DEATH RECEPTORS ARE A SUBSET OF THE TUMOR NECROSIS FACTOR RECEPTOR FAMILY
So far, we have considered a pathway of apoptosis, the mitochondrial pathway, which is activated in response to cellular stress and some developmental cues. In this review, we discuss a different pathway of apoptosis that is triggered by the interaction of extracellular ligands with receptors on the cell surface.
On the surfaces of some mammalian cells (and probably those of other vertebrates as well) are receptors that, when bound by their ligands, engage a pathway of caspase activation and apoptosis that can be distinct from the mitochondrial pathway that we have discussed. These surface molecules are referred to as “death receptors,” and their mode of caspase activation is called the death receptor pathway of apoptosis.
The death receptors and the proteins that activate them (“death ligands”) are all closely related molecules. The death ligands are members of a larger family of proteins, the tumor necrosis factor (TNF) family, and the receptors for these ligands are members of the TNF receptor (TNFR) family.1
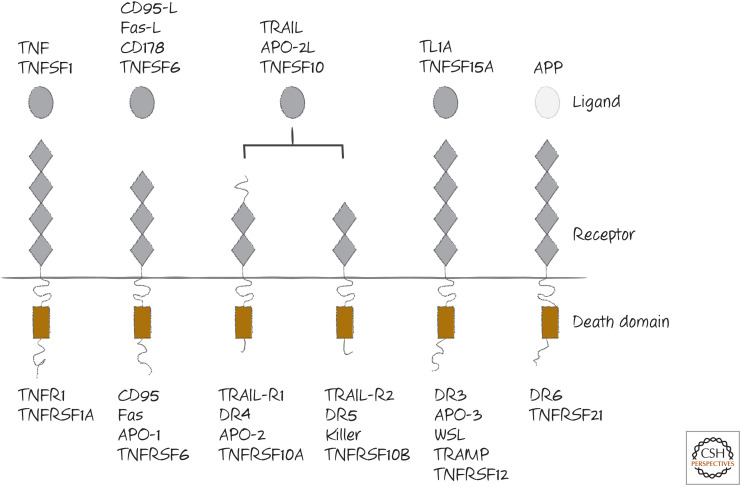
The major death receptors are tumor necrosis factor receptor-1 (TNFR1), CD95 (also called Fas and APO-1), the TRAIL receptors (TRAIL receptor-1, also called DR4, and TRAIL receptor-2, also called DR5 in humans; rodents have only one, which resembles DR5), DR3 (death receptor 3), and DR6 (death receptor 6). These are shown in Figure 1. The death ligands are also shown and include TNF, CD95-ligand (CD95-L or Fas-L), and TRAIL (TNF-related apoptosis-inducing ligand, also called APO-2L). Although a ligand for DR3 has been identified, this does not readily trigger apoptosis, and therefore there could be other ways DR3 is engaged or it might not normally function in apoptosis induction. The apoptosis-inducing ligand for DR6 has been identified as the cell-bound amyloid precursor protein, APP. Unlike the other ligands, this is not a member of the TNF family, and its function as a DR6 ligand is somewhat controversial.
Figure 1.
The death receptors and their ligands. Several names for each are shown. Of all of the ligands, only APP is not a member of the tumor necrosis factor (TNF) family.
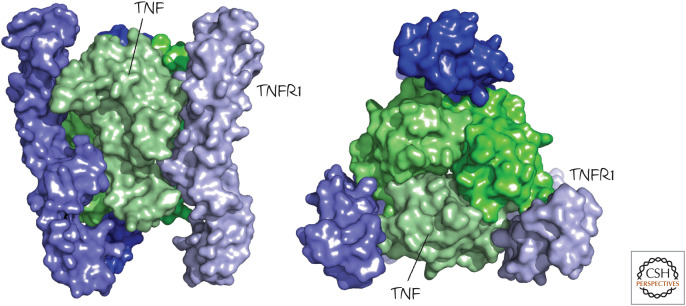
All of the TNF-family ligands, including the death ligands, are trimers (an example is shown in Fig. 2). This is also true of the receptors. It is often mistakenly suggested that the unligated death receptors are monomeric and assemble into trimers when bound, but there is very good evidence that the receptors are already trimeric on the cell surface before engaging their ligands. On binding a death ligand, the trimers form higher-order complexes and expose a region located in the intracellular portion of the receptor. This region contains a “death domain” (DD; see Green 2022a). An exception is the interaction between APP and DR6. In this case, the receptor appears to be monomeric, and it is dimerized by interaction with its ligand.
Figure 2.
Death receptors and death ligands are trimeric. Shown are two views of the structure of tumor necrosis factor (TNF; green) bound to the extracellular regions of the TNF receptor (TNFR1, blue). (PDB 1TNR [Banner et al. 1993].)
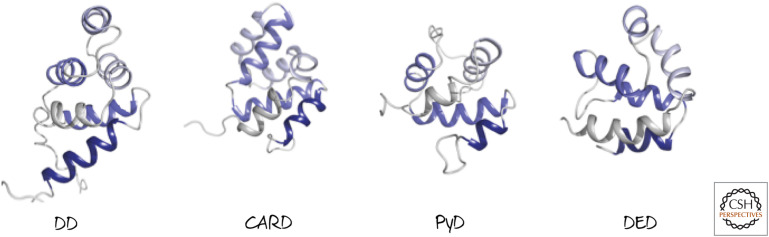
DDs are found in the death receptors but not in any of the other TNFR-family members. Recall that, structurally, they show the death fold also adopted by the death effector domains (DEDs) and caspase-recruitment domains (CARDs) found in caspases and their adapters (Green 2022a) (Fig. 3). The death domains share no sequence homology with the other death fold domains, but they interact with proteins that share the same domain (giving rise to DD–DD interactions).
Figure 3.
Ribbon diagrams of the representative structures of distinct death folds. DD, death domain; CARD, caspase-recruitment domain; PyD, pyrin domain; DED, death effector domain.
Different death receptors bind to different DD-containing adapter proteins as the next step in the signaling pathway. We begin with the simplest version of this pathway, seen in the case of the death receptor CD95.
HOW CD95 TRIGGERS APOPTOSIS
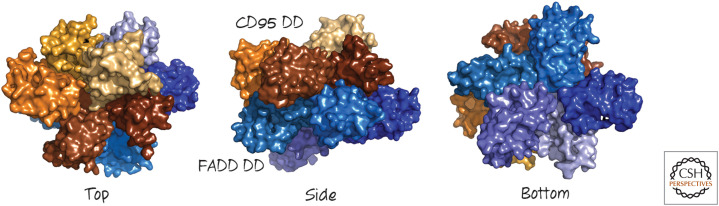
When CD95 is bound by its ligand CD95-L, or by some antibodies against CD95 that mimic this, the resulting conformational change in the intracellular DD allows it to interact with a small cytosolic protein called FADD (FAS-associated death domain protein). FADD contains a DD through which it binds to CD95 through a DD–DD interaction. The DDs bind to each other at three different interfaces, producing a two-layer structure (Fig. 4).
Figure 4.
The structure of the CD95 receptor death domain (DD) binding to the DD of the FAS-associated death domain protein (FADD) viewed from top, side, and underside. (Redrawn from Wang et al. 2010. PDB kindly provided by Dr. Hao Wu.)
These assemble into arrays, as shown in Figure 5. This explains why at least two trimers of CD95 must be brought together to produce the complex that leads to apoptosis.
Figure 5.
Model for the clustering of the CD95 receptor and FADD, based on the structure in Figure 4. The three different types of interface between different death domains (DDs) are shown by different lines. (Redrawn from Wang et al. 2010. PDB kindly provided by Dr. Hao Wu.)
The binding of the CD95 DD to the FADD DD causes FADD to expose a different death fold, termed a death effector domain (DED). In turn, the exposed DED on FADD now binds to the prodomain of the initiator caspase, caspase-8, through one of the two DEDs in this caspase. In this way, ligated CD95 binds to FADD, which exposes its DED to recruit caspase-8. This results in the oligomerization of caspase-8. This activates the caspase, which then cleaves itself, stabilizing caspase-8 as dimers, as we saw in Green et al. (2022a).
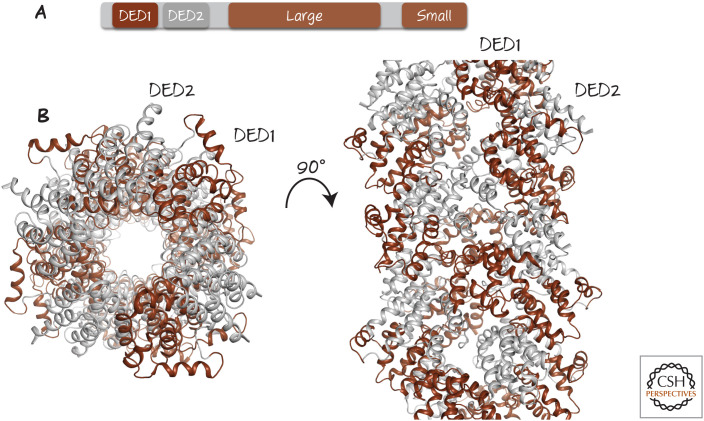
Actually, this is a bit more complicated. Caspase-8 has two DED domains (Fig. 6) and only one binds to FADD (DED1). The second one (DED2) is then exposed, allowing it to bind to the DED1 of another caspase-8 monomer, which exposes its own DED2. This results in a chain reaction, resulting in an extended filament of caspase-8, unless something else stops it (Fig. 6). We consider this “something else” below. As a result, one FADD protein recruits a complex of up to eight caspase-8 molecules. Caspase-8 can now cleave and activate the executioner caspases-3 and -7, which in turn orchestrate apoptosis (Fig. 7).
Figure 6.
Caspase-8 oligomerizes into filaments. (A) Domains in caspase-8. DED, death effector domain; Large, large subunit; Small, small subunit. (B) Cryoelectron microscopy structure of filament formed when caspase-8 prodomains are brought into proximity by FADD. Only death effector domain (DED) regions (1 and 2, colored as in A) are shown. (Left) “Top-down” view. (Right) Side view.
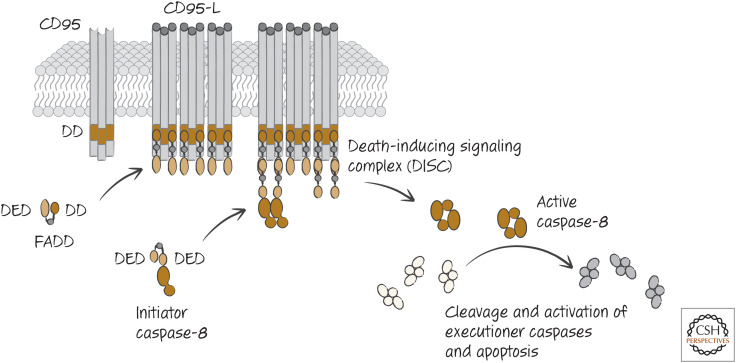
Figure 7.
The CD95-induced apoptotic pathway, showing the principal players from the initial binding of the CD95 ligand (CD95-L) to the clustered receptors at the cell plasma membrane to formation of the death-inducing signaling complex (DISC), leading to eventual activation of executioner caspases.
The complex comprising ligated CD95, FADD, and caspase-8 is called the CD95 death-inducing signaling complex (DISC). The DISC forms within seconds of CD95 ligation, and apoptosis can proceed within tens of minutes, presumably limited only by the rate of executioner caspase activation and substrate cleavage. However, in some cells, as we will see, this pathway can be slower and somewhat more complex.
In humans, the DISC can also include caspase-10, which appears to be similarly activated by binding to FADD through DED–DED interactions (note that caspase-10 is not found in rodents). However, at present, there is no compelling evidence that caspase-10 can replace caspase-8 for CD95-induced apoptosis. Human cells lacking caspase-8 are resistant to CD95 ligation (despite the presence of caspase-10).
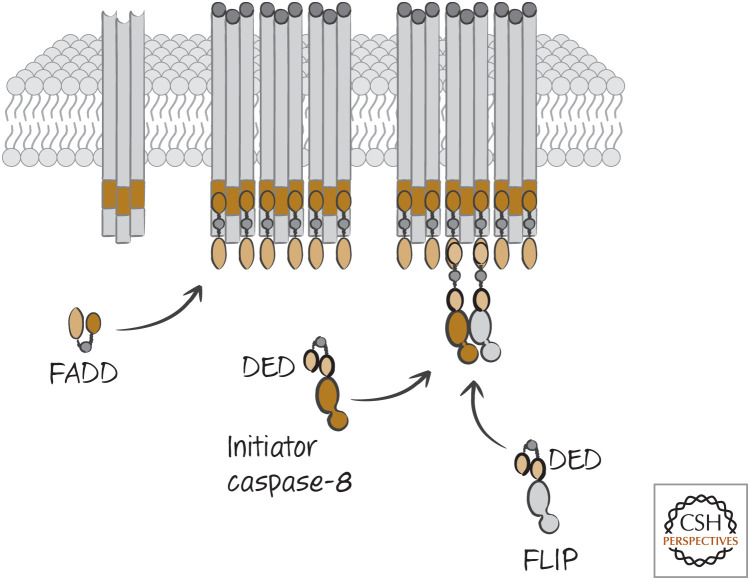
Not all cells expressing CD95 are sensitive to CD95-induced apoptosis. Another protein, FLICE-like inhibitory protein (FLIP), which is related to caspase-8 but lacks an active cysteine, can also bind to FADD in the DISC through a DED–DED interaction (see Green 2022a). If caspase-8 and FLIP are dimerized, caspase-8 becomes activated and cleaves FLIP, but the caspase-8 molecule does not require cleavage for enzymatic activity of this complex. Nevertheless, apoptosis does not ensue (Fig. 8). The reason for this is that although FLIP, like caspase-8, contains two DEDs, the second does not bind to the DED1 of caspase-8 (or of FLIP). FLIP, then, is the “something else” that is able to prevent formation of the caspase-8 filament, thereby restricting its activity and preventing apoptosis.
Figure 8.
FLIP inhibits apoptotic signaling. When FLICE-like inhibitory protein (FLIP) is present, the formation of the death-inducing signaling complex (DISC) recruits it, and this prevents caspase-8 from forming an active filament that would lead to apoptosis (as illustrated in Fig. 6). DED, death effector domain; FADD, FAS-associated DD protein.
The requirement for caspase-8 cleavage for apoptosis to occur is supported by the observation that replacing this caspase with a noncleavable form fails to restore CD95-induced apoptosis, even though the noncleavable caspase-8 dimer is enzymatically active. Cleavage of FLIP might generate a signal with other roles in the cell; alternatively, the caspase-8–FLIP dimer might interact with some substrates but not with others (such as the executioner caspases, which are not activated when FLIP is expressed). We return to FLIP and its interaction with caspase-8 when we consider another form of cell death in Green (2022b).
Some herpes viruses express a short form of FLIP (v-FLIP) that blocks CD95-induced apoptosis by binding to FADD and preventing caspase-8 filament formation and activation. There is also a short isoform of mammalian FLIP (called FLIPS), which similarly prevents caspase-8 activation (but does not form a proteolytically active dimer).
DEFECTS IN CD95 SIGNALING CAUSE A LYMPHOACCUMULATIVE DISEASE
Inactivating mutations in CD95, its ligand, or caspase-8 can cause the childhood disease acute lymphoproliferative syndrome (ALPS). This is marked by accumulation of T and B lymphocytes, including a massive increase in an unusual T-cell subset not found in normal individuals (for aficionados, this subset is CD3+, CD4−, CD8−, and B220+), presumably because apoptosis that would normally control the numbers of these cells does not occur. Autoimmunity and lymphoid tumors are not uncommon in people with ALPS, and the disease is usually lethal if not treated.
Mice with naturally occurring mutations in CD95 (lymphoproliferative, “lpr”) or CD95-L (generalized lymphoproliferative disorder, “gld”) show the same lymphocyte accumulation seen in ALPS, including accrual of the peculiar T-cell type and often become autoimmune (Fig. 9). Genetic deletion of CD95 or CD95-L has the same effects, but this is not seen when the deletion of CD95 is restricted to only T cells; it appears that loss of CD95 in another cell type, dendritic cells, is also involved in the disease. Such observations tell us that interactions between CD95 and its ligand are important for homeostasis in the immune system.2
Figure 9.
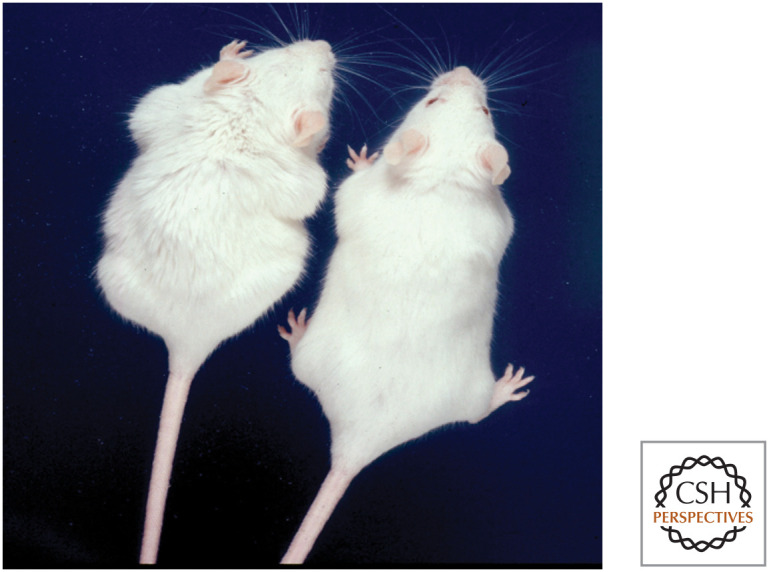
Lymphoproliferative lpr disease. The mouse on the left carries the lpr mutation in the Cd95 (Fas) gene, resulting in massive enlargement of the lymphoid organs. The control mouse on the right is wild type. (Image courtesy of Dr. Ralph C. Budd, University of Vermont, Burlington.)
TRAIL AND APOPTOSIS
TRAIL is a TNF-family ligand produced by T cells and other cell types. Like CD95-L, it is a trimer that can trigger apoptosis in target cells bearing the appropriate death receptors. In addition, like its relative, not all cells bearing receptors for TRAIL are sensitive to TRAIL-induced apoptosis, but in this case the mechanisms of resistance are less well understood (although one mechanism probably involves FLIP).
The mechanism of apoptosis induced by ligation of a TRAIL receptor appears similar to that induced by CD95; ligation of the receptor recruits FADD to the DD on the intracellular region of the TRAIL receptor, and this binds to and dimerizes caspase-8 to activate it. Cells lacking either FADD or caspase-8 are resistant to TRAIL-induced apoptosis.
At least one of the TRAIL receptors (DR5) can engage apoptotic signaling in a manner that is independent of its ligand, TRAIL. Endoplasmic reticulum (ER) stress can induce DR5 gene expression and cause DR5 protein in the ER to spontaneously oligomerize, resulting in caspase-8 activation and apoptosis. This is not the only way in which ER stress can cause apoptosis (other mechanisms involve engagement of the Bcl-2 family proteins, such as BIM, among others; see Green 2022c).
APOPTOTIC SIGNALING BY THE TNFR1 IS COMPLEX
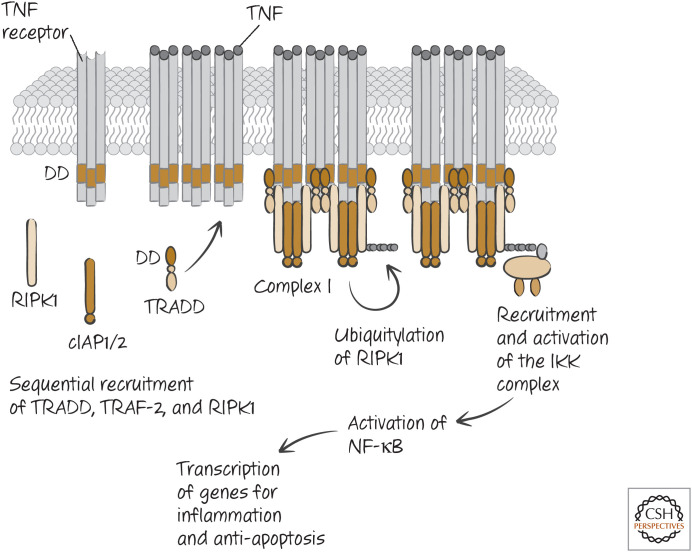
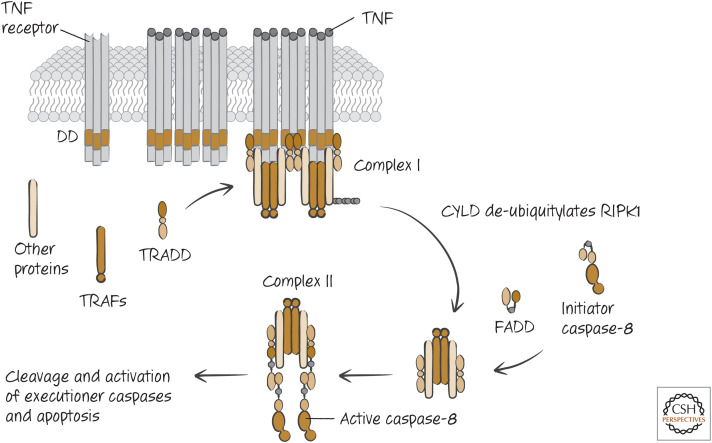
Two different, but related, ligands bind to the death receptor TNFR1. These are TNF itself and another TNF-family ligand called lymphotoxin.3 Ligation of TNFR1 by either causes it to expose its DD and interaction sites for another type of signaling molecule, TNF-receptor-associated factor-2 (TRAF-2). The DD of TNFR1 does not bind to FADD but, instead, to another DD-containing adapter protein, TRADD (tumor necrosis factor receptor type 1–associated death domain). TRADD helps to stabilize the binding of TRAF-2 and also recruits other molecules to the complex, RIPK1, cIAP, and cIAP2.
RIPK1 is a kinase, but here it is not the kinase activity that concerns us; the following steps do not depend on its enzymatic function. (We return to RIPK1 kinase activity in another form of cell death in Green [2022c].)
The cIAPs are members of the inhibitor of apoptosis protein family (IAP family; see Green 2022a), but they do not inhibit caspases. Instead, they act as E3-ubiquitin ligases to polyubiquitylate RIPK1. This involves lysine 63 of the ubiquitin chain and so has a function different from degradation (characteristic of lysine 48 linkages). The lysine-63-linked polyubiquitin on RIPK1 recruits other proteins, including a complex called LUBAC, which then creates a linear ubiquitylation on the protein. This, in turn, recruits NEMO (also known as I-κB kinase-γ, IKKγ). This recruits and activates the rest of the IKK complex, which in turn activates the transcription factor NF-κB (Fig. 10).
Figure 10.
Tumor necrosis factor (TNF) signaling for NF-κB, antiapoptosis, and inflammation. Other proteins and modifications involved in the process are not shown.
NF-κB induces the transcription of a number of genes involved in cell survival, including FLIP. When NF-κB is activated by TNFR1 ligation, apoptosis does not occur, and instead the cell responds in other ways—for example, participating in inflammatory responses by producing cytokines.
There are additional signaling consequences of this complex. For example, ligation of TNFR1 induces the activation of c-Jun amino-terminal kinase (JNK). This phosphorylates the protein Jun, which forms part of another transcription factor, AP-1. TRAIL ligation can also activate JNK. JNK has other targets in the cell, and its activation can promote apoptosis in some cell types, as discussed below.
If NF-κB is not functional or if survival genes induced by NF-κB are not active, a second signaling step can lead to apoptosis. This happens in some cell types or under conditions in which cell signaling is experimentally altered. An enzyme that removes ubiquitin chains (a deubiquitinase, or DUB), called CYLD, removes ubiquitin from RIPK1. The modified TRADD–RIPK1 complex (called TNFR1 signaling complex I) disengages from the receptor and becomes cytosolic. The DD of TRADD, previously associated with TNFR1, is now exposed, and this recruits FADD. FADD, in turn, binds and activates caspase-8 (forming TNFR1-signaling complex IIa). The caspase-8 then precipitates apoptosis, as we have seen (Fig. 11). Alternatively, RIPK1, which also has a DD, can recruit FADD (TNFR1-signaling complex IIb).
Figure 11.
The pathway of apoptosis induced by tumor necrosis factor (TNF). CYLD, ubiquitin carboxy-terminal hydrolase CYLD; DD, death domain; FADD, FAS-associated death domain protein; RIPK1, receptor-interacting serine/threonine-protein kinase 1; TRAF, TNF-receptor-associated factor; TRADD, tumor necrosis factor receptor type 1–associated death domain protein.
Mice that display defective NF-κB function (because parts of the NF-κB pathway have been knocked out) die during development because of extensive apoptosis in the liver. However, if these animals also lack TNFR1, they survive beyond birth. This tells us that it is apoptotic signaling from TNFR1 that is responsible for liver failure in NF-κB-defective embryos.
The rescue of these mice by TNFR1 deficiency tells us that other death ligands, such as TRAIL or CD95-L, do not contribute significantly to the lethal effects of loss of NF-κB signaling during development. Note, however, that activation of NF-κB can produce resistance to apoptosis induced by ligation of other death receptors by inducing expression of FLIP and other proteins.
There is evidence that other death receptors, including CD95 and the TRAIL receptors, might also form cytosolic signaling complexes following ligation that resemble complex II in TNFR signaling. At this point, it is not clear that this is necessary for caspase-8 activation and apoptosis in these cases. Furthermore, these and other death receptors can also activate JNK or NF-κB in some cells, although the precise mechanisms are less well defined.
DEATH RECEPTOR SIGNALING CAN ENGAGE THE MITOCHONDRIAL PATHWAY OF APOPTOSIS
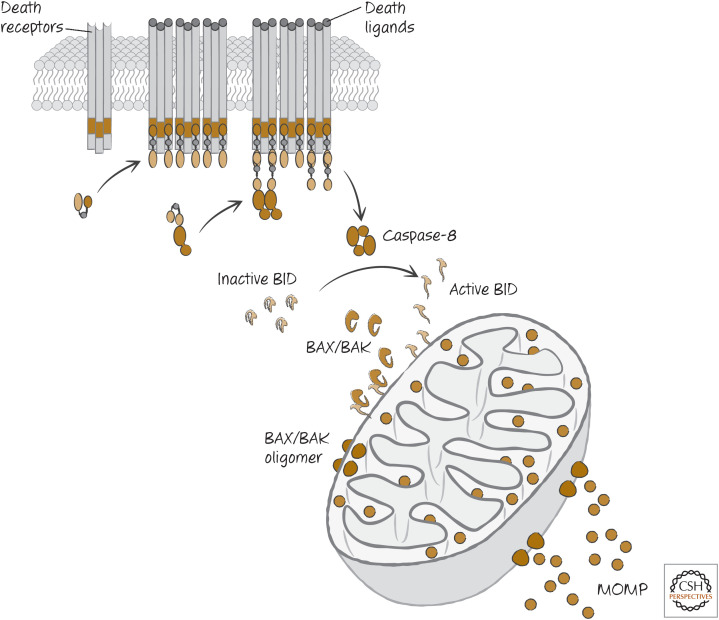
In Green (2022c), we discussed how the BH3-only protein BID is a sensor for proteases, because cleavage of the protein can activate it to engage the mitochondrial pathway of apoptosis by activating BAX and BAK. When death receptors are ligated and activate caspase-8, this causes mitochondrial outer membrane permeabilization (MOMP) and engages the mitochondrial pathway of apoptosis. This is because caspase-8 is very efficient at cleaving BID and activating this BH3-only protein (Fig. 12).
Figure 12.
The pathway of death receptor signaling giving rise to mitochondrial outer membrane permeabilization (MOMP) through caspase-8-mediated cleavage of BID. TNF, tumor necrosis factor; FADD, FAS-associated DD protein; TRAF, TNF-receptor-associated factor; TRADD, tumor necrosis factor receptor type 1–associated DD protein.
In some cells, inhibition of MOMP by antiapoptotic BCL-2 proteins has little or no effect on death receptor–mediated apoptosis. These have been called type I cells in terms of their response to death ligands.4 Lymphocytes and lymphoid tumors are examples of type I cells. In other cells, inhibition of MOMP effectively blocks apoptosis, and these are referred to as type II cells. Hepatocytes are an example of type II cells, as we will see.
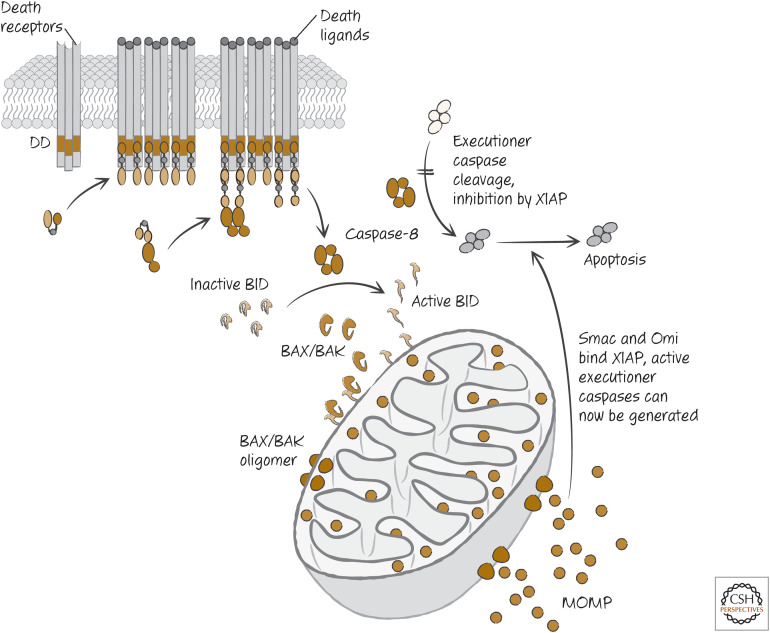
The crucial difference between type I and type II cells in the response to death receptor ligation could be the expression of XIAP. This protein blocks the activities of caspase-9 and the executioner caspases-3 and -7 (see Green 2022a) but does not inhibit caspase-8. When MOMP is triggered by cleaved BID, antagonists of XIAP, such as Smac and Omi, are released from the mitochondrial intermembrane space and disrupt XIAP function, allowing apoptosis to proceed. This effect can be seen in cells lacking APAF1 (which therefore cannot activate caspases as a result of cytochrome c release), and this tells us that the function of MOMP to block XIAP can be crucial for caspase-8 to activate caspases-3 and -7 and promote apoptosis by this pathway. This scheme is illustrated in Figure 13.
Figure 13.
Pathway of death receptor–induced apoptosis in type II cells. BID-induced mitochondrial outer membrane permeabilization (MOMP) can also engage apoptosome formation and caspase activation, but this does not appear to be necessary for apoptosis by this mechanism. DD, death domain.
One example is the cells of the liver—the hepatocytes. Injection of mice with CD95-ligand or with antibodies that activate CD95 causes a rapidly lethal destruction of the liver. Expression of BCL-2 in hepatocytes prevents this lethal effect. Mice that lack BID are also resistant to these treatments. Therefore, these cells are type II cells that require engagement of the mitochondrial pathway following CD95 ligation to cause apoptosis. However, mice lacking both BID and XIAP are sensitive.
CD95 ligation on hepatocytes therefore engages caspase-8, but, although caspase-8 can activate the executioner caspases, it seems that XIAP blocks them and preserves survival of both the cells and the animals. However, when caspase-8 cleaves and activates BID, the resulting MOMP releases proteins such as Smac and Omi that neutralize XIAP, and death ensues. At least in the case of hepatocytes, it is the expression and function of XIAP that make these type II cells; and MOMP must be engaged to inhibit XIAP in order for apoptosis to proceed.
There could be another way in which some death receptors engage apoptosis. As mentioned above, signaling from the TNFR1 and TRAIL receptors can activate JNK. As we saw in Green (2022c), JNK can phosphorylate the BH3-only protein BIM, promoting its activity. Signaling from some death receptors might therefore trigger apoptosis in this way, and there is evidence to support this idea. If this is the case, we have to reconcile this with the finding that caspase-8 is required for apoptosis induced by death receptors. Unfortunately, at present, we know of no way for caspase-8 to participate in the activation of JNK or BIM. This could change, however. Recently, another function for caspase-8 was identified that is independent of its caspase activity, instead acting as a scaffold for additional signaling events following death receptor ligation. If correct, this might provide some insights into how caspase-8 might be involved in apoptosis via JNK and BIM.
DEATH RECEPTORS IN OTHER ANIMALS
Although there is a TNFR-family protein in Drosophila, its ligation does not appear to activate caspases or apoptosis in any situation. Drosophila also have a homolog of FADD (called dFADD) and a homolog of caspase-8 containing DEDs (Dredd). If these proteins are not involved in apoptosis, what do they do?
It could be that the role of this pathway in Drosophila (and presumably other insects) is in host defense. Dredd and dFADD participate in the production of antibacterial peptides in the fly. Intriguingly, this is because Dredd cleaves and thereby activates a protein related to NF-κB called Relish. This has led to the idea that caspase-8 in mammals might have a similar role in a NF-κB pathway, but currently this is controversial.
In contrast to Drosophila, a TNFR-like receptor has been identified in corals, and remarkably this interacts with human TNF to trigger apoptosis, both in coral cells and in human cells in which it is experimentally expressed. It might be that the death receptor pathway, like the mitochondrial pathway, is more common in animals than we once thought.
Footnotes
From the recent volume Cell Death: Apoptosis and Other Means to an End by Douglas R. Green
Additional Perspectives on Cell Death available at www.cshperspectives.org
The TNF and TNFR superfamilies have been given the designations TNFSF and TNFRSF, respectively, followed by a number (those for death ligands and receptors are shown in Fig. 1). We have chosen to employ the most widely used names in the text, rather than these accepted designations.
Intriguingly, background genes influence the susceptibility to the disease, which manifests differently in different mouse strains. This is also true in people. Families that carry dominant-negative forms of CD95 (which can act in a partially dominant manner) can sometimes show ALPS in some children, whereas others are generally normal.
Both of these also bind to another TNFR-family member that is not a death receptor; TNF binds to TNFR2, and lymphotoxin binds to the lymphotoxin receptor. However, because these are not death receptors, we do not further discuss them nor other non-death-receptor members of this extended receptor family.
It is unfortunate (and confusing) that the forms of cell death are referred to as “type 1,” “type II,” and “type III,” whereas the cells that respond differently to death receptor signaling are also referred to as “type 1” and “type 2” cells. In this chapter, any reference to “type 1 cells” or “type II cells” is to the latter.
ADDITIONAL READING
Death Receptors
Banner DW, D'Arcy A, Janes W, Gentz R, Schoenfeld HJ, Broger C, Loetscher H, Lesslauer W. 1993. Crystal structure of the soluble human 55 kd TNF receptor-human TNF β complex: implications for TNF receptor activation. Cell 73: 431–445.
Hymowitz SG, Christinger HW, Fuh G, Ultsch M, O'Connell M, Kelley RF, Ashkenazi A, de Vos AM. 1999. Triggering cell death: the crystal structure of Apo2L/TRAIL in a complex with death receptor 5. Mol Cell 4: 563–571.
The structure of TRAIL shows a role for zinc that is not seen in other TNF-family ligands.
Quistad SD, Traylor-Knowles N. 2016. Precambrian origins of the TNFR superfamily. Cell Death Discov 2: 16058.
Evolution of the TNFR family, including death receptors.
Death Receptor Signaling for Apoptosis
Tummers B, Green DR. 2017. Caspase-8: regulating life and death. Immunol Rev 277: 76–89.
Muzio M, Stockwell BR, Stennicke HR, Salvesen GS, Dixit VM. 1998. An induced proximity model for caspase-8 activation. J Biol Chem 273: 2926–2930.
The activation of caspase-8 by FADD-mediated induced by ligation of death receptors.
Dickens LS, Boyd RS, Jukes-Jones R, Hughes MA, Robinson GL, Fairall L, Schwabe JW, Cain K, Macfarlane M. 2012. A death effector domain chain DISC model reveals a crucial role for caspase-8 chain assembly in mediating apoptotic cell death. Mol Cell 47: 291–305.
Fu TM, Li Y, Lu A, Li Z, Vajjhala PR, Cruz AC, Srivastava DB, DiMaio F, Penczek PA, Siegel RM, et al. 2016. Cryo-EM structure of caspase-8 tandem DED filament reveals assembly and regulation mechanisms of the death-inducing signaling complex. Mol Cell 64: 236–250.
Insights into the activation of caspase-8 to form filaments.
Hughes MA, Powley IR, Jukes-Jones R, Horn S, Feoktistova M, Fairall L, Schwabe JW, Leverkus M, Cain K, MacFarlane, M. 2016. Co-operative and hierarchical binding of c-FLIP and caspase-8: a unified model defines how c-FLIP isoforms differentially control cell fate. Mol Cell 61: 834–849.
Lu M, Lawrence DA, Marsters S, Acosta-Alvear D, Kimmig P, Mendez AS, Paton AW, Paton JC, Walter P, Ashkenazi, A. 2014. Opposing unfolded-protein-response signals converge on death receptor 5 to control apoptosis. Science 345: 98–101.
Describes how endoplasmic reticulum stress can cause TRAIL receptor activation and apoptosis in the absence of its ligand, TRAIL.
Defects in the CD95 Pathway Cause ALPS
Bidere N, Su HC, Lenardo MJ. 2006. Genetic disorders of programmed cell death in the immune system. Annu Rev Immunol 24: 321–352.
An overview of diseases in mice and humans caused by defects in CD95, CD95L, and associated caspases.
Rieux-Laucat, F. 2017. What's up in the ALPS. Curr Opin Immunol 49: 79–86.
Further insights into ALPS.
Watanabe-Fukunaga R, Brannan CI, Copeland NG, Jenkins NA, Nagata S. 1992. Lymphoproliferation disorder in mice explained by defects in Fas antigen that mediates apoptosis. Nature 356: 314–317.
Defects in CD95 (called “Fas antigen”) are responsible for a lymphoaccumulative disease in mice (originally thought to be a lymphoproliferation defect).
Lynch DH, Watson ML, Alderson MR, Baum PR, Miller RE, Tough T, Gibson M, Davis-Smith T, Smith CA, Hunter K, et al. 1994. The mouse Fas-ligand gene is mutated in gld mice and is part of a TNF family gene cluster. Immunity 1: 131–136.
Defects in CD95L (called “Fas ligand”) are responsible for a lymphoaccumulative disease in mice.
TNFR Signaling
Brenner D, Blaser H, Mak TW. 2015. Regulation of tumour necrosis factor signalling: Live or let die. Nat Rev Immunol 15: 362–374.
A useful review of TNFR signaling, including NF-κB activation, apoptosis, and the process of necroptosis (discussed in Green 2021b).
Death Receptors and the Mitochondrial Pathway
Li H, Zhu H, Xu CJ, Yuan J. 1998. Cleavage of BID by caspase 8 mediates the mitochondrial damage in the Fas pathway of apoptosis. Cell 94: 491–501.
Two papers identified BID as a target for caspase-8, linking the death receptor and mitochondrial pathways of apoptosis.
Luo X, Budihardjo I, Zou H, Slaughter C, Wang X. 1998. Bid, a Bcl2 interacting protein, mediates cytochrome c release from mitochondria in response to activation of cell surface death receptors. Cell 94: 481–490.
See above.
Scaffidi C, Fulda S, Srinivasan A, Friesen C, Li F, Tomaselli KJ, Debatin KM, Krammer PH, Peter ME. 1998. Two CD95 (APO-1/Fas) signaling pathways. EMBO J 17: 1675–1687.
The description of two cell types (“type I and type II”) in which the mitochondrial pathway is dispensable (type I) or required (type II) for death receptor–induced apoptosis.
Jost PJ, Grabow S, Gray D, McKenzie MD, Nachbur U, Huang DC, Bouillet P, Thomas HE, Borner C, Silke J, et al. 2009. XIAP discriminates between type I and type II FAS-induced apoptosis. Nature 460: 1035–1039.
Inhibition of XIAP is required for apoptosis induced by CD95 (called “Fas”) in type II cells in mice.
FIGURE CREDITS
- Banner DW, D'Arcy A, Janes W, Gentz R, Schoenfeld HJ, Broger C, Loetscher H, Lesslauer W. 1993. Crystal structure of the soluble human 55 kd TNF receptor-human TNF beta complex: implications for TNF receptor activation. Cell 73: 431–445. 10.1016/0092-8674(93)90132-a [DOI] [PubMed] [Google Scholar]
- Wang L, Yang JK, Kabaleeswaran V, Rice AJ, Cruz AC, Park AY, Yin Q, Damko E, Jang SB, Raunser S, et al. 2010. The Fas-FADD death domain complex structure reveals the basis of DISC assembly and disease mutations. Nat Struct Biol 17: 1324–1329. 10.1038/nsmb.1920 [DOI] [PMC free article] [PubMed] [Google Scholar]
REFERENCES
*Reference is also in this collection.
- *.Green DR. 2022a. Caspase activation and inhibition. Cold Spring Harb Perspect Biol 10.1101/cshperspect.a041020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Green DR. 2022b. Nonapoptotic cell death pathways. Cold Spring Harb Perspect Biol 10.1101/cshperspect.a041079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Green DR. 2022c. The mitochondrial pathway of apoptosis, Part II: the BCL-2 protein family. Cold Spring Harb Perspect Biol 10.1101/cshperspect.a041046 [DOI] [PMC free article] [PubMed] [Google Scholar]