Abstract
Objective
This systematic review aimed to evaluate the antiviral effect of mouthwashes against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2).
Material and methods
An electronic search was performed on PubMed, Scopus, Web of Science, Cochrane Library, LILACS, ProQuest, and Google Scholar, and was complemented by a manual search. Both clinical and in vitro studies that focused on the antiviral effect of mouthwashes against SARS-CoV-2 were included. Risk of bias assessment was performed only on the clinical studies using the RoB-2 and ROBINS-I tools.
Results
A total of 907 records were found; after initial selection by title and abstract, 33 full-text articles were selected to be evaluated for eligibility. Finally, a total of 27 studies were included for the qualitative synthesis, including 16 in vitro studies and 11 clinical trials. Antiviral effects were evaluated separately for the in vitro and clinical studies. In vitro studies included mouthwashes containing hydrogen peroxide, chlorhexidine digluconate, povidone-iodine, essential oils, cetylpyridinium chloride, and other compounds; in vivo studies included mouthwashes containing hydrogen peroxide, chlorhexidine digluconate, povidone-iodine, cetylpyridinium chloride, essential oils, chlorine dioxide, β-cyclodextrin-citrox, and sorbitol with xylitol. Povidone-iodine, cetylpyridinium chloride, and essential oils were effective in vitro, while hydrogen peroxide, chlorhexidine digluconate, povidone-iodine, cetylpyridinium chloride, β-cyclodextrin-citrox, and sorbitol with xylitol were effective in vivo. Unclear or high risk of bias was found for almost all clinical studies, and only one study presented with a low risk of bias. No further quantitative analysis was performed.
Conclusion
Although povidone-iodine, cetylpyridinium chloride, and essential oils may be an alternative to reduce the viral load in vitro and in vivo, more studies are needed to determine the real antiviral effect of these different mouthwashes against SARS-CoV-2.
This work was not funded. The protocol was registered in PROSPERO (identification number: CRD42021236134).
Keywords: COVID-19, Mouthwashes, Oral hygiene, Viral load, Coronavirus
Abbreviations: RCT, randomized controlled trials; non-RCT, non-randomized controlled trials; H2O2, hydrogen peroxide; CHX, chlorhexidine digluconate; PVP-I, povidone-iodine; CPC, cetylpyridinium chloride; OCT, octenidine dihydrochloride; APD, anionic phthalocyanine derivate; CDCM, β-cyclodextrin-citrox; PBS, phosphate-buffered saline; TCID50/mL, 50% of tissue culture infective dose; CCID50, 50% of cell culture infectious dose; RT-qPCR, quantitative reverse transcription polymerase chain reaction; pfu/mL, plaque forming units per milliliter
1. Introduction
The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is mainly transmitted by respiratory droplets expelled when speaking, breathing, coughing, and sneezing, and by contact between objects contaminated by these droplets and the mucosa (J. Xu et al., 2020, R. Xu et al., 2020).
The virus accumulates and replicates in the upper respiratory tract, as high viral loads can be found in the oral cavity, nose, and oropharynx in patients affected with the 2019 coronavirus disease (COVID-19) (Wölfel et al., 2020, Zou et al., 2020). A prolonged viral load is found in the sputum of infected patients (Wölfel et al., 2020), as saliva is a viral reservoir in patients with asymptomatic to mild COVID-19 (Florence Carrouel et al., 2021b). As saliva can play a role in the transmission of this disease (R. Xu et al., 2020), a possible method to decrease the amount of SARS-CoV-2 in saliva could be through mouthwash use, as some reagents target the outer lipid membrane of the virus (Carrouel et al., 2021, Gottsauner et al., 2020).
Mouthwashes containing chlorhexidine digluconate (CHX), cetylpyridinium chloride (CPC), povidone-iodine (PVP-I), and essential oils have been shown to reduce the viral load of SARS-CoV-2 in vitro and clinically (Elzein et al., 2021, Meister et al., 2020, Mohamed et al., 2020, Seneviratne et al., 2021, Statkute et al., 2020), highlighting their potential for use against COVID-19.
Although mouthwash use is practical and affordable, scientific evidence is urgently needed to support its use against COVID-19 spread. Hence, the present systematic review aimed to evaluate the antiviral effect of mouthwashes against SARS-CoV-2.
2. Materials & methods
This systematic review was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA-2020) guidelines (Page et al., 2021). The protocol was registered in PROSPERO after the preliminary search was performed (identification number: CRD42021236134).
The following review question was addressed: Does the use of certain mouthwashes have an antiviral effect on SARS-CoV-2?
The PICO strategy was as follows:
-
-
Population:
-
-
Clinical studies: Adult patients with or without COVID-19; samples of saliva, sputum, oral plaque, or oral tissue.
In vitro studies: SARS-CoV-2 strains.
-
-
Intervention: Use of any mouthwash, including hydrogen peroxide (H2O2), CHX, PVP-I, CPC, or another antiviral compound, at any concentration.
-
-
Comparison: Without use of any mouthwash.
-
-
Outcome: Decrease in SARS-CoV-2 viral load, or the antiviral/virucidal effect of mouthwashes against SARS-CoV-2.
2.1. Search strategy
The following databases were assessed for the article search: PubMed, Scopus, Web of Science, Cochrane Library, and LILACS. ProQuest and Google Scholar were also searched. The electronic search was complemented by a manual search of the list of references of the items included. The final search was conducted until April 12th, 2021. A complementary update of the search was performed until September 30th, 2021. There were no limitations, publication date restrictions, or language restrictions.
Keywords used for the search comprised MeSH and free text terms: 'hydrogen peroxide', ‘acetylpyridine’, 'cetylpyridinium chloride', 'chlorhexidine digluconate', 'povidone iodine', ‘mouthwash’, 'mouth rinse', ‘rinse’, 'oral rinse', 'mouth bath', 'mouth wash', 'mouth washes', 'oral collutory', ‘COVID-19’, ‘SARS-CoV-2’, and ‘coronavirus’.
The following search strategy was used in PubMed without any limit or filter, and then adapted for the other databases: (COVID-19 OR SARS-COV-2 OR coronavirus) AND (“hydrogen peroxide” OR “cetylpyridinium chloride” OR acetylpyridine OR “chlorhexidine digluconate” OR chlorhexidine OR “povidone iodine” OR iodopovidone OR “mouth rinse” OR rinse OR “oral rinse” OR “mouth bath” OR “mouth wash*” OR mouthwash OR collutory).
Study selection was based on the predefined eligibility criteria, considering both published and unpublished studies. The web application Rayyan QCRI was used for the study selection process. Reviewer calibration was performed previously, obtaining a suitable inter-rater reliability value (K = 0.71).
Study selection by title and abstract was independently performed by two reviewers (GTSB and BPTU). In cases of disagreement, a third reviewer (JPIMM) would participate in the final decision when necessary. The final study selection by full-text article was performed by the initial two reviewers, based on the selection criteria. Disagreements were discussed with the same third reviewer and consensus was sought.
2.2. Selection criteria
Inclusion criteria:
-
-
Randomized controlled trials (RCT); non-randomized controlled trials (non-RCT); and cohort, case-control, and cross-sectional studies evaluating the antiviral effect, virucidal effect, or decrease in viral load against SARS-CoV-2 after mouthwash use.
-
-
Clinical studies that included adult patients with or without COVID-19; studies using saliva, sputum, oral plaque, or oral tissue samples.
-
-
In vitro studies with a detailed protocol that studied the antiviral effect, virucidal effect, or decrease in viral load of SARS-CoV-2 after mouthwash use.
-
-
In vitro studies that evaluated the action of mouthwashes against SARS-CoV-2 strains.
Exclusion criteria:
-
-
Case report studies, experts’ opinions, animal studies, literature reviews.
-
-
Studies only in children or adolescent patients.
-
-
Studies with patients diagnosed with any systemic disease that could affect the results.
-
-
Studies with disabled patients with difficulties in performing oral care.
-
-
In vitro studies using microorganisms other than SARS-CoV-2.
2.3. Data extraction
Data extraction was performed independently by four reviewers (JPIMM, RPCP, PSGHL, and DAPR), considering the following parameters: author; year of publication; country; sample number; patient age; intervention and control group; virus strain; mouthwash concentration; mouthwash dosing; decrease in viral load, antiviral, or virucidal effect; decrease in viral count; and percentage of viral inactivation. Data extraction was analyzed separately for in vitro and clinical studies.
2.4. Risk of bias assessment
The tools for assessing the risk of bias in interventional studies (RoB-2 for RCT (Sterne et al., 2019) and ROBINS-I for non-RCT (Sterne et al., 2016)) were used. No risk of bias assessment was performed for in vitro studies.
The risk of bias assessment was performed independently by two reviewers (KHUK, JM), considering a high, unclear, or low risk of bias. In the case of insufficient or unclear data, the study author was contacted for clarification. Discrepancies were identified and resolved through a discussion by the reviewers. The RevMan (Review Manager Software version 5.4, Cochrane Collaboration, Copenhagen, Denmark) program was used to analyze the risk of bias figures.
2.5. Strategy for data synthesis
A narrative analysis of the included studies was conducted, dividing the studies by their design into in vitro and clinical studies. No quantitative analysis was performed.
Study outcomes, such as the decrease in viral load, antiviral effect, virus count, or virucidal effect against SARS-CoV-2 after mouthwash use were considered, and were expressed as cycle threshold (Ct) reduction, percentage of virus inactivation, plaque forming unit count, log reduction, or any other representative value to evaluate virus reduction before and after treatment.
3. Results
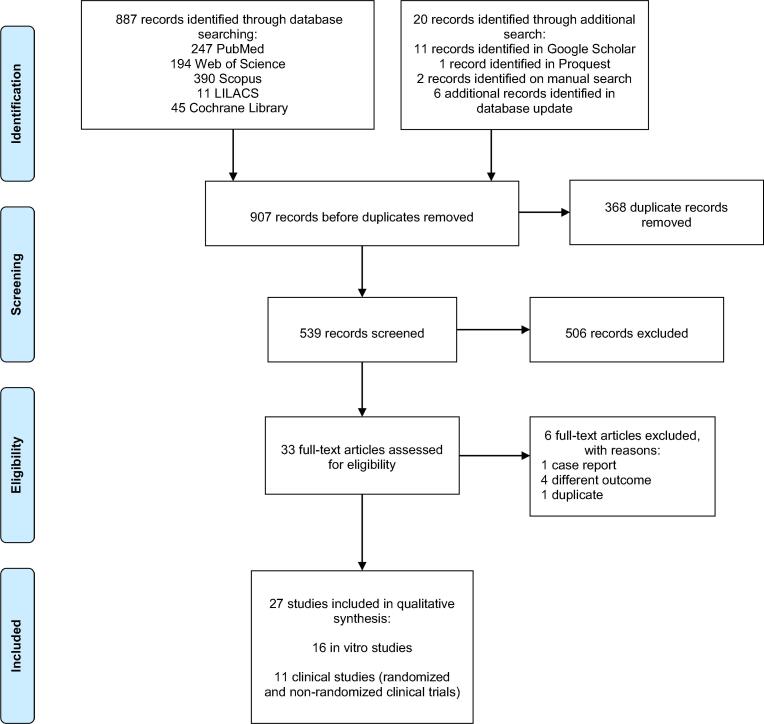
The total search resulted in 907 records, including the articles found upon searching the databases and in other resources. A total of 368 duplicates were removed, leaving 539 records for title and abstract assessment. Then, 33 articles were selected by title and abstract for their full text to be evaluated for eligibility. Six articles were excluded, leading to a total of 27 titles included in the qualitative synthesis. The PRISMA flowchart is shown in Fig. 1.
Fig. 1.
PRISMA flow chart.
The final studies included 16 in vitro studies (Anderson et al., 2020, Bidra et al., 2020b, Bidra et al., 2020a, Davies et al., 2021, Hassandarvish et al., 2020, Jain et al., 2021, Koch-Heier et al., 2021, Komine et al., 2021, Meister et al., 2020, Muñoz-Basagoiti et al., 2021, Pelletier et al., 2021, C.A. Santos et al., 2021, P.S. da S. Santos et al., 2021, Statkute et al., 2020, Steinhauer et al., 2021, Xu et al., 2021), and 11 clinical studies, including 9 RCTs (Avhad et al., 2020; Florence Carrouel et al., 2021a, Chaudhary et al., 2021, Choudhury et al., 2020, Eduardo et al., 2021, Elzein et al., 2021, Guenezan et al., 2021, Mohamed et al., 2020, Seneviratne et al., 2021) and two non-RCTs (Gottsauner et al., 2020, Schürmann et al., 2021). Due to the moderate to high risk of bias obtained in most clinical studies, no further quantitative analysis was performed.
3.1. Measurement of exposures and outcomes
Table 1 shows the summary of data from the in vitro studies. Mouthwashes with H2O2, CHX, PVP-I, essential oils, CPC, CPC + H2O2, CHX + CPC, octenidine dihydrochloride, anionic phthalocyanine derivate (APD), dequalinium chloride + benzalkonium chloride, polyaminopropyl biguanide (polyhexanide), ethanol + ethyl lauroyl arginate, delmopinol, dipotassium oxalate, and stabilized hypochlorous acid were studied. Results varied for mouthwashes of different concentrations.
Table 1.
Summary of data from in vitro studies.
| Study | SARS-CoV-2 strain | Sample | Mouthwash | Time | Measurement | Results | Study remarks |
|---|---|---|---|---|---|---|---|
| Meister et al., 2020(Meister et al., 2020) | Strain 1: UKEssen strain Strain 2: BetaCoV/Germany/Ulm/01/2020 Strain 3: BetaCoV/Germany/Ulm/02/2020 (Germany) |
n = 3 | Group A: H2O2 – Cavex Oral Pre Rinse Group B: CHX – Chlorhexamed Forte Group C: Dequalinium chloride, benzalkonium chloride – Dequonal Group D: CHX – Dynexidine Forte 0.2% Group E: PVP-I – Iso-Betadine mouthwash 1.0% Group F: Ethanol, essential oils – Listerine Cool Mint Group G: Octenidine dihydrochloride – Octenident mouthwash Group H: Polyaminopropyl biguanide (polyhexanide) – ProntOral mouthwash Control: organic secretion |
30 sec | Quantitative suspension test: tissue culture infective dose (TCID50/mL) | Significant reduction of strains 1–3 Group C: Dequalinium chloride, benzalkonium chloride log reduction: 2.61–3.11 Group E: Polyvidone-iodine log reduction: 2.61–3.11 Group F: Ethanol, essential oils log reduction: 2.61–3.11 Moderate reduction of strains 1–3: Group A: Hydrogen peroxide log reduction: 0.33–0.78 Group B: Clorhexidinebis (D-gluconate) log reduction: 0.78–1.17 Group D: Clorhexidinebis (D-gluconate) log reduction: 0.5–0.56 Group G: Octenidine dihydrochloride log reduction: 0.61–1.11 Group H (Polyaminopropyl biguanide): strain 1: moderately reduced (log reduction: 0.61) strains 2–3: significantly reduced (log reduction: 1.61–1.78) |
Different strains of SARS-CoV-2 can be inactivated efficiently by commercial mouth rinses in vitro. |
| Bidra et al., 2020(Bidra et al., 2020a) | USA- WA1/2020 strain (USA) |
n = 3 | Group 1: PVP-I 0.5% oral rinse – Veloce BioPharma Group 2: PVP-I 1.25% oral rinse – Veloce BioPharma Group 3: PVP-I 1.5% oral rinse – Veloce BioPharma Group 4: H2O2 1.5% – Sigma-Aldrich Group 5: H2O2 3.0% – Sigma-Aldrich Positive control: Ethanol 70% Negative control: Water |
15 sec, 30 sec | Standard end-point dilution assay: 50% cell culture infectious dose (CCID50) of virus per 0.1 mL | Group 1: PVP-I 0.5% 15 sec: log10 reduction: >4.33 30 sec: log10 reduction: >3.63 Group 2: PVP-I 1.25% 15 sec: log10 reduction: >4.33 30 sec: log10 reduction: >3.63 Group 3: PVP-I 1.5% 15 sec: log10 reduction: >4.33 30 sec: log10 reduction: >3.63 Group 4: H2O2 1.5% 15 sec: log10 reduction: 1.33 30 sec: log10 reduction: 1.0 Group 5: H2O2 3.0% 15 sec: log10 reduction: 1.00 30 sec: log10 reduction: 1.8 Ethanol group: 15 sec: log10 reduction: >4.33 30 sec: log10 reduction: >3.63 |
PVP-I mouth rinse could reduce the SARS-CoV-2 viral load at all concentrations at 15 and 30 s. H2O2 at 1.5% and 3.0% showed minimal virucidal activity against SARS-CoV-2 after at 15 and 30 s. |
| Bidra et al., 2020(Bidra et al., 2020b) | USA-WA1/2020 strain (USA) |
n = 3 | Group 1: PVP-I 1.5% oral rinse – Veloce BioPharma Group 2: PVP-I 0.75% oral rinse – Veloce BioPharma Group 3: PVP-I 0.5% oral rinse – Veloce BioPharma Positive control: Ethanol 70% Negative control: Water |
15 sec, 30 sec | Standard end-point dilution assay: 50% cell culture infectious dose (CCID50) of virus per 0.1 mL | Group 1: PVP-I 1.5% 15 sec: log10 reduction: 3.0 30 sec: log10 reduction: 3.33 Group 2: PVP-I 0.75% 15 sec: log10 reduction: 3.0 30 sec: log10 reduction: 3.33 Group 3: PVP-I 0.5% 15 sec: log10 reduction: 3.0 30 sec: log10 reduction: 3.33 Ethanol group 15 sec: log10 reduction: 2.17 30 sec: log10 reduction: 3.33 |
PVP-I mouth rinse could reduce the SARS-CoV-2 viral load at all concentrations after 15 and 30 s in vitro. |
| Anderson et al., 2020(Anderson et al., 2020) | hCoV-19/Singapore/2/2020 (Singapore) |
n = 3 | Group 1: PVP-I 10% antiseptic solution – BETADINE Group 2: PVP-I 0.45% throat spray – BETADINE Group 3: PVP-I 7.5% antiseptic skin cleanser – BETADINE Group 4: PVP-I 1.0% gargle and mouth wash – BETADINE Group 5: PVP-I 1.0% (1:2 dilution) gargle and mouth wash – BETADINE Control: PBS |
30 sec | Viral kill time assay: median tissue culture infectious dose (TCID50/mL) | Group 1: PVP-I 10% Antiseptic solution log10 reduction: ≥4.00 Group 2: PVP-I 0.45% Throat spray log10 reduction: ≥4.00 Group 3: PVP-I 7.5% Antiseptic skin cleanser log10 reduction: ≥4.00 Group 4: PVP-I 1.0% Gargle and mouth wash log10 reduction: ≥4.00 Group 5: PVP-I 1.0% (1:2 dilution) Gargle and mouth wash log10 reduction: ≥4.00 |
All PVP-I solutions showed great virucidal activity against SARS-CoV-2 after 30 s, corresponding to a ≥ 99.99% kill for all products. |
| Hassandarvish et al., 2020(Hassandarvish et al., 2020) | SARS-COV-2/MY/UM/6–3; TIDREC (Malaysia) |
Not mentioned | Group 1: PVP-I 1.0% gargle and mouth wash – BETADINE Group 2: PVP-I 0.5% gargle and mouth wash – BETADINE Control: Distilled water |
15 sec, 30 sec, 60 sec | Virus time-kill assay: Median tissue culture infectious dose (TCID50/ mL). |
Clean condition (bovine serum albumin): Group 1: PVP-I 1.0% Gargle and mouth wash 15 sec: log10 reduction: >5.00 30 sec: log10 reduction: >5.00 60 sec: log10 reduction: >5.00 Group 2: PVP-I 0.5% Gargle and mouth wash 15 sec: log10 reduction: >4.00 30 sec: log10 reduction: >5.00 60 sec: log10 reduction: >5.00 Dirty condition (bovine serum albumin + human erythrocytes): Group 1: PVP-I 1.0% Gargle and mouth wash 15 sec: log10 reduction: >5.00 30 sec: log10 reduction: >5.00 60 sec: log10 reduction: >5.00 Group 2: PVP-I 0.5% Gargle and mouth wash 15 sec: log10 reduction: >4.00 30 sec: log10 reduction: >5.00 60 sec: log10 reduction: >5.00 |
Both concentrations of PVP-I showed potent and rapid virucidal activity against SARS-CoV-2 at 15, 30 and 60 s. |
| Statkute et al., 2020(Statkute et al., 2020) | England2 strain (UK) |
Not mentioned | Group 1: Ethanol 7%, CHX 0.2% – Corsodyl Group 2: CPC 0.05%-0.1% – Dentyl Dual Action Group 3: CPC 0.05%-0.1% – Dentyl Fresh Protect Group 4: Ethanol 21%, essential oils – Listerine Cool Mint Group 5: Ethanol 23%, ethyl lauroyl arginate 0.147% – Listerine Advanced Gum Treatment Group 6: CPC 0.07–0.1%, sodium citric acid 0.05% – SCD Max Group 7: PVP-I 0.5% – Videne Group 8: Ethanol 21% Group 9: 23% Control: |
30 sec | Plaque assay: visual inspection of monolayer integrity | Complete virus eradication: (log10 reduction: >5) Group 2: CPC 0.05%-0.1% – Dentyl Dual Action Group 3: CPC 0.05%-0.1% – Dentyl Fresh Protect Group 5: Ethanol 23%, ethyl lauroyl arginate – Listerine Advanced Gum Treatment Moderate effect: (log10 reduction: ∼3) Group 4: Ethanol 21%, essential oils – Listerine Cool Mint Group 6: CPC 0.07–0.1%, sodium citric acid 0.05% – SCD Max Group 7: PVP-I 0.5% – Videne Low effect: (log10 reduction: <2) Group 1: Ethanol 7%, CHX 0.2% – Corsodyl |
Two CPC mouth rinses (Dentyl) and ethanol /ethyl lauroyl arginate (Listerine Advanced) showed high virus elimination. Moderate elimination was shown on ethanol/essential oils (Listerine Cool Mint), CPC with sodium citric acid (SCD Max), and PVP-I. CHX or ethanol alone showed little or no effect. |
| Pelletier et al., 2021(Pelletier et al., 2021) | USA-WA1/2020 strain (USA) |
n = 3 | Group 1: PVP-I 2.5% nasal antiseptic – Veloce BioPharma Group 2: PVP-I 1.25% nasal antiseptic – Veloce BioPharma Group 3: PVP-I 0.50% nasal antiseptic – Veloce BioPharma Group 4: PVP-I 1.5% oral rinse antiseptic – Veloce BioPharma Group 5: PVP-I 0.75% oral rinse antiseptic – Veloce BioPharma Group 6: PVP-I 0.5% oral rinse antiseptic – Veloce BioPharma Positive control: Ethanol 70% Negative control: Water |
60 sec | Standard end-point dilution assay: 50% cell culture infectious dose (CCID50) of virus per 0.1 mL | Group 1: PVP-I 2.5% nasal antiseptic log10 reduction: 4.63 Group 2: PVP-I 1.25% nasal antiseptic log10 reduction: 4.63 Group 3: PVP-I 0.50% nasal antiseptic log10 reduction: 4.63 Group 4: PVP-I 1.5% oral rinse antiseptic log10 reduction: 4.63 Group 5: PVP-I 0.75% oral rinse antiseptic log10 reduction: 4.63 Group 6: PVP-I 0.5% oral rinse antiseptic log10 reduction: 4.63 Group 7: Ethanol 70% log10 reduction: 4.63 |
All PVP-I concentrations of nasal and oral rinse antiseptics completely inactivated the SARS-CoV-2 after 60 s. |
| Jain et al., 2021(Jain et al., 2021) | Strain isolated from an Indian patient (India) |
Not mentioned | Group 1:CHX 0.12% – Sigma-Aldrich Group 2:CHX 0.2% – Sigma-Aldrich Group 3: PVP-I 1% |
30 sec, 60 sec |
Ct values obtained from RT-qPCR | Relative Ct change (Percent SARS-CoV-2 inactivation): Group 1:CHX 0.12% 30 sec: Ct change: 10.5 ± 0.5 (99.9% inactivation) 60 sec: Ct change 11 ± 1.0 (99.9% inactivation) Group 2:CHX 0.2% 30 sec: Ct change: 12.5 ± 0.5 (>99.9% inactivation) 60 sec: Ct change 13 ± 0 (>99.9% inactivation) Group 3: PVP-I 1% 30 sec: Ct change: 9.5 ± 0.5 (99.8% inactivation) 60 sec: Ct change 11 ± 2 (>99.9% inactivation) |
Both CHX and PVP-I showed high level of antiviral effect against SARS-CoV-2 at 30 and 60 s. |
| Koch-Heier et al., 2021(Koch-Heier et al., 2021) | Isolate “FI-100” strain (Germany) |
n = 2 | Group 1: CPC 0.05%, H2O2 1.5% – ViruProX® Group 2: CHX 0.1%, CPC 0.05%, sodium fluoride (F-) 0.005% – BacterX® pro Group 3: CHX 0.1% + CPC 0.05% Group 4: CPC 0.05% Group 5: CHX 0.1% Group 6: H2O2 1.5% |
30seg | Plaque assay: counting of plaque forming units per milliliter (pfu/mL) | Group 1: CPC 0.05%, H2O2 1.5% Reduction by ≥ 6.8 × 106 pfu/mL (≥1.9 log10 fold) Group 2: CHX 0.1%, CPC 0.05%, sodium fluoride (F-) 0.005% Reduction by ≥ 8.4 × 106 pfu/mL (≥2.0 log10 fold) Group 3: CHX 0.1% + CPC 0.05% Reduction by: 6.7 × 106 pfu/mL (1.2 log10 fold) Group 4: CPC 0.05% Reduction by: 5.6 × 106 pfu/mL (0.7 log10 fold) Group 5: CHX 0.1% no reduction Group 6: H2O2 1.5% no reduction |
Both ViruProX® and BacterX®, along with CPC + CHX combination, and CPC alone showed a significant reduction on the SARS-CoV-2. H2O2 and CHX alone had no virucidal effect against SARS-CoV-2. |
| Komine et al., 2021(Komine et al., 2021) | JPN/TY/WK-521 strain (Japan) |
n = 3 | Group 1: CPC 0.0125% toothpaste – GUM® WELL PLUS Dental paste Group 2: CPC 0.05% mouthwash – GUM® WELL PLUS Dental rinse (alcoholic type) Group 3: CPC 0.05% mouthwash – GUM® WELL PLUS Dental rinse (non-alcoholic type) Group 4: CPC spray – GUM® Disinfection spray for mouth/throat Group 5: CHX 0.06% + CPC 0.05% mouthwash – GUM® PAROEX (0.06% CHX) Group 6: CHX 0.12% + CPC 0.05% mouthwash – GUM® PAROEX (0.12% CHX) Group 7: CPC 0.075% mouthwash – GUM® Oral Rinse Group 8: CHX 0.12% mouthwash – GUM® PAROEX (0.12% CHX) Group 9: Delmopinol 0.20% mouthwash – GUM® PerioShield Group 10: CPC 0.04% mouthwash – GUM® MOUTH- WASH HERB 2020 Positive control: Ethanol 70% Negative control: PBS |
20sec, 30sec 3 min |
Plaque assay: plaque forming units per milliliter (pfu/mL) Virus suspension dilution measured per 0.1 mL |
Group 1: CPC 0.0125% toothpaste 3 min: log10 pfu/mL reduction: 3.3 (99.94% reduction) Group 2: CPC 0.05% mouthwash (alcoholic type) 20 sec: log10 pfu/mL reduction: 4.2 (99.994% reduction) Group 3: CPC 0.05% mouthwash (non-alcoholic type) 20 sec: log10 pfu/mL reduction: 4.1 (99.992% reduction) Group 4: CPC spray 20 sec: log10 pfu/mL reduction: >3.4 (>99.96% reduction) Group 5: CHX 0.06% + CPC 0.05% mouthwash 30 sec: log10 pfu/mL reduction: >4.3 (>99.995% reduction) Group 6: CHX 0.12% + CPC 0.05% mouthwash 30 sec: log10 pfu/mL reduction: >4.3 (>99.995% reduction) Group 7: CPC 0.075% mouthwash 30 sec: log10 pfu/mL reduction: >4.3 (>99.995% reduction) Group 8: CHX 0.12% mouthwash 30 sec: log10 pfu/mL reduction: 0.2 (42.5% reduction) Group 9: Delmopinol 0.20% mouthwash 30 sec: log10 pfu/mL reduction: >5.3 (>99.9995% reduction) Group 10: CPC 0.04% mouthwash 20 sec: log10 pfu/mL reduction: >4.4 (>99.996% reduction) Ethanol 70% 20 sec: log10 pfu/mL reduction: >5.4 (>99.9996% reduction) |
All dental care products containing 0.0125 to 0.30% CPC, as well as the mouthwash containing 0.20% delmopinol hydrochloride inactivated the SARS-CoV-2 in vitro. The mouthwash containing only 0.12% CHX did not inactivate sufficiently the SARS-CoV-2 in vitro. |
| Steinhauer et al., 2021(Steinhauer et al., 2021) | Not mentioned | n = 2 | Group A: CHX 0.1% – Chlorhexamed fluid 0.1% Group B: CHX 0.2% – Chlorhexamed forte alkoholfrei 0.2% Group C: Octenidine dihydrochloride 0.1%, phenoxyethanol 2% – Octenisept |
15 sec, 30 sec 1 min, 5 min, 10 min |
Quantitative suspension test: tissue culture infective dose (TCID50/mL) | Group A: CHX 0.1% (80% v/v) 5 min, 10 min: log10 reduction: <1 Group B: CHX 0.2% (80% v/v) 1 min, 5 min: log10 reduction: <1 Group C: Octenidine dihydrochloride + phenoxyethanol (80% v/v) 15 sec, 30 sec, 1 min: log10 reduction: ≥4.38 |
Octenidine dihydrochloride mouthwash was effective within 15 sec against SARS-CoV2. Both CHX mouthrinses had limited efficacy against SARS-CoV2. |
| Xu et al., 2021(Xu et al., 2021) | USA_WA1/2020 strain (USA) |
n = 2 | Group 1:20–30% ethanol, essential oils – Listerine Antiseptic original Group 2: CHX 0.12% – Chlorhexidine gluconate Xttrium Laboratories Group 3: H2O2 1.5% – Colgate Peroxyl Group 4: PVP-I 10% (1% available iodine) – PVP-I CVS Pharmacy |
30 min | Plaque assay: measure of fluorescence intensity |
Group 1:20–30% ethanol, essential oils 50% (v/v): complete inactivation (relative light unitsx104) 5% (v/v): moderate antiviral effect (relative light unitsx104) Group 2: CHX 0.12% 50% (v/v): complete inactivation (relative light unitsx104) 5% (v/v): moderate antiviral effect (relative light unitsx104) Group 3: H2O2 1.5% 50% (v/v): complete inactivation (relative light unitsx104) 5% (v/v): complete inactivation (relative light unitsx104) Group 4: PVP-I 10% (1% available iodine) 5% (v/v): complete inactivation (relative light unitsx104) 0.5% (v/v): no inactivation |
All mouthwashes inactivated the SARS-CoV2 without prolonged incubation. |
| Davies et al., 2021(Davies et al., 2021) | England 2 strain (UK) |
n = 3 | Group 1: CHX 0.2% – Chlorhexidine Gluconate Antiseptic Mouthwash (with ethanol) Group 2: CHX 0.2% – Corsodyl (alcohol free) Group 3: dipotassium oxalate 1.4% – Listerine Advanced Defence Sensitive (alcohol free) Group 4: essential oils, sodium fluoride, zinc fluoride – Listerine Total Care Group 5: stabilized hypochlorous acid 0.01–0.02% – OraWize+ Group 6: H2O2 1.5% – Peroxyl Group 7: PVP-I 0.58% – Povident |
1 min | Quantitative suspension test: tissue culture infective dose (TCID50/mL) | Tissue culture fluid unconcentrated Group 1: CHX 0.2% (with ethanol) log10 reduction: 0.5 (0.1–0.9) Group 2: CHX 0.2% (alcohol free) log10 reduction: 0.2 (-0.2–0.7) Group 3: dipotassium oxalate 1.4% (alcohol free) log10 reduction: ≥3.5 (3.2–3.8) Group 4: essential oils, sodium fluoride, zinc fluoride log10 reduction: ≥4.1 (3.8–4.4) Group 5: stabilized hypochlorous acid 0.01–0.02% log10 reduction: ≥5.5 (5.2–5.8) Group 6: H2O2 1.5% log10 reduction: 0.2 (-0.1–0.5) Group 7: PVP-I 0.58% log10 reduction: ≥4.1 (3.8–4.4) Tissue culture fluid concentrated Group 3: dipotassium oxalate 1.4% (alcohol free) log10 reduction: ≥4.2 (3.9–4.4) Group 4: essential oils, sodium fluoride, zinc fluoride log10 reduction: ≥5.2 (4.9–5.4) Group 5: stabilized hypochlorous acid 0.01–0.02% log10 reduction: 0.4 (0.0–0.8) Group 7: PVP-I 0.58% log10 reduction: ≥5.2 (4.9–5.4) |
Mouthwashes with 0,01–0,02% stabilized hypochlorous acid, 0.58% PVP-I, and both alcohol-based and non-alcohol-based products (both Listerine) were effective against the SARS-CoV-2 in vitro. H2O2 1.5% and 0.2% CHX were ineffective against the SARS-CoV-2 in vitro. |
| Munoz-Basagoiti et al., 2021(Muñoz-Basagoiti et al., 2021) | B.1.1.7 variant and D614G variant (Spain) |
n = 3 | Group 1: 1.47 mM CPC – Vitis Encias Group 2: 1.47 mM CPC + 1.33 mM CHX – Perio Aid Intensive Care Group 3: 2.063 mM CPC – Vitis CPC Protect |
30 sec, 1 min, 2 min |
ELISA, dynamic light scattering analysis, Tissue Culture Infectious Dose 50% (TCID50/mL) | D614G strain: Group 1: 1.47 mM CPC 2 min: decreased about 1000 times TCID50/mL Group 2: 1.47 mM CPC + 1.33 mM CHX 2 min: decreased about 1000 times viral TCID50/mL Group 3: 2.063 mM CPC 2 min: decreased about 1000 times viral TCID50/mL 1 min: decreased about 1000 times viral TCID50/mL B.1.1.7 strain Group 3: 2.063 mM CPC 1 min: decreased about 1000 times viral TCID50/mL 30 sec with sterilized saliva: decreased 10 fold TCID50/mL |
CPC inhibits the entrance of SARS-CoV-2. CPC mouthwashes decreased more than a thousand times the infectivity of SARS-CoV-2 in vitro. CPC is effective against SARS-CoV-2 variants, also in the presence of sterilized saliva. |
| Santos et al, 2021. (C. A. Santos et al., 2021) | Not mentioned | n = 3 | Group 1: anionic phtalocyanine derivate (APD) dental gel Group 2: anionic phtalocyanine derivate (APD) mouthwash Positive control Negative control |
30 sec, 1 min, 5 min |
Plaque assay: Median tissue culture infection dose (TCID50) |
Group 1: anionic phtalocyanine derivate (APD) dental gel 30 sec, 1 min, 5 min: 99.99% inactivation Group 2: anionic phtalocyanine derivate (APD) mouthwash 30 sec, 1 min, 5 min: 90% inactivation |
Both anionic phtalocyanine derivate (APD) mouthwash and dental gel can reduce the viability of SARS-CoV-2 in vitro in 30 s. |
| Santos et al., 2021(P. S. da S. Santos et al., 2021) | Not mentioned | n = 4 | Group 1: APD 1:2 dilution (1.0 mg/mL) Group 2: APD 1:4 dilution (0.5 mg/mL) Group 3: APD 1:8 dilution (0.25 mg/mL) Group 4: APD 1:16 dilution (0.125 mg/mL) Group 5: APD 1:32 dilution (0.0625 mg/mL) Group 6: APD 1:64 dilution (0.03125 mg/mL) Group 7: APD 1:128 dilution (0.0156 mg/mL) Positive control Negative control |
30 min | Plaque assay, RT-PCR |
Group 1: APD 1:2 dilution 99.96% reduction of viral load Group 2: APD 1:4 dilution 99.88% reduction of viral load Group 3: APD 1:8 dilution 99.84% reduction of viral load Group 4: APD 1:16 dilution 92.65% reduction of viral load Group 5: APD 1:32 dilution 77.42% reduction of viral load Group 6: APD 1:64 dilution 11.06% reduction of viral load Group 7: APD 1:128 dilution No viral neutralization |
APD in the 1.0 mg/mL to 0.125 mg/mL range was highly effective for the reduction of SARS-CoV-2 viral load, without causing any cytotoxicity. |
1 H2O2: Hydrogen peroxide; CHX: Chlorhexidine digluconate; PVP-I: Povidone-iodine; CPC: Cetylpyridinium chloride; OCT: octenidine dihydrochloride; APD: Anionic phtalocyanine derivate; PBS: Phosphate-buffered saline.
Table 2 presents the summary of data from the clinical studies. Mouthwashes containing H2O2, CHX, PVP-I, CPC, CPC + zinc lactate, H2O2 + CHX, essential oils, chlorine dioxide, β-cyclodextrin-citrox (CDCM), and sorbitol + xylitol were used. Results varied for mouthwashes of different concentrations.
Table 2.
Summary of data from clinical studies.
| Study | Country | Study design | Sample | Age | Mouthwash | Dosage | Treatment length | Detection method | Results | Study remarks | Risk of bias |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Gottsauner et al., 2020(Gottsauner et al., 2020) | Germany | Non-randomized clinical trial | 12 hospitalized patients positive to Sars-CoV-2 | 55 years (22–81 years) | H2O2 1% (gargling mouth and throat) |
20 mL for 30 sec | 1 time | RT-PCR | RT-PCR at baseline: 1.8 × 103 (3.1 × 102;4.7 × 104) copies/mL RT-PCR 30 min after procedure 1.5 × 103 (8.3 × 102;3.4 × 104) copies/mL No significant differences (p = 0.96) |
A H2O2 1% mouthrinse did not reduce the intraoral viral load of SARS-CoV-2. | Critical (high) risk |
| Avhad et al., 2020(Avhad et al., 2020) | India | Randomized clinical trial | 40 patients positive to SARS-CoV-2 | 19–49 years | Control group (n = 20): CHX 0.2% (rinse and gargle) Study group (n = 20): chlorine dioxide 0.1% (rinse and gargle) |
10 mL | 3 times a day for 7 days | RT-PCR | RT-PCR after one week: Control group: CHX 0.2% Positive cases: 12 Negative cases: 8 Study group: chlorine dioxide (0.1%) Positive cases: 8 Negative cases: 12 |
Chlorine dioxide mouthwash presented more cases with reduction of intensity of symptoms and negativity for COVID-19 in the patients. | Unclear risk |
| Choudhury et al., 2020(Choudhury et al., 2020) | Bangladesh | Randomized clinical trial | 606 patients positive to SARS-CoV-2 | 11–90 years | Group A (n = 303): PVP-I 1% (mouthwash/gargle, nasal drops and eye drops) Group B (n = 303): lukewarm water (mouthwash/gargle, nasal drops and eye drops) |
1 mL of PVP-I in 10 mL of sterile water/purified water 30 sec oral rinse, 30 sec gargle, 4–5 drop nasal, 2 eye drops |
4 hourly for 4 weeks | RT-PCR | RT-PCR positive: Group A: PVP-I 1% 3rd day: 11.55% 5th day: 7.92% 7th day: 2.64% Group B: lukewarm water 3rd day: 96.04% 5th day: 88.45% 7th day: 70.30% |
PVP-I 1% as mouthwash/gargle, nasal drop and eye drop, reduced mortality and morbidity by COVID-19, as well as reduce positivity cases at the 3rd, 5th and 7th day. |
High risk |
| Mohamed et al., 2020(Mohamed et al., 2020) | Malaysia | Randomized clinical trial | 20 patients positive to SARS-CoV-2 | 22–56 years | Group A (n = 5): PVP-I 1% – Betadine® (gargle) Group B (n = 5): essential oils, ethanol – Listerine Original (gargle) Group C (n = 5): tap water (gargle) Group D (n = 5): no intervention |
Group A: 10 mL for 30 sec Group B: 20 mL for 30 sec Group C: 100 mL for 30 sec |
3 times a day for 7 days | RT-PCR | RT-PCR results: Group A: PVP-I 1% 4th day: 100% negative 6th day: 100% negative 12th day: 100% negative Group B: essential oils 4th day: 80% negative, 20% positive 6th day: 80% negative, 20% positive 12th day: 80% negative, 20% positive Group C: tap water 4th day: 40% negative, 60% positive 6th day: 40% negative, 20% positive, 40% indeterminate 12th day: 40% negative, 40% positive, 20% indeterminate Group D: no intervention 4th day: 20% negative, 40% positive, 40% indeterminate 6th day: 60% positive, 40% indeterminate 12th day: 20% negative, 60% positive, 20% indeterminate |
PVP-I 1% PCR results were significantly reduced (p < 0.05) after the 4th, 6th and 12th day, when compared to the control. High rate of viral reduction after 4 days of PVP-I 1% and essential oil mouthwashes was achieved. |
High risk |
| Seneviratne et al., 2021(Seneviratne et al., 2021) | Singapore | Randomized clinical trial | 16 patients positive to SARS-CoV-2 | Group 1: 40.7 ± 11.5 Group 2: 43.6 ± 8.6 Group 3: 35.7 ± 8.5 Group 4: 36 ± 14.1 |
Group 1 (n = 4): PVP-I 0.5% – Betadine® (mouthwash) Group 2 (n = 6): CHX 0.2% (mouthwash) Group 3(n = 4): CPC 0.075% (mouthwash) Group 4 (n = 2): sterile water |
PVP-I: 5 mL for 30 sec CHX: 15 mL for 30 sec CPC: 20 mL for 30 sec Water: 15 mL for 30 sec |
1 time | RT-PCR | Relative fold change of cycle threshold: Group 1: PVP-I 5 min: fold change: 1.1 3 h: fold change: 1.2 6 h: fold change: 1 (p < 0.01) Group 2: CHX 0.2% 5 min: 0.9 (varied effect) 3 h: fold change: 1 6 h: fold change: 0.9 Group 3: CPC 5 min: fold change: 1 (p < 0.05) 3 h: fold change: 0.9 6 h: fold change: 0.9 (p < 0.05) |
There were not significant differences within all 3 mouthwashes. When comparing the mouthwashes with the water group, there was a significant increase in fold change for CPC after 5 min at 6 h, and for PVP-I at 6 h. The decrease of salivary load was maintained after 6 h for CPC and PVP-I mouthwashes. |
Unclear risk |
| Guenezan et al., 2021(Guenezan et al., 2021) | France | Randomized clinical trial | 24 ambulatory patients positive to SARS-CoV-2 | Control: 57 (45–68 years) Intervention: 33 (23–46 years) |
Control group (n = 12): no intervention Intervention group (n = 12): PVP-I 1% (mouthwash, gargles, nasal pulverization) + PVP-I10% (nasal ointment) |
25 mL for mouthwash and gargles, 0.5 mL for nasal pulverization |
4 times a day for 5 days | RT-PCR, TCID50 |
Mean relative difference in viral titers: Baseline – Day 1: Control: 32% (95% CI, 10%-65%) Intervention: 75% (95% CI, 43%-95%) No statistical differences between groups over time. |
The use of PVP-I had no influence on the changes of viral RNA quantification over time. | Unclear risk |
| Elzein et al., 2021(Elzein et al., 2021) | Lebanon | Randomized clinical trial | 61 patients positive to SARS-CoV-2 | 45.3 ± 16.7 years-old | Group A(n = 11): distilled water (mouth rinse) Group B (n = 33): CHX 0.2% (mouth rinse) Group C(n = 33): PVP 1% (mouth rinse) |
15 mL for 30 sec |
1 time | RT-PCR | A significant difference of Ct values between water group and CHX: (p = 0.0024) PVP-I: (p = 0.012) No significant difference between: CHX and PVP-I: p = 0.24 Differences before and after mouthwash: CHX Ct difference: 5.69 increase (p < 0.0001) PVP-I: Ct difference: 4.45 increase (p < 0.0001) No difference for water group (p = 0.566) |
Both CHX 0.2% and PVP-I 1% are effective against salivary SARS-CoV-2. | Unclear risk |
| Carrouel et al., 2021(Florence Carrouel et al., 2021a) |
France | Randomized clinical trial | 176 ambulatory patients positive to SARS-Cov-2 | Control: 44.08 ± 16.16 years Intervention: 42.06 ± 14.97 |
Control group (n = 88): Placebo(mouthwash) Intervention group (n = 88): CDCM (β-cyclodextrin-citrox) (mouthwash) |
30 mL for 1 min | 3 times a day (at 09.00, 14.00 and 19.00), for 7 days |
RT-PCR | % decrease T1-T2 (log10 copies/mL): Control group: −6.74% (-21.16% to 10.44%) Intervention group: −12.58% (-29.55% to −0.16%) % decrease T1-T3 (log10 copies/mL): Control group: −9.79% (-28.53% to 9.21%) Intervention group: −10.67% (-37.30% to 3.25%) % decrease T1-day 7 (log10 copies/mL): Control group: −50.62% (-100% to −27.66%) Intervention group: −58.62% (-100% to −34.36%) Only statistical difference at T1-T2 difference |
CDCM had a significant beneficial effect on reducing SARS-CoV-2 salivary viral load in adults with asymptomatic or mild COVID-19, 4 h after the initial dose. |
Low risk |
| Eduardo et al., 2021(Eduardo et al., 2021) |
Brazil | Randomized clinical trial | 60 patients positive to SARS-Cov-2 | 18–90 years-old | Group A (n = 9): Placebo (distilled water rinse) Group B (n = 7): CPC 0.075% + Zinc lactate 0.28% (Colgate Total 12® rinse) Group C (n = 7): H2O2 1.5% (Peroxyl® rinse) Group D (n = 8): CHX 0.12% (PerioGard® rinse) Group E (n = 12): H2O21.5% + CHX 0.12% (Peroxyl® + PerioGard® rinse) |
Group A: 20 mL for 1 min Group B: 20 mL for 30 s Group C: 10 mL for 1 min Group D: 15 mL for 30 sec Group E: 10 mL of H2O2 for 1 min, followed by 15 mL of CHX for 30 sec |
1 time | RT-PCR | Group A (placebo): minor changes Group B (CPC + Zinc): 20.4 ± 3.7-fold reduction Group C (H2O2): 15.8 ± 0.08-fold reduction Group D (CHX): ≥ 2-fold reduction Group E (H2O2 + CHX): ≥ 2-fold reduction |
CPC + Zinc and CHX mouthwashes reduced significantly SARS-CoV-2 viral load in saliva up to 60 min after rinsing·H2O2 reduced significantly the viral load up to 30 min after rinsing. H2O2 + CHX presented minimal reduction in the salivary viral load. |
Unclear risk |
|
Chaudhary et al., 2021(Chaudhary et al., 2021) |
US | Randomized clinical trial | 40 patients positive to SARS-Cov-2 | 21–80 years-old | Group 1 (n = 10): normal saline (mouth rinse) Group 2 (n = 10): H2O2 1% (mouth rinse) Group 3 (n = 10): CHX 0.12% (mouth rinse) Group 4 (n = 10): PVP-I 0.5% (mouth rinse) |
15 mL (total): rinse with 7.5 mL for 30 sec and expectorate, and then, rinse with the remaining 7.5 mL for 30 sec |
1 time | RT-PCR | Median reduction after 15 min: 61% − 89% for all groups (CHX, H2O2, normal saline, PVP-I) Median reduction at 45 min: 70% − 97% for all groups (CHX, H2O2, normal saline, PVP-I) No statistical difference between groups at neither 15-minute nor 45-minute (P > 0.05). |
Mouthrinses are a simple and highly efficacious for the reduction of the virus on the oral environment for up to 45 min. |
High risk |
| Schürmann et al., 2021(Schürmann et al., 2021) | Germany | Non-randomized clinical trial | 34 SARS-CoV-2 positive hospitalized patients | Not mentioned | Sorbitol and xylitol (Linolasept ® mouthwash) |
1 min | 1 time | RT-qPCR | Mean Ct values after rinsing: Increase of 3.1 (standard deviation 3.6). Reduction of viral load of 90%. |
Mouthwashing can reduce the viral load by 90%. |
Critical (high) risk |
1 H2O2: Hydrogen peroxide; CHX: Chlorhexidine digluconate; PVP-I: Povidone-iodine; CPC: Cetylpyridinium chloride.
3.2. Risk of bias of clinical studies
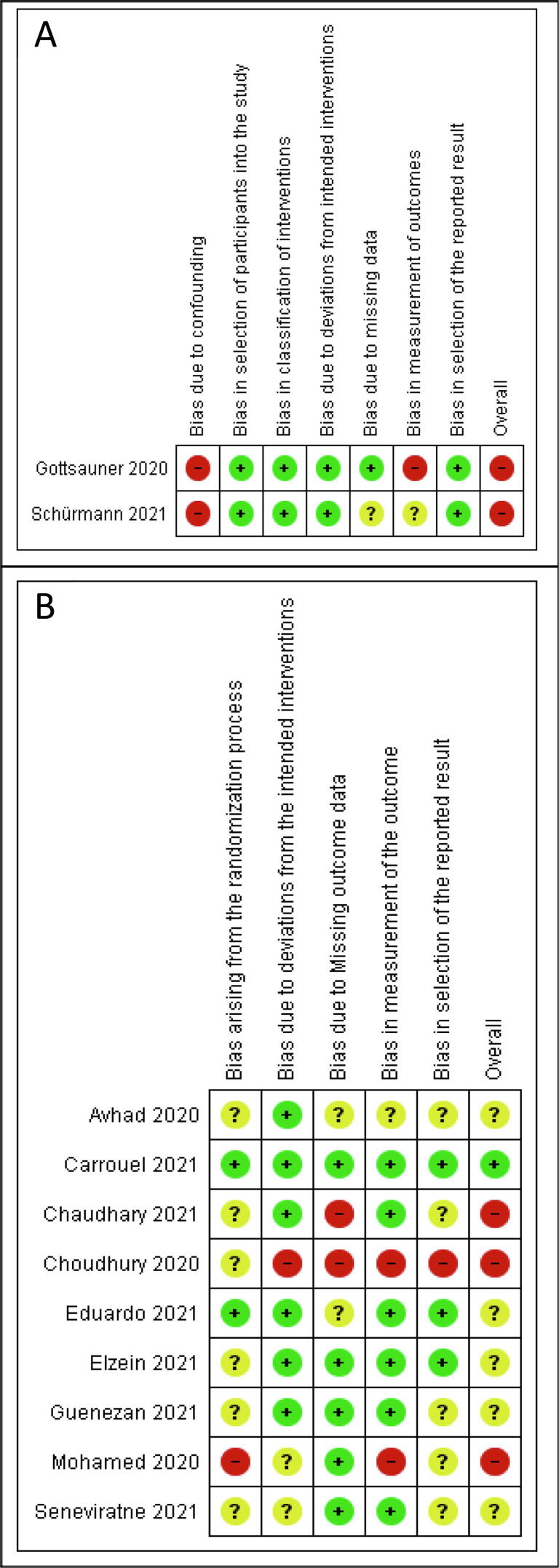
The risk of bias summary of clinical studies is shown in Fig. 2. Both non-RCTs showed a high risk of bias. For the RCTs, one study showed a low risk, five studies showed unclear bias, and three studies showed a high risk of bias.
Fig. 2.
Risk of bias summary of clinical studies: (A) Risk of bias of non-randomized clinical studies assessed with the ROBINS-I tool, (B) Risk of bias of randomized clinical studies assessed with the RoB-2 tool. Green images represent a low risk of bias, yellow images represent an unclear risk of bias, and red images represent a high risk of bias.
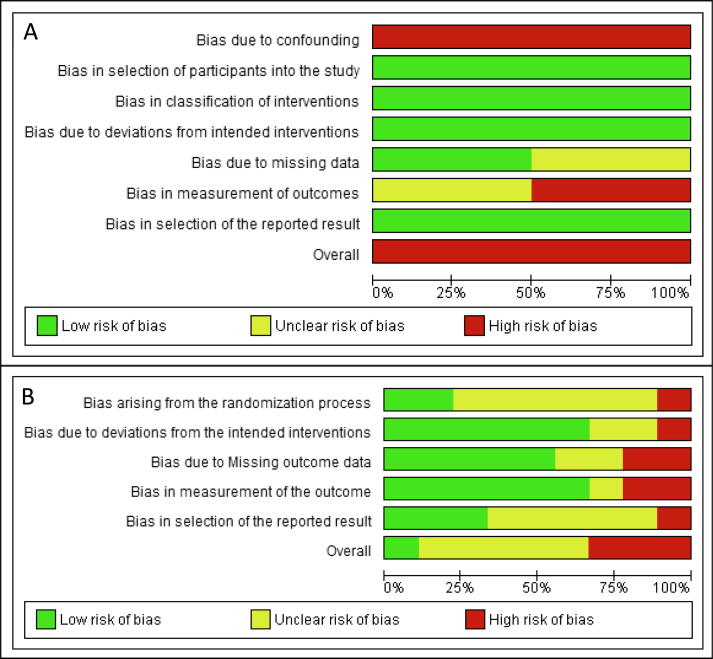
The combined risk of bias graph of the clinical trials is shown in Fig. 3. The non-RCTs presented a high risk of bias. The RCTs presented approximately 10% low risk, 60% unclear risk, and 30% high risk of bias.
Fig. 3.
Risk of bias graph of clinical studies: A) Risk of bias of non-randomized clinical studies assessed with the ROBINS-I tool, (B) Risk of bias of randomized clinical studies assessed with the RoB-2 tool. The green color represents a low risk of bias, yellow represents an unclear risk of bias, and red represents a high risk of bias.
3.3. Antiviral effect of mouthwashes
Table 3 shows the summary of all mouthwashes in vitro and clinically·H2O2 showed low to no effect in vitro, but a varied effect clinically. CHX showed a varied effect or no effect in vitro, and a varied effect clinically·H2O2 + CHX had minimal effect clinically. PVP-I showed a moderate to high effect in vitro and was mostly effective in patients. The essential oils and CPC were effective clinically, with a moderate to high effect in vitro. CPC + zinc lactate was effective clinically; CPC + H2O2 and CPC + CHX were highly effective in vitro. Chlorine dioxide was clinically more effective than CHX. CDCM and sorbitol + xylitol were also clinically effective. In vitro, octenidine dihydrochloride and polyaminopropyl biguanide showed a moderate to high effect; APD, dequalinium chloride, ethanol + ethyl lauroyl arginate, delmopinol, and dipotassium oxalate showed a high effect; and stabilized hypochlorous acid had a varied effect.
Table 3.
Antiviral effect against SARS-CoV-2 in in vitro and clinical studies.
| Mouthwash | Study type | N° of studies | Concentration | Dosage | Antiviral effect against SARS-CoV-2 | Overall effect |
|---|---|---|---|---|---|---|
| H2O2 | Clinical study | 2 (Chaudhary et al., 2021, Gottsauner et al., 2020) (High risk) | H2O2 1% | 20 mL for 30 sec / 15 mL for 1 min | Varied effect | Varied effect |
| 1 (Eduardo et al., 2021) (Unclear risk) | H2O2 1.5% | 10 mL for 1 min |
Effective | |||
| In vitro study | 5 (Bidra et al., 2020a, Davies et al., 2021, Koch-Heier et al., 2021, Meister et al., 2020, Xu et al., 2021) | H2O2 1.5%, | 15 sec, 30 sec | Low effect to no effect | Low to no effect in vitro | |
| 1 (Bidra et al., 2020a) | H2O2 3.0% | 15 sec to 30 sec | Low effect | |||
| CHX | Clinical study | 2(Chaudhary et al., 2021, Eduardo et al., 2021) (Unclear and high risk) | CHX 0.12% | 15 mL for 30 sec | Effective | Varied effect, to effective in patients |
| 3 (Avhad et al., 2020, Elzein et al., 2021, Seneviratne et al., 2021) (unclear risk) | CHX 0.2% | 15 mL for 30 sec |
Varied effect, to effective | |||
| In vitro study | 2 (Koch-Heier et al., 2021, Steinhauer et al., 2021) | CHX 0.1% | 30 sec | No effect | Variable to no effect in vitro | |
| 3 (Jain et al., 2021, Komine et al., 2021, Xu et al., 2021) | CHX 0.12% | 30 sec to 60 sec | Variable effect | |||
| 4 (Davies et al., 2021, Jain et al., 2021, Meister et al., 2020, Steinhauer et al., 2021) | CHX 0.2% | 30 sec to 60 sec | Variable effect | |||
| 2 (Davies et al., 2021, Statkute et al., 2020) | CHX 0.2% + Ethanol | 30 sec to 60 sec | Low to no effect | |||
| H2O2 + CHX | Clinical study | 1 (Eduardo et al., 2021) (Unclear risk) | H2O21.5% + CHX 0.12% |
10 mL of H2O2 for 1 min, followed by 15 mL of CHX for 30 sec |
Minimal effect | Minimal effect |
| PVP-I | Clinical study | 2 (Chaudhary et al., 2021, Seneviratne et al., 2021) (Unclear and high risk) | PVP-I 0.5% | 5 mL for 30 sec / 15 mL for 1 min |
Effective | Varied effect, mostly effective in patients |
| 4 (Choudhury et al., 2020, Elzein et al., 2021, Guenezan et al., 2021, Mohamed et al., 2020) (Unclear and high risk) | PVP-I 1% |
10–15 mL for 30 sec, 3–4 times a day | Varied effect, mostly effective | |||
| In vitro study | 7 (Anderson et al., 2020, Bidra et al., 2020a, Bidra et al., 2020b, Davies et al., 2021, Hassandarvish et al., 2020, Pelletier et al., 2021, Statkute et al., 2020) | PVP-I 0.5% | 15 sec to 60 sec | Moderate to high effect | Moderate to high effect in vitro | |
| 2(Bidra et al., 2020b, Pelletier et al., 2021) | PVP-I 0.75% | 15 sec to 60 sec | High effect | |||
| 5(Anderson et al., 2020, Hassandarvish et al., 2020, Jain et al., 2021, Meister et al., 2020, Xu et al., 2021) | PVP-I 1.0% | 15 sec to 60 sec | Mostly high effect | |||
| 1 (Bidra et al., 2020a) | PVP-I 1.25% | 15 sec to 30 sec | High effect | |||
| 3 (Bidra et al., 2020a, Bidra et al., 2020b, Pelletier et al., 2021) | PVP-I 1.5% | 15 sec to 60 sec | High effect | |||
| Essential oils | Clinical study | 1 (Mohamed et al., 2020) (High risk) | Ethanol + essential oils (Eucalyptol, Menthol, Methyl salicylate, Thymol) | 20 mL for 30 sec, 3 times a day | Effective | Effective in patients |
| In vitro study | 4 (Davies et al., 2021, Meister et al., 2020, Statkute et al., 2020, Xu et al., 2021) | Ethanol + essential oils (Eucalyptol, Menthol, Methyl salicylate, Thymol) | 30 sec to 60 sec | Moderate to high effect | Moderate to high effect in vitro | |
| CPC | Clinical study | 1 (Seneviratne et al., 2021) (Unclear risk) | CPC 0.075% | 20 mL for 30 sec |
Effective | Effective in patients |
| In vitro study |
1 (Komine et al., 2021) | CPC 0.04% mouthwash | 20 sec | High effect | Moderate to high effect in vitro | |
| 1 (Komine et al., 2021) | CPC 0.05% (alcoholic type) | 20 sec to 30 sec | High effect | |||
| 4 (Koch-Heier et al., 2021, Komine et al., 2021, Muñoz-Basagoiti et al., 2021, Statkute et al., 2020) | CPC 0.05% (non-alcoholic type) | 20 sec-2 min | High effect | |||
| 2 (Komine et al., 2021, Muñoz-Basagoiti et al., 2021) | CPC 0.075% | 30 sec to 1 min | High effect | |||
| 1 (Statkute et al., 2020) | CPC 0.07–0.1% + sodium citric acid 0.05% | 30 sec | Moderate effect | |||
| CPC + Zinc | Clinical study | 1 (Eduardo et al., 2021) (Unclear risk) | CPC 0.075% + Zinc lactate 0.28% | 20 mL for 30 sec |
Effective | Effective in patients |
| CPC + H2O2 | In vitro study | 1 (Koch-Heier et al., 2021) | CPC 0.05%, H2O2 1.5% |
30 sec | High effect | High effect in vitro |
| CHX + CPC | In vitro study | 1 (Komine et al., 2021) | CHX 0.06% + CPC 0.05% | 30 sec | High effect | High effect in vitro |
| 1 (Koch-Heier et al., 2021) | CHX 0.1% + CPC 0.05% | 30sec | High effect | |||
| 2 (Komine et al., 2021, Muñoz-Basagoiti et al., 2021) | CHX 0.12% + CPC 0.05% | 30 sec to 2 min | High effect | |||
| Octenidine dihydrochloride | In vitro study | 2 (Meister et al., 2020, Steinhauer et al., 2021) | Octenidine dihydrochloride 0.1%, phenoxyethanol 2% | 15 sec, 30 sec, 1 min | Moderate to high effect | Moderate to high effect in vitro |
| APD | In vitro study | 2 (C. A. Santos et al., 2021; P. S. da S. Santos et al., 2021) | APD | 30 sec, 1 min, 5 min, 30 min | High effect | High effect in vitro |
| Chlorine dioxide | Clinical study | 1 (Avhad et al., 2020) (Unclear risk) | Chlorine dioxide 0.1% | 10 mL 3 times a day |
More effective than CHX | Variable effect in patients |
| Dequalinium chloride | In vitro study | 1 (Meister et al., 2020) | Dequalinium chloride 1.5 mg, benzalkonium chloride 3.5 mg | 30 sec | High effect | High effect in vitro |
| Polyaminopropyl biguanide | In vitro study | 1 (Meister et al., 2020) | Polyaminopropyl biguanide (polyhexanide) 0,1 - < 0,25% | 30 sec | Moderate to high effect | Moderate to high effect in vitro |
| Ethanol + ethyl lauroyl arginate | In vitro study | 1 (Statkute et al., 2020) | Ethanol 23%, ethyl lauroyl arginate 0.147% | 30 sec | High effect | High effect in vitro |
| Delmopinol | In vitro study | 1 (Komine et al., 2021) | Delmopinol 0.20% mouthwash | 30 sec | High effect | High effect in vitro |
| Dipotassium oxalate | In vitro study | 1 (Davies et al., 2021) | Dipotassium oxalate 1.4% | 1 min | High effect | High effect in vitro |
| Stabilized hypochlorous acid | In vitro study | 1 (Davies et al., 2021) | Stabilized hypochlorous acid 0.01–0.02% | 1 min | Variable effect | Variable effect in vitro |
| CDCM (β-cyclodextrin-citrox) | Clinical study | 1 (Florence Carrouel et al., 2021a) (Low risk) | CDCM (β-cyclodextrin-citrox) |
30 mL for 1 min, 3 times a day | Effective | Effective in patients |
| Sorbitol and xylitol | Clinical study | 1 (Schürmann et al., 2021) (High risk) | Sorbitol and xylitol | 1 min | Effective | Effective in patients |
1 H2O2: Hydrogen peroxide; CHX: Chlorhexidine digluconate; PVP-I: Povidone-iodine; CPC: Cetylpyridinium chloride; APD: Anionic phtalocyanine derivate.
4. Discussion
The transmission of COVID-19 is mainly by contact with respiratory droplets, as the virus can be found in the sputum and saliva of infected people (Florence Carrouel et al., 2021b, Wölfel et al., 2020, J. Xu et al., 2020, R. Xu et al., 2020). SARS-CoV-2 is a single-stranded enveloped RNA virus that binds to angiotensin-converting enzyme 2 (ACE-2) receptors to enter the host cell (Shang et al., 2020). The oral cavity acts as an entry point and reservoir for this virus, as ACE-2 receptors are spread in the salivary glands, tongue, and oral mucosa; thus, good oral hygiene could be effective against COVID-19 (Gottsauner et al., 2020, Sampson et al., 2020).
Mouthwash use has been suggested to decrease the salivary viral load (F. Carrouel et al., 2021), as both clinical and in vitro studies have demonstrated their antiviral effect (Bidra et al., 2020b, Elzein et al., 2021, Komine et al., 2021, Meister et al., 2020, Mohamed et al., 2020, Seneviratne et al., 2021). Because prevention methods to help reduce the spread of COVID-19 are urgently needed, it is important to evaluate the current literature regarding the role of mouthwashes to reduce the viral load of SARS-CoV-2 (Carrouel et al., 2020, Moosavi et al., 2020). Therefore, this systematic review aimed to evaluate the antiviral effect of different mouthwashes against SARS-CoV-2.
Regarding the in vitro studies, H2O2 showed mostly minimal to no effect (Bidra et al., 2020a, Davies et al., 2021, Koch-Heier et al., 2021), suggesting it might not be that effective against SARS-CoV-2. CHX showed inconsistent results, with some studies finding a strong (Jain et al., 2021, Xu et al., 2021), weak (Komine et al., 2021, Statkute et al., 2020, Steinhauer et al., 2021), or even no (Davies et al., 2021, Koch-Heier et al., 2021) effect against different strains of SARS-CoV-2. It is possible that both H2O2 and CHX alone are not that effective as mouthwashes, as these in vitro results were mostly negative.
PVP-I showed positive results in vitro, as most studies reported a strong antiviral effect against various SARS-CoV-2 strains at different doses (Anderson et al., 2020, Bidra et al., 2020a, Bidra et al., 2020b, Hassandarvish et al., 2020, Jain et al., 2021, Meister et al., 2020, Pelletier et al., 2021). CPC alone (Koch-Heier et al., 2021, Komine et al., 2021, Muñoz-Basagoiti et al., 2021, Statkute et al., 2020) and in combination with other reagents (Koch-Heier et al., 2021) also showed high viral reduction in vitro at different doses. Both PVP-I, and CPC alone and in combination (CPC + H2O2 and CPC + CHX) could help to reduce the spread of SARS-CoV-2.
The essential oils (eucalyptol, menthol, methyl salicylate, and thymol) combined with ethanol showed a moderate to high effect in all in vitro studies (Davies et al., 2021, Meister et al., 2020, Statkute et al., 2020, Xu et al., 2021), suggesting that they could be effective against SARS-CoV-2. Mouthwashes with octenidine dihydrochloride also showed a moderate to high effect in vitro (Meister et al., 2020, Steinhauer et al., 2021). APD (C.A. Santos et al., 2021, P.S. da S. Santos et al., 2021), dequalinium chloride (Meister et al., 2020), polyaminopropyl biguanide (Meister et al., 2020), ethyl lauroyl arginate with ethanol (Statkute et al., 2020), delmopinol (Komine et al., 2021), and dipotassium oxalate (Davies et al., 2021) showed a moderate to high effect in vitro; however, few studies supported these results.
Regarding the clinical studies, both H2O2 and CHX showed a varied effect in patients with COVID-19; some studies reported an antiviral effect for H2O2 (Chaudhary et al., 2021, Eduardo et al., 2021) and CHX (Chaudhary et al., 2021, Eduardo et al., 2021, Elzein et al., 2021), but their combination had minimal effect clinically. PVP-I was mostly effective against SARS-CoV-2 clinically (Choudhury et al., 2020, Elzein et al., 2021, Mohamed et al., 2020, Seneviratne et al., 2021). The essential oils (Mohamed et al., 2020), CPC (Seneviratne et al., 2021), CDCM (Florence Carrouel et al., 2021a), and sorbitol + xylitol (Schürmann et al., 2021) were effective in reducing the viral load in patients with COVID-19. In one study, mouthwash with chlorine dioxide showed a greater effect than CHX clinically (Avhad et al., 2020), but these results were limited by a lack of negative control.
In patients with COVID-19, H2O2, CHX, PVP-I, CPC (alone and combined), CDCM, sorbitol + xylitol, and essential oils were found to be effective; however, these studies presented an unclear or high risk of bias, except for the study of CDCM (Florence Carrouel et al., 2021a), which was assessed to have a low risk. More clinical studies of higher quality and less bias are still needed.
Regarding previous systematic reviews, Burton (Burton et al., 2020) could not include any clinical trials, so no further conclusion was achieved. Ortega (Ortega et al., 2020) focused on H2O2 and also lacked clinical studies. Pérez-Errázuriz (Pérez-Errázuriz et al., 2021) focused only on CPC, and concluded that more research was needed. Finally, Stathis (Stathis et al., 2021) found that oral and nasal antiseptics, including PVP-I, CHX, Listerine, and iota-carrageenan, showed an in vitro effect against SARS-CoV-2, while no completed clinical trials were found. Cavalcante-Leão (Cavalcante-Leão et al., 2021) included two in vitro studies of Severe acute respiratory syndrome coronavirus 1 (SARS-CoV-1) and Middle-East Respiratory Syndrome coronavirus (MERS-CoV), suggesting that PVP-I at 1–7% could be the most effective against SARS-CoV-2. Ultimately, as COVID-19 is still a new disease, there is limited evidence to suggest any final standardized clinical protocol regarding the use of mouthwashes against SARS-CoV-2.
The SARS-CoV-2 is comprised of a lipid envelope with spike glycoproteins that help bind the virus to its host cell (O’Donnell et al., 2020, Shang et al., 2020). A known virucidal strategy against many coronavirus species is to disrupt this envelope, which may be the mechanism of action of many of the mouthwash reagents. Viral envelopes are composed of host cell proteins, and for the coronaviruses, the composition of this structure may be related to the endoplasmic reticulum membrane (O’Donnell et al., 2020).
PVP-I is a common antiseptic used safely as mouthwash with in vitro antiviral effects to SARS-CoV-1 and MERS-CoV (Eggers et al., 2018). PVP-I is composed of iodine and polyvinylpyrrolidone; when converted to free iodine, it penetrates microorganisms by oxidizing nucleic acids and disrupting proteins. This may provoke viral destruction by disorganization of the cell membrane, thus altering their metabolic pathway and causing irreversible damage (Bidra et al., 2020b, Carrouel et al., 2021, Choudhury et al., 2020). Our findings suggest that PVP-I may be effective against SARS-CoV-2 both in vitro and clinically.
CPC is a quaternary ammonium compound that may interact with the viral envelope, making it effective against SARS-CoV-2 (Gottsauner et al., 2020, Seneviratne et al., 2021). CPC affects proteins and lipids on the bacterial surface, and has antiviral effects against other viruses like influenza in vivo and in vitro (O’Donnell et al., 2020, Popkin et al., 2017). CPC, alone and in combination with other reagents, could be effective against SARS-CoV-2.
The essential oils are usually combined with 21–26% ethanol, although low concentrations of ethanol may impact the viral envelope. Moreover, both thymol and eucalyptol have been shown to interfere with the lipid envelope of the herpesvirus, suggesting a possible effect in this viral structure of SARS-CoV-2 (Astani et al., 2010, O’Donnell et al., 2020). Essential oils with ethanol may be an option to reduce viral spread, as found in our results.
CHX is a cationic bisguanide antiseptic with broad antimicrobial activity and antiviral effects against enveloped viruses, though its role against SARS-CoV-2 is still controversial (Bernstein et al., 1990, Bidra et al., 2020b, Carrouel et al., 2021, Sampson et al., 2020). Its mechanism of action is mainly due to its positive charge, which allows entry into the cell by interacting with the negative charge of the microbial surface, thus causing leakage (O’Donnell et al., 2020). As CHX is usually combined with low concentrations of ethanol, this would help achieve its antiviral effect (O’Donnell et al., 2020). Based on the mixed results found in vitro and clinically, CHX alone may not be sufficiently effective against SARS-CoV-2, so combinations with ethanol or CPC may present better results.
While H2O2 is not widely used due to its possible adverse effects, it is a good disinfectant (Gottsauner et al., 2020, Seneviratne et al., 2021). H2O2 disrupts the viral envelope by liberating oxygen-free radicals (Peng et al., 2020). Although the clinical studies showed that H2O2 had some antiviral effect, the in vitro studies did not.
COVID-19 can be transmitted through small droplets of expelled saliva; after inhalation of these droplets, host cells can be infected and symptoms of the disease can appear (J. Xu et al., 2020, R. Xu et al., 2020). SARS-CoV-2 can be found not only in saliva, but also in dental plaque (Gomes et al., 2021, To et al., 2020). As the saliva and oral cavity are considered reservoirs of the virus, the use of mouthwashes could help in the decrease of COVID-19 transmission.
PVP-I, CPC (alone and combined), and essential oil mouthwashes were the most effective against SARS-CoV-2 both in vitro and clinically. Based on these results, PVP-I at 0.5–1.0% for 30 sec, CPC at 0.04–0.075% for 20–30 sec, and essential oils with ethanol for 30 sec may be effective in decreasing the viral load in infected patients. These compounds may be useful in reducing the spread of COVID-19, as mouthwashes are cheap and simple to use, though these results are not conclusive.
This review highlighted several in vitro and clinical studies found in the literature. Nevertheless, the different reagents, concentrations, doses, and outcome analysis methods used, along with the unclear and high risk of bias present, highlighted that more studies—especially clinical research studies—are needed to clearly define the antiviral effect of mouthwashes against the different SARS-CoV-2 strains.
5. Limitations
In vitro studies are limited as their results cannot be extrapolated to humans. Most of the clinical studies presented an unclear or high risk of bias, and data from these studies was considered too limited to inform clinical recommendations. Finally, a meta-analysis of these findings would not be possible due to the different reagents, different outcome analyses, and the bias of the clinical studies.
6. Conclusion
The in vitro studies showed that mouthwashes containing PVP-I, CPC, and essential oils may have an antiviral effect against different strains of SARS-CoV-2.
The evidence from clinical studies found that mouthwashes with H2O2, CHX, PVP-I, CPC, CDCM, sorbitol + xylitol, or essential oils had an antiviral effect against SARS-CoV-2; however, because most studies were assessed to have an unclear to high risk of bias, these results should not be a determinant for clinical recommendations.
Based on both clinical and in vitro studies, PVP-I, CPC, and essential oils with ethanol may present the best results against SARS-CoV-2. Therefore, more studies with these products may be beneficial.
As the COVID-19 pandemic is still a major health problem worldwide, more high-quality clinical studies investigating the real antiviral effect of different mouthwash compounds against SARS-CoV-2 are urgently needed.
CRediT author Contribution Statement
Jhon Paul Iakov Mezarina Mendoza: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Resources, Software, Visualization, Writing – original draft, Writing – review & editing. Briggitte Patricia Trelles Ubillús: Data curation, Formal analysis, Investigation, Methodology, Writing – original draft. Gabriela Tazziana Salcedo Bolívar – Data curation, Formal analysis, Investigation, Methodology, Writing – original draft. Rosa Del Pilar Castañeda Palacios: Data curation, Formal analysis, Investigation, Methodology, Writing – original draft. Paulo Sergio Gilmar Herrera Lopez: Data curation, Formal analysis, Investigation, Methodology, Writing – original draft. David Alex Padilla Rodríguez: Data curation, Formal analysis, Investigation, Methodology, Writing – original draft. Karin Harumi Uchima-Koecklin: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Resources, Software, Supervision, Visualization, Writing – original draft, Writing – review & editing.
Ethics Information
The present study is a systematic review, which utilized data from previous existing studies. The authors did not perform any experiments for this studies that involved human or animal beings. This study presents an original work analyzed in a truthful manner that does not require any further ethical consideration.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Peer review under responsibility of King Saud University.
References
- Anderson D.E., Sivalingam V., Kang A.E.Z., Ananthanarayanan A., Arumugam H., Jenkins T.M., Hadjiat Y., Eggers M. Povidone-Iodine Demonstrates Rapid In Vitro Virucidal Activity Against SARS-CoV-2, The Virus Causing COVID-19 Disease. Infect. Dis. Ther. 2020;9:669–675. doi: 10.1007/s40121-020-00316-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Astani A., Reichling J., Schnitzler P. Comparative study on the antiviral activity of selected monoterpenes derived from essential oils. Phyther. Res. 2010;24:673–679. doi: 10.1002/ptr.2955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avhad S.K., Bhanushali M., Sachdev S.S., Save S.S. Comparison of Effectiveness of Chlorine Dioxide Mouthwash and Chlorhexidine Gluconate Mouthwash in Reduction of Oral Viral Load in Patients with COVID-19. Indian J. Public Heal. Res. Dev. 2020 doi: 10.37506/ijphrd.v11i11.11343. [DOI] [Google Scholar]
- Bernstein D., Schiff G., Echler G., Prince A., Feller M., Briner W. In vitro Virucidal Effectiveness of a 0.12%-Chlorhexidine Gluconate Mouthrinse. J. Dent. Res. 1990;69:874–876. doi: 10.1177/00220345900690030901. [DOI] [PubMed] [Google Scholar]
- Bidra A.S., Pelletier J.S., Westover J.B., Frank S., Brown S.M., Tessema B. Comparison of In Vitro Inactivation of SARS CoV-2 with Hydrogen Peroxide and Povidone-Iodine Oral Antiseptic Rinses. J. Prosthodont. 2020;29:599–603. doi: 10.1111/jopr.13220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bidra A.S., Pelletier J.S., Westover J.B., Frank S., Brown S.M., Tessema B. Rapid In-Vitro Inactivation of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Using Povidone-Iodine Oral Antiseptic Rinse. J. Prosthodont. 2020;29:529–533. doi: 10.1111/jopr.13209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton M.J., Clarkson J.E., Goulao B., Glenny A.-M., McBain A.J., Schilder A.G., Webster K.E., Worthington H.V. Use of antimicrobial mouthwashes (gargling) and nasal sprays by healthcare workers to protect them when treating patients with suspected or confirmed COVID-19 infection. Cochrane Database Syst. Rev. 2020 doi: 10.1002/14651858.CD013626.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrouel F., Conte M.P., Fisher J., Gonçalves L.S., Dussart C., Llodra J.C., Bourgeois D. COVID-19: A recommendation to examine the effect of mouthrinses with β-cyclodextrin combined with citrox in preventing infection and progression. J. Clin. Med. 2020;9:1–8. doi: 10.3390/jcm9041126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrouel F., Gonçalves L.S., Conte M.P., Campus G., Fisher J., Fraticelli L., Gadea-Deschamps E., Ottolenghi L., Bourgeois D. Antiviral Activity of Reagents in Mouth Rinses against SARS-CoV-2. J. Dent. Res. 2021;100:124–132. doi: 10.1177/0022034520967933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrouel F., Valette M., Gadea E., Esparcieux A., Illes G., Langlois M.E., Perrier H., Dussart C., Tramini P., Ribaud M., Bouscambert-Duchamp M., Bourgeois D. Use of an antiviral mouthwash as a barrier measure in the SARS-CoV-2 transmission in adults with asymptomatic to mild COVID-19: a multicentre, randomized, double-blind controlled trial. Clin. Microbiol. Infect. 2021 doi: 10.1016/j.cmi.2021.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrouel F., Valette M., Perrier H., Bouscambert-Duchamp M., Dussart C., Tramini P., Bourgeois D. Performance of self-collected saliva testing compared with nasopharyngeal swab testing for the detection of sars-cov-2. Viruses. 2021;13:1–9. doi: 10.3390/v13050895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavalcante-Leão B.L., de Araujo C.M., Basso I.B., Schroder, Liga A.G.D., Guariza-Filho O., Ravazzi G.C., Gonçalves F.M., Zeigelboim B.S., Santos R.S., Stechman-Neto J. Is there scientific evidence of the mouthwashes effectiveness in reducing viral load in Covid-19? A systematic review. J. Clin. Exp. Dent. 2021;13:179–189. doi: 10.4317/JCED.57406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhary P., Melkonyan A., Meethil A., Saraswat S., Hall D.L., Cottle J., Wenzel M., Ayouty N., Bense S., Casanova F., Chaney M., Chase H., Hermel R., McClement M., Sesson C., Woolsey B., Kumar P. Estimating salivary carriage of severe acute respiratory syndrome coronavirus 2 in nonsymptomatic people and efficacy of mouthrinse in reducing viral load. J. Am. Dent. Assoc. 2021;21:1–9. doi: 10.1016/j.adaj.2021.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choudhury M.I.M., Shabnam N., Ahsan T., Kabir M.S., Khan R.M., Ahsan S.M.A. Effect of 1% Povidone Iodine Mouthwash/Gargle, Nasal and Eye Drop in COVID-19 patient. Biores. Commun. 2020;7:919–923. [Google Scholar]
- Davies K., Buczkowski H., Welch S.R., Green N., Mawer D., Woodford N., Roberts A.D.G., Nixon P.J., Seymour D.W., Killip M.J. Effective in vitro inactivation of SARS-CoV-2 by commercially available mouthwashes. J. Gen. Virol. 2021;102 doi: 10.1099/jgv.0.001578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eduardo, F. de P., Corrêa, L., Heller, D., Daep, C.A., Benitez, C., Malheiros, Z., Stewart, B., Ryan, M., Machado, C.M., Hamerschlak, N., Rebello Pinho, J.R., Bezinelli, L.M., 2021. Salivary SARS-CoV-2 load reduction with mouthwash use: A randomized pilot clinical trial. Heliyon 7, 1–7. https://doi.org/10.1016/j.heliyon.2021.e07346. [DOI] [PMC free article] [PubMed]
- Eggers M., Koburger-Janssen T., Eickmann M., Zorn J. In Vitro Bactericidal and Virucidal Efficacy of Povidone-Iodine Gargle/Mouthwash Against Respiratory and Oral Tract Pathogens. Infect. Dis. Ther. 2018;7:249–259. doi: 10.1007/s40121-018-0200-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elzein R., Abdel-Sater F., Fakhreddine S., Hanna P.A., Feghali R., Hamad H., Ayoub F. In vivo evaluation of the virucidal efficacy of Chlorhexidine and Povidone-iodine mouthwashes against salivary SARS-CoV-2. A randomized-controlled clinical trial. J. Evid. Based Dent. Pract. 2021;101584 doi: 10.1016/j.jebdp.2021.101584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes S.C., Fachin S., Fonseca J.G., Angst P.D.M., Lamers M.L., Silva I.S.B., Nunes L.N. Dental biofilm of symptomatic COVID-19 patients harbours SARS-CoV-2. J. Clin. Periodontol. 2021 doi: 10.1111/jcpe.13471. jcpe.13471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottsauner M.J., Michaelides I., Schmidt B., Scholz K.J., Buchalla W., Widbiller M., Hitzenbichler F., Ettl T., Reichert T.E., Bohr C., Vielsmeier V., Cieplik F. A prospective clinical pilot study on the effects of a hydrogen peroxide mouthrinse on the intraoral viral load of SARS-CoV-2. Clin. Oral Investig. 2020;24:3707–3713. doi: 10.1007/s00784-020-03549-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guenezan J., Garcia M., Strasters D., Jousselin C., Lévêque N., Frasca D., Mimoz O. Povidone Iodine Mouthwash, Gargle, and Nasal Spray to Reduce Nasopharyngeal Viral Load in Patients With COVID-19: A Randomized Clinical Trial. JAMA Otolaryngol. Neck Surg. 2021;147:400. doi: 10.1001/jamaoto.2020.5490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassandarvish P., Tiong V., Mohamed N.A., Arumugam H., Ananthanarayanan A., Qasuri M., Hadjiat Y., Abubakar S. In vitro virucidal activity of povidone iodine gargle and mouthwash against SARS-CoV-2: implications for dental practice. Br. Dent. J. 2020;13–16 doi: 10.1038/s41415-020-2402-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain A., Grover V., Singh C., Sharma A., Das D., Singh P., Thakur K., Ringe R. Chlorhexidine: An effective anticovid mouth rinse. J. Indian Soc. Periodontol. 2021;25:86–88. doi: 10.4103/jisp.jisp_824_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch-Heier J., Hoffmann H., Schindler M., Lussi A., Planz O. Inactivation of sars-cov-2 through treatment with the mouth rinsing solutions viruprox® and bacterx® pro. Microorganisms. 2021;9:1–10. doi: 10.3390/microorganisms9030521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komine A., Yamaguchi E., Okamoto N., Yamamoto K. Virucidal activity of oral care products against SARS-CoV-2 in vitro. J. Oral Maxillofac. Surgery, Med. Pathol. 2021;2–4 doi: 10.1016/j.ajoms.2021.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meister T.L., Brüggemann Y., Todt D., Conzelmann C., Müller J.A., Groß R., Münch J., Krawczyk A., Steinmann J., Steinmann J., Pfaender S., Steinmann E. Virucidal efficacy of different oral rinses against severe acute respiratory syndrome Coronavirus 2. J. Infect. Dis. 2020;222:1289–1292. doi: 10.1093/infdis/jiaa471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohamed, N.A., Baharom, N., Wan Sulaiman, W.S., Rashid, Z.Z., Ken, W.K., Ali, U.K., Othman, S.N., Samat, M.N.A.B.D., Kori, N., Periyasamy, P., Zakaria, N.A., Sugurmar, A.N.K., Mohammad Kazmin, N.E., Khee, C.X., Saniman, S.M., Isahak, I., 2020. Early viral Clearance among COVID-19 patients when gargling with povidone-iodine and essential oils – A clinical trial. medRxiv. https://doi.org/10.1101/2020.09.07.20180448.
- Moosavi M.S., Aminishakib P., Ansari M. Antiviral mouthwashes: possible benefit for COVID-19 with evidence-based approach. J. Oral Microbiol. 2020;12 doi: 10.1080/20002297.2020.1794363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muñoz-Basagoiti J., Perez-Zsolt D., León R., Blanc V., Raïch-Regué D., Cano-Sarabia M., Trinité B., Pradenas E., Blanco J., Gispert J., Clotet B., Izquierdo-Useros N. Mouthwashes with CPC Reduce the Infectivity of SARS-CoV-2 Variants In Vitro. J. Dent. Res. 2021;100:1265–1272. doi: 10.1177/00220345211029269. [DOI] [PubMed] [Google Scholar]
- O’Donnell V.B., Thomas D., Stanton R., Maillard J.-Y., Murphy R.C., Jones S.A., Humphreys I., Wakelam M.J.O., Fegan C., Wise M.P., Bosch A., Sattar S.A. Potential Role of Oral Rinses Targeting the Viral Lipid Envelope in SARS-CoV-2 Infection. Function. 2020;1:1–12. doi: 10.1093/function/zqaa002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortega K.L., Rech B.O., El Haje G.L.C., Gallo C.B., Pérez-Sayáns M., Braz-Silva P.H. Do hydrogen peroxide mouthwashes have a virucidal effect? A systematic review. J. Hosp. Infect. 2020;106:657–662. doi: 10.1016/j.jhin.2020.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page M.J., McKenzie J.E., Bossuyt P.M., Boutron I., Hoffmann T.C., Mulrow C.D., Shamseer L., Tetzlaff J.M., Akl E.A., Brennan S.E., Chou R., Glanville J., Grimshaw J.M., Hróbjartsson A., Lalu M.M., Li T., Loder E.W., Mayo-Wilson E., McDonald S., McGuinness L.A., Stewart L.A., Thomas J., Tricco A.C., Welch V.A., Whiting P., Moher D. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ. 2021;372 doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelletier J.S., Tessema B., Frank S., Westover J.B., Brown S.M., Capriotti J.A. Efficacy of Povidone-Iodine Nasal and Oral Antiseptic Preparations Against Severe Acute Respiratory Syndrome-Coronavirus 2 (SARS-CoV-2) Ear, Nose Throat J. 2021;100:192S–196S. doi: 10.1177/0145561320957237. [DOI] [PubMed] [Google Scholar]
- Peng X., Xu X., Li Y., Cheng L., Zhou X., Ren B. Transmission routes of 2019-nCoV and controls in dental practice. Int. J. Oral Sci. 2020;12:1–6. doi: 10.1038/s41368-020-0075-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez-Errázuriz S., Velasco-Ortega E., Jiménez-Guerra Á., Aguilera-Navarro E. Cetylpyridinium Chloride as a Tool Against COVID-19. Int. J. Odontostomatol. 2021;15:27–30. doi: 10.4067/s0718-381x2021000100027. [DOI] [Google Scholar]
- Popkin D.L., Zilka S., Dimaano M., Fujioka H., Rackley C., Salata R., Griffith A., Mukherjee P.K., Ghannoum M.A., Esper F. Cetylpyridinium Chloride (CPC) Exhibits Potent, Rapid Activity Against Influenza Viruses in vitro and in vivo. Pathog. Immun. 2017;2:253. doi: 10.20411/pai.v2i2.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampson V., Kamona N., Sampson A. Could there be a link between oral hygiene and the severity of SARS-CoV-2 infections? Br. Dent. J. 2020;228:971–975. doi: 10.1038/s41415-020-1747-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos, C.A., Orcina, B. da F., Reia, V.B.C.B., Ribeiro, L.G., Grotto, R.M.T.M.T., Prudenciatti, A.M., Moraes, L.N. de, Zangrando, M.R.S.R., Vilhena, F.V., Santos, P.S. da S., de Moraes, L.N., Zangrando, M.R.S.R., Vilhena, F.V., Santos, P.S. da S., 2021. Virucidal activity of the antiseptic mouthwash and dental gel containing anionic phthalocyanine derivative: In vitro study. Clin. Cosmet. Investig. Dent. 13, 269–274. https://doi.org/10.2147/CCIDE.S315419. [DOI] [PMC free article] [PubMed]
- Santos, P.S. da S., Orcina, B., Machado, R.R., Vilhena, F., Alves, L., Zangrando, M., de Oliveira, R., Soares, M., Simão, A., Pietro, E., Kuroda, J., Benjamin, I., Araújo, D., Toma, S., Flor, L., Araki, K., Durigon, E., 2021. Beneficial effects of a mouthwash containing an antiviral phthalocyanine derivative on the length of hospital stay for COVID-19: Randomised trial. Res. Sq. https://doi.org/10.21203/rs.3.rs-365425/v1. [DOI] [PMC free article] [PubMed]
- Schürmann, M., Aljubeh, M., Tiemann, C., Sudhoff, H., 2021. Mouthrinses against SARS-CoV-2: anti-inflammatory effectivity and a clinical pilot study. Eur. Arch. Oto-Rhino-Laryngology. https://doi.org/10.1007/s00405-021-06873-8. [DOI] [PMC free article] [PubMed]
- Seneviratne C.J., Balan P., Ko K.K.K., Udawatte N.S., Lai D., Ng D.H.L., Venkatachalam I., Lim K.S., Ling M.L., Oon L., Goh B.T., Sim X.Y.J. Efficacy of commercial mouth-rinses on SARS-CoV-2 viral load in saliva: randomized control trial in Singapore. Infection. 2021;49:305–311. doi: 10.1007/s15010-020-01563-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shang J., Wan Y., Luo C., Ye G., Geng Q., Auerbach A., Li F. Cell entry mechanisms of SARS-CoV-2. Proc. Natl. Acad. Sci. USA. 2020;117 doi: 10.1073/pnas.2003138117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stathis C., Victoria N., Loomis K., Nguyen S.A., Eggers M., Septimus E., Safdar N. Review of the use of nasal and oral antiseptics during a global pandemic. Future Microbiol. 2021;16:119–130. doi: 10.2217/fmb-2020-0286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Statkute, E., Rubina, A., O’Donnell, V.B., Thomas, D.W., Stanton, R.J., 2020. The Virucidal Efficacy of Oral Rinse Components Against SARS-CoV-2 In Vitro. bioRxiv 21, 1–9. https://doi.org/10.1101/2020.11.13.381079.
- Steinhauer K., Meister T.L., Todt D., Krawczyk A., Paßvogel L., Becker B., Paulmann D., Bischoff B., Pfaender S., Brill F.H.H., Steinmann E. Comparison of the in-vitro efficacy of different mouthwash solutions targeting SARS-CoV-2 based on the European Standard EN 14476. J. Hosp. Infect. 2021;4–7 doi: 10.1016/j.jhin.2021.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterne J.A., Hernán M.A., Reeves B.C., Savović J., Berkman N.D., Viswanathan M., Henry D., Altman D.G., Ansari M.T., Boutron I., Carpenter J.R., Chan A.W., Churchill R., Deeks J.J., Hróbjartsson A., Kirkham J., Jüni P., Loke Y.K., Pigott T.D., Ramsay C.R., Regidor D., Rothstein H.R., Sandhu L., Santaguida P.L., Schünemann H.J., Shea B., Shrier I., Tugwell P., Turner L., Valentine J.C., Waddington H., Waters E., Wells G.A., Whiting P.F., Higgins J.P. ROBINS-I: A tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355:4–10. doi: 10.1136/bmj.i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterne J.A.C., Savović J., Page M.J., Elbers R.G., Blencowe N.S., Boutron I., Cates C.J., Cheng H.Y., Corbett M.S., Eldridge S.M., Emberson J.R., Hernán M.A., Hopewell S., Hróbjartsson A., Junqueira D.R., Jüni P., Kirkham J.J., Lasserson T., Li T., McAleenan A., Reeves B.C., Shepperd S., Shrier I., Stewart L.A., Tilling K., White I.R., Whiting P.F., Higgins J.P.T. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:1–8. doi: 10.1136/bmj.l4898. [DOI] [PubMed] [Google Scholar]
- To K.K.W., Tsang O.T.Y., Yip C.C.Y., Chan K.H., Wu T.C., Chan J.M.C., Leung W.S., Chik T.S.H., Choi C.Y.C., Kandamby D.H., Lung D.C., Tam A.R., Poon R.W.S., Fung A.Y.F., Hung I.F.N., Cheng V.C.C., Chan J.F.W., Yuen K.Y. Consistent detection of 2019 novel coronavirus in saliva. Clin. Infect. Dis. 2020;71:841–843. doi: 10.1093/cid/ciaa149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wölfel R., Corman V.M., Guggemos W., Seilmaier M., Zange S., Müller M.A., Niemeyer D., Jones T.C., Vollmar P., Rothe C., Hoelscher M., Bleicker T., Brünink S., Schneider J., Ehmann R., Zwirglmaier K., Drosten C., Wendtner C. Virological assessment of hospitalized patients with COVID-2019. Nature. 2020;581:465–469. doi: 10.1038/s41586-020-2196-x. [DOI] [PubMed] [Google Scholar]
- Xu C., Wang A., Hoskin E.R., Cugini C., Fine D.H., Markowitz K., Chang T.L. Differential effects of antiseptic mouth rinses on sars-cov-2 infectivity in vitro. Pathogens. 2021;10:1–14. doi: 10.3390/pathogens10030272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J., Li Y., Gan F., Du Y., Yao Y. Salivary Glands: Potential Reservoirs for COVID-19 Asymptomatic Infection. J. Dent. Res. 2020;99:989. doi: 10.1177/0022034520918518. [DOI] [PubMed] [Google Scholar]
- Xu R., Cui B., Duan X., Zhang P., Zhou X., Yuan Q. Saliva: potential diagnostic value and transmission of 2019-nCoV. Int. J. Oral Sci. 2020;12 doi: 10.1038/s41368-020-0080-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou L., Ruan F., Huang M., Liang L., Huang H., Hong Z., Yu J., Kang M., Song Y., Xia J., Guo Q., Song T., He J., Yen H.-L., Peiris M., Wu J. SARS-CoV-2 Viral Load in Upper Respiratory Specimens of Infected Patients. N. Engl. J. Med. 2020;382:1177–1179. doi: 10.1056/NEJMc2001737. [DOI] [PMC free article] [PubMed] [Google Scholar]