Abstract
Objective:
To compare changes in enamel microhardness adjacent to orthodontic brackets after using bonding agents containing various compositions of bioactive glass compared to a traditional resin adhesive following a simulated caries challenge.
Materials and Methods:
Extracted human third molars (n = 10 per group) had orthodontic brackets bonded using one of four novel bioactive glass (BAG)-containing orthodontic bonding agents (BAG-Bonds) or commercially available Transbond-XT. The four new adhesives contained BAG in varying percentages incorporated into a traditional resin monomer mixture. Teeth were cycled through low-pH demineralizing and physiologic-pH remineralizing solutions once each day over 14 days. Microhardness was measured on longitudinal sections of the teeth 100, 200, and 300 µm from the bracket edge and beneath the brackets, at depths of 25 to 200 µm from the enamel surface. Normalized hardness values were compared using three-way analysis of variance.
Results:
Significantly less reduction in enamel microhardness was found with the experimental adhesives at depths of 25 and 50 µm at all distances from the bracket edge. In all groups, there were no significant changes in enamel microhardness past 125-µm depth. Results varied with the different BAG-Bonds, with 81BAG-Bond showing the smallest decrease in enamel microhardness.
Conclusions:
The BAG-Bonds tested in this study showed a reduction in the amount of superficial enamel softening surrounding orthodontic brackets compared to a traditional bonding agent. The results indicate that clinically, BAG-Bonds may aid in maintaining enamel surface hardness, therefore helping prevent white spot lesions adjacent to orthodontic brackets.
Keywords: Bioactive glass, Orthodontic adhesive, White spot lesions, Microhardness
INTRODUCTION
Formation of incipient caries, commonly called white spot lesions (WSLs), is an unesthetic, common side effect of orthodontic treatment with fixed appliances.1 WSLs have been defined as clinically detectable manifestations of subsurface enamel demineralization, representing the first stage of caries formation.2 Mineral loss in these lesions can be up to 50% of the inorganic content1 and can result in changes in hardness and refractive index of the enamel, causing a scattering of light and giving the enamel a chalky, opaque appearance.3 WSLs are both an esthetic concern and a disconcerting visible sign of enamel demineralization secondary to orthodontic treatment.
The prevalence of WSLs during orthodontic treatment (nearly 50% of patients after 12 months)4,5 is due to many factors. First, fixed orthodontic appliances make oral hygiene more difficult, predisposing patients to an increase in plaque build-up on tooth surfaces at the gingival margin and adjacent to attachments.6 Second, the addition of orthodontic appliances in the mouth creates a rapid increase in bacterial flora, predominantly Streptococcus mutans and lactobacilli.7 This reduces the pH at the plaque/enamel interface, causing calcium and phosphate ions to migrate from enamel apatite into solution, resulting in mineral loss.8 Together, these factors create an environment that favors demineralization of enamel, compromises the esthetic result, and in severe cases, requires restorative treatment.5,9
Preventing development of WSLs during orthodontic treatment has been attempted through various approaches. Methods involving fluoride-containing mouth rinses, gels, varnishes, and dentifrices, have reduced the prevalence of caries during orthodontic treatment, but compliance is unpredictable and the ability to supply fluoride to areas where it is needed presents challenges.10–12
Conceptually, fluoride-releasing bonding agents have great potential to minimize decalcification around orthodontic brackets.13 However, traditional fluoride-releasing cements, glass-ionomer cements, and resin-modified glass ionomer cements have bond strengths that are substantially lower than those of conventional resins.14,15 Moreover, with recently introduced amorphous calcium phosphate–based remineralization materials, such as MI Paste™ (GC America, Alsip, IL), clinical trials have found insufficient evidence to make a recommendation regarding their long-term effectiveness.16A noncompliance-based material with sustained ion release near the brackets may provide an ideal preventive solution for WSL formation.
Bioactive glass (BAG) materials have recently been incorporated into the field of dentistry and are surface-active materials known to successfully release ions in simulated body fluid.17 Sol-gel BAG, prepared in our laboratory, is a three-dimensional cross-linked matrix of hydrolyzed alkoxides of SiO2, CaO, and P2O5 that releases ions such as calcium, phosphate, and fluoride.18,19 This release of ions into surrounding solution is a process that has the potential to prevent demineralization of enamel and thereby to prevent WSL formation around orthodontic brackets.
Previous investigations of the BAG-containing orthodontic resin bonding agents (BAG-Bonds) used in this study showed significantly higher calcium and phosphate ion release in acidic media than conventional resin adhesive controls.20 This capacity of BAG-Bonds to release ions and buffer acidic environments may help to prevent demineralization surrounding orthodontic brackets.
The aims of this study were (1) to evaluate the ability of BAG-Bonds to inhibit superficial enamel demineralization surrounding orthodontic brackets after being exposed to an in vitro caries challenge, and (2) to test the hypothesis that these novel adhesives will result in reduced demineralization surrounding orthodontic brackets compared to a conventional bonding resin.
MATERIALS AND METHODS
Preparation of BAG-Bond
Four BAG-Bonds (62BAG-Bond, 65BAG-Bond, 81BAG-Bond, and 85BAG-Bond) were developed in our laboratory (Table 1). The BAG-Bonds were prepared by mixing resin monomers with bioactive glass, an accelerator and an amine, as previously described.20 BAGs were synthesized by sol-gel methods18 and added to the monomer mixture until the workability of each product was similar to the control material, Transbond-XT (3M Unitek, Monrovia, Calif), as judged by an experienced clinician.
Table 1. .
Bioactive Glass (BAG)-Bonds: Composition, Mol%, Surface Area, and BAG:Monomer Ratiosa
Sample Preparation
Fifty extracted, caries-free, nonerupted human third molars were collected and stored in 0.5% chloramine-T solution at 4°C. They were free of white spot lesions or other enamel defects. Teeth were rinsed with deionized water and randomly assigned to five groups (n = 10 each), as shown in Table 1.
The enamel was cleaned with a prophylaxis cup at slow speed using nonfluoridated pumice and water. For each tooth, the bonding site was based on the best visual adaptation of the bracket pad. To protect adjacent enamel surfaces during etching, tape with a window the size of the bracket base was applied to each tooth.13
The window region was etched with 37% phosphoric acid gel (3M Unitek) for 30 seconds and copiously rinsed with deionized water. The tape was removed and the tooth was dried with compressed air.
After application of primer (Transbond-XT Primer, 3M Unitek), enough resin adhesive to cover the entire surface was applied to the mesh pad of each bracket (Victory Series, 3M Unitek). Excess primer and resin were removed with a sharp scaler and adhesives were light-cured (20 seconds) from the mesial and distal sides (Ortholux LED, 3M Unitek). Acid-resistant varnish was applied to each tooth, leaving a 1-mm rim of exposed enamel surrounding the bracket. Teeth were stored overnight in distilled water prior to pH cycling.
pH Cycling
A 14-day caries challenge was created, based on a modification of a pH cycling protocol described by Toda and Featherstone.21 Each tooth was immersed in 40 mL of artificial saliva at pH 7.0 (1.5 mmol/L Ca, 0.9 mmol/L PO4, 0.1 5 mol/L KCl, and 20 mmol/L cacodylate buffer) for 18 hours, followed by 6 hours in 40 mL of buffered artificial caries challenge solution at pH 4.4 (2.0 mmol/L Ca, 2.0 mmol/L PO4, 0.075 mol/L acetate). Fresh solutions were prepared each week. Between fluid changes, teeth were rinsed with deionized water. The cycle was repeated 5 days a week, with teeth remaining in artificial saliva during weekends.
Assessment of Demineralization
Teeth were embedded in clear epoxy resin (Epoxicure, Buehler, Lakebluff, Ill) and sectioned parallel to the long axis, buccolingually through the brackets using a water-cooled diamond wafering blade on a low-speed rotary saw, producing one 2-mm section for each tooth (Accutom-5, Struers Inc, Westlake, Ohio). Sections were serially polished through 4000-grit silicon carbide polishing paper.
Knoop microhardness testing (Duramin-5, Struers ) was used for the microhardness analysis (25 p/5 sec). On each tooth, 48 indentations were made occlusal and cervical to the adhesive: at 100, 200, and 300 µm from the adhesive edge, and at eight depths from the external surface of the enamel: 25, 50, 75, 100, 125, 150, 175, and 200 µm (Figure 1). Three sets of indentations were also made in the isolated enamel directly beneath the bracket at the same eight depths, to create a baseline microhardness measurement at each depth. The three Knoop hardness values obtained for each tooth, at each depth in enamel covered by the bracket, were averaged and used to normalize the other data points by dividing them by the corresponding average Knoop hardness values at each depth. Data are presented as percent change in microhardness vs baseline.
Figure 1. .
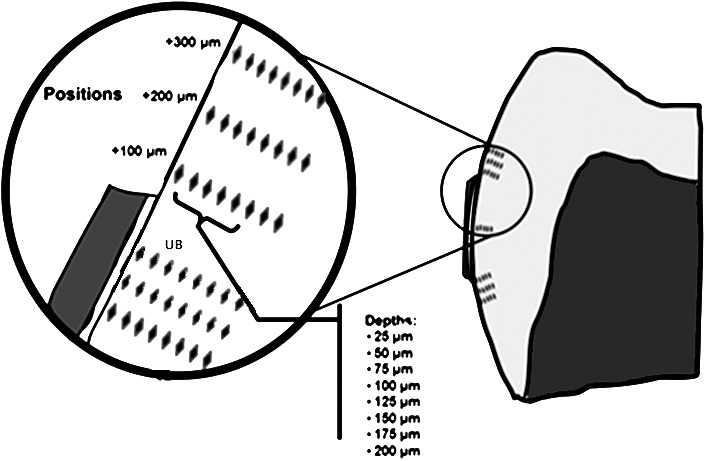
Location of indentations demonstrating distances from bracket edge and depths into enamel. UB indicates indents taken underneath the bracket and used to normalize data measurements from each tooth.
Statistical Analysis
Three-way analysis of variance (ANOVA) with Tukey post-hoc test (SAS, SAS, Cary, NC) compared the percentage change in microhardness vs distance and depth (α = .05).
RESULTS
Locations that showed significant change in microhardness for each adhesive are shown in Figure 2. Enamel in the 81BAG-Bond group was significantly softened at a depth of 25 µm from the surface at all three distances from the adhesive edge, and at a depth of 50 µm at distances of 100 and 300 µm. The 62BAG-Bond and 85BAG-Bond groups showed similar results, with significant reductions in hardness occurring to 75 µm from the surface at all three distances, as well as to 100 µm deep, 100 µm from the bracket. The 65BAG-Bond and Transbond-XT groups had reductions in enamel hardness that extended the deepest, with significant softening occurring to depths between 100 and 150 µm.
Figure 2. .
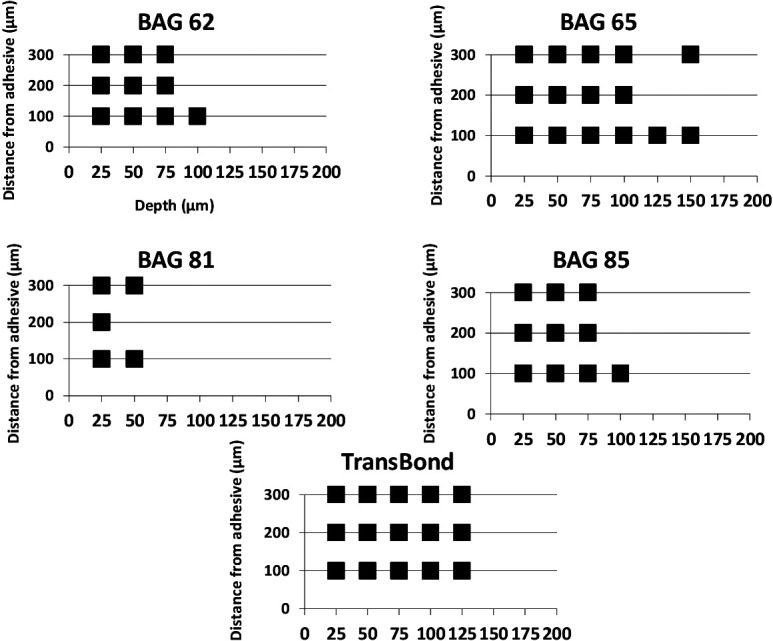
Square symbols indicate locations where significant reductions in microhardness (P ≤ .01) were found after normalizing.
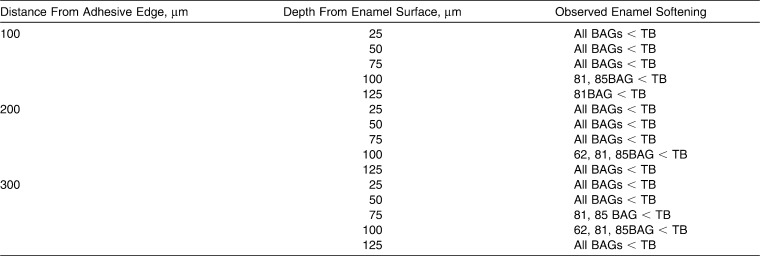
Comparison of changes in microhardness values for each group at the recorded location across the WSL are shown in Figure 3 and Table 2. At 25 and 50 µm deep at all distances from the bracket edge, all BAG-Bond adhesives showed significantly less reduction in hardness than Transbond-XT (P < .05).
Figure 3. .

Mean (standard error) percentage change in enamel microhardness at 100 µm, 200 µm, and 300 µm from the bracket edge. * P ≤ .05.
Table 2. .
Comparison of Significant Decreases in Microhardness (P ≤ .05 for Bioactive Glass [BAG]-Bond Groups) Compared to the Transbond XT (TB) Group at Depths up to 125 µm
Results varied for the intermediate depths up to 125 µm. Beyond 125-µm depth at all distances, no significant differences in enamel microhardness were found among the five adhesive groups.
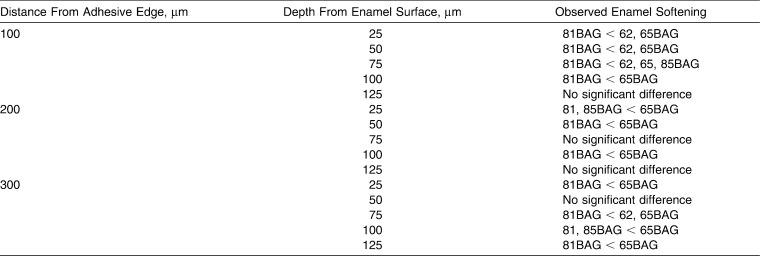
Results comparing the BAG-Bond adhesive groups to each other, at all three distances and at depths up to 125 µm are shown in Table 3. At 100 µm from the bracket edge and at depths of 25, 50, and 75 µm, 81BAG-Bond had less reduction in enamel microhardness than 62BAG-Bond and 65BAG-Bond.
Table 3. .
Comparison of Significant Changes in Microhardness (P ≤ .05) Among Bioactive Glass (BAG)-Bond Groups at Depths up to 125 µm
DISCUSSION
WSLs form in superficial enamel due to outward diffusion of calcium and phosphate ions, eventually leading to cavitation if the process continues.22,23 Our BAG-containing adhesives have previously shown calcium ion-release into surrounding solution.20 This study evaluated the potential use of these novel BAG-containing orthodontic bonding agents for the prevention of WSLs. Four different BAG-Bond adhesive resins were used to bond orthodontic brackets to extracted human teeth and were pH-cycled through a caries challenge that has been shown to correlate well with conditions associated with 1 month of fixed orthodontic appliance use in vivo.24 Comparisons in the present study were made to the popular, commercial adhesive, Transbond-XT.
Demineralization around orthodontic brackets has been assessed using various methods. In this study, areas of mineral loss due to acidic challenge were evaluated using cross-sectional microhardness values. This method can be correlated to caries as there exists a strong correlation (r = 0.91) between enamel microhardness and percentage of mineral in the caries lesion.22 Since WSLs are areas of low mineral content, microhardness measurements provide a reliable indication of enamel demineralization and potential WSL development.
We used an in vitro pH-cycling model for the evaluation of demineralization surrounding novel BAG-containing adhesives. This widely used protocol of exposing enamel to combinations of demineralization and remineralization is designed to mimic the dynamics of mineral loss and gain involved in caries formation.25 However, this in vitro protocol has important limitations, as it is not able to completely simulate the complex intraoral conditions leading to caries development such as bacterial biofilms and saliva, nor is it able to simulate clearance of the products from the oral cavity.26 These limitations should be kept in mind when evaluating the results. Nonetheless, previous in vitro investigations of an experimental glass ionomer cement that contained BAG found the bonding agent inhibited growth of cariogenic bacteria.27 Thus, this anticariogenic effect may also contribute to BAG-Bond's potential for preventing WSLs.
Teeth in the 81BAG-Bond group were significantly demineralized only to depths of 25 and 50 µm. The 81BAG-Bond contains BAG with the highest surface area (320 m2/g). Previous studies have shown that BAGs with high-specific surface area and pore volume contribute to a high release of ions.28 BAG's capacity to be a reservoir of available ions held by weak ionic bonds allows for easier release of calcium to the surroundings than from the tooth surface. In addition to its higher surface area, 81BAG-Bond also contains fluoride. Released fluoride ions may become incorporated in the tooth mineral and promote formation of highly insoluble fluoroapatite.20 In the current study, 81BAG-Bond demonstrated superior ability to prevent demineralization, perhaps due to an increased release of ions and a higher bioactivity rate when compared to the other adhesives.
Teeth bonded with 62BAG-Bond and 85BAG-Bond displayed significant demineralization from 25 to 75 µm deep at all three distances from the adhesive edge, and at 100 µm deep at 100 µm distant from the bracket edge. 62BAG-Bond contains fluoride and 31 mol% calcium, yet has the lowest surface area of all BAGs (75 m2/g). The 85BAG-Bond contains no fluoride and only 11 mol% calcium, yet has the second highest surface area (268 m2/g). Seemingly, these two BAG-Bond resins do not have the combination of high surface area and fluoride that allows for maximal demineralization prevention, as seen with 81BAG-Bond. While ion release increases with increased surface area, our results indicate that incorporation of fluoride in the BAG-Bond is needed for maximal WSL prevention.
Teeth in both the 65BAG-Bond and Transbond-XT groups demonstrated significant demineralization from depths up to 125 µm at all distances. Although 65BAG-Bond has a high calcium content, it lacks fluoride and has the second lowest surface area of all the BAGs tested. Transbond-XT is a non–ion-releasing composite resin without detectable fluoride release, and in the absence of daily fluoride exposure, has been associated with extensive erosive enamel lesions.29 Thus, combining the results of all groups indicates that key factors for combating demineralization include the release of fluoride and increased BAG surface area, whereas increasing the calcium content has less impact.
Microhardness tests on all five adhesives showed the greatest decrease in enamel microhardness at the superficial depths of 25 and 50 µm. All four BAG-Bonds demonstrated significantly less demineralization than the Transbond-XT control at these depths at all distances from the bracket. This result is encouraging as others have shown that preventive measures are most effective when the WSL formation is in its earliest stage because remineralization can take place if the lesions are less than 65 µm in depth.30 Others have also demonstrated that a WSL after 3 months in vivo typically extends to around 100 µm deep.1 Since the BAG-bond adhesives in this study reduced demineralization at these superficial depths, these adhesives hold potential for preventing WSL formation within the boundaries of what occurs in vivo.
At the middle depths of 75, 100, and 125 µm from the enamel surface, results varied. At 75-µm depth and 100 and 200 µm from the bracket, and 125 µm deep at 200 and 300 µm from the bracket, all teeth within the BAG-Bond groups had less demineralization than the control. These results suggest that the WSLs created by pH cycling were most likely shallow lesions in their initial stages. At 125-µm depth and deeper, all five groups showed no significant differences. The enamel at these depths remained largely unaffected by the demineralization challenge, most likely due to the brevity of the acidic exposure.
Brown et al.20 investigated the ion release profiles of each of the novel BAG-Bonds tested in this study. Their results indicated that BAG-Bonds may decrease the rate of enamel demineralization by increasing the pH in the milieu adjacent to the bracket/tooth interface, as well as by releasing a large amount of calcium ions into solution.20 These results, consistent with our current findings, suggest that incorporating BAG into resin adhesives provides a reservoir of calcium, phosphate, and fluoride ions that help to prevent enamel demineralization surrounding orthodontic brackets. Investigations are currently underway to determine whether periodic use of calcium and fluoride recharging solutions may extend the ion-release lifetimes of the adhesives beyond the times reported here.
CONCLUSIONS
All BAG-Bond adhesives outperformed Transbond-XT at maintaining superficial enamel hardness surrounding orthodontic brackets.
81BAG-Bond, containing fluoride and a high BAG surface area, had the best preventive effect against WSL formation.
Combining an ideal bioactive glass into resin adhesives helps to reduce superficial enamel softening surrounding orthodontic brackets compared to a conventional resin adhesive.
REFERENCES
- 1.Ogaard B, Rolla G, Arends J. Orthodontic appliances and enamel demineralization. Part 1. Lesion development. Am J Orthod Dentofacial Orthop. 1988;94:68–73. doi: 10.1016/0889-5406(88)90453-2. [DOI] [PubMed] [Google Scholar]
- 2.Chang HS, Walsh LJ, Freer TJ. Enamel demineralization during orthodontic treatment. Aetiology and prevention. Aust Dent J. 1997;42:322–327. doi: 10.1111/j.1834-7819.1997.tb00138.x. [DOI] [PubMed] [Google Scholar]
- 3.Mayne E, Cochrane N, Cai F, Woods M, Reynolds E. In-vitro study of the effect of casein phosphopeptide amorphous calcium fluoride phosphate on iatrogenic damage to enamel during orthodontic adhesive removal. Am J Orthod Dentofacial Orthop. 2011;139:543–551. doi: 10.1016/j.ajodo.2010.09.027. [DOI] [PubMed] [Google Scholar]
- 4.Gorelick L, Geiger AM, Gwinnett AJ. Incidence of whitespot formation after bonding and banding. Am J Orthod Dentofacial Orthop. 1982;81:93–98. doi: 10.1016/0002-9416(82)90032-x. [DOI] [PubMed] [Google Scholar]
- 5.Tufekci E, Dixon JS, Gunsolley JC, Lindauer SJ. Prevalence of white spot lesions during orthodontic treatment with fixed appliances. Angle Orthod. 2011;81:206–210. doi: 10.2319/051710-262.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gomes SC, Varela CC, deVeiga SL, Rosing CK, Opperman RV. Periodontal conditions in subjects following orthodontic therapy. Eur J Orthod. 2007;29:477–481. doi: 10.1093/ejo/cjm050. [DOI] [PubMed] [Google Scholar]
- 7.Lundstrom F, Krasse B. Streptococcus mutans and lactobacilli frequency in orthodontic patients: the effect of chlorhexidine treatments. Eur J Orthod. 1987;9:109–116. doi: 10.1093/ejo/9.2.109. [DOI] [PubMed] [Google Scholar]
- 8.Featherstone JD. The science and practice of caries prevention. J Am Dent Assoc. 2000;131:887–899. doi: 10.14219/jada.archive.2000.0307. [DOI] [PubMed] [Google Scholar]
- 9.Chapman J, Roberts W, Eckert G, Kula K, Gonzalez-Cabezas C. Risk factors for incidence and severity of white spot lesions during treatment with fixed orthodontic appliances. Am J Orthod Dentofacial Orthop. 2010;138:188–195. doi: 10.1016/j.ajodo.2008.10.019. [DOI] [PubMed] [Google Scholar]
- 10.O'Reilly MM, Featherstone JD. Demineralization and remineralization around orthodontic appliances: an in vivo study. Am J Orthod Dentofacial Orthop. 1987;92:33–40. doi: 10.1016/0889-5406(87)90293-9. [DOI] [PubMed] [Google Scholar]
- 11.Ogaard B, Rolla G, Arends J, ten Cate JM. Orthodontic appliances and enamel demineralization. Part 2. Prevention and treatment of lesions. Am J Orthod Dentofacial Orthop. 1988;94:123–128. doi: 10.1016/0889-5406(88)90360-5. [DOI] [PubMed] [Google Scholar]
- 12.Geiger AM, Gorelick L, Gwinnett AJ, Griswold PG. The effect of a fluoride program on white spot formation during orthodontic treatment. Am J Orthod Dentofacial Orthop. 1988;93:29–37. doi: 10.1016/0889-5406(88)90190-4. [DOI] [PubMed] [Google Scholar]
- 13.Todd MA, Staley RN, Kanellis MJ, Donly KJ, Wefel JS. Effect of a fluoride varnish on demineralization adjacent to orthodontic brackets. Am J Orthod Dentofacial Orthop. 1999;116:159–167. doi: 10.1016/s0889-5406(99)70213-1. [DOI] [PubMed] [Google Scholar]
- 14.Gaworski M, Weinstein M, Borislow AJ, Braitman LE. Decalcification and bond failure: a comparison of a glass ionomer and a composite resin bonding system in vivo. Am J Orthod Dentofacial Orthop. 1999;116:518–521. doi: 10.1016/s0889-5406(99)70182-4. [DOI] [PubMed] [Google Scholar]
- 15.Millett DT, McCabe JF. Orthodontic bonding with glass ionomer cement. Eur J Orthod. 1996;18:385–399. doi: 10.1093/ejo/18.4.385. [DOI] [PubMed] [Google Scholar]
- 16.Azarpazhooh A, Limeback H. Clinical efficacy of casein derivatives: a systematic review of the literature. J Am Dent Assoc. 2008;139:915–924. doi: 10.14219/jada.archive.2008.0278. [DOI] [PubMed] [Google Scholar]
- 17.Hench LL, Wilson J. Surface-active biomaterials. Science. 1984;226:630–636. doi: 10.1126/science.6093253. [DOI] [PubMed] [Google Scholar]
- 18.Hench LL. The story of bioglass. J Mater Sci Mater Med. 2006;17:967–978. doi: 10.1007/s10856-006-0432-z. [DOI] [PubMed] [Google Scholar]
- 19.Mitchell JC. In-vivo aging of bioactive glasses and other graft materials. In: Eliades G, Eliades T, Brantley W, Watts D, editors. Dental Materials In Vivo Aging and Related Phenomena. Chicago, Ill : Quintessence; 2003. pp. 263–276. [Google Scholar]
- 20.Brown ML, Davis HB, Tufekci E, Crowe JJ, Covell DA, Mitchell JC. Ion release from a novel bioactive orthodontic bonding agent for the prevention of white spot lesions: an in-vitro study. Angle Orthod. 2011;81:1014–1020. doi: 10.2319/120710-708.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Toda S, Featherstone JD. Effects of fluoride dentrifices on enamel lesion formation. J Dent Res. 2008;87:224–227. doi: 10.1177/154405910808700303. [DOI] [PubMed] [Google Scholar]
- 22.Featherstone JD, ten Cate JM, Shariati M, Arends J. Comparison of artificial caries-like lesions by quantitative microradiography and microhardness profiles. Caries Res. 1983;17:385–391. doi: 10.1159/000260692. [DOI] [PubMed] [Google Scholar]
- 23.Featherstone JD. The continuum of dental caries—evidence for a dynamic disease process. J Dent Res. 2004;83(special issue C):C39–C42. doi: 10.1177/154405910408301s08. [DOI] [PubMed] [Google Scholar]
- 24.Featherstone JD, O'Reilly MM, Shariati M, Brugler S. Enhancement of remineralization in vitro and in vivo. In: Leach SA, editor. Factors Relating to Demineralization and Remineralization of the Teeth. Oxford, UK : IRL Press; 1986. pp. 23–24. [Google Scholar]
- 25.White DJ. The application of in vitro models to research on demineralization and remineralization of the teeth. Adv Dent Res. 1995;9:175–193. doi: 10.1177/08959374950090030101. [DOI] [PubMed] [Google Scholar]
- 26.Buzalaf M, Hannas A, Magalhaes A, Rios D, Honorio H, Delbem A. pH-cycling models for in vitro evaluation of the efficacy of fluoridated dentrifices for caries control: strengths and limitations. J Appl Oral Sci. 2010;18:316–334. doi: 10.1590/S1678-77572010000400002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mitchell JC, Astashkina A, Park S, Baumgartner C. Antimicrobial effect of sol-gel bioactive glasses. J Dent Res. 2006;85(special issue A):0541. [Google Scholar]
- 28.Misra SK, Mohn D, Brunner TJ, Stark WJ, Philip SE, Roy I, Salih V, Knowles JC, Boccaccini AR. Comparison of nanoscale and microscale bioactive glass on the properties of P(3HB)/Bioglass composites. Biomaterials. 2008;29:1750–1761. doi: 10.1016/j.biomaterials.2007.12.040. [DOI] [PubMed] [Google Scholar]
- 29.Chin M, Sandham A, Rumachik E, Ruben J, Huysmans M. Fluoride release and cariostatic potential of orthodontic adhesives with and without daily fluoride rinsing. Am J Orthod Dentofacial Orthop. 2009;136:547–553. doi: 10.1016/j.ajodo.2007.10.053. [DOI] [PubMed] [Google Scholar]
- 30.Joyston-Bechal S, Kidd EA. Histopathological appearance of artificially produced caries-like lesions of enamel treated with APF during lesion formation in vitro. Caries Res. 1980;14:45–49. doi: 10.1159/000260433. [DOI] [PubMed] [Google Scholar]