Abstract
Purpose:
Patient derived xenografts (PDXs) are a research tool for studying cancer biology and drug response phenotypes. While engraftment rates are higher for tumors with more aggressive characteristics, it is uncertain whether engraftment is prognostic for cancer recurrence.
Experimental Design:
In a prospective study of breast cancer patients treated with neoadjuvant chemotherapy (NAC) with taxane+/−trastuzumab followed by anthracycline-based chemotherapy, we report the association between breast cancer events and PDX engraftment using tumors derived from treatment naïve (pre-NAC biopsies from 113 patients) and treatment resistant (post-NAC at surgery from 34 patients). Gray’s test was used to assess whether the cumulative incidence of a breast cancer event differs with respect to either pre-NAC PDX engraftment or post-NAC PDX engraftment.
Results:
With a median follow up of 5.7 years, the cumulative incidence of breast cancer relapse did not differ significantly according to pre-NAC PDX engraftment (5-year rate: 13.6% versus 13.4%; p=0.89). However, the incidence of a breast event was greater for patients with post-NAC PDX engraftment (5-year rate: 50.0% versus 19.6%), but this did not achieve significance (p=0.11).
Conclusions:
In treatment-naive breast cancer receiving standard NAC, PDX engraftment was not prognostic for breast cancer recurrence. Further study is needed to establish whether PDX engraftment in the treatment-resistant setting is prognostic for cancer recurrence.
Patient derived xenografts (PDXs) are a research tool for studying cancer biology and drug response phenotypes. However, small case series suggest that the rate of breast cancer recurrence may be associated with PDX engraftment. In a prospective study of breast cancer patients treated with neoadjuvant chemotherapy (NAC) wherein tumor cells derived from needle core biopsies were implanted into immune compromised mice, we found no differences in the cumulative rates of breast cancer recurrence according to pre-NAC PDX engraftment. However, a non-statistically significant higher incidence of breast events was observed in patients with post-NAC PDX engraftment (5-year rate: 50.0% versus 19.6%). These data suggest that in newly diagnosed patients treated with standard local and systemic therapy, PDX engraftment is not prognostic for cancer recurrence. However, further study is indicated in patients with chemotherapy resistant tumors regarding the role of PDX engraftment and cancer recurrence
Patient-derived xenografts (PDX) recapitulate the cell morphology, architecture, microenvironment and molecular signatures of patient tumors1,2, and additionally faithfully reproduce drug response phenotypes seen in patients.2 Thus, PDXs are a standard research tool used across multiple different solid tumors.
While many factors affect PDX engraftment, including the amount of tissue injected, site of injection (subcutaneous versus orthotopic), and mouse strain, the aggressiveness of the patient malignancy has been associated with higher engraftment. For example, in breast cancer, higher engraftment are seen in tumors with higher grade and ER negative status.2 These observations have led to the hypothesis that engraftment may be prognostic for disease outcomes. For example, a retrospective study of 24 patients with newly diagnosed breast cancer without prior cancer treatment and followed for a median time of 28 months found engraftment to be associated with relapse.3
We previously reported results of the prospective multi-center Breast Cancer Genome Guided Therapy Study (BEAUTY) clinical trial.4 Of the 140 patients enrolled, 113 were treated at centers with established PDX programs and had percutaneous tumor biopsies (PTB) obtained for PDX prior to neoadjuvant chemotherapy (weekly taxane +/− trastuzumab followed by anthracycline-based chemotherapy) and 34 had surgical tissue samples obtained from resection following chemotherapy.4,5 In this prospective study, we obtained 6–8 image guided percutaneous biopsies from the tumor (>90% were T2 or larger) and the cores were used for pathological confirmation, tumor sequencing and PDX generation. Within an hour of sample collection, one to two cores were implanted with Matrigel (BD Biosciences, Heidelberg, Germany) in the flanks of 6–8-week-old female immunodeficient mice which were pretreated and maintained with 17β-estradiol. Samples were implanted subcutaneously, and mice were monitored daily. The process handling was consistent across the entire study period. A histological evaluation of the tissue was not performed to assess for necrosis prior to implanting the tumor.
We defined PDX engraftment as the percent of patients with at least one stably transplantable xenograft pathologically confirmed as breast cancer and passed at least for four generations. pre-NAC PDX engraftment was 27.4% (31/113), and differs significantly by clinical molecular subtype [triple negative breast cancer (TNBC) 51.3% (20/39); HER2+, 26.5% (9/34); and ER+/HER2- at 5.0% (2/40)] as well as grade, type of mouse strain (NSG vs NOD-SCID mice), but not by baseline tumor Ki-67.4,5 Chemotherapy response (pCR) was not found to differ significantly by pre-NAC engraftment.5 Herein, we report on the association between PDX engraftment and breast cancer outcomes.
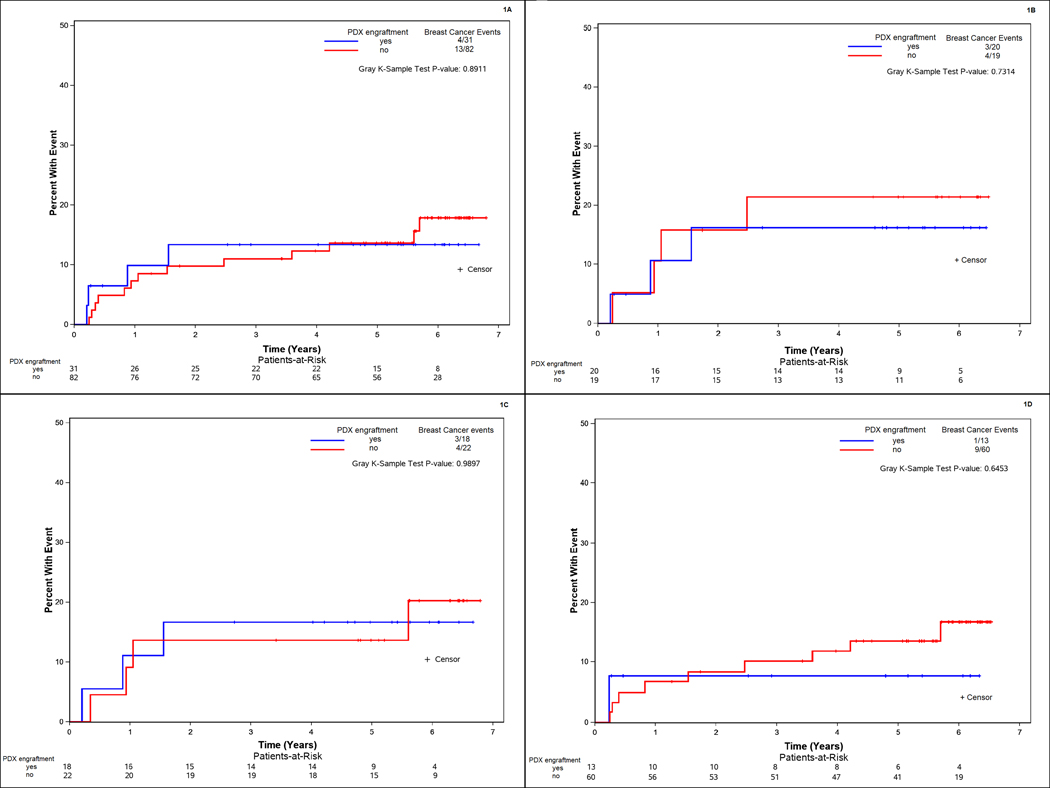
With a median follow up of 5.7 years, local, regional or distant disease events (either disease progression during NAC or recurrence post NAC) were reported in 17 patients. Gray’s test was used to assess whether the cumulative incidence of a breast cancer event (CI-BCE),differs with respect to either pre-NAC PDX engraftment or post-NAC PDX engraftment. Breast cancer events (outcomes) were defined as progression on chemotherapy prior to surgery or local, regional, or distant recurrence after surgery. Contralateral breast cancer and second primary disease were considered competing events. Time at risk started the day of registration (at most 21 days prior to start of NAC) when assessing pre-NAC PDX engraftment and the day of surgery when assessing post-NAC PDX engraftment. The CI-BCE was not found to differ according to pre-NAC PDX engraftment (5-year rate [95% CI]: 13.6% [7.2–22.1%] no engraftment; 13.4% [4.1–28.2%] engraftment; p=0.89). (Figure 1 A)
Figure 1:
Time to breast cancer event according to PDX engraftment in A) all patients prior to neoadjuvant chemotherapy; B) in triple negative breast cancer patients prior to neoadjuvant chemotherapy; and all patients prior to neoadjuvant chemotherapy according to type of mouse strain C) NSG and D) NOD-SCID
In the 39 patients with TNBC, the subgroup with the highest pre-NAC engraftment, CI-BCE was not found to differ significantly between those without PDX engraftment (5 year rate [CI]: 21.4% [6.3–42.3%]) and those with PDX engraftment (16.2% [3.8–36.4%], p=0.73).(Figure 1 B).
We previously evaluated the association between mouse strain and engraftment and found that pre-NAC engraftment were higher in NSG versus NOD-SCID (65.4% vs 25.5%; p=0.001).5 Therefore, we determined whether CI-BCE differed with respect to engraftment for each mouse strain. CI-BCE was not found to differ significantly between patients with pre-NAC PDX engraftment and patients without pre-NAC PDX engraftment according to type of mouse strain used. In the NOD-SCID mice 5-year CI-BCE rate was 7.7% [0.4–30.3%] in those with pre-NAC engraftment and 13.7% [6.3–23.8%] in those without pre-NAC engraftment (p=0.65). In the NSG mice 5-year CI-IBR rate was 16.7% [3.9–37.2%] in those with pre-NAC PDX engraftment and 13.6% [3.3–31.4%] in those without pre-NAC engraftment (p=0.99). (Figure 1 C and D).
PDXs were established from residual tissue from surgery (post-NAC) in 6 of 34 patients (17.6%), specifically, TNBC: 5/9; Her2+: 1/8; and Luminal: 0/17. The CI-BCE was higher among those with post-NAC PDX engraftment relative to those without post-NAC PDX engraftment (5 year rate [CI]: 19.6% [6.9–37.0%] no engraftment; 50.0% [7.7–82.9%] engraftment) but this did not reach statistical significance (p=0.11; Figure 2).
Figure 2:
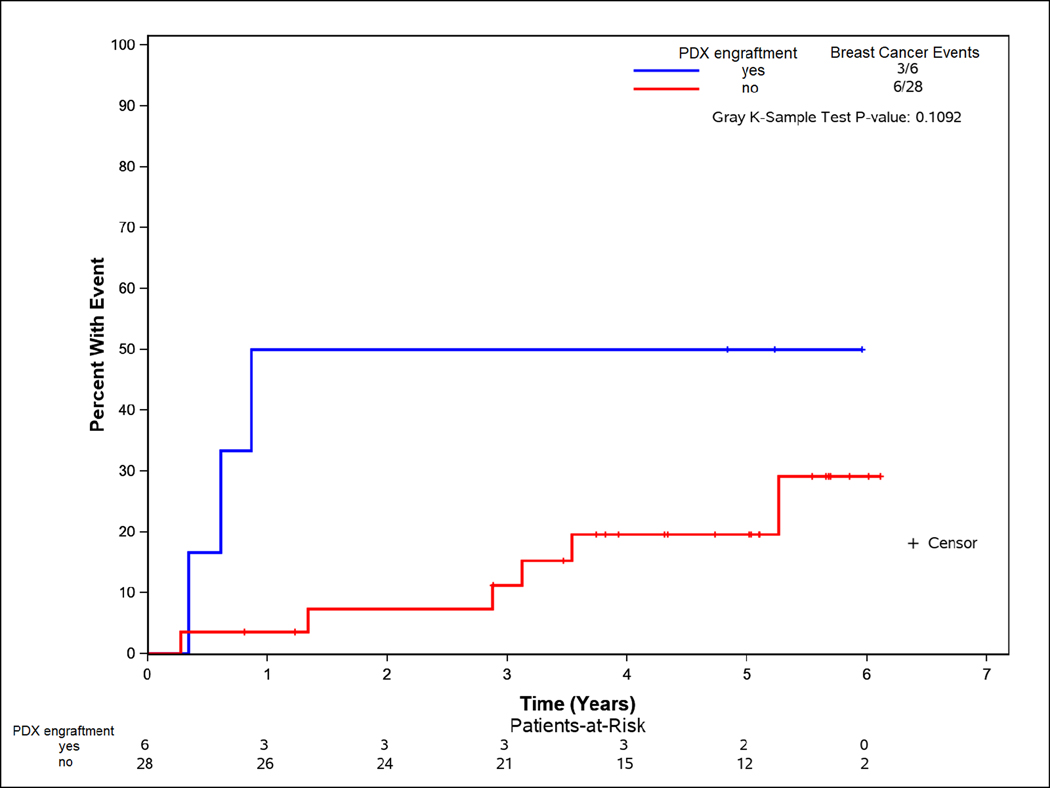
Time to breast cancer event according to PDX engraftment in patients with residual disease after neoadjuvant chemotherapy
The process of generating a PDX model results in the selection of tumors that engraft and propagate in mice. As demonstrated in the BEAUTY study as well as in prior reports, more aggressive tumors have a higher engraftment.2,6,7 A series of prior studies have reported that tumor engraftment was prognostic for recurrence and survival.8–11 However, these studies are small series of retrospectively collected patients who received a variety of treatments who had sufficient tumor (from breast, nodes or metastatic site) to attempt implantation. In this prospective study collecting PTB from patients receiving standard of care anthracycline and taxane based chemotherapy, including trastuzumab for HER2+ breast cancer, we found that the CI-BCE did not differ according to success of pre-NAC tumor engraftment into an immunocompromised mouse. Because tumor engraftment tends to be highest in TNBC, we further analyzed the association between tumor engraftment and outcome. Again, in this group, the CI-BCE between those with and without pre-NAC PDX engraftment did not significantly differ p=0.73).
In contrast, in patients with residual disease following neoadjuvant chemotherapy whose tumors exhibited chemotherapy resistance and who are at higher risk of local and distant recurrence (compared to those with pathologic complete response), post-NAC PDX engraftment tended to not only be associated with tumor subtype but also with a higher CI-BCE. These data suggest that for chemotherapy-resistant tumors that remain in the breast at the time of surgical resection, engraftment may identify a subset of patients at greater risk of recurrence.
Taken together, PDXs derived from pre-NAC and post-NAC settings provides an important tool to interrogate cancer biology and drug response. However, PDX engraftment as a biomarker of prognosis has not been definitively validated and remains a research question, in part due to the limited ability to study only in larger academic centers and lack of standardization of the methodologies surrounding PDX engraftment. Given the limitations of this study size, overall take rate and event rate, larger cohorts of PDXs collected in a standardized fashion from patients homogenously treated with appropriate therapies are needed to establish whether PDX engraftment in both treatment-naïve and treatment-resistant settings is prognostic for cancer recurrence.
Acknowledgments
Funding:
This work was supported by: Mayo Clinic Center for Individualized Medicine, Nadia’s Gift Foundation, John P. Guider, The Eveleigh Family, George M. Eisenberg Foundation for Charities, Pharmacogenomics Research Network (U19 GM61388, M. Goetz, L. Wang, R. Weinshilboum, R. Kalari, J. Ingle), National Institutes of Health (R01 CA196648, L. Wang), Mayo Clinic Cancer Center (CA15083–40A2), Mayo Clinic Breast Specialized Program of Research Excellence (SPORE P50CA116201, M. Goetz, V. Suman; K. Kalari, J. Carter, L. Wang, and J. Ingle); J. Boughey is the W.H. Odell Professor of Individualized Medicine; R. Weinshilboum is the Mary Lou and John H. Dasburg Professor of Cancer Genomics Research; M. Goetz is the Erivan K. Haub Family Professor of Cancer Research Honoring Richard F. Emslander, M.D.
Footnotes
Conflicts of Interest Statement: The authors have no relevant conflicts of interest to declare.
References
- 1.Weroha SJ, Becker MA, Enderica-Gonzalez S, et al. Tumorgrafts as in vivo surrogates for women with ovarian cancer. Clin Cancer Res 2014;20:1288–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hidalgo M, Amant F, Biankin AV, et al. Patient-derived xenograft models: an emerging platform for translational cancer research. Cancer Discov 2014;4:998–1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.DeRose YS, Wang G, Lin YC, et al. Tumor grafts derived from women with breast cancer authentically reflect tumor pathology, growth, metastasis and disease outcomes. Nat Med 2011;17:1514–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goetz MP, Kalari KR, Suman VJ, et al. Tumor Sequencing and Patient-Derived Xenografts in the Neoadjuvant Treatment of Breast Cancer. J Natl Cancer Inst 2017;109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yu J, Qin B, Moyer AM, et al. Establishing and characterizing patient-derived xenografts using pre-chemotherapy percutaneous biopsy and post-chemotherapy surgical samples from a prospective neoadjuvant breast cancer study. Breast Cancer Res 2017;19:130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang X, Claerhout S, Prat A, et al. A renewable tissue resource of phenotypically stable, biologically and ethnically diverse, patient-derived human breast cancer xenograft models. Cancer Res 2013;73:4885–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marangoni E, Vincent-Salomon A, Auger N, et al. A new model of patient tumor-derived breast cancer xenografts for preclinical assays. Clin Cancer Res 2007;13:3989–98. [DOI] [PubMed] [Google Scholar]
- 8.Némati F, Sastre-Garau X, Laurent C, et al. Establishment and characterization of a panel of human uveal melanoma xenografts derived from primary and/or metastatic tumors. Clin Cancer Res 2010;16:2352–62. [DOI] [PubMed] [Google Scholar]
- 9.Li S, Shen D, Shao J, et al. Endocrine-therapy-resistant ESR1 variants revealed by genomic characterization of breast-cancer-derived xenografts. Cell Rep 2013;4:1116–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Garrido-Laguna I, Uson M, Rajeshkumar NV, et al. Tumor engraftment in nude mice and enrichment in stroma- related gene pathways predict poor survival and resistance to gemcitabine in patients with pancreatic cancer. Clin Cancer Res 2011;17:5793–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kleine W. Prognostic significance of growth characteristics of xenotransplanted ovarian carcinomas into nude mice. Gynecologic Oncology 1986;25:65–72. [DOI] [PubMed] [Google Scholar]