Abstract
SARS-CoV-2 infection, responsible for COVID-19 outbreak, can cause cardiac complications, worsening outcome and prognosis. In particular, it can exacerbate any underlying cardiovascular condition, leading to atherosclerosis and increased plaque vulnerability, which may cause acute coronary syndrome. We review current knowledge on the mechanisms by which SARS-CoV-2 can trigger endothelial/myocardial damage and cause plaque formation, instability and deterioration. The aim of this review is to evaluate current non-invasive diagnostic techniques for coronary arteries evaluation in COVID-19 patients, such as coronary CT angiography and atherosclerotic plaque imaging, and their clinical implications. We also discuss the role of artificial intelligence, deep learning and radiomics in the context of coronary imaging in COVID-19 patients.
Keywords: COVID-19, Atherosclerosis, Coronary imaging, Coronary CT angiography
1. Atherosclerosis and COVID-19: Pathophysiology
Cardiovascular diseases (CVDs) are the leading cause of death worldwide. Among CVDs, ischemic heart disease is responsible for 16% of the world’s total deaths [1], [2]. Even though it is widely assumed that the incidence of myocardial infarction (MI) is related to traditional cardiovascular (CV) risk factors, it is known that the risk of MI increases during acute infections, such as influenza [3]. More recently, laboratory tests showed that the highest risk occurs within the first three days of influenza [4].
The infection caused by SARS-CoV-2, declared global pandemic by WHO in March 2020, is responsible for a potentially fatal syndrome called COVID-19, which is mainly a respiratory disease, but it can also have cardiac complications with worsening prognosis [5].
The pathophysiological mechanisms underlying cardiac involvement can be summarized as follows:
-
1.
The respiratory syndrome can cause severe hypoxemia with consequent multi-organ failure and cardiac injury [6]
-
2.
SARS-CoV-2 binds to ACE2 to gain intracellular entry; this can cause endothelial dysfunction and myocardial damage [7]
-
3.
SARS-CoV-2 increases inflammation by stimulating the release of cytokines (IL-6, IL-1B and TNF) and chemokines (CCL-2, CCL-3 and CCL-5) from respiratory epithelial cells, dendritic cells (DCs) and macrophages [8]. SARS-CoV-2 also promotes accumulation of epicardial adipose tissue (EAT) and perivascular adipose tissue (PVAT). This leads to local vascular inflammation by paracrine signaling, causing endothelial disfunction, plaque formation and deterioration [9]. EAT and PVAT, in turn, sustain systemic inflammation by cytokine-release into the general circulation [10]. In COVID- 19 patients, the severity of the cytokine storm has been recognized as an important factor in predicting the clinical course of extrapulmonary organ failure and mortality [11]. In addition, high levels of pro-inflammatory cytokines may lead to macrophage infiltration of the myocardium [12].
Any of these mechanisms may exacerbate underlying pathological cardiovascular conditions, promoting plaque vulnerability, leading to increased risk of acute coronary syndrome (ACS) [13], [14]. An increase of troponin serum levels has been observed in 8–12% of COVID-19 patients, which is associated with higher mortality [15]. In general, ACS is due to inflammation, rupture or erosion of the plaque, causing acute thrombosis of the atherosclerotic plaque [16]. Moreover, the vulnerability of the plaque has broader clinical implications [17]. SARS-CoV-2 infection can promote/determine coronary atherosclerotic plaque vulnerability: high levels of pro-inflammatory cytokines and chemokines can lead to thrombus formation, inducing either tissue factor secretion or coronary vasospasm, which increase shear stress and lead to platelet activation [18]. Increased inflammation can also induce neutrophil and monocyte activation, which is responsible for endothelial damage, rupture of the fibrous cap of the plaque and consequent thrombus formation. Furthermore, CD4 + T cells stimulate smooth muscle cells to migrate into the intima and generate collagen and fatty streaks, facilitating the progression of atherosclerotic lesions [19]. PVAT/EAT involvement in atherogenesis and CAD is a well-known process, supported by in vitro, ex vivo, animal and clinical studies. These reports highlight how adipose tissue can secrete and express adipokines and cytokines, cause inflammation [20], and clarify the outside-in signaling in vascular pathophysiology [21]. Moreover, SARS-CoV-2 both disseminates into PVAT through viremic blood stream and induces PVAT inflammation and consequent activation via the systemic cytokine-storm [22].
Acute infections such as COVID-19 can lead to both type 1 and type 2 MI: the former due to thin-cap rupture, which leads to inflammatory cells and fibrin release into the lumen, causing platelets, neutrophils and fibrin accumulation, responsible for acute obstruction of coronary arteries. The latter, due to acute stressors in patients with stable known or presumed CAD, which lead to higher oxygen demand resulting in insufficient blood flow to the ischemic myocardium to meet the increased myocardial oxygen demand. In particular, an acute infection may lead to IL-1, TNF-((and catecholamines’ release resulting in a mismatch between oxygen supply and demand [23]. Moreover, in addition to type 1 and type 2 MI, in COVID-19 patients there has been an increased incidence of MINOCA (myocardial infarction with non-obstructive coronary arteries) [24] which includes plaque (causing < 50% stenosis) rupture or erosion, coronary embolism and dissection, and coronary artery spasm. From a clinical point of view, differential diagnosis between different causes of ACS and acute cardiac injury in patients with COVID-19 can be difficult [25], [26]. In this setting, after excluding type 1 MI with coronary angiography demonstrating a culprit lesion, non-invasive cardiac imaging represents a useful diagnostic tool.
2. Non-invasive imaging in coronary evaluation
(Table 1 )Even though the role of imaging remains controversial and varies depending on country and institution [27], it has been recommended worldwide the use of imaging (computed tomography (CT) or chest radiography if the access to CT is limited) in patients with moderate to severe features of COVID-19, regardless of test results, and in positive patients with worsening respiratory status [28]. Thus, chest CT is a key tool for diagnosis and staging of COVID-19, but it can also give relevant information regarding extra-pulmonary manifestations. Precisely, as regards to cardiovascular involvement, the role of cardiovascular CT (CCT) in COVID-19 is continuously under investigations, with new data appearing monthly as noted in “The Journal of Cardiovascular Computer Tomography: 2020 Year in review” [29]; the exact clinical indications when to use CT or cardiac magnetic resonance (CMR) are yet to be defined but they both have promising implications in clinical practice. With regards to CCT, it can be used to evaluate coronary artery calcification (CAC), which can be easily detected during scans, and which seems to be an integrative marker of worse prognosis in patients with COVID-19 without previous CV disease. Dillinger et al. [30] evaluated 209 patients with no history of CVD who underwent CT within 24 h of admission. The median age was 62 years and CAC was detected in 106 patients (CAC + patients = 50.7%). 50.0% of CAC + subjects had primary outcome (first occurrence of noninvasive or invasive mechanical ventilation, ECMO, or death within 30 days after admission), compared to 17.5% of CAC- patients. Hence, CAC was significantly associated with the primary outcome, even in multivariate analysis, suggesting that the presence and extent of CAC are associated with a worse prognosis. Similarly, a smaller study by Nai Fovino et al. [31] evaluated 53 hospitalized COVID-19 patients without known coronary artery disease (CAD), who underwent CT, in which CAC score was calculated. In this study, the endpoints were in-hospital mortality and intensive care unit admission. A significant increase was found among patients with high (>400) CAC score, suggesting that CAC might be a helpful tool to stratify patients, and identify those at higher risk of worse outcome.
Table 1.
Studies regarding coronary imaging in COVID-19 patients.
| Authors | Vascular segments | Number (patients) | Date published | Research | Main results |
|---|---|---|---|---|---|
| Dillinger et al. (28) | Coronary arteries | 209 | 2020 | Evaluation of coronary artery calcification (CAC) using CT within 24 h of admission in patients with no history of CVD | CAC is significantly associated with a primary outcome (noninvasive or invasive mechanical ventilation, ECMO, death within 30 days after admission) |
| Nai Fovino (29) | Coronary arteries | 53 | 2020 | CAC evaluation in hospitalized COVID- 19 patients without known CAD | CAC > 400 is significantly associated with in-hospital mortality and ICU admission |
| Scoccia et al. (30) | Coronary arteries | 1625 | 2021 | Prognostic impact of clinical and subclinical CAD, as assessed by CAC score | Presence and extent of CAD are associated with in-hospital mortality and MI/CVAamong hospitalized patients and they appear to be a better prognostic gauge as compared to a clinical CV risk assessment |
| Grodecki et al. (51) | Epicardial adipose tissue (EAT) | 109 | 2021 | Association of EAT quantified on CT with the extent of pneumonia and adverse outcomes in patients with COVID- 19. | EAT is independently associated with extent of pneumonia and adverse outcomes in patients with COVID-19 |
| Hui et al. (52) | EAT | 41 | 2020 | Correlation between clinical characteristics and cardiac injury of COVID-2019 pneumonia |
Critical and severe COVID-19 patients show lower EAT density values, indicatingcardiac inflammation |
| Kotanidis et al. (54) | Perivascular adipose tissue | 435 | 2021 | Radiotranscriptomic signature (C19-RS), quantifying cytokine- driven vascular inflammation in patients with acute COVID-19, as a risk stratification tool | COVID-19 patients have high C19-RS values; B.1.1.7 variant has higher C19-RS values. C19-RS has prognostic value for in- hospital mortality. |
| Kotanidis et al. (55) | Perivascular adipose tissue (PVAT) | 201 | 2020 | CTA-based radiotranscriptomic phenotyping of PVAT may quantify COVID- 19-induced vascular inflammation, predicting clinical outcomes |
C19-RS was significantly associated with in-hospital death and a composite endpointofin- hospital deathandICU admission |
| Stefanini et al (61) |
Coronary arteries |
28 | 2020 | Incidence, clinical presentation, angiographic findings, and clinical outcomes evaluation of STEMI in COVID-19 patients |
STEMI may represent the first clinical manifestation ofCOVID-19,butin approximately40%of cases a culprit lesion is not identifiable by coronary angiography. |
Moreover, Scoccia et al. [32] tested the hypothesis that combining clinical history with CAC score routinely could stratify prognosis of COVID-19 patients, studying in-hospital mortality and in- hospital myocardial infarction and cerebrovascular accident (MI/CVA) as primary and secondary endpoints respectively. They divided patients into three groups: clinical CAD (with a positive history), subclinical CAD (with CAC > 0) and no CAD (CAC = 0). This study found a significant increase of both in-hospital mortality and MI/CVA rates among patients with clinical and subclinical CAD. In addition, through an area under the curve improvement, a multivariate model with “CAD stratification” was found to be superior to a model based on the clinical cardiovascular risk profile. Thus, this study suggests that CAC score might be a useful tool to complement COVID-19 risk stratification tools. Moreover, the presence and extension of CAD, assessed by CAC, appears as a better parameter than clinical cardiovascular risk assessment for COVID-19 prognosis.
In case of diagnosed or suspected cardiac complications, cardiac imaging is indicated, especially when it is likely to substantially change patient management or be lifesaving [33]. To evaluate CAD, coronary CT angiography (CCTA) is the preferred non-invasive imaging modality since it reduces exposure time of patients and personnel (Fig. 1 ). Taken into account that patients with COVID-19 and CV risk factors have higher morbidity and mortality [34], CCTA represents a valid imaging modality for assessment of patients with chronic coronary syndromes. It can also exclude or confirm an ACS in COVID-19 pneumonia with elevated troponin serum levels [33]. Moreover, because NSTEMI management depends on the patient CV risk, CCTA can speed-up risk stratification. In particular, as Licu et al. [35] showed in their study, CCTA can also identify the phenotype of vulnerable plaques at higher risk of rupture, leading to future ACS (Fig. 2 ). Presence of low attenuation (focal central area of plaque with attenuation density of < 30 HU), positive remodeling (outer vessel diameter 10% greater than the mean diameter of the segments immediately proximal and distal to the plaque), spotty calcification (focal calcification within the vessel wall < 3 mm in maximum diameter) and napkin-ring sign (central area of low attenuation surrounded by a ring-shaped area of higher attenuation representing lipid-rich atheroma) seem to be the CCTA features of atheromatous plaques producing ACS (Table 2 ). The presence of these features identifies very high-risk plaques, thus, very high- risk patients, who might benefit from primary or secondary prevention. The former with healthy diet, exercise, smoking cessation and effective hypolipidemic therapy. The latter, in case of symptomatic patients, could be obtained with rapid invasive coronary angiography. In the scenario of primary prevention, plaque features on CCTA may be used to facilitate the allocation of more aggressive and costly treatment regimens (e.g. new hypolipidemic or anti-inflammatory agents) to selected patients, depending on the underlying mechanism of atherosclerosis, resulting in a tailored management of ACS.
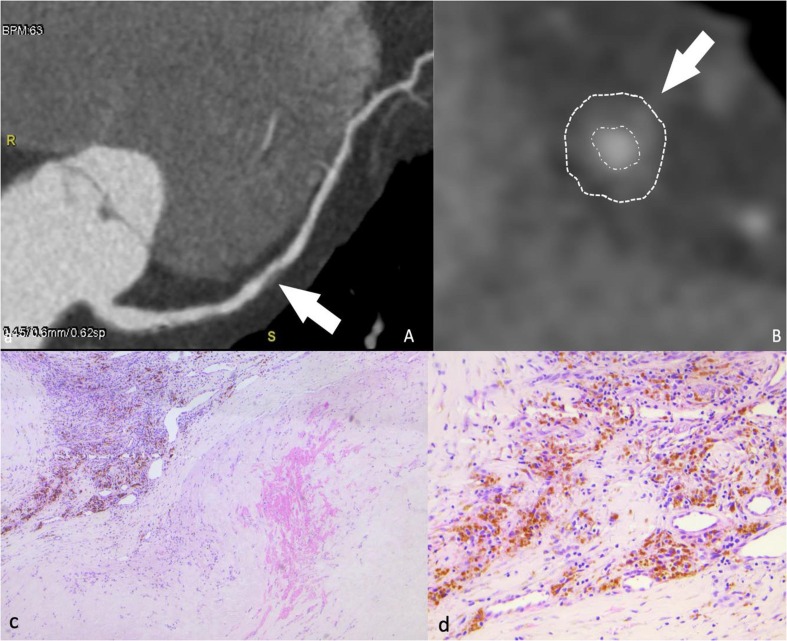
Fig. 1.
49 years old male patient underwent a CCTA (coronary CT angiography) for atipical chest pain. Images show mid- LAD soft, moderate plaque on multiplanar reconstruction (A, arrow) and cross-sectional view (B, arrow); this plaque does not present high risk features (positive remodeling, low attenuation, spotty calcification, napkin-ring sign). Histopathology on H&E is shown in C and D. C: atherosclerotic plaque with intraplaque hemorrhage (arrowhead) and neoangiogenesis with severe inflammation (arrow). D: Higher magnification of neoangigenesis inside the atherosclerotic plaque. The hemosiderin deposits, appearing as yellow–brown granules, are the sign of previous intraplaque hemorrhages.
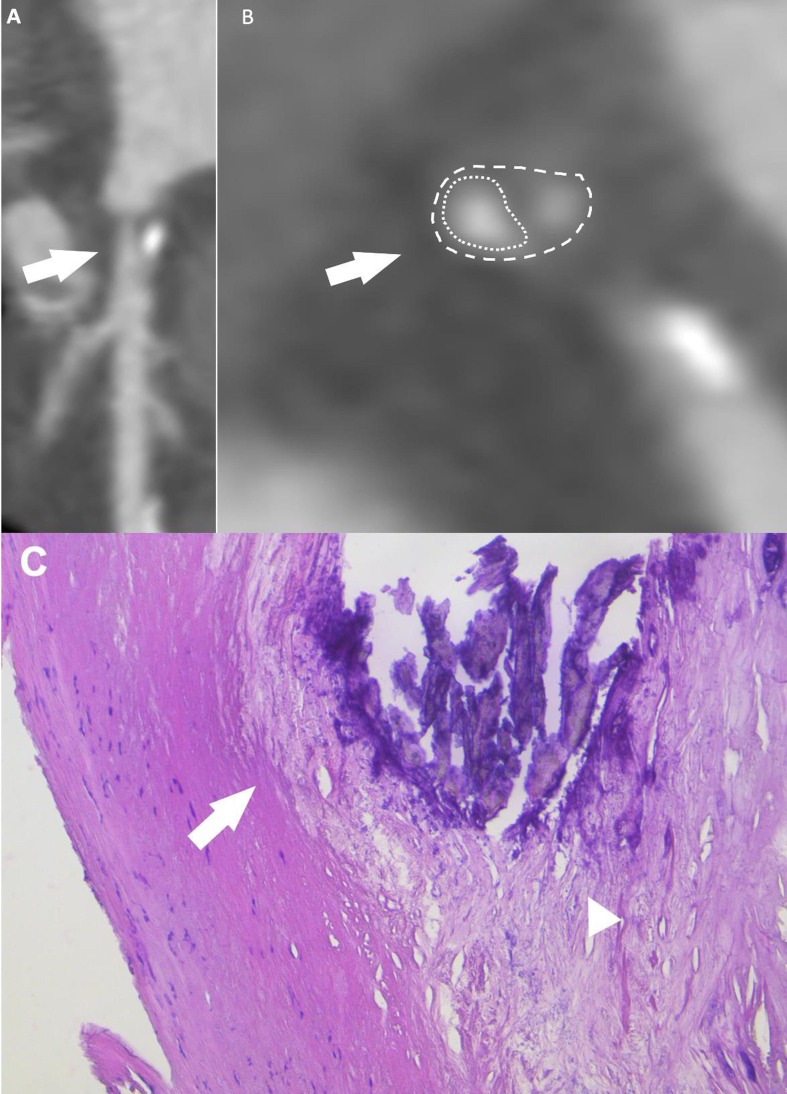
Fig. 2.
65 years old male patient underwent to CCTA for ventricular extrasystole and chest pain. Images show proximal LAD soft plaque with spotty calcification (feature of higher risk of rupture) on multiplanar reconstruction (A, arrow), cross sectional view (B, arrow) and on H&E (C) which shows the fibrous cap containing scattered inflammatory cells (C, arrows) a necrotic core contains a large calcification (C, arrowhead). Dusty calcium deposits are scattered throughout the necrotic core.
Table 2.
Vulnerable atherosclerotic plaque phenotype on CT.
| Reference | Feature | Meaning |
|---|---|---|
| Licu et al. (33) | Low attenuation plaque (LAP) | Vulnerability degree was significantly associated with higher rate of LAP, which depicts a larger necrotic core |
| Napkin-ring sign (NRS) | No significant association with plaque rupture has been found | |
| Positive remodeling (PR) | Outward PR and higher remodeling index were significantly associated with acute coronary syndrome (ACS) | |
| Spotty calcifications (SCs) | Incidence of SCs was significantly lower in patients with ACS | |
| Antonopoulos et al. (50) | Fat attenuation index (FAI) | FAI is inversely correlated with adipocyte size and expression of adipogenic markers, indicating adipose tissue inflammation |
| Dillinger et al. (28) | Coronary artery calcification (CAC) score | Presence and extent of CAC (a marker of atherosclerotic burden) is associated with worse prognosis in COVID-19 patients |
| Nai Fovino et al (29) | CAC | CAC > 400 is a marker of higher risk of worse outcome in hospitalized COVID-19 patients |
| Scoccia et al. (30) | CAC | Presence and extent of CAD, assessed by CAC, are associated with higher mortality and MI/CVA rates among hospitalized COVID-19 patients |
| Madonna et al (12) | Epicardial adipose tissue (EAT) | EAT volume correlates with CAC score, indicating total coronary plaque burden, and predicts early CAD |
| Hui et al. (52) | EAT | EAT density can be used as an indicator of cardiac inflammation in COVID-19. Lower density has been detected in severe and critical patients |
While little has changed in the management of STEMI during COVID-19 pandemic [36], for NSTE-ACS the European Society of Cardiology recommends non-invasive testing for patients included among intermediate and low risk categories, favoring CCTA if equipment and expertise are available in order to shorten hospital stay [37]. As the VERDICT trial shows, CCTA is a valid imaging modality, able to rule out clinically significant CAD (stenosis > 50%) in NSTE-ACS, with its negative predictive value of 90.9% and positive predictive value of 87.9%. Thus, the trial suggests that CCTA should be utilized to quickly identify patients in whom invasive testing will be futile. The implications are multiple and of great relevance: a clinical impact, reducing the duration of antithrombotic medical therapy; a safety impact for COVID-19 containment, avoiding unnecessary exposure and shortening hospitalization [38]; an economic impact, considering that over a lifetime, CCTA is cost effective because it allows an early and accurate detection of true CAD status, reducing mortality thanks to a more appropriate preventive medical therapy [39].
For patients with low pre-test probability, CCTA may be combined with myocardial late iodine enhancement (LIE), in order to detect myocardial inflammation, fibrosis or diffuse ischemia [40]. Even though cardiac MRI with late gadolinium enhancement (LGE) is the gold standard to detect myocardial fibrosis/edema/necrosis, LIE cardiac CT represents an effective alternative, particularly because of its fast acquisition times, accessibility and suitability for patients for whom MRI is contraindicated. In their study, Oda et al. [41] showed excellent agreement between MRI-LGE and CT-LIE; for this reason, they suggested that cardiac CT can assess late enhancing lesions with results comparable to those obtained with cardiac MRI, giving adequate information about myocardial characterization. Similarly, Otha et al. assessed the diagnostic performance of dual-energy CT with myocardial LIE using MRI-LGE as standard of reference and they obtained high accuracy, high diagnostic performance and excellent agreement [42]. In the context of SARS-CoV-2 infection, LIE can be a valuable additional tool, especially when MRI cannot be performed because of its long examination time which results in long exposure time. As Pontone et al. [43] showed in a single-case report and further explained in their review [44], in patients with COVID-19 specific scan protocols for CT allow to identify pneumonia and obstructive CAD (dual rule out), as well as pulmonary embolism (triple rule out) and also myocarditis through LIE, creating the new concept of quadruple rule out (Fig. 3, Fig. 4 ). In addition, as Feuchtner et al. [45] suggested in a single-case report, CCTA can also show suggestive findings for vasculitis, which may trigger endothelial dysfunction and vasospasm, explaining MINOCA in the context of NSTE-ACS.
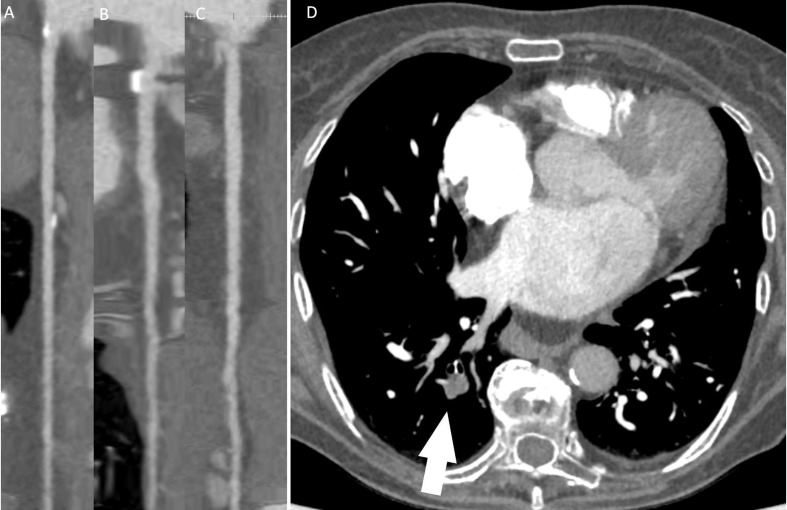
Fig. 3.
81 years old female patient with history of COVID19 three months earlier, underwent cardiac computed tomography for atypical chest pain. LAD (A), LCx (B) and RCA (C) did not show any significant stenosis. However pulmonary embolism was observed in superior segment of right inferior lobe (arrow, D).
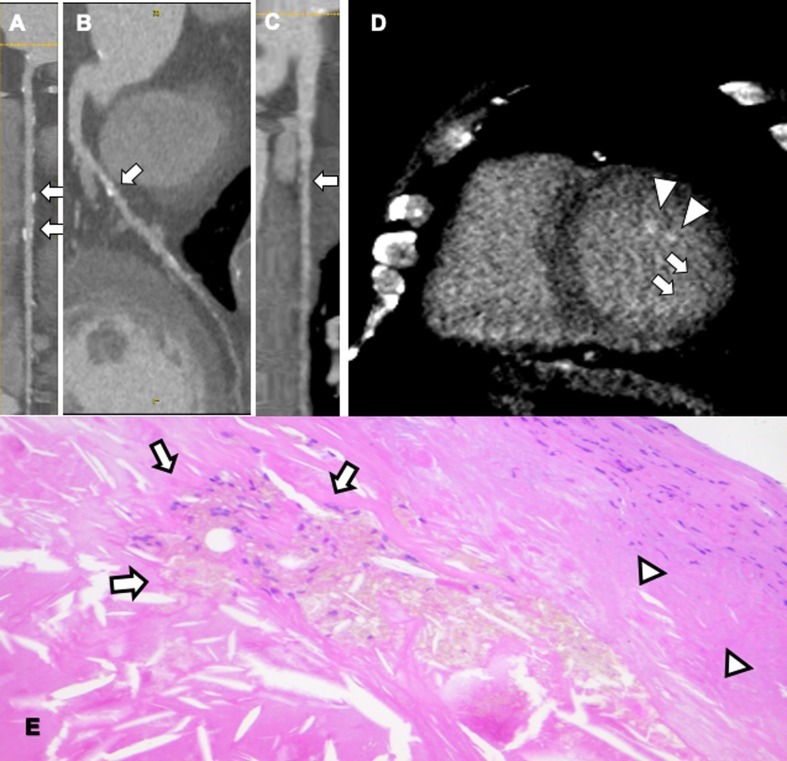
Fig. 4.
65 years old male patient with history of dilated cardiomyopathy, multiple cardiovascular risk factors that was not able to perform MR due to old metal femoral stem. Calcified moderate stenosis was observed on mid-RCA (A), LAD (B) and moderate mixed plaque on proximal first marginal (C). Late iodine enhancement showed hyperenhancement of subendocradium in inferolateral wall (arrows, D) and papillary muscles (arrowheads, D), indicating necrosis/fibrosis/inflammation. H&E view of unstable atherosclerotic plaque (E), with necrotic core and spindle lipid deposits (arrows) and fibrous cap with diffuse inflammatory infiltrate (arrowheads).
Since the advent of deep learning (DL)/machine learning (ML) techniques, there has been a huge and meaningful impact on radiology and cardiac radiology as well. In general, artificial intelligence (AI) technologies are those that make educated decisions regarding diagnosis, monitoring, treatment and management of a patient’s condition. Among AI, ML architecture is a two-step process in which features are extracted and used within a training system to create offline coefficients, which are then transformed by test data giving an intelligent classification. Meanwhile, DL architecture is similar to a visual cortex that imitates multiple layers of neural network applied to image data to directly extract features and to characterize tissues [46]. As Litjens et al. [47] examined in their review, many types of DL algorithms can be applied to cardiovascular imaging, the most common being convolutional neural networks (CNNs). CNNs are a type of artificial neural networks commonly used in image analysis, especially when the tasks required are classification (for example plaque risk assessment), regression (e.g. calcium scoring) or anatomical segmentation. Moreover, as Saba et al. [48] noted in their review, considering the rapid pace of growth of DL methods and their clinical and economic advantages, it is inevitable that they will have an increasingly relevant role in radiology.
In the COVID-19 pandemic setting, since the prevalence is high, AI, mainly with DL technologies, works well and represents a useful tool for diagnostic and prognostic purposes. Wang et al. [49] in their study proposed a fully automatic DL system that detects COVID-19 features on CT images, predicting the patient’s probability of having COVID-19. They also stratified patients into high-risk and low-risk groups, identifying those who need urgent medical care. Thus, they suggested DL as a convenient tool for fast screening COVID-19. Moreover, in a study that compared six AI paradigms used to classify COVID pneumonia and non-COVID pneumonia, Saba et al. [50] showed that all six AI models successfully classified COVID pneumonia against non-COVID. In the same study, DL was shown to have higher accuracy than ML. Similar results have been obtained by Li et al. [51] who developed a 3D DL framework for COVID- 19 detection. For a complete review on AI-based image analysis for COVID-19 see Dong et al. work [52].
AI may be applied to CCTA as well. As Han et al. [53] assessed in their study, AI-based CCTA significantly reduces time for post-processing and diagnosis compared to traditional methods. Moreover, CCTA-AI, when compared to traditional CCTA, appeared to have sensitivity of 88% versus 59%, accuracy of 86 versus 83% and negative predictive value of 94% versus 83% in identifying > 50% of stenotic vessels. Similarly, Muscogiuri et al. [54] in their study tried to develop a CNN to classify CCTA in the correct CAD reporting and data system (CAD-RADS) category. CAD-RADS classification is used to identify patients that may require further testing or invasive imaging, but also to evaluate the patient’s prognosis. In this classification, the final score of CCTA is based on patient-based analysis, in which each vessel is evaluated using a scale from 0 (absence of stenosis) to 5 (total occlusion), with 4 being either stenosis between 70 and 99% or > 50% left main or three vessels > 70%. The main findings obtained are represented by the high diagnostic accuracy of CNN in differentiating patients with CAD-RADS 0 from those with CAD-RADS > 0. Collectively, the application of the CAD-RADS classification allows ruling out CAD in a short time, and significantly decreases time of analysis for on-site physicians reading images compared to the CNN method.
The combination of CCTA and AI has been used to evaluate another promising non-invasive imaging marker, the perivascular fat attenuation index (FAI), a marker of adipocyte lipid content and size. In this field, considerable progress has been made by the study of Antonopoulos et al. [55], who hypothesized that the PVAT phenotype might change depending on different degrees of inflammation. In this work, PVAT gradient was measured with CCTA and quantified by the transcriptomic profile using AI. They obtained promising results, indicating that FAI has great sensitivity and specificity for detecting tissue inflammation and predicting future cardiovascular events. In the context of COVID-19 pandemic, considering the role of PVAT in the pathophysiology of atherosclerosis and CAD, EAT/PVAT volume and attenuation, measured with CT, have been used as indicators of extent of pneumonia and predictors of adverse outcomes. As Grodecki et al. [56] showed in their study, EAT volume and attenuation, automatically quantified with a DL algorithm from 3D fat voxels between −190 and −30 HU, associate with quantitative burden of COVID-19 pneumonia and independently predict clinical deterioration or death. Moreover, Hui et al. [57] suggested that EAT, defined as fat within the pericardial sac, is associated with increased inflammatory cytokine expression in patients with CAD. They also showed in their study that EAT density, measured in a layer range between the bifurcation of pulmonary artery and the diaphragm, has lower values in severe and critical COVID-19 patients when compared to light and mild groups. These findings suggest that low EAT density might be used as an indicator of cardiac inflammation in COVID-19 patients.
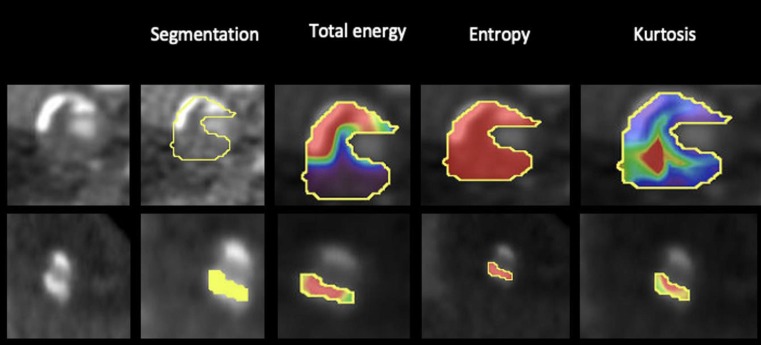
Among AI, recent research moved toward radiomics, a group of techniques in which highly abstract quantitative features are automatically extracted from medical images and then processed by statistical classifiers that select which features are relevant to the task. This method is particularly useful for coronary plaque characterization. Since coronary lesions are composed by different histologic components, each tissue will be depicted with different attenuation values on CT, giving quantitative image parameters, including shape, volume, heterogeneity, lesion length and remodeling index (Fig. 5 ). These characteristics have been shown to be predictive markers of future cardiac events [58]. During COVID-19 pandemic, this technique has been applied to PVAT in order to quantify inflammation. Kotanidis et al. [59], [60] developed a radiotranscriptomic signature (C19-RS) that can detect cytokine-driven vascular inflammation and demonstrated its prognostic value for in- hospital mortality and ICU admission. Moreover, they showed that SARS-CoV-2B.1.1.7 variant (“UK variant”) has higher C19-RS values compared to the original variant, suggesting a higher degree of inflammation.
Fig. 5.
Example of coronary CT angiography cross sections with radiomics analysis in two sample patients. Fig. 5a showed a 61-year-old man presented at our department with typical chest pain with a vulnerable plaque in the mid left anterior descending artery. From left to right, first we have segmented the coronary arteries to locate the coronary plaque, then we have shown three representative first order texture parameters (total energy, entropy and kurtosis, which represent heterogeneity, randomness and flatness of an image respectively), describing the distribution of HU values within a target lesion. Conversely, Fig. 2b demonstrated an 82-years-old woman free of symptoms with an eccentric calcified plaque in the mid right coronary artery. Postprocessing imaging data was performed with dedicated software (Olea Sphere 3.0; Olea Medical, La Ciotat, France).
AI can also be used for public health management. As Vaishya et al. [61] summarized in their review, AI can be applied for early detection of the infection, for monitoring and prediction of the spread of the virus, for contact tracing and for projection of cases and mortality.
3. Discussion
The public health and economic burdens of CAD are substantial worldwide. Since the beginning of COVID-19 pandemic, even more importance should be given to CAD considering the impact on mortality and prognosis that this group of diseases has on COVID-19 patients [62]. As many authors studied and explained, SARS-CoV-2 infection and CAD are mutually linked: not only pre-existing chronic CAD is associated with worse clinical outcomes, but also SARS-CoV-2 itself is associated with a higher risk of atherosclerosis and greater plaque vulnerability leading to a deterioration of the patient’s clinical condition. Thus, assessment of CAD is crucial in patients with CV risk factors presenting with COVID-19. In this context, non-invasive imaging techniques can play a valuable role in speeding-up risk stratification, while reducing exposure time to SARS-CoV- 2.
The link between CAD and COVID-19 and its impact on patients who do recover from the infection has not been clarified yet. The Center of Disease Control and Prevention started reporting long-term effects of SARS-CoV-2: many patients continue to report cardiopulmonary symptoms (e.g. chest pain, dyspnea, fatigue), colloquially known as “long COVID”, suggesting that the cardiovascular consequences of COVID-19 may extend beyond acute infection. Several registries and studies have been created to track the cardiac impact of the infection, but the results are still ongoing. For example, the post hospitalization COVID-19 study of the British Heart Foundation (https://www.phosp.org) will follow-up patients with questionnaires, laboratory tests, functional tests, cardiac MRI and chest CT. The latter will analyze for evidence of inflammation in the heart and the use of AI will help quantifying its level, comparing it to the one calculated before and during infection.
As we can read in The Journal of Cardiovascular Computer Tomography: 2020 Year in review [29], the field of CCTA advances at a remarkable pace with expanding clinical implications. Firstly, CAC score, assessed by CAC-DRS (Data and Reporting System), seems to have high prognostic accuracy, as well as CAD-RADS, which provides additional prognostic discrimination for prediction of future coronary heart diseases [63]. Moreover, using CAC score as a screening tool to assess CVD risk seems to have important consequences on treatment management. As Van der Aalst et al. [64] suggested in their study, CAC score compared to Systematic COronary Risk Evaluation (SCORE) classifies significantly fewer men and women at increased risk, thus less preventive treatment (ACE-inhibitors + statins) is indicated in this asymptomatic population, which may increase compliance and decrease costs for unnecessary treatment.
In addition, now that considerable progress has been made towards a better management of COVID-19, ACS represents a serious concern currently affecting global healthcare. ACS incidence is trending downward worldwide, having important consequences and implications. The Italian Society of Cardiology registered, during the pandemic, a significant reduction of the 48,4% of MI incidence in a week (for both STEMI and NSTEMI), compared to the equivalent period in 2019. Nevertheless, they observed an increase in STEMI fatality rate and complications during the pandemic compared to 2019 [65]. These data suggest that either patients are waiting longer between symptoms onset and hospital referral, or healthcare resources/medical effort are relocated to manage the pandemic, or both. Whichever the reason is, the consequences have great impact on global healthcare: late presenting MIs lead to chronic heart failure and sudden cardiac death, causing an increase of early and late morbidity and mortality. Thus, the global registration of lower rates of admitted and treated patients with ACS is a problem that has to be addressed. There are two possible ways to face it: promoting patients’ compliance and education on ACS, in order to avoid unnecessary delays in STEMI management, such as symptom-to-door time, which during the pandemic is deeply influenced by patient perception and fear of contracting COVID-19. Secondly, with regards to NSTEMI, every attempt should be made to avoid clinicians’ delayed or missed diagnosis. For this purpose, CCTA might be a useful tool to differentiate the causes of troponin-elevation and to identify patients who do not need invasive testing. In this setting, guidance and guidelines from international societies represent valuable tools to standardize protocols for management and treatment of ACS.
In line with the current recommendations from the European Society of Cardiology (ESC), the European Association of Cardiovascular Imaging (EACVI) and the Society of Cardiovascular Computed Tomography (SCCT), cardiac CT in COVID-19 patients should be limited to hospitalized patients, where it is likely to change patient management or be lifesaving [33], [37], [66]. In particular, CCTA is useful for, and should be reserved to, patients with low and intermediate risk of NSTE-ACS, in order to rule-out ACS without undergoing invasive coronary angiography (ICA), which is associated to higher risk for both the patient (procedural risks) and the healthcare providers (exposure risk). Thus, in the clinical setting, an ideal diagnostic algorithm might be performing a CCTA with a “quadruple rule-out” protocol and CAC score evaluation in patients with suspected or diagnosed COVID-19, who presents with chest pain/mild troponin increase/normal ECG or non-ST elevation, with low-to-intermediate CV risk, in order to quickly exclude the need of early invasive coronary angiography. In all the other cases of diagnosed SARS-CoV-2 infection, the mere CAC score evaluation might be included in routine chest-CT in order to better assess CV risk and COVID-19 prognosis.
Moreover, in the setting of ACS all efforts should be made to differentiate type 1 MI from type 2 MI and other causes of troponin elevation or ST changes, in order to avoid unnecessary ICA [67]. As Stefanini et al. [24] analyzed in their study, approximately 40% of their COVID-19 patients presenting with STEMI, did not have obstructive CAD and no culprit lesion was identifiable on ICA, excluding type 1 MI.
In this context, CCTA represents a valid clinical and diagnostic modality, thanks to its numerous advantages summarized as follows:
-
-
Coronary CT Angiography (CCTA) is a rapid non-invasive imaging modality for cardiovascular risk evaluation in patients during and after exposure to COVID-19;
-
-
CCTA with a 'quadruple rule out' protocol can identify pneumonia, pulmonary embolism, CAD and myocardial injury in acute chest pain;
-
-
CCTA is a non-invasive imaging modality with high accuracy and high negative predictive value, enabling identification of acute coronary syndromes without ST-segment elevation (NSTE-ACS) for which invasive testing will be futile;
-
-
Coronary artery calcification (CAC) seems a promising tool to evaluate CAD risks in COVID-19 patients;
-
-
CCTA has the potential to identify the phenotype of vulnerable plaques at higher risk of rupture and may be used to select patients at very-high risk for adequate invasive imaging;
-
-
Perivascular adipose tissue (PVAT) evaluated with CCTA may provide comprehensive information on inflammation grade and has the potential to provide predictive markers for pneumonia, in-hospital mortality and ICU admission;
-
-
Artificial intelligence (AI) and deep learning (DL) algorithms provide tools to reduce post-processing time and increase diagnostic accuracy.
However, CCTA has also some limitations: performing the exam in a symptomatic COVID-19 patient may be difficult, due to tachycardia and lack of breath-hold, leading to the need of beta-blockade and adjustment of the scanning protocol, in order to ensure optimal image quality and accurate diagnosis. That’s because CCTA should be performed only in equipped and qualified centers. Even though CCTA is not free of challenges, it is a well-established, effective imaging technique, which can be extremely useful during COVID-19 pandemic, particularly in the context of coronary artery diseases.
The role of this promising imaging modality should be further evaluated in studies based on large numbers of patients.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
All authors contributed as authors to this work.
References
- 1.Vos T, Lim SS, Abbafat Ci, et al.: Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019 Lancet, 2020;396:1204-1222. [DOI] [PMC free article] [PubMed]
- 2.WHO - World Health Organization. (2020, December 9th). Retrieved from who.int: https://www.who.int/news-room/fact-sheets/detail/the-top-10-causes-of-death.
- 3.Collins SD: Excess mortality from causes other than influenza and pneumonia during influenza epidemics. Public Health Rep (1896-1970) 1932;47:2159-79.
- 4.Smeeth L., Thomas S.L., Hall A.J., Hubbard R., Farrington P., Vallance P. Risk of myocardial infarction and stroke after acute infection or vaccination. N Engl J Med. 2004;351(25):2611–2618. doi: 10.1056/NEJMoa041747. [DOI] [PubMed] [Google Scholar]
- 5.Shi S., Qin M.u., Shen B.o., Cai Y., Liu T., Yang F., Gong W., Liu X.u., Liang J., Zhao Q., Huang H.e., Yang B.o., Huang C. Association of cardiac injury with mortality in. hospitalized patients with COVID-19 in Wuhan, China. JAMA Cardiol. 2020;5(7):802. doi: 10.1001/jamacardio.2020.0950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., Zhang L., Fan G., Xu J., Gu X., Cheng Z., Yu T., Xia J., Wei Y., Wu W., Xie X., Yin W., Li H., Liu M., Xiao Y., Gao H., Guo L., Xie J., Wang G., Jiang R., Gao Z., Jin Q., Wang J., Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. The Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zheng Y.-Y., Ma Y.-T., Zhang J.-Y., Xie X. COVID-19 and the cardiovascular system. Nature Rev Cardiol. 2020;17(5):259–260. doi: 10.1038/s41569-020-0360-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Law H.K.W., Cheung C.Y., Ng H.Y., Sia S.F., Chan Y.O., Luk W., Nicholls J.M., Peiris J.S.M., Lau Y.L. Chemokine up-regulation in SARS-coronavirus- infected, monocyte-derived human dendritic cells. Blood. 2005;106(7):2366–2374. doi: 10.1182/blood-2004-10-4166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mester A, Benedek I, Rat N, Tolescu C, Polexa SA, Benedek T. Imaging Cardiovascular Inflammation in the COVID-19 Era. Diagnostics (Basel). 2021;11(6):1114. Published 2021 Jun 18. doi:10.3390/diagnostics11061114. [DOI] [PMC free article] [PubMed]
- 10.Madonna R., Massaro M., Scoditti E., Pescetelli I., De Caterina R. The epicardial adipose tissue and the coronary arteries: dangerous liaisons. Cardiovasc Res. 2019;115(6):1013–1025. doi: 10.1093/cvr/cvz062. [DOI] [PubMed] [Google Scholar]
- 11.Wang H., Ma S. The cytokine storm and factors determining the sequence and se- verity of organ dysfunction in multiple organ dysfunction syndrome. Am J Emerg Med. 2008;26(6):711–715. doi: 10.1016/j.ajem.2007.10.031. [DOI] [PubMed] [Google Scholar]
- 12.Basso C, Leone O, Rizzo S et al.: Pathological features of COVID-19-associated myocardial injury: a multicentre cardiovascular pathology study. European Heart Journal (2020) 00, 1–9 doi:10.1093/eurheartj/ehaa664. [DOI] [PMC free article] [PubMed]
- 13.Xiong TY, Redwood S, Prendergast B, Chen M. Coronaviruses and the cardio-vascular system: acute and long-term implications. Eur Heart J 2020;41:1798–1800. [DOI] [PMC free article] [PubMed]
- 14.Saba L., Gerosa C., Wintermark M., Hedin U., Fanni D., Suri J.S., Balestrieri A., Faa G. Can COVID19 trigger the plaque vulnerability-a Kounis syndrome warning for “asymptomatic subjects”. Cardiovasc Diagn Ther. 2020;10(5):1352–1355. doi: 10.21037/cdt-20-561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Babapoor-Farrokhran S., Gill D., Walker J., Rasekhi R.T., Bozorgnia B., Amanullah A. Myocardial injury and COVID-19: Possible mechanisms. Life Sciences. 2020;253:117723. doi: 10.1016/j.lfs.2020.117723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Libby P. Mechanisms of Acute Coronary Syndromes and Their Implications for Therapy. N Engl J Med. 2013;368(21):2004–2013. doi: 10.1056/NEJMra1216063. [DOI] [PubMed] [Google Scholar]
- 17.Naghavi M., Libby P., Falk E., Casscells S.W., Litovsky S., Rumberger J., Badimon J.J., Stefanadis C., Moreno P., Pasterkamp G., Fayad Z., Stone P.H., Waxman S., Raggi P., Madjid M., Zarrabi A., Burke A., Yuan C., Fitzgerald P.J., Siscovick D.S., de Korte C.L., Aikawa M., Juhani Airaksinen K.E., Assmann G., Becker C.R., Chesebro J.H., Farb A., Galis Z.S., Jackson C., Jang I.-K., Koenig W., Lodder R.A., March K., Demirovic J., Navab M., Priori S.G., Rekhter M.D., Bahr R., Grundy S.M., Mehran R., Colombo A., Boerwinkle E., Ballantyne C., Insull W., Schwartz R.S., Vogel R., Serruys P.W., Hansson G.K., Faxon D.P., Kaul S., Drexler H., Greenland P., Muller J.E., Virmani R., Ridker P.M., Zipes D.P., Shah P.K., Willerson J.T. From Vulnerable Plaque to Vulnerable Patient: A Call for New Definitions and Risk Assessment Strategies: Part I. Circulation. 2003;108(14):1664–1672. doi: 10.1161/01.CIR.0000087480.94275.97. [DOI] [PubMed] [Google Scholar]
- 18.Sheth AR, Udhayvir S. Grewal US, Patel HP et al.: Possible mechanisms responsible for acute coronary events in COVID-19 Medical Hypotheses 2020;143: 110125. [DOI] [PMC free article] [PubMed]
- 19.Corrales-Medina V.F., Madjid M., Musher D.M. Role of acute infection in triggering acute coronary syndromes. Lancet Infect Dis. 2010;10(2):83–92. doi: 10.1016/S1473-3099(09)70331-7. [DOI] [PubMed] [Google Scholar]
- 20.Verhagen S.N., Visseren F.L. Perivascular adipose tissue as a cause of atherosclerosis. Atherosclerosis. 2011;214(1):3–10. doi: 10.1016/j.atherosclerosis.2010.05.034. [DOI] [PubMed] [Google Scholar]
- 21.Kim H.W., Shi H., Winkler M.A., Lee R., Weintraub N.L. Perivascular Adipose Tissue and Vascular Perturbation/Atherosclerosis. Arterioscler Thromb Vasc Biol. 2020;40(11):2569–2576. doi: 10.1161/ATVBAHA.120.312470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yin J., Wang S., Liu Y., Chen J., Li D., Xu T. Coronary microvascular dysfunction pathophysiology in COVID-19 [published online ahead of print, 2021 May 20] Microcirculation. 2021;28(7) doi: 10.1111/micc.v28.710.1111/micc.12718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Longo D.L., Musher D.M., Abers M.S., Corrales-Medina V.F. Acute Infection and Myocardial Infarction. N Engl J Med. 2019;380(2):171–176. doi: 10.1056/NEJMra1808137. [DOI] [PubMed] [Google Scholar]
- 24.Stefanini G.G., Montorfano M., Trabattoni D., Andreini D., Ferrante G., Ancona M., Metra M., Curello S., Maffeo D., Pero G., Cacucci M., Assanelli E., Bellini B., Russo F., Ielasi A., Tespili M., Danzi G.B., Vandoni P., Bollati M., Barbieri L., Oreglia J., Lettieri C., Cremonesi A., Carugo S., Reimers B., Condorelli G., Chieffo A. ST-elevation myocardial infarction in patients with COVID-19: clinical and angiographic outcomes. Circulation. 2020;141(25):2113–2116. doi: 10.1161/CIRCULATIONAHA.120.047525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Peretto G., Sala S., Caforio A.L.P. Acute myocardial injury, MINOCA, or myocarditis? Improving characterization of coronavirus-associated myocardial involvement. Eur Heart J. 2020 Jun 7;41(22):2124–2125. doi: 10.1093/eurheartj/ehaa396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Manolis A.S., Manolis A.A., Manolis T.A., Melita H. COVID-19 and Acute Myocardial Injury and Infarction: Related Mechanisms and Emerging Challenges. J Cardiovasc Pharmacol Ther. 2021;26(5):399–414. doi: 10.1177/10742484211011026. [DOI] [PubMed] [Google Scholar]
- 27.Kanne J.P., Bai H., Bernheim A., Chung M., Haramati L.B., Kallmes D.F., Little B.P., Rubin G.D., Sverzellati N. COVID-19 Imaging: What We Know Now and What Remains Unknown. Radiology. 2021;299(3):E262–E279. doi: 10.1148/radiol.2021204522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rubin G.D., Ryerson C.J., Haramati L.B., Sverzellati N., Kanne J.P., Raoof S., Schluger N.W., Volpi A., Yim J.-J., Martin I.B.K., Anderson D.J., Kong C., Altes T., Bush A., Desai S.R., Goldin J., Goo J.M., Humbert M., Inoue Y., Kauczor H.-U., Luo F., Mazzone P.J., Prokop M., Remy-Jardin M., Richeldi L., Schaefer-Prokop C.M., Tomiyama N., Wells A.U., Leung A.N. The Role of Chest Imaging in Patient Management During the COVID-19 Pandemic. Chest. 2020;158(1):106–116. doi: 10.1016/j.chest.2020.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Villines T.C., Al’Aref S.J., Andreini D., Chen M.Y., Choi A.D., De Cecco C.N., Dey D., Earls J.P., Ferencik M., Gransar H., Hecht H., Leipsic J.A., Lu M.T., Marwan M., Maurovich-Horvat P., Nicol E., Pontone G., Weir-McCall J., Whelton S.P., Williams M.C., Arbab-Zadeh A., Feuchtner G.M. The Journal of Cardiovascular Computed Tomography: 2020 Year in review. J Cardiovasc Comput Tomogr. 2021;15(2):180–189. doi: 10.1016/j.jcct.2021.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dillinger J.G., Benmessaoud F.A., Pezel T., Voicu S., Sideris G., Chergui N., Hamzi L., Chauvin A., Leroy P., Gautier J.F., Sène D., Henry P. Coronary Artery Calcification and Complications in Patients With COVID-19. JACC: Cardiovascular Imaging. 2020;13(11):2468–2470. doi: 10.1016/j.jcmg.2020.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nai Fovino L., Cademartiri F., Tarantini G. Subclinical coronary artery disease in COVID-19 patients. Eur Heart J Cardiovasc Imaging. 2020 Sep 1;21(9):1055–1056. doi: 10.1093/ehjci/jeaa202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Scoccia A., Gallone G., Cereda A., Palmisano A., Vignale D., Leone R., Nicoletti V., Gnasso C., Monello A., Khokhar A., Sticchi A., Biagi A., Tacchetti C., Campo G., Rapezzi C., Ponticelli F., Danzi G.B., Loffi M., Pontone G., Andreini D., Casella G., Iannopollo G., Ippolito D., Bellani G., Patelli G., Besana F., Costa C., Vignali L., Benatti G., Iannaccone M., Vaudano P.G., Pacielli A., De Carlini C.C., Maggiolini S., Bonaffini P.A., Senni M., Scarnecchia E., Anastasio F., Colombo A., Ferrari R., Esposito A., Giannini F., Toselli M. Impact of clinical and subclinical coronary artery disease as assessed by coronary artery calcium in COVID-19. Atherosclerosis. 2021;328:136–143. doi: 10.1016/j.atherosclerosis.2021.03.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Skulstad H., Cosyns B., Popescu B.A., Galderisi M., Salvo G.D., Donal E., Petersen S., Gimelli A., Haugaa K.H., Muraru D., Almeida A.G., Schulz-Menger J., Dweck M.R., Pontone G., Sade L.E., Gerber B., Maurovich-Horvat P., Bharucha T., Cameli M., Magne J., Westwood M., Maurer G., Edvardsen T. COVID-19 pandemic and cardiac imaging: EACVI recommendations on precautions, indications, prioritization, and protection for patients and healthcare personnel. Eur Heart J Cardiovasc Imaging. 2020 Jun 1;21(6):592–598. doi: 10.1093/ehjci/jeaa072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Clerkin K.J., Fried J.A., Raikhelkar J., Sayer G., Griffin J.M., Masoumi A., Jain S.S., Burkhoff D., Kumaraiah D., Rabbani L., Schwartz A., Uriel N. COVID-19 and Cardiovascular Disease. Circulation. 2020 May 19;141(20):1648–1655. doi: 10.1161/CIRCULATIONAHA.120.046941. Epub 2020 Mar 21 PMID: 32200663. [DOI] [PubMed] [Google Scholar]
- 35.Licu R.-A., Blîndu E., Opincariu D., Benedek T. Vulnerable Plaques Producing an Acute Coronary Syndrome Exhibit a Different CT Phenotype than Those That Remain Silent. Journal Of Cardiovascular Emergencies. 2020;6(2):26–34. doi: 10.2478/jce-2020-0008. [DOI] [Google Scholar]
- 36.Mahmud E, Dauerman HL, Welt FGP, Messenger JC, Rao SV, Grines C, Mattu A, Kirtane AJ, Jauhar R, Meraj P, Rokos IC, Rumsfeld JS, Henry TD. Management of Acute Myocardial Infarction During the COVID-19 Pandemic: A Position Statement From the Society for Cardiovascular Angiography and Interventions (SCAI), the American College of Cardiology (ACC), and the American College of Emergency Physicians (ACEP). J Am Coll Cardiol. 2020 Sep 15;76(11):1375-1384. doi: 10.1016/j.jacc.2020.04.039. Epub 2020 Apr 21. PMID: 32330544; PMCID: PMC7173829. [DOI] [PMC free article] [PubMed]
- 37.The European Society for Cardiology. ESC Guidance for the Diagnosis and Management of CV Disease during the COVID-19 Pandemic. https://www.escardio.org/Education/COVID-19-and- Cardiology/ESC-COVID-19-Guidance. (Last update: 10 June 2020).
- 38.Linde J.J., Kelbæk H., Hansen T.F., Sigvardsen P.E., Torp-Pedersen C., Bech J., Heitmann M., Nielsen O.W., Høfsten D., Kühl J.T., Raymond I.E., Kristiansen O.P., Svendsen I.H., Vall-Lamora M.H.D., Kragelund C., de Knegt M., Hove J.D., Jørgensen T., Fornitz G.G., Steffensen R., Jurlander B., Abdulla J., Lyngbæk S., Elming H., Therkelsen S.K., Jørgensen E., Kløvgaard L., Bang L.E., Hansen P.R., Helqvist S., Galatius S., Pedersen F., Abildgaard U., Clemmensen P., Saunamäki K., Holmvang L., Engstrøm T., Gislason G., Køber L.V., Kofoed K.F. Coronary CT Angiography in Patients With Non-ST-Segment Elevation Acute Coronary Syndrome. J Am Coll Cardiol. 2020 Feb 11;75(5):453–463. doi: 10.1016/j.jacc.2019.12.012. PMID: 32029126. [DOI] [PubMed] [Google Scholar]
- 39.Goehler A., Mayrhofer T., Pursnani A., Ferencik M., Lumish H.S., Barth C., Karády J., Chow B., Truong Q.A., Udelson J.E., Fleg J.L., Nagurney J.T., Gazelle G.S., Hoffmann U. Long-term health outcomes and cost-effectiveness of coronary CT angiography in patients with suspicion for acute coronary syndrome. J Cardiovasc Comput Tomogr. 2020;14(1):44–54. doi: 10.1016/j.jcct.2019.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Citro R., Pontone G., Bellino M., Silverio A., Iuliano G., Baggiano A., Manka R., Iesu S., Vecchione C., Asch F.M., Ghadri J.R., Templin C. Role of multimodality imaging in evaluation of cardiovascular involvement in COVID-19. Trends in Cardiovascular Medicine. 2021;31(1):8–16. doi: 10.1016/j.tcm.2020.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Oda S., Emoto T., Nakaura T., Kidoh M., Utsunomiya D., Funama Y., Nagayama Y., Takashio S., Ueda M., Yamashita T., Tsujita K., Ando Y., Yamashita Y. Myocardial Late Iodine Enhancement and Extracellular Volume Quantification with Dual-Layer Spectral Detector Dual-Energy Cardiac CT. Radiol Cardiothorac. Imaging. 2019;1(1):e180003. doi: 10.1148/ryct.2019180003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ohta Y., Kitao S., Yunaga H., Fujii S., Mukai N., Yamamoto K., Ogawa T. Myocardial Delayed Enhancement CT for the Evaluation of Heart Failure: Comparison to MRI. Radiology. 2018;288(3):682–691. doi: 10.1148/radiol.2018172523. [DOI] [PubMed] [Google Scholar]
- 43.Pontone G., Baggiano A., Conte E., Teruzzi G., Cosentino N., Campodonico J., Rabbat M.G., Assanelli E., Palmisano A., Esposito A., Trabattoni D. “Quadruple rule out” with cardiac computed tomography in COVID-19 patient with equivocal acute coronary syndrome presentation. JACC: Cardiovascular Imaging. 2020 doi: 10.1016/j.jcmg.2020.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pontone G., Scafuri S., Mancini M.E., Agalbato C., Guglielmo M., Baggiano A., Muscogiuri G., Fusini L., Andreini D., Mushtaq S., Conte E., Annoni A., Formenti A., Gennari A.G., Guaricci A.I., Rabbat M.R., Pompilio G., Pepi M., Rossi A. Role of computed tomography in COVID-19. J. Cardiovascular Computed Tomography. 2021;15(1):27–36. doi: 10.1016/j.jcct.2020.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Feuchtner G.M., Barbieri F., Luger A., Skalla E., Kountchev J., Widmann G., Plank F. Myocardial injury in COVID-19: The role of coronary computed tomography angiography (CTA) J. Cardiovascular Computed Tomography. 2021;15(1):e3–e6. doi: 10.1016/j.jcct.2020.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Suri J.S., Puvvula A., Biswas M., Majhail M., Saba L., Faa G., Singh I.M., Oberleitner R., Turk M., Chadha P.S., Johri A.M., Sanches J.M., Khanna N.N., Viskovic K., Mavrogeni S., Laird J.R., Pareek G., Miner M., Sobel D.W., Balestrieri A., Sfikakis P.P., Tsoulfas G., Protogerou A., Misra D.P., Agarwal V., Kitas G.D., Ahluwalia P., Kolluri R., Teji J., Maini M.A., Agbakoba A., Dhanjil S.K., Sockalingam M., Saxena A., Nicolaides A., Sharma A., Rathore V., Ajuluchukwu J.N.A., Fatemi M., Alizad A., Viswanathan V., Krishnan P.R., Naidu S. COVID-19 pathways for brain and heart injury in comorbidity patients: A role of medical imaging and artificial intelligence-based COVID severity classification: A review. Comput. Biology Medicine. 2020;124:103960. doi: 10.1016/j.compbiomed.2020.103960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Litjens G., Ciompi F., Wolterink J.M., de Vos B.D., Leiner T., Teuwen J., Išgum I. State-of-the-Art Deep Learning in Cardiovascular Image Analysis. JACC Cardiovasc Imaging. 2019 Aug;12(8 Pt 1):1549–1565. doi: 10.1016/j.jcmg.2019.06.009. PMID: 31395244. [DOI] [PubMed] [Google Scholar]
- 48.Saba L., Biswas M., Kuppili V., Cuadrado Godia E., Suri H.S., Edla D.R., Omerzu T., Laird J.R., Khanna N.N., Mavrogeni S., Protogerou A., Sfikakis P.P., Viswanathan V., Kitas G.D., Nicolaides A., Gupta A., Suri J.S. The present and future of deep learning in radiology. Eur J Radiol. 2019 May;114:14–24. doi: 10.1016/j.ejrad.2019.02.038. Epub 2019 Mar 2 PMID: 31005165. [DOI] [PubMed] [Google Scholar]
- 49.Wang S., Zha Y., Li W., Wu Q., Li X., Niu M., Wang M., Qiu X., Li H., Yu H., Gong W., Bai Y., Li L., Zhu Y., Wang L., Tian J. A fully automatic deep learning system for COVID-19 diagnostic and prognosticanalysis. Eur Respir J. 2020;56(2):2000775. doi: 10.1183/13993003.00775-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Saba L., Agarwal M., Patrick A., et al. Six artificial intelligence paradigms for tissue characterisation and classification of non-COVID-19 pneumonia against COVID-19 pneumonia in computed tomography lungs. Int J Comput Assist Radiol Surg. 2021;16(3):423–434. doi: 10.1007/s11548-021-02317-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Li L., Qin L., Xu Z., Yin Y., Wang X., Kong B., Bai J., Lu Y., Fang Z., Song Q., Cao K., Liu D., Wang G., Xu Q., Fang X., Zhang S., Xia J., Xia J. Using Artificial Intelligence to Detect COVID-19 and Community-acquired Pneumonia Based on Pulmonary CT: Evaluation of the Diagnostic Accuracy. Radiology. 2020;296(2):E65–E71. doi: 10.1148/radiol.2020200905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dong D., Tang Z., Wang S., Hui H., Gong L., Lu Y., Xue Z., Liao H., Chen F., Yang F., Jin R., Wang K., Liu Z., Wei J., Mu W., Zhang H., Jiang J., Tian J., Li H. The Role of Imaging in the Detection and Management of COVID-19: A Review. IEEE Rev Biomed Eng. 2021;14:16–29. doi: 10.1109/RBME.2020.2990959. Epub 2021 Jan 22 PMID: 32356760. [DOI] [PubMed] [Google Scholar]
- 53.Han D., Liu J., Sun Z., Cui Y., He Y., Yang Z. Deep learning analysis in coronary computed tomographic angiography imaging for the assessment of patients with coronary artery stenosis. Comput Methods Programs Biomed. 2020 Nov;196 doi: 10.1016/j.cmpb.2020.105651. Epub 2020 Jul 9 PMID: 32712571. [DOI] [PubMed] [Google Scholar]
- 54.Muscogiuri G., Chiesa M., Trotta M., Gatti M., Palmisano V., Dell’Aversana S., Baessato F., Cavaliere A., Cicala G., Loffreno A., Rizzon G., Guglielmo M., Baggiano A., Fusini L., Saba L., Andreini D., Pepi M., Rabbat M.G., Guaricci A.I., De Cecco C.N., Colombo G., Pontone G. Performance of a deep learning algorithm for the evaluation of CAD-RADS classification with CCTA. Atherosclerosis. 2020;294:25–32. doi: 10.1016/j.atherosclerosis.2019.12.001. [DOI] [PubMed] [Google Scholar]
- 55.Antonopoulos A.S., Sanna F., Sabharwal N., Thomas S., Oikonomou E.K., Herdman L., Margaritis M., Shirodaria C., Kampoli A.-M., Akoumianakis I., Petrou M., Sayeed R., Krasopoulos G., Psarros C., Ciccone P., Brophy C.M., Digby J., Kelion A., Uberoi R., Anthony S., Alexopoulos N., Tousoulis D., Achenbach S., Neubauer S., Channon K.M., Antoniades C. Detecting human coronary inflammation by imaging perivascular fat. Sci Transl Med. 2017;9(398) doi: 10.1126/scitranslmed.aal2658. [DOI] [PubMed] [Google Scholar]
- 56.Grodecki K., Lin A., Razipour A., Cadet S., McElhinney P.A., Chan C., Pressman B.D., Julien P., Maurovich-Horvat P., Gaibazzi N., Thakur U., Mancini E., Agalbato C., Menè R., Parati G., Cernigliaro F., Nerlekar N., Torlasco C., Pontone G., Slomka P.J., Dey D. Epicardial adipose tissue is associated with extent of pneumonia and adverse outcomes in patients with COVID-19. Metabolism. 2021;115:154436. doi: 10.1016/j.metabol.2020.154436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hui H, Zhang Y, Yang X, et al. Clinical and radiographic features of cardiac injury in patients with 2019 novel coronavirus pneumonia. medRxiv 2020.02.24.20027052; doi: https://doi.org/10.1101/2020.02.24.20027052.
- 58.Kolossváry M., Kellermayer M., Merkely B., Maurovich-Horvat P. Cardiac Computed Tomography Radiomics: A Comprehensive Review on Radiomic Techniques. J Thorac Imaging. 2018;33(1):26–34. doi: 10.1097/RTI.0000000000000268. [DOI] [PubMed] [Google Scholar]
- 59.Kotanidis C.P., Xie C., Adlam D., et al. Radiotranscriptomic analysis of perivascular adipose tissue quantifies vascular inflammation in COVID-19 from toutine CT angiograms: stratification of “new UK variant” infection and prediction of in-hospital outcomes. Heart. 2021;107(Suppl 1):A1–A185. doi: 10.1136/heartjnl-2021-BCS.238. [DOI] [Google Scholar]
- 60.Kotanidis C.P., Xie C., Kotronias R., et al. A Novel CT-derived Radiotranscriptomic Signature of Perivascular Adipose Tissue Stratifies COVID-19 Vascular Cytokine Burst and Predicts in Hospital Outcomes. Circulation. 2020;142:A16467. doi: 10.1161/circ.142.suppl_3.16467. [DOI] [Google Scholar]
- 61.Vaishya R., Javaid M., Khan I.H., Haleem A. Artificial Intelligence (AI) applications for COVID-19 pandemic. Diabetes & Metabolic Syndrome: Clinical Research & Reviews. 2020;14(4):337–339. doi: 10.1016/j.dsx.2020.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Buicu A.L., Cernea S., Benedek I., Buicu C.F., Benedek T. Systemic Inflammation and COVID-19 Mortality in Patients with Major Noncommunicable Diseases: Chronic Coronary Syndromes, Diabetes and Obesity. J Clin Med. 2021;10(8):1545. doi: 10.3390/jcm10081545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Williams M.C., Moss A., Dweck M., Hunter A., Pawade T., Adamson P.D., Shah A.S.V., Alam S., Maroules C.D., van Beek E.JR., Cury R., Nicol E.D., Newby D.E., Roditi G. Standardized reporting systems for computed tomography coronary angiography and calcium scoring: A real-world validation of CAD-RADS and CAC-DRS in patients with stable chest pain. Journal of Cardiovascular Computed Tomography. 2020;14(1):3–11. doi: 10.1016/j.jcct.2019.07.010. [DOI] [PubMed] [Google Scholar]
- 64.Van der Aalst, C. M., Denissen, S. J. A. M., Vonder, M., Gratama, J. W. C., Adriaansen, H. J., Kuijpers, D., … de Koning, H. J. (2020). Screening for cardiovascular disease risk using traditional risk factor assessment or coronary artery calcium scoring: the ROBINSCA trial. European Heart Journal - Cardiovascular Imaging.doi:10.1093/ehjci/jeaa168. [DOI] [PubMed]
- 65.De Rosa S, Spaccarotella C, Basso C, Calabrò MP, Curcio A, Filardi PP, et al. Società Italiana di Cardiologia and the CCU Academy investigators group. Reduction of hospitalizations for myocardial infarction in Italy in the COVID-19 era. Eur Heart J. (2020) 41:2083–8. doi: 10.1093/eurheartj/ehaa610. [DOI] [PMC free article] [PubMed]
- 66.Choi A.D., Abbara S., Branch K.R., Feuchtner G.M., Ghoshhajra B., Nieman K., Pontone G., Villines T.C., Williams M.C., Blankstein R. Society of Cardiovascular Computed Tomography guidance for use of cardiac computed tomography amidst the COVID-19 pandemic Endorsed by the American College of Cardiology. Journal of Cardiovascular Computed Tomography. 2020;14(2):101–104. doi: 10.1016/j.jcct.2020.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chieffo A., Stefanini G.G., Price S., Barbato E., Tarantini G., Karam N., Moreno R., Buchanan G.L., Gilard M., Halvorsen S., Huber K., James S., Neumann F.J., Möllmann H., Roffi M., Tavazzi G., Mauri Ferré J., Windecker S., Dudek D., Baumbach A. EAPCI Position Statement on Invasive Management of Acute Coronary Syndromes during the COVID-19 pandemic. Eur Heart J. 2020;41(19):1839–1851. doi: 10.1093/eurheartj/ehaa381. PMID: 32405641; PMCID: PMC7239193. [DOI] [PMC free article] [PubMed] [Google Scholar]