Abstract
1. INTRODUCTION
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) responsible for the coronavirus disease 2019 (COVID-19) continues to cause considerable morbidity and mortality worldwide. Different personalized responses among patients have been widely demonstrated, with some individuals developing rapid deterioration, while others having mild symptoms. One of the factors accounting for the prominent differences in the severity of symptoms and mortality appears to be biological sex. In fact, early on in the pandemic, it was noted that infection rates, complications, and crude fatality rates associated with COVID-19 showed a pronounced sex bias. However, due to the overall lack of sex-disaggregated data, definitive conclusions cannot be widely reached.
2. SEX-SPECIFIC CONDITIONS AND COMORBIDITIES
On one hand, male COVID-19 patients appear to be at higher risk of more severe outcomes and mortality than female patients (1). On the other hand, conditions unique to women, such as pregnancy, birth, and breastfeeding, may make female patients more vulnerable. Along this line, a remarkable review article published by Wastnedge et al. (2) in Physiological Reviews as part of the COVID-19 mini-reviews collection pointed out that the risk of severe COVID-19 disease in pregnancy may be higher than that in the general population. This could be due to elevated plasmin(ogen) levels in pregnant women cleaving the S protein of SARS-CoV-2, thereby enhancing the virulence and infectivity of the virus, as elegantly discussed by Ji et al. (3) in another review article published in the aforementioned collection of Physiological Reviews focusing on plasmin(ogen) as a common risk factor for COVID-19 susceptibility.
In addition, considering underlying diseases, a significant proportion of COVID-19 patients has cardiac and cardiometabolic disorders. Along this line, early on, it was highlighted that patients with cardiovascular comorbidities suffer more from COVID-19 having a worse clinical outcome and are at higher risk of death (3). Therefore, a lot of effort has focused on understanding and predicting the outcome of SARS-CoV-2 infection in patients with prior cardiovascular disease. In this context, the identification of sex-based biomarkers for prediction of hospitalization and severity of disease or death in COVID-19 patients is of great relevance and would contribute to a more personalized medical care.
3. CARDIOVASCULAR COMPLICATIONS
The effects of SARS-CoV-2 on the cardiovascular system, direct or indirect as discussed in the following section, and how these may be influenced by biological sex remain poorly understood. A considerable proportion of COVID-19 patients suffers cardiovascular damage, and acute myocardial injury evidenced by elevated cardiac biomarkers, such as increased troponin T and N-terminal pro-brain natriuretic protein levels, is associated with increased cardiovascular symptoms, including cardiac dysfunction and arrhythmias. Nevertheless, other cardiovascular complications are not frequently diagnosed during hospitalization, and despite the indication of myocardial ischemia, acute occlusion of the coronary vasculature seems relatively uncommon. Even so, in early reports from the US, the majority of COVID-19 patients presenting myocardial injury with ST-segment elevation and obstructive coronary artery disease were male. This follows the typical pattern of ischemic heart disease, where men present more frequently with obstructive coronary artery disease, while women exhibit more frequently a nonobstructive coronary artery disease or microvascular dysfunction. To add to the complexity, the clinical manifestation of acute complications may differ from that following months after infection, since long-term effects on the cardiovascular system are currently unclear. To this extent, a significant proportion of COVID-19 survivors present with chest pain several months after infection, which raises the possibility that this might be due to new incident of cardiovascular disease associated with SARS-CoV-2 infection. Whether one sex is more prone than the other is yet to be determined. Further research is warranted.
4. SEX-BIASED PATHOPHYSIOLOGICAL MECHANISMS OF SARS-COV-2 INFECTION IN THE CARDIOVASCULAR SYSTEM
4.1. Viral Entry and Localization
SARS-CoV-2 uses angiotensin-converting enzyme 2 (ACE2) as a host cell entry receptor (4), the expression of which differs between the sexes. Nevertheless, as we are in the beginning of understanding the pathophysiology underlying the disease, the role of ACE2 in cardiovascular complications of SARS-CoV-2 infection is incompletely understood and how circulating and tissue concentrations of ACE2 could influence SARS-CoV-2 susceptibility and virulence in the heart is currently unclear (5). Since ACE2 is expressed in the heart, including cardiac myocytes, fibroblasts, and endothelial cells, SARS-CoV-2 is considered a cadiotropic virus. Given the impact of sex and 17β-estradiol on the renin-angiotensin-aldosterone system (6–8), a plausible hypothesis has been that increased ACE2 levels in male cardiac cells may facilitate SARS-CoV-2 entry and virulence. Consequently, direct myocardial infection of SARS-CoV-2 would lead to a higher proportion of male patients presenting with myocardial complications, including an acute form of viral myocarditis. However, autopsy studies have not revealed clear results and the literature presently lacks data confirming viral invasion in the heart, with the current view being that myocardial localization of SARS-CoV-2 is primarily due to infected immune cells invading the myocardial tissue. This highlights the notion that systemic inflammation due to SARS-CoV-2 infection may impact the cardiovascular system in a sex-dependent manner through indirect mechanisms (FIGURE 1), linked to the underlying pathophysiology of the disease. This would not be surprising, as sex differences in immune responses have been well characterized and sex-biased regulation of inflammatory factors has been previously reported in cardiovascular patients (9, 10). In this context, systemic inflammation may lead to instability and rupture of atherosclerotic plaques, hence male COVID-19 patients may be more susceptible to myocardial infarction in this setting.
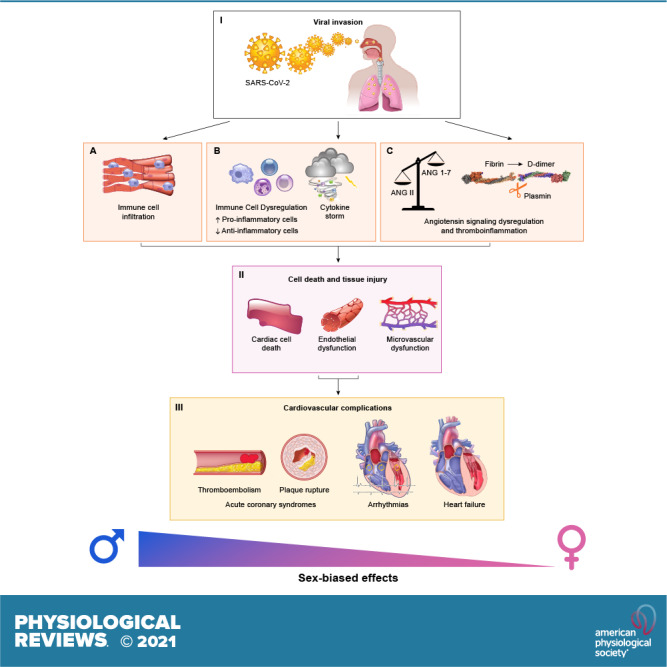
FIGURE 1.
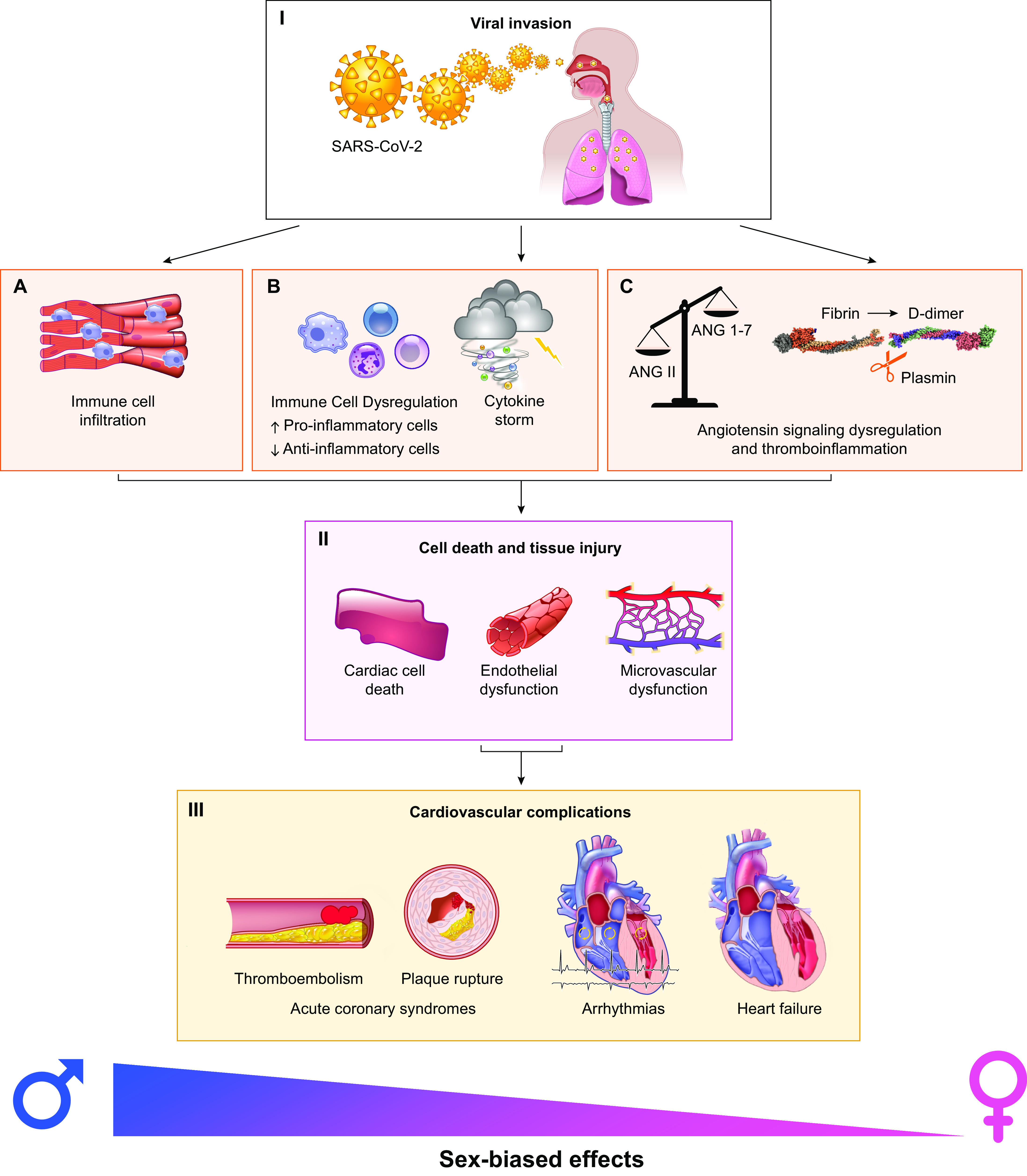
Sex-biased mechanisms of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection in the cardiovascular system. Following entry (I), SARS-CoV-2 is expected to be cadiotropic. However, the current view is that its myocardial localization is primarily due to infected immune cells invading the myocardial tissue (A). The host responds with systemic inflammation and cytokine storm (B), which contribute to cardiac and vascular injury (II). Dysregulation of angiotensin signaling, thromboinflammation and plasmin-associated hyperactive fibrinolysis-induced D-dimer increases (C) result in pronounced cell death and tissue injury, including microvascular dysfunction (II). Ultimately, these pathophysiological mechanisms modulated by sex lead to major cardiovascular complications (III) in a sex-dependent manner.
4.2. Systemic Inflammation
Respiratory failure-induced hypoxia and inflammation in airways lead to systemic inflammation and cytokine storm in the third phase of the disease (hyperinflammation phase), as elegantly explained in the second review article published by Romagnoli et al. (11) in the COVID-19 mini-reviews collection of Physiological Reviews. This host inflammatory response is a major contributor to cardiac and vascular injury. Several types of immune cells, including T cells and neutrophils, produce proinflammatory cytokines, which, in turn, attract further inflammatory cells, such as monocytes producing high levels of interleukin-6, for example, thereby contributing further to the inflammatory response. Consequently, cardiomyocyte function may be affected, including altered intracellular homeostasis, thereby contributing to cell death and extensive tissue damage, ultimately leading to reduced cardiac function and heart failure. Therefore, immune dysregulation plays a major role in target organ damage and failure. In this context, excessive systemic inflammation resulting from dysregulated host T cell-mediated immune responses has been associated with adverse clinical outcomes in patients with COVID-19 (12, 13). However, reports on differences between male and female COVID-19 patients in the levels and activity of T cells in SARS-CoV-2 infection are currently limited. Given the impact of sex and 17β-estradiol on T-cell phenotypes and function, a plausible hypothesis would be that a decreased ability of male COVID-19 patients to reduce this hyper-inflammatory response may contribute to more severe outcomes than in female COVID-19 patients. In support of this notion, it was reported that female COVID-19 patients have more abundant activated and terminally differentiated T-cell populations than male patients and that a poor T cell response is associated with worse disease outcome in male patients only (14). Additional studies and larger populations are needed to investigate this further.
4.3. Dysregulation of Angiotensin Signaling and Thromboinflammation
Various thrombotic complications have also been reported in COVID-19 patients, which indicate substantial endothelial injury and microvascular permeability. However, inflammatory activation of the coagulation system may also serve the purpose of containing the infection. A comprehensive review of literature recently published by Sriram et al. (15) in Physiological Reviews reports that the interaction of SARS-CoV-2 with ACE2, resulting in dysregulation of angiotensin signaling, increases thrombin-mediated and purinergic-mediated activation of platelets and exerts pathological effects on other cell types, e.g., endothelial cells, epithelial cells, and fibroblasts, ultimately further enhancing inflammation (15). This thromboinflammation has systemic effects resulting in pronounced cell death and tissue injury. Given the impact of sex and 17β-estradiol on the renin-angiotensin-aldosterone system (6–8), an abrogated dysregulation of angiotensin signaling with a particular increase in angiotensin 1–7 signaling would be linked to lower cardiovascular risk in female patients. On the other hand, aberrant activation leading to altered coagulation and coagulopathy may result in the occurrence of thrombotic microangiopathy in male patients. In particular, the significant increase in D-dimer levels of COVID-19 patients may lead to microvascular dysfunction, coronary thrombosis or embolism. Mechanistically, the mini-review on plasmin(ogen) preconditioning COVID-19 published in Physiological Reviews reported that extremely increased D-dimer in COVID-19 patients results from plasmin-associated hyperactive fibrinolysis (3). Interestingly, a retrospective case series with sex-stratified data and analyses reported that elevated D-dimer is an independent predictor of intensive care unit admission, invasive mechanical ventilation and in-hospital death in men only, suggesting that D-dimer could be a greater predictor of lethal COVID-19 coagulopathy in men than in women (16).
5. CONCLUDING REMARKS
The role of biological sex has yet underestimated consequences for the pathophysiology of SARS-CoV-2 infection. Sex-biased collateral cardiovascular damage associated with SARS-CoV-2 infection represents important biological phenomena that need further investigation, thereby highlighting the importance of understanding the underlying mechanisms. Similarly, the identification of novel sex-based biomarkers may help to identify those patients at higher risk, thereby preventing future and long-term consequences of the disease in a more personalized manner. For this purpose, prospective, large studies for long-term follow-up with sufficient participation of female patients are needed.
GRANTS
The author acknowledges laboratory support provided by grants from the European Union (101024324), Icelandic Research Fund (217946-051), Icelandic Cancer Society Research Fund, and University of Iceland Research Fund (1237-1232583).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author.
AUTHOR CONTRIBUTIONS
G.K. conceived and designed editorial; G.K. prepared figures; G.K. drafted manuscript; G.K. edited and revised manuscript; G.K. approved final version of manuscript.
REFERENCES
- 1.Peckha H, de Gruijter NM, Raine C, Radziszewska A, Ciurtin C, Wedderburn LR, Rosser EC, Webb K, Deakin CT. Male sex identified by global COVID-19 meta-analysis as a risk factor for death and ITU admission. Nat Commun 11: 6317, 2020. doi: 10.1038/s41467-020-19741-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wastnedge EA, Reynolds RM, van Boeckel SR, Stock SJ, Denison FC, Maybin JA, Critchley HO. Pregnancy and COVID-19. Physiol Rev 101: 303–318, 2021. doi: 10.1152/physrev.00024.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ji HL, Zhao R, Matalon S, Matthay MA. Elevated plasmin(ogen) as a common risk factor for COVID-19 susceptibility. Physiol Rev 100: 1065–1075, 2020. doi: 10.1152/physrev.00013.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hoffmann M, Kleine-Weber H, Schroeder S, Kruger N, Herrler T, Erichsen S, Schiergens TS, Herrler G, Wu NH, Nitsche A, Muller MA, Drosten C, Pohlmann S. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell 181: 271–280, 2020. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ritter O, Kararigas G. Sex-biased vulnerability of the heart to COVID-19. Mayo Clin Proc 95: 2332–2335, 2020. doi: 10.1016/j.mayocp.2020.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Al-Lami RA, Urban RJ, Volpi E, Algburi AM, Baillargeon J. Sex hormones and novel corona virus infectious disease (COVID-19). Mayo Clin Proc 95: 1710–1714, 2020. doi: 10.1016/j.mayocp.2020.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sabbatini AR, Kararigas G. Estrogen-related mechanisms in sex differences of hypertension and target organ damage. Biol Sex Differ 11: 31, 2020. doi: 10.1186/s13293-020-00306-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sabbatini AR, Karariga G. Menopause-related estrogen decrease and the pathogenesis of HFpEF: JACC review topic of the week. J Am Coll Cardiol 75: 1074–1082, 2020. doi: 10.1016/j.jacc.2019.12.049. [DOI] [PubMed] [Google Scholar]
- 9.Gaignebet L, Kańduła MM, Lehmann D, Knosalla C, Kreil DP, Kararigas G. Sex-specific human cardiomyocyte gene regulation in left ventricular pressure overload. Mayo Clin Proc 95: 688–697, 2020. doi: 10.1016/j.mayocp.2019.11.026. [DOI] [PubMed] [Google Scholar]
- 10.Kararigas G, Dworatzek E, Petrov G, Summer H, Schulze TM, Baczko I, Knosalla C, Golz S, Hetzer R, Regitz-Zagrosek V. Sex-dependent regulation of fibrosis and inflammation in human left ventricular remodelling under pressure overload. Eur J Heart Fail 16: 1160–1167, 2014. doi: 10.1002/ejhf.171. [DOI] [PubMed] [Google Scholar]
- 11.Romagnoli S, Peris A, De Gaudio AR, Geppetti P. SARS-CoV-2 and COVID-19: from the bench to the bedside. Physiol Rev 100: 1455–1466, 2020. doi: 10.1152/physrev.00020.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Laing AG, Lorenc A, Del Molino Del Barrio I, Das A, Fish M, Monin L, et al. A dynamic COVID-19 immune signature includes associations with poor prognosis. Nat Med 26: 1623–1635, 2020. doi: 10.1038/s41591-020-1038-6. [DOI] [PubMed] [Google Scholar]
- 13.Mathew D, Giles JR, Baxter AE, Oldridge DA, Greenplate AR, Wu JE, The UPenn COVID Processing Unit, et al. Deep immune profiling of COVID-19 patients reveals distinct immunotypes with therapeutic implications. Science 369: eabc8511, 2020. doi: 10.1126/science.abc8511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Takahashi T, Ellingson MK, Wong P, Israelow B, Lucas C, Klein J, Yale IMPACT Research Team, et al. Sex differences in immune responses that underlie COVID-19 disease outcomes. Nature 588: 315–320, 2020. doi: 10.1038/s41586-020-2700-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sriram K, Insel PA. Inflammation and thrombosis in COVID-19 pathophysiology: proteinase-activated and purinergic receptors as drivers and candidate therapeutic targets. Physiol Rev 101: 545–567, 2021. doi: 10.1152/physrev.00035.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yoshida Y, Gillet SA, Brown MI, Zu Y, Wilson SM, Ahmed SJ, Tirumalasetty S, Lovre D, Krousel-Wood M, Denson JL, Mauvais-Jarvis F. Clinical characteristics and outcomes in women and men hospitalized for coronavirus disease 2019 in New Orleans. Biol Sex Differ 12: 20, 2021. doi: 10.1186/s13293-021-00359-2. [DOI] [PMC free article] [PubMed] [Google Scholar]


