the first run of PCR. The second round of PCR was performed using second sets of primers GTFB-FIN,-RIN, GTFI-FIN,-RIN, and 0.1 µL of the first run mixture as a template. Cycling conditions for the first and second PCR run were 6 minutes of denaturation at 95°C followed by 30 cycles of 30 seconds of denaturation at 95°C, 30 seconds of annealing at 59°C, and 1 minute of extension at 72°C, with a final extension of 7 minutes at 72°C. As previously described, using this method we are able to identify both S mutans and S sobrinus at a level of ≥104 CFU/ml saliva.12 PCR products were stained with ethidium-bromide and analyzed on 1.8% agarose gels.
Table 1. .
PCR Primers Modified from Oho et al.14
Complete microbiological treatment was performed at the Department of Clinical and Molecular Microbiology, University Hospital Centre Zagreb, Croatia.
Data analysis was performed by SPSS version 10.0 (SPSS Inc, Chicago, Ill). Differences in age, OHI-S, SS, and DMFS between bracket groups were analyzed by Mann-Whitney test, and differences within the group between two time periods were analyzed by Wilcoxon test. Chi-square test was applied for difference assessment in bacteria count, with the cutoff point at 104 CFU/ml for cultivation and PCR method. Cohen Kappa was used for agreement assessment of bacteria detection between cultivation and PCR method, with a cutoff point 104 CFU/ml.
RESULTS
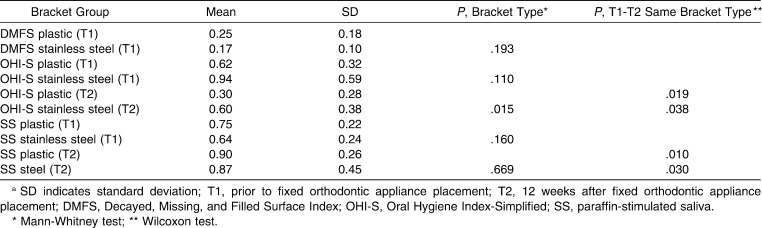
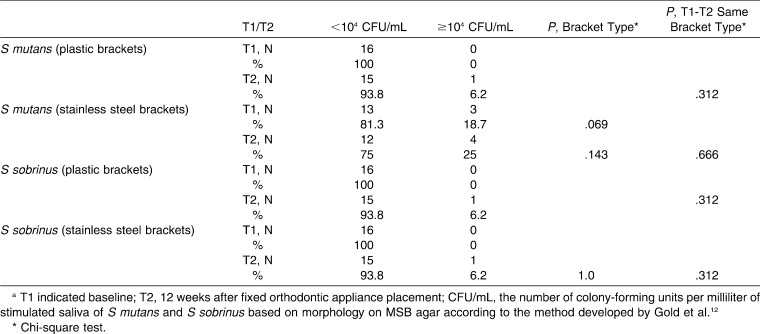
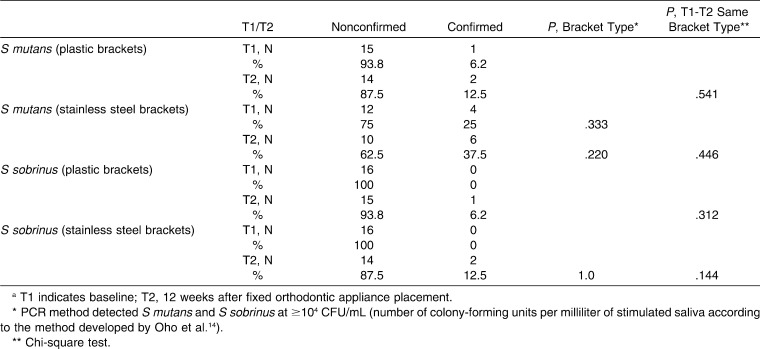
Gender distribution did not show a statistically significant difference in the two bracket groups; there were 10 female (62.5%) and 6 male patients (37.5%) in each group. Age distribution showed statistically significant differences in the two bracket groups; patients with plastic brackets were older (25.8 ± 4.9) than patients with stainless steel brackets (18.7 ± 5.3; P < .001). There were no statistically significant differences between the two bracket groups regarding oral hygiene variables (OHI-S and DMFS) at T1 (Table 2). Results indicate statistically significant improvement in OHI-S in both groups (plastic brackets: P = .019, stainless steel brackets: P = .038; Table 2). SS flow did not show statistically significant differences among different brackets groups at either T1 or T2. A statistically significant increase in stimulated saliva flow was determined in both groups between T1 and T2 (plastic brackets: P = .010, stainless steel brackets: P = .030; Table 2). Cohen Kappa showed good correlation between the two methods of detection (cultivation and PCR method) of S mutans and S sobrinus with detection level at 104 CFU/ml (κ = 0.718 for S mutans and 0.792 for S sobrinus, P < .001). Chi-square test revealed statistically insignificant differences in the number of colony-forming units per milliliter of S mutans and S sobrinus between the two bracket groups at either T1 or T2 (Tables 3 and 4).
Table 2. .
DMFS, OHI-S, and SS Resultsa
Table 3. .
Summary Data for S mutans and S sobrinus Isolation Using the Cultivation Methoda
Table 4. .
Summary Data for S mutans and S sobrinus Isolation Using Nested PCRa
DISCUSSION
Although some studies1,8,15 reported a negative influence of fixed orthodontic appliances on quantitative and qualitative distribution of oral microbiota, an unequivocal and consistent conclusion regarding changes of oral microbiota during orthodontic treatment is still lacking.
We were unable to detect any negative effect on microbial flora (S mutans and S sobrinus) 12 weeks after fixed orthodontic appliance placement, which is consistent with the results of Pandis et al.,5 Lara-Carrillo et al.,16 and Mota et al.17
In order to obtain a more uniform conclusion regarding the influence of fixed orthodontic appliances on oral microbiota, it is necessary to conduct clinical studies for a longer duration. Jordan and LeBlanc18 reported similar results in their four month longitudinal study. They linked the inconsistent pattern of mutans Streptococci prevalence observed among their patients to the oral hygiene practices of individual patients, something that may be attributable to the patients in this study as well. However, at 12 weeks we were unable to detect any plaque on the teeth of our subjects, attributable to the rigid and continuous instructions that were performed at all checkups. The positive effects of oral hygiene instructions for patients with fixed orthodontic appliances have been recognized,1 and significant improvement of the OHI-S index was observed in our study as well; this is in accordance with the results by Al-Jewair et al.,19 who reported good OHI compliance in 73% of patients. Improvement in OHI-S can be explained by the precise instructions in oral hygiene measures during each checkup and the resolving of crowding during the first 12 weeks of orthodontic treatment, but it can also be attributable to the Hawthorne effect (patients' awareness of being examined and evaluated).20
It is mainly in vitro studies that have been conducted to analyze the adhesion pattern of microbiota on different orthodontic materials, but without uniform conclusions for implications in clinical practice.2,21–22,23 Since organic acids produced by investigated bacteria have been recognized as the main pathological factors in dental caries and enamel demineralization,24 we wanted to compare if there is any difference in the prevalence of these microbial species between patients with different types of brackets.
Because of the difficulty in obtaining and controlling the size of the plaque sample, we used stimulated saliva as the representative sample to quantitatively determine levels of these microorganisms. Togelius et al.25 and Dasanayake et al.26 showed excellent correlation between stimulated saliva samples and plaque samples for quantitative assessment of mutans Streptococci. Results from our study indicate that there is no statistically significant difference in levels of S mutans and S sobrinus from samples of paraffin-stimulated saliva between patients with different bracket material, although Eliades et al.21 identified stainless steel as a surface material with increased potential for microbial attachment after measuring free surface energy and work of adhesion of raw materials and compared it to polycarbonate and ceramic material. In contrast, results from Fournier et al.22 indicate weaker in vitro affinity of S mutans for metallic brackets than for plastic brackets, which is in accordance with the results of a study conducted by Ahn et al.,2 who made in vitro multiple comparisons of cariogenic adhesion amounts on stainless steel, plastic, ceramic, and titanium brackets. Besides significant differences in adhesion pattern of different cariogenic strains, their results showed higher adherence of cariogenic streptococci on plastic brackets than on four other types of brackets. This was explained by the surface characteristics of plastic brackets that were modified with filler, which consequently led to increased adhesion of cariogenic streptococci.
Contrary to the results from aforementioned studies, microbiological data from our study did not indicate any statistically significant difference in total cariogenic Streptococci in paraffin-stimulated whole saliva of orthodontic patients with different bracket material. Similar results were reported by Papaioannou et al.,3 who were unable to find statistically significant differences regarding adhesion patterns among stainless steel, plastic, and ceramic brackets. In their in vitro study they underscored the important role of the salivary pellicle, which might negate any differences in cariogenic surface characteristics (surface free energy), together with the presence of histatins and lyzozymes in saliva that possess antibacterial activity and can contribute to decreased amounts of microbiota adhesion.
CONCLUSIONS
The numbers of colony-forming units of S mutans and S sobrinus per milliliter of paraffin-stimulated saliva were not influenced at a statistically significant level by fixed orthodontic appliance placement during the first 12 weeks of orthodontic treatment.
The numbers of colony forming units of S mutans and S sobrinus per milliliter of paraffin-stimulated saliva were not significantly altered by bracket type, either stainless steel or plastic.
REFERENCES
- 1.Smiech-Slomkowska G, Jablonska-Zrobek J. The effect of oral health education on dental plaque development and the level of caries-related Streptococcus mutans and Lactobacillus spp. Eur J Orthod. 2007;29:157–160. doi: 10.1093/ejo/cjm001. [DOI] [PubMed] [Google Scholar]
- 2.Ahn SJ, Lee SJ, Lim BS, Nahm DS. Quantitative determination of adhesion patterns of cariogenic streptococci to various orthodontic brackets. Am J Orthod Dentofacial Orthop. 2007;132:815–821. doi: 10.1016/j.ajodo.2005.09.034. [DOI] [PubMed] [Google Scholar]
- 3.Papaioannou W, Gizani S, Nassika M, Kontou E, Nakou M. Adhesion of Streptococcus mutans to different types of brackets. Angle Orthod. 2007;77:1090–1095. doi: 10.2319/091706-375.1. [DOI] [PubMed] [Google Scholar]
- 4.Sandham HJ, Nadeau L, Phillips HI. The effect of chlorhexidine varnish treatment on salivary mutans streptococcal levels in child orthodontic patients. J Dent Res. 1992;71:32–35. doi: 10.1177/00220345920710010501. [DOI] [PubMed] [Google Scholar]
- 5.Pandis N, Papaioannou W, Kontou E, Nakou M, Makou M, Eliades T. Salivary Streptococcus mutans levels in patients with conventional and self-ligating brackets. Eur J Orthod. 2010;32:94–99. doi: 10.1093/ejo/cjp033. [DOI] [PubMed] [Google Scholar]
- 6.Forsberg CM, Brattström V, Malmberg E, Nord CE. Ligature wires and elastomeric rings: two methods of ligation, and their association with microbial colonization of Streptococcus mutans and lactobacilli. Eur J Orthod. 1991;13:416–420. doi: 10.1093/ejo/13.5.416. [DOI] [PubMed] [Google Scholar]
- 7.Attin R, Thon C, Schlagenhauf U, Werner C, Wiegand A, Hannig C, Attin T. Recolonization of mutans steptococci on teeth with orthodontic appliances after antimicrobial therapy. Eur J Orthod. 2005;27:489–493. doi: 10.1093/ejo/cji018. [DOI] [PubMed] [Google Scholar]
- 8.Ristic M, Vlahovic Svabic M, Sasic M, Zelic O. Clinical and microbiological effects of fixed orthodontic appliances on periodontal tissues in adolescents. Orthod Craniofac Res. 2007;10:187–195. doi: 10.1111/j.1601-6343.2007.00396.x. [DOI] [PubMed] [Google Scholar]
- 9.Navazesh M. Methods for collecting saliva. Ann N Y Acad Sci. 1993;694:72–77. doi: 10.1111/j.1749-6632.1993.tb18343.x. [DOI] [PubMed] [Google Scholar]
- 10.World Health Organization. Oral Health Surveys Basic Methods 4th ed. World Health Organization; Geneva: 1997. [Google Scholar]
- 11.Greene JC, Vermillion JR. The simplified oral hygiene index. J Am Dent Assoc. 1964;68:7–13. doi: 10.14219/jada.archive.1964.0034. [DOI] [PubMed] [Google Scholar]
- 12.Gold OG, Jordan HV, Van Houte J. A selective medium for Streptococcus mutans. Arch Oral Biol. 1973;18:1357–1364. doi: 10.1016/0003-9969(73)90109-x. [DOI] [PubMed] [Google Scholar]
- 13.Watanabe K, Frommel TO. Detection of Porphyromonas gingivalis in oral plaque samples by use of the polymerase chain reaction. J Dent Res. 1993;72:1040–1044. doi: 10.1177/00220345930720060801. [DOI] [PubMed] [Google Scholar]
- 14.Oho T, Yamashita Y, Shimazaki Y, Kushiyama M, Koga T. Simple and rapid detection of Streptococcus mutans and Streptococcus sobrinus in human saliva by polymerase chain reaction. Oral Microbiol Immunol. 2000;15:258–262. doi: 10.1034/j.1399-302x.2000.150408.x. [DOI] [PubMed] [Google Scholar]
- 15.Topaloglu-Ak A, Ertugrul F, Eden E, Ates M, Bulut H. Effect of orthodontic appliances on oral microbiota—6 month follow-up. J Clin Pediatr Dent. 2011;35:433–436. doi: 10.17796/jcpd.35.4.61114412637mt661. [DOI] [PubMed] [Google Scholar]
- 16.Lara-Carrillo E, Montiel-Bastida NM, Sánchez-Pérez L, Alanís-Tavira J. Effect of orthodontic treatment on saliva, plaque and the levels of Streptococcus mutans and Lactobacillus. Med Oral Patol Oral Cir Bucal. 2010;15:924–920. doi: 10.4317/medoral.15.e924. [DOI] [PubMed] [Google Scholar]
- 17.Mota SM, Enoki C, Ito IY, Elias AM, Matsumoto MA. Streptococcus mutans counts in plaque adjacent to orthodontic brackets bonded with resin-modified glass ionomer cement or resin-based composite. Braz Oral Res. 2008;22:55–60. doi: 10.1590/s1806-83242008000100010. [DOI] [PubMed] [Google Scholar]
- 18.Jordan C, LeBlanc DJ. Influences of orthodontic appliances on oral populations of mutans streptococci. Oral Microbiol Immunol. 2002;17:65–71. doi: 10.1046/j.0902-0055.2001.00083.x. [DOI] [PubMed] [Google Scholar]
- 19.Al-Jewair TS, Suri S, Tompson BD. Predictors of adolescent compliance with oral hygiene instructions during two-arch multibracket fixed orthodontic treatment. Angle Orthod. 2011;81:525–531. doi: 10.2319/092010-547.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Feil PH, Grauer JS, Gadbury-Amyot CC, Kula K, McCunniff MD. Intentional use of the Hawthorne effect to improve oral hygiene compliance in orthodontic patients. J Dent Educ. 2002;66:1129–1135. [PubMed] [Google Scholar]
- 21.Eliades T, Eliades G, Brantley WA. Microbial attachment on orthodontic appliances. I. Wettability and early pellicle formation on bracket materials. Am J Orthod Dentofacial Orthop. 1995;108:351–360. doi: 10.1016/s0889-5406(95)70032-3. [DOI] [PubMed] [Google Scholar]
- 22.Fournier A, Payant L, Bouclin R. Adherence of Streptococcus mutans to orthodontic brackets. Am J Orthod Dentofacial Orthop. 1998;114:414–417. doi: 10.1016/s0889-5406(98)70186-6. [DOI] [PubMed] [Google Scholar]
- 23.Anhoury P, Nathanson D, Hughes CV, Socransky S, Feres M, Chou LL. Microbial profile on metallic and ceramic bracket materials. Angle Orthod. 2002;72:338–343. doi: 10.1043/0003-3219(2002)072<0338:MPOMAC>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 24.Hirose H, Hirose K, Isogai E, Miura H, Ueda I. Close association between Streptococcus sobrinus in the saliva of young children and smooth-surface caries increment. Caries Res. 1993;27:292–297. doi: 10.1159/000261553. [DOI] [PubMed] [Google Scholar]
- 25.Togelius J, Kristoffersson K, Anderson H, Bratthall D. Streptococcus mutans in saliva: intraindividual variations and relation to the number of colonized sites. Acta Odontol Scand. 1984;42:157–163. doi: 10.3109/00016358408993867. [DOI] [PubMed] [Google Scholar]
- 26.Dasanayake AP, Caufield PW, Cutter GR, Roseman JM, Köhler B. Differences in the detection and enumeration of mutans streptococci due to differences in methods. Arch Oral Biol. 1995;40:345–351. doi: 10.1016/0003-9969(94)00164-7. [DOI] [PubMed] [Google Scholar]