Supplemental Digital Content is Available in the Text.
OBJECTIVE
To review postmarketing data for delayed (≥14 days post-treatment) adverse events (AEs) of interest (inflammatory and noninflammatory nodules, hypersensitivity, granulomas) for newer hyaluronic acid (HA) fillers FDA-approved within the last 5 years (2016–2020).
METHODS
Reports from the Manufacturer and User Facility Device Experience (MAUDE) database were extracted for HAREF, HADEF, HAKYS, HAVER, HAVLR, HAVOB, HARH2, HARH3, and HARH4 from January 2016 to January 2021. Keywords from event narratives were used to identify and categorize AEs and then verified through inclusion/exclusion criteria. Percentages are based on the total combined events of interest to provide an overall perspective of the events reported during the search period.
RESULTS
Of 585 MAUDE reports, there were 195 (33.3%) delayed AEs of interest. Of those, 71.8% were nodules (42.1% inflammatory and 29.7% noninflammatory), 21.5% hypersensitivity, and 6.7% granulomas. The combined total events of interest, ordered by frequency reported, were HAVLR (74.4%), HAVOB (12.3%), HADEF (5.1%), HARH4 (3.6%), HAREF (2.6%), and HARH2 (2.1%), with no reports for HARH3, HAVER, and HAKYS.
CONCLUSION
Although delayed nodules and inflammatory events are rare, reports for these events were extracted from the MAUDE database from 2016 to 2020 for HAVLR, HAVOB, HADEF, HARH4, HAREF, and HARH2 (most to least frequent).
The most popular aesthetic fillers are derived from hyaluronic acid (HA) due to their safety profile.1 However, adverse events (AEs) still occur and are generally categorized as early-onset or delayed-onset. Early-onset (<14 days post-treatment) AEs (e.g., bruising, swelling, lumps/bumps) are more likely related to injection technique (e.g., superficial placement, rapid injection/flow rates, higher volumes).2 Delayed-onset (≥14 days post-treatment) events are more likely to be product related and concerning for providers because they can appear months to years after the treatment.3,4 Delayed-onset nodules and inflammatory events are of special interest to aesthetic providers because they are often immune mediated (noninfectious) and typically require removal of the product.3,4
In contrast to the older generation, the long-term safety profile of the newer generation HA fillers is not well-established. Thus, more recently FDA-approved HA fillers (2016–2020) are of interest in this review: Juvederm Volbella (HAVOB; Allergan plc), Restylane Refyne (HAREF; Galderma SA), and Restylane Defyne (HADEF; Galderma SA), all approved in 2016; Juvederm Vollure (HAVLR; Allergan plc), Teosyal RHA2/3/4 (HARH2/3/4; Teoxane SA), and Revanesse Versa (HAVER; Prollenium Medical Technologies), approved in 2017; and Restylane Kysse (HAKYS; Galderma SA) approved in 2020. Although the FDA approved HARH2/3/4 in 2017 and available in Europe since 2015, they were not available in the United States until mid 2020.
Each product uses various manufacturing technologies, providing them with unique properties that maximize versatility. HAVOB and HAVLR use Vycross technology, which combines low- molecular-weight and high-molecular-weight HA to improve the crosslinking efficiency of the HA chains5; HARH2/3/4 use Preserved Network technology, designed with reduced synthetic crosslinking due to preserved natural HA polymers6; HAVER uses Thixofix technology, designed to maximize the effectiveness of the crosslinked HA chains present in the gel7; and HAREF, HADEF, and HAKYS use XpresHAn technology, which has varying degrees of crosslinking to provide different levels of flexibility and support while maintaining natural movement in areas of dynamic expression.8–11
Several clinical trials have established the efficacy and safety of each product.12–20 Additionally, each product has postmarketing surveillance (PMS) data available in the Manufacturer and User Facility Device Experience (MAUDE) FDA database.
Although safety reviews with the MAUDE database have been conducted to understand events reported for older HA fillers,21–23 these analyses were not inclusive of those more recently approved. The aim of this safety review was to summarize delayed events of interest reported in the MAUDE database from January 2016 to January 2021 for FDA-approved HA fillers 2016 to 2020. The events of special interest in this review include hypersensitivity reactions, nodules (both inflammatory and non-inflammatory), and granulomas.
Methods
The MAUDE database compiles PMS data submitted to the FDA of potential device-related safety issues that are derived from mandatory reports (manufacturer) and voluntary reports (health care professionals [HCP] and consumers).24 This includes events assessed as related to the product and/or procedure collected from spontaneous reports, the literature, and health authorities (including ex-US data). The ‟Event Text” field in the reports contain information, such as the treatment date, time of event onset and duration, event description, and any interventions. This information can be incomplete for individual reports due to patient privacy, the reporter, or reporter follow-up.
The MAUDE database was queried for complications related to injection of HAREF, HADEF, HAKYS, HAVER, HAVLR, HAVOB, HARH2, HARH3, and HARH4 from January 1, 2016 to January 31, 2021. Duplicated reports under multiple report numbers were consolidated into single representative reports.
Keywords summarized in Table 1 were generated to search and categorize events of interest from each report. Categorizations were based on the ‟lay terms” most used to describe the events of interest. Because MAUDE does not classify the type of event in each report, the ‟event text” (narrative) section was queried for the keywords. Once events were categorized, event descriptions were used as a basis to either include or exclude event reports consistent with the criteria below. This ensures consistency of results across all products analyzed. Note that individual event reports may contain multiple AEs of interest.
TABLE 1.
Keywords Used to Identify and Categorize Delayed Events From MAUDE Reports
| Event | Extracted ‟Keywords” |
| Nodules | Nodule, papule, mass, lump, bump, induration |
| Granuloma | Granuloma, foreign body |
| Hypersensitivity | Hypersensitivity, swelling/inflammation |
Inclusion criteria were as follows: manufacturer or HCP reports, “delayed AE” identified by HCP or reported as ≥14 days after treatment, and verified events of interest (hypersensitivity, nodules [inflammatory or noninflammatory], and granulomas) based on descriptions.3,4 Hypersensitivity was confirmed if the description included HCP-reported hypersensitivity, diffuse facial swelling/inflammation, and persistent facial swelling. Nodules were confirmed if the description included palpable lumps/bumps or specifically identified them as nodules. Inflammatory nodules were differentiated from noninflammatory nodules if the description included tenderness, erythema, swelling/inflammation, pain, or irritation. Granulomas were confirmed if a biopsy demonstrated a “granulomatous” or “foreign body reaction”; otherwise, they were reclassified as the “other events of interest” if they met the criteria described.
Exclusion criteria were as follows: early-onset AE (<14 days) or no confirmation of a delayed event, patient or consumer reports with no HCP confirmation, and events that may have been caused by a different product or a combination of products based on narrative.
For the purposes of reporting relative frequencies, percentages were based on the total combined events of interest identified. The nodules reported by injection site were analyzed relative to the combined total number of nodules identified.
Results
Between 2016 and 2020, there were a combined total of 585 MAUDE reports extracted for each HA filler. Of these, there were 195 (33.3%) confirmed delayed AEs of interest; 71.8% were nodules (42.1% inflammatory and 29.7% noninflammatory), followed by hypersensitivity (21.5%), and granulomas (6.7%). Ordered by frequency, these were with HAVLR (74.4%), HAVOB (12.3%), HADEF (5.1%), HARH4 (3.6%), HAREF (2.6%), and HARH2 (2.1%), with no reports for HARH3, HAVER, and HAKYS (Figure 1 and See, Supplemental Digital Content 1, Table S1, http://links.lww.com/DSS/A972 displaying the total number of events and percentages relative to the total combined events of interest identified for each product).
Figure 1.

Percentage of delayed events of interest relative to the combined total for all HA fillers.
Of the 140 reports of nodules, 41.4% occurred in the lips, followed by nasolabial folds (NLFs; 23.6%), marionette lines (MLs; 22.1%), perioral areas (19.3%), tear troughs (12.1%), chin (5.7%), cheeks (4.3%), and prejowl (3.6%) (Figure 2). Note that nodules reported in multiple sites per patient counted as one event.
Figure 2.
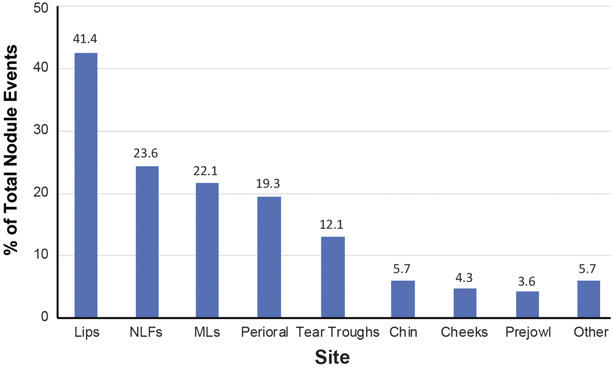
Location of nodules relative to total number of nodule events identified. Other includes neck, glabella, forehead, temple, nose, ear, and full face. One event equals one report. Nodules could be in multiple locations per event. MLs, marionette lines; NLFs, nasolabial folds.
XpresHAn Technology
Of the total events of interest, there was 1 (0.5%) report of granuloma and noninflammatory nodules for both HADEF and HAREF. HAREF had 1 (0.5%) report of hypersensitivity reaction and 2 (1.0%) inflammatory nodules, whereas HADEF had 4 (2.1%) hypersensitivity reactions and inflammatory nodules each (Figure 1). There were no events of interest reported for HAKYS during the search period.
Events had a time of onset ranging from 2 weeks to 4.5 months, with a median of 2 months. Nodules were reported in the lips and MLs for both products and in the chin, prejowl sulcus, and cheeks with HADEF.
Vycross Technology
Of the total events of interest, there were 56 (28.7%) reports of inflammatory nodules, 50 (25.6%) noninflammatory nodules, 31 (15.9%) hypersensitivity reactions, and 8 (4.1%) granulomas for HAVLR (Figure 1). For HAVOB, there were 13 (6.7%) reports of inflammatory nodules, 6 (3.1%) noninflammatory nodules, 3 (1.5%) hypersensitivity reactions, and 2 (1.0%) granulomas (Figure 1).
Events had a time of onset ranging from 2 weeks to 19 months after injection, with a median of 3 months. Of note, there was one report of inflammatory nodules with hypersensitivity (same patient) with a time of onset of 19 months, and 2 events of noninflammatory nodules with a time of onset of ≥1 year after injection of HAVLR. Nodules were most reported in the lips, followed by NLFs and MLs for HAVLR, and in the lips and perioral areas for HAVOB.
Preserved Network Technology
Of the total events of interest, HARH2 had 3 (1.5%) reports of inflammatory nodules and 1 (0.5%) hypersensitivity reaction, and HARH4 had 4 (2.1%) reports of inflammatory nodules, 2 (1.0%) hypersensitivity reactions, and 1 (0.5%) granuloma (Figure 1). There were no events of interest reported for HARH3 during the MAUDE search period. Report dates ranged from 2018 to 2020. Although not explicitly stated in reports for the other HA fillers, it was specified in the ‟event text” field that all but one occurred outside of the United States.
Events had a time of onset after injection ranging from 1 to 8 months, with a median of 3.5 months. Nodules were reported in the lips, chin, and nose for HARH4 and in the lips, chin, NLFs, MLs, and glabella for HARH2.
Treatment
With limited information in each report, documented treatments for the AEs of interest were generalized. Noninflammatory nodules were treated with hyaluronidase, and inflammatory nodules were treated with some combination of hyaluronidase, antihistamines, corticosteroids, anti-inflammatories, and antibiotics. Hypersensitivity was treated with some combination of corticosteroids, antihistamines, and anti-inflammatories, whereas granulomas were treated with antibiotics and corticosteroids.
Discussion
The US HA filler market continues to expand with 9 new FDA approvals within the past 5 years. Each product uses various manufacturing technologies, providing them with unique characteristics, which maximize versatility and aesthetic outcomes. Clinical trials have established the safety and efficacy of the fillers in this review12–20; however, AEs occur in clinical practice for all medical devices, and thus, there is a need for postmarketing safety surveillance to establish a long-term safety profile.
Delayed-onset nodules and inflammatory events were the focus of this analysis due to the likelihood of these events being product related.2,3 These events can appear months to years after treatment, and because they are usually immune mediated (noninfectious), treatment is difficult and may even require product removal.3,4
The estimated incidence of delayed nodules and inflammatory reactions in the literature generally ranges from 0.02% to 4.0%.25–29 Delayed inflammatory reactions with HA fillers are likely due to a Type IV hypersensitivity reaction and are typically treated with antihistamines, corticosteroids, and anti-inflammatory drugs.3,4,28 Inflammatory nodules are frequently reported as granulomas because they are treated similarly; however, biopsies are rarely taken to confirm diagnosis.3,4 Consequently, the incidence of true granulomas is estimated to be less than nodules.3,4,29
In this PMS retrospective review, nodules were the most frequently reported delayed event, of which, most were inflammatory in nature. Hypersensitivity reactions and granulomas were the second and third most frequently reported delayed event, respectively. Most of these events had a time of onset of 2 to 4 months, and documented treatments were consistent with consensus guidelines.30
Interestingly, nodules were most reported in the lips, and the relative frequencies were consistent with previously published literature, suggesting that the lips appear to be more prone to developing nodules.26
Of the delayed events identified in the MAUDE database during the 5-year search period, there were more reports for HAVLR and HAVOB (Vycross) overall (12.3%–74.4%), and most were noninflammatory (3.1%–25.6%) and inflammatory (6.7%–28.7%) nodules. Consistent with these findings, other published literature suggests that Vycross technology has been associated with higher rates of delayed-onset nodules in clinical practice (0.5%–4.25%).25,31,32 There were fewer reports for other HA fillers overall (2.1%–5.1%) and for nodules (0.5% noninflammatory; 1.0%–2.1% inflammatory). No events were found for newer agents, HAVER (Thixofix), HAKYS (XpresHAn), and HARH3 (Preserved Network). A likely explanation is that these products were not available in the United States for as long as others (after December 2017), thus limiting the number of total AEs reported.
The number of events of interest for each HA filler is likely influenced by the frequency of use (information not available) and the length of time available. It is also important to note that these events have been identified solely from the event description field in the MAUDE reports, which is often limited in nature. For this reason, strict inclusion and exclusion criteria were developed to help characterize events in the reports to the best of the authors' ability.
Importantly, because the MAUDE database is a passive surveillance system and heavily reliant on voluntary reporting, the true number of events is likely significantly larger than reported.33 For instance, the American Society of Plastic Surgeons reported more than 8 million dermal filler procedures in the United States from 2016 to 2019.34 Based on this and the estimated rates of delayed-onset AEs in the literature, more events are expected in clinical practice than seen in these databases. Therefore, the number of reports cannot be used to determine national incidence, and no definitive conclusions regarding the relative frequency of AEs between specific products can be made.
Despite the limitations of the current methodology, the MAUDE database is one of the few large data sources available for postmarket surveillance of HA dermal fillers. This review is meant to provide visibility on the most frequent delayed-onset events linked to HA fillers reported in this database. Similar methodology has been used to provide information about the relative number of events reported each year and the types of events being reported more frequently.21–23
For example, a 10-year retrospective analysis (January 2007–July 2017) of the MAUDE database reports that the most common complication reported for aesthetic dermal filler use was nodule formation.21 These included both early-onset and delayed-onset nodules. Of the total MAUDE reports extracted for overall AEs (N = 5,204), 20.9% were for Juvederm Voluma XC (HAVOL), 2.9% HAVOB, 0.8% HAVLR, 0.06% HADEF, and 0.03% HAREF).
A 21-year retrospective MAUDE analysis (June1993–August 2014) found that the most frequently reported AEs associated with injectables were lumps (39.2%), infection (12.9%), and swelling (10.2%).22 Of the total MAUDE reports extracted (N = 3,782), the Juvederm brand had 517 reports (13.7%) and the Restylane brand had 236 reports (6.2%).
Finally, a 2-year retrospective MAUDE analysis (January 2014–December 2016) found that the most common complications associated with HA-based fillers were swelling, infection, nodules, and pain.23 Of the total MAUDE reports extracted (N = 1748); 839 (48.0%) for HAVOL, 633 (36.2%) for Juvederm, and 128 for Restylane (7.3%).
Postmarketing safety surveillance can provide information on real-world use of products in patient populations normally excluded or not studied during clinical trials, as well as providing insight into less common and/or rare AEs not observed during clinical trials.35 This allows for a better understanding of the events that are occurring. Comparisons of delayed nodules and inflammatory events reported for both older and newer HA fillers would be useful for clinicians and are of interest for future analyses.
Conclusion
Although delayed-onset nodules and inflammatory events may be relatively uncommon in clinical practice, this retrospective database review from 2016 to 2020 demonstrates that they do occur for HA fillers with XpresHAn, Vycross, and Preserved Network technologies, with the most common being nodules. This review benefits the practicing clinician by providing visibility on the most frequent delayed-onset events linked to HA fillers reported in the MAUDE database and how these events were treated in practice.
Supplementary Material
Acknowledgments
The authors thank MedSense Ltd for their assistance in the preparation of this manuscript and Marc Mikhael for assisting in data analyses.
Footnotes
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the full text and PDF versions of this article on the journal's Web site (www.dermatologicsurgery.org).
Supported by Galderma Laboratories, L.P., Fort Worth, TX.
J. L. Cohen has served as a consultant and clinical trial investigator for Galderma; Jessica Hicks, Alessandra Nogueira, and Bill Andriopoulos are employees of Galderma. The remaining authors have indicated no significant interest with commercial supporters.
Contributor Information
Jessica Hicks, Email: Jessica.HICKS@galderma.com.
Alessandra Nogueira, Email: alessandra.nogueira@galderma.com.
Vanessa Lane, Email: vanessa@medicalwritingltd.co.uk.
Bill Andriopoulos, Email: bill.andriopoulos@galderma.com.
References
- 1.Rohrich RJ, Bartlett EL, Dayan E. Practical approach and safety of hyaluronic acid fillers. Plast Reconstr Surg Glob Open 2019;7:e2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Glogau RG, Kane MA. Effect of injection techniques on the rate of local adverse events in patients implanted with nonanimal hyaluronic acid gel dermal fillers. Dermatol Surg 2008;34(Suppl 1):S105–9. [DOI] [PubMed] [Google Scholar]
- 3.Sclafani AP, Fagien S. Treatment of injectable soft tissue filler complications. Dermatol Surg 2009;35(Suppl 2):1672–80. [DOI] [PubMed] [Google Scholar]
- 4.DeLorenzi C. Complications of injectable fillers, Part I. Aesthet Surg J 2013;33:561–75. [DOI] [PubMed] [Google Scholar]
- 5.Sattler G, Philipp-Dormston WG, Van Den Elzen H, Van Der Walt C, et al. A prospective, open-label, observational, postmarket study evaluating VYC-17.5L for the correction of moderate to severe nasolabial folds over 12 months. Dermatol Surg 2017;43:238–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Micheels P, Sarazin D, Besse S, Elias B. Comparison of two swiss-designed hyaluronic acid gels: six‐month clinical follow‐up. J Drugs Dermatol 2017;16:154‐161. [PubMed] [Google Scholar]
- 7.Barr SK, Benchetrit A, Sapijaszko M, Andriessen A. Clinical evaluation of a cross-linked hyaluronic Acid dermal filler applied for facial augmentation. J Drugs Dermatol 2015;14:19–23. [PubMed] [Google Scholar]
- 8.Segura S, Anthonioz L, Fuchez F, Herbage B. A complete range of hyaluronic acid filler with distinctive physical properties specifically designed for optimal tissue adaptations. J Drugs Dermatol 2012;11(Suppl 1):S5–8. [PubMed] [Google Scholar]
- 9.Solish N, Bertucci V, Percec I, Wagner T, et al. Dynamics of hyaluronic acid fillers formulated to maintain natural facial expression. J Cosmet Dermatol 2019;18:738–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lundgren B, Sandkvist U, Bordier N, Gauthier B. Using a new photo scale to compare product integration of different hyaluronan-based fillers after injection in human ex vivo skin. J Drugs Dermatol 2018;17:982–6. [PubMed] [Google Scholar]
- 11.Philipp-Dormston WG, Schuster B, Podda M. Perceived naturalness of facial expression after hyaluronic acid filler injection in nasolabial folds and lower face. J Cosmet Dermatol 2020;19:1600–6. [DOI] [PubMed] [Google Scholar]
- 12.Philipp-Dormston WG, Hilton S, Nathan M. A prospective, open-label, multicenter, observational, postmarket study of the use of a 15 mg/mL hyaluronic acid dermal filler in the lips. J Cosmet Dermatol 2014;13:125–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Monheit G, Beer K, Hardas B, Grimes PE, et al. Safety and effectiveness of the hyaluronic acid dermal filler VYC-17.5L for nasolabial folds: results of a randomized, controlled study. Dermatol Surg 2018;44:670–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dayan S, Maas CS, Grimes PE, Beer K, et al. Safety and effectiveness of VYC-17.5L for long-term correction of nasolabial folds. Aesthet Surg J 2020;40:767–77. [DOI] [PubMed] [Google Scholar]
- 15.Gold MH, Baumann LS, Clark CP, III, Schlessinger J. A multicenter, double-blinded, randomized, split-face study of the safety and efficacy of a novel hyaluronic acid gel for the correction of nasolabial folds. J Drugs Dermatol 2018;17:66–73. [PubMed] [Google Scholar]
- 16.Fagien S, Monheit G, Jones D, Bank D, et al. Hyaluronic acid gel with (HARRL) and without lidocaine (HAJU) for the treatment of moderate‐to‐severe nasolabial folds: a randomized, evaluator‐blinded, phase III study. Dermatol Surg 2018;44:549–56. [DOI] [PubMed] [Google Scholar]
- 17.Baumann L, Weiss RA, Grekin S, Narins R, et al. Comparison of hyaluronic acid gel with (HARDL) and without lidocaine (HAJUP) in the treatment of moderate‐to‐severe nasolabial folds: a randomized, evaluator blinded study. Dermatol Surg 2018;44:833–40. [DOI] [PubMed] [Google Scholar]
- 18.Weiss R, Beer K, Cox SE, Palm M, et al. A randomized, controlled, evaluator-blinded, multi-center study of hyaluronic acid filler effectiveness and safety in lip fullness augmentation. Dermatol Surg 2021;47:527–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kaufman-Janette J, Taylor SC, Cox SE, Weinkle SH, et al. Efficacy and safety of a new resilient hyaluronic acid dermal filler, in the correction of moderate-to-severe nasolabial folds: a 64-week, prospective, multicenter, controlled, randomized, double-blind and within-subject study. J Cosmet Dermatol 2019;18:1244–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Monheit G, Kaufman-Janette J, Joseph JH, Shamban A, et al. Efficacy and safety of two resilient hyaluronic acid fillers in the treatment of moderate-to-severe nasolabial folds: a 64-week, prospective, multicenter, controlled, randomized, double-blinded, and within-subject study. Dermatol Surg 2020;46:1521–9. [DOI] [PubMed] [Google Scholar]
- 21.Povolotskiy R, Oleck NC, Hatzis CM, Paskhover B. Adverse events associated with aesthetic dermal fillers: a 10-year retrospective study of FDA data. Am J Cosmet Surg 2018;3:143–51. [Google Scholar]
- 22.Ortiz AE, Ahluwalia J, Song SS, Avram MM. Analysis of U.S. Food and Drug Administration data on soft-tissue filler complications. Dermatol Surg 2020;46:958–61. [DOI] [PubMed] [Google Scholar]
- 23.Rayess HM, Svider PF, Hanba C, Patel VS, et al. A cross-sectional analysis of adverse events and litigation for injectable fillers. JAMA Facial Plast Surg 2018;20:207–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.MAUDE: Manufacturer and User Facility Device Experience. Available from: accessdata.fda.gov/scripts/cdrh/cfdocs/cfMAUDE/search.CFM. Accessed March 1, 2021. [Google Scholar]
- 25.Artzi O, Loizides C, Verner I, Landau M. Resistant and recurrent late reaction to hyaluronic acid–based gel. Dermatol Surg 2016;42:31–7. [DOI] [PubMed] [Google Scholar]
- 26.King M, Bassett S, Davies E, King S. Management of delayed onset nodules. J Clin Aesthet Dermatol 2016;9:E1–5. [PMC free article] [PubMed] [Google Scholar]
- 27.Chung KL, Convery C, Ejikeme I, Ghanem AM. A systematic review of the literature of delayed inflammatory reactions after hyaluronic acid filler injection to estimate the incidence of delayed type hypersensitivity reaction. Aesthet Surg J 2020;40:NP286–300. [DOI] [PubMed] [Google Scholar]
- 28.Bhojani-Lynch T. Late-onset inflammatory response to hyaluronic acid dermal fillers. Plast Reconstr Surg Glob Open 2017;5:e1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee JM, Kim YJ. Foreign body granulomas after the use of dermal fillers: pathophysiology, clinical appearance, histologic features, and treatment. Arch Plast Surg 2015;42:232–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Urdiales-Gálvez F, Delgado NE, Figueiredo V, Lajo-Plaza JV, et al. Treatment of soft tissue filler complications: expert consensus recommendations. Aesth Plast Surg 2018;42:498–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sadeghpour M, Quatrano NA, Bonati LM, Arndt KA, et al. Delayed-onset nodules to differentially crosslinked hyaluronic acids: comparative incidence and risk assessment. Dermatol Surg 2019;45:1085–94. [DOI] [PubMed] [Google Scholar]
- 32.Beleznay K, Carruthers JD, Carruthers A, Mummert ME, et al. Delayed-onset nodules secondary to a smooth cohesive 20 mg/mL hyaluronic acid filler: cause and management. Dermatol Surg 2015;41:929–39. [DOI] [PubMed] [Google Scholar]
- 33.Sawaya J, Champlain A, Cohen J, Avram M. Barriers to reporting: limitations of the maude database. Dermatol Surg 2021;47:424–5. [DOI] [PubMed] [Google Scholar]
- 34.American Society of Plastic Surgeons (ASPS). Plastic Surgery Statistics Report 2019. Available from: plasticsurgery.org/documents/News/Statistics/2019/plastic-surgery-statistics-full-report-2019.pdf. Accessed March 1, 2021. [Google Scholar]
- 35.Kennedy DGS, Lillie R. Spontaneous reporting in the United States. In: Strom BL, editor. Pharmacoepidemiology (3rd ed). Chichester, United Kingdom: Wiley; 2000; pp. 151–74. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


