Abstract
The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) delta variant transmits much more rapidly than prior SARS-CoV-2 viruses. The primary mode of transmission is via short range aerosols that are emitted from the respiratory tract of an index case. There is marked heterogeneity in the spread of this virus, with 10% to 20% of index cases contributing to 80% of secondary cases, while most index cases have no subsequent transmissions. Vaccination, ventilation, masking, eye protection, and rapid case identification with contact tracing and isolation can all decrease the transmission of this virus.
Keywords: COVID-19, SARS-CoV-2, Delta, Transmission, Vaccination, Aerosol, Superspreading, Overdispersion
Key points
-
•
Severe acute respiratory syndrome coronavirus 2 has an intense but discrete infectious period.
-
•
Respiratory transmission is the dominant mode of transmission, with viral particles suspended on fine aerosols emitted from the respiratory tract. Risk for transmission is highest at close distance and in poorly ventilated indoor settings.
-
•
Viral factors are associated with increased transmissibility.
-
•
Transmission dynamics are heterogeneous, with the majority of secondary cases arising from a small minority of index cases and most index cases leading to no secondary transmissions.
-
•
Vaccines dramatically decrease transmission by decreasing the risk of infection among the vaccinated and by decreasing the chance of transmission from vaccinated individuals who become infected.
Introduction
Understanding the transmission characteristics of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is essential to designing effective mitigation strategies. The virus spreads predominantly through shared air between an index and a secondary case during a relatively brief period of infectiousness.1 Detailed assessments of transmission have revealed deep flaws in the droplet–aerosol dichotomy that has been emphasized for decades as a model for transmission of respiratory pathogens.2 Host, viral, and environmental factors all influence risk of transmission of SARS-CoV-2, with marked heterogeneity a key feature of its spread.
In the first year of the pandemic, ancestral SARS-CoV-2 virus that emerged in Wuhan, China (termed Wuhan-Hu-1), was slowly replaced by virus containing the D614G mutation.3 In experimental models, the D614G-containing virus replicates more efficiently and transmits more rapidly than ancestral virus.4 , 5 As D614 G became dominant, experts predicted other variants with a competitive advantage were likely to emerge thereafter.
All RNA viruses accrue mutations, and mutations that confer a fitness advantage are likely to expand at a population level.6 The base mutation rate for SARS-CoV-2 is 4 × 10−4 nucleotide substitutions per site per year, or approximately 1 to 2 mutations per month based on its large genome size and the presence of a proofreading exoribonuclease that ensures relatively high fidelity transcription.7 , 8 Although active viral replication in an immunocompetent human host occurs for a relatively short period, prolonged infection is well-described in some hosts, particularly those with severe B-cell immunodeficiencies.9, 10, 11 In these immunocompromised hosts, mutations may accumulate more rapidly than expected owing to the significantly higher amount of viral replication, and it is thought that this is the context in which more transmissible variants may have emerged.12
A variant of concern eventually called alpha was first recognized in the United Kingdom in December of 2020.13 It was defined by 17 mutations, including 8 in the spike protein, and it rapidly became dominant in the UK and much of the world, with researchers estimating it was 43% to 90% more transmissible than its predecessor virus.14 A Japanese study found that the secondary attack rate in households was significantly higher for alpha versus prior SARS-CoV-2 lineages (38.7% vs 19.3%; P<.001).15
The delta variant of concern was first identified in the state of Maharashtra, India, in late 2020 and subsequently spread rapidly around the globe, causing large surges of cases and hospitalizations.16 It has a higher replication efficiency than alpha in experimental human airway epithelial systems.17 In India, where alpha and delta first competed, small outbreaks associated with alpha were followed by much larger delta outbreaks in the same regions, and delta was estimated to be 1.3 to 1.7 times more transmissible than alpha.18 In a matched household cluster study later conducted in the UK, including a total of 2586 delta and 3390 alpha index cases, the adjusted odds ratio of household transmission was 1.70 for delta compared with alpha (95% confidence interval [CI], 1.48–1.95).19 Delta dominated across the vast majority of the world for the majority of 2021 and has been associated with large outbreaks, even in settings with relatively high vaccine coverage.
In this review, we describe the important factors influencing SARS-CoV-2 transmission, with particular attention to unique features of the delta era. We outline modes of transmission and determinants of infectiousness of SARS-CoV-2. We also review the nature of the heterogeneity that defines the transmission dynamics of this virus. We describe the role of vaccines in preventing transmission both directly, by decreasing cases, and indirectly, by decreasing the likelihood of secondary transmission when a vaccinated individual develops infection. Finally, we review the evidence for other transmission mitigation strategies, including masking, social distancing, rapid case identification and contact tracing, and improved ventilation.
Modes of transmission
The modes of transmission of SARS-CoV-2 have been elucidated through detailed case contact studies in a variety of contexts. Although there was initial concern about the potential role of fomite or indirect transmission, this mechanism of spread is not important for SARS-CoV-2, if it occurs at all.1 Although SARS-CoV-2 remains viable for hours on contaminated surfaces under ideal experimental conditions, in real-world settings replication-competent virus is only rarely recovered from surfaces and then only at extremely low levels.20, 21, 22 In the few case reports where fomite transmission has been suggested, respiratory transmission cannot be excluded.1
Respiratory transmission, with SARS-CoV-2 carried on tiny particles emitted from the respiratory tract of an index case to a contact, is the clear and dominant route of spread.1 From early in the pandemic, it has been evident that proximity is a key determinant of transmission risk. For instance, a contact tracing study of train passengers in China before universal masking that included 2334 index cases and 72,093 close contacts found that the risk of transmission was directly related to the distance between the seats and the amount of shared time on the train.23 A detailed contact tracing study of the Diamond Princess cruise ship outbreak found that passengers with SARS-CoV-2 infection were either infected in shared public spaces in close contact or in their cabins when they were lodging with another infected passenger, but did not find evidence of transmissions between rooms.24 In an outbreak of 14 confirmed and 6 probable cases on a plane in Japan, being seated within 2 rows of the index case was associated with an adjusted odds ratio for infection of 7.47 (95% CI, 2.06–27.2).25
Before the COVID-19 pandemic, respiratory transmission of respiratory viruses and bacteria was categorized widely in a dichotomous way, with some pathogens, like tuberculosis, spread on smaller particles called aerosols and others spread on larger particles called droplets.26, 27, 28 Pathogens spread on larger droplets were not thought to reach individuals more than about 6 feet away because they would fall to the ground owing to gravitation effect, whereas smaller aerosols could remain suspended over longer distances and times. Designating a pathogen as droplet or aerosol spread implied the relative importance of different personal protective equipment, with surgical masks thought to suffice in the context of pathogens with droplet transmission (droplet precautions) and respirators needed to prevent aerosol transmission (airborne precautions). Because proximity was so important and surgical masks reasonably effective at preventing spread (particularly in hospital settings), droplet spread within the traditional model was initially presumed to be the most important mechanism of transmission.29 , 30 However, it has become clear that the predominant mode of transmission of SARS-CoV-2 (as well as most other respiratory pathogens) is aerosol transmission, with short range aerosols the most important.31 The risk of aerosol transmission is greatest at short range because the concentration, and therefore infectious dose, is highest there, whereas aerosols are diluted over larger distances.32 Confusion about this topic has led some experts to call for a change in the terminology used to describe transmission of respiratory pathogens, and for a shift to focusing on inhalation as the major mode of transmission (Table 1 ).33
Table 1.
Traditional versus updated understanding of airborne transmission
| Traditional: droplet vs aerosol dichotomy | Updated: inhalation | |
|---|---|---|
| Relative importance of droplets and aerosols | Droplets are thought to be responsible for most transmission of respiratory viruses; aerosols are important for certain pathogens like tuberculosis or measles.32 | Both droplets and aerosols contribute to transmission, though short range aerosols are the most important vehicle for most respiratory viruses.33 |
| Role of proximity | Most aerosol transmissions are thought to happen at longer distances. | Proximity is important for droplets and aerosols, with concentrations decreased by gravity and dilution for droplets and dilution for aerosols. |
| Role of masking | Surgical masking is sufficient for preventing droplet transmission; respirator/N95 masks are needed to prevent aerosol transmission.34 | Surgical masks (especially when worn by source) provide some (but not complete) protection against aerosols.35 There is a theoretic benefit to a respirator/N95, although the incremental benefit has not been clearly demonstrated in clinical trials or real-world studies to date. |
| Role of ventilation | Not necessary for droplet spread; needed for aerosols or pathogens primarily transmitted via droplets when index cases undergo aerosol generating procedures. | An important tool that can be used to decrease risk of most respiratory pathogens through dilutional mechanism. |
Evidence supporting the importance of aerosol transmission of SARS-CoV-2 includes numerous experimental and clinical studies.31 For instance, a mathematical model showing the high risk associated with distances of less than 6 feet,36 a study showing that viral RNA was found in fine aerosols (particles ≤5 μm) 85% of the time rather than larger particles,37 and real-world and experimental animal studies showing transmission is possible through the air at distances far greater than 6 feet.38, 39, 40 In health care settings, there are now many well-documented human-to-human transmissions at distances of more than 6 feet, for instance in shared patient hospital rooms.41 Longer range transmissions tend to occur in poorly ventilated settings or when the air flow is directed from an index case to secondary cases.42 , 43 In a very detailed description of an outbreak at a hospital in Boston, positive pressure in patient rooms relative to a nursing station on the unit was a proposed as a mechanism of spread beyond a patient room.41 A detailed cluster report with sequencing of virus genomes at an isolation facility in New Zealand with closed circuit television monitoring found 3 linked secondary cases who were never in the same room an d always more than 2 m away from the index case, with aerosol transmission the only plausible mechanism of spread.44 , 45 As discussed elsewhere in this article, despite the overwhelming evidence for the predominance of aerosol transmission, the benefit of higher filtration masks over routine surgical masks in the community and in health care settings has yet to be demonstrated conclusively.
Determinants of infectiousness
Host, virologic, and environmental factors all impact infectiousness of SARS-CoV-2. Apart from vaccination, which we discuss elsewhere in this review, the clearest host factor impacting transmission risk is whether the index case eventually develops symptoms. Several studies and systematic reviews have shown that persistently asymptomatic index cases are much less likely to lead to secondary cases compared with symptomatic index cases. For example, a household contact study from the original outbreak in Wuhan that included 27,101 affected households found that asymptomatic index cases were much less likely to transmit, with an adjusted odds ratio of 0.21 (95% CI, 0.14–0.31).46 A study from Singapore of 628 people with SARS-CoV-2 infection and 3790 close contacts found the transmission risk was 3.85 times higher for symptomatic versus asymptomatic index cases (95% CI, 2.1–7.2).47 A systematic review found the secondary attack rate was lower for people with persistently asymptomatic infection (relative risk, 0.35; 95% CI, 0.10–1.27).48 To date, there are no detailed studies of transmission risk by symptom status of individuals harboring the delta variant.
The respiratory tract viral load in the host at the time of an exposure is also clearly associated with infectiousness, with higher viral loads associated with greater likelihood of transmission.49 In a pre-delta era cohort study in Spain that included 282 index cases and 753 close contacts, secondary attack rate was directly related to the respiratory tract viral load of the index case at diagnosis.50 A study of 1058 students with SARS-CoV-2 infection at the University in Colorado, 860 of whom lived in multiple occupancy rooms, found that the average viral load in the 116 index cases who transmitted to a roommate was 6.5 times higher compared with the 414 who did not.51 In a Danish household contact study that included 66,311 index cases and 213,576 household contacts, the risk of transmission was also directly related to the viral load.52
Other host factors that may impact infectiousness include immune status and the age of the index case. Certain immunocompromised hosts may be more likely to transmit, but few studies have quantified this risk. A household contact study of 58 households in the United States found that immunocompromised index cases had a higher risk of transmission; however, just 2 of the 58 index cases were considered immunocompromised.53 Early in the pandemic, there was some evidence suggesting that young children aged less than 10 years were less susceptible to infection by ancestral SARS-CoV-2.1 , 54 , 55 Whether this decreased susceptibility persists in the era of the delta variant is currently unknown, although it is notable that large numbers of unvaccinated children have developed delta infection in settings where many adults are vaccinated like the United States and United Kingdom.56 , 57 A household study of delta transmission in Singapore found that older age was associated with a greater likelihood of transmission, although this finding may relate to contact patterns within households rather than inherent host factors.58
Viral factors also impact infectiousness. As described, the in vitro replication rate of delta is higher than that for alpha.16 The spike protein of delta more efficiently binds to the host cell membrane angiotensin-conerting enzyme 2 protein, which is the key host cell entry receptor.59 This may correlate with significantly higher in vivo respiratory tract viral loads for delta. In an outbreak of delta in Guangdong province in China, the peak viral load was much higher compared with the ancestral virus with a median peak cycle threshold of 20.6 for delta infections versus 34.0 for a historical cohort (P<.001).60 Although the increased transmissibility and fitness of delta compared with prior variants is not disputed, studies have been mixed about whether the peak viral load is in fact higher for delta.61, 62, 63, 64, 65 Additional mutations outside of the spike region may enhance viral replication in other ways. Researchers showed that mutations in the nucleocapsid protein found in delta and other more transmissible variants enhance messenger RNA (mRNA) delivery and packaging into virions and are associated with a more rapid viral replication.66
A number of environmental factors also predict the likelihood of transmission. The most important is ventilation, with outdoor transmission almost never identified.1 , 67 Indoor environments were noted to be important very early after the emergence of SARS-CoV-2 based on associations with clusters of transmission in Japan during its first wave.68 Environmental factors like lower ambient temperatures and higher relative humidity may also be associated with an increased transmission risk.69 Socioeconomic deprivation has repeatedly been shown to be associated with an increased risk for infection, likely because it is associated with an increased probability of more frequent and higher risk exposures.70, 71, 72, 73, 74
The period of infectiousness and serial interval
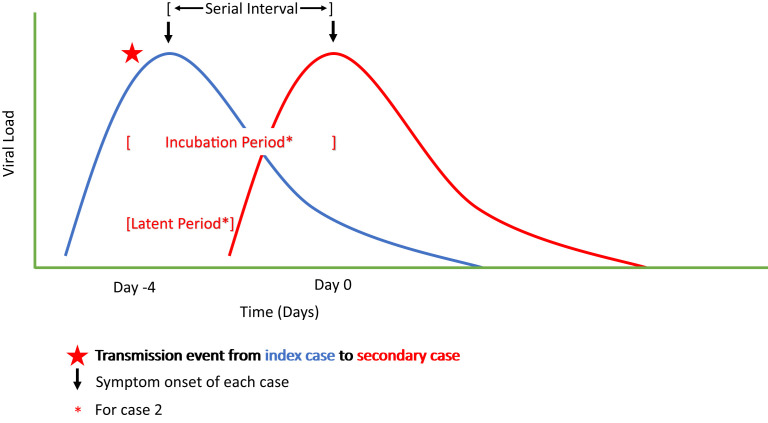
A person with SARS-COV-2 infection has a discrete period of infectiousness that has been well-defined for immunocompetent hosts. After an individual is exposed, there is an incubation period, defined as the time from exposure to symptom onset. Two key early papers examining the early cases in Wuhan, China, in the pre-delta era estimated the incubation period as 5.2 days (95% CI, 4.1–7.0) with 97.5% developing symptoms by 12.5 days (when reviewing the first 425 known cases)75 and 5.1 days (95% CI, 4.5–5.8 days), with 97.5% developing symptoms by 11.5 days after exposure (for 181 cases with a known exposure and symptom onset) (Fig. 1 ).76 The incubation period for delta seems to be significantly shorter than for prior SARS-CoV-2 viruses (Table 2 ).60 An analysis of 68 infections from 24 clusters from a contained delta outbreak in Guangdong, China, found the mean incubation period was 4.4 days (95% CI, 3.9–5.0).77 The incubation period for SARS-CoV-2, including the delta variant, is significantly longer and more variable than the incubation period for influenza. In 1 example, the incubation period for pandemic H1N1 influenza A virus in 2009 was approximately 2 days, with a standard deviation of approximately 2 days.78
Fig. 1.
Virologic characteristics of a transmission.
Table 2.
Incubation period, latent period, and serial interval for Wuhan-Hu-1 and the delta variant
Because 20% to 30% of people never develop symptoms, the latent period, which is the period from exposure to first detectable polymerase chain reaction (PCR), is also useful for understanding the transmission risk.48 , 79 Among 101 confirmed delta cases from the Guangdong outbreak, the mean latent period was 4.0 days (95% CI, 3.5–4.4), with 95% of cases having detectable viral RNA by 8.2 days (95% CI, 7.1–9.3).80 This interval is shorter than the mean latent period estimated in the pre-delta period, which was 5.5 days (95% CI, 5.1–5.9), with 95% of cases having detectable viral RNA by 10.6 days (95% CI, 9.6–11.6).79
These viral characteristics lead to the observed serial interval, or the time between symptom onset in a primary and secondary case. A meta-analysis in the pre-delta era estimated the serial interval as 5.4 days (95% CI, 5.19–5.61).81 Some studies have observed a shorter serial interval for delta compared with earlier variants,77 , 80 , 82 although others have not.83 The long and variable incubation period and significant proportion of presymptomatic transmission make it difficult to estimate a mean serial interval for recently emerged variants, because it takes data from numerous well-defined transmission pairs to generate a reliable estimate. The degree to which a possible shorter serial interval contributes to the more rapid spread of delta, which is also more transmissible than prior SARS-CoV-2 viruses (discussed in the section on Transmissibility and heterogeneity), is not known at this time.
For those who develop symptoms, the infectious period begins before symptom onset, with presymptomatic transmission a major driver of the COVID-19 pandemic (see Fig. 1). A detailed study of 25,381 people with SARS-CoV-2 infection in Germany from February 2020 through March 2021 found that, among those who develop symptoms, the respiratory tract viral load peaked 1 to 3 days before symptom onset with higher viral loads among sicker patients.64 Detailed viral load data from an analysis of the delta outbreak in Guangdong suggest that viral loads peak around the time of symptom onset60 and researchers estimated that 73.9% of transmissions may have occurred before symptom onset in the index case.80
Although individuals may remain PCR positive for weeks after infection, late transmissions occur very rarely, if at all. An early rigorous contact tracing study from Taiwan in the pre-delta era that included nearly 3000 close contacts of 100 cases found no linked cases from exposures occurring after an index case had symptoms for 6 days.84 The National Basketball Association had a closed environment for their 2020 season, with systematic and frequent testing, allowing for detailed descriptions of transmission in this setting that included nearly 4000 individuals.85 Their policies allowed individuals with infection to discontinue isolation at 10 days after symptom onset or first positive PCR test. They found no secondary infections after that time, despite 36 individuals remaining persistently PCR positive on nasopharyngeal testing.
The period of infectiousness is related to SARS-CoV-2 viral load dynamics, which are quite different than those of other severe coronavirus infections like SARS-CoV-1 and MERS-CoV. As noted elsewhere in this article, index case viral load is a key determinant of transmission risk.49 , 50 For SARS-CoV-2, transmissions occur starting 1 to 2 days before symptom onset as the viral load increases and peaks around or just after symptoms onset, before decreasing thereafter.9 The viral load, therefore, peaks well before most people with severe cases of SARS-CoV-2 are hospitalized and explains why more SARS-CoV-2 aerosols are found in homes of index cases than critical care wards for affected patients.86 In contrast, in both SARS-CoV-1 and MERS-CoV, the respiratory tract viral load peaks after inevitable symptom onset (neither are known to have asymptomatic cases), peaking around day 10 for SARS-CoV-1 and days 7 to 10 for MERS-CoV.9 The transmission risk is greater later in infection, after symptom onset for SARS-CoV-1 and MERS-CoV, making transmission mitigation of those infections easier than for SARS-CoV-2.
Transmissibility and heterogeneity
The basic reproductive number (R0) of an infectious disease is a measure of its transmissibility. The R0 is defined as the mean number of secondary infections resulting from an infected person in a susceptible population. The R0 is influenced by the rate of contacts within a given population, the probability of transmission during a given contact, and the duration of infectiousness. Infectious diseases with an R0 of greater than 1 can result in epidemics depending in part on the degree of population immunity. Estimates of the R0 are, thus, useful for a general understanding of the epidemic threat of a given pathogen, but vary significantly by setting and the methodology used for estimation.87 For SARS-CoV-2, approximations of the R0 have increased as the dominant virus has evolved from the ancestral strain (R0 ≈ 3) to the alpha variant (R0 ≈ 4.5) to the delta variant (R0 ≈ 8) (Table 3 ).88, 89, 90
Table 3.
Basic reproductive number (R0) for various pathogens
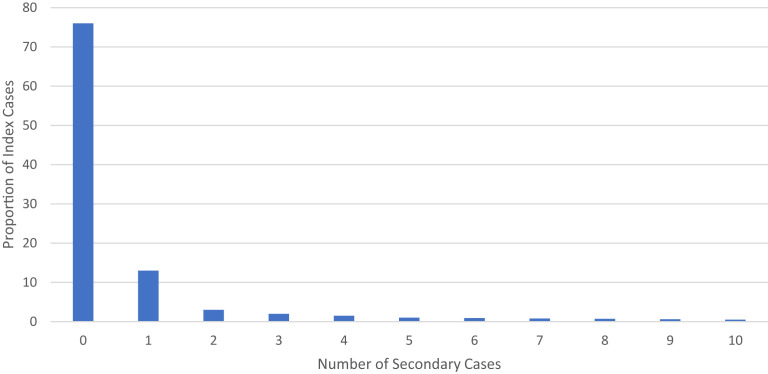
Because the R0 is an average, it does not describe individual variation in transmission. This individual variation, or heterogeneity, can be an important feature of some infectious diseases, with implications for epidemic control. Heterogeneity is typically described using the dispersion parameter of a negative binomial distribution, or k.100 When the k is very small, transmission displays overdispersion, meaning that a relatively high proportion of secondary infections result from a relatively low proportion of index cases. Highly overdispersed pathogens are characterized by superspreading events—discrete transmission events with unusually large numbers of secondary cases. Investigations early during the pandemic using a variety of methodologies documented high degrees of overdispersion with SARS-CoV-2 transmission, with 10% to 20% of index cases leading to approximately 80% of secondary infections,101, 102, 103, 104 and numerous examples of superspreading events (Fig. 2 ).105, 106, 107, 108, 109, 110 Despite the delta variant’s increased overall transmissibility, manifested by an increased R0, there is early evidence that transmission continues to display a similar degree of heterogeneity.82 , 111 Superspreading events continue to be identified in the delta era, including in highly vaccinated populations.112 Although superspreading occurs for other highly transmissible respiratory pathogens like influenza and the measles, it is generally thought to be less important as compared with SARS-CoV-2, with higher dispersion parameters for these other pathogens (Table 4 ).113 Note that increased overdispersion for measles has been reported in the postvaccination era, likely owing to heterogeneity of susceptibility.114 , 115
Fig. 2.
Example of proportion of secondary cases from SARS-CoV-2 index cases.
Table 4.
Estimated dispersion parameter (k) for selected highly transmissible respiratory pathogens
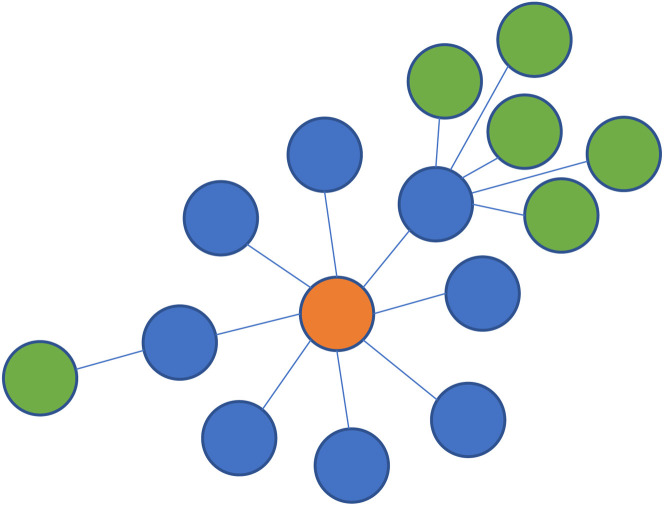
Many factors can contribute to the likelihood of a superspreading event, including those related to the virus (eg, timing relative to peak viral load), host (eg, presence of symptoms), environment (eg, ventilation, proximity), and behavior (eg, singing, masking).117 Transmission heterogeneity is also heavily driven by the concentration of exposure risk factors within specific networks, including among frontline and low-wage workers, and in congregate settings such as nursing homes, prisons, and homeless shelters.118 The heterogeneity of transmission clusters can be visualized when transmission chains are depicted pictorially (Fig. 3 ).
Fig. 3.
Chains and clustering of SARS-CoV-2 transmission.
Although superspreading events are rare, they make an outsized contribution to epidemic growth. As a result, public health interventions that reduce superspreading events (so-called cutting the tail interventions) can meaningfully decrease transmission, with modeling suggesting that elimination of transmission events with greater than 10 secondary infections could result in a reduction in the R0 of ancestral virus from 3.00 to 1.09.119
Transmission prevention with vaccination
Prevention of SARS-CoV-2 through vaccination can be achieved through direct protection (prevention of infection or disease among vaccinated individuals) and indirect protection (prevention of infection among all community members through decreases in transmission). In the early months after vaccination and before the delta era, randomized controlled trials and large-scale observational studies demonstrated high degrees of direct protection from vaccines using a variety of platforms, particularly against symptomatic and severe disease.120 , 121 Similarly, infection-acquired immunity showed substantial protection against reinfection of at least 80%.122
Indirect protection can be generated through 2 distinct mechanisms. First, vaccines may decrease the overall risk of infection by protecting against both symptomatic and asymptomatic infections—put simply, if a person never becomes infected, they cannot transmit the virus to another person. Second, a vaccine may decrease the transmission potential of a vaccinated person who does become infected, leading to a lower secondary attack rate compared with unvaccinated people with infection. During the early months of the vaccine roll out, before the global dominance of delta, the sum of the evidence from well-designed studies using a variety of methodologies suggested a large decrease in transmission through both of these mechanisms.49 Although the exact amount of the decrease varied by vaccine and transmission context, the overall protection against infection was at least 50%, and the decrease in transmission potential among the vaccinated relative to the unvaccinated was also 50% or greater, equivalent to an approximate transmission reduction of at least 75%.49 In the delta era, however, we must consider 2 additional factors that may influence the effects of vaccines on transmission—that is, the impact of the delta variant itself and changes in immunity over time since vaccination.
Delta’s Impact on Vaccine Protection against Transmission
When compared with alpha, direct protection by the vaccines against symptomatic delta infections seems to be modestly decreased—by about 10% to 20%—with no change in the relative protection against severe outcomes.123 , 124 An increased risk of symptomatic reinfection also seems to be greater with delta compared with alpha, with surveillance data from the United Kingdom showing an adjusted odds ratio of 1.46 (95% CI, 1.03–2.05) for symptomatic reinfection for delta compared with alpha.125 Importantly, even though the relative protection by vaccines against illness and severe disease is largely preserved when compared with pre-delta viruses, the increased overall transmissibility seen with delta results in a substantially increased absolute risk of these outcomes among vaccinated people.
Understanding how vaccine effectiveness against all infections changes in the context of delta is critical to informing expectations about the vaccines’ continued indirect protection. To reliably estimate this change, observational studies must use systematic or regular testing regardless of symptom status. To date, the most rigorous study to use this approach was a population representative survey of randomly selected households in the United Kingdom that included 384,543 individuals, conducted scheduled PCR testing of participants, and adjusted for a number of important potential confounding variables.126 After 2 doses of the ChAdOx1 vaccine, they found a lower vaccine effectiveness against infection of 67% (95% CI, 62%–71%) during a period dominated by delta, relative to 79% (95% CI, 56%–90%) during a period dominated by alpha. For the mRNA-1273 vaccine, they found no difference in protection against infection by delta compared with alpha, with an 82% vaccine effectiveness (95% CI, 75%–87%) during the delta period and 77% vaccine effectiveness (95% CI, 66%–84%) during the alpha period. One other study of 4217 frontline workers in the United States similarly used regular testing and control of confounders, finding a combined vaccine effectiveness against overall infection for mRNA-1273, BNT162b2, and (to a much lesser extent) Ad26.COV2.S, against overall infection of 91% (95% CI, 81%–96%) before delta dominance and 66% (95% CI, 26%–84%) during a period of delta dominance.127 The limited reliable data to date thus suggest substantial preservation in relative vaccine effectiveness against all infections by delta, with a decrease of 0% to 25% compared with pre-delta viruses. As before, this metric needs to be distinguished from the absolute risk of delta infection for vaccinated people, which is considerably increased because of delta’s increased overall transmissibility relative to earlier variants.
The impact of delta’s emergence on the second component of indirect protection—the decrease in transmission potential among vaccinated people who become infected—has been scrutinized intensely ever since it was shown that the amount of SARS-CoV-2 viral RNA present at diagnosis (as measured semiquantitatively by the cycle threshold of PCR assays) did not differ by vaccination status for people with delta infection.128 For pre-delta viruses, the cycle threshold value at diagnosis had been consistently found to be higher (meaning lower viral levels) for vaccinated people,49 so this finding suggested that the transmission potential had possibly become more similar between vaccinated and unvaccinated people in the context of delta. However, a subsequent study in Singapore used longitudinal sampling to show that, although cycle threshold values at diagnosis were similar between vaccinated and unvaccinated people, there was a much more rapid decay in the cycle threshold value among those who had been vaccinated,129 a finding that has since been replicated.63 They also found that vaccinated people with delta infection had far fewer symptoms relative to unvaccinated people, which has also been associated with reduced transmission potential.47 , 130 Several studies have attempted to examine whether the relationship between cycle threshold values and presence of replication-competent virus differs by vaccination status, with mixed findings.131 , 132 One study of 24,706 health care workers found that infectious virus was present in 69% of 162 infections after vaccination (91% of which were delta), relative to 85% of infections among the unvaccinated.132 There was a significantly lower probability of culture positivity with vaccination after adjusting for the cycle threshold value. Another study found infectious virus in 37 of 39 vaccinated people (95%) infected with delta, relative to 15 of 17 specimens (88%) from unvaccinated people, with both groups having similar cycle threshold values at diagnosis.131
Regardless of these virologic findings, rigorously designed contact tracing studies provide the most direct evidence about the transmission potential of people with delta infection after vaccination. The largest such study used contact tracing data from England and included 108,498 index cases with 146,243 contacts.133 After adjusting for potential confounders, investigators found that 2 BNT162b2 doses (adjusted risk ratio, 0.;, 95% CI, 0.39–0.65) and 2 ChAdOx1 doses (adjusted risk ratio, 0.76; 95% CI, 0.70–0.83) reduced delta transmission, but that this was less than for alpha transmission (BNT162b2 adjusted risk ratio, 0.32; 95% CI, 0.21–0.48; ChAdOx1 adjusted risk ratio, 0.48; 95% CI, 0.30–0.78). Of note, these estimated decreases were early after vaccination and transmission reduction attenuated over time, as we discuss elsewhere in this review. Another household contact tracing study of 4921 index cases and 7771 contacts in the Netherlands found a decrease in the transmission potential for delta after full vaccination of the index case of 63% (95% CI, 46%–75%).124 A much smaller contact tracing study of a delta outbreak in China similarly found a similar decrease in transmission after 2 doses of an inactivated SARS-CoV-2 vaccine (65% decrease; 95% CI, 16%–88%).80 In contrast, a contact tracing study of 1024 household contacts linked to 301 index cases in Singapore did not find a significant difference in the transmission risk based on index case vaccination status (adjusted odds ratio, 0.73; 95% CI, 0.38–1.40), although the confidence interval was relatively wide.58 Finally, a contact tracing study of 471 delta index cases and 602 contacts in the United Kingdom that collected daily upper respiratory tract samples for up to 20 days found similar secondary attack rates in contacts of vaccinated (25%; 95% CI, 15%–35%) and unvaccinated (23%; 95% CI, 15%–31%) index cases.63 The discrepant findings between this study and the larger contact tracing studies may relate to its smaller sample size, intensive sampling strategy, and/or greater time since vaccination among the index cases.
Importantly, these contact tracing studies are likely to underestimate the reduction in transmission potential resulting from vaccination for people infected with delta for 2 reasons. First, index cases with a greater number and severity of symptoms are more likely to be identified and included in these studies. Because vaccines decrease the severity of symptoms, and more severe symptoms are associated with an increased risk of transmission, there is likely to be selection bias present. Second, some contacts may have been infected outside the household, potentially even during the same exposure as the index case.134
Transmission Risk in the Context of Waning Immunity
Although the majority of observational analyses assessing changes in vaccine protection over time since vaccination are highly vulnerable to biases inherent to their study design,135 there are several reliable lines of evidence showing modest decreases in protection against symptomatic and overall infection over time, as well as an attenuation over time in the reduction in transmission potential after vaccination. The strongest evidence comes from the randomized controlled trials of mRNA-1273 and BNT162b2, which continued to follow participants after the placebo group crossed over to receive the vaccine about 5 to 6 months after the trials began, resulting in early and late vaccinated groups. Symptomatic infection rates were modestly higher in the early vaccine group for both vaccines nearly 1 year after initial vaccination, when the delta variant was dominant in the United States. Vaccine efficacy against symptomatic infection at close to 1 year after vaccination can be approximated after considering the change in protection caused by delta for the more recently vaccinated group (Table 5 ).
Table 5.
Vaccine efficacy against symptomatic delta
| Assumed baseline vaccine efficacy vs symptomatic delta124 | Vaccine efficacy vs symptomatic delta, 10–12 mo after vaccination, BNT162b2136 | Vaccine efficacy vs symptomatic delta, 10–12 mo after vaccination, mRNA-1273137 |
|---|---|---|
| 0.9 | 0.87 | 0.84 |
| 0.85 | 0.8 | 0.76 |
| 0.8 | 0.73 | 0.69 |
| 0.75 | 0.66 | 0.61 |
| 0.7 | 0.6 | 0.53 |
| 0.65 | 0.53 | 0.45 |
Further evidence for waning protection against infection regardless of symptom status comes from the previously described community-based study in the United Kingdom, which used representative population sampling and systematic testing.126 These investigators found a modest decrease in vaccine effectiveness against infection (as above, the critical outcome for transmission prevention) from 14 to 90 days after the second dose for BNT162b2 (85% at 14 days [95% CI, 79%–90%]; 75% at 90 days [95% CI, 70%–80%]) and ChAdOx1 (68% at 14 days [95% CI, 61%–73%]; 61% at 90 days [95% CI, 53%–68%]). The previously described study of 4217 frontline workers in the United States who underwent regular testing found a vaccine effectiveness after full vaccination of 85% (95% CI, 68%–93%) at 14 to 119 days, 81% (95% CI, 34%–95%) at 120 to 149 days, and 73% (95% CI, 49%–86%) at 150 days or more.127
The decrease in the transmission potential resulting from vaccination among people who become infected also seems to attenuate over time, as shown by the detailed contact tracing study of 108,498 index cases and 146,243 contacts in England.133 This analysis found no transmission decrease at 12 weeks after vaccination for index cases vaccinated with ChAdOx1 (2%, 95% CI, –2% to 6%) and a significantly attenuated decrease for those vaccinated with BNT162b2 (24%, 95% CI, 20%–28%). This finding is further supported by virologic data from Israel, where viral loads at diagnosis were found to be lower among vaccinated individuals with delta infection within 2 months of vaccination relative to unvaccinated people, but that this difference disappeared by 6 months after vaccination.138
In summary, the considerable indirect protection seen during the early months of the vaccine roll out, although still substantial in the delta era, has likely been diminished to some extent because of a modest decrease in the relative protection against infection and increased transmission potential of vaccinated individuals who become infected. The durable impact of booster doses on transmission remains to be seen, though they have shown early promise in short-term follow-up.138, 139, 140, 141
Transmission prevention
Extensive accumulated evidence supports multiple additional strategies for effective transmission prevention. Of note, most of the evidence supporting these strategies comes from the pre-delta era. Besides vaccination, the other major tools to prevent transmission of SARS-CoV-2 include ventilation, physical distancing, rapid case contact tracing and isolating, and effective personal protective equipment.
The role of improved ventilation in preventing transmission of SARS-CoV-2 has been shown in a variety of ways. In a crossover study looking at the effect of portal air/UV filtration devices in hospital wards with patients with COVID-19, SARS-CoV-2 was detected in aerosols when the filters were not in use, but not when the devices were turned on.142 In a study of household transmission in China, opening a window to allow for better ventilation was associated with a decreased infection risk.143 In a study that included 169 primary schools in the state of Georgia, schools that had improved ventilation had a lower incidence of SARS-CoV-2 cases, with an adjusted relative risk of 0.61 (95% CI, 0.43–0.87).144 Dilution methods alone, including opening doors and windows, or combining dilution with filtration (with installation of HEPA air filters) were protective against incident SARS-CoV-2 infections. The importance of ventilation in decreasing transmission of respiratory pathogens was widely recognized even before the COVID-19 pandemic; for example, opening windows in hospitals and homes was found to provide excellent ventilation and decrease tuberculosis transmission risk.145 , 146
The protective role of masking has been shown in multiple settings. In community settings, a large, prospective, cluster randomized controlled trial including nearly 350,000 people from 600 villages in Bangladesh from November 2020 through April 2021 found 11.2% lower cases (estimated via history of symptoms with a positive serology) in villages randomized to surgical masks.147 In the trial, people living in towns in the intervention arms were given free masks and information about the importance of masking, and observed masking was 13.3% in control villages and 42.3% in treatment villages, with a regression adjusted increase of 28.8% increase in masking associated with the intervention (95% CI, 27%–31%). The investigators found no statistically significant benefit for their primary outcome in villages randomized to cloth masking. The only other randomized control trial of community masking individually randomized 4862 Danish participants to recommendations to wear surgical masks outside the home or not and found that 1.8% of the mask group and 2.1% of the control group developed infection in the following month, a difference that was not statistically significant.148 The broader importance of this study is limited by individual randomization (because of the hypothesized importance of masks for source control) and the low prevalence of infection. A systematic review of 6 studies evaluating the impact of face masking found that wearing a mask was associated with a significantly decreased risk of SARS-CoV-2 infection with an adjusted odds ratio of 0.19 (95% CI, 0.11–0.33).149 Another systematic review that assessed the effect of masking on severe coronavirus infections from SARS-CoV-2, SARS-CoV-1, or MERS-CoV found that face masks were associated with a reduced risk of infection (adjusted odds ratio, 0.15; 95% CI, 0.07–0.34).150 Observational studies of mask mandate policies have also suggested a benefit to community masking.151 A study comparing 15 counties in Kansas with a mask mandate with 68 counties without mask mandates found a mean 60% decrease in cases and hospitalizations and a 65% decrease in deaths in counties with mask mandates.152 These observational studies must be interpreted with caution because of secular trends and other policies that were implemented concurrently.
Universal masking in health care settings has also played an important role in decreasing transmission in health care settings, which have the potential to be important sites of SARS-CoV-2 transmission.30 A systematic review found masking in health care settings may have decreased the infection risk by 70% (unadjusted odds ratio, 0.29; 95% CI, 0.18–0.44).149 Universal masking policies were also associated with fewer health care worker infections at large hospital systems.153, 154, 155 An outbreak at a Veterans Affairs hospital ended after the implementation of universal masking.156
Despite the clear dominance of aerosol transmission for SARS-CoV-2 and other respiratory viruses, the benefit of N95 masks over surgical masks has not been shown conclusively for preventing transmission. In the pre–COVID-19 era, a cluster randomized trial of nearly 3000 health care workers did not find a benefit for the prevention of influenza A infections for those who used N95 versus surgical masks.157 A meta-analysis examining masking for SARS-CoV-2 and other viral infections found greater benefits with N95 versus surgical masks, though the interaction was not significant (P = .09).150 In an unadjusted analysis of transmissions at a large hospital system in Michigan between April and May 2020, those wearing an N95 at time of exposure to a patient with COVID-19 were significantly less likely to be seropositive (10.2%) compared with those wearing surgical or cloth masks (13.1%) or no mask (17.5%).158
Observational studies in health care settings have suggested that eye protection may incrementally increase protection for health care workers. A systematic review of 13 studies from SARS-CoV-1, MERS-CoV, and SARS-CoV-2 found that eye protection was associated with a lower risk of infection (unadjusted relative risk, 0.34; 95% CI, 0.22–0.52).150 Numerous case and cluster reports have also suggested a potential role for eye protection, including in a detailed outbreak investigation at a hospital in Boston where staff members who developed infection were less likely to have worn eye protection during encounters with index cases, although the difference was not statistically significant (30% vs 67%; prevalence ratio, 0.44; 95% CI, 0.18–1.08).159
Although universal masking policies seem to be effective at substantially reducing the risk of transmission in health care settings, it does not bring the risk to zero. Most residual cases occur in settings where masking is impractical or not possible, like break rooms, shared work rooms, or shared patient rooms or open wards.30 , 41 , 160 However, a few cases of well-documented transmission between a masked source patient and masked health care worker wearing eye protection have been described.161 These cases seem to occur with prolonged exposure at close proximity with source patients with very high respiratory tract viral loads at the time of contact.
Rapid case identification and contact tracing with testing and isolation has been used to help control the COVID-19 pandemic. During the initial outbreak in South Korea, the roll out of rapid contact tracing with testing before symptom onset brought the effective R0 from 1.3 to 0.6 compared with the preceding period, when testing was symptom driven.162 In another pre-delta study, the launch of an immediate trace and test program on the Isle of Wight in the UK was associated with a decrease in the R0 from 1.3 to 0.5.163 In contrast a retrospective study in Portugal that compared 98 cases identified through contact tracing with 453 found through routine testing did not find a difference in the secondary attack rate (13.3% vs 17.2%; P = .406).164 A mathematical model suggests that although contact tracing can be helpful early in an outbreak, the benefit is lost once cases outnumber contact tracing capacity by more than 10 to 1.165
Given the efficiency with which SARS-CoV-2 can be transmitted, multiple mitigation strategies are typically used at times of outbreaks, including improving ventilation and encouraging physical distancing and indoor universal masking, as well as additional layers of protection for people in certain high-risk environments like health care settings.
Preliminary understanding of transmission dynamics of the omicron variant
In November 2021 the omicron variant, with more than 50 total mutations including 15 in the spike protein’s receptor binding domain, was identified in South Africa.166 It rapidly spread throughout South Africa and the globe, leading to massive surges in infections. Evidence of the characteristic features of omicron transmission are rapidly emerging but remain highly preliminary as of this writing.
Initial estimates suggested a 5.4-fold (95% CI, 3.1- to 10.0-fold) weekly growth advantage for omicron over delta.166 Although omicron’s R0 is estimated to be 3.19 (95% CI, 2.82–3.61) times greater than delta, much of the transmission advantage seems to be driven by an increased risk of infection among people with existing immunity.167 This finding is supported by a large Danish household transmission study, which found similar attack rates among unvaccinated people living in households with an omicron or delta index case, but much higher risk of infection for fully vaccinated and boosted household members for omicron compared with delta (adjusted odds ratio, 2.61 [95% CI, 2.34–2.90] and 3.66 [95% CI, 2.65–5.05], respectively).168 This finding corroborates in vitro data showing a substantially attenuated neutralization of omicron with sera from vaccinated or convalesced individuals.169 , 170 Whether omicron has intrinsically increased transmissibility over prior variants is unknown at this time.
Another characteristic feature of early omicron outbreak reports is a shorter incubation period of about 3 days, with very high rates of symptoms among vaccinated people.171 , 172 Viral load increases, peaks, and decreased over a period of about 10 days, similar to findings from prior variants, with somewhat lower peak viral loads compared with delta.173 , 174 Preliminary evidence from Japan suggests that the peak viral load may occur 3 to 6 days after symptom onset, which may be later than was seen with prior variants.175 Occasional long-range transmission continues to be documented along with superspreading events, although additional data are needed.168 , 171 , 176
These preliminary reports suggest that omicron is more rapidly transmitted than delta with a shorter incubation period and marked immune evasion that greatly increases risk of infection among vaccinated and recovered individuals. Very preliminary reports suggest that the viral load may peak later for omicron infections compared with those from prior variants. More data are needed to confirm these findings and to determine whether there are changes in the infectious period of each case and the exact degree of overdispersion characterizing transmission of omicron.
Summary
The SARS-CoV-2 delta variant transmits more rapidly and efficiently than prior SARS-CoV-2 viruses. The predominant mode of transmission of SARS-CoV-2 is via short-range aerosols. Vaccines prevent transmission both by blocking cases and by decreasing the risk of secondary cases from a vaccinated index case. However, the effect of vaccination on transmission reduction has been attenuated owing to the significantly increased transmissibility of the delta variant and waning protection for individuals remotely vaccinated. Besides vaccination, ventilation, masking, eye protection, and rapid case identification with contact tracing and isolation have all been shown to decrease transmission.
Disclosure
The authors have no relevant financial or commercial disclosures. There were no funding sources for this article.
References
- 1.Meyerowitz E.A., Richterman A., Gandhi R.T., et al. Transmission of SARS-CoV-2: a review of viral host, and environmental factors. Ann Intern Med. 2021;174:69–79. doi: 10.7326/M20-5008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fang F.C., Benson C.A., Del Rio C., et al. COVID-19-lessons learned and questions remaining. Clin Infect. 2021;72:2225–2240. doi: 10.1093/cid/ciaa1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Plante J.A., Liu Y., Liu J., et al. Spike mutation D614G alters SARS-CoV-2 fitness. Nature. 2021;592:116–121. doi: 10.1038/s41586-020-2895-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhou B., Thao T.T.N., Hoffmann D., et al. SARS-CoV-2 spike D614G change enhances replication and transmission. Nature. 2021;592:122–127. doi: 10.1038/s41586-021-03361-1. [DOI] [PubMed] [Google Scholar]
- 5.Hou Y.J., Chiba S., Halfmann P., et al. SARS-CoV-2 D614G variant exhibits efficient replication ex vivo and transmission in vivo. Science. 2020;370:1464–1468. doi: 10.1126/science.abe8499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kepler L., Hamins-Puertolas M., Rasmussen D.A. Decomposing the sources of SARS-CoV-2 fitness variation in the United States. Virus Evol. 2021;7 doi: 10.1093/ve/veab073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Majumdar P., Niyogi S. SARS-CoV-2 mutations: the biological trackway towards viral fitness. Epidemiol Infect. 2021;149 doi: 10.1017/s0950268821001060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu C., Shi W., Becker S.T., et al. Structural basis of mismatch recognition by a SARS-CoV-2 proofreading enzyme. Science. 2021;373:1142–1146. doi: 10.1126/science.abi9310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cevik M., Tate M., Lloyd O., et al. SARS-CoV-2, SARS-CoV, and MERS-CoV viral load dynamics, duration of viral shedding, and infectiousness: a systematic review and meta-analysis. Lancet Microbe. 2021;2:e13–e22. doi: 10.1016/S2666-5247(20)30172-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Choi B., Choudhary M.C., Regan J., et al. Persistence and Evolution of SARS-CoV-2 in an Immunocompromised Host. N Engl J Med. 2020;383:2291–2293. doi: 10.1056/NEJMc2031364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.D'Abramo A., Vita S., Maffongelli G., et al. Prolonged and severe SARS-CoV-2 infection in patients under B-cell-depleting drug successfully treated: a tailored approach. Int J Infect Dis. 2021;107:247–250. doi: 10.1016/j.ijid.2021.04.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Corey L., Beyrer C., Cohen M.S., et al. SARS-CoV-2 Variants in Patients with Immunosuppression. N Engl J Med. 2021;385:562–566. doi: 10.1056/NEJMsb2104756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Galloway S.E., Paul P., MacCannell D.R., et al. Emergence of SARS-CoV-2 B.1.1.7 Lineage - United States, December 29, 2020-January 12, 2021. MMWR Morb Mortal Wkly Rep. 2021;70:95–99. doi: 10.15585/mmwr.mm7003e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Davies N.G., Abbott S., Barnard R.C., et al. Estimated transmissibility and impact of SARS-CoV-2 lineage B.1.1.7 in England. Science. 2021:372. doi: 10.1126/science.abg3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tanaka H., Hirayama A., Nagai H., et al. Increased Transmissibility of the SARS-CoV-2 Alpha Variant in a Japanese Population. Int J Environ Res Public Health. 2021;18 doi: 10.3390/ijerph18157752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mlcochova P., Kemp S.A., Dhar M.S., et al. SARS-CoV-2 B.1.617.2 Delta variant replication and immune evasion. Nature. 2021;599:114–119. doi: 10.1038/s41586-021-03944-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li H., Liu T., Wang L., et al. SARS-CoV-2 Delta variant infects ACE2(low) primary human bronchial epithelial cells more efficiently than other variants. J Med Virol. 2021 doi: 10.1002/jmv.27372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dhar M.S., Marwal R., Vs R., et al. Genomic characterization and epidemiology of an emerging SARS-CoV-2 variant in Delhi, India. Science. 2021 doi: 10.1126/science.abj9932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Allen H., Vusirikala A., Flannagan J., et al. Household transmission of COVID-19 cases associated with SARS-CoV-2 delta variant (B.1.617.2): national case-control study. Lancet Reg Health Eur. 2021 doi: 10.1016/j.lanepe.2021.100252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.van Doremalen N., Bushmaker T., Morris D.H., et al. Aerosol and Surface Stability of SARS-CoV-2 as Compared with SARS-CoV-1. N Engl J Med. 2020;382:1564–1567. doi: 10.1056/NEJMc2004973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rocha A.L.S., Pinheiro J.R., Nakamura T.C., et al. Fomites and the environment did not have an important role in COVID-19 transmission in a Brazilian mid-sized city. Sci Rep. 2021;11:15960. doi: 10.1038/s41598-021-95479-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Harvey A.P., Fuhrmeister E.R., Cantrell M.E., et al. Longitudinal Monitoring of SARS-CoV-2 RNA on High-Touch Surfaces in a Community Setting. Environ Sci Technol Lett. 2021;8:168–175. doi: 10.1021/acs.estlett.0c00875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hu M., Lin H., Wang J., et al. Risk of coronavirus disease 2019 transmission in train passengers: an epidemiological and modeling study. Clin Infect Dis. 2021;72:604–610. doi: 10.1093/cid/ciaa1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xu P., Jia W., Qian H., et al. Lack of cross-transmission of SARS-CoV-2 between passenger's cabins on the Diamond Princess cruise ship. Build Environ. 2021;198:107839. doi: 10.1016/j.buildenv.2021.107839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Toyokawa T., Shimada T., Hayamizu T., et al. Transmission of SARS-CoV-2 during a 2-h domestic flight to Okinawa, Japan, March 2020. Influenza Other Respir Viruses. 2021 doi: 10.1111/irv.12913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xie X., Li Y., Chwang A.T., et al. How far droplets can move in indoor environments--revisiting the Wells evaporation-falling curve. Indoor Air. 2007;17:211–225. doi: 10.1111/j.1600-0668.2007.00469.x. [DOI] [PubMed] [Google Scholar]
- 27.Riley R.L. What nobody needs to know about airborne infection. Am J Respir Crit Care Med. 2001;163:7–8. doi: 10.1164/ajrccm.163.1.hh11-00. [DOI] [PubMed] [Google Scholar]
- 28.Riley R.L., Mills C.C., O'Grady F., et al. Infectiousness of air from a tuberculosis ward. Ultraviolet irradiation of infected air: comparative infectiousness of different patients. Am Rev Respir Dis. 1962;85:511–525. doi: 10.1164/arrd.1962.85.4.511. [DOI] [PubMed] [Google Scholar]
- 29.Klompas M., Baker M.A., Rhee C. Airborne transmission of SARS-CoV-2: theoretical considerations and available evidence. JAMA. 2020;324:441–442. doi: 10.1001/jama.2020.12458. [DOI] [PubMed] [Google Scholar]
- 30.Richterman A., Meyerowitz E.A., Cevik M. Hospital-acquired SARS-CoV-2 infection: lessons for public health. JAMA. 2020;324:2155–2156. doi: 10.1001/jama.2020.21399. [DOI] [PubMed] [Google Scholar]
- 31.Wang C.C., Prather K.A., Sznitman J., et al. Airborne transmission of respiratory viruses. Science. 2021:373 2021–37308/28. doi: 10.1126/science.abd9149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Klompas M., Milton D.K., Rhee C., et al. Current insights into respiratory virus transmission and potential implications for infection control programs: a narrative review. Ann Intern Med. 2021 doi: 10.7326/m21-2780. [DOI] [PubMed] [Google Scholar]
- 33.Marr L.C., Tang J.W. A paradigm shift to align transmission routes with mechanisms. Clin Infect Dis. 2021 doi: 10.1093/cid/ciab722. [DOI] [PubMed] [Google Scholar]
- 34.Meyerowitz E.A., Richterman A. A defense of the classical model of transmission of respiratory pathogens. Clin Infect Dis. 2021;73:1318. doi: 10.1093/cid/ciab016. [DOI] [PubMed] [Google Scholar]
- 35.Dharmadhikari A.S., Mphahlele M., Stoltz A., et al. Surgical face masks worn by patients with multidrug-resistant tuberculosis: impact on infectivity of air on a hospital ward. Am J Respir Crit Care Med. 2012;185:1104–1109. doi: 10.1164/rccm.201107-1190OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li Y., Cheng P., Jia W. Poor ventilation worsens short-range airborne transmission of respiratory infection. Indoor Air. 2021 doi: 10.1111/ina.12946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Coleman K.K., Tay D.J.W., Sen Tan K., et al. Viral load of SARS-CoV-2 in respiratory aerosols emitted by COVID-19 patients while breathing, talking, and singing. Clin Infect Dis. 2021 doi: 10.1093/cid/ciab691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kutter J.S., de Meulder D., Bestebroer T.M., et al. SARS-CoV and SARS-CoV-2 are transmitted through the air between ferrets over more than one meter distance. Nat Commun. 2021;12:1653. doi: 10.1038/s41467-021-21918-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chaintoutis S.C., Thomou Z., Mouchtaropoulou E., et al. Outbreaks of SARS-CoV-2 in naturally infected mink farms: impact, transmission dynamics, genetic patterns, and environmental contamination. Plos Pathog. 2021;17 doi: 10.1371/journal.ppat.1009883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Port J.R., Yinda C.K., Avanzato V.A., et al. Increased aerosol transmission for B.1.1.7 (alpha variant) over lineage A variant of SARS-CoV-2. bioRxiv. 2021 doi: 10.1101/2021.07.26.453518. [DOI] [Google Scholar]
- 41.Karan A., Klompas M., Tucker R., et al. The risk of SARS-CoV-2 transmission from patients with undiagnosed Covid-19 to roommates in a large academic medical center. Clin Infect Dis. 2021 doi: 10.1093/cid/ciab564. [DOI] [PubMed] [Google Scholar]
- 42.Kwon K.-S., Park J.-I., Park Y.J., et al. Evidence of long-distance droplet transmission of SARS-CoV-2 by direct air flow in a restaurant in Korea. J Korean Med Sci. 2020;35 doi: 10.3346/jkms.2020.35.e415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lu J., Gu J., Li K., et al. COVID-19 outbreak associated with air conditioning in restaurant, Guangzhou, China, 2020. Emerg Infect Dis. 2020;26:1628–1631. doi: 10.3201/eid2607.200764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fox-Lewis A., Williamson F., Harrower J., et al. Airborne transmission of SARS-CoV-2 delta variant within tightly monitored isolation facility, New Zealand (Aotearoa) Emerg Infect Dis. 2021;28:2021. doi: 10.3201/eid2803.212318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wong S.C., Au A.K., Chen H., et al. Transmission of omicron (B.1.1.529) - SARS-CoV-2 variant of concern in a designated quarantine hotel for travelers: a challenge of elimination strategy of COVID-19. Lancet Reg Health West Pac. 2021 doi: 10.1016/j.lanwpc.2021.100360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li F., Li Y.Y., Liu M.J., et al. Household transmission of SARS-CoV-2 and risk factors for susceptibility and infectivity in Wuhan: a retrospective observational study. Lancet Infect Dis. 2021;21:617–628. doi: 10.1016/S1473-3099(20)30981-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sayampanathan A.A., Heng C.S., Pin P.H., et al. Infectivity of asymptomatic versus symptomatic COVID-19. Lancet. 2021;397:93–94. doi: 10.1016/S0140-6736(20)32651-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Buitrago-Garcia D., Egli-Gany D., Counotte M.J., et al. Occurrence and transmission potential of asymptomatic and presymptomatic SARS-CoV-2 infections: a living systematic review and meta-analysis. Plos Med. 2020;17 doi: 10.1371/journal.pmed.1003346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Richterman A., Meyerowitz E.A., Cevik M. Indirect protection by reducing transmission: ending the pandemic with SARS-CoV-2 vaccination. Open Forum Infect Dis. 2021 doi: 10.1093/ofid/ofab259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Marks M., Millat-Martinez P., Ouchi D., et al. Transmission of COVID-19 in 282 clusters in Catalonia, Spain: a cohort study. Lancet Infect Dis. 2021;21:629–636. doi: 10.1016/S1473-3099(20)30985-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bjorkman K.K., Saldi T.K., Lasda E., et al. Higher viral load drives infrequent severe acute respiratory syndrome coronavirus 2 transmission between asymptomatic residence hall roommates. J Infect Dis. 2021;224:1316–1324. doi: 10.1093/infdis/jiab386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lyngse F.P., Mølbak K., Træholt Frank K., et al. Association between SARS-CoV-2 transmission risk, viral load, and age: a nationwide study in Danish households. medRxiv. 2021:2028. doi: 10.1101/2021.02.28.21252608. [DOI] [Google Scholar]
- 53.Lewis N.M., Chu V.T., Ye D., et al. Household transmission of severe acute respiratory syndrome coronavirus-2 in the United States. Clin Infect Dis. 2021;73:1805–1813. doi: 10.1093/cid/ciaa1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Goldstein E., Lipsitch M., Cevik M. On the effect of age on the transmission of SARS-CoV-2 in households, schools, and the community. J Infect Dis. 2021;223:362–369. doi: 10.1093/infdis/jiaa691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Davies N.G., Klepac P., Liu Y., et al. Age-dependent effects in the transmission and control of COVID-19 epidemics. Nat Med. 2020;26:1205–1211. doi: 10.1038/s41591-020-0962-9. [DOI] [PubMed] [Google Scholar]
- 56.Tonzel J.L., Sokol T. COVID-19 outbreaks at youth summer camps - Louisiana, June-July 2021. MMWR Morb Mortal Wkly Rep. 2021;70:1425–1426. doi: 10.15585/mmwr.mm7040e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lam-Hine T., McCurdy S.A., Santora L., et al. Outbreak associated with SARS-CoV-2 B.1.617.2 (Delta) variant in an elementary school - Marin County, California, May-June 2021. MMWR Morb Mortal Wkly Rep. 2021;70:1214–1219. doi: 10.15585/mmwr.mm7035e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ng O.T., Koh V., Chiew C.J., et al. Impact of delta variant and vaccination on SARS-CoV-2 secondary attack rate among household close contacts. Lancet Reg Health West Pac. 2021;17 doi: 10.1016/j.lanwpc.2021.100299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhang J., Xiao T., Cai Y., et al. Membrane fusion and immune evasion by the spike protein of SARS-CoV-2 delta variant. Science. 2021 doi: 10.1126/science.abl9463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wang Y., Chen R., Hu F., et al. Transmission, viral kinetics and clinical characteristics of the emergent SARS-CoV-2 Delta VOC in Guangzhou, China. EClinicalMedicine. 2021;40 doi: 10.1016/j.eclinm.2021.101129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Luo C.H., Morris C.P., Sachithanandham J., et al. Infection with the SARS-CoV-2 delta variant is associated with higher infectious virus loads compared to the alpha variant in both unvaccinated and vaccinated individuals. medRxiv. 2021 doi: 10.1101/2021.08.15.21262077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Teyssou E., Delagrèverie H., Visseaux B., et al. The delta SARS-CoV-2 variant has a higher viral load than the beta and the historical variants in nasopharyngeal samples from newly diagnosed COVID-19 patients. J Infect. 2021;83:e1–e3. doi: 10.1016/j.jinf.2021.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Singanayagam A., Hakki S., Dunning J., et al. Community transmission and viral load kinetics of the SARS-CoV-2 delta (B.1.617.2) variant in vaccinated and unvaccinated individuals in the UK: a prospective, longitudinal, cohort study. Lancet Infect Dis. 2021 doi: 10.1016/S1473-3099(21)00648-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jones T.C., Biele G., Mühlemann B., et al. Estimating infectiousness throughout SARS-CoV-2 infection course. Science. 2021:373 2021–37305/27. doi: 10.1126/science.abi5273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Li B., Deng A., Li K., et al. Viral infection and transmission in a large, well-traced outbreak caused by the SARS-CoV-2 Delta variant. medRxiv. 2021:21260122. doi: 10.1101/2021.07.07.21260122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Syed A.M., Taha T.Y., Tabata T., et al. Rapid assessment of SARS-CoV-2 evolved variants using virus-like particles. Science. 2021 doi: 10.1126/science.abl6184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Dixon B.C., Fischer R.S.B., Zhao H., et al. Contact and SARS-CoV-2 infections among college football athletes in the southeastern conference during the COVID-19 pandemic. JAMA Netw Open. 2021;4 doi: 10.1001/jamanetworkopen.2021.35566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Furuse Y., Sando E., Tsuchiya N., et al. Clusters of coronavirus disease in communities, Japan, January-April 2020. Emerg Infect Dis. 2020;26:2176–2179. doi: 10.3201/eid2609.202272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Raines K.S., Doniach S., Bhanot G. The transmission of SARS-CoV-2 is likely comodulated by temperature and by relative humidity. PLoS One. 2021;16:e0255212. doi: 10.1371/journal.pone.0255212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cevik M., Marcus J.L., Buckee C., et al. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) transmission dynamics should inform policy. Clin Infect Dis. 2021;73:S170–S176. doi: 10.1093/cid/ciaa1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Esaryk E.E., Wesson P., Fields J., et al. Variation in SARS-CoV-2 infection risk and socioeconomic disadvantage among a Mayan-Latinx population in Oakland, California. JAMA Netw Open. 2021;4 doi: 10.1001/jamanetworkopen.2021.10789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mazzilli S., Chieti A., Casigliani V., et al. Risk of SARS-CoV-2 infection and disease severity in people at socioeconomic disadvantage in Italy. Eur J Public Health. 2021;31 doi: 10.1093/eurpub/ckab164.552. [DOI] [Google Scholar]
- 73.Allan-Blitz L.T., Goldbeck C., Hertlein F., et al. Association of lower socioeconomic status and SARS-CoV-2 positivity in Los Angeles, California. J Prev Med Public Health. 2021;54:161–165. doi: 10.3961/jpmph.21.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Rodriguez-Diaz C.E., Guilamo-Ramos V., Mena L., et al. Risk for COVID-19 infection and death among Latinos in the United States: examining heterogeneity in transmission dynamics. Ann Epidemiol. 2020;52:46–53. doi: 10.1016/j.annepidem.2020.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Li Q., Guan X., Wu P., et al. Early Transmission Dynamics in Wuhan, China, of Novel Coronavirus-Infected Pneumonia. N Engl J Med. 2020;382:1199–1207. doi: 10.1056/NEJMoa2001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lauer S.A., Grantz K.H., Bi Q., et al. The Incubation Period of Coronavirus Disease 2019 (COVID-19) From Publicly Reported Confirmed Cases: Estimation and Application. Ann Intern Med. 2020;172:577–582. doi: 10.7326/m20-0504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Meng Z., Jianpeng X., Aiping D., et al. Notes from the Field: Transmission Dynamics of an Outbreak of the COVID-19 Delta Variant B.1.617.2 — Guangdong Province, China, May–June 2021. China CDC Weekly. 2021;3:584–586. doi: 10.46234/ccdcw2021.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tom B.D., Van Hoek A.J., Pebody R., et al. Estimating time to onset of swine influenza symptoms after initial novel A(H1N1v) viral infection. Epidemiol Infect. 2011;139:1418–1424. doi: 10.1017/S0950268810002566. [DOI] [PubMed] [Google Scholar]
- 79.Xin H., Li Y., Wu P., et al. Estimating the latent period of coronavirus disease 2019 (COVID-19) Clin Infect Dis. 2021 doi: 10.1093/cid/ciab746. [DOI] [PubMed] [Google Scholar]
- 80.Kang M., Xin H., Yuan J., et al. Transmission dynamics and epidemiological characteristics of Delta variant infections in China. medRxiv. 2021;2021:21261991. doi: 10.1101/2021.08.12.21261991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Rai B., Shukla A., Dwivedi L.K. Estimates of serial interval for COVID-19: A systematic review and meta-analysis. Clin Epidemiol Glob Health. 2021;9:157–161. doi: 10.1016/j.cegh.2020.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hari H., Jun-Sik L., Sun-Ah S., et al. Transmission dynamics of the delta variant of SARS-CoV-2 infections in Daejeon, South Korea. Res Square. 2021 doi: 10.21203/rs.3.rs-934350/v1. [DOI] [Google Scholar]
- 83.Pung R., Mak T.M., Kucharski A.J., et al. Serial intervals in SARS-CoV-2 B.1.617.2 variant cases. Lancet. 2021;398:837–838. doi: 10.1016/S0140-6736(21)01697-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Cheng H.Y., Jian S.W., Liu D.P., et al. Contact Tracing Assessment of COVID-19 Transmission Dynamics in Taiwan and Risk at Different Exposure Periods Before and After Symptom Onset. JAMA Intern Med. 2020;180:1156–1163. doi: 10.1001/jamainternmed.2020.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Mack C.D., DiFiori J., Tai C.G., et al. SARS-CoV-2 Transmission Risk Among National Basketball Association Players, Staff, and Vendors Exposed to Individuals With Positive Test Results After COVID-19 Recovery During the 2020 Regular and Postseason. JAMA Intern Med. 2021;181:960–966. doi: 10.1001/jamainternmed.2021.2114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.de Man P., Ortiz M., Bluyssen P.M., et al. Airborne SARS-CoV-2 in home- and hospital environment investigated with a high-powered air sampler. J Hosp Infect. 2021 doi: 10.1016/j.jhin.2021.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Delamater P.L., Street E.J., Leslie T.F., et al. Complexity of the Basic Reproduction Number (R(0)) Emerg Infect Dis. 2019;25:1–4. doi: 10.3201/eid2501.171901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Billah M.A., Miah M.M., Khan M.N. Reproductive number of coronavirus: A systematic review and meta-analysis based on global level evidence. PLoS One. 2020;15:e0242128. doi: 10.1371/journal.pone.0242128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Xia F., Yang X., Cheke R.A., et al. Quantifying competitive advantages of mutant strains in a population involving importation and mass vaccination rollout. Infect Dis Model. 2021;6:988–996. doi: 10.1016/j.idm.2021.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Dagpunar J. Interim estimates of increased transmissibility, growth rate, and reproduction number of the Covid-19 B.1.617.2 variant of concern in the United Kingdom. medRxiv. 2021:21258293. doi: 10.1101/2021.06.03.21258293. [DOI] [Google Scholar]
- 91.Breban R., Riou J., Fontanet A. Interhuman transmissibility of Middle East respiratory syndrome coronavirus: estimation of pandemic risk. Lancet. 2013;382:694–699. doi: 10.1016/S0140-6736(13)61492-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Cauchemez S., Fraser C., Van Kerkhove M.D., et al. Middle East respiratory syndrome coronavirus: quantification of the extent of the epidemic, surveillance biases, and transmissibility. Lancet Infect Dis. 2014;14:50–56. doi: 10.1016/S1473-3099(13)70304-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Fisman D., Khoo E., Tuite A. Early epidemic dynamics of the West African 2014 Ebola outbreak: estimates derived with a simple two-parameter model. Plos Curr. 2014;6 doi: 10.1371/currents.outbreaks.89c0d3783f36958d96ebbae97348d571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Khan A., Naveed M., Dur E.A.M., et al. Estimating the basic reproductive ratio for the Ebola outbreak in Liberia and Sierra Leone. Infect Dis Poverty. 2015;4 doi: 10.1186/s40249-015-0043-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Balcan D., Hu H., Goncalves B., et al. Seasonal transmission potential and activity peaks of the new influenza A(H1N1): a Monte Carlo likelihood analysis based on human mobility. BMC Med. 2009;7 doi: 10.1186/1741-7015-7-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Andreasen V., Viboud C., Simonsen L. Epidemiologic characterization of the 1918 influenza pandemic summer wave in Copenhagen: implications for pandemic control strategies. J Infect Dis. 2008;197:270–278. doi: 10.1086/524065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lipsitch M., Cohen T., Cooper B., et al. Transmission dynamics and control of severe acute respiratory syndrome. Science. 2003;300:1966–1970. doi: 10.1126/science.1086616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Guerra F.M., Bolotin S., Lim G., et al. The basic reproduction number (R(0)) of measles: a systematic review. Lancet Infect Dis. 2017;17:e420–e428. doi: 10.1016/s1473-3099(17)30307-9. [DOI] [PubMed] [Google Scholar]
- 99.Anderson R.M., May R.M. Directly transmitted infections diseases: control by vaccination. Science. 1982;215:1053–1060. doi: 10.1126/science.7063839. [DOI] [PubMed] [Google Scholar]
- 100.Lloyd-Smith J.O., Schreiber S.J., Kopp P.E., et al. Superspreading and the effect of individual variation on disease emergence. Nature. 2005;438:355–359. doi: 10.1038/nature04153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Miller D., Martin M.A., Harel N., et al. Full genome viral sequences inform patterns of SARS-CoV-2 spread into and within Israel. Nat Commun. 2020;11:5518. doi: 10.1038/s41467-020-19248-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Endo A., Abbott S., Kucharski A.J., et al. Estimating the overdispersion in COVID-19 transmission using outbreak sizes outside China. Wellcome Open Res. 2020;5:67. doi: 10.12688/wellcomeopenres.15842.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Adam D.C., Wu P., Wong J.Y., et al. Clustering and superspreading potential of SARS-CoV-2 infections in Hong Kong. Nat Med. 2020;26:1714–1719. doi: 10.1038/s41591-020-1092-0. [DOI] [PubMed] [Google Scholar]
- 104.Bi Q., Wu Y., Mei S., et al. Epidemiology and transmission of COVID-19 in 391 cases and 1286 of their close contacts in Shenzhen, China: a retrospective cohort study. Lancet Infect Dis. 2020;20:911–919. doi: 10.1016/S1473-3099(20)30287-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Lemieux J.E., Siddle K.J., Shaw B.M., et al. Phylogenetic analysis of SARS-CoV-2 in Boston highlights the impact of superspreading events. Science. 2021:371. doi: 10.1126/science.abe3261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Park S.Y., Kim Y.M., Yi S., et al. Coronavirus Disease Outbreak in Call Center, South Korea. Emerg Infect Dis. 2020;26:1666–1670. doi: 10.3201/eid2608.201274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Yusef D., Hayajneh W., Awad S., et al. Large Outbreak of Coronavirus Disease among Wedding Attendees, Jordan. Emerg Infect Dis. 2020;26:2165–2167. doi: 10.3201/eid2609.201469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Hamner L., Dubbel P., Capron I., et al. High SARS-CoV-2 Attack Rate Following Exposure at a Choir Practice - Skagit County, Washington, March 2020. MMWR Morb Mortal Wkly Rep. 2020;69:606–610. doi: 10.15585/mmwr.mm6919e6. [DOI] [PubMed] [Google Scholar]
- 109.Szablewski C.M., Chang K.T., Brown M.M., et al. SARS-CoV-2 Transmission and Infection Among Attendees of an Overnight Camp - Georgia, June 2020. MMWR Morb Mortal Wkly Rep. 2020;69:1023–1025. doi: 10.15585/mmwr.mm6931e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Payne D.C., Smith-Jeffcoat S.E., Nowak G., et al. SARS-CoV-2 Infections and Serologic Responses from a Sample of U.S. Navy Service Members - USS Theodore Roosevelt, April 2020. MMWR Morb Mortal Wkly Rep. 2020;69:714–721. doi: 10.15585/mmwr.mm6923e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ryu S., Kim D., Lim J.-S., et al. Serial interval and transmission dynamics during the SARS-CoV-2 Delta variant predominance in South Korea. medRxiv. 2021:21262166. doi: 10.1101/2021.08.18.21262166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Siddle K.J., Krasilnikova L.A., Moreno G.K., et al. Evidence of transmission from fully vaccinated individuals in a large outbreak of the SARS-CoV-2 Delta variant in Provincetown, Massachusetts. medRxiv. 2021 doi: 10.1101/2021.10.20.21265137. [DOI] [Google Scholar]
- 113.Chen P.Z., Koopmans M., Fisman D.N., et al. Understanding why superspreading drives the COVID-19 pandemic but not the H1N1 pandemic. Lancet Infect Dis. 2021;21:1203–1204. doi: 10.1016/S1473-3099(21)00406-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Becker A.D., Birger R.B., Teillant A., et al. Estimating enhanced prevaccination measles transmission hotspots in the context of cross-scale dynamics. Proc Natl Acad Sci U S A. 2016;113:14595–14600. doi: 10.1073/pnas.1604976113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ackley S.F., Hacker J.K., Enanoria W.T.A., et al. Genotype-Specific Measles Transmissibility: A Branching Process Analysis. Clin Infect Dis. 2018;66:1270–1275. doi: 10.1093/cid/cix974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Fraser C., Cummings D.A., Klinkenberg D., et al. Influenza transmission in households during the 1918 pandemic. Am J Epidemiol. 2011;174:505–514. doi: 10.1093/aje/kwr122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Frieden T.R., Lee C.T. Identifying and Interrupting Superspreading Events-Implications for Control of Severe Acute Respiratory Syndrome Coronavirus 2. Emerg Infect Dis. 2020;26:1059–1066. doi: 10.3201/eid2606.200495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Cevik M., Baral S.D. Networks of SARS-CoV-2 transmission. Science. 2021;373:162–163. doi: 10.1126/science.abg0842. [DOI] [PubMed] [Google Scholar]
- 119.Althouse B.M., Wenger E.A., Miller J.C., et al. Superspreading events in the transmission dynamics of SARS-CoV-2: Opportunities for interventions and control. Plos Biol. 2020;18 doi: 10.1371/journal.pbio.3000897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Dagan N., Barda N., Kepten E., et al. BNT162b2 mRNA Covid-19 Vaccine in a Nationwide Mass Vaccination Setting. N Engl J Med. 2021;384:1412–1423. doi: 10.1056/NEJMoa2101765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.McDonald I., Murray S.M., Reynolds C.J., et al. Comparative systematic review and meta-analysis of reactogenicity, immunogenicity and efficacy of vaccines against SARS-CoV-2. NPJ Vaccin. 2021;6 doi: 10.1038/s41541-021-00336-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Kojima N., Shrestha N., Klausner J. A Systematic Review of the Protective Effect of Prior SARS-CoV-2 Infection on Repeat Infection. medRxiv. 2021:21262741. doi: 10.1101/2021.08.27.21262741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Cevik M., Grubaugh N.D., Iwasaki A., et al. COVID-19 vaccines: Keeping pace with SARS-CoV-2 variants. Cell. 2021;184:5077–5081. doi: 10.1016/j.cell.2021.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.de Gier B., Andeweg S., Joosten R., et al. Vaccine effectiveness against SARS-CoV-2 transmission and infections among household and other close contacts of confirmed cases, the Netherlands. Euro Surveill. 2021;26 doi: 10.2807/1560-7917.Es.2021.26.31.2100640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.England P.H. SARS-CoV-2 variants of concern and variants under investigation in England: Technical briefing 19. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/1005517/Technical_Briefing_19.pdf Available at: Accessed October 1, 2021.
- 126.Pouwels K.B., Pritchard E., Matthews P.C., et al. Effect of Delta variant on viral burden and vaccine effectiveness against new SARS-CoV-2 infections in the UK. Nat Med. 2021 doi: 10.1038/s41591-021-01548-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Fowlkes A., Gaglani M., Groover K., et al. Effectiveness of COVID-19 Vaccines in Preventing SARS-CoV-2 Infection Among Frontline Workers Before and During B.1.617.2 (Delta) Variant Predominance - Eight U.S. Locations, December. MMWR Morb Mortal Wkly Rep. 2021;70:1167–1169. doi: 10.15585/mmwr.mm7034e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Brown C.M., Vostok J., Johnson H., et al. Outbreak of SARS-CoV-2 Infections, Including COVID-19 Vaccine Breakthrough Infections, Associated with Large Public Gatherings - Barnstable County, Massachusetts. MMWR Morb Mortal Wkly Rep. 2021;70:1059–1062. doi: 10.15585/mmwr.mm7031e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Chia P.Y., Xiang Ong S.W., Chiew C.J., et al. Virological and serological kinetics of SARS-CoV-2 Delta variant vaccine-breakthrough infections: a multi-center cohort study. medRxiv. 2021:21261295. doi: 10.1101/2021.07.28.21261295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Qiu X., Nergiz A.I., Maraolo A.E., et al. The role of asymptomatic and pre-symptomatic infection in SARS-CoV-2 transmission-a living systematic review. Clin Microbiol Infect. 2021;27:511–519. doi: 10.1016/j.cmi.2021.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Riemersma K.K., Grogan B.E., Kita-Yarbro A., et al. Shedding of Infectious SARS-CoV-2 Despite Vaccination. medRxiv. 2021:21261387. doi: 10.1101/2021.07.31.21261387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Shamier M.C., Tostmann A., Bogers S., et al. Virological characteristics of SARS-CoV-2 vaccine breakthrough infections in health care workers. medRxiv. 2021:21262158. doi: 10.1101/2021.08.20.21262158. [DOI] [Google Scholar]
- 133.Eyre D.W., Taylor D., Purver M., et al. Effect of Covid-19 Vaccination on Transmission of Alpha and Delta Variants. N Engl J Med. 2022 doi: 10.1056/NEJMoa2116597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Accorsi E.K., Qiu X., Rumpler E., et al. How to detect and reduce potential sources of biases in studies of SARS-CoV-2 and COVID-19. Eur J Epidemiol. 2021;36:179–196. doi: 10.1007/s10654-021-00727-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Scott J., Richterman A., Cevik M. Covid-19 vaccination: evidence of waning immunity is overstated. Bmj. 2021;374:n2320. doi: 10.1136/bmj.n2320. [DOI] [PubMed] [Google Scholar]
- 136.U.S. Food & Drug Administration (2021) Vaccines and Related Biological Products Advisory Committee Meeting September 17, 2021: Application for licensure of a booster dose for COMIRNATY (COVID-19 Vaccine, mRNA). FDA Briefing Document.
- 137.Baden L.R., El Sahly H.M., Essink B., et al. Phase 3 Trial of mRNA-1273 during the Delta-Variant Surge. N Engl J Med. 2021 doi: 10.1056/NEJMc2115597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Levine-Tiefenbrun M., Yelin I., Alapi H., et al. Viral loads of Delta-variant SARS-CoV-2 breakthrough infections after vaccination and booster with BNT162b2. Nat Med. 2021 doi: 10.1038/s41591-021-01575-4. [DOI] [PubMed] [Google Scholar]
- 139.Bar-On Y.M., Goldberg Y., Mandel M., et al. Protection of BNT162b2 Vaccine Booster against Covid-19 in Israel. N Engl J Med. 2021;385:1393–1400. doi: 10.1056/NEJMoa2114255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Barda N., Dagan N., Cohen C., et al. Effectiveness of a third dose of the BNT162b2 mRNA COVID-19 vaccine for preventing severe outcomes in Israel: an observational study. Lancet. 2021 doi: 10.1016/s0140-6736(21)02249-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Pfizer. Pfizer and BioNTech announce phase 3 trial data showing high efficacy of a booster dose of their covid-19 vaccine. https://wwwpfizercom/news/press-release/press-release-detail/pfizer-and-biontech-announce-phase-3-trial-data-showing 2021 Available at:
- 142.Conway Morris A., Sharrocks K., Bousfield R., et al. The removal of airborne SARS-CoV-2 and other microbial bioaerosols by air filtration on COVID-19 surge units. Clin Infect Dis. 2021 doi: 10.1093/cid/ciab933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Wang Y., Tian H., Zhang L., et al. Reduction of secondary transmission of SARS-CoV-2 in households by face mask use, disinfection and social distancing: a cohort study in Beijing, China. BMJ Glob Health. 2020;5 doi: 10.1136/bmjgh-2020-002794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Gettings J., Czarnik M., Morris E., et al. Mask Use and Ventilation Improvements to Reduce COVID-19 Incidence in Elementary Schools - Georgia, November 16-December 11, 2020. MMWR Morb Mortal Wkly Rep. 2021;70:779–784. doi: 10.15585/mmwr.mm7021e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Escombe A.R., Oeser C.C., Gilman R.H., et al. Natural ventilation for the prevention of airborne contagion. Plos Med. 2007;4 doi: 10.1371/journal.pmed.0040068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Lygizos M., Shenoi S.V., Brooks R.P., et al. Natural ventilation reduces high TB transmission risk in traditional homes in rural KwaZulu-Natal, South Africa. BMC Infect Dis. 2013;13 doi: 10.1186/1471-2334-13-300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Abaluck J., Kwong L.H., Styczynski A., et al. The Impact of Community Masking on COVID-19. A Cluster Randomized Trial in Bangladesh. 2021;31:2021. doi: 10.3386/w28734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Bundgaard H., Bundgaard J.S., Raaschou-Pedersen D.E.T., et al. Effectiveness of Adding a Mask Recommendation to Other Public Health Measures to Prevent SARS-CoV-2 Infection in Danish Mask Wearers : A Randomized Controlled Trial. Ann Intern Med. 2021;174:335–343. doi: 10.7326/M20-6817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Li Y., Liang M., Gao L., et al. Face masks to prevent transmission of COVID-19: A systematic review and meta-analysis. Am J Infect Control. 2021;49:900–906. doi: 10.1016/j.ajic.2020.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Chu D.K., Akl E.A., Duda S., et al. Physical distancing, face masks, and eye protection to prevent person-to-person transmission of SARS-CoV-2 and COVID-19: a systematic review and meta-analysis. Lancet. 2020;395:1973–1987. doi: 10.1016/S0140-6736(20)31142-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Lyu W., Wehby G.L. Community Use Of Face Masks And COVID-19: Evidence From A Natural Experiment Of State Mandates In The US. Health Aff (Millwood) 2020;39:1419–1425. doi: 10.1377/hlthaff.2020.00818. [DOI] [PubMed] [Google Scholar]
- 152.Ginther D.K., Zambrana C. Association of Mask Mandates and COVID-19 Case Rates, Hospitalizations, and Deaths in Kansas. JAMA Netw Open. 2021;4 doi: 10.1001/jamanetworkopen.2021.14514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Baker M.A., Fiumara K., Rhee C., et al. Low Risk of Coronavirus Disease 2019 (COVID-19) Among Patients Exposed to Infected Healthcare Workers. Clin Infect Dis. 2021;73:e1878–e1880. doi: 10.1093/cid/ciaa1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Rhee C., Baker M., Vaidya V., et al. Incidence of Nosocomial COVID-19 in Patients Hospitalized at a Large US Academic Medical Center. JAMA Netw Open. 2020;3 doi: 10.1001/jamanetworkopen.2020.20498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Kociolek L.K., Patel A.B., Hultquist J.F., et al. Viral whole-genome sequencing to assess impact of universal masking on SARS-CoV-2 transmission among pediatric healthcare workers. Infect Control Hosp Epidemiol. 2021:1–5. doi: 10.1017/ice.2021.415. [DOI] [PubMed] [Google Scholar]
- 156.Thompson E.R., Williams F.S., Giacin P.A., et al. Universal masking to control healthcare-associated transmission of severe acute respiratory coronavirus virus 2 (SARS-CoV-2) Infect Control Hosp Epidemiol. 2021:1–7. doi: 10.1017/ice.2021.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Radonovich L.J., Jr., Simberkoff M.S., Bessesen M.T., et al. N95 Respirators vs Medical Masks for Preventing Influenza Among Health Care Personnel: A Randomized Clinical Trial. Jama. 2019;322:824–833. doi: 10.1001/jama.2019.11645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Sims M.D., Maine G.N., Childers K.L., et al. Coronavirus Disease 2019 (COVID-19) Seropositivity and Asymptomatic Rates in Healthcare Workers Are Associated with Job Function and Masking. Clin Infect Dis. 2021;73:S154–S162. doi: 10.1093/cid/ciaa1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Klompas M., Baker M.A., Rhee C., et al. A SARS-CoV-2 Cluster in an Acute Care Hospital. Ann Intern Med. 2021;174:794–802. doi: 10.7326/M20-7567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Lindsey B.B., Villabona-Arenas C.J., Campbell F., et al. Characterising within-hospital SARS-CoV-2 transmission events: a retrospective analysis integrating epidemiological and viral genomic data from a UK tertiary care setting across two pandemic waves. medRxiv. 2021;2021:21260537. doi: 10.1101/2021.07.15.21260537. [DOI] [Google Scholar]
- 161.Klompas M., Baker M.A., Griesbach D., et al. Transmission of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) From Asymptomatic and Presymptomatic Individuals in Healthcare Settings Despite Medical Masks and Eye Protection. Clin Infect Dis. 2021;73:1693–1695. doi: 10.1093/cid/ciab218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Park Y., Huh I.S., Lee J., et al. Application of Testing-Tracing-Treatment Strategy in Response to the COVID-19 Outbreak in Seoul, Korea. J Korean Med Sci. 2020;35:e396. doi: 10.3346/jkms.2020.35.e396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Kendall M., Milsom L., Abeler-Dörner L., et al. Epidemiological changes on the Isle of Wight after the launch of the NHS Test and Trace programme: a preliminary analysis. Lancet Digit Health. 2020;2:e658–e666. doi: 10.1016/s2589-7500(20)30241-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Malheiro R., Figueiredo A.L., Magalhães J.P., et al. Effectiveness of contact tracing and quarantine on reducing COVID-19 transmission: a retrospective cohort study. Public Health. 2020;189:54–59. doi: 10.1016/j.puhe.2020.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Gardner B.J., Kilpatrick A.M. Contact tracing efficiency, transmission heterogeneity, and accelerating COVID-19 epidemics. Plos Comput Biol. 2021;17:e1009122. doi: 10.1371/journal.pcbi.1009122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Viana R., Moyo S., Amoako D.G., et al. Rapid epidemic expansion of the SARS-CoV-2 Omicron variant in southern Africa. medRxiv. 2021:21268028. doi: 10.1101/2021.12.19.21268028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Ito K., Piantham C., Nishiura H. Relative Instantaneous Reproduction Number of Omicron SARS-CoV-2 variant with respect to the Delta variant in Denmark. J Med Virol. 2021 doi: 10.1002/jmv.27560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Lyngse F.P., Mortensen L.H., Denwood M.J., et al. SARS-CoV-2 Omicron VOC Transmission in Danish Households. medRxiv. 2021;2021:21268278. doi: 10.1101/2021.12.27.21268278. [DOI] [Google Scholar]
- 169.Carreño J.M., Alshammary H., Tcheou J., et al. Activity of convalescent and vaccine serum against SARS-CoV-2 Omicron. Nature. 2021 doi: 10.1038/s41586-022-04399-5. [DOI] [PubMed] [Google Scholar]
- 170.Schmidt F., Muecksch F., Weisblum Y., et al. Plasma Neutralization of the SARS-CoV-2 Omicron Variant. New Engl J Med. 2021 doi: 10.1056/NEJMc2119641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Brandal L.T., MacDonald E., Veneti L., et al. Outbreak caused by the SARS-CoV-2 Omicron variant in Norway, November to December 2021. Euro Surveill. 2021 doi: 10.2807/1560-7917.Es.2021.26.50.2101147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Helmsdal G., Hansen O.K., Møller L.F., et al. Omicron outbreak at a private gathering in the Faroe Islands, infecting 21 of 33 triple-vaccinated healthcare workers. medRxiv. 2021:21268021. doi: 10.1101/2021.12.22.21268021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Hay J.A., Kissler S.M., Fauver J.R., et al. Viral dynamics and duration of PCR positivity of the SARS-CoV-2 Omicron variant. medRxiv. 2022:22269257. doi: 10.1101/2022.01.13.22269257. [DOI] [Google Scholar]
- 174.Puhach O., Adea K., Hulo N., et al. Infectious viral load in unvaccinated and vaccinated patients infected with SARS-CoV-2 WT, Delta and Omicron. medRxiv. 2022 doi: 10.1101/2022.01.10.22269010. [DOI] [PubMed] [Google Scholar]
- 175.Torjesen I. Covid-19: Peak of viral shedding is later with omicron variant, Japanese data suggest. Bmj. 2022;376 doi: 10.1136/bmj.o89. [DOI] [PubMed] [Google Scholar]
- 176.Gu H., Krishnan P., Ng D.Y.M., et al. Probable Transmission of SARS-CoV-2 Omicron Variant in Quarantine Hotel, Hong Kong, China, November 2021. Emerg Infect Dis. 2021;28 doi: 10.3201/eid2802.212422. [DOI] [PMC free article] [PubMed] [Google Scholar]