Visual Abstract
Keywords: PSMA, PET/CT, prostate cancer, biochemical relapse
Abstract
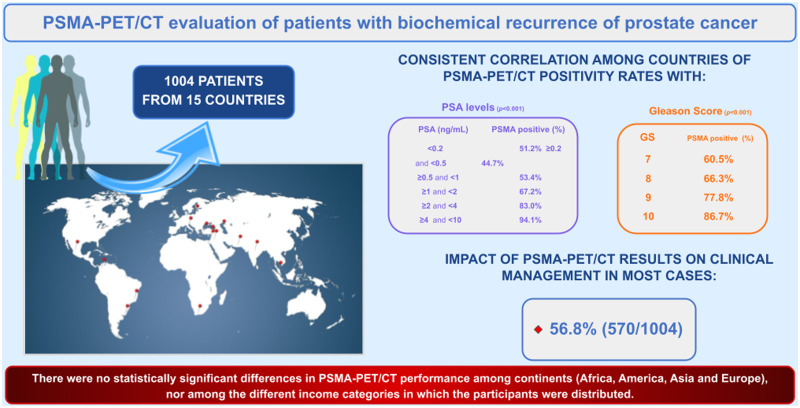
Biochemical recurrence (BCR) is a clinical challenge in prostate cancer (PCa) patients, as recurrence localization guides subsequent therapies. The use of PET with prostate-specific membrane antigen (PSMA) provides better accuracy than conventional imaging practice. This prospective, multicenter, international study was performed to evaluate the diagnostic performance and clinical impact of PSMA PET/CT for evaluating BCR in PCa patients in a worldwide scenario. Methods: Patients were recruited from 17 centers in 15 countries. Inclusion criteria were histopathologically proven prostate adenocarcinoma, previous primary treatment, clinically established BCR, and negative conventional imaging (CT plus bone scintigraphy) and MRI results for patients with PSA levels of 4–10 ng/mL. All patients underwent PET/CT scanning with 68Ga-PSMA-11. Images and data were centrally reviewed. Multivariate logistic regression analysis was applied to identify the independent predictors of PSMA-positive results. Variables were selected for this regression model on the basis of significant associations in the univariate analysis and previous clinical knowledge: Gleason score, the PSA level at the time of the PET scan, PSA doubling time, and primary treatment strategy. All patients were monitored for a minimum of 6 mo. Results: From a total of 1,004 patients, 77.7% were treated initially with radical prostatectomy and 22.3% were treated with radiotherapy. Overall, 65.1% had positive PSMA PET/CT results. PSMA PET/CT positivity was correlated with the Gleason score, PSA level at the time of the PET scan, PSA doubling time, and radiotherapy as the primary treatment (P < 0.001). Treatment was modified on the basis of PSMA PET/CT results in 56.8% of patients. PSMA PET/CT positivity rates were consistent and not statistically different among countries with different incomes. Conclusion: This multicenter, international, prospective trial of PSMA PET/CT confirmed its capability for detecting local and metastatic recurrence in most PCa patients in the setting of BCR. PSMA PET/CT positivity was correlated with the Gleason score, PSA level at the time of the PET scan, PSA doubling time, and radiotherapy as the primary treatment. PSMA PET/CT results led to changes in therapeutic management in more than half of the cohort. The study demonstrated the reliability and worldwide feasibility of PSMA PET/CT in the workup of PCa patients with BCR.
Prostate cancer (PCa) is the second most common cancer in men, accounting for 7.8% of all cancers in this population (1). Greater life expectancy worldwide and improved access to screening and diagnostic methods in developing nations are mainly responsible for the current trend of increasing incidence (2).
Initial treatment with curative intent is feasible, with radical prostatectomy or radiotherapy; nevertheless, early recurrence occurs in up to 50% of patients within 10 y (3–5). Biochemical recurrence (BCR) is defined as increasing serum prostate-specific antigen (PSA) levels after initial treatment, under specific criteria (6–8).
The key question for proper treatment planning in BCR remains whether the rise in PSA levels is reflective of local, regional, or distant recurrence. With increasing rates of success of early salvage therapy, the diagnosis of local tumor recurrence at the earliest possible stage has become pertinent. Salvage radiotherapy after radical prostatectomy has been shown to be most effective—reaching a durable response—when the postoperative PSA level is preferably below 0.5 ng/mL, with better outcomes when the PSA level is below 0.2 ng/mL (4,9).
Despite guidelines indicating that prostate-specific membrane antigen (PSMA) PET/CT is the imaging modality of choice in BCR (10–17), in some countries—especially those with lower incomes—conventional imaging with CT and bone scintigraphy are still being used, even if the diagnostic yield of these techniques is low, especially for patients with low PSA levels (11).
Most PSMA PET/CT studies have been performed at a single institution or were retrospectively planned. Furthermore, most reported studies have been conducted at academic centers in highly developed countries; thus, to our knowledge, there are no data from large prospective international trials. The International Atomic Energy Agency initiated a Coordinated Research Project to evaluate the feasibility and usefulness of PSMA PET/CT for studying PCa patients with BCR in 15 countries to inform international practice.
The primary aim of this prospective study was to evaluate the diagnostic performance of PSMA PET/CT in PCa patients with BCR worldwide, through an international multicenter effort, and the impact of PSMA PET/CT on clinical management.
MATERIALS AND METHODS
Study Design
Two investigators’ meetings were held, in 2017 and 2019. The first defined the study protocol, whereas in the second, an interim evaluation was performed, together with image and data review. The study followed a prospective, multicenter, international design, encompassing 17 centers in 15 countries (Azerbaijan, Brazil, Colombia, India, Israel, Italy, Jordan, Lebanon, Malaysia, Mexico, Pakistan, Poland, South Africa, Turkey, and Uruguay). Standard forms for data registration were developed and agreed on by the investigators. Data were collected for PSMA PET/CT positivity rate, localization of positive findings, and impact on patient management (Supplemental Fig. 1) (supplemental materials are available at http://jnm.snmjournals.org). All centers obtained local ethics clearance for prospective recruitment of patients and data collection, according to national regulations. All subjects signed an informed consent form.
Patients
Patients who had histopathologically proven prostate adenocarcinoma, who had undergone primary definitive treatment (radical prostatectomy or radiotherapy), and who had BCR were recruited. All patients were monitored for a minimum of 6 mo after PSMA PET/CT.
The 6 inclusion criteria were an age of greater than 18 y; histopathologically proven prostatic adenocarcinoma; previous primary treatment for PCa (radical prostatectomy or radiotherapy); BCR, defined as a PSA level above 0.2 ng/mL, confirmed by 2 subsequent consecutive measurements, after radical prostatectomy, or as an absolute increase in the PSA level of 2 ng/mL above the nadir after radiotherapy; negative conventional imaging (CT plus bone scintigraphy) and MRI results for patients with PSA levels of 4–10 ng/mL; and written informed consent.
The 3 exclusion criteria were a history of any malignancy other than PCa; a history of Paget disease; and BCR and PSA levels of greater than or equal to 10 ng/mL.
PET/CT Imaging
All patients underwent PSMA PET/CT with the same radiopharmaceutical, 68Ga-PSMA-11 (18–21), which was synthesized at the radiopharmaceutical laboratory of each participating center. PET studies were performed on dedicated PET/CT scanners, and image quality was evaluated by board-certified nuclear medicine physicians.
According to the methodology proposed in the medical literature (10), patients were administered 68Ga-PSMA-11 (2 MBq/kg; a minimum of 125 MBq) by slow intravenous injection. At 60 to 90 min after injection, standard image acquisition was performed. Low-dose (diagnostic) CT images were obtained from the midthigh to above the orbitomeatal line. Three-dimensional PET images were acquired for the same body extension, for at least 2 min/bed position. Real true-body images (images from head to toes), contrast-enhanced CT, and diuretic and late images were allowed.
PET/CT studies were assessed by 2 board-certified nuclear medicine physicians with extensive experience in PSMA PET/CT oncologic imaging at each center, and all scans were later centrally reviewed. Discordant findings were addressed at consensus meetings, and final results were used for analysis.
PET/CT Image Analysis
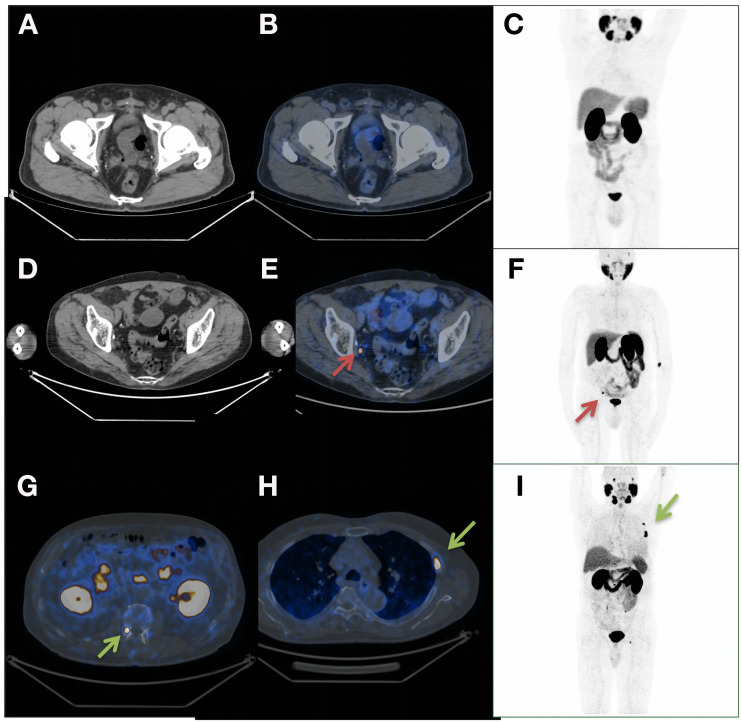
The studies were classified as either positive or negative with regard to the identification of findings suggestive of recurrence on the basis of procedure guidelines for PCa imaging (Fig. 1) (10). The anatomic sites of the lesions were registered.
FIGURE 1.
(A–C) Negative PSMA PET/CT results for 65-y-old patient who had undergone radical prostatectomy plus PNLD and had T3bN0 BCR (PSA, 0.55 ng/mL). Treatment plan was not altered by PSMA PET/CT results (radiotherapy) (A: axial CT; B: axial fusion; C: maximum-intensity projection [MIP]). (D–F) Positive PSMA PET/CT results for 67-y-old patient who had undergone radical prostatectomy plus PNLD and who had T2aN1 BCR (PSA, 0.4 ng/mL). Treatment plan was modified from radiotherapy to ADT (D: axial CT; E: axial fusion; F: MIP) for 0.4-cm lymph nodes (red arrows). (G–I) Positive PSMA PET/CT results for 65-y-old patient who had undergone radical prostatectomy plus PNLD and who had T3aN0 BCR (PSA, 0.2 ng/mL). Treatment plan was modified from radiotherapy to chemotherapy (G: axial CT; H: axial fusion; I: MIP) for metastatic bone lesions (green arrows). PNLD = pelvic lymph node dissection.
PSMA PET/CT findings were compared with histology (when necessary, in the judgment of the clinician); correlative imaging methods, such as CT with contrast, MRI, whole-body MRI, and bone scanning; and clinical and laboratory data (PSA behavior). All data were obtained in the normal care pathway.
Given the composite nature of the standard of reference, we could not calculate sensitivity or specificity; furthermore, a proper evaluation of negative findings was beyond the scope of the present study, which focused on assessing the PSMA PET/CT detection rate (positivity rate), defined as the proportion of patients with positive PSMA PET/CT results.
Intention to Treat
Before PSMA PET/CT, an intention-to-treat questionnaire was completed by the assistant urooncology teams by the time of referral for evaluation; the treatment categories were radiotherapy only, radiotherapy and antiandrogenic therapy (ADT), salvage lymphadenectomy, ADT only, active surveillance, bilateral orchiectomy, second-generation ADT (abiraterone or enzalutamide), radionuclide therapy, and chemotherapy (taxane).
After the PSMA PET/CT results were made available, the assistant urooncology teams completed the same questionnaire on the basis of the actual treatments used.
Statistical Analyses
The demographic and clinical variables were tabulated using descriptive analysis. Continuous variables were assessed for the gaussian distribution of the data and presented as mean ± SD, if normally distributed, or median (25th percentile, 75th percentile) if not normally distributed. Comparisons of patients with positive PSMA results and those with negative PSMA results were performed using the t test or Wilcoxon–Mann–Whitney test. Discrete variables were presented as proportions and compared between groups using the χ2 test. A multivariate logistic regression analysis was performed to identify the independent predictors of positive PSMA results. Variables were selected for the regression model on the basis of significant associations in the univariate analysis and previous clinical knowledge. The level of significance was set at a P value of less than 0.05. Analyses were performed using Stata version 15.1 (Stata Corp.).
RESULTS
Patient Characteristics
A total of 1,198 PCa patients referred for PSMA PET/CT because of BCR between November 2017 and December 2019 were enrolled; 194 were subsequently excluded because of missing information or loss of follow-up data. Therefore, a cohort of 1,004 patients could be analyzed, here divided by country: Azerbaijan (48), Brazil (165), Colombia (29), India (86), Israel (16), Italy (172), Jordan (26), Lebanon (65), Malaysia (35), Mexico (91), Pakistan (19), Poland (111), South Africa (42), Turkey (57), and Uruguay (42). For 2 nations (India and Turkey), data from 2 contributing centers were pooled together for the present study (see the list of participant centers and contributors in the supplemental materials). The distribution of patients according to the Gleason score (GS) was as follows: for a GS of 7, there were 613 patients (61.1%); for a GS of 8, there were 196 patients (19.5%); for a GS of 9, there were 180 patients (17.9%); and for a GS of 10, there were 15 patients (1.5%). The distribution of patients according to PSA levels at PET/CT was as follows: for PSA levels of less than 0.2 ng/mL, there were 41 patients (4.1%); for PSA levels between greater than or equal to 0.2 ng/mL and less than 0.5 ng/mL, there were 188 patients (18.7%); for PSA levels between greater than or equal to 0.5 ng/mL and less than 1 ng/mL, there were 232 patients (23.1%); for PSA levels between greater than or equal to 1 ng/mL and less than 2 ng/mL, there were 235 patients (23.4%); for PSA levels between greater than or equal to 2 ng/mL and less than 4 ng/mL, there were 206 patients (20.5%); and for PSA levels between greater than or equal to 4 ng/mL and less than 10 ng/mL, there were 102 patients (10.2%). The mean PSA doubling time was 11.18 mo (SD, 13.15 mo) (Table 1). Overall, 780 patients (77.7%) were treated initially with radical prostatectomy and 224 (22.3%) were treated with radiotherapy. At the time of the PET scan, the mean time from PCa diagnosis to BCR was 15.6 mo (range, 0.6–43.7 mo); 248 patients (24.7%) were receiving ongoing ADT; and 630 patients (62.7%) had a PSA doubling time of less than or equal to 10 mo.
TABLE 1.
Patient Characteristics Based on PSMA PET Results
| Characteristic | All patients (n = 1,004)* | Patients with negative PSMA PET/CT results (n = 350)* | Patients with positive PSMA PET/CT results (n = 654)* | P |
|---|---|---|---|---|
| Age† | 67.29 ± 7.48 | 66.37 ± 7.36 | 67.77 ± 7.51 | 0.005 |
| PSA level at time of PET scan | <0.001 | |||
| <0.2 | 41 (4.1) | 20 (5.7) | 21 (3.2) | |
| 0.2–0.5 | 188 (18.7) | 104 (29.7) | 84 (12.8) | |
| 0.5–1.0 | 232 (23.1) | 108 (30.9) | 124 (19.0) | |
| 1–2 | 235 (23.4) | 77 (22.0) | 158 (24.2) | |
| 2–4 | 206 (20.5) | 35 (10.0) | 171 (26.1) | |
| >4 | 102 (10.2) | 6 (1.7) | 96 (14.7) | |
| PSA doubling time† | 11.18 ± 13.15 | 12.97 ± 14.04 | 10.22 ± 12.56 | 0.002 |
| Initial PSA before therapy† | 17.27 ± 22.10 | 14.63 ± 17.69 | 18.69 ± 24.02 | 0.006 |
| TNM | <0.001 | |||
| T1 | 4 (0.5) | 2 (0.6) | 2 (0.4) | |
| T2 | 439 (56.0) | 208 (65.8) | 231 (49.4) | |
| T3 | 333 (42.5) | 103 (32.6) | 230 (49.1) | |
| T4 | 8 (1.0) | 3 (0.9) | 5 (1.1) | |
| Ongoing ADT | 248 (24.7) | 62 (17.7) | 186 (28.4) | <0.001 |
| Radiotherapy as first treatment | 224 (22.3) | 35 (10.0) | 189 (28.9) | <0.001 |
| Time to relapse‡ | 23.0 (8.0, 49.0) | 22.5 (8.0, 48.0) | 24.0 (9.0, 51.0) | 0.57 |
| GS | <0.001 | |||
| 7 | 613 (61.1) | 242 (69.1) | 371 (56.7) | |
| 8 | 196 (19.5) | 66 (18.9) | 130 (19.9) | |
| 9 | 180 (17.9) | 40 (11.4) | 140 (21.4) | |
| 10 | 15 (1.5) | 2 (0.6) | 13 (2.0) | |
| Country income | 0.07 | |||
| High income | 390 (38.8) | 149 (42.6) | 241 (36.9) | |
| Upper middle income | 509 (50.7) | 160 (45.7) | 349 (53.4) | |
| Lower middle income | 105 (10.5) | 41 (11.7) | 64 (9.8) | |
| Continent | 0.73 | |||
| Africa | 42 (4.2) | 18 (5.1) | 24 (3.7) | |
| Asia | 182 (18.1) | 64 (18.3) | 118 (18.0) | |
| Europe | 388 (38.6) | 132 (37.7) | 256 (39.1) | |
| Latin America | 392 (39.0) | 136 (38.9) | 256 (39.1) |
Data are reported as numbers of patients, with percentages of patients in parentheses, unless otherwise indicated.
†Data are reported as mean ± SD.
‡Data are reported as median (25th percentile, 75th percentile).
The mean age of patients was 67.3 y (range, 45–87 y); 908 men (90.4%) met the eligibility requirement because they had PSA levels of less than 4 ng/mL, whereas 96 men (9.6%) had PSA concentrations of 4–10 ng/mL and negative MRI, CT, and bone scintigraphy results. The mean PSA level at the time of the PET scan was 1.55 ng/mL. Regarding the stage at presentation, 443 men (44.1%) had clinical stages T1–T2 and 341 (34.0%) had clinical stages T3–T4; in 220 men (21.9%), the T stage was unknown. The mean duration of follow-up after PSMA PET/CT was 16.8 mo (SD, 9.3 mo).
Regarding income, 105, 509, and 390 patients were in the lower middle-income, upper middle-income, and high-income groups, respectively. PSA differences were not significant among them (P = 0.94). Of note, there were statistically significant differences regarding PSA doubling time, ongoing ADT, and radiotherapy as the primary treatment among the different income groups. For these 3 groups, the mean PSA doubling times were 9.14, 9.98, and 13.3 mo (P < 0.001). There were 40 patients (38.1%), 131 patients (25.7%), and 77 patients (19.7%) receiving ongoing ADT (P < 0.001). Radiotherapy was the primary treatment in 42 patients (40.0%), 129 patients (25.3%), and 53 patients (13.6%) (P < 0.001).
PSMA PET/CT
At least 1 malignant lesion was found in 65.1% of the patients (654/1,004), whereas 34.9% (350/1,004) had negative PSMA PET/CT results, with no detectable disease. A summary of the PSMA PET/CT results is shown in Table 1.
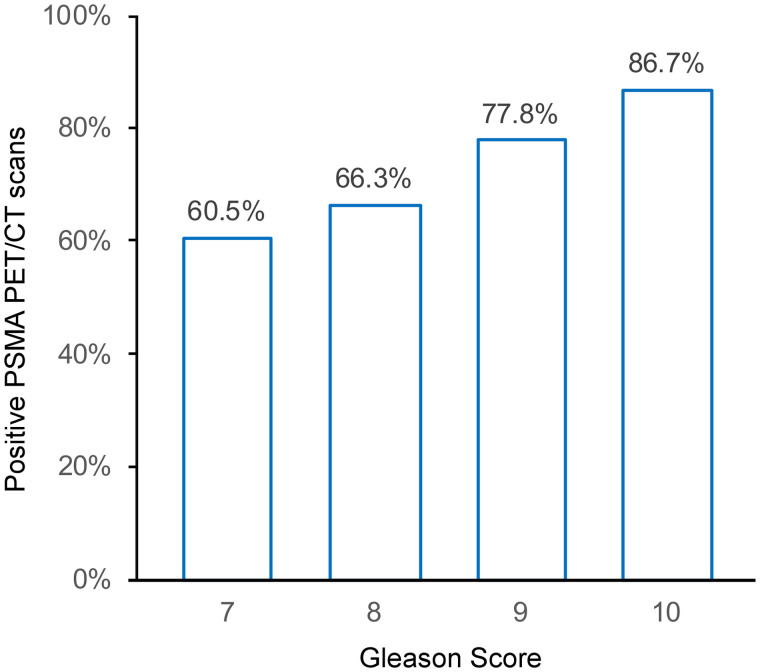
There was a correlation between PSMA PET/CT and the GS (P < 0.001). Detection rates were 60.5% (371/613) for patients with a GS of 7; 66.3% (130/196) for those with a GS of 8; 77.8% (140/180) for those with a GS of 9; and 86.7% (13/15) for those with a GS of 10 (Fig. 2).
FIGURE 2.
Correlation between PSMA PET/CT positivity and GS.
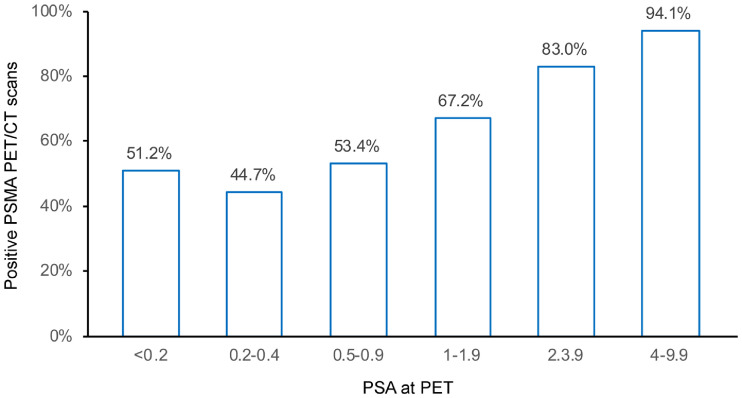
We also found a significant correlation between PSMA PET/CT positivity and PSA values (P < 0.001). Detection rates were 51.2% (21/41) for PSA values of less than 0.2; 44.7% (84/188) for PSA values between greater than or equal to 0.2 and less than 0.5; 53.4% (124/232) for PSA values between greater than or equal to 0.5 and less than 1; 67.2% (158/235) for PSA values between greater than or equal to 1 and less than 2; 83.0% (171/206) for PSA values between greater than or equal to 2 and less than 4; and 94.1% (96/102) for PSA values between greater than or equal to 4 and less than 10 (Fig. 3).
FIGURE 3.
Correlation between PSMA PET/CT positivity and PSA values.
PSMA PET/CT results were positive for 69.4% of the patients (437/630) whose PSA doubling times were less than or equal to 10 mo and for 58.0% of the patients (217/374) whose PSA doubling times were greater than 10 mo (P = 0.003) (Fig. 4).
FIGURE 4.
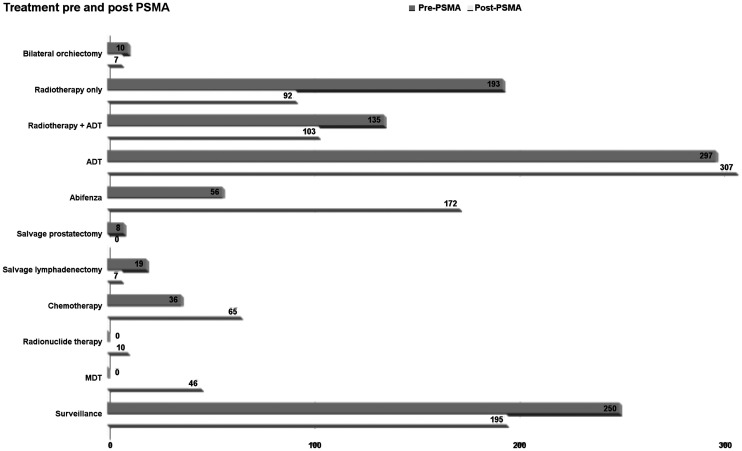
Impact of PSMA PET/CT on clinical management. MDT = metastasis-directed therapy.
The PSMA PET/CT positivity rates per anatomic site were 13.7% (138/1,004) in the prostate or prostatic bed only; 3.9% (39/1,004) in the prostate or prostatic bed and pelvic lymph nodes; 20.5% (206/1,004) in the pelvic lymph nodes only; and 27.0% (271/1,004) in a metastasis at any site (bone only in 10.0% [100/1,004]) (Table 2). In a univariate analysis, factors associated with positive PSMA PET/CT results were age, the PSA level at the time of the PET scan, PSA doubling time, the initial PSA level before therapy, TNM, GS, ongoing ADT, and radiotherapy as the first treatment. Logistic regression showed that PSMA PET/CT positivity was associated with the GS, the PSA level at the time of the PET scan, decreasing PSA doubling time, and radiotherapy as the primary treatment (Table 3).
TABLE 2.
Positive PSMA PET/CT Studies per Anatomic Site
| Anatomic site | Result for positive PSMA PET/CT studies* |
|---|---|
| Prostate or prostatic bed only | 138 (13.7) |
| Prostate or prostatic bed + lymph nodes | 39 (3.9) |
| Lymph nodes only | 206 (20.5) |
| Metastasis at any site | 271 (27.0) |
| Bone only | 100 (10.0) |
Data are reported as numbers of patients, with percentages of patients in parentheses.
TABLE 3.
Association of Clinical Covariates with Likelihood of Detection by PSMA PET/CT
| Covariate | Odds ratio | z | P | 95% CI | |
|---|---|---|---|---|---|
| Lower | Upper | ||||
| Age | 1.01 | 1.69 | 0.091 | 0.99 | 1.03 |
| PSA level at PCa diagnosis | 0.99 | −0.05 | 0.958 | 0.99 | 1.01 |
| GS | 1.37 | 3.30 | 0.001 | 1.25 | 1.65 |
| at time of PSMA PET/CT | 1.72 | 7.57 | 0.001 | 1.47 | 1.97 |
| PSA doubling time | 0.98 | −3.30 | 0.001 | 0.97 | 0.99 |
| Ongoing ADT | 1.23 | 1.14 | 0.255 | 0.93 | 1.76 |
| Radiotherapy first | 2.17 | 3.56 | 0.001 | 1.42 | 3.34 |
Of the 1,004 cases included in the present study, 12.4% (124 patients) had doubtful PET findings (as reported by local readers). Of these, 90 patients had other positive findings, regardless of the indeterminate one(s); thus, their scans were already defined as positive PSMA PET/CT scans. Of the remaining 34 patients (3.4%) in whom the indeterminate lesion at PSMA PET/CT was the sole finding, 3 were confirmed to have true-positive results on the basis of follow-up data, whereas 31 (3.1%) were regarded as having false-positive results (encompassing reactive lymph nodes, bone fractures, trauma, and benign pulmonary lesions).
Impact of PSMA PET/CT on Clinical Management
Disease management changed in 56.8% of our cohort (570/1,004) after PSMA PET/CT information was obtained. The following changes occurred as a result of PSMA PET/CT: 77 patients underwent active surveillance, 35 underwent radiotherapy only, 55 underwent radiotherapy and ADT, 152 underwent ADT only, 48 underwent salvage lymphadenectomy, 5 underwent bilateral orchiectomy, 140 underwent second-generation ADT (abiraterone or enzalutamide), 10 underwent radionuclide therapy, and 48 (patients with polymetastatic disease) started taxane chemotherapy.
Of the patients for whom there was no management change motivated by PSMA PET/CT results (434/1,004; 43.2%), 118 remained under active surveillance, 57 underwent radiotherapy only, 48 underwent radiotherapy and ADT, 5 underwent salvage lymphadenectomy, 155 underwent ADT, 2 underwent bilateral orchiectomy, 32 underwent second-generation ADT (abiraterone or enzalutamide), and 17 (patients with polymetastatic disease) started taxane chemotherapy (Fig. 4).
PSMA PET/CT Worldwide
The centers were grouped in 2 distinct ways: by country income (high income: Israel, Italy, Poland, and Uruguay; upper middle income: Azerbaijan, Brazil, Colombia, Jordan, Lebanon, Malaysia, Mexico, South Africa, and Turkey; and lower middle income: India and Pakistan) and by continent (Africa, America, Asia, and Europe). There were no significant differences in PSMA PET/CT positivity by lower middle income, upper middle income, and high income (61%, 69%, and 62%, respectively) or by continent (Africa: 57%; Asia: 65%; Europe: 66%; and Latin America: 65%) (P = 0.07 and P = 0.73, respectively) (Table 1).
DISCUSSION
Our findings resonate with the available literature on the use of PSMA PET/CT in the evaluation of PCa patients in the scenario of BCR (3–8,10,20–44). We analyzed 4 main aspects of PSMA PET/CT in this setting: positivity rate, clinical factors associated with PSMA positivity, differences in performance with regard to continents and incomes, and impact on clinical management. The PSMA PET/CT positivity rate was 65.1%, similar to the positivity rates reported in other studies, ranging overall from 63% to 75% (10,14,16,21,22). Also, increasing PSA levels at the time of the scan were associated with higher PSMA PET/CT positivity, with rates similar to those previously reported (Supplemental Table 1); the exception was higher PSMA PET/CT positivity in the group with PSA levels of less than 0.2 ng/mL compared with the mean in the available literature: 51.2% versus 36.8% (3–8,10,20–44). This difference might be explained by the small number of patients in this group in our cohort (41) but also by the small number of patients evaluated in the cohort of all patients (316). Nevertheless, 51.2% falls into the range observed in the literature (11.3%–58.3%). In the other scenarios (PSA levels of <0.5, <1.0, and <2.0 ng/mL), the positivity rates were quite similar (44.7% vs. 43.3%; 53.4% vs. 52.2%, and 67.2% vs. 58.9%, respectively).
The observed location of malignant lesions is in agreement with those in previous reports, with lymph nodes being the principal site of recurrence (24.4%), followed by local recurrence in the prostate bed (17.6%), and with any metastatic disease in 27.0% (9,45).
Furthermore, higher positivity rates were also associated with features of advanced or aggressive disease other than increasing PSA levels: a shorter PSA doubling time (≤10 mo) and a higher GS. These findings are also in line with the current available literature (42,46) and are likely due to the presence of more neoplastic lesions and to higher tumoral cell turnover, which provide more available sites for PSMA ligand binding and, thus, lead to positive PET/CT results.
One interesting finding was the association of radiotherapy as a primary radical treatment with PSMA PET/CT positivity in the BCR setting. Although patients receiving radiotherapy represented only 22.3% of all patients, they comprised 28.9% of patients with positive PSMA PET/CT results (P < 0.001). It is already known that, in comparison to radical prostatectomy, radiotherapy is associated with higher BCR rates (46). Our results suggest that in addition to having more frequent residual/recurrent disease, these patients are also more likely to have positive PSMA PET/CT scans in the BCR setting.
The most relevant finding is that there were no statistically significant differences in PSMA PET/CT performance among continents or among the different income categories in which the participants were distributed. This finding is important because it highlights the fact that the great heterogeneities among nations do not seem to interfere with each country’s capacity to provide high-quality PSMA PET/CT studies in the appropriate medical centers.
PSMA PET/CT affected clinical management in more than half of our cohort, as the therapeutic strategy was altered by PSMA PET/CT results 56.8% of the time, similar to previous reports in different studies (13,16,21).
Regarding the limitations of the present study, a major one is that histopathology as a gold standard was available only in a few cases. It is well known that histopathologic confirmation in all patients is not feasible because of practical and ethical issues. Hence, in most patients, a composite standard of reference (histopathology and clinical and laboratory evaluations) was used. Another important limitation is the relatively small percentage of patients included in low-income countries and in Africa. Furthermore, South Africa’s income and PSMA PET/CT availability are not representative of the continent. Moreover, regarding the impact of PSMA PET/CT on clinical management, the available data unfortunately do not permit an evaluation of its effects on survival rates.
The endeavor of performing this multicenter, international study, enrolling more than 1,000 patients from many countries was made possible only through the combined efforts of several different researchers and the support of the International Atomic Energy Agency, a nonprofit agency, which enabled gathering of this large and diverse cohort.
CONCLUSION
This multicenter, international, prospective trial of PSMA PET/CT confirms its capability for detecting local and metastatic recurrences in most PCa patients in the setting of BCR. PSMA PET/CT positivity was correlated with the GS, the PSA level at the time of the PET scan, PSA doubling time, and radiotherapy as the primary treatment. PSMA PET/CT results led to changes in therapeutic management in more than half of the cohort. The present study demonstrates the reliability and feasibility of PSMA PET/CT in the workup of PCa patients with BCR.
DISCLOSURE
This research was partially funded by IAEA. No personal grants, consulting fees, or honoraria were involved in the present work. No other potential conflict of interest relevant to this article was reported.
ACKNOWLEDGMENTS
We thank the following individuals for their contributions: from the National Centre of Oncology, Baku, Azerbaijan—Jamil A. Aliyev, Fuad Guliyev, Elnur Mehdi, Mirsaleh Valiyev, and Leyla Mehmetbeyli; from Brazil—Margaret Masukawa, Jonatas L. Pereira, Murilo A. Luz, Rodrigo J. Cerci, and Miguel Morita; from Azienda Ospedaliero-Universitaria di Bologna, Bologna, Italy—Andrea Farolfi, Paolo Castellucci, Veronica Cervati, Francesca Serani, and Riccardo Schiavina; from King Hussein Cancer Center, Amman, Jordan—Ula Al-Rasheed and Samer Salah; from the University of Pretoria, Pretoria, South Africa—Mike Sathekge; from Ankara University Medical school—Çiğdem Soydal; and from the Centro Uruguayo de Imagenologıa Molecular, Montevideo, Uruguay—G. dos Santos, E. Silvera, and M. Rodríguez.
KEY POINTS.
QUESTION: In a large international cohort of PCa patients in the setting of BCR, how similar are PSMA PET/CT positivity rates and impact on clinical management among countries on different continents and with different incomes?
PERTINENT FINDINGS: PSMA PET/CT positivity was correlated with the GS, serum PSA levels, and radiotherapy as the primary treatment. An impact of PSMA PET/CT results on clinical management was observed in most cases, and all findings were similarly consistent regardless of the country.
IMPLICATIONS FOR PATIENT CARE: Our results confirm the worldwide feasibility and usefulness of PSMA PET/CT in the setting of PCa BCR.
REFERENCES
- 1. Ferlay JEM, Lam F, Colombet M, et al.Global cancer observatory: cancer tomorrow. Lyon, France: International Agency for Research on Cancer. https://gco.iarc.fr/today/data/factsheets/cancers/40-All-cancers-excluding-non-melanoma-skin-cancer-fact-sheet.pdf. Accessed December 21, 2021.
- 2. Rawla P. Epidemiology of prostate cancer. World J Oncol. 2019;10:63–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gupta SK, Watson T, Denham J, et al. Prostate-specific membrane antigen positron emission tomography-computed tomography for prostate cancer: distribution of disease and implications for radiation therapy planning. Int J Radiat Oncol Biol Phys. 2017;99:701–709. [DOI] [PubMed] [Google Scholar]
- 4. Meredith G, Wong D, Yaxley J, et al. The use of 68Ga-PSMA PET CT in men with biochemical recurrence after definitive treatment of acinar prostate cancer. BJU Int. 2016;118(suppl 3):49–55. [DOI] [PubMed] [Google Scholar]
- 5. Morigi JJ, Stricker PD, van Leeuwen PJ, et al. Prospective comparison of 18F-fluoromethylcholine versus 68Ga-PSMA PET/CT in prostate cancer patients who have rising psa after curative treatment and are being considered for targeted therapy. J Nucl Med. 2015;56:1185–1190. [DOI] [PubMed] [Google Scholar]
- 6. Afshar-Oromieh A, Avtzi E, Giesel FL, et al. The diagnostic value of PET/CT imaging with the 68Ga-labelled PSMA ligand HBED-CC in the diagnosis of recurrent prostate cancer. Eur J Nucl Med Mol Imaging. 2015;42:197–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Afshar-Oromieh A, Holland-Letz T, Giesel FL, et al. Diagnostic performance of 68Ga-PSMA-11 (HBED-CC) PET/CT in patients with recurrent prostate cancer: evaluation in 1007 patients. Eur J Nucl Med Mol Imaging. 2017;44:1258–1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Calais J, Czernin J, Cao M, et al. 68Ga-PSMA-11 PET/CT mapping of prostate cancer biochemical recurrence after radical prostatectomy in 270 patients with a PSA level of less than 1.0 ng/mL: impact on salvage radiotherapy planning. J Nucl Med. 2018;59:230–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dietlein F, Kobe C, Neubauer S, et al. PSA-stratified performance of 18F- and 68Ga-PSMA PET in patients with biochemical recurrence of prostate cancer. J Nucl Med. 2017;58:947–952. [DOI] [PubMed] [Google Scholar]
- 10. Fendler WP, Eiber M, Beheshti M, et al. 68Ga-PSMA PET/CT: joint EANM and SNMMI procedure guideline for prostate cancer imaging—version 1.0. Eur J Nucl Med Mol Imaging. 2017;44:1014–1024. [DOI] [PubMed] [Google Scholar]
- 11. Eiber M, Maurer T, Souvatzoglou M, et al. Evaluation of hybrid 68Ga-PSMA ligand PET/CT in 248 patients with biochemical recurrence after radical prostatectomy. J Nucl Med. 2015;56:668–674. [DOI] [PubMed] [Google Scholar]
- 12. Hofman MS, Hicks RJ, Maurer T, Eiber M. Prostate-specific membrane antigen PET: clinical utility in prostate cancer, normal patterns, pearls, and pitfalls. Radiographics. 2018;38:200–217. [DOI] [PubMed] [Google Scholar]
- 13. Han S, Woo S, Kim YJ, Suh CH. Impact of 68Ga-PSMA PET on the management of patients with prostate cancer: a systematic review and meta-analysis. Eur Urol. 2018;74:179–190. [DOI] [PubMed] [Google Scholar]
- 14. Bashir U, Tree A, Mayer E, et al. Impact of Ga-68-PSMA PET/CT on management in prostate cancer patients with very early biochemical recurrence after radical prostatectomy. Eur J Nucl Med Mol Imaging. 2019;46:901–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Albisinni S, Artigas C, Aoun F, et al. Clinical impact of 68Ga-prostate-specific membrane antigen (PSMA) positron emission tomography/computed tomography (PET/CT) in patients with prostate cancer with rising prostate-specific antigen after treatment with curative intent: preliminary analysis of a multidisciplinary approach. BJU Int. 2017;120:197–203. [DOI] [PubMed] [Google Scholar]
- 16. Mattiolli AB, Santos A, Vicente A, et al. Impact of 68GA-PSMA PET/CT on treatment of patients with recurrent/metastatic high risk prostate cancer - a multicenter study. Int Braz J Urol. 2018;44:892–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Matushita CS, da Silva AMM, Schuck PN, et al. 68Ga-prostate-specific membrane antigen (PSMA) positron emission tomography (PET) in prostate cancer: a systematic review and meta-analysis. Int Braz J Urol. 2021;47:705–729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. National Cancer Institute. Cancer reference information. https://www.cancer.gov/publications/dictionaries/cancer-drug/def/gallium-ga-68-labeled-psma-11. Accessed September 24, 2020.
- 19. Ceci F, Oprea-Lager DE, Emmett L, et al. E-PSMA: the EANM standardized reporting guidelines v1.0 for PSMA-PET. Eur J Nucl Med Mol Imaging. 2021;48:1628–1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Markowski MC, Chen Y, Feng Z, et al. PSA doubling time and absolute PSA predict metastasis-free survival in men with biochemically recurrent prostate cancer after radical prostatectomy. Clin Genitourin Cancer. 2019;17:470–475.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hope TA, Aggarwal R, Chee B, et al. Impact of 68Ga-PSMA-11 PET on management in patients with biochemically recurrent prostate cancer. J Nucl Med. 2017;58:1956–1961. [DOI] [PubMed] [Google Scholar]
- 22. Tan N, Bavadian N, Calais J, et al. Imaging of prostate specific membrane antigen targeted radiotracers for the detection of prostate cancer biochemical recurrence after definitive therapy: a systematic review and meta-analysis. J Urol. 2019;202:231–240. [DOI] [PubMed] [Google Scholar]
- 23. Fendler WP, Calais J, Eiber M, et al. Assessment of 68Ga-PSMA-11 PET accuracy in localizing recurrent prostate cancer: a prospective single-arm clinical trial. JAMA Oncol. 2019;5:856–863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sachpekidis C, Eder M, Kopka K, et al. 68Ga-PSMA-11 dynamic PET/CT imaging in biochemical relapse of prostate cancer. Eur J Nucl Med Mol Imaging. 2016;43:1288–1299. [DOI] [PubMed] [Google Scholar]
- 25. Schmuck S, Nordlohne S, von Klot CA, et al. Comparison of standard and delayed imaging to improve the detection rate of [68Ga]PSMA I&T PET/CT in patients with biochemical recurrence or prostate-specific antigen persistence after primary therapy for prostate cancer. Eur J Nucl Med Mol Imaging. 2017;44:960–968. [DOI] [PubMed] [Google Scholar]
- 26. Kranzbühler B, Nagel H, Becker AS, et al. Clinical performance of 68Ga-PSMA-11 PET/MRI for the detection of recurrent prostate cancer following radical prostatectomy. Eur J Nucl Med Mol Imaging. 2018;45:20–30. [DOI] [PubMed] [Google Scholar]
- 27. Miksch J, Bottke D, Krohn T, et al. Interobserver variability, detection rate, and lesion patterns of 68Ga-PSMA-11-PET/CT in early-stage biochemical recurrence of prostate cancer after radical prostatectomy. Eur J Nucl Med Mol Imaging. 2020;47:2339–2347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sonni I, Eiber M, Fendler WP, et al. Impact of 68Ga-PSMA-11 PET/CT on staging and management of prostate cancer patients in various clinical settings: a prospective single-center study. J Nucl Med. 2020;61:1153–1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sanli Y, Kuyumcu S, Sanli O, et al. Relationships between serum PSA levels, Gleason scores and results of 68Ga-PSMA PET/CT in patients with recurrent prostate cancer. Ann Nucl Med. 2017;31:709–717. [DOI] [PubMed] [Google Scholar]
- 30. Lengana T, van de Wiele C, Lawal I, et al. 68Ga-PSMA-HBED-CC PET/CT imaging in black versus white South African patients with prostate carcinoma presenting with a low volume, androgen dependent biochemical recurrence: a prospective study. Nucl Med Commun. 2018;39:179–185. [DOI] [PubMed] [Google Scholar]
- 31. Rauscher I, Düwel C, Haller B, et al. Efficacy, predictive factors, and prediction nomograms for 68Ga-labeled prostate-specific membrane antigen-ligand positron-emission tomography/computed tomography in early biochemical recurrent prostate cancer after radical prostatectomy. Eur Urol. 2018;73:656–661. [DOI] [PubMed] [Google Scholar]
- 32. Berliner C, Tienken M, Frenzel T, et al. Detection rate of PET/CT in patients with biochemical relapse of prostate cancer using [68Ga]PSMA I&T and comparison with published data of [68Ga]PSMA HBED-CC. Eur J Nucl Med Mol Imaging. 2017;44:670–677. [DOI] [PubMed] [Google Scholar]
- 33. Derlin T, Schmuck S, Juhl C, et al. PSA-stratified detection rates for [68Ga]THP-PSMA, a novel probe for rapid kit-based 68Ga labeling and PET imaging, in patients with biochemical recurrence after primary therapy for prostate cancer. Eur J Nucl Med Mol Imaging. 2018;45:913–922. [DOI] [PubMed] [Google Scholar]
- 34. Grubmüller B, Baltzer P, D’Andrea D, et al. 68Ga-PSMA 11 ligand PET imaging in patients with biochemical recurrence after radical prostatectomy: diagnostic performance and impact on therapeutic decision-making. Eur J Nucl Med Mol Imaging. 2018;45:235–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Deandreis D, Guarneri A, Ceci F, et al. 68Ga-PSMA-11 PET/CT in recurrent hormone-sensitive prostate cancer (HSPC): a prospective single-centre study in patients eligible for salvage therapy. Eur J Nucl Med Mol Imaging. 2020;47:2804–2815. [DOI] [PubMed] [Google Scholar]
- 36. Farolfi A, Ceci F, Castellucci P, et al. 68Ga-PSMA-11 PET/CT in prostate cancer patients with biochemical recurrence after radical prostatectomy and PSA <0.5 ng/mL: efficacy and impact on treatment strategy. Eur J Nucl Med Mol Imaging. 2019;46:11–19. [DOI] [PubMed] [Google Scholar]
- 37. Hoffmann MA, Buchholz HG, Wieler HJ, et al. PSA and PSA kinetics thresholds for the presence of 68Ga-PSMA-11 PET/CT-detectable lesions in patients with biochemical recurrent prostate cancer. Cancers (Basel). 2020;12:398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kraft P, Maurer T, Gafita A, et al. Pre-test 68Ga-PSMA-ligand PET/CT positivity in early biochemical recurrent prostate cancer after radical prostatectomy: validation of a prediction model. EJNMMI Res. 2020;10:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Bianchi L, Borghesi M, Schiavina R, et al. Predictive accuracy and clinical benefit of a nomogram aimed to predict 68Ga-PSMA PET/CT positivity in patients with prostate cancer recurrence and PSA < 1 ng/ml external validation on a single institution database. Eur J Nucl Med Mol Imaging. 2020;47:2100–2105. [DOI] [PubMed] [Google Scholar]
- 40. Calais J, Ceci F, Eiber M, et al. 18F-fluciclovine PET-CT and 68Ga-PSMA-11 PET-CT in patients with early biochemical recurrence after prostatectomy: a prospective, single-centre, single-arm, comparative imaging trial. Lancet Oncol. 2019;20:1286–1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. McCarthy M, Francis R, Tang C, Watts J, Campbell A. A multicenter prospective clinical trial of 68gallium PSMA HBED-CC PET-CT restaging in biochemically relapsed prostate carcinoma: oligometastatic rate and distribution compared with standard imaging. Int J Radiat Oncol Biol Phys. 2019;104:801–808. [DOI] [PubMed] [Google Scholar]
- 42. Verburg FA, Pfister D, Heidenreich A, et al. Extent of disease in recurrent prostate cancer determined by [68Ga]PSMA-HBED-CC PET/CT in relation to PSA levels, PSA doubling time and Gleason score. Eur J Nucl Med Mol Imaging. 2016;43:397–403. [DOI] [PubMed] [Google Scholar]
- 43. Fourquet A, Aveline C, Cussenot O, et al. 68Ga-PSMA-11 PET/CT in restaging castration-resistant nonmetastatic prostate cancer: detection rate, impact on patients’ disease management and adequacy of impact. Sci Rep. 2020;10:2104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Treglia G, Annunziata S, Pizzuto DA, Giovanella L, Prior JO, Ceriani L. Detection rate of 18F-labeled PSMA PET/CT in biochemical recurrent prostate cancer: a systematic review and a meta-analysis. Cancers (Basel). 2019;11:710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Barbosa FG, Queiroz MA, Nunes RF, et al. Revisiting prostate cancer recurrence with PSMA PET: atlas of typical and atypical patterns of spread. Radiographics. 2019;39:186–212. [DOI] [PubMed] [Google Scholar]
- 46. Perera M, Papa N, Christidis D, et al. Sensitivity, specificity, and predictors of positive 68Ga-prostate-specific membrane antigen positron emission tomography in advanced prostate cancer: a systematic review and meta-analysis. Eur Urol. 2016;70:926–937. [DOI] [PubMed] [Google Scholar]