Abstract
Objective:
To determine changes in mouse myosin heavy chain (MyHC) protein expression that may occur with a clinically relevant vertical dimension of occlusion (VDO) increase.
Materials and Methods:
Six CD-1 male mice (age: 6 weeks) underwent a 10% bite opening to replicate the clinical condition using composite on the maxillary molars and were compared to six age-matched controls. Mice were sacrificed at day 7 and 14 after bite opening. A representative masseter transverse cryosection from each animal was examined in selected sampling regions (anterior, posterior, posterior-deep, and posterior-intermediate) to assay fiber phenotype proportions and fiber size.
Results:
In control masseter muscles, the proportion of muscle fibers containing MyHC IIb increased in the posterior-intermediate and posterior-deep regions between 7 and 14 days (ANOVA, P < .05). The increase in the proportion of MyHC IIb fibers in the bite opening group did not occur when compared to the control group (P < .05). In addition, after 14 days of bite opening, the proportion of fibers positive for MyHC IIa was decreased in the anterior region compared to control masseter muscles. Muscle fiber diameter remained unchanged in both groups (experimental and control) and over time (P > .10).
Conclusion:
These data are consistent with a selective plasticity of the expression of MyHC IIb protein in the deep regions of the male masseter muscle in response to a clinically relevant VDO increase.
Keywords: Masticatory muscle, Vertical dimension of occlusion
INTRODUCTION
The treatment of select orthodontic cases is facilitated by increasing the vertical dimension of occlusion (VDO). Orthodontic treatment modalities that open the VDO are implemented to alleviate crossbites, to allow the placement of mandibular brackets in deep bite cases, to disclude the dentition facilitating tooth movement, or to help level the Curve of Spee.1 The increase in VDO stretches jaw-closing muscles and, thus, may have a more direct effect on vertically-oriented fibers in this group of muscles compared to other jaw muscles.2 Studies using adult animal models have examined the effect of passively elongating limb muscle on their contractile properties.3,4 However, it is unclear whether or not jaw closing musculature responds to stretch in the same manner with a new vertical opening endpoint. To complicate the assessment of the effects of an increase in VDO, many patients treated by an increase in VDO in orthodontics are adolescents and are still growing, and it is unclear what effect growth has on this response.
A muscle's contractile properties are in part governed by the protein myosin. Muscle speed of contraction depends on the myosin heavy chain (MyHC) isoforms, and the major types of adult MyHC isoforms in mammalian skeletal muscles can be categorized as slow (MyHC I/β) or fast (MyHC II).5,6 Fast fiber types can further be separated into MyHC IIa, IIx/d, or IIb based on their contraction speeds (fibers with MyHC IIb are fastest contracting). The adult human masseter is composed of slow and fast, fatigue-resistant (IIa) fiber types, while the adult mouse masseter consists of only fast MyHC II fibers. Slow and fast muscles are preprogrammed; however, the final phenotypes of these muscles can vary due to the plasticity of muscle to functional demands, muscle stretch, and hormonal influences.7–10 In addition to MyHC plasticity, the cross-sectional area of muscle fibers can also change with functional demands to increase or decrease force production of the muscle fiber.
Muscle stretching can alter MyHC phenotype within muscle fibers. The effect of bite opening (or muscle stretch) on masseter MyHC fiber phenotype has not been examined in depth. Only a few studies have evaluated VDO and masseter MyHC plasticity11–13; however, in all studies, the authors opened the bite beyond an orthodontic clinical relevance (20%–50%). In humans the average maximum opening is about 45–50 mm,14 and orthodontic procedures open the anterior bite by about 3–4 mm, which is 10% or less of the maximum bite opening at the incisors.14 A smaller, yet clinically applicable, bite opening has not been investigated.
The purpose of this study was to examine the effects of a clinically relevant bite opening on the muscle fiber MyHC content in the adolescent male mouse masseter during maturation. Two hypotheses were tested: (1) the myosin heavy chain phenotype of the masseter muscle fibers will change to a slower (MyHC IIa) phenotype in response to the new, increased VDO; and (2) increasing the VDO will increase the mean diameter of muscle fibers containing MyHC IIa.
MATERIALS AND METHODS
Animals
CD-1 male mice (Charles Rivers Laboratories, Wilmington, Mass) were maintained in the animal facilities at the University of Florida Health Science Center. Twelve mice, 6 weeks of age, were used in the study. Sample size estimation was based on power-sample size calculations from previous data. Approval for this study was obtained from the Institutional Animal Care & Use Committee at the University of Florida, and all procedures complied with the Guide for Care and Use of Laboratory Animals.
Groups
The mice were divided into two groups: six mice were assigned to the control group and six mice were assigned to the treatment group. Both control (ctl) and experimental (exp) groups experienced the same anesthesia (ketamine and xylazine). The experimental group was subjected to an increase of vertical dimension using composite placed over all maxillary molars for 7 or 14 days, while the control group only had a sham manipulation consisting of mandibular retraction without composite placement. The incisors had no contact in the experimental group. Animal alimentation was the same for all groups, and weight was monitored during the course of the experiment. Mice were sacrificed after 7 days (ctl-7, n = 3; exp-7, n = 3) and 14 days (ctl-14, n = 3; exp-14, n = 3), and masseter muscles were harvested bilaterally, flash frozen, and stored at −20°C until sectioned.
Bite Opening
The maximum bite opening of a 6-week-old mouse was measured to be 10 mm using a caliper. Therefore, a 1-mm vertical separator was placed between the incisors to produce a 10% vertical opening. The right and left maxillary molars were dried; etched and flowable composite was applied and set at the 1-mm VDO increase. The control mice had the same manipulations including acid etching, but no composite was placed. All animals received an analgesic for the first 48 hours to manage postoperative discomfort.
MyHC Immunolabeling
One masseter from each animal was cryosectioned in the transverse plane. Serial 14 µm transverse sections of masseter were placed consecutively on sets of contiguous slides to allow the direct comparison of different antibody labeling. Sections were incubated overnight at 4°C in primary antibodies: mouse anti-IIa MyHC IgG (clone SC715); mouse anti-IIb MyHC IgM (clone BFF35); and rat anti-collagen IV (Biodesign) to delineate fibers, followed by appropriate secondary antibodies for 1 hour at room temperature. Fibers with MyHC IIx/d were not identified due to the lack of a reliable antibody. Sections were mounted in glycerol and viewed on a Nikon FXA photomicroscope. Controls for nonspecific antibody binding included sections incubated without primary antibodies and with secondary antibodies only. The immunolabeled transverse muscle section just superior to the aponeurosis of the superficial layer of the masseter was acquired using a digitizing camera (Zeiss Mrc5); the individual images comprising the section were assembled into collages (Photoshop CS2) and analyzed using Image-Pro Plus software (Media Cybernetics).
Muscle Fiber Sampling and Assessment
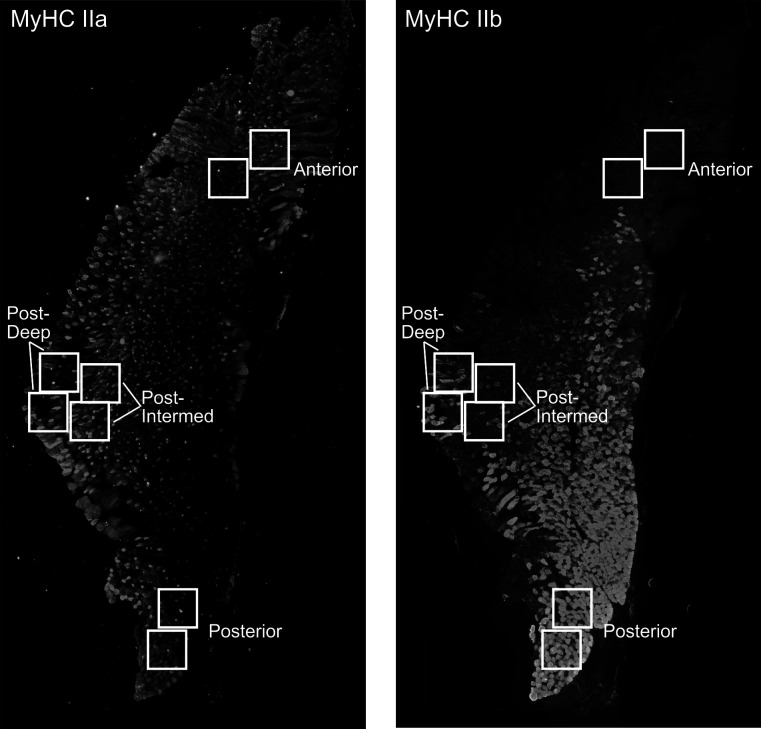
To obtain a representative sampling of the masseter section, four regions (anterior, posterior, posterior-intermediate, posterior-deep) were chosen based on the spatial distributions of MyHC isoforms and anatomy of the muscle (Figure 1).15 The anterior region represents an area with a high proportion of MyHC IIa fibers, while the posterior region has a high proportion of MyHC IIb fibers. The posterior-intermediate and posterior-deep regions have been identified as areas of fiber type differences between male and female.15
Figure 1. .
Representative images of transverse sections of the masseter immunostained for MyHC IIa and IIb showing the regions sampled. Squares delineate two standardized areas of interest per region (anterior, posterior, posterior-intermediate, and posterior-deep). (A) IIa fibers. (B) IIb fibers.
Each region was sampled using two 450 × 450 µm areas of interest. All fiber counts and fiber identifications were completed by a single investigator who was blinded to the source of the images to minimize bias. Each area of interest image was thresholded to remove background fluorescence, and the muscle fibers were outlined and then designated as IIa, IIb, IIa/b combination, or unlabeled. The fiber counts from the two areas of interest were pooled, and proportions of each fiber type for a designated region were then calculated. The diameter of each muscle fiber in the two areas of interest for each region was also pooled to characterize fiber size for each region. A test-retest of fiber type assessment was calculated and found to be 98% for fiber types MyHC IIa and IIb.
Statistical Analyses
MyHC phenotype proportions (IIa and IIb) and muscle fiber diameters were analyzed for differences across time (7 and 14 days), occlusal condition (increased vertical dimension of occlusion and sham manipulated), and location (four regions) utilizing a mixed model analysis of variance (ANOVA) and a probability level of P < .05. When the ANOVA was found to be significant, multiple comparisons were conducted using the least significant difference (LSD) test.
RESULTS
Both experimental and control mice gained weight during the period of study (7 or 14 days) (day 14 > day 7, P < .05), and no significant differences in weight were found between groups (P > .1).
Differences Between Control Groups Over Time
After calculation of the ANOVA for differences in the proportion of MyHC IIa and IIb, a statistically significant interaction was observed among all factors (regions, MyHC, group, and time). Post hoc comparisons were calculated for all combinations of factors for each region of the transverse section (LSD test; significant differences shown in Figure 2).
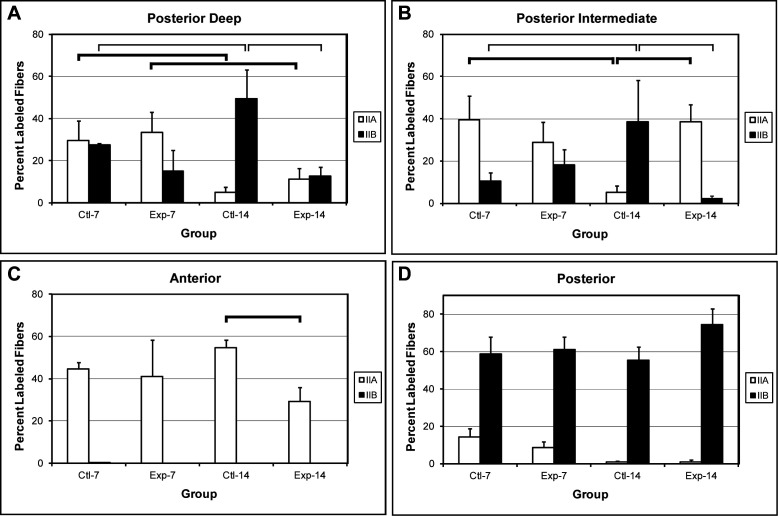
Figure 2. .
Bar graphs illustrating percent labeled MyHC IIa and IIb fibers in the four groups, control and experimental groups at 7 days and 14 days. Statistically significant differences are denoted between groups by horizontal lines: thick lines (MyHC IIa group differences) and thin lines (MyHC IIb group differences) (LSD test, * P < .05). (A) Posterior-deep region. (B) Posterior-intermediate region. (C) Anterior region. (D) Posterior region.
Statistically significant differences were observed in the proportion of fiber types in the posterior-deep region between control groups at 7 and 14 days (ctl-7 and ctl-14 groups). The proportion of IIa fibers decreased and the proportion of IIb fibers increased (Figure 2A, ctl-7 vs ctl-14). A similar significant change in the proportion of fiber types was found in the posterior-intermediate region (Figure 2B, ctl-7 vs ctl-14). No significant changes in fiber type proportions were detected in the anterior and posterior regions.
Effects of a VDO Increase
To evaluate the effects of an increase in VDO on MyHC fiber type proportions, statistical comparisons between the control and experimental groups were made after 7 days and 14 days of bite opening. No significant differences were found in the proportions of MyHC IIa or IIb fibers after 7 days of bite opening; however, significant differences were observed after 14 days of bite opening (Figure 2).
In the posterior-deep region, a significant difference between the control and experimental groups was found in the proportion of MyHC IIb fibers (49% vs 13%), while no difference was observed in the proportion of MyHC IIa fibers (Figure 2A). An effect of bite opening was also found in the posterior-intermediate region (Figure 2B). The proportion of MyHC IIb fibers (39% vs 2%) and MyHC IIa fibers (5% vs 39%) differed significantly between the control and experimental groups, respectively.
In the anterior region, no MyHC IIb fibers were identified. However, a significant reduction in the proportion of MyHC IIa fibers (55%–29%) between control and experimental groups was observed after 14 days of bite opening (Figure 2C). In the posterior region, no significant differences were found in the proportion of IIa or IIb fibers between 14 day control and experimental groups (Figure 2D). The posterior region contains predominantly MyHC IIb fibers with a very small proportion of IIa fibers. Bite opening did not have an obvious effect on the proportion of muscle fiber MyHC IIa or IIb phenotypes in this region.
Cross-Sectional Diameter
The cross-sectional diameter of the MyHC IIa and IIb muscle fibers did not vary across the control or experimental groups. However, a statistically significant difference was observed between the sizes of the IIa and IIb fibers, with the IIa fibers being consistently smaller (P < .05).
DISCUSSION
Using male adolescent mice to model the age of orthodontic patients, this study assessed the plasticity of the masseter muscle to a small, short-term vertical bite opening. The rapid decrease in the proportion of MyHC IIb protein in the experimental bite opening group over a 14-day period in this study was consistent with other reports in the literature that have evaluated MyHC responses in fast-contracting muscles. Chronic, low-frequency electrical stimulation of the rat tibialis anterior muscle reached a significant change in MyHC IIb protein levels after 8 days.16 In cranial nerve innervated extraocular muscle, a surgical shortening of the muscle caused a significant decrease in MyHC IIb protein 3 days after surgery.17
One advantage of this study was the examination of regions of the masseter muscle to assess potential local plastic changes after a VDO increase. It has been shown previously in the rabbit masseter that this muscle has multiple functional compartments that are activated at different times in the chewing cycle, depending on the task.18 In addition, the orientation of muscle fibers varies throughout the muscle, and those fibers with a more vertical orientation (posterior-intermediate and posterior-deep regions) would be more highly affected by a muscle stretch than oblique fibers. In fact, the mouse masseter posterior-intermediate and posterior-deep regions were found to be responsive to a small increase in VDO with a relative proportional decrease in MyHC IIb fibers and an increase in MyHC IIa fibers when compared to controls at 14 days. Interestingly, a different response was found in the anterior region with a relative decrease in the proportion of MyHC IIa fibers, while no change was found in the posterior region of the muscle. These findings are clinically relevant in that the significant changes in fiber type phenotype observed in VDO may influence bite force production and speed of contraction.
One possible explanation for the maintenance of a relatively slower phenotype with increased bite opening in the posterior-intermediate and posterior-deep regions might be fiber stretch.8,9 Muscle stretch of the rabbit extensor digitorum longus muscle causes a MyHC transition to a slower phenotype.9 Additionally, in rat masseter studies that used an approximate 20% increase in the VDO, a relative decrease in MyHC IIb and increase in MyHC IIa message was also observed.12,13 However, a second possible explanation for the maintenance of a slower phenotype in our bite opening group is an increase in muscle function. Although, the experimental mice were eating and gaining weight, they may have changed their chewing patterns and masticatory muscle activity. Motor units in the posterior-intermediate and posterior-deep areas may have been preferentially recruited to chew or brux due to composite placement on maxillary molars. Increased regional muscle activation has been shown in adult rat tibialis anterior muscle to promote a slower phenotype.19 The change in motor activation strategies also may have affected the anterior masseter by reduced activation of this region. The anterior region of the masseter muscle is typically recruited during incising,20 and the ability to incise would theoretically be reduced with the increase in vertical dimension due to the increase in gape. The reduced activation would preferentially favor the presence of faster contracting fibers and would result in the relative reduction in the proportion of MyHC IIa fibers. Since no MyHC IIb positive fibers were identified in the anterior region, the faster phenotype could be MyHC IIx, a phenotype that was not evaluated in this study.
In the control mice, a transition to a faster phenotype represented by increases in the proportion of MyHC IIb and decreases in the proportion of MyHC IIa occurred in the posterior-deep and posterior-intermediate regions of the masseter muscle between 7 and 14 days. MyHC IIb positive fibers have been found in higher proportions in the male mouse masseter compared to the female, and sexual dimorphism has been observed in these two regions.15,21
In summary, our study demonstrated that in control male mouse masseter 2 weeks of maturation was accompanied by transition towards a faster (IIb) phenotype. This maturation shift towards a faster phenotype in the posterior-deep and posterior-intermediate regions did not occur in masseter muscles from male mice in which bite opening was increased by 10%. The long-term effects of bite opening are currently unclear and require further examination.
CONCLUSIONS
A short-term (7–14 days) increase in the VDO results in changes in muscle fiber phenotype in the mouse masseter. In specific regions of the muscle, there is a shift from the faster contracting IIb fiber type to the slower contracting IIa fiber type.
These findings support the conclusion that bite-opening procedures such as those used in orthodontics can have a rapid effect on the muscle fiber contractile characteristics. The reversibility of these changes has not yet been evaluated.
REFERENCES
- 1.Muratore F, Varvara G, Tripodi D, De Simone R, Pascetta C, Festa F. Cerec 3 for orthodontics: a tool for treating deep bite [in English, Italian] Int J Comput Dent. 2002;5:25–31. [PubMed] [Google Scholar]
- 2.Lindauer SJ, Gay T, Rendell J. Effect of jaw opening on masticatory muscle EMG-force characteristics. J Dent Res. 1993;72:51–55. doi: 10.1177/00220345930720010701. [DOI] [PubMed] [Google Scholar]
- 3.Matano T, Tamai K, Kurokawa T. Adaptation of skeletal muscle in limb lengthening: a light diffraction study on the sarcomere length in situ. J Orthop Res. 1994;12:193–196. doi: 10.1002/jor.1100120207. [DOI] [PubMed] [Google Scholar]
- 4.Coutinho EL, Gomes AR, Franca CN, Oishi J, Salvini TF. Effect of passive stretching on the immobilized soleus muscle fiber morphology. Braz J Med Biol Res. 2004;37:1853–1861. doi: 10.1590/s0100-879x2004001200011. [DOI] [PubMed] [Google Scholar]
- 5.Schiaffino S, Reggiani C. Myosin isoforms in mammalian skeletal muscle. J Appl Physiol. 1994;77:493–501. doi: 10.1152/jappl.1994.77.2.493. [DOI] [PubMed] [Google Scholar]
- 6.Pette D, Staron RS. Myosin isoforms, muscle fiber types, and transitions. Microsc Res Tech. 2000;50:500–509. doi: 10.1002/1097-0029(20000915)50:6<500::AID-JEMT7>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 7.Agbulut O, Noirez P, Beaumont F, Butler-Browne G. Myosin heavy chain isoforms in postnatal muscle development of mice. Biol Cell. 2003;95:399–406. doi: 10.1016/s0248-4900(03)00087-x. [DOI] [PubMed] [Google Scholar]
- 8.Morgan MJ, Loughna PT. Work overload induced changes in fast and slow skeletal muscle myosin heavy chain gene expression. FEBS Lett. 1989;255:427–430. doi: 10.1016/0014-5793(89)81138-x. [DOI] [PubMed] [Google Scholar]
- 9.Yang H, Alnaqeeb M, Simpson H, Goldspink G. Changes in muscle fibre type, muscle mass and IGF-I gene expression in rabbit skeletal muscle subjected to stretch. J Anat. 1997;190(Pt 4):613–622. doi: 10.1046/j.1469-7580.1997.19040613.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reader M, Schwartz G, English A. W. Brief exposure to testosterone is sufficient to induce sex differences in the rabbit masseter muscle. Cells Tissues Organs. 2001;169:210–217. doi: 10.1159/000047884. [DOI] [PubMed] [Google Scholar]
- 11.Faulkner JA, McCully KK, Carlson DS, McNamara JA Contractile properties of the muscles of mastication of rhesus monkeys (Macaca mulatta) following increase in muscle length. Arch Oral Biol. 1982;27:841–845. doi: 10.1016/0003-9969(82)90039-5. [DOI] [PubMed] [Google Scholar]
- 12.Ohnuki Y, Saeki Y, Yamane A, Yanagisawa K. Quantitative changes in the mRNA for contractile proteins and metabolic enzymes in masseter muscle of bite-opened rats. Arch Oral Biol. 2000;45:1025–1032. doi: 10.1016/s0003-9969(00)00085-6. [DOI] [PubMed] [Google Scholar]
- 13.Arai C, Ohnuki Y, Umeki D, Saeki Y. Effects of bite-opening and cyclosporin a on the mRNA levels of myosin heavy chain and the muscle mass in rat masseter. Jpn J Physiol. 2005;55:173–179. doi: 10.2170/jjphysiol.R2123. [DOI] [PubMed] [Google Scholar]
- 14.Dworkin SF, Huggins KH, Le Resche L, von Korff MR, Howard J, Truelove E, Sommers E. Epidemiology of signs and symptoms in temporomandibular disorders: clinical signs in cases and controls. J Am Dent Assoc. 1990;120:273–281. doi: 10.14219/jada.archive.1990.0043. [DOI] [PubMed] [Google Scholar]
- 15.Widmer CG, Morris-Wiman JA, Nekula C. Spatial distribution of myosin heavy-chain isoforms in mouse masseter. J Dent Res. 2002;81:33–38. doi: 10.1177/002203450208100108. [DOI] [PubMed] [Google Scholar]
- 16.Termin A, Pette D. Changes in myosin heavy-chain isoform synthesis of chronically stimulated rat fast-twitch muscle. Eur J Biochem. 1992;204:569–573. doi: 10.1111/j.1432-1033.1992.tb16669.x. [DOI] [PubMed] [Google Scholar]
- 17.Park SC, Kim YT, Kim SA, Oh SY. Changes in muscle fiber size and in the composition of myosin heavy chain isoforms of rabbit extraocular rectus muscle following recession surgery. Jpn J Ophthalmol. 2008;52:386–392. doi: 10.1007/s10384-008-0568-0. [DOI] [PubMed] [Google Scholar]
- 18.Widmer CG, Carrasco DI, English AW. Differential activation of neuromuscular compartments in the rabbit masseter muscle during different oral behaviors. Exp Brain Res. 2003;150:297–307. doi: 10.1007/s00221-003-1464-y. [DOI] [PubMed] [Google Scholar]
- 19.Kirschbaum BJ, Schneider S, Izumo S, Mahdavi V, Nadal-Ginard B, Pette D. Rapid and reversible changes in myosin heavy chain expression in response to increased neuromuscular activity of rat fast-twitch muscle. FEBS Lett. 1990;268:75–78. doi: 10.1016/0014-5793(90)80976-p. [DOI] [PubMed] [Google Scholar]
- 20.Weijs WA, Dantuma R. Functional anatomy of the masticatory apparatus in the rabbit. Neth J Zool. 1981;31:99–147. [Google Scholar]
- 21.Eason JM, Schwartz GA, Pavlath GK, English AW. Sexually dimorphic expression of myosin heavy chains in the adult mouse masseter. J Appl Physiol. 2000;89:251–258. doi: 10.1152/jappl.2000.89.1.251. [DOI] [PubMed] [Google Scholar]