Abstract
Leiomyosarcoma (LMS) is the most common soft tissue sarcoma in adults and can occur in any part of the body. Uterine leiomyosarcoma (uLMS) is the most common location for LMS, making up 2% to 5% of all uterine malignancies. It is an aggressive tumor that is challenging to treat because of its resistance to standard therapy. The majority of patients (60%) are diagnosed with early-stage disease. However, regardless of the stage, uLMS has a poor prognosis. Surgical resection is the cornerstone of treatment for patients with localized LMS independent of the site of origin. Adjuvant chemotherapy for early-stage disease remains controversial as multiple clinical trials have failed to demonstrate benefit on overall survival. Progress has been made in therapy for advanced and recurrent disease. This case study will highlight the current and emerging data regarding novel therapies for women with uLMS.
CASE STUDY
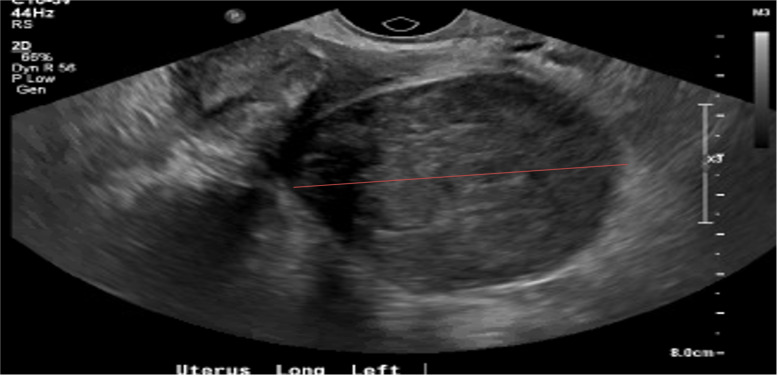
LM is a 51-year-old African American female (gravida 5, para 5) who presented to her primary care provider with complaints of postmenopausal bleeding for 3 days and associated pelvic pain radiating to the left hip. An ultrasound revealed an enlarged uterus (9.9 × 6.1 × 5.6 cm) with multiple uterine fibroids (Figure 1) and questionable pedunculated or broad ligament fibroid measuring 7.2 × 5.4 × 5.4 cm. Endometrial thickness was 4.9 mm. CEA, CA-125, and CA 19-9 were all within normal limits. MRI of the pelvis demonstrated multiple fibroids with deviation of the uterus and no convincing adnexal masses. A patient-centered discussion took place regarding surgical management vs. observation since her symptoms were minimal at that time. LM chose observation.
Figure 1.
Ultrasound of uterus. Enlarged uterus (9.9 × 6.1 × 5.6 cm) with multiple uterine fibroids.
A few months later, LM presented to the emergency department with a left lower extremity occlusive deep venous thrombosis. Vascular surgery was consulted, and she underwent antithrombolytic treatment with minimal improvement. It was felt that the clot would not improve until excision of the fibroid because of the compression that this was causing in the pelvis. She underwent exploratory laparotomy, total abdominal hysterectomy, and bilateral salpingo-oophorectomy. The mass was separate from the uterus, located between the external iliac artery and vein, and compressing the vein medially as opposed to laterally. Vascular surgery assisted in removal that resulted in a venotomy. Final pathology revealed a stage IIB high-grade spindle cell leiomyosarcoma with mitotic activity more than 10/10 high power fields.
CT scan of the chest, abdomen, and pelvis demonstrated no evidence of metastatic disease. The multidisciplinary tumor board recommended 4 cycles of gemcitabine and docetaxel. Radiation was not recommended for local control because of her recent venous injury, current thrombosis, and significant edema affecting her mobility. Following the first cycle of chemotherapy, she was hospitalized with neutropenic fever. Her second cycle was delayed 1 week. Following her second cycle, she was admitted with sepsis secondary to port infection with Serratia marcescens. Her port was removed, and she completed a 14-day course of antibiotics. LM went on to receive her remaining 2 cycles of chemotherapy without further issues. A post-treatment CT revealed no evidence of recurrent disease. She is now undergoing surveillance every 3 months for 2 years, every 6 months until 5 years, and then annual visits.
Uterine fibroids (leiomyomas) are the most common benign pelvic tumors in women and are the major indication for hysterectomy. Ultrasound evidence shows that more than 80% of African American women and 70% of White women will have uterine fibroids by age 50; however, only 20% to 50% of all women with fibroids experience symptoms, and this incidence is underestimated (Eltoukhi et al., 2014). For women over 40, abnormal uterine bleeding or pelvic pain are the most common symptoms seen with fibroids for which women seek gynecologic evaluation. Hysterectomy remains the most common intervention in the United States; however, other options may include pharmaceutical management, minimally invasive surgery, and uterine artery embolization (Eltoukhi et al., 2014).
Leiomyosarcoma (LMS) is the most common soft tissue sarcoma (STS) in adults and can occur in any part of the body (George et al., 2018). Malignant change in a leiomyoma is termed LMS. Uterine leiomyosarcoma (uLMS) is the most common location for LMS and the most common subtype of uterine sarcomas, making up 2% to 5% of all uterine malignancies (Cao et al., 2019; George et al., 2018; Roberts et al., 2018; Vellanki et al., 2010). It differs from endometrial carcinoma in prognosis and management. The median age at diagnosis is the early 50s (Cao et al., 2019).
Uterine LMS often presents with abnormal vaginal bleeding (56%), palpable pelvic mass (54%), and/or pelvic pain (22%; Cao et al., 2019; Roberts et al., 2018). It is an aggressive tumor that is challenging to treat because of its resistance to standard therapy, as evidenced by high rates of both recurrence and progression. The majority of patients (60%) are diagnosed with early-stage disease (Roberts et al., 2018). However, regardless of the stage, uLMS has a poor prognosis. Even patients with uterus-limited disease have 50% to 70% risk for recurrence due to a propensity for early hematogenous dissemination (George et al., 2018; Roberts et al., 2018). The most common sites for metastasis is to the lungs (74%) followed by peritoneum (41%), bone (33%), and liver (27%; Roberts et al., 2018; Tirumani et al., 2014). The time to recurrence varies widely, with a median of 12 to 24 months. Mortality with metastatic disease is typically within 2 years (Roberts et al., 2018).
ETIOLOGY
Most patients have no predisposing factors for developing uLMS. The potential risk factors are prior radiation therapy to the pelvis (10%–25%), long-term tamoxifen (1%–2%), or certain inherited genetic syndromes, including retinoblastoma and Li-Fraumeni syndrome (George et al., 2018; Roberts et al., 2018; Vellanki et al., 2010). Additional risk factors include postmenopausal status and African American race. Patients taking tamoxifen should be advised to have a pelvic exam every year and report any abnormal vaginal bleeding as soon as possible.
PATHOLOGY
By gross examination, uLMS are usually solitary and large (> 5 cm) in 75% of cases (Roberts et al., 2018). Tumors are composed of malignant mesenchymal cells that show distinct features of smooth muscle lineage (George et al., 2018; Mangla & Yadav, 2019). The typical histologic pattern of uLMS is large spindle cells with pleomorphic nuclei and high levels of mitotic activity (George et al., 2018; Mangla & Yadav, 2019; Vellanki et al., 2010).
Case Study
LM's pathology demonstrated a high-grade tumor that was 11.8 cm in size with the periphery of the neoplasm well-circumscribed/smooth with a zone of benign soft tissue/smooth muscle between the neoplasm and the periphery. The mitotic activity was more than 10/10 high power fields.
DIAGNOSIS
The clinical presentation of uLMS is often associated with nonspecific symptoms caused by the displacement of structures—rather than invasion—of specific anatomic locations of the primary tumor and its metastases (George et al., 2018; Mangla & Yadav, 2019). There is no single preoperative test that can reliably differentiate benign from malignant uterine disease (George et al., 2018; Mangla & Yadav, 2019; Roberts et al., 2018). Intrauterine masses that are considered to be benign fibroids but continue to increase in size after menopause should raise suspicion for malignancy (Roberts et al., 2018). Uterine LMS is typically diagnosed by pathology after hysterectomy. In rare cases, it is diagnosed with endometrial sampling preoperatively or with frozen section intraoperatively (Roberts et al., 2018).
Thirty-three percent of patients will present with distant metastatic disease; therefore, all patients should undergo imaging to rule out metastatic disease following pathologic confirmation of the diagnosis (Mangla & Yadav, 2019; Roberts et al., 2018). There are no data on the ideal imaging evaluation for women with LMS. While MRI remains the optimal imaging modality to characterize pelvic masses originating from the uterus, it is difficult to distinguish uLMS from a leiomyoma (Roberts et al., 2018). After the diagnosis is made, further imaging with CT (chest, abdomen, pelvis) or PET scans are all used to evaluate for metastatic disease (Mangla & Yadav, 2019; Roberts et al., 2018).
PROGNOSTIC FACTORS
The International Federation of Gynecology and Obstetrics (FIGO) and the American Joint Committee on Cancer (AJCC) have designated staging to define carcinoma of the corpus uteri, which applies to uterine sarcoma; the FIGO 2009 system is most commonly used (Kim & Song, 2009). The prognosis of uLMS depends on the histologic grade, tumor size, and tumor depth (Vellanki et al., 2010). Uterine LMS staged by the FIGO 2009 staging system (Table 1) does not include tumor grading (Prat, 2009). Five-year survival estimates FIGO stage I is 76%; stage II, 60%; stage III, 45%; and stage IV, 29% (Prat, 2009). Estrogen receptors (ER) and progesterone receptors (PR) have been reported to be positive in 40% to 70% of patients and may have a prognostic significance (George et al., 2018).
Table 1. FIGO Staging for Uterine Leiomyosarcoma.
| Stage | Definition |
|---|---|
| I | Tumor limited to uterus |
| IA | < 5 cm in greatest dimension |
| IB | > 5 cm in greatest dimension |
| II | Tumor extends beyond the uterus, within the pelvis |
| IIA | Adnexal involvement |
| IIB | Involvement of other pelvic tissues |
| III | Tumor invades abdominal tissues |
| IIIA | 1 site |
| IIIB | > 1 site |
| IIIC | Involves pelvic and/or para-aortic lymph nodes |
| IV | Tumor invades pelvic organs and/or distant metastasis |
| IVA | Invasion of bladder or rectum |
| IVB | Distant metastases |
Note. Adapted with permission from Prat (2009).
For women with leiomyosarcomas, some investigators consider tumor size (> 5 cm) to be the most important prognostic factor for a poorer prognosis (Franzetti Pellanda et al., 2017). Other factors that have been evaluated for their potential prognostic effect include tumor fragmentation, extrauterine spread, mitotic index, and tumor grade, although tumor grade in uLMS remains an area of controversy and is not routinely applied to diagnostic or staging procedures (Roberts et al., 2018).
Case Study
In this case, LM's tumor measured 11.8 cm and extended beyond the uterus but within the pelvis with involvement of other pelvic tissues making it a FIGO stage IIB.
SURGICAL MANAGEMENT
Surgical resection is the cornerstone of treatment for patients with localized LMS independent of the site or origin (Mangla & Yadav, 2019). Whenever possible, women with uLMS should undergo surgery and management with a gynecologic oncologist (Mangla & Yadav, 2019). The standard surgical approach for uLMS is a hysterectomy for patients whose disease is confined to the uterus. However, oophorectomy and lymphadenectomy remain controversial (Roberts et al., 2018). A National Cancer Database study found that bilateral salpingo-
oophorectomy is reasonable in perimenopausal and postmenopausal women, although there is no data to indicate that oophorectomy improves survival (Mangla & Yadav, 2019). Retrospective data have demonstrated longer overall survival among women whose disease is completely resected compared with those with residual disease and supports an attempt to resect all disease even if locally advanced if feasible (Mangla & Yadav, 2019).
Routine lymphadenectomy should not be performed in women with uLMS because the risk of occult metastatic disease to lymph nodes is low; however, lymph nodes that appear enlarged and/or suspicious should undergo resection (Mangla & Yadav, 2019). This was illustrated by Leitao and colleagues’ study of 59 women with clinical stage I or II uLMS (all of whom underwent surgical staging) in which the incidence of lymph node metastases was less than 5% (Leitao et al., 2012; Mangla & Yadav, 2019).
TREATMENT
Observation
Observation is the standard of care following resection of a uterus-limited, intact specimen. While chemotherapy or pelvic radiation is sometimes considered following surgery for uLMS, no form of adjuvant therapy has demonstrated improvement in survival outcomes compared with observation (George et al., 2018; Roberts et al., 2018). Thus, the current standard of care after resection of early-staged uLMS is observation (George et al., 2018; Roberts et al., 2018).
Radiation Therapy
The role of adjuvant radiation therapy in nonmetastatic disease is controversial. Although this therapy reduces the rate of local recurrences, it has no significant impact on overall survival, as most patients with recurrent disease have distant failures (Gadducci et al., 2008; George et al., 2018). Adjuvant radiation therapy is not recommended for patients with FIGO stage I uLMS (George et al., 2018). A phase III prospective randomized trial from EORTC (protocol 55874) evaluated the role of adjuvant radiation therapy in patients with stage I and II uLMS and randomly assigned them to treatment with postoperative radiation therapy or observation. Among women with uLMS (n = 103), pelvic radiation therapy resulted in no significant differences in either local or distant progression rates or overall survival compared with observation. Due to the lack of obvious benefit of adjuvant radiation therapy, it is not recommended in an optimally resected uLMS. In the advanced stage that is not completely resected or metastatic disease, radiation therapy is most useful when used in palliative treatment to distant sites (Gadducci et al., 2008; Mangla & Yadav, 2019).
Systemic Therapy
The role of adjuvant chemotherapy for early-stage disease is poorly defined; however, it has been used because of the high risk of systemic relapse (Roberts et al., 2018). Some experts recommend giving adjuvant chemotherapy after surgery for stage II cancers (Hensley et al., 2009, 2013). Combination docetaxel and gemcitabine has been proven as an effective regimen in patients with metastatic uLMS; therefore, it has been investigated as an adjuvant regimen (George et al., 2018).
Hensley and colleagues (2013) conducted a prospective phase II study of uterus-confined leiomyosarcoma in patients who had undergone complete surgical resection of their disease. Following cytoreduction, patients received 4 cycles of adjuvant gemcitabine plus docetaxel followed by 4 cycles of doxorubicin. At 2 years, 78% were disease-free (95% confidence interval [CI] = 67%–91%), and at 3 years, 57% were disease-free (95% CI = 44%–74%). Median time to recurrence was 27.4 months. In this study, progression-free survival was not reached and exceeded 36 months (Hensley et al., 2009, 2013).
Single-agent doxorubicin has shown to be an active agent for uLMS and is less toxic than combination therapies (Novetsky & Powell, 2013). If systemic therapy is used for treating high-grade uterine sarcoma, preferred therapies include single-agent doxorubicin or gemcitabine/docetaxel (Hensley et al., 2009, 2013; Novetsky & Powell, 2013).
Case Study
Due to the increased risk of relapse, the multidisciplinary conference recommended 4 cycles of adjuvant chemotherapy with gemcitabine 900 mg/m2 IV (days 1 and 8) and docetaxel 75 mg/m2 IV gemcitabine (day 1) given every 21 days. Radiation was not recommended for local control because of LM's recent venous injury, current thrombosis, and significant edema affecting her mobility. Following the first cycle of chemotherapy, she was hospitalized with neutropenic fever. Her second cycle was delayed 1 week. Following her second cycle, she was admitted with sepsis secondary to port infection with Serratia marcescens. Her port was removed, and she completed a 14-day course of antibiotics. She went on to receive her remaining 2 cycles of chemotherapy without further issues.
Targeted Therapy
Results from studies designed for soft tissue sarcoma that include patients with uLMS are applicable to treatment decisions for advanced uLMS. The oral multi-kinase inhibitor pazopanib (Votrient) was approved for treatment of soft tissue sarcoma that has progressed after prior cytotoxic therapy based on results of a phase III trial comparing pazopanib to placebo. Progression-free survival was 4.6 months among patients assigned to pazopanib compared with 1.6 months among those assigned to placebo. Objective response was observed in 6% of patients on pazopanib. There was no difference in overall survival (George et al., 2018; Roberts et al., 2018).
Immunotherapy
Immunotherapy strategies with the use of nivolumab (Opdivo) and pembrolizumab (Keytruda), which are both anti–PD-1 antibodies, are under active investigation. These checkpoint inhibitors are demonstrating promise as some LMS tumors express the T-cell checkpoint protein PD-1 (Roberts et al., 2018).
ADVANCED/METASTATIC DISEASE
While the majority of women present with disease confined to the uterus, about 30% to 35% will present with metastatic disease and have a poor prognosis (Roberts et al., 2018). Among the active agents for the treatment of advanced, metastatic LMS are fixed-dose-rate gemcitabine plus docetaxel, doxorubicin (with or without ifosfamide), single-agent gemcitabine, ifosfamide, trabectedin, pazopanib, and dacarbazine (National Comprehensive Cancer Network [NCCN], 2020). A prospective, phase III trial (GOG 250) demonstrated that the addition of bevacizumab to gemcitabine/docetaxel did not improve response rates or progression-free survival (Hensley et al., 2015).
Trabectedin is approved in patients with uLMS who have received a prior anthracycline-containing regimen (George et al., 2018). A phase III trial comparing trabectedin to dacarbazine in patients who had received prior anthracycline therapy demonstrated improved progression-free survival among patients treated with trabectedin (4.2 months) vs. dacarbazine (1.5 months) with no difference in overall survival (Demetri et al., 2016; Hensley et al., 2017; Martin-Broto et al., 2016; Roberts et al., 2018). Trabectedin also demonstrated activity in patients with no prior therapy (GOG 87M) either alone or in combination with doxorubicin in a prospective, phase II study; however, it should be noted that there was significant toxicity of febrile neutropenia (24%). A subsequent randomized trial of trabectedin plus doxorubicin compared with doxorubicin alone did not show superior response rates (17% in both arms) and a progression-free survival of 5.7 months vs. 5.5 months (Martin-Broto et al., 2016).
For women with metastatic disease who are not surgical candidates, treatment is given with palliative intent. Chemotherapy is a reasonable option for women with metastatic uLMS who maintain a good performance status and in whom organ function permits the use of cytotoxic chemotherapy.
SURVEILLANCE
Uterine LMS is an aggressive tumor with a high risk of relapse, even when confined to the uterus at diagnosis (NCCN, 2020). Post-treatment surveillance per NCCN Guidelines recommends a history and physical exam every 3 to 4 months for the first 2 to 3 years and then every 6 to 12 months thereafter. Imaging surveillance should include CT chest, abdomen, and pelvis every 3 to 6 months for the first 3 years and then every 6 to 12 months for the next 2 years. Depending on histology, grade, and initial stage, annual to biannual imaging can be considered for an additional 5 years. MRI abdomen/pelvis and CT chest can also be considered, with PET/CT or other imaging as needed to clarify findings or upon clinical concern for metastasis.
Patients should receive education regarding the symptoms of recurrent disease. Patients with bleeding (vaginal, bladder or rectal), decreased appetite, weight loss, pain (pelvis, abdomen, hip or back), cough, shortness of breath, and swelling (abdomen or legs) should seek prompt evaluation.
Case Study
LM is undergoing surveillance every 3 months for 2 years then every 6 months until 5 years, and then will go on to annual visits. A decision of treatment of recurrent disease is made according to site and nature of the recurrence. In LM's case, if there is a local recurrence, radiation therapy with or without brachytherapy are options. For metastatic recurrence, palliative cytotoxic chemotherapy, targeted therapy, or immunotherapy could be considered.
CONCLUSION
Uterine LMS is a rare uterine malignancy that arises from the smooth muscle of the uterine wall. Uterine LMS most commonly metastasizes to the lungs, liver, abdomen, pelvis, and pelvic or para-aortic lymph nodes. Compared with other types of uterine cancers, uLMS is an aggressive tumor associated with a high risk of recurrence regardless of stage at presentation. Whenever possible, women with uLMS should undergo surgery and management with a gynecologic oncologist (Mangla & Yadav, 2019). For all women with newly diagnosed early-stage uLMS following surgery, surveillance is recommended. Adjuvant pelvic radiation does not improve pelvic recurrence rates or survival outcomes. Adjuvant chemotherapy for early-stage disease remains controversial as multiple clinical trials have failed to demonstrated benefit on overall survival. Progress has been made in therapy for advanced and recurrent disease (Mangla & Yadav, 2019). For all women with advanced stage disease that is completely resected, chemotherapy should be considered. Novel chemotherapies, targeted therapies such as pazopanib, and new immunotherapies such as nivolumab or pembrolizumab have demonstrated promise in previously difficult, drug-resistant disease.
Footnotes
The authors have no conflicts of interest to disclose.
References
- Cao, S., Liu, Y., Bai, X., & Wang, L. (2019). A case report of uterine leiomyosarcoma. OncoTargets and Therapy, 12, 8583–8586. 10.2147/ott.s218222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demetri, G. D., von Mehren, M., Jones, R. L., Hensley, M. L., Schuetze, S. M., Staddon, A.,…Patel, S. R. (2016). Efficacy and safety of trabectedin or dacarbazine for metastatic liposarcoma or leiomyosarcoma after failure of conventional chemotherapy: Results of a phase III randomized multicenter clinical trial. Journal of Clinical Oncology, 34(8), 786–793. 10.1200/jco.2015.62.4734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eltoukhi, H. M., Modi, M. N., Weston, M., Armstrong, A. Y., & Stewart, E. A. (2014). The health disparities of uterine fibroid tumors for African American women: A public health issue. American Journal of Obstetrics and Gynecology, 210(3), 194–199. 10.1016/j.ajog.2013.08.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franzetti Pellanda, A., De Bari, B., Deniaud-Alexandre, E., Krengli, M., Van Houtte, P., Richetti, A.,…Bernier, J. (2017). Outcome and prognostic factors in 110 consecutive patients with primary uterine leiomyosarcoma: A rare cancer network study. Chinese Journal of Cancer Research, 29(6), 521–532. 10.21147/j.issn.1000-9604.2017.06.06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadducci, A., Cosio, S., Romanini, A., & Genazzani, A. R. (2008). The management of patients with uterine sarcoma: A debated clinical challenge. Critical Reviews in Oncology/Hematology, 65(2), 129–142. 10.1016/j.critrevonc.2007.06.011 [DOI] [PubMed] [Google Scholar]
- George, S., Serrano, C., Hensley, M. L., & Ray-Coquard, I. (2018). Soft tissue and uterine leiomyosarcoma. Journal of Clinical Oncology, 36(2), 144–150. 10.1200/jco.2017.75.9845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hensley, M. L., Ishill, N., Soslow, R., Larkin, J., Abu-Rustum, N., Sabbatini, P.,…Aghajanian, C. A. (2009). Adjuvant gemcitabine plus docetaxel for completely resected stages I–IV high grade uterine leiomyosarcoma: Results of a prospective study. Gynecologic Oncology, 112(3), 563–567. 10.1016/j.ygyno.2008.11.027 [DOI] [PubMed] [Google Scholar]
- Hensley, M. L., Miller, A., O’Malley, D. M., Mannel, R. S., Behbakht, K., Bakkum-Gamez, J. N., & Michael, H. (2015). Randomized phase III trial of gemcitabine plus docetaxel plus bevacizumab or placebo as first-line treatment for metastatic uterine leiomyosarcoma: An NRG Oncology/Gynecologic Oncology Group Study. Journal of Clinical Oncology, 33(10), 1180–1185. 10.1200/jco.2014.58.3781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hensley, M. L., Patel, S. R., von Mehren, M., Ganjoo, K., Jones, R. L., Staddon, A.,…Demetri, G. D. (2017). Efficacy and safety of trabectedin or dacarbazine in patients with advanced uterine leiomyosarcoma after failure of anthracycline-based chemotherapy: Subgroup analysis of a phase 3, randomized clinical trial. Gynecologic Oncology, 146(3), 531–537. 10.1016/j.ygyno.2017.06.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hensley, M. L., Wathen, J. K., Maki, R. G., Araujo, D. M., Sutton, G., Priebat, D. A.,…Baker, L. H. (2013). Adjuvant therapy for high-grade, uterus-limited leiomyosarcoma: Results of a phase 2 trial (SARC 005). Cancer, 119(8), 1555–1561. 10.1002/cncr.27942 [DOI] [PubMed] [Google Scholar]
- Kim, H. S., & Song, Y. S. (2009). International Federation of Gynecology and Obstetrics (FIGO) staging system revised: What should be considered critically for gynecologic cancer? Journal of Gynecologic Oncology, 20(3), 135–136. 10.3802/jgo.2009.20.3.135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leitao, M. M., Zivanovic, O., Chi, D. S., Hensley, M. L., O’Cearbhaill, R., Soslow, R. A., & Barakat, R. R. (2012). Surgical cytoreduction in patients with metastatic uterine leiomyosarcoma at the time of initial diagnosis. Gynecologic Oncology, 125(2), 409–413. 10.1016/j.ygyno.2012.02.014 [DOI] [PubMed] [Google Scholar]
- Mangla, A., & Yadav, U. (2019). Cancer, leiomyosarcoma. https://www.ncbi.nlm.nih.gov/books/NBK551667
- Martin-Broto, J., Pousa, A. L., de las Peñas, R., García del Muro, X., Gutierrez, A., Martinez-Trufero, J.,…Balaña, C. (2016). Randomized phase II study of trabectedin and doxorubicin compared with doxorubicin alone as first-line treatment in patients with advanced soft tissue sarcomas: A Spanish Group for Research on Sarcoma Study. Journal of Clinical Oncology, 34(19), 2294–2302. 10.1200/jco.2015.65.3329 [DOI] [PubMed] [Google Scholar]
- National Comprehensive Cancer Network. (2020). NCCN Clinical Practice Guidelines in Oncology: Uterine neoplasms. https://www.nccn.org/professionals/physician_gls/pdf/uterine.pdf
- Novetsky, A. P., & Powell, M. A. (2013). Management of sarcomas of the uterus. Current Opinion in Oncology, 25(5), 546–552. 10.1097/cco.0b013e328363e0ef [DOI] [PubMed] [Google Scholar]
- Prat, J. (2009). FIGO staging for uterine sarcomas. International Journal of Gynecology & Obstetrics, 104(3), 177–178. 10.1016/j.ijgo.2008.12.008 [DOI] [PubMed] [Google Scholar]
- Roberts, M. E., Aynardi, J. T., & Chu, C. S. (2018). Uterine leiomyosarcoma: A review of the literature and update on management options. Gynecologic Oncology, 151(3), 562–572. 10.1016/j.ygyno.2018.09.010 [DOI] [PubMed] [Google Scholar]
- Tirumani, S. H., Deaver, P., Shinagare, A. B., Tirumani, H., Hornick, J. L., George, S., & Ramaiya, N. H. (2014). Metastatic pattern of uterine leiomyosarcoma: Retrospective analysis of the predictors and outcome in 113 patients. Journal of Gynecologic Oncology, 25(4), 306–312. 10.3802/jgo.2014.25.4.306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vellanki, V. S., Rao, M., Sunkavalli, C. B., Chinamotu, R. N., & Kaja, S. (2010). A rare case of uterine leiomyosarcoma: A case report. Journal of Medical Case Reports, 4(1), article number: 222. 10.1186/1752-1947-4-222 [DOI] [PMC free article] [PubMed] [Google Scholar]