Visual Abstract
Keywords: infectious disease, PET/CT, respiratory, 18F-FDG, COVID-19, PET/CT
Abstract
The aim of this study was to assess the temporal evolution of pulmonary 18F-FDG uptake in patients with coronavirus disease 2019 (COVID-19) and post–COVID-19 lung disease (PCLD). Methods: Using our hospital’s clinical electronic records, we retrospectively identified 23 acute COVID-19, 18 PCLD, and 9 completely recovered 18F-FDG PET/CT patients during the 2 peaks of the U.K. pandemic. Pulmonary 18F-FDG uptake was measured as a lung target-to-background ratio (TBRlung = SUVmax/SUVmin) and compared with temporal stage. Results: In acute COVID-19, less than 3 wk after infection, TBRlung was strongly correlated with time after infection (rs = 0.81, P < 0.001) and was significantly higher in the late stage than in the early stage (P = 0.001). In PCLD, TBRlung was lower in patients treated with high-dose steroids (P = 0.003) and in asymptomatic patients (P < 0.001). Conclusion: Pulmonary 18F-FDG uptake in COVID-19 increases with time after infection. In PCLD, pulmonary 18F-FDG uptake rises despite viral clearance, suggesting ongoing inflammation. There was lower pulmonary 18F-FDG uptake in PCLD patients treated with steroids.
Throughout the United Kingdom, during February and March 2020 there was a rapid spread of coronavirus disease 2019 (COVID-19), which may result in viral pneumonitis and acute respiratory distress syndrome (1). The median time from symptom onset to intensive care admission was 10 d, although only 5% of patients were admitted (1). This is when antiviral responses are at a peak, suggesting that pneumonitis is a consequence of adaptive immunity (2).
Persistent respiratory symptoms affect at least one third of hospitalized COVID-19 patients, some of whom will have post–COVID-19 lung disease (PCLD) (3). Steroids are critical in reducing mortality from COVID-19, but their role in PCLD is less clear, and identifying those who might benefit may be difficult.
Currently, 18F-FDG PET/CT has no role in the management of patients with COVID-19 (4), and there has been little investigation into the quantification and evolution of 18F-FDG uptake in COVID-19 (Supplemental Table 1; supplemental materials are available at http://jnm.snmjournals.org). Given the growing role of 18F-FDG PET/CT in interstitial lung diseases, the primary aim of this preliminary study was to assess the temporal evolution of 18F-FDG uptake in COVID-19 and to correlate this evolution with clinical progression and recovery. A secondary aim was to investigate whether steroids could alter this evolution.
MATERIALS AND METHODS
The Institutional Review Board approved this retrospective study and waived the requirement to obtain informed consent. The challenges of the pandemic constrained the methodologic design, necessitating a retrospective approach.
Patient Selection
All studies performed in the department over the first U.K. peak of the coronavirus pandemic (March–April 2020) and from September 2020 to February 2021 (second peak) were assessed for acute COVID-19 by following the British Society of Thoracic Imaging guidelines or a confirmed history of COVID-19 in the electronic health record system (5). These studies included some of patients without positive polymerase chain reaction (PCR) test results, because of the poor availability of PCR tests in the early period. Also included were studies performed for persistent (>4 wk) respiratory symptoms, in keeping with PCLD, and studies of patients who had recovered from COVID-19 after the initial period. Ongoing treatment with steroids and other immunosuppressive drugs was recorded. Formal lung function tests were not performed because of infection risks. Acute studies between May and September 2020 were not examined because of the low prevalence and incidence of COVID-19 in London during that time (Supplemental Fig. 1; Supplemental Table 2).
18F-FDG PET/CT Imaging Protocol
Patients fasted for at least 6 h, and blood glucose levels were recorded before injection of 400 MBq of 18F-FDG adjusted for weight in keeping with the guidelines of the Administration of Radioactive Substances Advisory Committee (6). After an uptake time of 63.1 ± 10.9 min, whole-body PET scans of supine patients with their arms above their head were acquired at a rate of 2 min per bed position using a GE Healthcare Discovery 710 PET/CT scanner. A nonenhanced low-dose CT scan was acquired for anatomic coregistration and attenuation correction. Images were reconstructed using a resolution recovery iterative algorithm.
All images were reviewed by at least one dually accredited radiologist–nuclear medicine physician. Quantification was performed by investigators with at least 10 y of experience in quantifying PET/CT images of diffuse lung disease. PET analysis was performed with masking of clinical history and CT analysis.
Determination of Temporal Stage
After review of the clinical, CT, and electronic health records, the number of days since disease onset was estimated, and the acute COVID-19 cases were assigned to 1 of 2 temporal groups: early or late COVID-19 (7). Early COVID-19 (approximately ≤1 wk after disease onset) was defined predominantly as CT findings of ground-glass opacities with or without associated interlobular thickening. Late COVID-19 (>1 wk to ≤4 wk after disease onset) was defined as CT findings of increasing consolidation and signs of resolution marked by subpleural sparing, development of a fibrous stripe, and crescentic consolidation or a reversed halo or atoll sign. Patients who were asymptomatic after 28 d were classed as recovered patients. In addition, patients who were imaged because of persistent symptoms after 28 d were described as having PCLD. The CT component was correlated with other cross-sectional images to reduce the likelihood of incorrect classification due to breathing artifacts.
Quantitative 18F-FDG PET Analysis
All images were processed using a standard protocol on a dedicated imaging workstation (ADW Volume Viewer, version 4.6; GE Healthcare), which calculated the lung target-to-background ratio (TBRlung = SUVmax/SUVmins) following methods described previously (8–10).
Statistics
The difference in 18F-FDG PET uptake measures within the lung against temporal staging and pretreatment with steroids were assessed using the nonparametric Mann–Whitney test. Results were depicted using box-and-whisker plots. All statistical analyses were performed using SPSS, version 25.0 (IBM).
RESULTS
Of the 3,112 18F-FDG PET/CT studies screened, 50 met the criteria for study entry, including 18 patients referred for 18F-FDG PET/CT for investigation of PCLD. Of these 50 patients (median age, 61 y; range, 18–87 y), 32 were male (64%), 27 were of ethnic minority background (54%), and 23 (46%) had acute COVID-19. None were intentionally imaged for COVID-19. Nine patients had asymptomatic recovered COVID-19 as confirmed by the electronic health record system (Supplemental Tables 3–5).
In 18 of the 50 patients, imaging was performed because of persistent shortness of breath and respiratory symptoms in keeping with PCLD. All 18 had been admitted to the hospital and had required oxygen. Fifteen of these patients previously had PCR tests positive for COVID-19, and COVID-19 was clinically diagnosed in the others. Nine had ongoing treatment with steroids for PCLD; the other 9 were not receiving treatment for their PCLD. All PCLD patients underwent repeated PCR testing confirming that they were PCR-negative before PET imaging (Supplemental Table 5).
Temporal Stage
After review of the CT component of the PET/CT (lung windows) and available clinical history, 8 (35%) of the 23 acute COVID-19 patients were determined to represent early COVID-19 and 15 (65%) late (Fig. 1; Supplemental Table 5).
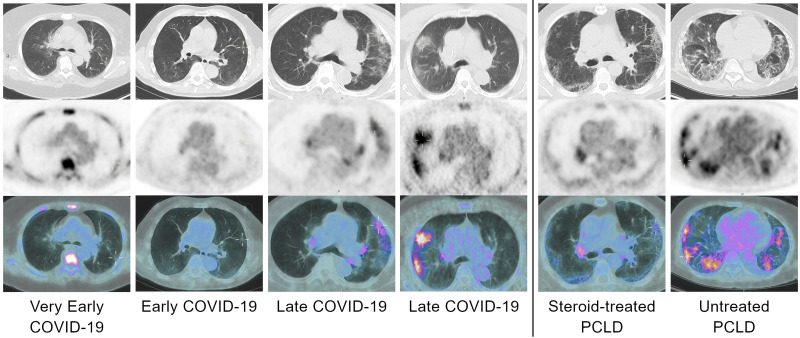
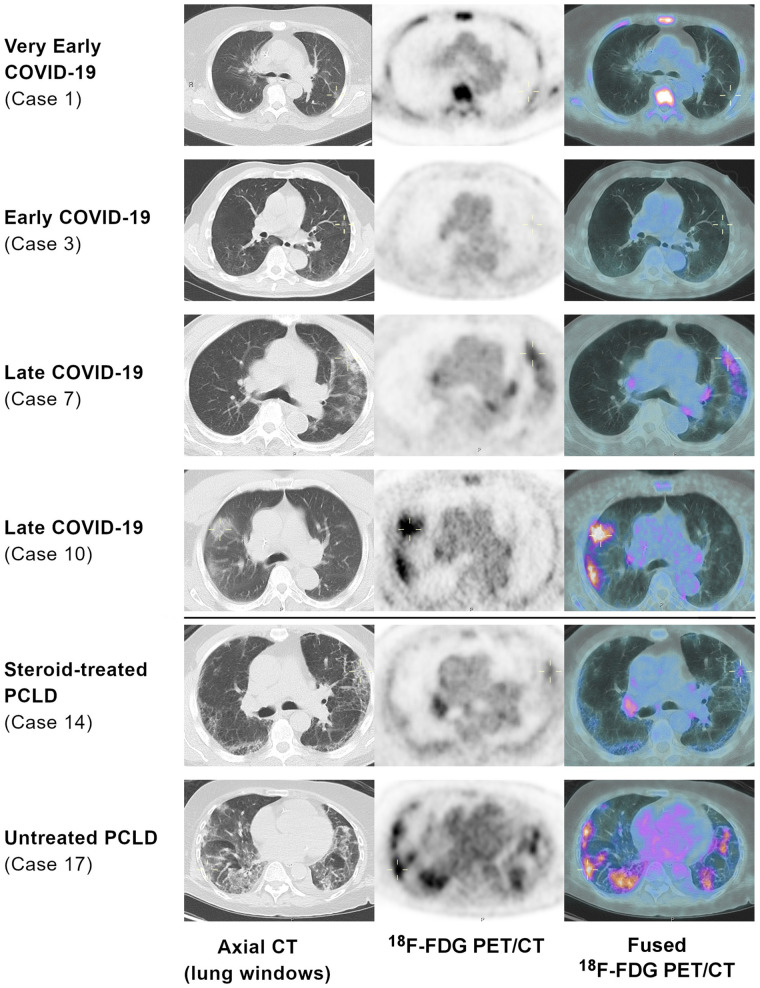
FIGURE 1.
Exemplar images demonstrating increasing 18F-FDG uptake with temporal stage and lower 18F-FDG uptake in steroid-treated PCLD (lung-windowed axial CT, 18F-FDG PET [SUV 0–5], and 18F-FDG PET/CT images). Medullary uptake in case 1 was due to leukemia and not COVID-19.
Association of Pulmonary 18F-FDG Uptake with Temporal Staging in Early- and Late-Stage Disease
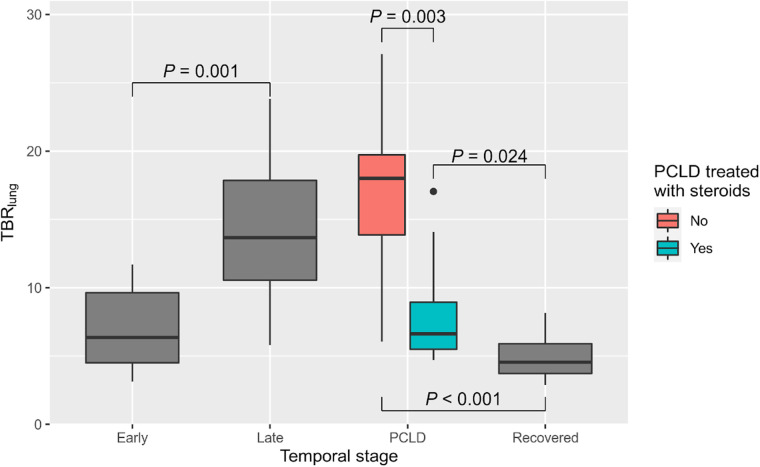
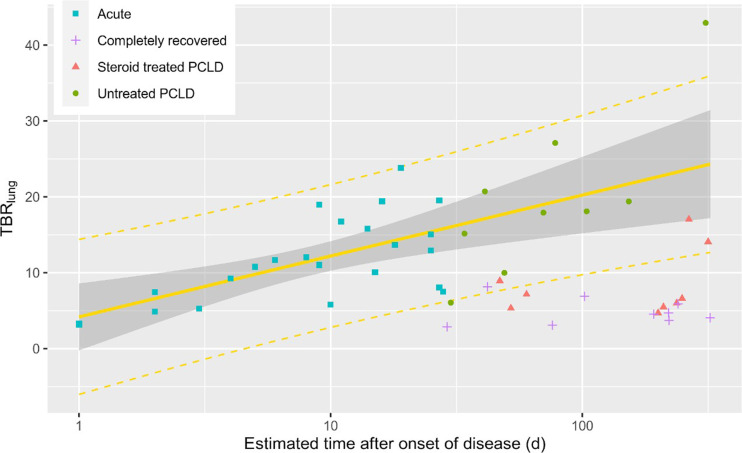
18F-FDG uptake analysis of lung lesions in acute-disease patients demonstrated an increasing TBRlung over time, with progression from low-avidity ground-glass changes in the early stage to avid consolidation during the late stage (median values in the early stage: SUVmax, 1.6, and TBRlung, 6.4; median values in the late stage: SUVmax, 4.0, and TBRlung, 13.7). In acute-disease patients, TBRlung differed significantly between the early and late stages, with late-stage patients having a higher TBRlung than early-stage patients (P = 0.001; Fig. 2). Among these acute-disease patients, a significant positive correlation was observed between TBRlung and estimated time since onset (rs = 0.60, P = 0.003; Fig. 3). This correlation was stronger when limited to acute-disease patients estimated to be in the first 3 wk of infection (n = 18, rs = 0.81, P < 0.001).
FIGURE 2.
18F-FDG uptake (TBRlung) by temporal stage.
FIGURE 3.
18F-FDG uptake (TBRlung) against estimated time after onset of disease (on logarithmic scale), with superimposed regression using 23 acute (early and late) patients (F1,23 = 14.94, P < 0.001; Spearman rs = 0.595, P = 0.003). Steroid treated = at least 10 d of high-dose steroid treatment.
Pulmonary 18F-FDG Uptake in PCLD
There was a lower TBRlung in patients who had received treatment with high-dose steroids (P = 0.003) (Fig. 2) (median values in steroid-treated patients: SUVmax, 2.4, and TBRlung, 6.62; median values in untreated patients: SUVmax, 5.8, and TBRlung, 18.1).
TBRlung was lower in asymptomatically recovered patients (median SUVmax, 1.2; median TBRlung, 4.6) than in either untreated PCLD patients or those treated with steroids (P < 0.001 and P = 0.020, respectively; P < 0.001 on Kruskal–Wallis testing for all 3 groups).
DISCUSSION
To our knowledge, this study was the first attempt to characterize the evolution of pulmonary 18F-FDG uptake in patients with COVID-19 assigned a temporal stage (early stage to late stage to PCLD) based on clinical context and CT findings.
The increase in lung avidity with time suggests increasing lung inflammation (11,12) in acute COVID-19. In most cases, 18F-FDG uptake would then be expected to decrease with viral clearance and establishment of immunity. There is, however, a subset of COVID-19 patients with delayed recovery who continue to show significant 18F-FDG uptake, reminiscent of our findings in interstitial lung disease (8,9,13,14), and raising the possibility that COVID-19 pneumonitis is associated with an activated host immune response rather than direct viral pathology (12,15,16). It would be useful to understand the ability of lung avidity to predict the clinical course or the likelihood that post–COVID-19 interstitial lung disease will develop in this patient cohort.
The RECOVERY study (Randomized Evaluation of COVID-19 Therapy), which this study predates, demonstrated a survival benefit from steroid use in hypoxic patients with COVID-19 (15). In our study, several patients went on to develop an inflammatory organizing pneumonia characterized by persistent and increasing 18F-FDG uptake. Steroid therapy is a recognized treatment for organizing pneumonia and other inflammatory interstitial lung diseases (15), and 18F-FDG uptake was consistently lower in those cases treated with postdischarge steroids. Our findings raise the question of whether steroid administration has a role not just in acute hypoxia but in the later stages of COVID-19 and in PCLD. This question has been debated (15), with calls for a randomized, controlled trial to define the role of steroid therapy more widely. Although imaging may be useful, it is hard to determine from CT whether parenchymal changes indicate reversible inflammation or irreversible fibrosis. It is possible that 18F-FDG PET/CT may offer a sensitive and specific biomarker to guide and rationalize steroid treatment.
Given the challenges of nuclear medicine imaging in the pandemic, this study has methodologic limitations. They are directly related to the infectious and emergent epidemic, the workload and severe capacity restraints of PET/CT departments, the need to protect staff and sterilize equipment, and the medical instability of seriously ill COVID-19 patients. These challenges limit patient numbers, preventing the use of a control group and longitudinal 18F-FDG PET/CT imaging. Diagnostic CT will likely remain the most practical way to investigate acute COVID-19, although PET imaging may give potential mechanistic insights. However, PCLD patients are not currently believed to be an infection risk, and performing longitudinal 18F-FDG PET/CT studies in this population may thus be realistic and feasible. This study was not prospectively designed to examine the use of steroids in PCLD; however, statistically significant lower 18F-FDG uptake was observed in PCLD patients who received steroids than in those who did not. Finally, the lack of PCR testing in the first wave, as well as the high incidence of asymptomatic cases throughout the pandemic, creates uncertainties about prevalence, and retrospective analyses may therefore suffer from selection bias. Despite the design limitations, the findings of this study offer some insight into the development of pulmonary disease in COVID-19 patients and can help provide the evidence to justify performing formal prospective studies on this topic in the future.
CONCLUSION
18F-FDG uptake in COVID-19 patients increases with time after infection and correlates with severity. Persistent 18F-FDG uptake is seen in patients with PCLD disease. These findings suggest that future studies may be directed at the use of 18F-FDG PET/CT to clarify the disease trajectory and may aid management of those patients with persistent respiratory symptoms
DISCLOSURE
Helen Garthwaite was funded by Breathing Matters. This work was undertaken at University College London Hospitals/University College London (UCLH/UCL), which receives funding from the U.K. Department of Health’s National Institute for Health Research Biomedical Research Centre’s funding scheme and the UCL Experimental Cancer Medicine Centre. No other potential conflict of interest relevant to this article was reported.
KEY POINTS.
QUESTION: What is the temporal evolution of 8F-FDG uptake in COVID-19 and in PCLD?
PERTINENT FINDINGS: 18F-FDG uptake was shown to increase with time after COVID-19 infection. Steroid treatment was associated with reduced uptake in PCLD.
IMPLICATIONS FOR PATIENT CARE: 18F-FDG PET/CT may help us understand the disease trajectory and aid in management of PCLD.
REFERENCES
- 1. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Young BE, Ong SWX, Kalimuddin S, et al. Epidemiologic features and clinical course of patients infected with SARS-CoV-2 in Singapore. JAMA. 2020;323:1488–1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Carfì A, Bernabei R, Landi F, et al. Persistent symptoms in patients after acute COVID-19. JAMA. 2020;324:603–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zhao Y, Shang Y-M, Song W-B, et al. Follow-up study of the pulmonary function and related physiological characteristics of COVID-19 survivors three months after recovery. EClinicalMedicine. 2020;25:100463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.**Updated** version 2 BSTI COVID-19 guidance for the reporting radiologist. The British Society of Thoracic Imaging website. https://www.bsti.org.uk/standards-clinical-guidelines/clinical-guidelines/bsti-covid-19-guidance-for-the-reporting-radiologist/. Updated March 16, 2020. Accessed September 21, 2021.
- 6. Notes for guidance on the clinical administration of radiopharmaceuticals and use of sealed radioactive sources. Gov.uk website. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/1018160/ARSAC_Notes_for_guidance_on_the_clinical_administration_of_radiopharmaceuticals_and_use_of_sealed_radioactive_sources.pdf. Published September 2021. Accessed September 21, 2021.
- 7. Shi H, Han X, Jiang N, et al. Radiological findings from 81 patients with COVID-19 pneumonia in Wuhan, China: a descriptive study. Lancet Infect Dis. 2020;20:425–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Groves AM, Win T, Screaton NJ, et al. Idiopathic pulmonary fibrosis and diffuse parenchymal lung disease: implications from initial experience with 18F-FDG PET/CT. J Nucl Med. 2009;50:538–545. [DOI] [PubMed] [Google Scholar]
- 9. Win T, Screaton NJ, Porter JC, et al. Pulmonary 18F-FDG uptake helps refine current risk stratification in idiopathic pulmonary fibrosis (IPF). Eur J Nucl Med Mol Imaging. 2018;45:806–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Giraudo C, Evangelista L, Fraia AS, et al. Molecular imaging of pulmonary inflammation and infection. Int J Mol Sci. 2020;21:894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Braune A, Hofheinz F, Bluth T, et al. Comparison of static and dynamic 18F-FDG PET/CT for quantification of pulmonary inflammation in acute lung injury. J Nucl Med. 2019;60:1629–1634. [DOI] [PubMed] [Google Scholar]
- 12. Bouadma L, Lescure F-X, Lucet J-C, Yazdanpanah Y, Timsit J-F. Severe SARS-CoV-2 infections: practical considerations and management strategy for intensivists. Intensive Care Med. 2020;46:579–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sudre CH, Murray B, Varsavsky T, et al.Attributes and predictors of long-COVID: analysis of COVID cases and their symptoms collected by the Covid Symptoms Study App. medRxiv website. https://www.medrxiv.org/content/10.1101/2020.10.19.20214494v1. Published October 2020. Accessed September 21, 2021.
- 14. Win T, Screaton NJ, Porter J, et al. Novel positron emission tomography/computed tomography of diffuse parenchymal lung disease combining a labeled somatostatin receptor analogue and 2-deoxy-2[18F]fluoro-D-glucose. Mol Imaging. 2012;11:91–98. [PubMed] [Google Scholar]
- 15. Horby P, Lim WS, Emberson J, et al.Effect of dexamethasone in hospitalized patients with COVID-19: preliminary report. medRxiv website. https://www.medrxiv.org/content/10.1101/2020.06.22.20137273v1. Published June 2020. Accessed September 21, 2021.
- 16. Ackermann M, Verleden SE, Kuehnel M, et al. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in Covid-19. N Engl J Med. 2020;383:120–128. [DOI] [PMC free article] [PubMed] [Google Scholar]