Reconstruction of scalp defects is challenging. The use of Leukocyte- and platelet-rich fibrin (L-PRF) in closing scalp defects has not yet been described in the literature. The use of L-PRF is well known in dentoalveolar surgery: L-PRF enhances periodontal healing and may contribute to bony regeneration in maxillary sinus floor augmentation and dental implant osseointegration.1 Leukocyte-rich and platelet-rich fibrin has also been shown to induce blood vessel formation in vivo.2 Several medical disciplines have seen the benefits of L-PRF for wound healing; it is being used for dura closure in neurosurgery3 and in the treatment of refractory leg ulcers.4
Materials and Methods
This study was evaluated and approved by the ethics committee (OG 205). All procedures were in accordance with the 1975 Declaration of Helsinki. Patients who underwent excision of a skin malignancy or nonhealing wound on the scalp and subsequent reconstruction with L-PRF at our department between January 2014 and December 2020 were included.
Operative Technique
All patients were treated under combined local anesthesia and intravenous sedation. Preoperatively, peripheral blood samples were obtained from a peripheral vein into a 9 mL IntraSpin blood tube (Intra-lock, Birmingham,). These samples were immediately placed in an IntraSpin centrifuge (Intra-lock) at 2,700 rotations per minute (rpm) for 12 minutes. The L-PRF clot was then separated from the red blood cells and placed in an Xpression L-PRF-box (Intra-lock). Minimal compression was applied on each L-PRF clot to transform it into an L-PRF membrane. Surgical excision was performed as required in each patient. In some cases, the underlying periosteum was removed (Figure 1). In cases where invasive growth of the malignancy was noted, the underlying external table of the calvarium was removed using a small 3-mm round carbide burr water-cooled at 40,000 rpm until a viable surface was obtained. Leukocyte- and platelet-rich fibrin was applied to the wound bed (Figure 2), after which a Mepitel silicone sheet (Mölnycke, Berchem, Belgium) and Melolin nonabsorbing bandage (Smith & Nephew, Watford, United Kingdom) were applied. An absorbent bandage was placed as the final layer. Patients were discharged after an observation period of 2 hours. Antibiotics (i.e., amoxicillin–clavulanic acid) were prescribed for 1 week, and the bandages were left in situ for 7 days.
Figure 1.
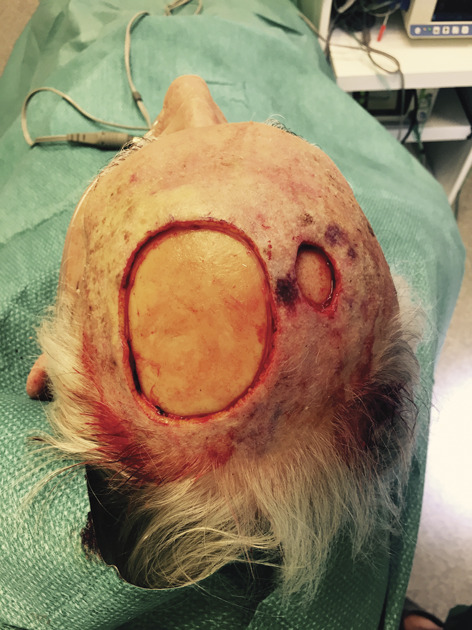
Scalp defect. The photograph shows 2 scalp defects, and periosteum was removed.
Figure 2.
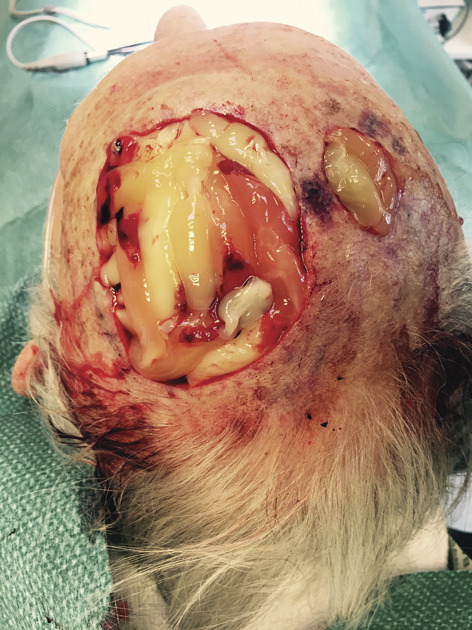
L-PRF membranes. The photograph shows the L-PRF membranes on both defects. L-PRF, leukocyte-rich and platelet-rich fibrin.
Follow-up
Follow-ups were conducted on the seventh postoperative day. At every follow-up, the wound was gently cleaned and evaluated. New L-PRF membranes were only applied to the regions without granulation. A new bandage was applied, and a follow-up was conducted on the seventh postoperative day. Leukocyte-rich and platelet-rich fibrin membranes were not applied in cases where excessive wound exudation was observed; in these cases, 10% povidone–iodine gel (Isobetadine) was applied and repeated by home nursing every 2 days. A follow-up was conducted after 7 days. As soon as the wound was fully covered with granulation tissue, no additional L-PRF applications were performed (Figure 3). Wound care was performed by home nursing with 10% povidone–iodine gel (Isobetadine) every 2 days. A follow-up was conducted after 14 days. Follow-ups continued until the wound was fully epithelialized (Figure 4).
Figure 3.

Full granulation. The photograph shows full granulation of both defects.
Figure 4.
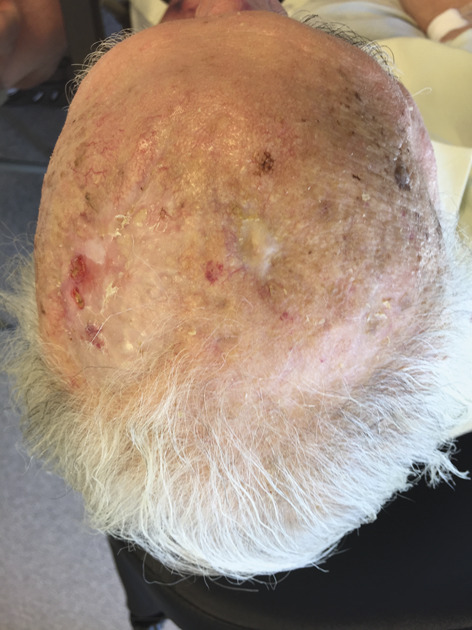
Full epithelialization.
Results
Twenty-one patients were included in this study: 17 patients presented with a skin malignancy on the scalp and 4 were referred for a nonhealing scalp wound. The average age at the time of excision was 79 years (range, 46–95 years). One patient presented with 2 separate malignancies of the scalp. Overall, 22 scalp defects were reconstructed. The average size of the defect was 13 cm2 (range, 0.65–41.25 cm2). The underlying periosteum was removed in 16 cases (73%). In 2 cases in which the underlying periosteum was removed, a rotated temporal fascia was used to cover the calvarium before applying L-PRF. The external table of the calvarium was partially removed in 6 cases (27%). In 8 cases, the entire underlying periosteum was removed without using a flap coverage or removal of the external table of the calvarium. An average of 3 L-PRF applications (range, 1–9) were necessary before full-wound granulation was obtained. The scalp defect was fully granulated at an average time of 7.4 weeks (range, 1–37 weeks). A full epithelization was achieved at an average time of 14.6 weeks (range, 3–44 weeks). In 20 cases (91%), full epithelization of the defect was obtained; in 2 cases (9%), the patient passed away before full epithelization of the wound could be obtained.
Conclusion
Our study showed that the use of L-PRF in the reconstruction of scalp defects seem to be an effective method for scalp coverage in patients with small-to-large defects, even when the L-PRF is placed on the external table of calvarium without vascularized base. L-PRF is superior to other reconstruction techniques because it can be performed in the outpatient setting, in a single procedure together with resection and is overall available and inexpensive. The major disadvantages include frequent and more follow-ups for larger defects and poor aesthetic results in hairy scalps.
Footnotes
The authors have indicated no significant interest with commercial supporters.
Contributor Information
Tim Van Cleemput, Email: vancleemput@hotmail.be.
Sylvie Hendrikx, Email: sylvie.hendrikx@noorderhart.be.
Constantinus Politis, Email: stan@politis.be.
Yannick Spaey, Email: yannick.spaey@noorderhart.be.
References
- 1.Castro AB, Meschi N, Temmerman A, Pinto N, et al. Regenerative potential of leucocyte- and platelet-rich fibrin. Part B: sinus floor elevation, alveolar ridge preservation and implant therapy. A systematic review. J Clin Periodontol 2017;44:225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ratajczak J, Vangansewinkel T, Gervois P, Merckx G, et al. Angiogenic properties of ‘leukocyte- and platelet-rich fibrin. Sci Rep 2018;8:14632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Theys T, Hoylandt AV, Broeckx CE, Gerven LV, et al. Plasma-rich fibrin in neurosurgery: a feasibility study. Acta Neurochir (Wien) 2018;160:1497. [DOI] [PubMed] [Google Scholar]
- 4.Pinto NR, Ubilla M, Zamora Y, Rio VD, et al. Leucocyte- and platelet-rich fibrin (L-PRF) as a regenerative medicine strategy for the treatment of refractory leg ulcers: a prospective cohort study. Platelets 2018;29:468. [DOI] [PubMed] [Google Scholar]


