Abstract
Background:
A precision medicine approach to bipolar disorder (BD) requires greater knowledge of neural mechanisms, especially within the BD phenotype. The present study evaluated differences in resting state functional connectivity (RSFC) between young adults followed longitudinally since childhood with full-threshold type I BD (BD-I)—characterized by distinct manic episodes—or a more sub-syndromal presentation of BD (BD Not Otherwise Specified [BD-NOS]), compared to one another and to healthy controls (HC). Independent Components Analysis (ICA), a multivariate data-driven method, and dual regression were used to explore whether connectivity within resting state networks (RSNs) differentiated the groups, especially for characteristic fronto-limbic alterations in BD.
Methods:
Young adults (ages 18–30) with BD-I (n=28), BD-NOS (n=14), and HCs (n=52) underwent structural and RSFC neuroimaging. ICA derived 30 components from RSFC data; a subset of these components, representing well-characterized RSNs, was used for between-group analyses.
Results:
Participants with BD-I had significantly greater connectivity strength between the executive control network and right caudate vs. HCs. Participants with BD-NOS had significantly greater connectivity strength between the sensorimotor network and left precentral gyrus vs. HCs, which was significantly related to psychiatric symptoms.
Limitations:
Limitations included small BD-NOS sample size and variation in BD mood state and medication status.
Conclusions:
Results for BD-I participants support prior findings of fronto-limbic alterations characterizing BD. Alterations in the sensorimotor network for adults with BD-NOS aligns with the small but growing body of evidence that sensorimotor network alterations may represent a marker for vulnerability to BD. Further study is required to evaluate specificity.
Keywords: bipolar disorder, resting state functional connectivity, sensorimotor, executive control, young adults
Bipolar disorder (BD) is the most expensive and impairing psychiatric illness, with high rates of associated suicide and substance use (Axelson et al., 2006; Peele et al., 2003). BD is characterized by episodes of mania, including elevated, expansive or irritable mood plus simultaneous changes—decreased need for sleep, increased goal directed activity, and/or increased risk-taking behavior. However, clinicians and researchers struggle when assessing children to determine if they have either full-duration manic or hypomanic episodes, or a more sub-syndromal course of manic symptoms. Such decisions have important consequences, including determining what medication, therapy, and academic interventions a child does or does not receive, and guiding parents about outcome as young adults.
To address this need, the Course and Outcome of Bipolar Illness in Youth (COBY) Study began in 2000 as a longitudinal phenomenological study of children with BD. COBY enrolled children meeting full criteria for either a manic or hypomanic episode (BD-I, or BD-II, respectively). COBY also enrolled those with sub-syndromal BD “Not Otherwise Specified” (BD-NOS), defined as children with distinct periods of abnormally elevated, expansive or irritable mood during which individuals experienced two or more manic symptoms (three if irritable mood) present for at least four hours within a 24-hour period (Birmaher et al., 2006). The abnormal mood and concomitant symptoms had to represent a change in functioning and occur over four days.
COBY enrolled primarily BD-I and BD-NOS children and followed them into young adulthood—when they are more capable of speaking for themselves about their symptoms, and when we know using prospective data whether or not they have developed a full manic episode characteristic of BD-I by adulthood (≥ age 18). The COBY study has contributed several key findings to the field. First, COBY confirmed that BD exists in childhood and is associated with substantial impairment (suicide attempts, psychiatric hospitalization; Axelson et al., 2006). Second, children meeting COBY criteria for BD-NOS have comparable or more impairment as children with BD-I, including comorbidity and suicidality (Axelson et al., 2006; Birmaher et al., 2009). Third, with time as children became young adults, increasingly more participants initially classified as BD-NOS developed a manic or hypomanic episode—45% at average follow-up of five years—yet a unique predictor of conversion has not been identified (Axelson et al., 2011).
Greater understanding of the neural mechanisms underlying childhood-onset BD-I and BD-NOS is essential to a precision medicine approach to BD, whereby knowledge of bio-behavioral markers of phenotypes of BD illness augments clinical care, resulting in earlier and more precise diagnosis, treatment, and prognosis (Gordon, 2016). However, the COBY study itself was not a neuroimaging study. Moreover, most functional magnetic resonance imaging (fMRI) studies have focused on alterations in fronto-limbic circuitry in participants with BD-I, excluding those with sub-syndromal BD-NOS.
We sought to address this gap about distinct vs. shared neural mechanisms in BD phenotypes in a cross-sectional imaging study evaluating between-group differences in spontaneous neural resting state functional connectivity (RSFC) comparing young adults followed since childhood by the COBY study with childhood-onset of either a full-syndromal course including manic episodes (BD-I) vs. those with a sub-syndromal course of BD-NOS plus a newly recruited group of healthy controls (HCs). Thus, this study design avoided the potential confound of retrospective recall bias about participants’ BD status.
RSFC can evaluate task-independent neural coactivation in resting state networks (RSNs; Fox and Greicius, 2010), which include sensorimotor, visual, executive control, and default mode networks (DMN; Beckmann et al., 2005), some of which have exhibited RSFC differences in participants with BD (Doucet et al., 2017; Martino et al., 2016; Meda et al., 2012; Ongur et al., 2010; Yip et al., 2014). For our study, we used the innovative Independent Components Analysis (ICA) approach: a multivariate, data-driven method to identify RSFC alterations within networks in BD-I, BD-NOS, and HC participants. ICA derives maximally independent spatial components that represent RSNs common across a sample; these RSNs can then be tested for between-group differences using a dual regression technique (Filippini et al., 2009). Importantly, investigating whole-brain RSFC has differentiated other characteristics of BD (mood state, resilience) and therefore may characterize BD subtypes (Doucet et al., 2017; Martino et al., 2016).
Focusing our analyses on eight well-characterized whole brain RSNs, we used ICA/dual regression analyses to explore whether within-network connectivity differentiated groups. We hypothesized that within-RSN connectivity would differentiate BD-I from BD-NOS, and that findings would be consistent with prior work implicating fronto-limbic circuitry and DMN involvement in the neural pathophysiology of BD (Buckner et al., 2013; Martino et al., 2016; Phillips and Swartz, 2014). Post-hoc analyses evaluated whether regions with significant within-network connectivity covaried with clinical measures.
Methods
Participants
Participants were enrolled in our Institutional Review Board approved study after obtaining informed consent. Participants with BD were originally enrolled in Brown University’s COBY study site. We also enrolled a new group of young adult HCs. See Table 1 for demographic data.
Table 1.
Demographic Data in Participants with Bipolar Disorder I (BD-I), Bipolar Disorder Not Otherwise Specified (BD-NOS), and Healthy Controls (HC)
| BD-I Participants (n=28) | BD-NOS Participants (n=14) | HC (n=52) | Statistic | ||||
|---|---|---|---|---|---|---|---|
| Demographic measures | N | % | N | % | N | % | |
| Male | 18 | 64.3 | 8 | 57.1 | 28 | 53.8 | Pearson χ2=0.81, df=2, p=0.666 |
| Female | 10 | 35.7 | 6 | 42.9 | 24 | 46.2 | |
| White | 23 | 82.1 | 12 | 85.7 | 39 | 75.0 | p = 1.00b |
| Non-white | 5 | 17.9 | 2 | 14.3 | 9a | 17.3 | |
| Mean | SD | Mean | SD | Mean | SD | ||
| Age (years) | 20.62 | 2.40 | 20.89 | 2.90 | 21.50 | 2.01 | F=1.48, df=2, 91 p=0.233 |
| Full-scale IQ | 105.75 | 11.39 | 112.50 | 13.39 | 110.35 | 12.32 | F=1.85, df=2, 91 p=0.163 |
In HC group, there were participants whose race was unknown (n=2; 3.8%) or not reported (n=2; 3.8%).
Fisher’s Exact Test for count data was run to compare white vs. non-white participants to account for cells having less than n=5.
Inclusion criteria for all participants were: ages 18–30, English fluency, and no implanted metal that would prohibit an MRI. Exclusion criteria for all participants were: Wechsler Abbreviated Scale of Intelligence full-scale intelligence quotient (FSIQ) ≤ 70 and color blindness.
We included participants with BD if they met criteria for BD-I or BD-NOS1. BD-I required presence of a full-criteria manic episode (symptom presentation and duration), per DSM-IV, consisting of ≥ 7 days of abnormally elevated, expansive, or irritable mood, and ≥ 3 associated symptoms (if mood was irritable), with significant impairment. BD-NOS required ≥ 1 distinct period(s) of abnormally elevated, expansive or irritable mood, and ≥ 2 associated symptoms (3 if mood was irritable) for ≥ 4 hours within a 24-hour period. The abnormal mood with accompanied symptoms had to represent a change in functioning and occur over four days (consecutive not required).
Exclusion criteria for participants with BD were: autism or primary psychosis diagnoses, or medical/neurological conditions potentially mimicking BD.
Inclusion criteria for HCs were: no history in themselves or first-degree relatives of lifetime psychiatric diagnoses or substance abuse/dependence.
Exclusion criteria for HCs were: learning disorder diagnoses, or serious non-psychiatric medical disorders.
After data collection, we checked for outliers of greater than three standard deviations (SDs) in age and IQ, which resulted in the exclusion of one HC participant exceeding three SDs for age.
Clinical Assessments
Before the MRI visit, participants completed a psychiatric interview (Structured Clinical Interview for DSM-IV; First et al., 2001) with a board-certified psychiatrist or licensed clinical psychologist (inter-rater reliability: κ > 0.85) to determine the presence or absence of current and past psychiatric illness. At the MRI visit, a psychiatrist completed the Young Mania Rating Scale (YMRS; Young et al., 1978) and Hamilton Depression and Anxiety Rating Scales (HAM-D & HAM-A, respectively; Hamilton, 1967; Maier et al., 1988) with participants with BD, and the Global Assessment of Functioning (GAF) with all participants (Table 2). Higher scores indicate a greater amount of what was measured (e.g., depression, functioning ability).
Table 2.
Clinical Data in Participants with Bipolar Disorder I (BD-I), Bipolar Disorder Not Otherwise Specified (BD-NOS), and Healthy Controls (HC)
| BD-I Participants (n=28) | BD-NOS Participants (n=14) | HC (n=52) | Statistic | ||||
|---|---|---|---|---|---|---|---|
| Symptom Measuresa | Mean | SD | Mean | SD | Mean | SD | |
| Young Mania Rating Scale | 4.18 | 3.41 | 3.43 | 2.03 | -- | -- | t=0.76, df=40, p=0.454 |
| Hamilton Depression Rating Scale | 4.32 | 3.93 | 4.50 | 5.93 | -- | -- | t =-0.12, df=40, p=0.908 |
| Hamilton Anxiety Rating Scale | 6.50 | 4.85 | 5.43 | 6.10 | -- | -- | t=0.62, df=40, p=0.539 |
| Global Assessment of Functioningb | 66.36 | 13.13 | 70.11 | 12.30 | 90.02c | 5.42 | F=64.04, df=2, 89 p=0.000 |
| Current Comorbid SCID diagnoses | |||||||
| N | % | N | % | N | % | ||
| Attention deficit/hyperactivity disorder | 17 | 60.7 | 7 | 50.0 | -- | ||
| Generalized anxiety disorder | 4 | 14.3 | 2 | 14.3 | -- | ||
| Panic Disorder | 4 | 14.3 | 3 | 21.4 | -- | ||
| Social Phobia | 0 | 0 | 0 | 0 | -- | ||
| PTSD | 1 | 3.6 | 0 | 0 | -- | ||
| Substance Abuse History | 6 | 21.4 | 2 | 14.3 | |||
| Mood State | |||||||
| Euthymicc | 22 | 78.6 | 12 | 85.7 | -- | ||
| Depressedd | 5 | 17.9 | 2 | 14.3 | -- | ||
| Hypomanice | 1 | 3.6 | -- | -- | -- | ||
| Medications at MRI | |||||||
| No medications | 14 | 50.0 | 9 | 64.3 | |||
| Mood Stabilizer | 1 | 3.6 | 0f | 0 | -- | ||
| Antiepileptic | 6 | 21.4 | 2f | 14.3 | |||
| Antipsychotic | 1 | 3.6 | 0f | 0 | |||
| Atypical antipsychotic | 1 | 3.6 | 1f | 7.1 | -- | ||
| SSRI | 2 | 7.1 | 2f | 14.3 | |||
| Other Antidepressant | 2 | 7.1 | 1f | 7.1 | |||
| Stimulant medications | 9 | 32.1 | 1f | 7.1 | -- | ||
| Sedative | 0 | 0 | 0f | 0 | |||
In majority of cases, symptom assessment occurred on the day of the MRI. When that score was not available, we replaced it with one from within one month for patients (1/42 total), and for the HC GAF, with their baseline visit GAF score (7/52 total).
Based on the post-hoc Games-Howell test, there were significant mean differences between BD-I and HC (p < 0.000, d=2.4), and BD-NOS and HC (p < 0.000, d=2.1).
Defined as YMRS ≤ 12 and HAM-D ≤7.
Defined as YMRS ≤12 and HAM-D ≥8.
Defined as YMRS 13–26 and HAM-D ≤7.
1 report missing within the group.
Neuroimaging
MRI Data Acquisition.
At Brown University’s 3 Tesla Tim Trio MRI scanner, participants had both a resting state functional scan during which they were instructed to relax with their eyes open while remaining motionless (repetition time=2000 ms, echo time=25 ms, flip angle=90°, field of view=192 cm, 3×3×3mm; acquisition time 8.36 minutes) and a high-resolution structural MPRAGE scan (1×1×1mm resolution, acquisition time 7:36 minutes).
MRI Preprocessing.
Image pre-processing, using FMRIB Software Library (“FSL”), included the following steps. First, non-brain material was extracted from each participant’s structural image (Smith, 2002). Next, FSL FMRI Expert Analysis Tool (FEAT, version 6.0) was used to pre-process imaging data with the following settings: brain extraction of the functional image (Smith, 2002), motion correction using FSL MCFLIRT (Jenkinson et al., 2002), spatial smoothing with a Gaussian kernel of FWHM of 6.0mm, grand-mean intensity normalization of the entire 4D dataset by a single multiplicative factor, and registration of the functional image to structural image (full search, 12 degrees of freedom). To identify and remove head motion components for each participant, FSL’s ICA-based Automatic Removal Of Motion Artifacts (ICA-AROMA; Pruim et al., 2015) was run for each participant on their preprocessed functional image. ICA-AROMA generates participant-level components, identifies components contaminated with motion-related artifacts, and removes these components using FSL’s fsl_regfilt linear regression function. This method has been shown empirically to optimize the removal of motion-related artifacts while preserving data and is superior to other motion removal techniques (Parkes et al., 2018). Per FSL recommendation, after motion components were regressed from each participant’s functional image, high pass temporal filtering of 150 (Gaussian-weighted least-squares straight line fitting; sigma=75.0s) was applied to the functional image derived from ICA-AROMA. Lastly, all participants’ functional images were registered to a 2mm MNI152 standard image using FLIRT (Jenkinson et al., 2002; Jenkinson and Smith, 2001).
We excluded participants whose head motion exceeded two SDs of mean framewise displacement (Jenkinson; n=3) resulting in a final sample of 94 participants: n=28 BD-I, n=14 BD-NOS, and n=52 HCs. Participants included in the analyses had uncorrected motion parameters within the limits used by other researchers: root mean square absolute and relative motion were below 1.5 mm and 0.4 mm, respectively (Abram et al., 2017; McNabb et al., 2018). Absolute displacement refers to movement from a fixed position, whereas relative displacement is motion occurring between volumes of data acquisition (Power et al., 2015). The root mean square of relative displacement is recommended as an indicator of motion given that it results in more intractable signal distortions than absolute displacement (Power et al., 2015). We present analyses on group differences in motion in Results. As data were processed and analyzed, there were three steps in which the influence of motion-related artifacts were removed that have been empirically tested to remove the effect of head motion without removing excessive data: (1) FSL’s McFlirt, (2) ICA-AROMA, and (3) dual regression (Filippini et al., 2009; Nickerson et al., 2017; Parkes et al., 2018; Power et al., 2015).
Independent Components Analysis (ICA).
Following standard methods (Filippini et al., 2009; Smith et al., 2014), to identify RSNs after preprocessing, we used Probabilistic Independent Component Analysis (Beckmann and Smith, 2004) via FSL’s MELODIC (Multivariate Exploratory Linear Decomposition into Independent Components, Version 3.15) to derive ICA components from the whole sample. MELODIC preprocessing steps included: masking of non-brain voxels; voxel-wise de-meaning of data; normalization of voxel-wise variance. Next, data were whitened and projected into a 30-dimensional subspace using principal component analysis. The whitened observations were decomposed into sets of vectors that describe signal variation across the temporal domain (time-courses), the session/subject domain, and across the spatial domain (maps) by optimizing for non-Gaussian spatial source distributions using a fixed-point iteration technique (Hyvarinen, 1999). Lastly, estimated component maps were divided by the SD of the residual noise and thresholded by fitting a mixture model to the histogram of intensity values (Beckmann and Smith, 2004).
To identify which of our ICA components represented well-characterized RSNs, we used spatial correlation analyses (FSL’s fslcc function) to correlate our ICA image (30 volumes=30 spatial components) with an image containing eight spatial maps labeled as RSNs, derived by FSL from group-level ICA on 10 participants for use as a network atlas (Beckmann et al., 2005; hereafter referred to as “reference networks”): medial visual, lateral occipital, auditory, sensorimotor, DMN, executive control (ECN), right- and left-lateralized fronto-parietal. This function generated a list of the volumes in our group-ICA image that corresponded at p>0.204 to these eight reference networks (Table 3). Next, for the reference networks that correlated with more than one ICA component, we chose the component with the highest correlation to represent that RSN, plus kept additional ICA components based on visual matching to best approximate the reference RSNs (see Figure 1; Beckmann et al., 2005). The remaining ICA components were not used for further analysis; they generally represent noise in the data (motion, physiological artifacts).
Table 3.
Whole Brain Resting State Networks and Associated ICA Components
| Resting State Networka | ICA Component Numberb | p-value |
|---|---|---|
| Medial visual | 3 | 0.867 |
| Lateral occipital | 5 | 0.501 |
| 23 | 0.581 | |
| Auditory | 14 | 0.627 |
| 19 | 0.206 | |
| Sensorimotor | 6 | 0.704 |
| 21 | 0.385 | |
| Default mode | 1 | 0.225 |
| 8 | 0.688 | |
| Executive control | 10 | 0.511 |
| 15 | 0.325 | |
| Right-lateralized fronto-parietal | 7 | 0.539 |
| Left-lateralized fronto-parietal | 9 | 0.604 |
| 12 | 0.292 |
The name of a network provided in the image containing eight reference networks, available from http://www.fmrib.ox.ac.uk/datasets/royalsoc8/.
The components derived from the present sample’s ICA that correlate at p > 0.204 with the reference networks and that visually match the reference network. Some networks are made up of more than one ICA component.
Figure 1:
Whole-sample ICA Components for each Resting State Networka
a Components representing 8 well-characterized reference networks resulting from our Independent Components Analysis (ICA) in the present sample (n=94) following the example of Reineberg and colleagues (2015). Images shown with z-score values between 4 (red) and 15 (yellow). Coordinates in MNI Space.
Testing Between-Group Differences in RSNs.
Having derived ICA components and identified those representing reference networks, we used FSL’s analytic strategies of dual regression (Filippini et al., 2009; Nickerson et al., 2017) and non-parametric permutation testing (Randomise; Winkler et al., 2014) to determine how the three groups differed in within-network connectivity for planned contrasts using a general linear model inference. In the first two stages using dual regression: 1) group-spatial maps were regressed onto each participant’s functional data to derive time courses; and 2) those time courses were regressed onto the participant’s functional data to derive participant’s individualized spatial maps. These spatial maps indicate for each participant their whole brain voxels’ connectivity with the group-level ICA component representing an RSN, simultaneously controlling for influence from other ICA components (including nuisance effects like motion and physiological signal; Filippini et al., 2009; Smith et al., 2014). In the third stage, to identify brain regions of significant connectivity with RSNs, only the spatial maps for the ICA components of interest (i.e., those matching the reference networks; Table 3) were compared across groups for non-parametric permutation testing consisting of 5000 permutations (Winkler et al., 2014). The outcome is a t-stat image per contrast for each ICA component using alpha <.05, threshold-free cluster enhancement (TFCE; Smith and Nichols, 2009), and family-wise error (FWE) corrected for multiple comparisons across voxels within each component. There were six one-tailed planned contrasts comparing BD-I, BD-NOS, and HCs, including as covariates age (demeaned) and gender.
To determine anatomical regions with significant between-group differences in connectivity with RSNs, for each significant t-stat image per contrast (p< 0.05), we set the threshold for the contrast image at a significance of p<0.05. Then we used FSL cluster to identify the information about these thresholded clusters (location of peak intensity in MNI mm coordinates). Lastly, we used the Harvard-Oxford Cortical and Subcortical Structural atlases provided in FSL to determine anatomical regions that significantly differed between groups at p<0.05 (see Table 4, which displays probabilistic labels, consistent with other work; Liberg et al., 2014; Smith et al., 2014).
Table 4.
Significant Differences in Within-Network Connectivity for Executive Control and Sensorimotor Networks: Maximum Intensity Location Differences Among Bipolar Disorder I (BD-I), Bipolar Disorder Not Otherwise Specified (BD-NOS), and Healthy Control (HC) Groups
| Coordinatesc |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| Resting State Network | Hemisphere | Probabilistic anatomical labela | BAb | Cluster size | x | y | z | Between Group Differencesd | Cohen’s d |
|
| |||||||||
| Executive Control Network | BD-I > HC | ||||||||
| Right | Caudate (43%) | -- | 9 | 18 | −12 | 26 | p=0.044TFCE | 1.0 | |
| Sensorimotor | BD-NOS> HC | ||||||||
| Left | Precentral gyrus (58%) | 6 | 59 | −40 | −12 | 54 | p=0.021TFCE | 1.64 | |
Region determined by Harvard-Oxford Cortical and Subcortical Structural Atlases.
Brodmann Areas (BA) identified from Yale BioImage Suite Package.
Peak intensity reported in MNI mm.
TFCE: threshold-free cluster enhancement.
Post-hoc analyses tested whether regions that significantly differed in their connectivity with RSNs between groups were associated with clinical measures. Using the cluster information from the previous step, we created binary cluster masks, and used fslstats to extract mean values for non-zero voxels from individual participants’ second stage dual regression parameter estimate images (the individualized spatial maps) for each significant result. These extracted values were used for correlations with clinical and cognitive measures, and were also used to derive Cohen’s d effect sizes for each significant between-group difference (see Table 4; Cohen, 1988).
Results
Motion
To test whether groups differed on motion metrics prior to motion correction steps, as measured by root mean square relative motion (Power et al., 2015), we conducted a one-way ANOVA. We found no statistically significant difference among groups, F(2, 91)=2.89, p=0.06. There was a significant difference among groups on root mean square absolute motion, F(2, 91)=3.24, p=0.04. A test of homogeneity of variances was not significant (p=0.07); therefore, we conducted a post-hoc Tukey HSD test to assess differences between motion in participants with BD-I (M=0.33, SD=0.26), BD-NOS (M=0.37, SD=0.29), and HCs (M=0.23, SD=0.17). Post-hoc tests were non-significant (p=0.07), indicating the group means were all part of the same subset. As previously mentioned, relative root mean square framewise displacement is the preferred metric to assess uncorrected motion as it reflects difficult-to-correct signal distortions, and our groups did not differ on that metric.
Independent Components Analysis
We found representation of all eight networks in our data (Table 3; Figure 1). Several networks were comprised of more than one ICA component.
Dual Regression
Two of the eight RSNs contained regions in which groups had significantly different network connectivity strength in gray matter (Table 4 for Cohen’s d effect sizes from extracted parameter estimates). The auditory RSN (IC 14) was significantly different for participants with BD-I vs. HCs; however, the significant region was entirely in white matter, and therefore we do not interpret those findings.
Executive Control Network (ECN; ICA Component 10).
The significant ICA component representing the ECN has the following regions: the frontal pole, bilateral dorsolateral prefrontal cortex (DLPFC), bilateral superior frontal gyrus, anterior cingulate gyrus, paracingulate gyrus, left insula, and bilateral middle frontal gyri. BD-I exhibited significantly greater connectivity with the ECN vs. HCs in the right caudate (Table 4; Figure 2A).
Figure 2:
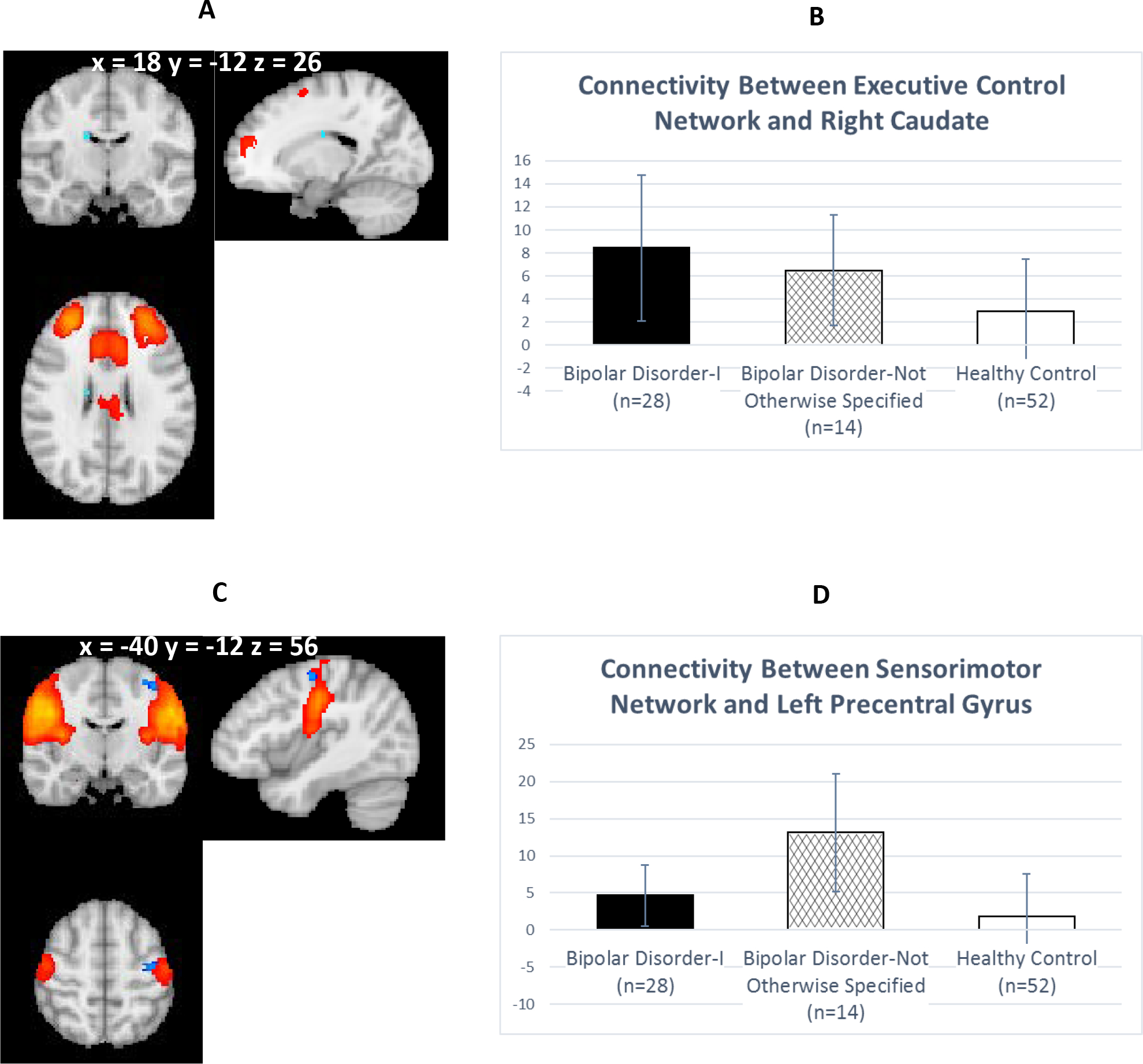
Between-Group Differences in Connectivity in Executive Control and Sensorimotor Networksa
a Panel A displays greater connectivity for Bipolar Disorder I (BD-I) vs. Healthy Controls (HCs) at p < 0.05Threshold Free Cluster Enhancement between the executive control network (red; z-score values ranging between 4 [red] to 15 [yellow]), and the right caudate (light blue; t-stat image overlay at threshold of p < 0.05). Panel B depicts the average parameter estimates by group. BD-I has significantly greater parameter estimates representing the connectivity between the executive control network and the right caudate (t=4.49, df=78, p < 0.001), relative to HCs. Panel C displays greater connectivity for Bipolar Disorder Not Otherwise Specified (BD-NOS) vs. HCs at p < 0.05Threshold Free Cluster Enhancement between sensorimotor network (red; z-score values ranging between 4 [red] to 15 [yellow]) and left precentral gyrus (blue; t-stat image overlay at threshold of p < 0.05). Panel D illustrates the average parameter estimates by group. BD-NOS has significantly greater parameter estimates representing the connectivity between the sensorimotor network and the left precentral gyrus (t=6.05, df=64, p < 0.001), relative to HCs. Coordinates in MNI space.
Sensorimotor (ICA Component 21).
The ICA component representing the sensorimotor network has the following regions: bilateral precentral and postcentral gyri, bilateral central opercular cortex, and right insular cortex. BD-NOS had significantly greater connectivity with the sensorimotor network vs. HC in the left precentral gyrus (Table 4; Figure 2C).
Post-Hoc Analyses: Correlations of results with clinical and cognitive characteristics
We assessed whether any of the above-referenced brain regions exhibiting significantly different connectivity with networks relative to other groups were related to clinical or cognitive characteristics. To do so, we examined correlations between the parameter estimates extracted from each participant’s individualized spatial maps and ratings of depression (HAM-D), mania (YMRS), anxiety (HAM-A), overall functioning (GAF), and intelligence (verbal IQ, performance IQ, and FSIQ). Correlations were only examined for the two groups part of the significant contrast, and were run separately by group.
Executive Control Network (Figure 2A).
Among HC participants, values from the region containing the right caudate were significantly negatively associated with performance IQ (r=−0.42, p=0.002) and FSIQ (r=−0.41, p=0.002).
Sensorimotor Network (Figure 2C).
Among BD-NOS participants, values from the region containing the left precentral gyrus were significantly positively associated with HAM-D (r=0.64, p=0.01) and HAM-A (r=0.64, p=0.01).
Post-Hoc Analyses: Medication and Mood State
To determine whether BD groups differed in number of individuals within categories of mood state and medications, we ran ranked ANOVA tests on mood states, dividing them into three categories (euthymic, depressed, or mania-related [manic, hypomanic, or mixed]), as well as medications reported on the day of the MRI (no medications, one drug, two drugs, or three or more drugs). Kruskal-Wallis H tests indicated no significant differences between patient groups in mood states (χ2[1]=0.34, p=0.56) or reported medications (χ2[1]=0.22, p=0.64) at the MRI. Next, we tested whether mood state or medication affected the RSFC dual regression results. Because most participants with BD were euthymic and not on medication, we used t-tests to investigate between-group differences in the extracted parameter estimates with only euthymic and non-medicated participants (i.e., excluding participants with BD-I and BD-NOS whose mood was not euthymic or who reported any medication use on the day of the MRI). The between-group differences for these analyses with medication and mood state for both RSNs remained significant, indicating that our results were not driven by mood state or medication use at the MRI.
Discussion
In our investigation of RSFC differences among young adults with BD-I and BD-NOS using ICA and dual regression, we identified greater connectivity strength between the ECN and right caudate for full-threshold BD-I, and between the sensorimotor RSN and left precentral gyrus for sub-syndromal BD-NOS, both compared to HCs. Furthermore, for BD-NOS participants, RSFC parameter estimates from the left precentral gyrus—an area significantly stronger in connectivity with the sensorimotor network—were also related to depression and anxiety symptoms, indicating greater sensorimotor connectivity with these regions is associated with symptom severity. While our findings for BD-I participants confirm prior study results characterizing BD as a disorder of fronto-limbic dysregulation (Chen et al., 2011; Vargas et al., 2013), adults with BD-NOS did not have these fronto-limbic connectivity patterns. Instead, BD-NOS participants had greater connectivity in the sensorimotor network, which is in line with other investigations suggesting that the neural substrate of vulnerability to BD may be manifest through alterations in the sensorimotor network (Cassano et al., 2012; Doucet et al., 2017; Stoddard et al., 2016).
Greater connectivity among BD-I vs. HC participants between the ECN and caudate aligns with prior task-dependent and -independent research demonstrating that individuals with BD display neural alterations consistent with difficulty regulating and inhibiting affect, attention, and motor behavior (Chen et al., 2011; Phillips and Swartz, 2014; Stoddard et al., 2016). In another whole-brain, data-driven RSFC investigation, individuals with BD-I and II (ages 10–50) had hyper-connectivity between prefrontal and dorsal striatal regions vs. HCs (Stoddard et al., 2016). Also, in a RSCF investigation of BD-I, unaffected siblings, and HCs using graph theory—which identifies brain connectivity at the global and local level using nodes and edges—the degree to which the right caudate influenced the ECN was greater for both individuals with BD-I and their unaffected siblings, compared to HCs (Doucet et al., 2017). Researchers have also found higher regional brain activity at rest in BD relative to HCs in the right caudate (Liu et al., 2012). A meta-analysis of both task-dependent and –independent studies also found that adults with BD had greater activity in the caudate in task-based studies (e.g., facial affect processing and working memory; Chen et al., 2011). In sum, there is evidence for greater connectivity/activity in fronto-limbic regions generally, and the caudate specifically, for individuals with BD (and unaffected siblings in Doucet et al., 2017) during rest and tasks.
Having situated our results for participants with BD-I within the convergence of findings on fronto-limbic alterations in BD, we briefly discuss why these abnormalities may be more prominent in individuals who have experienced a manic episode. A wealth of data, ranging from structural and functional MRI to behavioral and neuropsychological performance studies, among others, implicate fronto-limbic circuitry underpinning functional impairments in BD-I, especially in executive functioning (e.g., Cotrena et al., 2016; Li et al., 2012; Liu et al., 2010; Maller et al., 2014; Oertel-Knochel et al., 2015; Sweeney et al., 2000; Young et al., 2016). Our finding of a significant inverse relation between ECN-caudate connectivity parameter estimates with intellectual functioning for HCs within this contrast aligns with this, and may be due to the involvement of prefrontal regions, part of the ECN, that are responsible for executive functions such as regulating attention, memory, and decision-making. Additionally, there may be a potential neurotoxic effect of the experience of manic episodes (Abe et al., 2015; Moorhead et al., 2007). To illustrate, BD participants who had a manic episode during a 6-year follow-up period of a longitudinal study (vs. those without manic episode(s) during follow-up) had volume decreases in the DLPFC, which the researchers attributed to the hypothesized neurotoxic effects of a manic episode (Abe et al., 2015). Therefore, our ENC results support prior findings across study design of abnormalities in fronto-limbic circuitry in BD, which by extension may be attributable to the experience of a manic episode, potentially resulting in neural alterations as a compensatory mechanism and/or marker of dysfunction in BD.
While investigations characterizing fronto-limbic alterations in BD tend to dominate the literature, ostensibly due to the resulting functional impairments related to emotion dysregulation and reward-seeking behavior as defining features of BD, other neural networks also may be integral to understanding the whole spectrum of BD. Our findings that participants with BD-NOS had greater sensorimotor network connectivity with the left precentral gyrus than HCs highlights the importance of considering a) how other neural activity beyond cortico-striatal circuitry may contribute to dysregulation, and b) other fundamental neural processes as markers of BD illness. The characterization of BD as a disorder with altered sensory perception and motor activity is well-documented and integrated in diagnostic criteria (Dickstein et al., 2005; Parker, 2014; Parker et al., 2017; Scott et al., 2017); however, the neural substrate is investigated less frequently than other hypotheses of BD, complicating the interpretation of our findings. Drawing on prior research, we offer three explanations for what sensorimotor network differences in individuals with BD-NOS may look like functionally that warrant further study.
First, vulnerability to developing BD (i.e., being at high risk to experience a manic or hypomanic episode either due to having a first-degree relative with the disorder or by meeting criteria for the sub-threshold disorder) may be characterized by abnormal sensorimotor connectivity. Our results appear consistent with the aforementioned RSFC graph theory investigation of vulnerability to BD suggesting brain organization abnormalities: although the sensorimotor network is typically a cohesive self-contained network, it may be both less intra-connected and more abnormally inter-connected to other brain regions in BD (Doucet et al., 2017). Moreover, those researchers also found the degree to which the left precentral gyrus influenced the sensorimotor network was greater for individuals vulnerable to BD compared to HCs. Thinning of cortical grey matter in somatosensory regions was found for high-risk youth offspring of individuals with BD who exhibited psychiatric symptoms compared to those without psychiatric symptoms (Hanford et al., 2016). Further, in the superior longitudinal fasciculus (SLF)—a tract thought to subserve motor control by connecting motor regions that include the precentral gyrus and supplementary motor area—white matter connectivity is altered for children with BD, children at risk for BD (i.e., having a first degree relative with BD), and community adolescents with subthreshold BD symptoms (Frazier et al., 2007; Paillere Martinot et al., 2014).
Second, greater sensorimotor connectivity in the left precentral gyrus among BD-NOS participants vs. controls may represent another unique aspect of the neural mechanisms underlying emotion regulation—separate from that generated by fronto-limbic alterations. In the aforementioned RSFC investigation germane to our ECN results, researchers also found a temporal-parietal network exhibiting hyperconnectivity in BD that contained sensory regions, including the left precentral gyrus (Stoddard et al., 2016). Participants with major depressive disorder have altered, greater RSFC in the sensorimotor network and precentral gyrus, which researchers interpret as difficulty with executive memory related to emotions (Zhi et al., 2018). In another study, individuals with cognitive vulnerability to depression had reduced volume in the left precentral gyrus, which correlated with cognitive distortions that characterize depression (Zhang et al., 2012). Sensorimotor regions are implicated in emotion processing and recognition of facial affect (Adolphs et al., 2000; Phillips et al., 2008; Wood et al., 2016). In fact, in healthy participants, researchers demonstrated that the precentral/Brodmann Area 6 region is related to motor ability specific to recognition of facial expressions (Furl et al., 2010). Difficulties with facial emotion recognition in BD have been well documented (Wegbreit et al., 2014). In the present investigation, we found a positive association between depression and anxiety symptoms and connectivity strength between the sensorimotor network and the left precentral gyrus for participants with BD-NOS. Taken together, these findings suggest that our results of greater sensorimotor and precentral gyrus connectivity in BD-NOS could reflect both a vulnerability to a mood disorder like BD specifically and to aspects of emotional dysregulation in general. Finally, it is possible that contributing to BD-NOS neural connectivity is the longer illness duration and more time spent in subthreshold mood states vs. individuals with BD-I, as prior COBY studies have shown (Birmaher et al., 2009; Birmaher et al., 2006).
Third, greater connectivity between the precentral gyrus, associated with motor functions, and the sensorimotor network for BD-NOS individuals vs. HCs may be related to motor activity dysregulation associated with BD. A meta-analysis on BD motor behavior concluded that BD is characterized by impaired, disorganized motor control, finding that individuals with euthymic or depressed BD overall tend to exhibit less motor activity than HCs, and these motor abnormalities are a feature of BD that is distinct from mood (Scott et al., 2017). Furthermore, mean activity level during mania was not significantly different from HC activity, but may be greater than during depression. Based on prior research one might expect individuals with BD-I, having experienced distinct manic episodes comprised of increased agitation, goal-seeking behavior, and dysregulated activity, would exhibit greater connectivity; however, we did not find that. Rather, in our sample participants with BD-NOS had the highest absolute motion metric prior to removal of motion artifacts. Thus, those with BD-NOS may be uniquely predisposed to more restlessness and motion, but that hypothesis requires further investigation.
Our study had several limitations. First, the BD-NOS sample size was relatively small, potentially impacting post-hoc correlations. We could not add new participants with BD-NOS to our sample because they would have lacked the longitudinal follow-up since childhood that avoids the confound of retrospective recall bias on BD status. However, we calculated Cohen’s d effect size for all significant between-group differences (Table 4). Relatedly, the number of non-white participants in each group, especially BD-NOS, impeded our ability to conduct further analyses on race. However, similar to another recent RSFC study of participants with BD, our groups did not appear to significantly differ in the number of participants who were white vs. non-white (Table 1; Ellard et al., 2018). Second, participants varied in their mood state and medication status at the MRI. We conducted post-hoc exploratory analyses to evaluate potential effects of both mood state and medication on our results and found significant between group differences did not change when analyses were run with only euthymic or only non-medicated participants. It is possible the impact of mood state and medication was on brain regions that were not identified in our RSFC analyses. That investigation is beyond the scope of our study but has been probed by other researchers (e.g., Fleck et al., 2017; Strakowski et al., 2016; Zhang et al., 2018). Third, while we corrected for whole brain voxel-wise multiple comparisons within each analyzed network, we did not correct across networks, which is sometimes done as an additional multiple-comparisons step. However, ICA has been shown to be a statistically conservative method by which to analyze RSFC data (Calhoun and de Lacy, 2017) and our approach is consistent with other investigators (Janes et al., 2012; Reineberg et al., 2015). Lastly, while it is possible that psychosocial treatments may have influenced the participants’ illness course, and thus our ICA findings, we are unable to test that possibility because such data was not collected in our present study. This would be an important area of inquiry for future studies.
In conclusion, our study provides preliminary evidence that young adults with BD-I and BD-NOS exhibit differences in ECN and sensorimotor network connectivity, respectively, compared to HCs. Our investigation did not find networks in which BD-I and BD-NOS were significantly different from each other, nor were any of our findings related to the DMN. Future study is warranted to ascertain the specificity of these neural alterations within a larger sample of adults with BD compared to adults with other illnesses, such as depression. Ultimately, identifying specific vs. shared neural mechanisms of psychopathology is the bedrock on which personalized, precision-medicine diagnostic and treatment strategies can be based, thus reducing impairment for this serious disorder.
Acknowledgements
We are grateful to the young adults for their time and effort participating in this study, without which this research would not be possible. We are also grateful to the COBY Study Team for allowing us to approach their participants for this separate study. We also acknowledge the use of computational resources and services at Brown University’s Center for Computation and Visualization.
Role of the Funding Source
This study was supported by the National Institute of Mental Health grant Bio-Behavioral Research Award for Innovative New Scientist (NIMH BRAINS R01; R01MH087513; Principal Investigator DPD). Young adult participants with bipolar disorder were recruited from Brown University’s site of the Course and Outcome in Bipolar Youth (COBY) study (funded by a separate R01MH059929). Sarah A. Thomas is supported by National Institute of Mental Health grant T32MH078788. The funders had no role in the study design; in the collection, analysis, and interpretation of data; in the writing of the report; or in the decision to submit the paper for publication.
Footnotes
Conflicts of interest: none.
Although several participants with BD-II were included in the COBY longitudinal study (Birmaher et al., 2006), we only enrolled n=3 BD-II participants in our neuroimaging study. Because our analyses focused on comparing full-duration episodes to a subsyndromal course, we did not include BD-II participants because analyses would have been underpowered or confounded by participants who had experienced a hypomanic episode.
References
- Abe C, Ekman CJ, Sellgren C, Petrovic P, Ingvar M, Landen M, 2015. Manic episodes are related to changes in frontal cortex: a longitudinal neuroimaging study of bipolar disorder 1. Brain 138, 3440–3448. 10.1093/brain/awv266 [DOI] [PubMed] [Google Scholar]
- Abram SV, Wisner KM, Fox JM, Barch DM, Wang L, Csernansky JG, MacDonald AW, Smith MJ, 2017. Fronto-Temporal Connectivity Predicts Cognitive Empathy Deficits and Experiential Negative Symptoms in Schizophrenia. Hum. Brain Mapp. 38, 1111–1124. 10.1002/hbm.23439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adolphs R, Damasio H, Tranel D, Cooper G, Damasio AR, 2000. A role for somatosensory cortices in the visual recognition of emotion as revealed by three-dimensional lesion mapping. J. Neurosci. 20, 2683–2690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axelson D, Birmaher B, Strober M, Gill MK, Valeri S, Chiappetta L, Ryan N, Leonard H, Hunt J, Iyengar S, Bridge J, Keller M, 2006. Phenomenology of children and adolescents with bipolar spectrum disorders. Arch. Gen. Psychiatry 63, 1139–1148. 10.1001/archpsyc.63.10.1139 [DOI] [PubMed] [Google Scholar]
- Axelson DA, Birmaher B, Strober MA, Goldstein BI, Ha W, Gill MK, Goldstein TR, Yen S, Hower H, Hunt JI, Liao F, Iyengar S, Dickstein D, Kim E, Ryan ND, Frankel E, Keller MB, 2011. Course of subthreshold bipolar disorder in youth: diagnostic progression from bipolar disorder not otherwise specified. J. Am. Acad. Child Adolesc. Psychiatry 50, 1001–1016 e1003. 10.1016/j.jaac.2011.07.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckmann CF, DeLuca M, Devlin JT, Smith SM, 2005. Investigations into resting-state connectivity using independent component analysis. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 360, 1001–1013. 10.1098/rstb.2005.1634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckmann CF, Smith SM, 2004. Probabilistic independent component analysis for functional magnetic resonance imaging. IEEE Trans. Med. Imaging 23, 137–152. 10.1109/TMI.2003.822821 [DOI] [PubMed] [Google Scholar]
- Birmaher B, Axelson D, Goldstein B, Strober M, Gill MK, Hunt J, Houck P, Ha W, Iyengar S, Kim E, Yen S, Hower H, Esposito-Smythers C, Goldstein T, Ryan N, Keller M, 2009. Four-year longitudinal course of children and adolescents with bipolar spectrum disorders: the Course and Outcome of Bipolar Youth (COBY) study. Am. J. Psychiatry 166, 795–804. 10.1176/appi.ajp.2009.08101569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birmaher B, Axelson D, Strober M, Gill MK, Valeri S, Chiappetta L, Ryan N, Leonard H, Hunt J, Iyengar S, Keller M, 2006. Clinical course of children and adolescents with bipolar spectrum disorders. Arch. Gen. Psychiatry 63, 175–183. 10.1001/archpsyc.63.2.175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner RL, Krienen FM, Yeo BTT, 2013. Opportunities and limitations of intrinsic functional connectivity MRI. Nat. Neurosci. 16, 832–837. 10.1038/nn.3423 [DOI] [PubMed] [Google Scholar]
- Calhoun VD, de Lacy N, 2017. Ten Key Observations on the Analysis of Resting-state Functional MR Imaging Data Using Independent Component Analysis. Neuroimaging Clin. N. Am. 27, 561–+. 10.1016/j.nic.2017.06.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassano GB, Rucci P, Benvenuti A, Miniati M, Calugi S, Maggi L, Pini S, Kupfer DJ, Maj M, Fagiolini A, Frank E, 2012. The Role of Psychomotor Activation in Discriminating Unipolar From Bipolar Disorders: A Classification-Tree Analysis. J. Clin. Psychiat. 73, 22–28. 10.4088/JCP.11m06946 [DOI] [PubMed] [Google Scholar]
- Chen CH, Suckling J, Lennox BR, Ooi C, Bullmore ET, 2011. A quantitative meta-analysis of fMRI studies in bipolar disorder. Bipolar Disord. 13, 1–15. 10.1111/j.1399-5618.2011.00893.x [DOI] [PubMed] [Google Scholar]
- Cohen J, 1988. Statistical power analysis for the behavioral sciences, 2nd ed. Lawrence Earlbaum Associates, Hillsdale, NJ. [Google Scholar]
- Cotrena C, Branco LD, Shansis FM, Fonseca RP, 2016. Executive function impairments in depression and bipolar disorder: association with functional impairment and quality of life. J. Affect. Disord. 190, 744–753. 10.1016/j.jad.2015.11.007 [DOI] [PubMed] [Google Scholar]
- Dickstein DP, Garvey M, Pradella AG, Greenstein DK, Sharp WS, Castellanos FX, Pine DS, Leibenluft E, 2005. Neurologic examination abnormalities in children with bipolar disorder or Attention-deficit/hyperactivity disorder. Biol. Psychiatry 58, 517–524. 10.1016/j.biopsych.2004.12.010 [DOI] [PubMed] [Google Scholar]
- Doucet GE, Bassett DS, Yao NL, Glahn DC, Frangou S, 2017. The Role of Intrinsic Brain Functional Connectivity in Vulnerability and Resilience to Bipolar Disorder. Am. J. Psychiatry 174, 1214–1222. 10.1176/appi.ajp.2017.17010095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellard KK, Gosai AG, Bernstein EE, Kaur N, Sylvia LG, Camprodon JA, Dougherty DD, Nierenberg AA, Deckersbach T, 2018. Intrinsic functional neurocircuitry associated with treatment response to transdiagnostic CBT in bipolar disorder with anxiety. J. Affect. Disord. 238, 383–391. 10.1016/j.jad.2018.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filippini N, MacIntosh BJ, Hough MG, Goodwin GM, Frisoni GB, Smith SM, Matthews PM, Beckmann CF, Mackay CE, 2009. Distinct patterns of brain activity in young carriers of the APOE-epsilon4 allele. Proc. Natl. Acad. Sci. USA 106, 7209–7214. 10.1073/pnas.0811879106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- First M, Spitzer R, Gibbon M, Williams J, 2001. Structured Clinical Interview for the DSM-IV-TR Axis I Disorders. American Psychiatric Press, Washington, DC. [Google Scholar]
- Fleck DE, Ernest N, Adler CM, Cohen K, Eliassen JC, Norris M, Komoroski RA, Chu WJ, Welge JA, Blom TJ, DelBello MP, Strakowski SM, 2017. Prediction of lithium response in first-episode mania using the LITHium Intelligent Agent (LITHIA): Pilot data and proof-of-concept. Bipolar Disord. 19, 259–272. 10.1111/bdi.12507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Greicius M, 2010. Clinical applications of resting state functional connectivity. Front. Syst. Neurosci. 4, 19. 10.3389/fnsys.2010.00019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frazier JA, Breeze JL, Papadimitriou G, Kennedy DN, Hodge SM, Moore CM, Howard JD, Rohan MP, Caviness VS, Makris N, 2007. White matter abnormalities in children with and at risk for bipolar disorder. Bipolar Disord. 9, 799–809. 10.1111/j.1399-5618.2007.00482.x [DOI] [PubMed] [Google Scholar]
- Furl N, van Rijsbergen NJ, Kiebel SJ, Friston KJ, Treves A, Dolan RJ, 2010. Modulation of perception and brain activity by predictable trajectories of facial expressions. Cereb. Cortex 20, 694–703. 10.1093/cercor/bhp140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon JA, 2016. On being a circuit psychiatrist. Nat. Neurosci. 19, 1385–1386. 10.1038/nn.4419 [DOI] [PubMed] [Google Scholar]
- Hamilton M, 1967. Development of a rating scale for primary depressive illness. Br. J. Soc. Clin. Psychol. 6, 278–296. [DOI] [PubMed] [Google Scholar]
- Hanford LC, Sassi RB, Minuzzi L, Hall GB, 2016. Cortical thickness in symptomatic and asymptomatic bipolar offspring. Psychiatry Res. Neuroimaging 251, 26–33. 10.1016/j.pscychresns.2016.04.007 [DOI] [PubMed] [Google Scholar]
- Hyvarinen A, 1999. Fast and robust fixed-point algorithms for independent component analysis. IEEE Trans. Neural Netw. 10, 626–634. 10.1109/72.761722 [DOI] [PubMed] [Google Scholar]
- Janes AC, Nickerson LD, Frederick Bde B, Kaufman MJ, 2012. Prefrontal and limbic resting state brain network functional connectivity differs between nicotine-dependent smokers and non-smoking controls. Drug Alcohol Depend. 125, 252–259. 10.1016/j.drugalcdep.2012.02.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson M, Bannister P, Brady M, Smith S, 2002. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage 17, 825–841. 10.1006/nimg.2002.1132 [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Smith S, 2001. A global optimisation method for robust affine registration of brain images. Med. Image Anal. 5, 143–156. 10.1016/s1361-8415(01)00036-6 [DOI] [PubMed] [Google Scholar]
- Li CT, Hsieh JC, Wang SJ, Yang BH, Bai YM, Lin WC, Lan CC, Su TP, 2012. Differential relations between fronto-limbic metabolism and executive function in patients with remitted bipolar I and bipolar II disorder. Bipolar Disord. 14, 831–842. 10.1111/bdi.12017 [DOI] [PubMed] [Google Scholar]
- Liberg B, Ekman CJ, Sellgren C, Johansson A, Landen M, 2014. Vertex-based morphometry in euthymic bipolar disorder implicates striatal regions involved in psychomotor function. Psychiatry Res. Neuroimaging 221, 173–178. 10.1016/j.pscychresns.2014.01.007 [DOI] [PubMed] [Google Scholar]
- Liu CH, Li F, Li SF, Wang YJ, Tie CL, Wu HY, Zhou Z, Zhang D, Dong J, Yang Z, Wang CY, 2012. Abnormal baseline brain activity in bipolar depression: a resting state functional magnetic resonance imaging study. Psychiatry Res. 203, 175–179. 10.1016/j.pscychresns.2012.02.007 [DOI] [PubMed] [Google Scholar]
- Liu JX, Chen YS, Hsieh JC, Su TP, Yeh TC, Chen LF, 2010. Differences in white matter abnormalities between bipolar I and II disorders. J. Affect. Disord. 127, 309–315. 10.1016/j.jad.2010.05.026 [DOI] [PubMed] [Google Scholar]
- Maier W, Buller R, Philipp M, Heuser I, 1988. The Hamilton Anxiety Scale: reliability, validity and sensitivity to change in anxiety and depressive disorders. J. Affect. Disord. 14, 61–68. [DOI] [PubMed] [Google Scholar]
- Maller JJ, Thaveenthiran P, Thomson RH, McQueen S, Fitzgerald PB, 2014. Volumetric, cortical thickness and white matter integrity alterations in bipolar disorder type I and II. J. Affect. Disord. 169, 118–127. 10.1016/j.jad.2014.08.016 [DOI] [PubMed] [Google Scholar]
- Martino M, Magioncalda P, Huang Z, Conio B, Piaggio N, Duncan NW, Rocchi G, Escelsior A, Marozzi V, Wolff A, Inglese M, Amore M, Northoff G, 2016. Contrasting variability patterns in the default mode and sensorimotor networks balance in bipolar depression and mania. Proc. Natl. Acad. Sci. USA 113, 4824–4829. 10.1073/pnas.1517558113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNabb CB, Sundram F, Soosay I, Kydd RR, Russell BR, 2018. Increased sensorimotor network connectivity associated with clozapine eligibility in people with schizophrenia. Psychiatry Res. Neuroimaging 275, 36–42. 10.1016/j.pscychresns.2018.02.008 [DOI] [PubMed] [Google Scholar]
- Meda SA, Gill A, Stevens MC, Lorenzoni RP, Glahn DC, Calhoun VD, Sweeney JA, Tamminga CA, Keshavan MS, Thaker G, Pearlson GD, 2012. Differences in Resting-State Functional Magnetic Resonance Imaging Functional Network Connectivity Between Schizophrenia and Psychotic Bipolar Probands and Their Unaffected First-Degree Relatives. Biol. Psychiatry 71, 881–889. 10.1016/j.biopsych.2012.01.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moorhead TW, McKirdy J, Sussmann JE, Hall J, Lawrie SM, Johnstone EC, McIntosh AM, 2007. Progressive gray matter loss in patients with bipolar disorder. Biol. Psychiatry 62, 894–900. 10.1016/j.biopsych.2007.03.005 [DOI] [PubMed] [Google Scholar]
- Nickerson LD, Smith SM, Ongur D, Beckmann CF, 2017. Using Dual Regression to Investigate Network Shape and Amplitude in Functional Connectivity Analyses. Front. Neurosci. 11, 115. 10.3389/fnins.2017.00115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oertel-Knochel V, Reuter J, Reinke B, Marbach K, Feddern R, Alves G, Prvulovic D, Linden DEJ, Knochel C, 2015. Association between age of disease-onset, cognitive performance and cortical thickness in bipolar disorders. J. Affect. Disord. 174, 627–635. 10.1016/j.jad.2014.10.060 [DOI] [PubMed] [Google Scholar]
- Ongur D, Lundy M, Greenhouse I, Shinn AK, Menon V, Cohen BM, Renshaw PF, 2010. Default mode network abnormalities in bipolar disorder and schizophrenia. Psychiatry Res. Neuroimaging 183, 59–68. 10.1016/j.pscychresns.2010.04.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paillere Martinot ML, Lemaitre H, Artiges E, Miranda R, Goodman R, Penttila J, Struve M, Fadai T, Kappel V, Poustka L, Conrod P, Banaschewski T, Barbot A, Barker GJ, Buchel C, Flor H, Gallinat J, Garavan H, Heinz A, Ittermann B, Lawrence C, Loth E, Mann K, Paus T, Pausova Z, Rietschel M, Robbins TW, Smolka MN, Schumann G, Martinot JL, consortium I, 2014. White-matter microstructure and gray-matter volumes in adolescents with subthreshold bipolar symptoms. Mol. Psychiatry 19, 462–470. 10.1038/mp.2013.44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker G, 2014. The Suprasensory World of Bipolar II Disorder. Am. J. Psychiatry 171, 614–615. 10.1176/appi.ajp.2014.13121570 [DOI] [PubMed] [Google Scholar]
- Parker G, Paterson A, Romano M, 2017. Altered Sensory Phenomena Experienced in Bipolar Disorder. Am. J. Psychiatry 174, 1146–1150. 10.1176/appi.ajp.2017.16121379 [DOI] [PubMed] [Google Scholar]
- Parkes L, Fulcher B, Yucel M, Fornito A, 2018. An evaluation of the efficacy, reliability, and sensitivity of motion correction strategies for resting-state functional MRI. Neuroimage 171, 415–436. 10.1016/j.neuroimage.2017.12.073 [DOI] [PubMed] [Google Scholar]
- Peele PB, Xu Y, Kupfer DJ, 2003. Insurance expenditures on bipolar disorder: Clinical and parity implications. Am. J. Psychiatry 160, 1286–1290. 10.1176/appi.ajp.160.7.1286 [DOI] [PubMed] [Google Scholar]
- Phillips M, Ladouceur C, Drevets W, 2008. A neural model of voluntary and automatic emotion regulation: implications for understanding the pathophysiology and neurodevelopment of bipolar disorder. Mol. Psychiatry 13, 833–857. 10.1038/mp.2008.65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips ML, Swartz HA, 2014. A Critical Appraisal of Neuroimaging Studies of Bipolar Disorder: Toward a New Conceptualization of Underlying Neural Circuitry and a Road Map for Future Research. Am. J. Psychiatry 171, 829–843. 10.1176/appi.ajp.2014.13081008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power JD, Schlaggar BL, Petersen SE, 2015. Recent progress and outstanding issues in motion correction in resting state fMRI. Neuroimage 105, 536–551. 10.1016/j.neuroimage.2014.10.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruim RHR, Mennes M, Buitelaar JK, Beckmann CF, 2015. Evaluation of ICA-AROMA and alternative strategies for motion artifact removal in resting state fMRI. Neuroimage 112, 278–287. 10.1016/j.neuroimage.2015.02.063 [DOI] [PubMed] [Google Scholar]
- Reineberg AE, Andrews-Hanna JR, Depue BE, Friedman NP, Banich MT, 2015. Resting-state networks predict individual differences in common and specific aspects of executive function. Neuroimage 104, 69–78. 10.1016/j.neuroimage.2014.09.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott J, Murray G, Henry C, Morken G, Scott E, Angst J, Merikangas KR, Hickie IB, 2017. Activation in Bipolar Disorders A Systematic Review. JAMA Psychiatry 74, 189–196. 10.1001/jamapsychiatry.2016.3459 [DOI] [PubMed] [Google Scholar]
- Smith DV, Utevsky AV, Bland AR, Clement N, Clithero JA, Harsch AE, McKell Carter R, Huettel SA, 2014. Characterizing individual differences in functional connectivity using dual-regression and seed-based approaches. Neuroimage 95, 1–12. 10.1016/j.neuroimage.2014.03.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, 2002. Fast robust automated brain extraction. Hum. Brain Mapp. 17, 143–155. 10.1002/hbm.10062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Nichols TE, 2009. Threshold-free cluster enhancement: addressing problems of smoothing, threshold dependence and localisation in cluster inference. Neuroimage 44, 83–98. 10.1016/j.neuroimage.2008.03.061 [DOI] [PubMed] [Google Scholar]
- Stoddard J, Gotts SJ, Brotman MA, Lever S, Hsu D, Zarate C, Ernst M, Pine DS, Leibenluft E, 2016. Aberrant intrinsic functional connectivity within and between corticostriatal and temporal-parietal networks in adults and youth with bipolar disorder. Psychol. Med. 46, 1509–1522. 10.1017/s0033291716000143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strakowski SM, Fleck DE, Welge J, Eliassen JC, Norris M, Durling M, Komoroski RA, Chu WJ, Weber W, Dudley JA, Blom TJ, Stover A, Klein C, Strawn JR, DelBello MP, Lee JH, Adler CM, 2016. fMRI brain activation changes following treatment of a first bipolar manic episode. Bipolar Disord. 18, 490–501. 10.1111/bdi.12426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweeney JA, Kmiec JA, Kupfer DJ, 2000. Neuropsychologic impairments in bipolar and unipolar mood disorders on the CANTAB neurocognitive battery. Biol. Psychiatry 48, 674–684. [DOI] [PubMed] [Google Scholar]
- Vargas C, Lopez-Jaramillo C, Vieta E, 2013. A systematic literature review of resting state network--functional MRI in bipolar disorder. J. Affect. Disord. 150, 727–735. 10.1016/j.jad.2013.05.083 [DOI] [PubMed] [Google Scholar]
- Wegbreit E, Cushman GK, Puzia ME, Weissman AB, Kim KL, Laird AR, Dickstein DP, 2014. Developmental meta-analyses of the functional neural correlates of bipolar disorder. JAMA Psychiatry 71, 926–935. 10.1001/jamapsychiatry.2014.660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler AM, Ridgway GR, Webster MA, Smith SM, Nichols TE, 2014. Permutation inference for the general linear model. Neuroimage 92, 381–397. 10.1016/j.neuroimage.2014.01.060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood A, Rychlowska M, Korb S, Niedenthal P, 2016. Fashioning the Face: Sensorimotor Simulation Contributes to Facial Expression Recognition. Trends in Cogn. Sci. 20, 227–240. 10.1016/j.tics.2015.12.010 [DOI] [PubMed] [Google Scholar]
- Yip SW, Mackay CE, Goodwin GM, 2014. Increased temporo-insular engagement in unmedicated bipolar II disorder: an exploratory resting state study using independent component analysis. Bipolar Disord. 16, 748–755. 10.1111/bdi.12206 [DOI] [PubMed] [Google Scholar]
- Young KD, Bodurka J, Drevets WC, 2016. Differential neural correlates of autobiographical memory recall in bipolar and unipolar depression. Bipolar Disord. 18, 571–582. 10.1111/bdi.12441 [DOI] [PubMed] [Google Scholar]
- Young R, Biggs J, Ziegler V, Meyer D, 1978. A rating scale for mania: reliability, validity and sensitivity. Br. J. Psychiatry 133, 429–435. [DOI] [PubMed] [Google Scholar]
- Zhang W, Xiao Y, Sun H, Patino LR, Tallman MJ, Weber WA, Adler CM, Klein C, Strawn JR, Nery FG, Gong Q, Sweeney JA, Lui S, DelBello MP, 2018. Discrete patterns of cortical thickness in youth with bipolar disorder differentially predict treatment response to quetiapine but not lithium. Neuropsychopharmacology 43, 2256–2263. 10.1038/s41386-018-0120-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Yao S, Zhu X, Wang X, Zhu X, Zhong M, 2012. Gray matter volume abnormalities in individuals with cognitive vulnerability to depression: a voxel-based morphometry study. J. Affect. Disord. 136, 443–452. 10.1016/j.jad.2011.11.005 [DOI] [PubMed] [Google Scholar]
- Zhi D, Calhoun VD, Lv L, Ma X, Ke Q, Fu Z, Du Y, Yang Y, Yang X, Pan M, Qi S, Jiang R, Yu Q, Sui J, 2018. Aberrant Dynamic Functional Network Connectivity and Graph Properties in Major Depressive Disorder. Front. Psychiatry 9, 339. 10.3389/fpsyt.2018.00339 [DOI] [PMC free article] [PubMed] [Google Scholar]



