Abstract
Insights into the genetic basis of human disease are helping to address some of the key challenges in new drug development including the very high rates of failure. Here we review the recent history of an emerging, genomics-assisted approach to pharmaceutical research and development, and its relationship to Mendelian randomization (MR), a well-established analytical approach to causal inference. We demonstrate how human genomic data linked to pharmaceutically relevant phenotypes can be used for (1) drug target identification (mapping relevant drug targets to diseases), (2) drug target validation (inferring the likely effects of drug target perturbation), (3) evaluation of the effectiveness and specificity of compound-target engagement (inferring the extent to which the effects of a compound are exclusive to the target and distinguishing between on-target and off-target compound effects), and (4) the selection of end points in clinical trials (the diseases or conditions to be evaluated as trial outcomes). We show how genomics can help identify indication expansion opportunities for licensed drugs and repurposing of compounds developed to clinical phase that proved safe but ineffective for the original intended indication. We outline statistical and biological considerations in using MR for drug target validation (drug target MR) and discuss the obstacles and challenges for scaled applications of these genomics-based approaches.
GENOMICS-LED DRUG DEVELOPMENT IN CONTEXT
The modern era of drug development can be traced back to observations that certain natural substances from plants or animals had beneficial effects on the human body, which could be harnessed to treat illness (Pina et al. 2009). For example, willow bark extracts had been noted to reduce inflammation as early as the 5th century BC (Pina et al. 2009). With advances in organic chemistry came the ability to extract and modify active molecular entities and use knowledge of their structure to synthesize related chemicals with similar properties (Drews 2000). For example, the active ingredient in willow bark extract was eventually shown to be salicylic acid, and it was the acetyl salt of salicylic acid that was developed as aspirin by Bayer. Indeed, much of the early expertise in organic chemistry emerged from German and Swiss companies that applied their knowledge to the development of compounds with medicinal properties.
With the ability to synthesize large numbers of compounds and test them for biological activity in cell, tissue, and organ-based laboratory models of human disease, came the era of “phenotypic” screens for drug development (Moffat et al. 2017). Phenotypic screening investigated the impact of compounds on cells, tissues, or model organisms and selected efficacious compounds based on their ability to alter some aspect of the biochemistry, physiology, or pathological process in the model. In this approach, a compound could be pursued as a drug despite incomplete knowledge of the molecular basis of its action. Many licensed drugs emerged from this approach and it continues to the present day, recently leading to the development of memantine for Alzheimer's disease and levetiracetam and zonisamide for epilepsy (Moffat et al. 2017). However, if knowledge of mechanism of action and the therapeutic target remains elusive, it is difficult to anticipate the full repertoire of drug effects and the opportunity to develop improved compounds based on mechanistic knowledge is also constrained.
With increased understanding of the molecular basis of disease, efforts have gradually shifted toward a more target-based approach to drug development (Hughes et al. 2011; Swinney and Anthony 2011). By contrast with the phenotypic approach to drug development, a potential drug target is selected based on prior evidence that implicates it in the disease process. The target-based approach contrasts with the phenotypic approach by working from the potential mechanism toward the disease as opposed to starting with the disease and working back to a mechanism. The target-based approach to drug development was inspired by the receptor theory of drug action, growth of pharmacology as a scientific discipline, and an expanding knowledge base on the function of key protein classes, particularly enzymes, receptors, and their natural substrates and ligands. Many targets of this type formed the basis for what has also been referred to as rational drug development; notable examples include β-adrenoceptor blockers and agonists and histamine H1- and H2-receptor blockers. With these advances came the growing recognition that proteins, encoded by the genome, are not only the major proximal effector molecules in biology, but also comprise the major category of drug targets (Finan et al. 2017).
TARGET-BASED DRUG DEVELOPMENT AND HIGH RATES OF DRUG DEVELOPMENT FAILURE
In the target-based model, drug development starts by attempting to identify proteins, “drug targets,” causally linked to a disease and whose function is amenable to the therapeutic action of small molecule drugs or monoclonal antibodies (Lindsay 2003). Until recently, programs based on this paradigm continued to be shaped by proof-of-concept laboratory experiments in (cell, tissue, and animal) disease models focusing on a small set of targets prioritized based on prior knowledge, with the aim of building evidence of a causal relationship between the target and the disease. Successful drugs continue to be developed using this approach, but there is a growing recognition that the process has been inefficient. Numerous reviews of the field have concluded that success rates in target-based drug development remain extremely low. Fewer than 5 in 100 initiated drug development programs yield a licensed drug; 90% of randomized clinical trials fail and around a half to two-thirds of such failures are due to lack of efficacy in the disease of interest (Munos 2009; Paul et al. 2010; Pammolli et al. 2011; Hay et al. 2014; Harrison 2016).
It has become apparent that the problem of late-stage failure in target-based drug development has its roots in the poor predictive utility of laboratory models and observational (nonrandomized) studies for human disease pathogenesis. Work in isolated systems (cells and tissues ex vivo) may not be representative of the situation in the whole organism; moreover, work in animal models may not be representative of human pathophysiology (Hingorani et al. 2019). Human observational studies (although set in the right organism) can be affected by confounding and reverse causation. This leads to a high false discovery rate that permeates through (pre-)clinical science, resulting in pursuing drug targets and related compounds with a high probability of failure, increasing the overall cost of drug development if these failures occur at late-stage clinical phase testing (Hingorani et al. 2019). Developing a solution to the problem of high rates of drug development failure has therefore become both a scientific challenge and an economic imperative.
GENOME-WIDE ASSOCIATION STUDIES FOR DRUG TARGET IDENTIFICATION
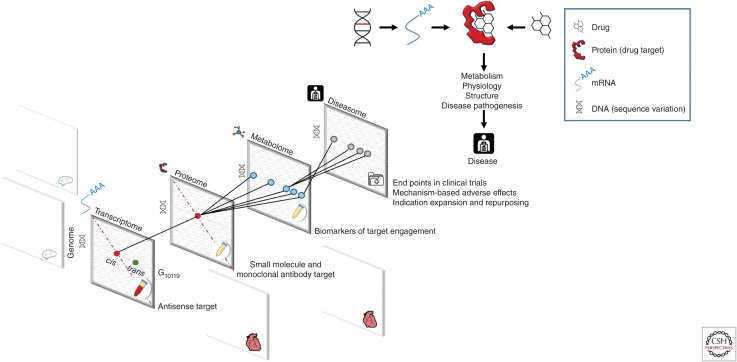
The sequencing of the human genome and identification of all protein coding genes now provides both a comprehensive list of potential key effectors in disease biology as well as potential therapeutic targets. With the development of comprehensive maps of human genetic sequence variation has come the ability to undertake genome-wide association studies (GWAS) in patients and populations. GWAS test relationships between natural sequence variation (genotype) and biomarkers (quantitative biological traits, e.g., blood pressure or circulating metabolite concentration) relevant to disease or to clinical end points (phenotype) (McCarthy et al. 2008; Visscher et al. 2017; Claussnitzer et al. 2020). Proteins mediate the effect of both genetic variation (according to Crick's central dogma [Crick 1970]) and drug action, and independent variation in the genome is inherited at random (according to Mendel's laws), much like treatment allocation in a clinical trial. Thus, the concept has emerged that variants in a gene encoding a drug target, that alter its expression or function, might be used as a tool to anticipate the effect of drug action on the same target (Fig. 1).
Figure 1.
Human genomics and drug development. (Right-hand panel) Relationship between human genomics and drug target identification and validation. Proteins mediate the effect of drugs and natural genetic variation on metabolism, physiology, organ structure, and disease pathogenesis. (Left-hand panel) Scalable approaches to interrogate all potential targets and diseases. Mapping the effect of genetic variation (genotype) on gene and protein expression (transcriptome and proteome) in different tissues, on metabolism (metabolome) and disease risk (diseasome), and applying drug target Mendelian randomization can help anticipate the effect of drug action on a target protein. This principle can help support drug target identification, validation, separation of “on-” from “off-target” effects, end-point selection for clinical trials and indication expansion, and repurposing priorities. Information contributing to the different data layers can be summary level and obtained in independent data sets.
This paradigm makes it feasible, for the first time, to match drug targets to disease end points (target identification) systematically and robustly through GWAS. For example, Finan, Gaulton, and colleagues showed that GWAS frequently “rediscover” genes encoding the targets of licensed drugs for the same disease (Finan et al. 2017). GWAS test common variation across all genes against a single phenotype, with stringent control over the false-positive rate and a philosophy of replication of positive findings to reduce the risk of false discoveries. Phenome-wide association analysis (PheWAS—in which associations from a single exposure, traditionally a gene, with many diseases and biomarkers are explored [Denny et al. 2016]) complement GWAS by helping to anticipate the mechanism-based adverse effects of drug action beyond the primary disease indication (target validation). Integrating evidence from GWAS of the proteome, metabolome, physiological, and imaging data with disease GWAS can also help map the mediating pathways to disease (Fig. 1) and identify biomarkers of target efficacy that can be used as a proxy to evaluate the effects of first-in-class compounds on target engagement and specificity (compound validation). The principle used here is that, in the absence of horizontal pleiotropy, a specific compound with no off-target effects should share the same pattern of effects on biomarkers as variants in a gene encoding the corresponding drug target that affect its expression or activity. An example of this consistency was demonstrated by Wurtz et al. in the comparison of the effects of variants in the HMGCR gene with the effects of taking a statin that targets the protein encoded by the same gene on circulating metabolites and lipoproteins (Würtz et al. 2016).
As the number of GWAS on common diseases has grown since 2007, it has become apparent that human genetics can provide a rich resource for the identification of genes encoding potential drug targets matched to the diseases, with potential to increase the success rate of drug development (Finan et al. 2017). At least three lines of evidence support this proposition: (1) human genetics has consistently “rediscovered” known drug target-disease indication pairings across a wide range of disease areas (Finan et al. 2017); (2) analysis of drug approvals have shown that approval rates were doubled among drug-indication pairs for which, in retrospect, there was prior genetic support; and (3) there are emerging examples of successful development programs being seeded by human genetic information (e.g., PCSK9 and ANGPLT3 for the treatment of coronary heart disease [CHD] as well as maraviroc for the treatment of HIV disease) (Wheeler et al. 2007).
The importance of human genetic evidence in informing numerous aspects of drug development is increasingly reflected in the emphasis given to human genetic target validation by the pharmaceutical industry (www.gsk.com/en-gb/media/press-releases/gsk-and-23andme-sign-agreement-to-leverage-genetic-insights-for-the-development-of-novel-medicines).
LIMITATIONS OF USING GWAS AS A RESOURCE FOR IDENTIFICATION OF DRUG TARGETS TO TREAT DISEASE
Although there are huge opportunities from the use of GWAS as a resource for human drug target identification, several challenges are also recognized, some of which have seeded new research initiatives and methodological developments.
Breadth and Depth of GWAS Studies
One key limitation is that GWAS have yet to be fully exploited as a resource for drug target identification. There are perhaps 10,000 complex diseases (those with both a polygenic and environmental contribution) but approximately only a few hundred have been studied in GWAS (Hingorani et al. 2019). Moreover, although there are many large meta-GWAS studies in common disease (e.g., AFGen, CARDIoGRAMplusC4D, or DIAGRAM), sample sizes for many conditions remain modest, limiting the ability to robustly detect the full spectrum of genetic loci influencing a given disorder. This shortfall is likely to be addressed by many growing initiatives around the globe to undertake GWAS in large prospective cohort studies and national biobanks linked to research-based measures (e.g., biomarkers, proteomics and metabolomics, and imaging data acquisition) as well as through linkage to primary and secondary care health records. Successful examples include UK, Estonian, and Japanese biobanks, as well as the FinnGen study. Comparable genomics initiatives have also been set within health care systems including the Million Veterans Program and Geisinger Health.
GWAS Identify Genomic Regions Not Causal Genes
Currently, most drug targets are proteins and, for target identification, the causal genes mediating the association need to be correctly identified. Many variants identified through GWAS are located in the vicinity of coding sequences, presumably in regulatory regions. Assignment of the causal gene in the region of a GWAS association can therefore be difficult, particularly when linkage disequilibrium (LD) between variants displaying disease associations in a region extend across several genes. Therefore, complementary approaches have been developed (Richardson et al. 2021) to help prioritize the likely causal gene in a region identified by a GWAS based on (1) fine mapping using dense genotyping arrays; (2) transancestry association studies exploiting smaller LD blocks in certain non-European populations; (3) statistical and pathway methods for gene prioritization (e.g., GoShifter [Trynka et al. 2015], PrixFixe [Taşan et al. 2015], and FUMA [Watanabe et al. 2017]); (4) colocalization of disease-associated signals with mRNA or protein expression (e.g., COLOC [Wallace 2020], ENLOC [Wen et al. 2017], eCAVIAR [Hormozdiari et al. 2016]); and (5) incorporation of functional annotation methods (e.g., STOPGAP [Shen et al. 2017]).
Orthogonal information for gene prioritization from GWAS can also come from comparisons with genes known to be responsible for monogenic disorders that display a similar phenotype to the complex condition being studied. Sequence variation responsible for a monogenic disease more typically resides in the coding rather than the regulatory region of the responsible gene so there is less ambiguity about the location of the causal gene than for a GWAS of a common disorder (Abifadel et al. 2003; Cohen et al. 2005). Assignment of a causal gene may also be aided by comparison with information on sets of highly curated gene functions (e.g., genes involved in metabolic processes, or from comparisons with genes encoding drug targets for compounds licensed for the same disease indication as the GWAS). Despite a widely perceived difficulty in assigning a causal gene from a GWAS signal, a recent survey of different approaches for gene prioritization from GWAS came to the conclusion that the proximity of a gene to the lead variant was the strongest predictor of the causal gene (Stacey et al. 2019).
Not All Genes Encode Druggable Proteins
After identifying a gene that is associated with a therapeutically relevant disease trait using GWAS, a subsequent challenge is understanding if the encoded protein might be amenable to drug development (i.e., “druggable”).
The current Ensembl/Havana35 annotated human genome (GRCh38 v103) contains 22,940 protein-coding genes with UniProtKB (v2021_01) Swiss-Prot (manually reviewed entries) containing 20,396 human proteins and TrEMBL (unreviewed) contains 174,126 proteins. Several attempts have been made to define the druggable portion of the human genome (Finan et al. 2017): the subset of protein-coding genes that encode drugged or potentially druggable proteins, so designated because they share sequence, structural, or functional properties with previously drugged proteins. Progressive iterations have seen an expansion in the number of proteins that have been included within the druggable set as more targets yield to drug development. In the latest iteration of the druggable genome, Finan et al. included potential targets for monoclonal antibodies for the first time (based on proteins that are secreted or which are targeted to the cell membrane) and removed olfactory receptors and phosphatases based on known difficulties targeting them, taking the set up to 4479 proteins. However, novel targeting modalities such as proteolysis targeting chimeras (PROTACs) (Sakamoto et al. 2001), which work by marking the target protein for degradation via E3 ligase and the development of antisense technologies to target mRNA species, not just proteins, will eventually widen the druggable component of the genome even further (Editorial 2017).
Tractability of an Identified Drug Target
In addition to druggability, a further consideration of the tractability of a potential drug target relates to consideration of the tissues in which the target is expressed, the relevance or not of that tissue to the disease process, and whether the therapeutic modality chosen renders the target accessible to the drug. For example, Mendelian randomization (MR) analyses have suggested that targeting PCSK9 to reduce low-density lipoprotein cholesterol (LDL-C) could lead to a mechanism-based increase in the risk of type 2 diabetes (Ference et al. 2016; Schmidt et al. 2017b) and Alzheimer's disease (Williams et al. 2020; Schmidt et al. 2021). However, these effects might not be observed in practice if PCSK9 is targeted by a monoclonal antibody therapeutic, which may not gain access (Plenge et al. 2013; Finan et al. 2017; Hingorani et al. 2019; Schmidt et al. 2021) to the β cell or the central nervous system, where these effects are possibly mediated.
Therapeutic Area
Whereas human genetics for drug development is generally applicable to many therapeutic areas, there are some important exceptions or limitations. Given that genetic target validation of the type described uses germline genetic variation, it is likely to be more limited for cancer than for other diseases, since many cancers are driven by somatic and not germline mutations. Nevertheless, findings from GWAS of prostate and breast cancer have identified variants in the genes encoding the androgen and estrogen receptors respectively, both of which are known to be effective therapeutic targets in these diseases, suggesting that GWAS can still play a part in cancer therapeutics where the mechanism of disease initiation is distinct from the treatment (Coignard et al. 2021; Sipeky et al. 2021). GWAS of human subjects is also not capable of identifying targets in an infectious disease pathogen, but may still find use in identifying host response genes that might make a substantial contribution to the infectious disease process (Newport and Finan 2011; Pietzner et al. 2020). Additionally, developmental diseases or those where the pathology is caused by irreversible damage prior to the presentation of disease, for example, type 1 diabetes, are less likely to be amenable to target identification through GWAS unless leveraging age-specific data of sufficient sample size.
Mechanistic Considerations
Most drugs act by modifying the function of a protein target rather than its level (Gaulton et al. 2017), but most variants identified by GWAS are intergenic, rather than protein coding (Buniello et al. 2019). Nevertheless, genetically validating clinically used drug target/disease mechanisms (positive control examples) have shown that even such variants associated with gene or protein expression (regulatory variants) can model the effects of licensed drugs (see Table 1; Millwood et al. 2016a,b).
Table 1.
Comparison of the findings from randomized controlled trials (RCTs) and Mendelian randomization (MR) of the corresponding therapeutic target
| Drug target | Orthodox drug development | MR | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Compound(s) evaluated | Developmental stage | Therapeutic area | Outcomes assessed in preclinical studies or RCTs of selective drug interventions | Findings from preclinical studies or RCTs of selective drug interventions | Encoding gene | Outcomes evaluated in MR | Findings from MR | Inferences drawn from comparison of the findings from preclinical studies or RCTs and MR | |
| Cholesteryl ester transfer protein (Sofat et al. 2010) | Torcetrapib | Phase III | Cardiovascular disease (CVD) | Blood lipids (total-, low-density lipoprotein cholesterol (LDL-C), and high-density lipoprotein cholesterol (HDL-C), triglycerides; blood pressure; CVD events | HDL elevation, triglyceride and LDL reduction; unintended blood pressure elevation; unintended increase in CVD events | CETP (Barter et al. 2007) | Blood lipids (total-, LDL-C, and HDL-C, triglycerides); blood pressure | Associations with blood lipids consistent with effects in RCTs; no genetic association with blood pressure | Blood pressure elevating effect of torcetrapib is off target |
| Hydroxy methyl (HMG)-coA reductase (Swerdlow et al. 2015) | Statins | Phase IV (post-marketing) | CVD | Blood lipid fractions, weight, type 2 diabetes risk | Statin treatment in RCTs linked to increased weight and risk of type 2 diabetes | HMGCR (Swerdlow et al. 2015) | Blood lipid fractions, anthropometric measures, glucose and insulin, type 2 diabetes risk | HMGCR single-nucleotide polymorphisms (SNPs) associated with lower LDL-C, higher weight, fasting glucose and insulin, and type 2 diabetes risk | Increased risk of type 2 diabetes is an unintended on-target effect of statins mediated in part through weight gain |
| Niemann–Pick C1-like 1 (Cannon et al. 2015) | Ezetimibe | Phase III | CVD | LDL-C, cardiovascular death, nonfatal myocardial infarction, unstable angina requiring hospitalization and revascularization | Ezetimibe added to statins produces modest additional benefit in cardiovascular outcomes in patients following an acute coronary syndrome (ACS) | NPC1L1 (The Myocardial Infarction Genetics Consortium Investigators et al. 2014) | Plasma lipid levels and risk of coronary heart disease (CHD) | Inactivating mutations in NPC1L1 are associated with lower LDL-C and protection from myocardial infarction risk | Niemann–Pick C1-like 1 is a validated target for LDL-C lowering and CHD prevention |
| Proprotein convertase subtilisin/kexin type 9 serine protease (Schmidt et al. 2017a) | Alirocumab, evolocumab | Phase II | Lipid lowering and CVD | LDL-C | Alirocumab and evolocumab reduce LDL-C among patients with heterozygous familial or polygenic hypercholesterolemia and reduce cardiovascular events in patients with or at high risk of CVD | PCSK9 (Cohen et al. 2006) | LDL-C and risk of CHD | Inactivating mutations in PCSK9 associated with reduced LDL-C and CHD risk | Proprotein convertase subtilisin/kexin type 9 serine protease is a validated target for LDL-C lowering and reduction in cardiovascular risk |
| Glucagon-like peptide-1 receptor (Marso et al. 2016) | Liraglutide | Phase III | Diabetes and CVD | Death from cardiovascular causes, nonfatal myocardial infarction, or nonfatal stroke | Liraglutide reduced risk of death from cardiovascular causes, nonfatal myocardial infarction, or nonfatal stroke among patients with type 2 diabetes mellitus | GLP1R (Scott et al. 2016) | Body weight, glycemic traits, lipids, blood pressure, risk of type 2 diabetes, and CHD | A low frequency, coding region missense variant in GLP1R is associated with lower fasting glucose, diabetes risk, and risk of CHD | GLP1R is a validated target for treatment of diabetes and reducing CHD risk |
| Lipoprotein- associated phospholipase A2 (Lp-PLA2) (O'Donoghue et al. 2014; STABILITY Investigators et al. 2014) | Darapladib | Phase III | CVD | Major cardiovascular events or major coronary events | No reduction in CVD events in patients with stable coronary disease or recent ACS, despite reductions in Lp-PLA2 mass and activity | PLA2G7 (Casas et al. 2010; Millwood et al. 2016a) | Lp-PLA2 concentration, blood lipids, inflammation markers, and CHD events | PLA2G7 variants were not associated with alterations in cardiovascular risk markers or CHD events | Lp-PLA2 is not involved in the development of CVD; low priority as therapeutic target for this indication |
| Interleukin 6 receptor (IL-6R) (Interleukin-6 Receptor Mendelian Randomisation Analysis (IL6R MR) Consortium et al. 2012) | Tocilizumab | Phase III | Autoimmune disease | Blood lipid fractions and inflammation markers including IL- 6, C-reactive protein (CRP), and fibrinogen | In patients with rheumatoid arthritis, tocilizumab-induced alterations in circulating inflammation markers characteristic of IL-6 blockade | IL6R (Interleukin-6 Receptor Mendelian Randomisation Analysis (IL6R MR) Consortium et al. 2012) | Blood lipid fractions and inflammation markers including IL-6, CRP, and fibrinogen; cardiovascular events including CHD events and abdominal aortic aneurysm | Variants in the IL6R gene that recapitulate the biomarker profile of IL-6R blockade were associated with a reduction in CHD events | IL-6R signaling is involved in the development of CHD; the IL-6R blocker tocilizumab could be repurposed for the treatment of CVD |
| CRP (Casas et al. 2008) | No CRP inhibitors yet available for clinical use | Preclinical | CVD | Effects of CRP on processes believed to contribute to atherosclerosis studied in vitro or in animals; associations of CRP with CVD in human observational studies | Observational associations of CRP with CVD events in humans, but studies prone to confounding; pro-atherogenic effect of CRP in vitro and in animals later proved to be artefactual | CRP (C Reactive Protein Coronary Heart Disease Genetics Collaboration (CCGC) et al. 2011) | Inflammation and coagulation markers, blood lipid fractions, and CHD events | SNPs in the CRP gene exclusively associated with CRP exhibited no association with CHD; no causal association of CRP with CHD based on instrumental variables analysis | CRP is not causal in CHD pathogenesis; priority as a therapeutic target for CHD prevention diminished |
| Secretory phospholipase A2 (sPLA2) (Nicholls et al. 2014) | Varespladib | Phase III | CVD | sPLA2 concentration, blood lipids, inflammation markers, and CVD events | No beneficial effect of varespladib on CVD events in patients with recent ACS, despite a drug- induced reduction in sPLA2 concentration and activity | PLA2G2A (Holmes et al. 2013) | sPLA2 mass and activity and major vascular events (MVEs) in general populations and patients with ACS | SNPs in the PLA2G2A gene were associated with substantial alterations in sPLA2 mass and activity but not with MVEs | sPLA2 is not involved in the development of CVD; dismissed as a therapeutic target in CVD |
| Potassium/sodium hyperpolarization-activated cyclic nucleotide-gated channel 4 (Martin et al. 2014) | Ivabradine | Phase IV (post-marketing) | CVD | Risk of atrial fibrillation | Developed for angina and heart failure, post hoc meta-analysis of RCTs (motivated by genetic findings (Casas et al. 2008; Interleukin-6 Receptor Mendelian Randomisation Analysis (IL6R MR) Consortium et al. 2012) indicated ivabradine treatment is associated with a higher risk of atrial fibrillation | HCN4 (Ellinor et al. 2012; den Hoed et al. 2013) | Atrial fibrillation (genome-wide association analysis) | Variants in the gene HCN4 encoding the target of ivabradine associated with a higher risk of atrial fibrillation | Atrial fibrillation is a mechanism-based adverse effect of ivabradine treatment |
| Tumor necrosis factor receptor 1 (TNFR1) and TNF (van Oosten et al. 1996; The Lenercept Multiple Sclerosis Study Group 1999) | Monoclonal antibodies against TNF-α | Phase III and phase IV | Neurological disease | Multiple sclerosis (MS) exacerbations | MS exacerbations | TNFRSF1A (Gregory et al. 2012) | MS | A variant in the TNFRSF1A that encodes the TNFR1 gene indicates expression of a soluble form of TNFR1 that blocks the effect of TNF, and associates with a higher risk of MS; the mechanism mimics that of monoclonal antibodies against TNF | Exacerbation of MS induced by anti-TNF monoclonal antibodies is mechanism based |
Surveys of GWAS rediscovery of clinically used drug target/disease parings have indicated such studies are capable of identifying the targets for a variety of drug mechanisms including inhibitors and antagonists, agonists and activators, as well as positive and negative allosteric modulators (Nelson et al. 2015; Finan et al. 2017). However, the majority (69%; ChEMBL v27) of drug target/mechanism pairs are the subject of inhibitory or blocking drugs because the effects of activators or agonists tend to be affected by down-regulation of the target (Gaulton et al. 2017). Genetic variants that increase disease risk via reduced expression or function of the encoded protein would naturally point to development of an agonist or activator of the target protein to achieve the desired therapeutic effect, which might be less tractable pharmacologically. In such cases, if the target of interest is inactivated by a second protein and the inactivator is druggable, a therapeutic option might then be to develop an inhibitor of the inactivating protein.
The failure to find significant genetic associations in or near a potential drug target for a disease also does not necessarily exclude the target from consideration (Minikel et al. 2020). One reason is that the effect of altering the expression or function of a target may only be seen beyond some threshold, such that a genetic variant of weak effect may not adequately model the effect of targeting the protein with a potent drug. The availability of common (weak effect) and rare (large effect) genetic variants in the same gene, which allows the construction of an allelic series (effectively a genetic dose-response curve [Plenge et al. 2013]), may go some way toward mitigating this possibility in specific cases (Dudbridge 2021). Another potential cause for not finding a genetic signal in or near a drug target encoding gene may be found in the fact that genetic influences on protein expression or activity are often present from early life. Such early and consistent effect may entrain developmental adaptive compensation (canalization [Richmond and Davey Smith 2021]) through changes in other pathways that mitigate any biologically adverse effect on the system as a whole. Furthermore, by design, GWAS minimize the false-positive rate (type 1 errors) ensuring that most findings are true. At the same time, stringent control of the type 1 error rate increases the false-negative (type 2 errors) rate manyfold, making GWAS a very poor design to show the absence of an effect (Altman and Bland 1995). Thus, the lack of genome-wide significant findings of variants in a gene encoding a drug target of interest in a particular disease need not exclude it as a therapeutic target. Next, we discuss how “drug target Mendelian randomization” can be used to provide a more focused analysis of the likely therapeutic consequence of on-target action of a drug.
MENDELIAN RANDOMIZATION AND DRUG TARGET VALIDATION
Having prioritized a protein as the likely cause for a disease, and having confirmed that the protein is druggable, an essential next step is to gather evidence on the likely range of on-target effects of pharmacologically perturbing the target, a process we refer to as drug target validation. Drug target validation has been traditionally addressed in preclinical studies involving cell and tissue experiments, as well as animal models. Additionally, if a potential target can be measured directly in humans, or its activity or expression inferred by measurement of downstream biomarkers, another aspect of target validation might involve assessing the association of the protein or related biomarkers with disease incidence in nonrandomized (i.e., observational) studies.
However, the same issues that compromise target identification in cell, tissue, and animal models also affect target validation. Moreover, while nonrandomized human observational studies on a biomarker association with disease (e.g., HDL-C with coronary disease) may provide a starting point for a therapeutic hypothesis, most drug targets (e.g., cholesteryl ester transfer protein or lipoprotein lipase) rarely affect a single biomarker. Therefore, a one-to-one assignment of biomarkers to drug targets may lead to oversimplistic and overoptimistic models of the potential relevance of a specific drug target. Furthermore, nonrandomized studies are often affected by reverse causation, residual and unmeasured confounding (Schmidt et al. 2016a), and/or selection bias (Hernán et al. 2004), potentially compromising the inferences that can be drawn from such studies for drug development. Next, we discuss how MR can address some of the key limitations of preclinical science for drug target validation, and how performing MR analyses where a drug target is the exposure of interest, can strengthen the existing (cumulative) evidence base necessary to make robust choices on which target to pursue for further development.
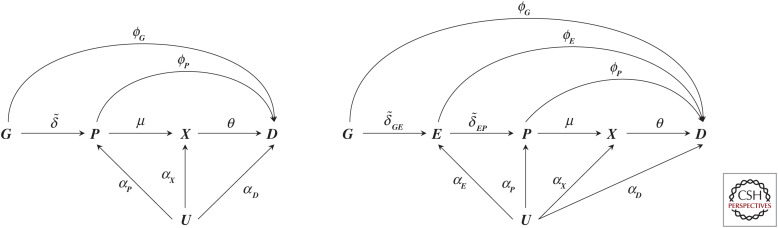
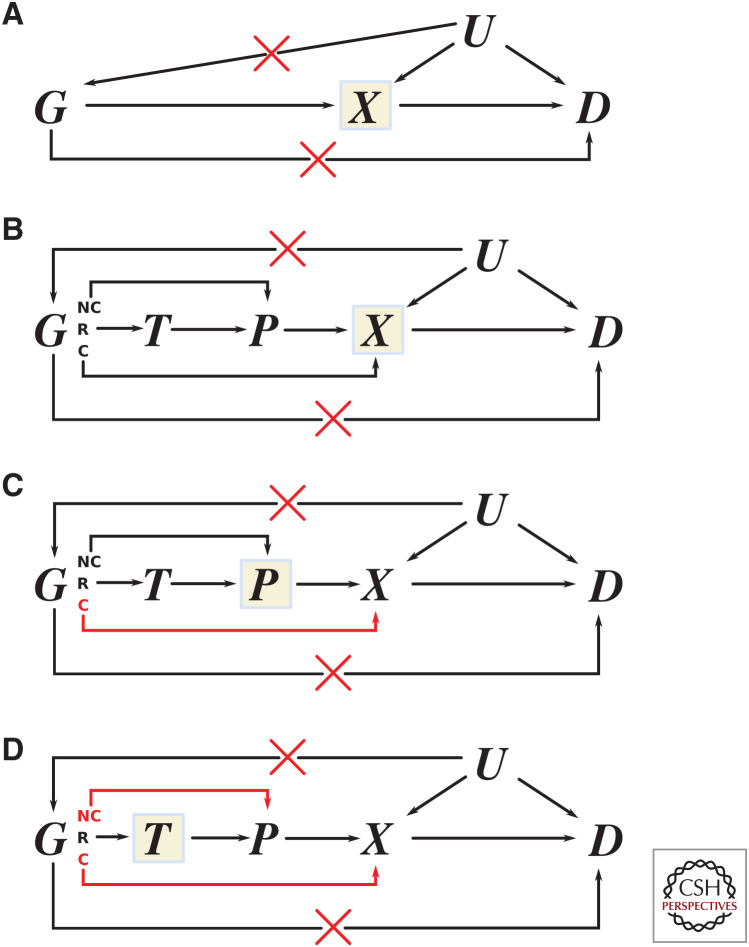
MR was developed as a novel research design for investigating causal relationships between risk factors and health outcomes using nonrandomized data; outside of genetics this is often referred to as instrumental variable analysis (Schmidt et al. 2016b) or as a quasi-randomized experiment, for example in pharmacoepidemiology (Martens et al. 2006). The premise is to identify one or multiple “instrumental variables” (e.g., genetic variants) that are strongly related to an exposure of interest (e.g., LDL-C), that affect the outcome (e.g., CHD) exclusively through the exposure of interest (the exclusion restriction assumption), and that do not share a common cause with the outcome of interest, which would otherwise lead to confounding (Richmond and Davey Smith 2021). This is shown in Figure 2, where the risk factor is depicted simply as X, the outcome as D, with their relationship confounded by U (which may represent multiple common causes), and G (representing a genetic variant or variants) may act as an instrument if ϕG = 0.
Figure 2.
A graph representation of two possible Mendelian randomization scenarios. N.B., the left-hand side of the graph represents a scenario where a single (or multiple) genetic variant is represented by node G, which has an effect (indicated by an arrow) on a protein P, where the protein affects a downstream biomarker X, and all previously defined nodes affect the outcome D. The effect magnitudes are indicated by arrow labels and may include a null effect (when there is no causal effect between two nodes). The right-hand side adds a node E, between G and P, reflecting the effect of mRNA expression on P and D. Finally, in both scenarios, all nodes except G may be affect by confounding, encoded by common cause U; of course, this node most often reflects multiple distinct causes. (Figure reprinted from Schmidt et al. 2020, courtesy of the Creative Commons Attribution 4.0 International License.)
By defining confounding as a common cause between an exposure and an outcome, it becomes clear that most genetic associations with traits are relatively protected against this source of bias. Whereas there will be many factors that influence a disease or a quantitative trait, hardly any of these factors will, in turn, influence the assignment of one's genotype. Nevertheless, confounding bias may still occur by, for example, inadvertently mixing ethnicities with distinct genotypes within the same study. Through shared environment, ethnicities may also differ in disease risk or quantitative trait levels, hence admixed populations may result in confounding or “population stratification” bias. By reducing the number of potential confounding factors from up to infinity to a much smaller number of ethnic (and familial) variables, genetic analyses can focus on accounting for these specific sources of bias, either analytically or by design (for example, leveraging within-family designs [Hwang et al. 2021]) and produce a result that is often robust to any remaining—that is residual—confounding. Similarly, unlike the directly observed associations between the risk factor and disease, genetic associations are protected from reverse causation, since the presence of the disease does not alter the sequence of the germline. Whether the presence of disease modifies the genetic association with another outcome is, of course, more plausible, and the topic of ongoing research (Patel et al. 2019).
Thus, these relatively robust genetic associations can be used to obtain inference on the effects protein perturbation might have on disease by collecting (aggregated) data on the genetic association with (1) a relevant drug target-related exposure (e.g., concentration of the protein forming the drug target, expression of mRNA encoding the target, or a downstream complex biomarker known to be affected by the protein of interest), and (2) an outcome of interest (e.g., disease incidence or a quantitative trait such as cholesterol). As an example, PCSK9 was first considered as a drug target after finding that mutations in PCSK9 were associated with a lower LDL-C concentration (15% reduction), as well as a decreased risk of CHD; hazard ratio 0.50 (95% CI: 0.32 to 0.79) (Cohen et al. 2006).
In the following section, we define “drug target MR” more formally and introduce important inferential considerations, in part based on Schmidt et al. (2020).
GENETIC WEIGHTS AND THE INFERENTIAL TARGET IN DRUG TARGET MR
To emphasize, the cited PCSK9 estimates for LDL-C and CHD pertain to the effect of a hypothetical change in one's genotype, which may be (partially) mediated by the encoded protein. For genetic associations to inform drug target validation, we need to be willing to assume that the effect of a variant in a gene (e.g., PCSK9) only acts on a disease end point (e.g., CHD) through its effect on the encoded protein, PCSK9 (i.e., assume the absence of pretranslational horizontal pleiotropy [the exclusion restriction assumption]).
Before discussing the PCSK9 example further, we will first formally define our inferential target. Similar as before, we denote a single, or multiple, genetic variants (e.g., in PCSK9) as G, the encoded protein P as the drug target we aim to validate (e.g., PCSK9), an outcome D such as CHD, and an intermediate biomarker X (e.g., LDL-C) potentially affected by P (left diagram of Fig. 2). Here the arrows indicate the direction of effect, and the edge labels the effect magnitude in a relevant unit (e.g., as mmol/L for LDL-C or hazard for CHD); we note that effect magnitudes may include zero for no effect. Furthermore, as we discuss below, genetic variants are typically preferentially selected from within or near a protein coding gene of interest acting in cis.
Using this diagram, we can formally define the inferential target as “the effect a unit change in protein concentration or activity has on an outcome,” and label this as: ω = ϕP + μθ, which itself consists of the following biologically relevant estimates:
Any direct effect the protein has on the outcome, sidestepping biomarker X: ϕP.
The protein effect on the biomarker: μ.
The biomarker effect on the outcome: θ.
The genetic effect on an outcome such as CHD: , is distinct from ω. In the absence of any horizontal pleiotropy pathways that might occur proximal to protein translation (e.g., ϕG = 0, referred to as pretranslational pleiotropy [Schmidt et al. 2020]) this expression simplifies to , which still does not equal ω. Nevertheless, under this no-pretranslational pleiotropy assumption, the genetic association with an outcome such as CHD (e.g., from a GWAS), that is, , provides clear evidence that the protein likely affects CHD. For example, if ϕG = 0, but this implies that ω ≠ 0, and hence under the same MR assumptions genetic analyses may provide indirect evidence on a drug targets effect on disease.
In this same setting, drug target MR can be used to additionally determine the effect direction of ω. Further, if we are willing to assume complete linearity of the drug target effect(s) on disease, as well as strict homogeneity in drug target effect(s) among current and future subjects, we can also obtain an estimate of ω. Specifically, we take the genetic effect on the outcome, the genetic effect on the protein drug target, and simply divide the two:
| (1) |
In the absence of linearity and homogeneity, which often may be unlikely, ω represents an average effect, and (as we discuss below) might provide robust evidence on the presence of an effect and its direction (e.g., if the protein increases or decreases disease risk).
As discussed below, estimates of associations between genetic variants and protein concentration are becoming more widely available. Nevertheless, sufficiently strong genetic associations with P (e.g., PCSK9) may not always be available. In the absence of genetic instruments for P, drug target MR can instead use the genetic association with X (e.g., LDL-C) as a proxy for protein concentration and activity. In this case, the denominator in Equation 1 includes μ and we are left with
| (2) |
Hence a biomarker weighted drug target MR analysis does not directly estimate ω but instead provides an estimate on ωbw; with “bw” for biomarker weighted. Notice that in a drug target MR context, we denote a biomarker as a metabolite level or other quantitative measure such as blood pressure distal in the causal pathway to the encoded protein.
It is important here, and in general, to note that neither ω ≠ 0 nor ωbw ≠ 0 provide any evidence on the causal effect of X on D (e.g., θ). To see this, set θ = 0, in which case the results from Equations 1 and 2 remain unaffected with ω = ϕP + μθ = ϕP. As such, and contrary to the PCSK9 example, we could even consider weighting the genetic effect on an outcome by the genetic effect on a biomarker that does not necessarily reside on the causal pathway but is merely affected by the drug target; see Schmidt et al. (2021). Finally, we also emphasize that the necessary no-horizontal pleiotropy assumption only pertains to pretranslational effects, such as ϕG, and that post-translational effects (Schmidt et al. 2020), such as ϕP, are part of the drug target effect and hence do not cause bias. This, of course, will change if our inferential target shifts from P to X; in that case, the horizontal pleiotropy assumption requires both ϕG and ϕP to be zero. Hence drug target MRs, by focusing on exposures more proximal to the effect of genetic variation, are more robust to horizontal pleiotropy than MR studies focusing on the causal effect of more distal biomarkers such as X. This also reflects drug targets often affecting multiple downstream biomarkers and disease, which does not violate the horizontal pleiotropy assumption required in drug target MR, which is exclusively concerned with pretranslational pathways (Schmidt et al. 2020).
The impact of horizontal pleiotropy can be further limited by considering the genomic position of variants associated with the exposure of interest, which in the case of a protein as the inferential target resolves to variants within or in the proximity of the encoding gene acting in cis versus variants located elsewhere (i.e., cis vs. trans acting).
Whereas X can represent any variable that is affected by a protein drug target, and as such is positioned post-translationally with respect to P, we can also think of a variable, which is instead positioned pre-translationally: mRNA expression of the encoding gene. The right diagram in Figure 2 depicts such a situation where, as an example of a pre-translational variable, mRNA expression is represented as E. Furthermore, we decomposed the pre-translational horizontal pleiotropy term ϕG into and , where the former might occur through LD and the latter representing a direct effect of mRNA expression on the outcome, sidestepping its effect on protein translation. If we follow the same derivations as above, it is clear we need to assume that and are both zero (i.e., the absence of pre-translational horizontal pleiotropy). Then we find that weighting a drug target MR by the genetic association with mRNA expression results in the following effect:
This simply reflects the mRNA effect on the outcome. Critical for this effect to provide robust inference on the protein to outcome effect, we need to assume (Fig. 2). That is, we need to be willing to assume that all of the mRNA effect on the outcome acts through its effect on protein expression. As such, drug target MR using pretranslational weights, where the inferential target remains protein P, need to make stronger assumptions (which may be very reasonable) than if we had simply been interested in the mRNA effect on the outcome itself. This further shows that the no-horizontal pleiotropy becomes more stringent the further the inferential target is positioned from the genetic variants themselves, and that the inferential target does not necessarily match the genetic information used.
In the preceding section we have generally assumed the inferential drug target was a protein, reflecting the majority case. However, it is more accurate to think of the gene product as the inferential target, that is, any mediator from the gene to the protein. For example, if one wanted to assess the potential effects for an antisense drug, altering mRNA expression, the drug-target MR paradigm could be used to assess the effect of modulating transcript level on the outcome, even if the effect is eventually mediated by the effect of transcript modulation on the encoded protein. This conceptualization uses Crick's central dogma of a unilateral flow of information from DNA to mRNA to protein.
INTERPRETING DRUG TARGET MR EFFECT ESTIMATES
As detailed in the previous section, if we are willing to assume complete linearity of the drug target effect(s) on disease, as well as strict homogeneity of drug target effect(s) in all patients, the effect magnitude can sometimes provide actionable insights, allowing drug target MR to inform drug development beyond a statistical test of the null hypothesis and a robust indication of effect direction.
There are some potential caveats that suggest that drug target MR analysis in general (irrespective of the sourced data), may be more useful as a test of effect direction rather than effect magnitude. This is because drugs that inhibit a target do so usually by modifying its function not its concentration, whereas genetic variants used in MR analysis usually affect protein expression and therefore concentration. Given the typically nonlinear drug dose-response, the often-modest explained variance genetic variants have on the level or function of a protein may misrepresent the potential treatment effect of a drug. MR analyses assess the effect of target modulation in any tissue, whereas certain tissues may be inaccessible to a drug either because of its chemistry or anatomical or physiological barriers. Furthermore, RCTs are closely monitored, and followed for a fixed period, allowing for exploration of induction times. While MR estimates are considered to reflect a lifelong exposure in the absence of serial assessment, possible changes across age are difficult to explore, as are disease induction times. For these reasons we suggest that drug target MR offers a robust indication of effect direction but may not directly anticipate the effect magnitude of pharmacologically interfering with a protein.
Empirical evidence has shown that effect estimates from drug target MRs often differ from effects from drug compounds affecting the same target (Ference 2018; Holmes et al. 2021). While various reasons have been proposed to explain this (e.g., lifetime exposure by a genetic variant versus a fraction of this in a drug trial [Holmes et al. 2021]), none have actually considered whether a drug target MR effect and drug compound effect should be equal. In the following we provide straightforward mathematical derivations to show that these effects should in fact be expected to differ.
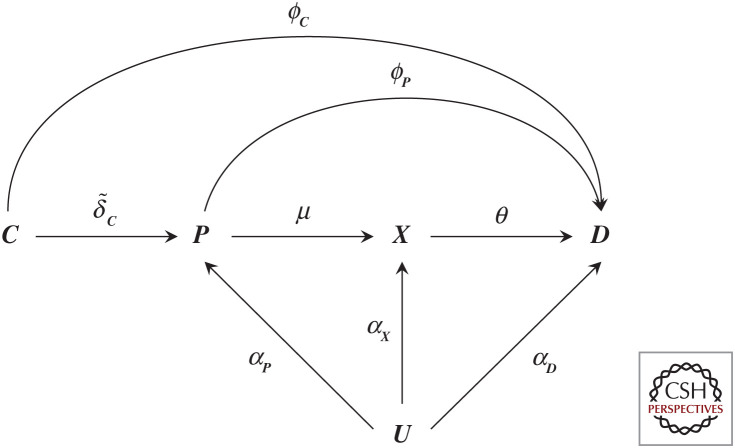
A drug compound (much like a genetic variant) elicits its effect on an outcome by perturbing a drug target; as such we can replace node G in Figure 2 by C representing the drug compounds. The effect of C on a drug target can be indicated by , and any potential off-target effect on outcome D as ϕC (Fig. 3). In this case, the effect of a drug compound on the outcome is , which is distinct from ω, the effect the drug target has on the outcome. It becomes straightforward to see that even when the drug compound affects the outcome exclusively through the drug target (e.g., the compound has no off-target effects ϕC = 0), its effect is . Now will only equal ω in the very specific setting when , that is, when the drug compound effect on its drug target is one and the drug compound has no off-target effects. Given that there is little reason for to equate to one, we can conclude the effects from drug compound and drug target effects are distinct and need not agree. Separating a drug compound effect (which may fully or partially act through a drug target) from the drug target effect itself of course does not invalidate the (causal) relevance of either.
Figure 3.
A graph representation of a randomized controlled drug trial. N.B., Node C represents a randomly allocated drug compound that has an effect (indicated by an arrow) on a protein P, where the protein affects a downstream biomarker X, and all previously defined nodes affect the outcome D. Here the effect magnitudes are indicated by arrow labels and may include a null effect (when there is no causal effect between two nodes). All nodes, except C (which we assume has been allocated at random) may be affected by confounding, encoded by common cause U, where of course this node most often reflects multiple distinct causes. Finally, we note that node C is sometimes further differentiated by identifying a randomization node “Z”, which has a (strong) effect on whether one takes a drug “C” (treatment adherence), where in such a case “C” is no longer randomized and potentially affected by confounders.
Whereas the above derivations clearly show that the difference between a compound effect and drug target effect (assuming the latter has no off-target effects) is determined by , researchers have instead used some version of ωbw (often also weighting the trial estimate by θ) in an attempt to compare trial estimates to MR estimates. Here we show, however, that if it is desirable for the drug target and drug compound effect to equate to one another, either the drug compound effect should be divided by its effect on the drug target , or the drug target effect should be multiplied by the same constant. The former scaling of course is similar to instrumental variable analyses adjusting compound estimates to account for any nonadherence (Schmidt and Groenwold 2018). To reiterate, however, such scaling (either by a genetic biomarker effect or by ) is only feasibly when the drug compound has no off-target effect(s), which is unlikely to generally hold.
USING PROTEOMICS DATA AS AN EXPOSURE IN DRUG TARGET MR
Given that the majority of current drug targets are proteins, it seems reasonable to assume that loci identified from genetic associations with protein concentration (protein quantitative trait loci [pQTL]) will offer an important category of exposures for drug target MR (Schmidt et al. 2021).
Current examples of MRs using protein exposures assess the effect of a change in protein level against a disease outcome. However, since most drugs act by altering the function of a protein, using MR of protein level changes implicitly assumes that an effect of variation in protein concentration is directly proportional to variation in protein function. Limited examples of available genetic associations with concentration and activity of the same protein suggest a high correlation between the two, although evidence is mainly for enzymes and the situation could, in theory, be different for other types of protein. The promise of PROTAC therapeutics also indirectly supports protein level variation being able to model disease (Sakamoto et al. 2001).
The main downsides of currently available pQTL data are that these are largely focused on circulating proteins. Among these, secreted proteins that have their action in the circulation could be an important category of drug targets and many are already the targets of monoclonal antibody therapeutics (Attwood et al. 2020). However, other proteins are present in the circulation due to cell damage or turnover. Although this is not their physiological site of action, it might be assumed that concentration in the blood reflects tissue-specific expression. If so, the utility of blood pQTLs will depend on relative contributions from the disease-relevant versus disease-irrelevant tissues to the blood pool. Consequently, the relevance of blood pQTLs will vary considerably between disease areas. In future, comparison of tissue-specific eQTL and blood pQTL data at scale may provide insights into the tissue of origin of proteins detectable in the blood. Additionally, as technologies improve, more tissue-specific pQTL data will become available, which can be incorporated in MR analyses. Assay heterogeneity is also a potential issue in the use of pQTL data from MR analysis. For example, more research is needed on the agreement between different assays of the same protein based on new proteomics technologies that measure many thousands of proteins in the circulation in a single sample (e.g., the SomaLogic aptamer based proteomics platform and the O-link antibody-based proximity extension assays, as well as with mass spectrometry and ELISA-based techniques (Pietzner et al. 2021).
CIS AND TRANS INSTRUMENTS IN DRUG TARGET MR
MR analysis of risk factor or biomarker exposures typically incorporate multiple variants selected from throughout the genome and can provide valuable insight on prioritizing generic therapeutic strategies (e.g., lowering LDL-C to prevent CHD). Traditionally, drug target MR has preferentially selected instruments from within a small cis region known to encode the (protein) drug target, in an attempt to guard against the influence of pre-translational horizontal pleiotropy. One of the advantages of using cis-acting variants in a drug target MR, is that there is a more robust hypothesis that these variants act through the target of interest, which can guard against the influence of pre-translational horizontal pleiotropy, although an exception arises if there is LD with a flanking gene that is the true causal gene.
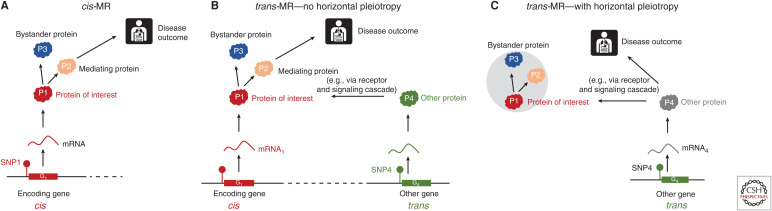
With the increase in the more highly powered GWAS of circulating proteins it has become apparent that the circulating concentration of many proteins is influenced by variants outside of the encoding gene region (i.e., acting in trans). If one assumes that the majority of trans associations reflect real biology (not assay artefacts), then the natural question is: could variants acting in trans be used as a source of genetic instruments for MR? While a seemingly attractive proposition, we sound some notes of caution. Variants associated in trans with the gene encoding the protein of interest, are likely acting in cis for a second gene in their immediate vicinity and through the gene product encoded by that gene. If the gene product from the second gene has a pathway to disease independent of the protein of interest, pre-translational horizontal pleiotropy occurs and a critical MR assumption is violated (Schmidt et al. 2020). Only in the absence of such horizontal pleiotropy would the trans variants be valid instruments for the protein of interest. However, with trans variants, there is no guarantee that the target of interest is on the causal pathway between the trans-acting instrument and the disease (Fig. 4).
Figure 4.
Comparison between cis- versus trans-Mendelian randomization (MR) for drug target validation. (A) cis-MR variant in cis-single-nucleotide polymorphism (SNP1) in the proximity of gene G1 is used as an instrument for the protein of interest (P1), which is causally linked to a disease. SNP1 is also associated with P2 (a mediator of the effect of P1 on a disease) and with P3 (a bystander protein residing off the causal pathway from P1 to disease. SNP1 is associated with P1, P2, and P3 and the disease outcome and is a valid instrument for P1. (B) Trans-MR with no horizontal pleiotropy. A variant in another gene (SNP4), which influences expression of P4, upstream in the causal pathway, is used as a trans instrument for P1. SNP4 associates with P4, P1, P2, and P3 and is a valid instrument for P1 because there is no direct causal pathway from P4 to disease. (C) Trans-MR with horizontal pleiotropy. In this scenario, P1 is not causal for disease but is a bystander protein. SNP4 is upstream of P1 and associates with disease because of a direct pathway through P4 (which may not have been measured). As in scenario B SNP4 associates with P1, P2, and P3 and disease, but it is not a valid instrument for P1 because there is a direct causal pathway from P4 to disease. (Figure reprinted from Schmidt et al. 2020, courtesy of a Creative Commons Attribution 4.0 International License.)
INTERPRETING THE UTILITY OF BIOMARKER-WEIGHTED DRUG TARGET MR ANALYSES
Whereas transcript and protein levels can themselves be regarded as biomarkers, in a drug target MR context, we denote a biomarker as a metabolite level or other quantitative measure such as blood pressure distal in the causal pathway to the encoded protein. It is worth contrasting a drug target MR analyses using biomarker exposures, with those using pQTLs and eQTLs. Whereas for eQTLs and pQTLs there is a natural dichotomy into genetic variants acting in cis (in the vicinity of the encoding gene and the gene product) and those acting in trans (distant from the encoding gene), no such natural dichotomy exists for variants influencing downstream traits such as circulating metabolites. However, the lack of a defined cis does not preclude the study of cis variants and cis MR analysis at a metabolite associated gene with an outcome, where the inference is on the effect of perturbing a protein that has an effect on a downstream metabolite (Schmidt et al. 2020; Holmes et al. 2021). Within the bounds of the MR assumptions, the inference that the gene product is causally associated with the outcome is valid; however, any inference that it is mediated by the biomarker exposure is not (see above). With a biomarker MR, the biomarker acts as a mechanism to indirectly measure the gene product only and associations between the biomarker level and the outcome status can, and probably are in many cases, bystander effects. As discussed above (Fig. 2), a biomarker weighted drug target MR does not estimate ω, the effect of the drug target on disease, and instead estimates
where μ represent the drug target effect on the biomarker. While this leads to a valid null-hypothesis test, it is clear that the sign of ωbw may differ from that of ω, and to appropriately interpret the effect direction one needs robust information on the effect direction of μ.
Despite the more challenging inference, there are some good reasons to conduct a biomarker weighted drug target MR, even in the presence of available pQTL data. Chiefly, many nonprotein biomarker GWAS are larger than most GWAS of proteomics to date, and therefore often highly powered with hundreds or thousands of participants using established assays. Additionally, whereas there are no studies directly assessing this, it is likely that biomarker MR estimates will indirectly incorporate the effect of coding sequence variation in a way that eQTLs and pQTLs may not. The reason is that both e/pQTLs influence transcript/protein level and not protein activity, whereas coding sequence variation is more likely to have an impact on protein activity (and not level), an effect that is then captured in the effect on the downstream biomarker (e.g., a metabolite level). That said, coding sequences may, in some cases, also contribute to protein concentration (e.g., where they lead to a large impact on structure that leads to nonsense-mediated decay).
BIOLOGICAL RELEVANCE OF PRE-TRANSLATIONAL PLEIOTROPY FOR DRUG TARGET VALIDATION
As we addressed before, horizontal pleiotropy in the context of drug target validation involves an assumption on the absence of pre-translational horizontal pleiotropy (i.e., ϕG = 0, in Figure 2). Since the seminal contribution by Bowden et al. (2015) introduced the MR-Egger method, which is appropriate when all instruments are affected by horizontal pleiotropy, there has been a growing body of methods that, under various assumptions, can provide valid MR estimates in the presence of horizontal pleiotropy (Richmond and Davey Smith 2021). Typically, these methods have not considered drug target MRs specifically, cis settings, nor pre-translational pleiotropy. For example, a biomarker weighted drug target MR may be highly heterogenous (e.g., have a large Q-statistic), which could either signal the presence of pre-translational horizontal pleiotropy (Bowden et al. 2017) or may simply be caused by the drug target effecting disease through multiple pathways (i.e., post-translation pleiotropy). We showed before that such post-translational pleiotropy is in fact part of the drug target effect and therefore does not invalidate drug target MR estimates (Schmidt et al. 2020).
Previously, we discussed the concept of horizontal pleiotropy in the context of trans-pQTL associations in a drug-target MR. It is worth also considering the mechanisms and implications of pre-translational pleiotropy in the cis setting. This is particularly relevant when performing drug target scans where there may not be a specific understanding of the genomic locus, or a prior hypothesis for the likely effect. LD between cis variants and variants within other genes surrounding the target locus, provide an obvious source of pre-translation pleiotropy. However, the presence of such LD, while complicating attributing any disease-causing effect to the selected cis protein under consideration, does provide potentially valuable information for drug development. Further exploration of the LD region might identify the appropriate gene–protein pair, which if druggable, could lead to further target leads.
Pleiotropy is usually inferred by heterogeneity of the MR estimate, but this needs to be considered in the biological context (Fig. 5). For example, if a coding sequence variant is used as an instrument, that influences protein function but not level, and an effect on a disease outcome might be observed when using a downstream biomarker as the exposure but not when using protein or transcript expression as the exposure. Therefore, coding variation that effects the outcome but not the transcript or protein expression will introduce heterogeneity in the analysis. Protein or transcript isoform-level variation is another theoretical mechanism through which heterogeneity can manifest in an MR estimate, for example, genes that have multiple transcripts or multiple protein isoforms, of which only a subset impacts the outcome. Many eQTLs represent associations with an entire transcript pool of the gene and the isoform-binding characteristics of protein assays are usually unknown. Given that variants used as instruments might be drawn from the entire genic region, there remains a possibility that a subset of these variants might operate on the outcome, but their impact might not be completely captured via the exposure assay, manifesting in increased heterogeneity of the MR estimate, which in this case could be inappropriately interpreted as horizontal pleiotropy (Fig. 5).
Figure 5.
Cis-acting variants with respect to different exposures and perceived heterogeneity. Multiple scenarios where cis-acting variants can impact the outcome but not via the exposure. (A) A conventional Mendelian randomization (MR) graph whereby instrumental variants in gene G are acting via biomarker exposure X on disease outcome D. (B) Mechanistically the same as part A but with greater resolution depicting potential (but unused) exposures of transcript level T and protein level P and with the instrumental genetic variants partitioned into noncoding (NC), regulatory (R), and coding (C) variants. Regulatory variants are more likely to impact the transcript level, whereas noncoding variants are more likely to impact protein level via translational efficiency and not the transcript level. Coding variants are more likely to impact on protein function/activity, which will alter downstream biomarker level but this effect will not be mediated via transcript or protein level. An exception to these assumptions is nonsense-mediated decay where aberrant insertion stop codons will lead to destruction of mRNA. (C) The exposure is changed from the biomarker to the protein encoded by gene G. (D) The exposure is changed from the level of protein to transcript level T. In C and D, pathways whereby instrumental genetic variants are impacting the outcome but not via the exposure are highlighted in red.
SCALING DRUG TARGET VALIDATION APPROACHES
Whereas a hypothesis-driven approach investigating a small subset of targets is relatively easy to perform, it is often desirable to investigate the broad landscape of targets against a disease. Scaling up drug target MR involves computational, statistical, and methodological coordination. The following sections explore some of the difficulties involved in performing large-scale analysis, with a particular focus on drug target MR.
Publicly Available GWAS Data
The vast majority of MR studies employ a two-sample MR approach, irrespective of the precise analytical method. The two-sample paradigm uses exposure and outcome data sets derived in different samples and can operate on summary-level genetic associations, and ensures any weak instrument bias acts toward a conservative, neutral effect estimate (Burgess and Thompson 2011). By accessing data from two (or more) independent sources, many of the obstacles encountered with sharing individual-level data are avoided, and will often allow for large, scaled analyses. Researchers, encouraged by journals, are now frequently sharing summary-level data upon publication, further increasing the potential for two-sample MR. This greater availability of aggregated data, however, also introduces the problem of data handling, where file format and information detail often differ between publications and research laboratories.
To efficiently and reliably conduct large-scale scans across multiple targets, GWAS summary data sets require a homogenous structure and a normalization of genome assembly, effect alleles and genomic coordinate representation (e.g., the difference between Ensembl and VCF representation INDELs), as well as normalization of variant identifiers. Several projects have attempted to do this (Hemani et al. 2018; Kamat et al. 2019), with some making normalized data sets available (Hemani et al. 2018). However, the lack of a common data-sharing standard for GWAS data can only be viewed as a missed opportunity that has slowed down the pace of large-scale research. Worse, in some cases, results are available but they appear to be deliberately obfuscated to limit their use. For example, the AMD Consortium (Fritsche et al. 2016) has released summary information where the effect size has been dichotomized to ±1.
Absence of Genetic Variation
As discussed, cis-MR analyses may be preferred due to the natural robustness against some sources of horizontal pleiotropy; clearly these MR analyses can only be applied in settings when there is variation in the drug target encoding gene. It is theoretically possible to use variation at other loci that also impacts the protein level (trans associations). However, the use of these variants in MR analyses increases the risk of horizontal pleiotropy. Fortunately, GWAS of mRNA and protein expression provide hundreds of empirical examples of genetic variants influencing the expression of nearby genes (acting in cis). Recently, the GTEx project and large pQTL analysis (Sun et al. 2018) have catalogued cis variants with functional effects for many thousands of genes suggesting that the absence of cis-acting regulatory variants should not generally be a limiting factor for conducting a drug-target MR (Sun et al. 2018; Yao et al. 2018; Folkersen et al. 2020). However, the presence or absence of coding genetic variation in known drug target encoding loci has rarely been systematically explored (Minikel et al. 2020). Possibly the absence, or presence, of coding genetic variation itself may provide valuable insight on the viability of a drug target for downstream development (Minikel et al. 2020). If a potential target cannot tolerate natural variation, does that make it a better drug target? That is, will it elicit a greater effect for a smaller concentration of drug? Or will the effect of targeting it be detrimental to the patient? Clearly in absence of genetic variation, drug target MR will simply overlook these targets, and this further shows why MR can only be one (likely important) source of evidence in preclinical drug development.
Evidence Prioritization
Multiple testing is a particular concern when considering scans of multiple drug target/disease combinations. As described above, GWAS are designed to minimize the false-positive rate (type 1 errors) at the cost of an increased false-negative rate (type 2 errors). As such, GWAS can robustly show that a genetic association is present but are inadequate to rule out the presence of an association.
One could consider a false-positive minimization approach for drug target validation. This clearly makes sense if one wants to focus on efficacy, ensuring target perturbation affects the intended trait(s). Especially in preclinical settings, however, drug target analyses using human genetics offer the chance to consider safety as well. Unlike validating intended effect, where the aim is to minimize false claims of efficacy, safety is more concerned with not overlooking potential signals, which requires an optimization of power (and minimization of false negatives). Given that sample size in most drug target MR analyses is fixed (unless, in the rare case where a de novo study is designed to genetically validate a drug target), stringent control of false positives will decrease power and often greatly limit the potential to detect safety signals. Hence, depending on the aim of drug target validation, researchers may wish to find a balance between stringent multiple testing control and sufficient power to offer an appropriate level of sensitively to detect important (safety) signals, with similar considerations for the identification of repurposing opportunities, for which there might be many.
It may also be beneficial to position genetic drug target validation within a program of existing preclinical experiments on cell, tissue, and animal models. Alignment of human genomic and standard preclinical evidence can be used to attempt to replicate or falsify findings and offer an efficient solution to building confidence in a target and disease. When scanning multiple drug targets, further gains might be made by looking for internal consistency and considering that proteins may be grouped by shared pathways, which might be anticipated to result in consistent MR estimates.
Due to the growing amount of available GWAS data, we are now able to independently replicate MR findings. For a type 1 error rate α and m-independent replications, the false-positive rate becomes αm; for example, for α = 0.05 and m = 3, the type 1 error rate is 0.053 = 0.000125. Alternatively, one may decide to forgo replication and optimize power instead by meta-analyzing independent GWAS. Due to the often cumulative nature of GWAS, where newer publications typically meta-analyze previous GWAS findings, completely independent data is rarely available. An ideal scenario would be a study repository that would enable users to deconvolute the cohorts used in a study and identify truly independent cohorts. Building such a resource would be time consuming unless studies were required to register their cohort-specific data prior to publication.
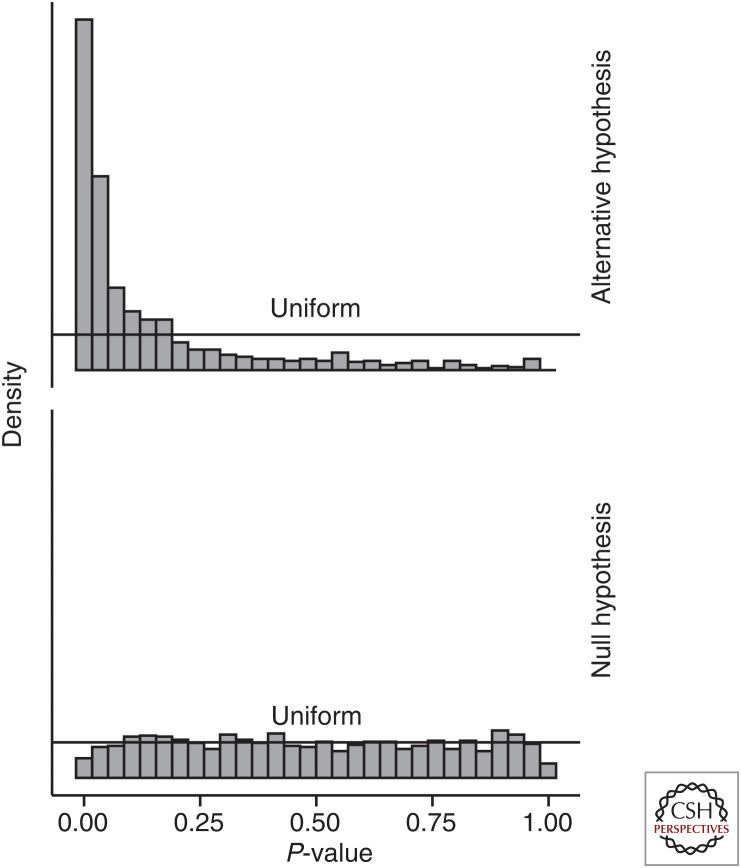
Finally, a shift in inferential perspective may be required when considering results from drug target scans. As suggested before, a drug target scan should be viewed as one component in a body of evidence. With this in mind, and noting that under the null distribution a set of P-values is expected to follow a uniform distribution (Storey 2002), rather than attempting to differentiate true and false-positive association based on a P-value cutoff (e.g., 0.05), testing the whole set of P-values for a significant deviation from the uniform distribution gives an indication of how different the results in the set are from an overall null distribution (Fig. 6). For example, in a preclinical setting one may wish to identify targets with a strong cardiometabolic fingerprint, where 30+ traits (including lipid and glucose measurements) might be relevant. In such a setting, the above detailed procedure may be employed to prioritize targets for general cardiometabolic enrichment, before considering individual disease associations.
Figure 6.
P-value distributions when the null-hypothesis is false (“alternative hypothesis”) or true. N.B., P-values under the null-hypothesis were generated by sampling z-statistics from a standard normal distribution, whereas P-values under the alternative distribution were sampled from a normal distribution with mean of 2 and standard deviation of 1.
EXAMPLES OF HOW DRUG TARGET MR CAN INCREASE DRUG DEVELOPMENT YIELD
In the previous sections, we have discussed the rationale, and the biological and methodological underpinnings of using human genetics for drug target identification and validation, with a specific focus on drug target MR. Next, we present specific cases where human genetics has supported clinical trial findings.
Preclinical Drug Target Prioritization on Anticipated In-Human Effects
For those first-in-class drug molecules in early clinical phase, MR studies could inform “stop or go” decisions (e.g., whether to proceed to human phase I–III clinical trial evaluations).
Two prior examples show the concept. In the first study, Holmes et al. (2013) evaluated whether secretory phospholipase A2 (PLA2) is a valid therapeutic target for CVD management. This MR study was conducted because a first-in-class sPLA2 inhibitor (varespladib) was already in advanced clinical development based on observational association between sPLA2-IIA mass and/or activity and incident CVD events in observational studies. For the MR study, Holmes and colleagues used variants in the gene (PLA2G2A) that encodes secretory sPLA2-IIA and showed that these variants did not meaningfully affect CVD, despite a large effect on sPLA2-IIA levels. Consistent with this, the VISTA-16 randomized trial evaluating the effect of a sPLA2 inhibitor (varespladib) on CVD outcomes was stopped prematurely for lack of efficacy. In a second example of an MR study in this category, Casas and colleagues showed that variants in the PLA2G7 gene that encodes the distinct drug target lipoprotein-associated PLA2 (Lp-PLA2), that were associated with differences in the circulating concentration of this marker, were not associated with altered risk of CVD (Casas et al. 2010). In agreement with these findings, a subsequent study used variants in the PLA2G7 gene that associated with Lp-PLA2 levels, which again did not show a convincing CHD effect (Millwood et al. 2016b). These MR analyses implied that reverse causation, confounding, or both affect the nonrandomized (observational) studies that before reported a risk increasing association of Lp-PLA2 mass or activity with CVD. Consistent with the MR analysis, an Lp-PLA2 inhibitor, darapladib, also failed to demonstrate efficacy in two phase III RCTs, one in patients with stable coronary artery disease, the other in patients who had recently suffered an acute coronary syndrome (O'Donoghue et al. 2014; STABILITY Investigators et al. 2014).
Identification of Safety and Efficacy Phenotypes for Evaluation Clinical Trials
The ability to undertake drug target MR analysis for many disease outcomes makes it feasible to anticipate the effect of perturbing a drug target on a wide range of traits. Theoretical arguments detailing the number of diseases likely to be influenced by any gene or protein, suggest that perturbation of any given drug target is likely to influence the risk of several diseases, and that the profile of effects is target specific. This is backed up by empirical observations of genetic pleiotropy (variants in the same gene being associated with several diseases [Sivakumaran et al. 2011; Solovieff et al. 2013]) and the parallel observation that same drug class can be effective in different diseases (therapeutic pleiotropy [Solovieff et al. 2013; Shih et al. 2018]).
Using genomics to prespecify the outcomes in clinical trials (see Ference et al. 2021) that are anticipated to be affected by pharmacological action on a particular target (target-specific outcomes of both efficacy) would represent a departure from the current norm where end points in a particular therapeutic area tend to be uniform regardless of the target being evaluated (Martin et al. 2014). For example, genetic information led to the discovery of atrial fibrillation as a mechanism-based adverse effect of ivabradine, licensed for angina and heart failure (Martin et al. 2014). This information could also help reduce the risk of an effective drug and target failing to demonstrate efficacy in a clinical trial because a clinical end point unaffected by target perturbation has been included in a composite outcome, thereby diluting the observed treatment effect. Furthermore, early information on a target's potential adverse effects, can ensure clinical trials are specifically designed to rule out such an adverse effect (e.g., through noninferiority designs [Giugliano et al. 2017]).
Indication Expansion and Repurposing Opportunities
Large-scale use of a new drug post-licensing sometimes provokes debate on possible unexpected benefits in different disease areas or unexpected harms. However, this evidence is often insufficient to draw firm conclusions. This is either because it comes from nonrandomized (phase IV) pharmacoepidemiological studies (that are prone to biases by for example immortal time bias [Levesque et al. 2010], and severe confounding by indication [Schmidt et al. 2016a]) or from trials where the outcome of concern was not a primary end point, which increases false-positive (e.g., for efficacy) and -negative (e.g., for safety) rates. An example of how drug target MR can provide additional evidence comes from evaluations of whether interleukin 6 receptor (IL-6R) is a valid drug target for prevention of coronary events (Interleukin-6 Receptor Mendelian Randomisation Analysis (IL6R MR) Consortium et al. 2012; Sarwar and Butterworth 2012). IL-6R is currently the target for the therapeutic monoclonal antibody tocilizumab, licensed for rheumatoid arthritis. Demonstration of a role for this target in coronary disease would provide an opportunity to expand the indications for this already licensed medication. Swerdlow and colleagues showed that variants in the IL6R gene exhibited effects on a wide range of inflammation and other markers that were consistent in profile with that seen during tocilizumab treatment of patients with rheumatoid arthritis. The same genetic variants were also associated with a reduced risk of CHD, a finding that has received independent corroboration (Sarwar and Butterworth 2012). These findings implicate IL-6R as a valid target for coronary disease prevention and suggest that tocilizumab might be repositioned perhaps initially as an adjunctive infusional therapy in acute coronary syndrome. This question is currently being addressed in ongoing clinical trials (Kleveland et al. 2016; Anstensrud et al. 2019). More recent genetic studies have also implicated IL-6R in abdominal aortic aneurysm (Harrison et al. 2013), atrial fibrillation, and inflammatory bowel diseases (Parisinos et al. 2018), flagging additional indication expansion opportunities. The same principle could be applied to develop genetically supported repurposing hypotheses for many first-in-class compounds and targets that proved safe in humans but that failed in their originally intended indication. A series of compounds and related targets have been the subject of asset sharing initiatives developed by the U.S. National Institutes of Health and the UK Medical Research Council (Hayes and Nutt 2019).
Delineating Compound Specificity
Proving that a drug engages its therapeutic target in humans and with no off-target actions has been a major challenge in clinical phase drug development. When a first-in-class molecule causes an adverse effect in clinical phase trials, or post-marketing, it can be difficult to decide whether this is mechanism-based or “off-target” (specific to the drug molecule and unlikely to be shared by other class members). In the past, clarification has been provided by undertaking a randomized trial of other (chemically dissimilar) agents from the same class, but this risks further harm if the adverse effect is mechanism-based rather than agent-specific. Variants in a gene that encode a drug target and that affect its expression or activity should lead to metabolic and physiological changes that profile the effects of a clean drug with no off-target actions (Sofat et al. 2010). Hence, comparison of the effects of variants in a gene encoding a drug target in population studies, and a drug targeting the same protein in a trial could help distinguish on- from off-target effects. For example, when torcetrapib, a first-in-class cholesteryl-ester transfer protein (CETP) inhibitor, was developed to raise HDL-C with the aim of preventing cardiovascular events, an unexpected blood-pressure–elevating effect was detected during a large phase III randomized trial. To disentangle whether this effect was an on- or off-target effect, variants in the CETP gene were used to reproduce the effect of CETP inhibition on the major blood lipid fractions (on-target actions) as well as reduce the risk of CHD, which showed a blood pressure–decreasing effect (Schmidt et al. 2021). These results argued that the blood pressure-elevating effect of torcetrapib, and subsequent compounds evacetrapib and anacetrapib, are off-target and should not be shared by CETP inhibitors that are sufficiently different (Schmidt et al. 2021). Indeed, the chemically distinct CETP inhibitor dalcetrapib showed the same blood pressure–decreasing effect as reported by the CETP MR (Schmidt et al. 2021).
The same principle was used to demonstrate that variants in the HMGCR gene that encode HMG-coA reductase, the target for statins, reproduce the effects of statin treatment on multiple metabolites and lipoprotein lipid subclasses measured by NMR spectroscopy (Würtz et al. 2016). The same genetic variants were used to show that the modestly increased risk of type 2 diabetes among statin users in clinical trials (which does not offset their clinical benefit in reducing CHD events even among patients with diabetes), is an on-target action, potentially mediated in part by increases in body weight and waist circumference (Swerdlow et al. 2015).
With the ability to undertake GWAS of numerous proteomics and metabolomics blood biomarkers, as well as physiological and imaging measures in large cohort studies, comes the ability to infer the effects of drug target perturbation not only on disease end points but also a vast range of variables that could serve as readouts for adequate and specific target engagement in early phase I–II clinical trials.
CONCLUDING REMARKS
The approaches we have described necessitate linking genotype to phenotype at scale. This is exposing the need for greater partnership between academia, health care systems, technology developers and providers, as well as the pharmaceutical industry. This is because large population and patient cohorts (and associated phenotypic and disease outcome data) reside mainly in the public sector, while new technologies, compound development expertise, and financial resources reside mainly in the commercial sector. Developing governance frameworks that allow equitable partnership among these players, and that recognize the contribution made by different stakeholders to value creation, is challenging.
Different models are emerging. Primarily publicly funded, academia-led initiatives include the UK Biobank, the eMERGE Consortium, All of Us, and the Million Veteran Program, as well as the recently completed UK 100,000 Genomes Project. Some studies (e.g., UK Biobank) have subsequently secured additional industry investment for sequencing or proteomics analysis. In other cases, a commercial vehicle has emerged from an academic one (e.g., Nashville Biosciences from Vanderbilt University BioVU). Initiatives with a major pharmaceutical industry component include an Amgen purchase of Decode Genetics, the Regeneron partnership with Geisinger Health, and the recent GSK investment in the consumer genetic testing company with a huge client database, 23andMe. The origin, funding, generation, control of, and access to the linked genotype and phenotype data differ among these initiatives. Should such resources result in new drugs, it remains unclear whether, and how, the contribution of the public sector or private citizens will be recognized. For instance, in the direct-to-consumer genomics arena, an opaque relationship exists between citizens (who pay consumer genomics providers for personal genome analysis for ancestry and disease risk information), and the pharmaceutical industry (who pay the same providers for access to aggregated personal genetic data linked to self-reported health outcomes). In this scenario, citizens risk paying twice: once for a genetic profile provided by the personal genomics company and then again for any drug developed by its pharma partners through insights generated from the aggregated genetic and health data to which citizens have already contributed at their own cost. In addition, in some health care settings, many of the citizens that contributed their data to the development might not be able to benefit from any developed therapeutics due to their cost. These emerging requirements for genomic and health care data for new drug development are likely to force a rethinking of the social contract and economic model for drug development.
Finally, throughout this contribution, we have discussed how human genetics and drug target MR can complement existing sources of (pre-)clinical evidence on anticipating the likely effect of drug target perturbation in humans to increase the yield of drug development. We see this as an adjunct in the drug development process but not as a substitute for randomized trials. These will continue to be required because (1) as we explained here drug compound and drug target are distinct exposures; (2) any new compound could have off-target actions that cannot be modeled genetically; (3) drug target effects modeled through MR, even tissue-specific analyses, reflect biological consequences of target perturbation, which does not guarantee (irrespective of absence or presence of off-target effects) that drug compounds will affect the target in the same manner in the same tissues (some of which are notoriously difficult to access using a drug); (4) by starting follow-up immediately after randomization, drug trials are protected against many of the biases that may still affect genetic studies (Richmond and Davey Smith 2021), which often only enroll subjects decades after gamete forming (that is, after “natural” randomization). Nevertheless, by providing early, in-human evidence on the anticipated on-target effects of drug target perturbation against an array of clinically relevant traits, drug target MR is likely to provide additional, accurate information on which target to pursue for clinical phase development. As such, integration of human genetics in (preclinical) drug development is expected to decrease cost and increase yield.
ACKNOWLEDGMENTS
A.F.S. is supported by BHF Grant PG/18/5033837 and the UCL BHF Research Accelerator AA/18/6/34223. C.F. and A.F.S. received additional support from the National Institute for Health Research University College London Hospitals Biomedical Research Centre. C.F. is supported by Health Data Research UK and the HDR UK Multi-omics Consortium. A.D.H. is an NIHR Senior Investigator, and additionally supported by a UKRI-NIHR Grant MR/V033867/1 for the Multimorbidity Mechanism and Therapeutics Research Collaborative.
Footnotes
Editors: George Davey Smith, Rebecca Richmond, and Jean-Baptiste Pingault
Additional Perspectives on Combining Human Genetics and Causal Inference to Understand Human Disease and Development available at www.perspectivesinmedicine.org
REFERENCES
*Reference is also in this collection.
- Abifadel M, Varret M, Rabès JP, Allard D, Ouguerram K, Devillers M, Cruaud C, Benjannet S, Wickham L, Erlich D, et al. 2003. Mutations in PCSK9 cause autosomal dominant hypercholesterolemia. Nat Genet 34: 154–156. 10.1038/ng1161 [DOI] [PubMed] [Google Scholar]
- Altman DG, Bland JM. 1995. Statistics notes: absence of evidence is not evidence of absence. BMJ 311: 485–485. 10.1136/bmj.311.7003.485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anstensrud AK, Woxhold S, Sharma K, Broch K, Bendz B, Aakhus S, Ueland T, Amundsen BH, Damås JK, Hopp E, et al. 2019. Rationale for the ASSAIL-MI-trial: a randomised controlled trial designed to assess the effect of tocilizumab on myocardial salvage in patients with acute ST-elevation myocardial infarction (STEMI). Open Heart 6: e001108. 10.1136/openhrt-2019-001108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attwood MM, Jonsson J, Rask-Andersen M, Schiöth HB. 2020. Soluble ligands as drug targets. Nat Rev Drug Discov 19: 695–710. 10.1038/s41573-020-0078-4 [DOI] [PubMed] [Google Scholar]
- Barter PJ, Caulfield M, Eriksson M, Grundy SM, Kastelein JJP, Komajda M, Lopez-Sendon J, Mosca L, Tardif JC, Waters DD, et al. 2007. Effects of Torcetrapib in patients at high risk for coronary events. N Engl J Med 357: 2109–2122. 10.1056/NEJMoa0706628 [DOI] [PubMed] [Google Scholar]
- Bowden J, Davey Smith G, Burgess S. 2015. Mendelian randomization with invalid instruments: effect estimation and bias detection through egger regression. Int J Epidemiol 44: 512–525. 10.1093/ije/dyv080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowden J, Del Greco MF, Minelli C, Davey Smith G, Sheehan N, Thompson J. 2017. A framework for the investigation of pleiotropy in two-sample summary data mendelian randomization. Stat Med 36: 1783–1802. 10.1002/sim.7221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buniello A, MacArthur JAL, Cerezo M, Harris LW, Hayhurst J, Malangone C, McMahon A, Morales J, Mountjoy E, Sollis E, et al. 2019. The NHGRI-EBI GWAS catalog of published genome-wide association studies, targeted arrays and summary statistics 2019. Nucleic Acids Res 47: D1005–D1012. 10.1093/nar/gky1120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess S, Thompson SG. 2011. Avoiding bias from weak instruments in mendelian randomization studies. Int J Epidemiol 40: 755–764. 10.1093/ije/dyr036 [DOI] [PubMed] [Google Scholar]
- Cannon CP, Blazing MA, Giugliano RP, McCagg A, White JA, Theroux P, Darius H, Lewis BS, Ophuis TO, Jukema JW, et al. 2015. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med 372: 2387–2397. 10.1056/NEJMoa1410489 [DOI] [PubMed] [Google Scholar]
- Casas JP, Shah T, Hingorani AD, Danesh J, Pepys MB. 2008. C-reactive protein and coronary heart disease: a critical review. J Intern Med 264: 295–314. 10.1111/j.1365-2796.2008.02015.x [DOI] [PubMed] [Google Scholar]
- Casas JP, Ninio E, Panayiotou A, Palmen J, Cooper JA, Ricketts SL, Sofat R, Nicolaides AN, Corsetti JP, Fowkes FGR, et al. 2010. PLA2G7 genotype, lipoprotein-associated phospholipase A2 activity, and coronary heart disease risk in 10 494 cases and 15 624 controls of European ancestry. Circulation 121: 2284–2293. 10.1161/CIRCULATIONAHA.109.923383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claussnitzer M, Cho JH, Collins R, Cox NJ, Dermitzakis ET, Hurles ME, Kathiresan S, Kenny EE, Lindgren CM, MacArthur DG, et al. 2020. A brief history of human disease genetics. Nature 577: 179–189. 10.1038/s41586-019-1879-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J, Pertsemlidis A, Kotowski IK, Graham R, Garcia CK, Hobbs HH. 2005. Low LDL cholesterol in individuals of African descent resulting from frequent nonsense mutations in PCSK9. Nat Genet 37: 161–165. 10.1038/ng1509 [DOI] [PubMed] [Google Scholar]
- Cohen JC, Boerwinkle E, Mosley TH Jr, Hobbs HH. 2006. Sequence variations in PCSK9, low LDL, and protection against coronary heart disease. N Engl J Med 354: 1264–1272. 10.1056/NEJMoa054013 [DOI] [PubMed] [Google Scholar]
- Coignard J, Lush M, Beesley J, O'Mara TA, Dennis J, Tyrer JP, Barnes DR, McGuffog L, Leslie G, Bolla MK, et al. 2021. A case-only study to identify genetic modifiers of breast cancer risk for BRCA1/BRCA2 mutation carriers. Nat Commun 12: 1078. 10.1038/s41467-020-20496-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- C Reactive Protein Coronary Heart Disease Genetics Collaboration (CCGC); Wensley F, Gao P, Burgess S, Kaptoge S, Di Angelantonio E, Shah T, Engert JC, Clarke R, Davey Smith G, et al. 2011. Association between C reactive protein and coronary heart disease: mendelian randomisation analysis based on individual participant data. BMJ 342: d548. 10.1136/bmj.d548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crick F. 1970. Central dogma of molecular biology. Nature 227: 561–563. 10.1038/227561a0 [DOI] [PubMed] [Google Scholar]
- den Hoed M, Eijgelsheim M, Esko T, Brundel BJJM, Peal DS, Evans DM, Nolte IM, Segrè V, Holm H, Handsaker RE, et al. 2013. Identification of heart rate-associated loci and their effects on cardiac conduction and rhythm disorders. Nat Genet 45: 621–631. 10.1038/ng.2610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denny JC, Bastarache L, Roden DM. 2016. Phenome-wide association studies as a tool to advance precision medicine. Annu Rev Genomics Hum Genet 17: 353–373. 10.1146/annurev-genom-090314-024956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drews J. 2000. Drug discovery: a historical perspective. Science 287: 1960–1964. 10.1126/science.287.5460.1960 [DOI] [PubMed] [Google Scholar]
- *.Dudbridge F. 2021. Polygenic Mendelian randomization. Cold Spring Harb Perspect Med 11: a039586. 10.1101/cshperspect.a039586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Editorial. 2017. It's all druggable. Nat Genet 49: 169–169. 10.1038/ng.3788 [DOI] [PubMed] [Google Scholar]
- Ellinor PT, Lunetta KL, Albert CM, Glazer NL, Ritchie MD, Smith AV, Arking DE, Muller-Nurasyid M, Krijthe BP, Lubitz SA, et al. 2012. Meta-analysis identifies six new susceptibility loci for atrial fibrillation. Nat Genet 44: 670–675. 10.1038/ng.2261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ference BA. 2018. How to use Mendelian randomization to anticipate the results of randomized trials. Eur Heart J 39: 360–362. 10.1093/eurheartj/ehx462 [DOI] [PubMed] [Google Scholar]
- Ference BA, Robinson JG, Brook RD, Catapano AL, Chapman MJ, Neff DR, Voros S, Giugliano RP, Davey Smith G, Fazio S, et al. 2016. Variation in PCSK9 and HMGCR and risk of cardiovascular disease and diabetes. N Engl J Med 375: 2144–2153. 10.1056/NEJMoa1604304 [DOI] [PubMed] [Google Scholar]
- *.Ference BA, Holmes MV, Davey Smith G. 2021. Using Mendelian randomization to improve the design of randomized trials. Cold Spring Harb Perspect Med 11: a040980. 10.1101/cshperspect.a040980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finan C, Gaulton A, Kruger FA, Lumbers RT, Shah T, Engmann J, Galver L, Kelley R, Karlsson A, Santos R, et al. 2017. The druggable genome and support for target identification and validation in drug development. Sci Transl Med 9: eaag1166. 10.1126/scitranslmed.aag1166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folkersen L, Gustafsson S, Wang Q, Hvidberg Hansen D, Hedman AK, Schork A, Page K, Zhernakova D, Wu Y, Peters J, et al. 2020. Genomic and drug target evaluation of 90 cardiovascular proteins in 30,931 individuals. Nat Metab 2: 1135–1148. 10.1038/s42255-020-00287-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritsche LG, Igl W, Cooke Bailey JN, Grassmann F, Sengupta S, Bragg-Gresham JL, Burdon KP, Hebbring SJ, Wen C, Gorski M, et al. 2016. A large genome-wide association study of age-related macular degeneration highlights contributions of rare and common variants. Nat Genet 48: 134–143. 10.1038/ng.3448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaulton A, Hersey A, Nowotka M, Bento AP, Chambers J, Mendez D, Mutowo P, Atkinson F, Bellis LJ, Cibrián-Uhalte E, et al. 2017. The ChEMBL database in 2017. Nucleic Acids Res 45: D945–D954. 10.1093/nar/gkw1074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giugliano RP, Mach F, Zavitz K, Kurtz C, Im K, Kanevsky E, Schneider J, Wang H, Keech A, Pedersen TR, et al. 2017. Cognitive function in a randomized trial of evolocumab. N Engl J Med 377: 633–643. 10.1056/NEJMoa1701131 [DOI] [PubMed] [Google Scholar]
- Gregory AP, Dendrou CA, Attfield KE, Haghikia A, Xifara DK, Falk B, Poschmann G, Gurman K, Lambert L, Leach OA, et al. 2012. TNF receptor 1 genetic risk mirrors outcome of anti-TNF therapy in multiple sclerosis. Nature 488: 508–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison RK. 2016. Phase II and phase III failures: 2013–2015. Nat Rev Drug Discov 15: 817–818. 10.1038/nrd.2016.184 [DOI] [PubMed] [Google Scholar]
- Harrison SC, Smith AJ, Jones GT, Swerdlow DI, Rampuri R, Bown MJ, Aneurysm Consortium, Folkersen L, Baas AF, de Borst GJ, et al. 2013. Interleukin-6 receptor pathways in abdominal aortic aneurysm. Eur Heart J 34: 3707–3716. 10.1093/eurheartj/ehs354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hay M, Thomas DW, Craighead JL, Economides C, Rosenthal J. 2014. Clinical development success rates for investigational drugs. Nat Biotechnol 32: 40–51. 10.1038/nbt.2786 [DOI] [PubMed] [Google Scholar]
- Hayes AG, Nutt DJ. 2019. Compound asset sharing initiatives between pharmaceutical companies, funding bodies, and academia: learnings and successes. Pharmacol Res Perspect 7: e00510. 10.1002/prp2.510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemani G, Zheng J, Elsworth B, Wade KH, Haberland V, Baird D, Laurin C, Burgess S, Bowden J, Langdon R, et al. 2018. The MR-base platform supports systematic causal inference across the human phenome. eLife 7: e34408. 10.7554/eLife.34408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernán MA, Hernández-Diaz S, Robins JM. 2004. A structural approach to selection bias. Epidemiology 15: 615–625. 10.1097/01.ede.0000135174.63482.43 [DOI] [PubMed] [Google Scholar]
- Hingorani AD, Kuan V, Finan C, Kruger FA, Gaulton A, Chopade S, Sofat R, MacAllister RJ, Overington JP, Hemingway H, et al. 2019. Improving the odds of drug development success through human genomics: modelling study. Sci Rep 9: 18911. 10.1038/s41598-019-54849-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes MV, Simon T, Exeter HJ, Folkersen L, Asselbergs FW, Guardiola M, Cooper JA, Palmen J, Hubacek JA, Carruthers KF, et al. 2013. Secretory phospholipase A2-IIA and cardiovascular disease: a Mendelian randomization study. J Am Coll Cardiol 62: 1966–1976. 10.1016/j.jacc.2013.06.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes MV, Richardson TG, Ference BA, Davies NM, Davey Smith G. 2021. Integrating genomics with biomarkers and therapeutic targets to invigorate cardiovascular drug development. Nat Rev Cardiol 18: 435–453. 10.1038/s41569-020-00493-1 [DOI] [PubMed] [Google Scholar]
- Hormozdiari F, van de Bunt M, Segrè AV, Li X, Joo JWJ, Bilow M, Sul JH, Sankararaman S, Pasaniuc B, Eskin E. 2016. Colocalization of GWAS and eQTL signals detects target genes. Am J Hum Genet 99: 1245–1260. 10.1016/j.ajhg.2016.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes JP, Rees S, Kalindjian SB, Philpott KL. 2011. Principles of early drug discovery. Br J Pharmacol 162: 1239–1249. 10.1111/j.1476-5381.2010.01127.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Hwang LD, Davies NM, Warrington NM, Evans DM. 2021. Integrating family-based and Mendelian randomization designs. Cold Spring Harb Perspect Med 11: a039503. 10.1101/cshperspect.a039503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Interleukin-6 Receptor Mendelian Randomisation Analysis (IL6R MR) Consortium; Swerdlow DI, Holmes MV, Kuchenbaecker KB, Engmann JE, Shah T, Sofat R, Guo Y, Chung C, Peasey A, et al. 2012. The interleukin-6 receptor as a target for prevention of coronary heart disease: a Mendelian randomisation analysis. Lancet 379: 1214–1224. 10.1016/S0140-6736(12)60110-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamat MA, Blackshaw JA, Young R, Surendran P, Burgess S, Danesh J, Butterworth AS, Staley JR. 2019. PhenoScanner V2: an expanded tool for searching human genotype–phenotype associations. Bioinformatics 35: 4851–4853. 10.1093/bioinformatics/btz469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleveland O, Kunszt G, Bratlie M, Ueland T, Broch K, Holte E, Michelsen AE, Bendz B, Amundsen BH, Epsevik T, et al. 2016. Effect of a single dose of the interleukin-6 receptor antagonist tocilizumab on inflammation and troponin T release in patients with non-ST-elevation myocardial infarction: a double-blind, randomized, placebo-controlled phase 2 trial. Eur Heart J 37: 2406–2413. 10.1093/eurheartj/ehw171 [DOI] [PubMed] [Google Scholar]
- Levesque LE, Hanley JA, Kezouh A, Suissa S. 2010. Problem of immortal time bias in cohort studies: example using statins for preventing progression of diabetes. BMJ 340: b5087. 10.1136/bmj.b5087 [DOI] [PubMed] [Google Scholar]
- Lindsay MA. 2003. Target discovery. Nat Rev Drug Discov 2: 831–838. 10.1038/nrd1202 [DOI] [PubMed] [Google Scholar]
- Marso SP, Daniels GH, Brown-Frandsen K, Kristensen P, Mann JF, Nauck MA, Nissen SE, Pocock S, Poulter NR, Ravn LS, et al. 2016. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med 375: 311–322. 10.1056/NEJMoa1603827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martens EP, Pestman WR, de Boer A, Belitser SV, Klungel OH. 2006. Instrumental variables: application and limitations. Epidemiology 17: 260–267. 10.1097/01.ede.0000215160.88317.cb [DOI] [PubMed] [Google Scholar]
- Martin RIR, Pogoryelova O, Koref MS, Bourke JP, Teare MD, Keavney BD. 2014. Atrial fibrillation associated with ivabradine treatment: meta-analysis of randomised controlled trials. Heart 100: 1506–1510. 10.1136/heartjnl-2014-305482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy MI, Abecasis GR, Cardon LR, Goldstein DB, Little J, Ioannidis JPA, Hirschhorn JN. 2008. Genome-wide association studies for complex traits: consensus, uncertainty and challenges. Nat Rev Genet 9: 356–369. 10.1038/nrg2344 [DOI] [PubMed] [Google Scholar]
- Millwood IY, Bennett DA, Walters RG, Clarke R, Waterworth D, Johnson T, Chen Y, Yang L, Guo Y, Bian Z, et al. 2016a. Lipoprotein-associated phospholipase A2 loss-of-function variant and risk of vascular diseases in 90,000 Chinese adults. J Am Coll Cardiol 67: 230–231. 10.1016/j.jacc.2015.10.056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millwood IY, Bennett DA, Walters RG, Clarke R, Waterworth D, Johnson T, Chen Y, Yang L, Guo Y, Bian Z, et al. 2016b. A phenome-wide association study of a lipoprotein-associated phospholipase A2 loss-of-function variant in 90,000 Chinese adults. Int J Epidemiol 45: 1588–1599. 10.1093/ije/dyw087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minikel EV, Karczewski KJ, Martin HC, Cummings BB, Whiffin N, Rhodes D, Alföldi J, Trembath RC, van Heel DA, Daly MJ, et al. 2020. Evaluating drug targets through human loss-of-function genetic variation. Nature 581: 459–464. 10.1038/s41586-020-2267-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moffat JG, Vincent F, Lee JA, Eder J, Prunotto M. 2017. Opportunities and challenges in phenotypic drug discovery: an industry perspective. Nat Rev Drug Discov 16: 531–543. 10.1038/nrd.2017.111 [DOI] [PubMed] [Google Scholar]
- Munos B. 2009. Lessons from 60 years of pharmaceutical innovation. Nat Rev Drug Discov 8: 959–968. 10.1038/nrd2961 [DOI] [PubMed] [Google Scholar]
- Nelson MR, Tipney H, Painter JL, Shen J, Nicoletti P, Shen Y, Floratos A, Sham PC, Li MJ, Wang J, et al. 2015. The support of human genetic evidence for approved drug indications. Nat Genet 47: 856–860. 10.1038/ng.3314 [DOI] [PubMed] [Google Scholar]
- Newport MJ, Finan C. 2011. Genome-wide association studies and susceptibility to infectious diseases. Brief Funct Genomics 10: 98–107. 10.1093/bfgp/elq037 [DOI] [PubMed] [Google Scholar]
- Nicholls SJ, Kastelein JP, Schwartz GG, Bash D, Rosenson RS, Cavender MA, Brennan DM, Koenig W, Jukema JW, Nambi V, et al. 2014. Varespladib and cardiovascular events in patients with an acute coronary syndrome: the VISTA-16 randomized clinical trial. JAMA 311: 252–262. 10.1001/jama.2013.282836 [DOI] [PubMed] [Google Scholar]
- O'Donoghue ML, Braunwald E, White HD, Lukas MA, Tarka E, Steg PG, Hochman JS, Bode C, Maggioni AP, Im KA, et al. 2014. Effect of darapladib on major coronary events after an acute coronary syndrome: the SOLID-TIMI-52 randomized clinical trial. JAMA 312: 1006–1015. 10.1001/jama.2014.11061 [DOI] [PubMed] [Google Scholar]
- Pammolli F, Magazzini L, Riccaboni M. 2011. The productivity crisis in pharmaceutical R&D. Nat Rev Drug Discov 10: 428–438. 10.1038/nrd3405 [DOI] [PubMed] [Google Scholar]
- Parisinos CA, Serghiou S, Katsoulis M, George MJ, Patel RS, Hemingway H, Hingorani AD. 2018. Variation in interleukin 6 receptor gene associates with risk of Crohn's disease and ulcerative colitis. Gastroenterology 155: 303–306.e2. 10.1053/j.gastro.2018.05.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel RS, Tragante V, Schmidt AF, McCubrey RO, Holmes MV, Howe LJ, Direk K, Akerblom A, Leander K, Virani SS, et al. 2019. Subsequent event risk in individuals with established coronary heart disease: design and rationale of the GENIUS-CHD consortium. Circ Genom Prec Med 12: e002470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul SM, Mytelka DS, Dunwiddie CT, Persinger CC, Munos BH, Lindborg SR, Schacht AL. 2010. How to improve R&D productivity: the pharmaceutical industry's grand challenge. Nat Rev Drug Discov 9: 203–214. 10.1038/nrd3078 [DOI] [PubMed] [Google Scholar]
- Pietzner M, Wheeler E, Carrasco-Zanini J, Raffler J, Kerrison ND, Oerton E, Auyeung VPW, Luan J, Finan C, Casas JP, et al. 2020. Genetic architecture of host proteins involved in SARS-CoV-2 infection. Nat Commun 11: 6397. 10.1038/s41467-020-19996-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietzner M, Wheeler E, Carrasco-Zanini J, Kerrison ND, Oerton E, Koprulu M, Luan J, Hingorani AD, Williams SA, Wareham NJ, et al. 2021. Cross-platform proteomics to advance genetic prioritisation strategies. bioRxiv 10.1101/2021.03.18.435919 [DOI] [Google Scholar]
- Pina AS, Hussain A, Roque ACA. 2009. An historical overview of drug discovery. Methods Mol Biol 572: 3–12. 10.1007/978-1-60761-244-5_1 [DOI] [PubMed] [Google Scholar]
- Plenge RM, Scolnick EM, Altshuler D. 2013. Validating therapeutic targets through human genetics. Nat Rev Drug Discov 12: 581–594. 10.1038/nrd4051 [DOI] [PubMed] [Google Scholar]
- *.Richardson TG, Zheng J, Gaunt TR. 2021. Computational tools for causal inference in genetics. Cold Spring Harb Perspect Med 11: a039248. 10.1101/cshperspect.a039248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Richmond RC, Davey Smith G. 2021. Mendelian randomization: concepts and scope. Cold Spring Harb Perspect Med 10.1101/cshperspect.a040501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakamoto KM, Kim KB, Kumagai A, Mercurio F, Crews CM, Deshaies RJ. 2001. Protacs: chimeric molecules that target proteins to the Skp1–Cullin–F box complex for ubiquitination and degradation. Proc Natl Acad Sci 98: 8554–8559. 10.1073/pnas.141230798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarwar N, Butterworth AS. 2012. Interleukin-6 receptor pathways in coronary heart disease: a collaborative meta-analysis of 82 studies. Lancet 379: 1205–1213. 10.1016/S0140-6736(11)61931-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt AF, Groenwold RHH. 2018. Adjusting for bias in unblinded randomized controlled trials. Stat Methods Med Res 27: 2413–2427. 10.1177/0962280216680652 [DOI] [PubMed] [Google Scholar]
- Schmidt AF, Klungel OH, Groenwold RHH. 2016a. Adjusting for confounding in early postlaunch settings: going beyond logistic regression models. Epidemiology 27: 133–142. 10.1097/EDE.0000000000000388 [DOI] [PubMed] [Google Scholar]
- Schmidt AF, Hingorani AD, Jefferis BJ, White J, Groenwold R, Dudbridge F; UCLEB Consortium. 2016b. Comparison of variance estimators for meta-analysis of instrumental variable estimates. Int J Epidemiol 45: 1975–1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt AF, Pearce LS, Wilkins JT, Overington JP, Hingorani AD, Casas JP. 2017a. PCSK9 monoclonal antibodies for the primary and secondary prevention of cardiovascular disease. Cochrane Database Syst Rev 4: CD011748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt AF, Swerdlow DI, Holmes MV, Patel RS, Fairhurst-Hunter Z, Lyall DM, Hartwig FP, Horta BL, Hyppönen E, Power C, et al. 2017b. PCSK9 genetic variants and risk of type 2 diabetes: a Mendelian randomisation study. Lancet Diabetes Endocrinol 5: 97–105. 10.1016/S2213-8587(16)30396-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt AF, Finan C, Gordillo-Marañón M, Asselbergs FW, Freitag DF, Patel RS, Tyl B, Chopade S, Faraway R, Zwierzyna M, et al. 2020. Genetic drug target validation using Mendelian randomisation. Nat Commun 11: 3255. 10.1038/s41467-020-16969-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt AF, Hunt NB, Gordillo-Maranon M, Charoen P, Drenos F, Casa JP, Kivimake M, Lawlor DA, Giambartolomei C, Papacosta O, et al. 2021. Cholesteryl ester transfer protein (CETP) as a drug target for cardiovascular disease. Nat Commun 10.1038/s41467-021-25703-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott RA, Freitag DF, Li L, Chu AY, Surendran P, Young R, Grarup N, Stancáková A, Chen Y, Varga TV, et al. 2016. A Genomic approach to therapeutic target validation identifies a glucose-lowering GLP1R variant protective for coronary heart disease. Sci Transl Med 8: 341ra76. 10.1126/scitranslmed.aad3744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen J, Song K, Slater AJ, Ferrero E, Nelson MR. 2017. STOPGAP: a database for systematic target opportunity assessment by genetic association predictions. Bioinformatics 33: 2784–2786. 10.1093/bioinformatics/btx274 [DOI] [PubMed] [Google Scholar]
- Shih HP, Zhang X, Aronov AM. 2018. Drug discovery effectiveness from the standpoint of therapeutic mechanisms and indications. Nat Rev Drug Discov 17: 19–33. 10.1038/nrd.2017.194 [DOI] [PubMed] [Google Scholar]
- Sipeky C, Tammela TLJ, Auvinen A, Schleutker J. 2021. Novel prostate cancer susceptibility gene SP6 predisposes patients to aggressive disease. Prostate Cancer Prostatic Dis 10.1038/s41391-021-00378-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sivakumaran S, Agakov F, Theodoratou E, Prendergast JG, Zgaga L, Manolio T, Rudan I, McKeigue P, Wilson JF, Campbell H. 2011. Abundant pleiotropy in human complex diseases and traits. Am J Hum Genet 89: 607–618. 10.1016/j.ajhg.2011.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sofat R, Hingorani AD, Smeeth L, Humphries SE, Talmud PJ, Cooper J, Shah T, Sandhu MS, Ricketts SL, Boekholdt SM, et al. 2010. Separating the mechanism-based and off-target actions of cholesteryl ester transfer protein inhibitors with CETP gene polymorphisms. Circulation 121: 52–62. 10.1161/CIRCULATIONAHA.109.865444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solovieff N, Cotsapas C, Lee PH, Purcell SM, Smoller JW. 2013. Pleiotropy in complex traits: challenges and strategies. Nat Rev Genet 14: 483–495. 10.1038/nrg3461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- STABILITY Investigators; White HD, Held C, Stewart R, Tarka E, Brown R, Davies RY, Budaj A, Harrington RA, Steg PG, et al. 2014. Darapladib for preventing ischemic events in stable coronary heart disease. N Engl J Med 370: 1702–1711. 10.1056/NEJMoa1315878 [DOI] [PubMed] [Google Scholar]
- Stacey D, Fauman EB, Ziemek D, Sun BB, Harshfield EL, Wood AM, Butterworth AS, Suhre K, Paul DS. 2019. ProGeM: a framework for the prioritization of candidate causal genes at molecular quantitative trait loci. Nucleic Acids Res 47: e3. 10.1093/nar/gky837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storey JD. 2002. A direct approach to false discovery rates. J R Stat Soc Series B Stat Methodol 64: 479–498. 10.1111/1467-9868.00346 [DOI] [Google Scholar]
- Sun BB, Maranville JC, Peters JE, Stacey D, Staley JR, Blackshaw J, Burgess S, Jiang T, Paige E, Surendran P, et al. 2018. Genomic atlas of the human plasma proteome. Nature 558: 73–79. 10.1038/s41586-018-0175-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swerdlow DI, Preiss D, Kuchenbaecker KB, Holmes MV, Engemann JEL, Shah T, Sofat R, Stender S, Johnson PCD, Scott RA, et al. 2015. HMG-coenzyme A reductase inhibition, type 2 diabetes, and bodyweight: evidence from genetic analysis and randomised trials. Lancet 385: 351–361. 10.1016/S0140-6736(14)61183-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swinney DC, Anthony J. 2011. How were new medicines discovered? Nat Rev Drug Discov 10: 507–519. 10.1038/nrd3480 [DOI] [PubMed] [Google Scholar]
- Taşan M, Musso G, Hao T, Vida M, MacRae CA, Roth FP. 2015. Selecting causal genes from genome-wide association studies via functionally coherent subnetworks. Nat Methods 12: 154–159. 10.1038/nmeth.3215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- The Lenercept Multiple Sclerosis Study Group. 1999. TNF neutralization in MS: results of a randomized, placebo-controlled multicenter study. The University of British Columbia MS/MRI Analysis Group. Neurology 53: 457–457. 10.1212/WNL.53.3.457 [DOI] [PubMed] [Google Scholar]
- The Myocardial Infarction Genetics Consortium Investigators; Stitziel NO, Won HH, Morrison AC, Peloso GM, Do R, Lange LA, Fontanillas P, Gupta N, Duga S, et al. 2014. Inactivating mutations in NPC1L1 and protection from coronary heart disease. N Engl J Med 371: 2072–2082. 10.1056/NEJMoa1405386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trynka G, Westra HJ, Slowikowski K, Hu X, Xu H, Stranger BE, Klein RJ, Jan B, Raychaudhuri S. 2015. Disentangling the effects of colocalizing genomic annotations to functionally prioritize non-coding variants within complex-trait loci. Am J Hum Genet 97: 139–152. 10.1016/j.ajhg.2015.05.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Oosten BW, Barkhof F, Truyen L, Boringa JB, Bertelsmann FW, von Blomberg BM, Woody JN, Hartung HP, Polman CH. 1996. Increased MRI activity and immune activation in two multiple sclerosis patients treated with the monoclonal anti-tumor necrosis factor antibody cA2. Neurology 47: 1531–1534. 10.1212/WNL.47.6.1531 [DOI] [PubMed] [Google Scholar]
- Visscher PM, Wray NR, Zhang Q, Sklar P, McCarthy MI, Brown MA, Yang J. 2017. 10 years of GWAS discovery: biology, function, and translation. Am J Hum Genet 101: 5–22. 10.1016/j.ajhg.2017.06.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace C. 2020. Eliciting priors and relaxing the single causal variant assumption in colocalisation analyses. PLoS Genet 16: e1008720. 10.1371/journal.pgen.1008720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe K, Taskesen E, van Bochoven A, Posthuma D. 2017. Functional mapping and annotation of genetic associations with FUMA. Nat Commun 8: 1826. 10.1038/s41467-017-01261-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen X, Pique-Regi R, Luca F. 2017. Integrating molecular QTL data into genome-wide genetic association analysis: probabilistic assessment of enrichment and colocalization. PLoS Genet 13: e1006646. 10.1371/journal.pgen.1006646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler J, McHale M, Jackson V, Penny M. 2007. Assessing theoretical risk and benefit suggested by genetic association studies of CCR5: experience in a drug development programme for maraviroc. Antiviral Ther 12: 233–245. [PubMed] [Google Scholar]
- Williams DM, Finan C, Schmidt AF, Burgess S, Hingorani AD. 2020. Lipid lowering and Alzheimer disease risk: a Mendelian randomization study. Ann Neurol 87: 30–39. 10.1002/ana.25642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Würtz P, Wang Q, Soininen P, Kangas AJ, Fatemifar G, Tynkkynen T, Tiainen M, Perola M, Tillin T, Hughes AD, et al. 2016. Metabolomic profiling of statin use and genetic inhibition of HMG-CoA reductase. J Am Coll Cardiol 67: 1200–1210. 10.1016/j.jacc.2015.12.060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao C, Chen G, Song C, Keefe J, Mendelson M, Huan T, Sun BB, Laser A, Maranville JC, Wu H, et al. 2018. Genome-wide mapping of plasma protein QTLs identifies putatively causal genes and pathways for cardiovascular disease. Nat Commun 9: 3268. 10.1038/s41467-018-05512-x [DOI] [PMC free article] [PubMed] [Google Scholar]