Abstract
Ductal carcinoma in situ (DCIS) is a nonobligate precursor of invasive cancer, and its detection, diagnosis, and management are controversial. DCIS incidence grew with the expansion of screening mammography programs in the 1980s and 1990s, and DCIS is viewed as a major driver of overdiagnosis and overtreatment. For pathologists, the diagnosis and classification of DCIS is challenging due to undersampling and interobserver variability. Understanding the progression from normal breast tissue to DCIS and, ultimately, to invasive cancer is limited by a paucity of natural history data with multiple proposed evolutionary models of DCIS initiation and progression. Although radiologists are familiar with the classic presentation of DCIS as asymptomatic calcifications at mammography, the expanded pool of modalities, advanced imaging techniques, and image analytics have identified multiple potential biomarkers of histopathologic characteristics and prognosis. Finally, there is growing interest in the nonsurgical management of DCIS, including active surveillance, to reduce overtreatment and provide patients with more personalized management options. However, current biomarkers are not adept at enabling identification of occult invasive disease at biopsy or accurately predicting the risk of progression to invasive disease. Several active surveillance trials are ongoing and are expected to better identify women with low-risk DCIS who may avoid surgery.
© RSNA, 2021
Learning Objectives:
After reading the article and taking the test, the reader will be able to:
■ Explain the uncertainty in the natural history of ductal carcinoma in situ (DCIS)
■ Describe the common and uncommon imaging presentations of DCIS
■ Identify the nonstandard treatment options for DCIS via active surveillance
Accreditation and Designation Statement
The RSNA is accredited by the Accreditation Council for Continuing Medical Education (ACCME) to provide continuing medical education for physicians. The RSNA designates this journal-based SA-CME activity for a maximum of 1.0 AMA PRA Category 1 Credit™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
Disclosure Statement
The ACCME requires that the RSNA, as an accredited provider of CME, obtain signed disclosure statements from the authors, editors, and reviewers for this activity. For this journal-based CME activity, author disclosures are listed at the end of this article.
Summary
Ductal carcinoma in situ is a controversial breast disease, and its detection, diagnosis, and management are the subjects of active research.
Essentials
■ The incidence of ductal carcinoma in situ (DCIS) steadily increased with the widespread adoption of screening mammography in the 1980s and 1990s and remained stable since.
■ The natural history and genomic evolution of DCIS are poorly understood due to a paucity of longitudinal data.
■ DCIS suffers from overdiagnosis and overtreatment, but current estimates are very imprecise.
■ DCIS classically manifests as calcifications on screening mammograms and clumped nonmass enhancement on MRI scans, with promising advanced imaging techniques under investigation.
■ Active surveillance is a nonsurgical management strategy for DCIS, but adoption is limited by the risk of occult invasive disease at the time of diagnosis and progression to invasive cancer during surveillance.
Introduction
Ductal carcinoma in situ (DCIS) exists at the border between benign breast disease and malignant cancer, resulting in notable challenges in detection, diagnosis, and management. Pathologists have variability in their diagnosis of DCIS, radiologists differ in how they describe DCIS to patients, and there are ongoing efforts aimed at renaming DCIS (1–3). As the incidence of DCIS increased with the introduction of organized breast cancer screening starting in the 1980s, an awareness of DCIS as a major driver of overdiagnosis and overtreatment in breast cancer screening programs has grown (4). Despite how commonly DCIS is diagnosed, our knowledge of the underlying tumor biology, factors influencing the imaging presentation, and prognosis are still relatively nascent. Multidisciplinary work in radiology, pathology, genetics, surgery, and tumor biology are all rapidly contributing to our understanding of the disease, but many questions remain unanswered. Simultaneously, new approaches to the treatment of DCIS with the introduction of nonsurgical management by means of active surveillance aim to reduce overtreatment and provide more personalized options for patients. In this review, we seek to provide a multidisciplinary update on DCIS centered on the radiologist and include the many factors that may influence breast imaging practices in the present and near future.
Epidemiologic Characteristics
There has been a dramatic increase in the incidence of DCIS following the introduction of organized breast cancer screening programs in the United States in the 1980s. Since the Surveillance, Epidemiology, and End Results, or SEER, program first started to collect data in 1975–1979 until 2000, the incidence of DCIS increased by 571% (4.9 vs 32.9 cases per 100 000 women) (5). The incidence of invasive ductal carcinoma increased by only 31% (56.7 vs 75.7 cases per 100 000 women) over the same time period, although it still represented two-thirds of new breast cancer diagnoses (5). Since 2000, the overall incidence rates of both DCIS and invasive cancer have been relatively stable; however, there have been differences in patient and pathology subgroups. From 2000 to 2014, the incidence of DCIS among women aged 20–44 years (1.3%) and 45–55 years (0.6%) increased, while there was a decrease for women aged 55–69 years (0.3%) (6). Overall, the incidence of DCIS is highest among non-Hispanic White (26.6 per 100 000 women) and Black (26.5 per 100 000) women; however, the incidence of DCIS increased for Black women (1.6%) from 2000 to 2014 while it was stable for White women (7,8). There has also been an increased proportion of estrogen receptor (ER) and progesterone receptor positivity, as well as intermediate nuclear grade DCIS over the same period (9). Finally, there is a strong correlation between the incidence of DCIS and both state-level prevalence of mammography screening as well as county-level poverty statistics (ie, higher incidence in low poverty locations) (7). The changes to DCIS incidence over time are thus a complicated combination of access to screening mammography services, as well as shifts in demographic and pathologic characteristics.
Pathologic Characteristics
DCIS encompasses a highly heterogeneous group of lesions demonstrating a spectrum of histopathologic features. The primary classification system is based on a three-tier system of nuclear grading that incorporates nuclear proliferation, mitotic figures, and architecture (10). Nuclear grade is approximately evenly split between high-grade (42%) and intermediate-grade (43%) DCIS, with a lesser percentage of low-grade DCIS (14%). Nuclear grade is one of the most important discriminating features of DCIS as it is associated with disease prognosis, risk of upstaging to invasive cancer at surgical excision, and eligibility for active surveillance (11–15). However, a major challenge to nuclear grading is a lack of reproducible measurements as multiple studies have shown notable interobserver variability among pathologists, even with use of a simplified two-tier system (high vs nonhigh grade) and/or majority opinion–based scores (16,17). Nuclear grade also influences the imaging manifestations of DCIS, such as a greater proportion of high-grade DCIS seen at MRI (18) and low-grade DCIS seen at US (19). Several investigators have identified radiomics features at mammography (20) and MRI (21) that correlate with nuclear grade. However, because nuclear grade is routinely established by means of pathologic assessment at the time of biopsy, there is limited clinical relevance to predicting nuclear grade from imaging.
Other important standard of care DCIS markers are hormone receptor status, especially ER, and necrosis, in particular comedo-like necrosis. Approximately 87% of DCIS cases are ER positive (22). ER status is important as it corresponds with invasive recurrence and prognosis and allows for adjuvant endocrine therapy (23). ER status also correlates with the imaging presentation, for example, ER-negative DCIS is more likely to be visible at US (24) and ER-positive DCIS is more likely to manifest as fine pleomorphic and fine-linear branching calcifications (25). Comedo-like necrosis refers to the presence of central expansile necrosis and is found in roughly one-third of DCIS diagnoses; it is most commonly associated with high-grade DCIS (10). Similar to nuclear grade, comedo-like necrosis is associated with disease prognosis, upstaging to invasive cancer, ineligibility for most active surveillance, and interobserver disagreement among pathologists (11–15,17). Calcifications are often found in areas of necrosis, and comedo-like necrosis is associated with fine-linear branching morphologic characteristics at mammography (24). Of note, although human epidermal growth factor receptor 2 is not routinely obtained on DCIS samples in the United States, it may be included at international sites.
Underinterpretation (eg, atypical ductal hyperplasia) and overinterpretation (eg, invasive cancer) of DCIS are also a diagnostic challenge for pathologists. A large study of 240 breast biopsies interpreted by 115 pathologists demonstrated that, while there was 96% concordance for invasive carcinoma cases, this declined to 84% for DCIS cases and 48% for cases with atypia (3). Undersampling during core-needle biopsies can also contribute to diagnostic uncertainty if there is insufficient representative material for the pathologist to interpret. Atypical ductal hyperplasia and low-grade DCIS share overlapping morphologic features, but a DCIS diagnosis requires at least two completely involved ducts or a size of at least 2 mm (10). As a result, a core-needle biopsy sample that includes only one small, involved duct may yield a diagnosis of atypical ductal hyperplasia that is upgraded to DCIS at surgical excision (Fig 1). Similarly, the use of a 14-gauge automated biopsy device (vs an 11-gauge vacuum-assisted device) and fewer biopsy samples is more likely to miss concomitant invasive cancer at initial core biopsy, which is then upstaged to invasive cancer at surgery (11).
Figure 1:

Photomicrograph (hematoxylin-eosin stain; low-power view) shows low-grade ductal carcinoma in situ with cribriform architecture. If only the boxed area is visible to the pathologist, then the diagnosis would be atypical ductal hyperplasia because fewer than two involved ducts are visible and the extent of disease is less than 2 mm.
One solution to the interobserver challenges that pathologists face is the adoption of automatic image analysis by means of pathomics (26). The recent expansion of digitized whole-slide pathologic images is facilitating the availability of larger data repositories for image analysis. The development of the field of pathomics can be viewed in parallel to radiomics, albeit with a notable lag for pathomics because the transition from analog to digital radiologic images happened many years earlier. Pathomics approaches with more traditional hand-crafted features and more recently advanced deep learning techniques have shown promise in the standardization of pathology measurements (26). The combined use of radiomics and pathomics to expand our understanding of the biology of disease and develop biomarkers is very exciting but has thus far been explored in only a very limited fashion in breast pathology and not yet for DCIS (27).
Progression from DCIS to Invasive Cancer
In pure DCIS lesions, the neoplastic cells are fully contained within the branches of the mammary ductal tree. The progression from DCIS to invasive breast cancer requires the breakdown of the ducts’ basement membrane and the migration of tumor cells into the stroma. The mechanism by which this process occurs is to date poorly understood.
Several cellular models of DCIS progression (Fig 2) have been proposed that can be divided into independent (independent evolution model) and direct (evolutionary bottleneck and multiclonal invasion models) lineages (4,28). In the independent evolution model, DCIS and invasive cancer evolve separately in the same location, possibly due to a field effect that promotes tumorigenesis. In this model, there should be minimal overlap in the genetic profiles of DCIS and invasive ductal carcinoma that are found in close anatomic proximity because they evolve separately (28). The bottleneck model posits that the neoplastic cells evolve within the ducts until a mutation, or other (epi)genomic event, gives rise to a new subpopulation of cells that are capable of breaking down the basement membrane and invading the stroma. Because acquisition of the invasive phenotype is the result of randomly occurring replication errors during cell division, the process constitutes an evolutionary bottleneck. Support for this model comes from similarities in the bulk sequencing of adjacent DCIS and synchronous invasive cancer with only a few mutations unique to the invasive cancer (4,29). More recently, a single cell sequencing study of DCIS and synchronous invasive cancer refuted the bottleneck model in favor of the so-called multiclonal invasion model (29,30). Using DNA copy number profiling of single cells, the study found that multiple genetic subpopulations were present both inside the ducts and the surrounding stroma, suggesting that multiple subclones first arose inside the ductal tree and then invaded the surrounding stroma in parallel.
Figure 2:
Evolutionary models of invasion in ductal carcinoma in situ (DCIS). (A) Independent evolution model shows DCIS (clones A and B) and invasive cancer (C) evolving from separate normal cells (N1 and N2). (B) Evolutionary bottleneck model shows DCIS evolving from a single ancestral cell (N1) and a single clone (C) evolves to invade and expand to form the invasive carcinoma. (C) Multiclonal invasion model shows DCIS evolving from a single ancestral cell (N1), and then multiple clones (A, B, and C) each evolve to form invasive cancer and comingle. Reprinted, with permission, from reference 28.
Despite these proposed models of how invasive progression occurs, it is unclear why some DCIS lesions progress to invasive cancer and others remain indolent for extended periods of time. Differences in the mutational profiles between pure DCIS lesions and those with synchronous invasive disease have been identified (32), but there is a paucity of actionable markers to stratify newly diagnosed DCIS lesions according to risk of invasive progression (32). Commercially available tools, such as the Oncotype DX Breast DCIS Score (Genomic Health) and DCISionRT (Prelude Dx), and validated metrics, such as the Van Nuys Prognostic Index and the Memorial Sloan-Kettering Cancer Center DCIS nomogram, are designed to predict the risk of cancer recurrence following treatment, but this is a different outcome than progression to invasive cancer for untreated DCIS (33). Recent studies have shown that the tumor microenvironment and cancer–immune cell interactions may play important roles in the invasion process (34). For example, more tumor-infiltrating lymphocytes in DCIS adjacent to invasive cancer versus pure DCIS indicates greater inflammation in the local tissue ecology (34). This line of work has the potential to expand the search for biomarkers beyond genomic signatures.
There is also a disconnect between the imaging appearance of DCIS as a putative precursor for invasive cancer. The most common manifestation of DCIS is calcifications (approximately 80%), but even though concomitant DCIS is found in 60% of invasive cancers, calcifications are only seen in 30% of invasive cancer cases (35,36). There are several hypotheses for this paradox. First, the calcifications that develop with DCIS may disappear during the progression to invasive cancer. Although calcification remodeling does occur, DCIS calcifications typically increase in size and extent over time (37). Second, a large proportion of noncalcified DCIS may be missed with mammography; thus, the reported prevalence of imaging presentations is wrong. However, screening with digital breast tomosynthesis, US, and MRI has not identified a hidden cohort of noncalcified DCIS (38–40). Third, many invasive cancers may have a very brief sojourn time as DCIS and thus the calcifications that would normally develop with DCIS do not have sufficient time to develop. However, noncalcified DCIS tends to be less biologically aggressive than calcified DCIS, with lower nuclear grade and human epidermal growth factor receptor 2 negativity, which suggests it is less likely to progress to invasive cancer (41). There is an overall paucity of data on the natural history of DCIS, especially early during genesis, and input from pathology, radiology, and genetics are all needed to better clarify the process.
Imaging Appearance
Mammographic Examination
DCIS most commonly (approximately 80%) manifests as asymptomatic calcifications at mammography (24). Approximately half of DCIS calcifications will have fine pleomorphic morphologic characteristics and a grouped distribution (24,42). Fine pleomorphic and fine-linear or fine-linear branching calcifications are more likely to be found in high-grade DCIS and DCIS with necrosis, whereas round calcifications are associated with low-grade DCIS (43). However, Breast Imaging Reporting and Data System descriptors alone are not specific enough to help differentiate nuclear grade. Noncalcified mammographically detected DCIS is present in approximately 10% of DCIS diagnoses, usually manifesting as a mass (67%) and less likely as a focal asymmetry (24%) or architectural distortion (15%) (24,44). Although noncalcified pure DCIS has a good prognosis, calcified DCIS with an associated mass, asymmetry, or architectural distortion is associated with upstaging to invasive cancer (11,24,44). Finally, among women with palpable DCIS there is a greater likelihood of a noncalcified presentation: 14% for palpable DCIS versus 4% for all DCIS in one series (45).
Despite the common appearance of calcifications at mammography, their genesis remains poorly understood. Breast calcifications are hypothesized to develop through both passive (degenerative or dystrophic) and active (secretory or bone matrix protease) processes with two primary chemical compositions: calcium oxalate (type I) and calcium hydroxyapatite (type II) (46,47). Type I calcifications are usually associated with benign processes, whereas type II calcifications are more frequently seen with malignancy processes (46,47). Differences in the underlying physiologic genesis of calcifications likely explain the wide variety of morphologic appearances as well as the association between calcification morphologic characteristics and distribution with DCIS nuclear grade and hormone receptor status. Breast calcifications have also been evaluated with Raman spectroscopy, which measures energy shifts in scattered light (46). Limited analysis to date has shown that Raman spectroscopy can help differentiate the chemical composition of calcifications (type I vs type II) as a means to differentiate DCIS and invasive cancer from benign lesions (46). However, current techniques can only image to a depth of 40 mm due to light scatter, which limits in vivo analysis.
US Examination
Although DCIS detection is generally associated with screening mammography, in women who undergo US approximately half of DCIS lesions can be identified (24). The most common manifestation is a hypoechoic irregular hypervascular mass, parallel in orientation without posterior features (Fig 3) (24). Alternatively, a mass with a "pseudomicrocystic" appearance, which has mixed solid and microcysts, is thought to reflect distention of the lobular portion of the terminal ductal lobular units (48). Ductal abnormalities including an increase in the number of ducts from neogenesis, ductal ectasia, or intraductal echogenic material may be present and subtle intraductal material with low-level echoes may be incorrectly dismissed as benign ductal ectasia (49). For unclear reasons, ER-negative DCIS is more likely to be sonographically visible than ER-positive DCIS (61% vs 46%, respectively) (24). For noncalcified DCIS, detection rates with US (95%) are superior to those with digital breast tomosynthesis (84%) and mammography (68%) (50). Mammographically occult DCIS that is detected with US is more likely to be lower grade and less likely to have comedonecrosis and human epidermal growth factor receptor 2 amplification (19). This may be due to a slower growth rate that conforms to the ductal anatomy and does not produce mass effect or cause necrosis-induced calcifications that are apparent at mammography, but the cause is uncertain.
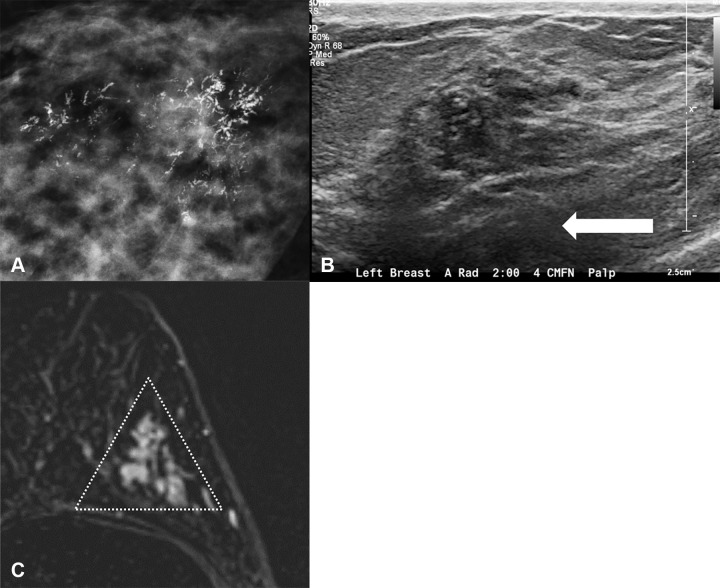
Figure 3:
Images in three different patients demonstrate classic imaging appearance of ductal carcinoma in situ (DCIS). (A) Mammogram shows DCIS as fine pleomorphic calcifications. (B) US scan shows DCIS as hypoechoic irregular mass without posterior features (arrow). (C) MRI scan shows DCIS as nonmass enhancement in a segmental distribution (triangle).
MRI Examination
DCIS most commonly manifests as nonmass enhancement at MRI, with masses or foci of enhancement decidedly less common (51). The enhancement in DCIS is believed to result from both vascular and basement membrane permeability as gadolinium collects within the ducts (52). Given its confinement to the milk ducts, it is intuitive that the majority of DCIS manifesting as nonmass enhancement (60%–80%) are segmentally or linearly distributed depending on the number of ducts and lobules involved (Fig 3) (53,54). It is likely that such distributions reflect typical DCIS proliferation, beginning with intraductal tumorogenesis and subsequent local proliferation through the ductal pathway. It has also been posited that internal morphologic features may shed additional light on the proliferative growth pattern of DCIS. For example, clumped internal enhancement may reflect patchy intraluminal carcinoma cell growth whereas a clustered ring may reflect a greater predilection for peripheral growth and periductal stromal angiogenesis and possibly a greater rate of upgrade to invasive disease (55,56). Compared with invasive counterparts, DCIS lesions exhibit peak contrast enhancement later than invasive cancers, with a lower fraction demonstrating delayed phase washout (57).
The MRI appearance of DCIS reflects the underlying biology and correlates with outcomes. For example, DCIS detected at MRI is more likely to be high nuclear grade than DCIS detected at mammography (18). Semiquantitative MRI features can be used to differentiate DCIS nuclear grades (58) and identify low-risk DCIS based on Van Nuys score (59). However, radiomics heterogeneity features of low gray-level run emphasis and short run low gray-level run emphasis approached statistical significance in the differentiation of high versus nonhigh nuclear grade DCIS (21). Quantitative measures of lesion size or volume (60,61), signal enhancement ratio (61), and surrounding lesion tissue enhancement (60,61) are also associated with ipsilateral recurrence. One limitation, however, is that almost all DCIS included in research studies and clinical trials is first detected with mammography, which creates a selection bias for inclusion (62). Further work on MRI-detected DCIS, such as from high-risk screening populations, will expand our knowledge further. Radiomics assessment of DCIS with MRI is an incredibly active area of research.
MRI is also the most accurate modality for determining disease extent, with a recent review demonstrating that MRI more closely matched pathologic size estimates compared with mammography in eight of nine studies (63). Despite the established benefits of preoperative size assessment with MRI, the reported benefits of preoperative breast MRI for surgical planning are mixed with conflicting impacts on mastectomy and reoperation rates (63). The Eastern Cooperative Oncology Group–American College of Radiology Imaging Network, or ECOG-ACRIN, E4112 trial demonstrated that standardized MRI and management plans can yield high overall and single (ie, no repeat excision) wide local excision success rates (96% and 79%, respectively) (54,64). Furthermore, the positive predictive value of MRI-prompted biopsies was 32%, yielding an additional cancer detection rate of 6.2% with a false-positive rate of 14.2% (54). Unsurprisingly, patients reported greater satisfaction when their DCIS was treated with a single surgery. More recent studies have shown that the use of 3.0-T scanners can also provide incremental benefits in determining disease extent (21,65).
Additional complementary MRI techniques such as diffusion-weighted imaging and ultrafast MRI could further improve DCIS characterization. Diffusion-weighted imaging provides information regarding breast lesion microstructure by measuring water mobility. DCIS lesions have been found to be visible on diffusion-weighted images, with apparent diffusion coefficients lower than those in normal breast tissue but higher than those in invasive cancers, and thus diffusion-weighted imaging may assist in the differentiation among DCIS grades (66–69). Ultrafast MRI is a contrast material–based technique that leverages a high-temporal-resolution sequence (<10 seconds) performed within the first 1–2 minutes after contrast material administration. Qualitatively, ultrafast techniques may help visualize important nonmass enhancement that differentiates DCIS from benign background parenchymal enhancement (70). Some quantitative metrics, including time to enhancement and maximum slope, have promise for improving MRI specificity (71,72). Further research is needed to understand how ultrafast sequences can assist with DCIS characterization.
Contrast-enhanced Mammography
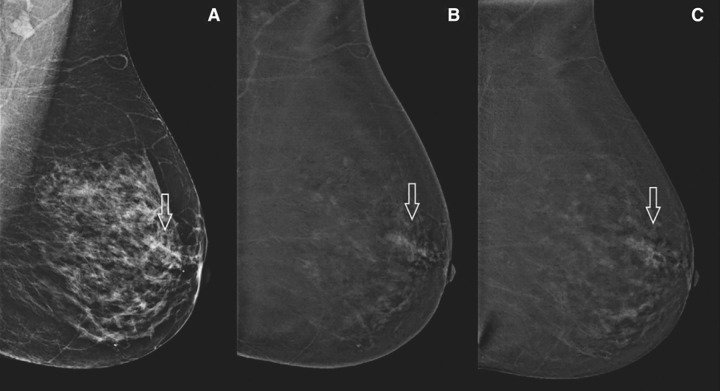
Contrast-enhanced mammography is a new imaging modality that is gaining acceptance for indications such as determining response to therapy, preoperative staging, and, possibly, screening (73). Contrast-enhanced mammography may be a useful tool for the diagnosis and management of DCIS because it compensates for the weaknesses of mammography (ie, lack of enhancement) and MRI (ie, inability to depict calcifications). In particular, contrast-enhanced mammography may prove helpful to better define extent of disease by identifying both calcified and noncalcified DCIS and also to identify occult invasive cancer by detecting enhancing invasive cancer in the setting of DCIS calcifications. Although only a small number of retrospective studies on contrast-enhanced mammography have included many DCIS cases to date (range, 19–37 DCIS cases), they demonstrate the promise of contrast-enhanced mammography in the detection of pure DCIS and mixed DCIS and/or invasive cancer (75%–100% of DCIS lesions showed enhancement) (Fig 4) (74,75).
Figure 4:
Images in a patient with pure ductal carcinoma in situ. (A) Low-energy mammogram shows an asymmetry (arrow). (B, C) Contrast-enhanced mammograms obtained in early (B) and late (C) phases show nonmass enhancement (arrow). Reprinted, with permission, from reference 74.
Overdiagnosis and Overtreatment
During the past few decades, it has become increasingly clear that a substantial fraction of mammographically detected DCIS lesions are slow progressing or indolent, leading to overdiagnosis of tumors that would not have caused symptoms during the patient’s remaining lifetime in the absence of screening (4,9,76). A review of autopsy studies found that 5.9%–18% of women who died of other causes had undetected DCIS (9). In 2009, a National Institutes of Health State of Science Conference provided a call to action for the breast oncology community to decrease DCIS overdiagnosis and overtreatment, although today DCIS incidence and treatment patterns remain essentially unchanged (77). Estimates of DCIS overdiagnosis are predominately based on modeling studies, which report incredibly wide ranges from 20% to 91% based on differences in model assumptions (22,78–80). To our knowledge, only one study has provided estimates based on nuclear grade: 61% for low grade, 57% for intermediate grade, and 45% for high grade (78). A survey of radiologists agreed that DCIS is the primary driver of breast cancer overdiagnosis and provided estimates of 30% for low nuclear grade and 4% for high nuclear grade (2). This realization is at odds with current practice, with more than 97% of patients choosing an immediate lumpectomy or mastectomy, often in combination with adjuvant radiation treatment in the case of a lumpectomy (81). Furthermore, the increase in mastectomy rates is not associated with any improvements in patient outcomes (81). The resulting overtreatment of nonprogressive DCIS can cause substantial harms and significantly reduce the patient’s quality of life without reducing breast cancer mortality (8,45,82).
Because the vast majority of patients receive treatment after diagnosis, there are limited data about DCIS natural history and the extent of overtreatment. A series of older studies in patients with missed DCIS lesions estimated that approximately 25% of patients who did not undergo surgical excision had an ipsilateral invasive breast event within 15 years of diagnosis (83–86). A more recent cancer registry study in women who did not undergo surgery after diagnosis of DCIS estimated the 15-year risk of ipsilateral invasive cancer to be lower, on the order of 10%–20% (76). The risk of subsequent invasive cancer in the same breast appears to depend on the lesion subtype, with nonhigh grade (nuclear grade I or II) and hormone receptor–positive lesions having a reduced risk (87).
Despite limited sample sizes and selection biases in these observational studies, available data support the notion that most patients with DCIS would not develop invasive breast cancer if they did undergo immediate surgery. The most definitive answer regarding the propensity to progress to invasive cancer, however, will result from ongoing randomized studies that are evaluating active surveillance as an alternative to immediate surgery (88–90).
Active Surveillance
Active surveillance is an alternative management strategy for DCIS that aims to de-escalate treatment to reduce overtreatment. Instead of initial surgery with possible radiation therapy, patients undergo close mammographic follow-up and may receive hormonal therapy (ie, tamoxifen) or aromatase inhibitors. This approach is meant to parallel changes to the management of early-stage prostate cancer, which demonstrated that more aggressive therapy did not necessarily improve mortality at the cost of morbidity (91). The goal of active surveillance as a management strategy is to identify women who will never need surgery or women for whom it is safe to delay surgery until a later date, often at the patient’s request.
Active Surveillance Trials
There are four current multicenter trials designed to determine which DCIS lesions are associated with future risk of invasive disease. The Comparison of Operative versus Medical Endocrine Therapy (COMET) for low-risk DCIS trial, based in the United States, is a randomized control trial comparing active surveillance to usual care for DCIS and is actively recruiting (12). Two additional studies initially designed as randomized control trials have had enrollment difficulties due to poor recruitment: The Low Risk DCIS (LORIS) trial in the United Kingdom (13) has stopped recruitment altogether, whereas the Low-Risk DCIS (LORD) trial in the Netherlands (14) has stopped randomizing patients but allows women to enroll in the active surveillance arm alone. Finally, the LORETTA trial is a single-arm confirmatory trial of endocrine therapy alone based in Japan and is actively recruiting (15). The inclusion and exclusion criteria of the trials vary, with the LORD trial being the most restrictive and the COMET trial being the most inclusive (Table). In general, only screening-age women with DCIS detected as asymptomatic calcifications without concomitant invasive cancer and low or low and intermediate nuclear grade are eligible. These criteria are designed to address the two major challenges of active surveillance: the risk of enrolling women with occult invasive disease and progression to invasive disease.
Current Active Surveillance Trials
Occult Invasive Disease
Some women with DCIS detected at core-needle biopsy will harbor occult invasive disease that normally would be diagnosed at surgical excision. However, in active surveillance, the occult invasive cancer is undetected and untreated, which could theoretically affect the patient’s prognosis. Pathologic and demographic factors are included in the active surveillance trial criteria to help reduce the rate of occult invasive cancer, notably nonhigh nuclear grade and asymptomatic calcifications (see Table). A meta-analysis reported an overall DCIS upstaging rate of 25.9% (11), but several retrospective series have demonstrated that the application of active surveillance trial criteria can reduce upstaging rates to as low as 7% (92–94). Predicting which women diagnosed with DCIS at core-needle biopsy will be diagnosed with invasive cancer (upstaged) at surgical excision is an active area of radiomics exploration. Computer vision and deep learning approaches applied to mammography and MRI have shown promising results (area under the receiver operating characteristic curve, approximately 0.7) to predict upstaging (95–97). Although not a task radiologists are specifically trained to perform, one study has shown that a focus group and educational intervention can improve fellowship-trained breast radiologists’ ability to predict upstaging at mammography (area under the receiver operating characteristic curve = 0.623 before intervention vs 0.765 after intervention) (98). Finally, even though MRI is the most sensitive imaging modality for the detection of DCIS and invasive cancer, it is not included in the eligibility criteria for any of the active surveillance trials; however, a survey of the Society of Breast Imaging membership indicated broad support to screening potential patients for active surveillance with both MRI and US (2).
Progression to Invasive Disease
All patients undergoing active surveillance are at some risk of progression to invasive cancer. The close imaging surveillance (ie, diagnostic mammography every 6–12 months) is designed to detect progression to invasive cancer as soon as possible and thus ameliorate any changes in patient prognosis. Predicting which patients will progress to invasive cancer at the time of active surveillance enrollment is limited by a paucity of natural history data for DCIS. As noted earlier, several studies have sought to estimate the progression risk by studying patients with missed DCIS (see Overdiagnosis and Overtreatment). Furthermore, a small number of studies have reported the outcomes in women undergoing active surveillance who were not in a trial, many of whom would not meet trial eligibility criteria (99,100). A retrospective review of 29 women demonstrated low rates of progression (9%), albeit with short follow-up periods (mean, 2.7 years) (99). A cohort study of 14 women from a single arm trial for endocrine therapy and delayed surgical excision for DCIS who opted instead for active surveillance demonstrated progression rates of 36% over a median follow-up of 10–70 months (100). Unfortunately, none of these studies have sufficient sample size to identify features that enable the reliable prediction of progression to invasive disease and so imaging features associated with occult invasive disease are often used as surrogates (eg, mass or asymmetry).
To identify patients at potential risk of progression, the trials all have criteria for a repeat biopsy. For the COMET trial, a biopsy is recommended for any new mass, architectural distortion, density, or increase in long-axis calcification extent of 5 mm; for the LORD trial, a biopsy is recommended for any new mass, asymmetry, or calcifications; and, for the LORIS trial, biopsy is recommended for any new suspicious finding per the radiologists or an increase in calcification long-axis length of 30%. These active surveillance trials will provide the best evidence to date on the natural history of DCIS, predictors of progression to invasive cancer, and patient acceptability of nonsurgical management. Unfortunately, the closure and/or scaling back of recruitment for the LORD and LORIS trials will limit the diversity and quantity of data for analysis.
Conclusion
The detection, diagnosis, and management of ductal carcinoma in situ (DCIS) remains a challenge for breast radiologists, pathologists, and surgeons alike—especially in an environment concerned about overdiagnosis and overtreatment. A major challenge is our very limited understanding of DCIS on the biologic spectrum from benign to invasive cancer and the natural history of untreated DCIS. Genomics and imaging provide limited insights into the progression of DCIS to invasive cancer. Radiologists are generally familiar with the most common imaging presentations of DCIS, but our understanding of the relationship between imaging features and pathology markers, new imaging techniques, and advanced image analytics continues to evolve. Meanwhile, active surveillance trials will soon provide a robust data source on DCIS prognosis for women diagnosed with low-risk DCIS, although these expectations are tempered by poor recruitment among the two European trials. Radiologists can play a major role in ensuring active surveillance enrollment is safer for patients and identifying when patients may be at risk for disease progression. Small improvements in the diagnosis and management of DCIS can have a major positive impact on patients given the high incidence of DCIS. Radiologists are thus well situated to take a proactive role in the multidisciplinary exploration of DCIS.
Supported by National Institutes of Health/National Cancer Institute grants R01CA203883, NCI R01CA203883, and R00CA207872.
Disclosures of Conflicts of Interest: L.J.G. Grants from ECOG/ACRIN (TMIST), Alliance for Clinical Trials in Oncology Foundation, AUR Breast Cancer Research Foundation, and MD Anderson Cancer Center; consulting fees from Hologic, Medscape, and Reference; payment for expert testimony from Hare Wynn, Thompson Miller, Simpson ZCK Law; leadership or fiduciary role in Society of Breast Imaging. H.R. Grant funding from GE Healthcare (not related to work). M.A. No relevant relationships. A.H.H. Grants from Cancer Research UK (Prime), Department of Defense, and Susan G. Komen for the Cure. M.D.R. No relevant relationships.
Abbreviations:
- COMET
- Comparison of Operative versus Medical Endocrine Therapy
- DCIS
- ductal carcinoma in situ
- ER
- estrogen receptor
- LORD
- Low-Risk DCIS
- LORIS
- Low Risk DCIS
References
- 1. Graff S . Ductal carcinoma in situ: should the name be changed? J Natl Cancer Inst 2010. ; 102 ( 1 ): 6 – 8 . [DOI] [PubMed] [Google Scholar]
- 2. Grimm LJ , Destounis SV , Rahbar H , Soo MS , Poplack SP . Ductal Carcinoma In Situ Biology, Language, and Active Surveillance: A Survey of Breast Radiologists’ . Knowledge and Opinions. J Am Coll Radiol 2020. ; 17 ( 10 ): 1252 – 1258 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Elmore JG , Longton GM , Carney PA , et al . Diagnostic concordance among pathologists interpreting breast biopsy specimens . JAMA 2015. ; 313 ( 11 ): 1122 – 1132 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. van Seijen M , Lips EH , Thompson AM , et al . Ductal carcinoma in situ: to treat or not to treat, that is the question . Br J Cancer 2019. ; 121 ( 4 ): 285 – 292 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cancer Stat Facts: Female Breast Cancer. Surveillance, Epidemiology, and End Results Program . https://seer.cancer.gov/statfacts/html/breast.html. Accessed July 12, 2021 .
- 6. Ryser MD , Hendrix LH , Worni M , Liu Y , Hyslop T , Hwang ES . Incidence of Ductal Carcinoma In Situ in the United States, 2000-2014 . Cancer Epidemiol Biomarkers Prev 2019. ; 28 ( 8 ): 1316 – 1323 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Special Section: Breast Carcinoma in Situ . American Cancer Society . https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2015/special-section-breast-carcinoma-in-situ-cancer-facts-and-figures-2015.pdf. Accessed July 12, 2021 .
- 8. Ryser MD , Worni M , Turner EL , Marks JR , Durrett R , Hwang ES . Outcomes of Active Surveillance for Ductal Carcinoma in Situ: A Computational Risk Analysis . J Natl Cancer Inst 2015. ; 108 ( 5 ): djv372 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Erbas B , Provenzano E , Armes J , Gertig D . The natural history of ductal carcinoma in situ of the breast: a review . Breast Cancer Res Treat 2006. ; 97 ( 2 ): 135 – 144 . [DOI] [PubMed] [Google Scholar]
- 10. WHO Classification of Tumours. Breast Tumours . 5th ed. Lyon, France: : International Agency for Research on Cancer, 2019. . [Google Scholar]
- 11. Brennan ME , Turner RM , Ciatto S , et al . Ductal carcinoma in situ at core-needle biopsy: meta-analysis of underestimation and predictors of invasive breast cancer . Radiology 2011. ; 260 ( 1 ): 119 – 128 . [DOI] [PubMed] [Google Scholar]
- 12. Comparison of Operative versus Medical Endocrine Therapy for Low Risk DCIS: The COMET Trial . Patient-Centered Outcomes Research Institute . https://www.pcori.org/research-results/2016/comparing-treatment-options-women-low-risk-ductal-carcinoma-situ-dcis-comet. August 8, 2016 .
- 13. LORIS: A phase III trial of surgery versus active monitoring for Low Risk Ductal Carcinoma in situ (DCIS) . University of Birmingham; . http://www.birmingham.ac.uk/research/activity/mds/trials/crctu/trials/loris/index.aspx. November 19, 2016 . [Google Scholar]
- 14. Management of low-risk DCIS (LORD) . The Netherlands Cancer Institute . https://clinicaltrials.gov/ct2/show/NCT02492607. November 19, 2016 .
- 15. Hiroji I . Single-arm confirmatory trial of endocrine therapy alone for estrogen receptor-positive, low-risk ductal carcinoma in situ of the breast (JCOG1505, LORETTA trial) . https://upload.umin.ac.jp/cgi-open-bin/ctr_e/ctr_view.cgi?recptno=R000032260. Accessed July 14, 2021 .
- 16. van Seijen M , Jóźwiak K , Pinder SE , et al . Variability in grading of ductal carcinoma in situ among an international group of pathologists . J Pathol Clin Res 2021. ; 7 ( 3 ): 233 – 242 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Groen EJ , Hudecek J , Mulder L , et al . Prognostic value of histopathological DCIS features in a large-scale international interrater reliability study . Breast Cancer Res Treat 2020. ; 183 ( 3 ): 759 – 770 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kuhl CK , Schrading S , Bieling HB , et al . MRI for diagnosis of pure ductal carcinoma in situ: a prospective observational study . Lancet 2007. ; 370 ( 9586 ): 485 – 492 . [DOI] [PubMed] [Google Scholar]
- 19. Moon HJ , Kim EK , Kim MJ , Yoon JH , Park VY . Comparison of Clinical and Pathologic Characteristics of Ductal Carcinoma in Situ Detected on Mammography versus Ultrasound Only in Asymptomatic Patients . Ultrasound Med Biol 2019. ; 45 ( 1 ): 68 – 77 . [DOI] [PubMed] [Google Scholar]
- 20. Hayes BD , Brodie C . O’CIS are associated with R5 rather than R3 calcifications in breast screening mammography . Breast J 2013. ; 19 ( 3 ): 319 – 324 . [DOI] [PubMed] [Google Scholar]
- 21. Chou SS , Gombos EC , Chikarmane SA , Giess CS , Jayender J . Computer-aided heterogeneity analysis in breast MR imaging assessment of ductal carcinoma in situ: Correlating histologic grade and receptor status . J Magn Reson Imaging 2017. ; 46 ( 6 ): 1748 – 1759 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. de Koning HJ , Draisma G , Fracheboud J , de Bruijn A . Overdiagnosis and overtreatment of breast cancer: microsimulation modelling estimates based on observed screen and clinical data . Breast Cancer Res 2006. ; 8 ( 1 ): 202 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Miligy IM , Toss MS , Shiino S , et al . The clinical significance of oestrogen receptor expression in breast ductal carcinoma in situ . Br J Cancer 2020. ; 123 ( 10 ): 1513 – 152 . [Published correction appears in Br J Cancer 2020;123(10):1584.]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Rauch GM , Kuerer HM , Scoggins ME , et al . Clinicopathologic, mammographic, and sonographic features in 1,187 patients with pure ductal carcinoma in situ of the breast by estrogen receptor status . Breast Cancer Res Treat 2013. ; 139 ( 3 ): 639 – 647 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Avdan Aslan A , Gültekin S , Esendağli Yilmaz G , Kurukahvecioğlu O . Is There Any Association Between Mammographic Features of Microcalcifications and Breast Cancer Subtypes in Ductal Carcinoma In Situ? Acad Radiol 2021. ; 28 ( 7 ): 963 – 968 . [DOI] [PubMed] [Google Scholar]
- 26. Bera K , Schalper KA , Rimm DL , Velcheti V , Madabhushi A . Artificial intelligence in digital pathology: new tools for diagnosis and precision oncology . Nat Rev Clin Oncol 2019. ; 16 ( 11 ): 703 – 715 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Tran WT , Jerzak K , Lu FI , et al . Personalized Breast Cancer Treatments Using Artificial Intelligence in Radiomics and Pathomics . J Med Imaging Radiat Sci 2019. ; 50 ( 4 Suppl 2 ): S32 – S41 . [DOI] [PubMed] [Google Scholar]
- 28. Casasent AK , Edgerton M , Navin NE . Genome evolution in ductal carcinoma in situ: invasion of the clones . J Pathol 2017. ; 241 ( 2 ): 208 – 218 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kim SY , Jung SH , Kim MS , et al . Genomic differences between pure ductal carcinoma in situ and synchronous ductal carcinoma in situ with invasive breast cancer . Oncotarget 2015. ; 6 ( 10 ): 7597 – 7607 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Casasent AK , Schalck A , Gao R , et al . Multiclonal Invasion in Breast Tumors Identified by Topographic Single Cell Sequencing . Cell 2018. ; 172 ( 1-2 ): 205 – 217.e12 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Pareja F , Brown DN , Lee JY , et al . Whole-Exome Sequencing Analysis of the Progression from Non-Low-Grade Ductal Carcinoma In Situ to Invasive Ductal Carcinoma . Clin Cancer Res 2020. ; 26 ( 14 ): 3682 – 3693 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Visser LL , Elshof LE , Van de Vijver K , et al . Discordant Marker Expression Between Invasive Breast Carcinoma and Corresponding Synchronous and Preceding DCIS . Am J Surg Pathol 2019. ; 43 ( 11 ): 1574 – 1582 . [DOI] [PubMed] [Google Scholar]
- 33. Lei RY , Carter DL , Antell AG , et al . A Comparison of Predicted Ipsilateral Tumor Recurrence Risks in Patients With Ductal Carcinoma in Situ of the Breast After Breast-Conserving Surgery by Breast Radiation Oncologists, the Van Nuys Prognostic Index, the Memorial Sloan Kettering Cancer Center DCIS Nomogram, and the 12-Gene DCIS Score Assay . Adv Radiat Oncol 2020. ; 6 ( 2 ): 100607 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Narayanan PL , Raza SEA , Hall AH , et al . Unmasking the immune microecology of ductal carcinoma in situ with deep learning . NPJ Breast Cancer 2021. ; 7 ( 1 ): 19 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Gajdos C , Tartter PI , Bleiweiss IJ , et al . Mammographic appearance of nonpalpable breast cancer reflects pathologic characteristics . Ann Surg 2002. ; 235 ( 2 ): 246 – 251 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Chen H , Bai F , Wang M , Zhang M , Zhang P , Wu K . The prognostic significance of co-existence ductal carcinoma in situ in invasive ductal breast cancer: a large population-based study and a matched case-control analysis . Ann Transl Med 2019. ; 7 ( 18 ): 484 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Grimm LJ , Miller MM , Thomas SM , et al . Growth Dynamics of Mammographic Calcifications: Differentiating Ductal Carcinoma in Situ from Benign Breast Disease . Radiology 2019. ; 292 ( 1 ): 77 – 83 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Friedewald SM , Rafferty EA , Rose SL , et al . Breast cancer screening using tomosynthesis in combination with digital mammography . JAMA 2014. ; 311 ( 24 ): 2499 – 2507 . [DOI] [PubMed] [Google Scholar]
- 39. Berg WA , Zhang Z , Lehrer D , et al . Detection of breast cancer with addition of annual screening ultrasound or a single screening MRI to mammography in women with elevated breast cancer risk . JAMA 2012. ; 307 ( 13 ): 1394 – 1404 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Saadatmand S , Geuzinge HA , Rutgers EJT , et al . MRI versus mammography for breast cancer screening in women with familial risk (FaMRIsc): a multicentre, randomised, controlled trial . Lancet Oncol 2019. ; 20 ( 8 ): 1136 – 1147 . [DOI] [PubMed] [Google Scholar]
- 41. Mun HS , Shin HJ , Kim HH , Cha JH , Kim H . Screening-detected calcified and non-calcified ductal carcinoma in situ: differences in the imaging and histopathological features . Clin Radiol 2013. ; 68 ( 1 ): e27 – e35 . [DOI] [PubMed] [Google Scholar]
- 42. Holmberg L , Wong YN , Tabár L , et al . Mammography casting-type calcification and risk of local recurrence in DCIS: analyses from a randomised study . Br J Cancer 2013. ; 108 ( 4 ): 812 – 819 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Barreau B , de Mascarel I , Feuga C , et al . Mammography of ductal carcinoma in situ of the breast: review of 909 cases with radiographic-pathologic correlations . Eur J Radiol 2005. ; 54 ( 1 ): 55 – 61 . [DOI] [PubMed] [Google Scholar]
- 44. Bragg A , Candelaria R , Adrada B , et al . Imaging of Noncalcified Ductal Carcinoma In Situ . J Clin Imaging Sci 2021. ; 11 34 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Sagara Y , Mallory MA , Wong S , et al . Survival Benefit of Breast Surgery for Low-Grade Ductal Carcinoma In Situ: A Population-Based Cohort Study . JAMA Surg 2015. ; 150 ( 8 ): 739 – 745 . [DOI] [PubMed] [Google Scholar]
- 46. O’Grady S , Morgan MP . Microcalcifications in breast cancer: From pathophysiology to diagnosis and prognosis . Biochim Biophys Acta Rev Cancer 2018. ; 1869 ( 2 ): 310 – 320 . [DOI] [PubMed] [Google Scholar]
- 47. Cox RF , Morgan MP . Microcalcifications in breast cancer: Lessons from physiological mineralization . Bone 2013. ; 53 ( 2 ): 437 – 450 . [DOI] [PubMed] [Google Scholar]
- 48. Wang LC , Sullivan M , Du H , Feldman MI , Mendelson EB . US appearance of ductal carcinoma in situ . RadioGraphics 2013. ; 33 ( 1 ): 213 – 228 . [DOI] [PubMed] [Google Scholar]
- 49. Watanabe T , Yamaguchi T , Tsunoda H , et al . Ultrasound Image Classification of Ductal Carcinoma In Situ (DCIS) of the Breast: Analysis of 705 DCIS Lesions . Ultrasound Med Biol 2017. ; 43 ( 5 ): 918 – 925 . [DOI] [PubMed] [Google Scholar]
- 50. Su X , Lin Q , Cui C , et al . Non-calcified ductal carcinoma in situ of the breast: comparison of diagnostic accuracy of digital breast tomosynthesis, digital mammography, and ultrasonography . Breast Cancer 2017. ; 24 ( 4 ): 562 – 570 . [DOI] [PubMed] [Google Scholar]
- 51. Shehata M , Grimm L , Ballantyne N , et al . Ductal Carcinoma in Situ: Current Concepts in Biology, Imaging, and Treatment . J Breast Imaging 2019. ; 1 ( 3 ): 166 – 176 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Jansen SA , Paunesku T , Fan X , et al . Ductal carcinoma in situ: X-ray fluorescence microscopy and dynamic contrast-enhanced MR imaging reveals gadolinium uptake within neoplastic mammary ducts in a murine model . Radiology 2009. ; 253 ( 2 ): 399 – 406 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Jansen SA , Newstead GM , Abe H , Shimauchi A , Schmidt RA , Karczmar GS . Pure ductal carcinoma in situ: kinetic and morphologic MR characteristics compared with mammographic appearance and nuclear grade . Radiology 2007. ; 245 ( 3 ): 684 – 691 . [DOI] [PubMed] [Google Scholar]
- 54. Chou SS , Romanoff J , Lehman CD , et al . Preoperative Breast MRI for Newly Diagnosed Ductal Carcinoma in Situ: Imaging Features and Performance in a Multicenter Setting (ECOG-ACRIN E4112 Trial) . Radiology 2021. ; 301 ( 1 ): 66 – 77 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Yamada T , Mori N , Watanabe M , et al . Radiologic-pathologic correlation of ductal carcinoma in situ . RadioGraphics 2010. ; 30 ( 5 ): 1183 – 1198 . [DOI] [PubMed] [Google Scholar]
- 56. Buadu LD , Murakami J , Murayama S , et al . Breast lesions: correlation of contrast medium enhancement patterns on MR images with histopathologic findings and tumor angiogenesis . Radiology 1996. ; 200 ( 3 ): 639 – 649 . [DOI] [PubMed] [Google Scholar]
- 57. Newstead GM . MR imaging of ductal carcinoma in situ . Magn Reson Imaging Clin N Am 2010. ; 18 ( 2 ): 225 – 240 , viii . [DOI] [PubMed] [Google Scholar]
- 58. Rahbar H , Partridge SC , Demartini WB , et al . In vivo assessment of ductal carcinoma in situ grade: a model incorporating dynamic contrast-enhanced and diffusion-weighted breast MR imaging parameters . Radiology 2012. ; 263 ( 2 ): 374 – 382 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Rahbar H , Parsian S , Partridge SC , Demartini WB , Kurland BF , Lehman CD . MR biomarkers at 3 Tesla for prediction of Van Nuys pathological classification of ductal carcinoma in situ . Radiological Society of North America; . Chicago, IL: , 2012. . [Google Scholar]
- 60. Kim SA , Cho N , Ryu EB , et al . Background parenchymal signal enhancement ratio at preoperative MR imaging: association with subsequent local recurrence in patients with ductal carcinoma in situ after breast conservation surgery . Radiology 2014. ; 270 ( 3 ): 699 – 707 . [DOI] [PubMed] [Google Scholar]
- 61. Luo J , Johnston BS , Kitsch AE , et al . Ductal Carcinoma in Situ: Quantitative Preoperative Breast MR Imaging Features Associated with Recurrence after Treatment . Radiology 2017. ; 285 ( 3 ): 788 – 797 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Kuhl CK . Let Us Move Out of Plato’s Cave: The Greater Reality of DCIS . Radiology 2021. ; 301 ( 1 ): 78 – 80 . [DOI] [PubMed] [Google Scholar]
- 63. Bartram A , Gilbert F , Thompson A , Mann GB , Agrawal A . Breast MRI in DCIS size estimation, breast-conserving surgery and oncoplastic breast surgery . Cancer Treat Rev 2021. ; 94 102158 . [DOI] [PubMed] [Google Scholar]
- 64. Lehman CD , Gatsonis C , Romanoff J , et al . Association of Magnetic Resonance Imaging and a 12-Gene Expression Assay With Breast Ductal Carcinoma In Situ Treatment . JAMA Oncol 2019. ; 5 ( 7 ): 1036 – 1042 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Pickles MD , Gibbs P , Hubbard A , Rahman A , Wieczorek J , Turnbull LW . Comparison of 3.0 T magnetic resonance imaging and X-ray mammography in the measurement of ductal carcinoma in situ: a comparison with histopathology . Eur J Radiol 2015. ; 84 ( 4 ): 603 – 610 . [DOI] [PubMed] [Google Scholar]
- 66. Rahbar H , Partridge SC , Eby PR , et al . Characterization of ductal carcinoma in situ on diffusion weighted breast MRI . Eur Radiol 2011. ; 21 ( 9 ): 2011 – 2019 . [DOI] [PubMed] [Google Scholar]
- 67. Rahbar H , Parsian S , Lam DL , et al . Can MRI biomarkers at 3 T identify low-risk ductal carcinoma in situ? Clin Imaging 2016. ; 40 ( 1 ): 125 – 129 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Partridge SC , DeMartini WB , Kurland BF , Eby PR , White SW , Lehman CD . Quantitative diffusion-weighted imaging as an adjunct to conventional breast MRI for improved positive predictive value . AJR Am J Roentgenol 2009. ; 193 ( 6 ): 1716 – 1722 . [DOI] [PubMed] [Google Scholar]
- 69. Iima M , Le Bihan D , Okumura R , et al . Apparent diffusion coefficient as an MR imaging biomarker of low-risk ductal carcinoma in situ: a pilot study . Radiology 2011. ; 260 ( 2 ): 364 – 372 . [DOI] [PubMed] [Google Scholar]
- 70. Kim SY , Cho N , Choi Y , et al . Ultrafast Dynamic Contrast-Enhanced Breast MRI: Lesion Conspicuity and Size Assessment according to Background Parenchymal Enhancement . Korean J Radiol 2020. ; 21 ( 5 ): 561 – 571 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Mus RD , Borelli C , Bult P , et al . Time to enhancement derived from ultrafast breast MRI as a novel parameter to discriminate benign from malignant breast lesions . Eur J Radiol 2017. ; 89 ( 90 ): 96 . [DOI] [PubMed] [Google Scholar]
- 72. Onishi N , Sadinski M , Hughes MC , et al . Ultrafast dynamic contrast-enhanced breast MRI may generate prognostic imaging markers of breast cancer . Breast Cancer Res 2020. ; 22 ( 1 ): 58 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Sogani J , Mango VL , Keating D , Sung JS , Jochelson MS . Contrast-enhanced mammography: past, present, and future . Clin Imaging 2021. ; 69 ( 269 ): 279 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Vignoli C , Bicchierai G , De Benedetto D , et al . Role of preoperative breast dual-energy contrast-enhanced digital mammography in ductal carcinoma in situ . Breast J 2019. ; 25 ( 5 ): 1034 – 1036 . [DOI] [PubMed] [Google Scholar]
- 75. Cheung YC , Juan YH , Lin YC , et al . Dual-Energy Contrast-Enhanced Spectral Mammography: Enhancement Analysis on BI-RADS 4 Non-Mass Microcalcifications in Screened Women . PLoS One 2016. ; 11 ( 9 ): e0162740 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Ryser MD , Weaver DL , Zhao F , et al . Cancer Outcomes in DCIS Patients Without Locoregional Treatment . J Natl Cancer Inst 2019. ; 111 ( 9 ): 952 – 960 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Allegra CJ , Aberle DR , Ganschow P , et al . NIH state-of-the-science conference statement: diagnosis and management of ductal carcinoma in situ (DCIS) . NIH Consens State Sci Statements 2009. ; 26 ( 2 ): 1 – 27 . [PubMed] [Google Scholar]
- 78. van Luijt PA , Heijnsdijk EA , Fracheboud J , et al . The distribution of ductal carcinoma in situ (DCIS) grade in 4232 women and its impact on overdiagnosis in breast cancer screening . Breast Cancer Res 2016. ; 18 ( 1 ): 47 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Yen MF , Tabár L , Vitak B , Smith RA , Chen HH , Duffy SW . Quantifying the potential problem of overdiagnosis of ductal carcinoma in situ in breast cancer screening . Eur J Cancer 2003. ; 39 ( 12 ): 1746 – 1754 . [DOI] [PubMed] [Google Scholar]
- 80. Seigneurin A , François O , Labarère J , Oudeville P , Monlong J , Colonna M . Overdiagnosis from non-progressive cancer detected by screening mammography: stochastic simulation study with calibration to population based registry data . BMJ 2011. ; 343 ( nov23 1 ): d7017 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Worni M , Akushevich I , Greenup R , et al . Trends in Treatment Patterns and Outcomes for Ductal Carcinoma In Situ . J Natl Cancer Inst 2015. ; 107 ( 12 ): djv263 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Ozanne EM , Shieh Y , Barnes J , Bouzan C , Hwang ES , Esserman LJ . Characterizing the impact of 25 years of DCIS treatment . Breast Cancer Res Treat 2011. ; 129 ( 1 ): 165 – 173 . [DOI] [PubMed] [Google Scholar]
- 83. Page DL , Dupont WD , Rogers LW , Landenberger M . Intraductal carcinoma of the breast: follow-up after biopsy only . Cancer 1982. ; 49 ( 4 ): 751 – 758 . [DOI] [PubMed] [Google Scholar]
- 84. Page DL , Dupont WD , Rogers LW , Jensen RA , Schuyler PA . Continued local recurrence of carcinoma 15-25 years after a diagnosis of low grade ductal carcinoma in situ of the breast treated only by biopsy . Cancer 1995. ; 76 ( 7 ): 1197 – 1200 . [DOI] [PubMed] [Google Scholar]
- 85. Sanders ME , Schuyler PA , Dupont WD , Page DL . The natural history of low-grade ductal carcinoma in situ of the breast in women treated by biopsy only revealed over 30 years of long-term follow-up . Cancer 2005. ; 103 ( 12 ): 2481 – 2484 . [DOI] [PubMed] [Google Scholar]
- 86. Eusebi V , Feudale E , Foschini MP , et al . Long-term follow-up of in situ carcinoma of the breast . Semin Diagn Pathol 1994. ; 11 ( 3 ): 223 – 235 . [PubMed] [Google Scholar]
- 87. Maxwell AJ , Clements K , Hilton B , et al . Risk factors for the development of invasive cancer in unresected ductal carcinoma in situ . Eur J Surg Oncol 2018. ; 44 ( 4 ): 429 – 435 . [DOI] [PubMed] [Google Scholar]
- 88. Hwang ES , Hyslop T , Lynch T , et al . The COMET (Comparison of Operative versus Monitoring and Endocrine Therapy) trial: a phase III randomised controlled clinical trial for low-risk ductal carcinoma in situ (DCIS) . BMJ Open 2019. ; 9 ( 3 ): e026797 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Francis A , Thomas J , Fallowfield L , et al . Addressing overtreatment of screen detected DCIS; the LORIS trial . Eur J Cancer 2015. ; 51 ( 16 ): 2296 – 2303 . [DOI] [PubMed] [Google Scholar]
- 90. Elshof LE , Tryfonidis K , Slaets L , et al . Feasibility of a prospective, randomised, open-label, international multicentre, phase III, non-inferiority trial to assess the safety of active surveillance for low risk ductal carcinoma in situ - The LORD study . Eur J Cancer 2015. ; 51 ( 12 ): 1497 – 1510 . [DOI] [PubMed] [Google Scholar]
- 91. Hamdy FC , Donovan JL , Lane JA , et al . 10-Year Outcomes after Monitoring, Surgery, or Radiotherapy for Localized Prostate Cancer . N Engl J Med 2016. ; 375 ( 15 ): 1415 – 1424 . [DOI] [PubMed] [Google Scholar]
- 92. Grimm LJ , Ryser MD , Partridge AH , et al . Surgical Upstaging Rates for Vacuum Assisted Biopsy Proven DCIS: Implications for Active Surveillance Trials . Ann Surg Oncol 2017. ; 24 ( 12 ): 3534 – 3540 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Pilewskie M , Stempel M , Rosenfeld H , Eaton A , Van Zee KJ , Morrow M. Do LORIS . Trial Eligibility Criteria Identify a Ductal Carcinoma In Situ Patient Population at Low Risk of Upgrade to Invasive Carcinoma? Ann Surg Oncol 2016. ; 23 ( 11 ): 3487 – 3493 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Oseni TO , Smith BL , Lehman CD , Vijapura CA , Pinnamaneni N , Bahl M . Do Eligibility Criteria for Ductal Carcinoma In Situ (DCIS) Active Surveillance Trials Identify Patients at Low Risk for Upgrade to Invasive Carcinoma? Ann Surg Oncol 2020. ; 27 ( 11 ): 4459 – 4465 . [DOI] [PubMed] [Google Scholar]
- 95. Shi B , Grimm LJ , Mazurowski MA , et al . Prediction of Occult Invasive Disease in Ductal Carcinoma in Situ Using Deep Learning Features . J Am Coll Radiol 2018. ; 15 ( 3 Pt B ): 527 – 534 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Shi B , Grimm LJ , Mazurowski MA , et al . Can Occult Invasive Disease in Ductal Carcinoma In Situ Be Predicted Using Computer-extracted Mammographic Features? Acad Radiol 2017. ; 24 ( 9 ): 1139 – 1147 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Harowicz MR , Saha A , Grimm LJ , et al . Can algorithmically assessed MRI features predict which patients with a preoperative diagnosis of ductal carcinoma in situ are upstaged to invasive breast cancer? J Magn Reson Imaging 2017. ; 46 ( 5 ): 1332 – 1340 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Grimm LJ , Neely B , Hou R , et al . Mixed-Methods Study to Predict Upstaging of DCIS to Invasive Disease on Mammography . AJR Am J Roentgenol 2021. ; 216 ( 4 ): 903 – 911 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Grimm LJ , Ghate SV , Hwang ES , Soo MS . Imaging Features of Patients Undergoing Active Surveillance for Ductal Carcinoma in Situ . Acad Radiol 2017. ; 24 ( 11 ): 1364 – 1371 . [DOI] [PubMed] [Google Scholar]
- 100. Meyerson AF , Lessing JN , Itakura K , et al . Outcome of long term active surveillance for estrogen receptor-positive ductal carcinoma in situ . Breast 2011. ; 20 ( 6 ): 529 – 533 . [DOI] [PMC free article] [PubMed] [Google Scholar]