Abstract
Background
The aim of this study was to examine the associations among depression, anxiety and health-related quality of life and predictors of improvement of quality of life in patients with inflammatory bowel disease.
Methods
This was a prospective cohort study conducted in the gastroenterology clinic at McMaster University Medical Center in Hamilton, Ontario, Canada from May 2014 to March 2015. We included 60 adult patients above the age of 18 years old with a diagnosis of inflammatory bowel disease. We assessed anxiety and depression using the Hospital Anxiety and Depression Scale (HADS) and Health Related Quality of Life (HRQoL) using the Short Inflammatory Bowel Disease questionnaire (SIBDQ) at baseline and after 6 months. Linear regression was performed to estimate the associations among depression, anxiety and predictors of improvement in health-related quality of life.
Results
The anxiety scores decreased over the span of 6 months (median HADS-A baseline 9.00 [interquartile range {IQR} 6 to 12], and median HADS-A 6 months 7.00 [IQR 3.75 to 7.00]). There was a moderate negative correlation between anxiety (baseline r = −0.510, and 6-month r = −0.620; P < 0.001), depression (baseline r = −0.630, and 6-month r = −0.670; P < 0.001) and HRQoL scores. Using a multivariate linear regression model, elevated HADS score were associated with lower SIBDQ scores at baseline (Beta coefficient −0.696 [95% confidence interval {CI} −1.51 to −0.842]; P < 0.001). Lower SIBDQ score at baseline predicted decreased SIBDQ at 6 months (Beta coefficient 0.712 [95% CI 0.486 to 1.02]; P < 0.001).
Conclusion
Anxiety and depression are frequently seen in inflammatory bowel disease patients and lead to poor HRQoL. Psychological comorbidities may contribute to maladaptive behaviours and difficult disease management.
Keywords: Anxiety disorders, Depressive disorders, Inflammatory bowel disease, Quality of life
Introduction
Inflammatory bowel disease (IBD) comprises a group of chronic autoimmune conditions affecting the gastrointestinal tract and is largely composed of ulcerative colitis (UC) and Crohn’s disease (CD). While there is a bimodal incidence pattern corresponding to early adulthood (25 to 34 years) and late adulthood (55 to 64 years), these diseases are more common in the young population (1). The chronic nature of IBD and its prevalence in early adulthood impose significant challenges associated with daily living (2–4). Psychological and physical health of affected individuals is often negatively affected by disease symptomology (2–4). Patients with IBD are burdened by challenges in finding employment with flexible hours due to the unpredictable nature of the disease, obtaining medical coverage, building healthy interpersonal relationships and striving for a successful future, all of which contribute to significant psychological distress (5).
Psychological distress has been associated with poor medication adherence leading to poor disease control (5). The bidirectional communication between the central nervous system and the enteric nervous system, known as the gut-brain axis (GBA), accounts for the reciprocal effects of emotion and mood on gastrointestinal function (6). While psychological factors are known to influence the functional state of an individual and reduce health-related quality of life (HRQoL) (3,7), the relationship between IBD, psychological distress and HRQoL is poorly understood (4). The purpose of this study was to examine the associations between depression, anxiety, HRQoL and predictors of HRQoL in IBD patients.
METHODS
This prospective study was conducted from May 2014 to March 2015 in the Digestive Diseases Clinic, McMaster University Medical Centre (MUMC), Hamilton, Ontario, Canada. This study was approved by Hamilton Integrated Research Ethics Board (HiREB Project Number 14–082).
Patients were asked to complete standardized questionnaires at the time of enrolment and at 6 months. The questionnaires were administered using LimeSurvey (LimeSurvey GmbH, Hamburg, Germany) software during the clinic visit (8). We collected information on demographics, diagnosis, disease duration, disease activity and medical therapy. C-reactive protein (CRP) levels were evaluated along with disease-specific activity indices to assess disease activity in CD and UC patients.
Evaluation of Anxiety and Depression
The Hospital Anxiety and Depression Scale (HADS) (3) was used to determine depression and anxiety at baseline and at 6 months (Supplementary Appendix A). The HADS is a 14-item questionnaire that measures degree of anxiety (7-item sub-scale) and depression (7-item sub-scale) in an outpatient setting. A total score of 8 or greater in each sub-scale HADS Anxiety (HADS-A) or HADS Depression (HADS-D) is consistent, respectively, with clinical anxiety or depression (3). Other psychological questionnaires have items such as weight loss and anorexia, which could be a consequence of IBD rather than anxiety or depression.
Evaluation of Quality of Life
General quality of life was measured using the Short-Form 12 (SF12), a 12-item survey with a physical component score (PCS) and a mental component score (MCS). The test-retest reliability scores are 0.89 (PCS) and 0.76 (MSC). A score of <50 is considered a low quality of life (9).
Disease-specific quality of life was measured using the Inflammatory Bowel Disease Questionnaire (IBDQ) (10). This questionnaire contains 32 items across four major domains, including bowel symptoms, systemic symptoms, social function and emotional scores. The Short Inflammatory Bowel Disease Questionnaire (SIBDQ) is the shortened version of this questionnaire for convenient clinical use, consisting of 10 items (Supplementary Appendix B). This questionnaire was developed and validated for both CD and UC, with high test–retest reliability. Each item is scored on a 7-point scale, from 1 (a severe problem) to 7 (not a problem at all), giving an absolute SIBDQ score range from 10 (poor HRQoL) to 70 (optimal HRQoL) (11).
Evaluation of Disease Activity
Disease activity was assessed using the validated Harvey Bradshaw Index (HBI) for CD, and Partial Mayo Score (PMS) for UC. Disease-specific serological biomarkers included complete blood count and CRP level (12).
Statistical Analysis
Statistical analysis was performed using IBM SPSS (Version 23) statistical software (13). Descriptive statistics were used to analyze demographic and clinical data. Continuous data were reported as median with interquartile range (IQR) or mean with standard deviation, and categorical data as proportions. Independent student T tests were used to compare demographics at baseline and 6 months. A P value of <0.05 was considered to be statistically significant. Spearman rho’s correlation coefficient was used to assess correlation between HRQoL and HADS-A and HADS-D scores. Fisher’s exact test or the Chi2 test was used to explore univariate associations between high and low SIBDQ scores, and HADS-A and HADS-D scores along with age, disease activity and CRP. Multivariate linear regression was performed to determine whether the presence of anxiety or depression (HADS-A or HADS-D) predicted decreased HRQoL (decrease in SIBDQ scores) at baseline and after 6 months. Total SIBDQ scores were used in this.
The SIBDQ scores were dichotomized into low and high SIBDQ score results based on 50% percentile. The HADS-A and HADS-D scores were evaluated in people with high versus low SIBDQ scores at baseline. We developed a model to determine whether anxiety and depression scores predict quality of life in IBD through the SIBDQ score using a multivariate linear regression or logistic regression model depending on whether the dependent variable was continuous or dichotomous. To assess whether higher HADS scores and lower disease activity predict better quality of life (SIBDQ >50%), the dependent variable was SIBDQ, and independent variables were HADS-A, HADS-D and disease activity (HBI and Mayo scores, CRP).
RESULTS
Sixty patients with CD and UC were enrolled in the study (median age 34 years [IQR 28–34], 38% males). Fifty-one (85%) patients were diagnosed with CD, with duration of disease of 11 to 15 years (Table 1). The majority of IBD patients had moderate to severe disease which was assessed using HBI and Mayo Score, and 90% of the patients were on biologic therapy. The mean CRP was 6.40 (±10.26) at baseline, with no significant change after 6 months 9.2 (±19.88; P > 0.05; Table 1).
Table 1.
Participant demographics
| Gender | |
|---|---|
| Male: n (%) | 23 (38.3%) |
| Female: n (%) | 37 (61.7%) |
| Age: mean years (SD) | 40 (16) |
| Disease: n (%) | |
| Ulcerative colitis | 7 (11.7%) |
| Crohn’s disease | 53 (88.3%) |
| Concomitant medications: | |
| α-TNF | 53 (88%) |
| No biologic therapy | 6 (4%) |
| C-reactive protein (SD) | |
| Baseline (n = 59) | 6.4 (10.26) |
| 6 months (n = 58) | 9.2 (19.88) |
Association Between Anxiety, Depression, and HRQoL at Baseline
At baseline, anxiety was present in 31 patients and depression was present in six patients. There was a moderate negative correlation between anxiety (r = −0.510; P < 0.001), depression (r = −0.630; P < 0.001) and HRQoL scores at baseline (measured by SIBDQ).
A multivariate linear regression model was performed. The increased HADS scores were associated with lower SIBDQ scores at baseline (Beta coefficient −0.696 [95% CI −1.51 to −0.842]; P < 0.001). The effect was similar when individual anxiety and depression scores were assessed, or even when anxiety and depression variables were dichotomized based on a cut-off of 8 (Table 2). Gender did not influence the association between HADS-A and HRQoL (measured by SIBDQ).
Table 2.
Linear regression model HADS score components versus SIBDQ
| Baseline SIBDQ | Standardized coefficients Βeta | 95% CI | P value |
|---|---|---|---|
| HADS Total baseline | −0.696 | −1.51 to −0.842 | <0.001 |
| HADS Anxiety baseline | −0.494 | −2.03 to −0.741 | <0.001 |
| HADS Depression baseline | −0.711 | −2.79 to −1.577 | <0.001 |
| HADS Depression dichotomized baseline | −0.615 | −22.2 to −9.67 | <0.001 |
| HADS Anxiety dichotomized baseline | −0.471 | −13.2 to −4.55 | <0.001 |
| 6 months SIBDQ | |||
| SIBDQ baseline | 0.712 | 0.486 to 1.02 | <0.001 |
| HADS Total baseline | −0.494 | −1.27 to −0.454 | <0.001 |
| HADS Anxiety baseline | −0.370 | −1.78 to −0.361 | 0.00400 |
| HADS Depression baseline | −0.492 | −2.35 to −0.822 | <0.001 |
| SF12 baseline | 0.322 | 0.254 to 2.25 | 0.0150 |
| HADS Depression dichotomized baseline | −0.545 | −22.2 to −8.11 | <0.001 |
HADS, Hospital Anxiety and Depression Scale; SF-12, Short-Form Survey 12 Items; SIBDQ, Short Inflammatory Bowel Disease Questionnaire.
A logistic regression model was performed to assess the association between HADS and disease activity on dichotomized SIBDQ. The SIBDQ scores were dichotomized into low and high SIBDQ score results based on 50% percentile. This SIBDQ cut-off was approximately 52. The strongest predictor for low SIBDQ was HADS-D score, which was independent of disease severity (odds ratio [OR] 0.568 [95% CI 0.381 to 0.846]). Baseline HADS-D scores were associated with low SIBDQ (HADS-D 5.03 ± 3.24; P < 0.001) and this was independent of disease severity (Table 3).
Table 3.
Difference in baseline HADS anxiety, and depression scores between patients with low SIBDQ score versus high SIBDQ score at baseline
| SIBDQ Score Baseline | |||
|---|---|---|---|
| Baseline | Low score Mean (SD) (n = 34) |
High Score Mean (SD) (n = 26) |
P value |
| Age (in years) | 40.5 (17.3) | 38.1 (15.3) | 0.586 |
| HADS Anxiety Score | 9.44 (2.76) | 6.96 (3.55) | 0.00300 |
| HADS Depression Score | 5.03 (3.24) | 1.96 (1.56) | <0.001 |
| CRP | 5.94 (6.62) | 6.99 (13.7) | 0.701 |
| CD HBI | 24 2.54 (2.26) |
24 1.54 (1.64) |
0.0850 |
| UC PMS | 5 3.60 (1.82) |
1 0 |
0.145 |
CD, Crohn’s disease; CRP, C-reactive protein; HADS, Hospital Anxiety and Depression Scale; HBI, Harvey-Bradshaw Index; PMS, Partial Mayo Score; SIBDQ, Short Inflammatory Bowel Disease Questionnaire; UC, Ulcerative colitis.
Association Between Anxiety, Depression, and HRQoL at 6 Months
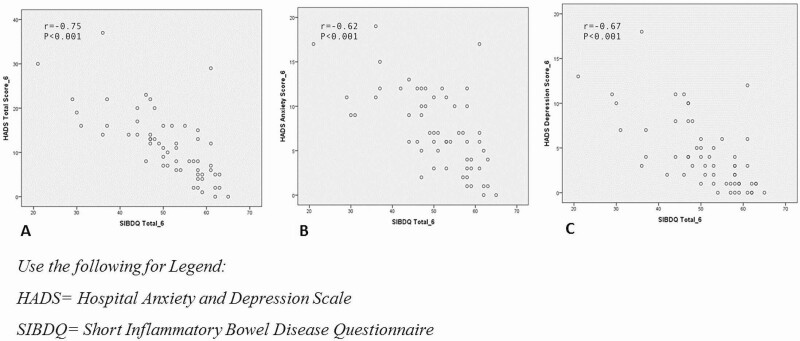
Anxiety was present in 24 patients and depression was present in 9 patients at 6 months. There were moderate negative correlations between both anxiety (r = −0.620; P < 0.001) and depression (r = −0.670; P < 0.001) scores and HRQoL scores at 6 months (measured by SIBDQ) (Figure 1).
Figure 1.
Correlation between anxiety, depression and HRQoL at 6 months.
A multivariate linear regression model was performed. After 6 months, lower SIBDQ score at baseline predicted decreased SIBDQ at 6 months (Beta coefficient 0.712 [95% CI 0.486 to 1.02]; P < 0.001). Increased anxiety and depression scores at baseline both predicted decreased HRQoL at 6 months (Table 2). There were decreases in HADS-A and HADS-D scores, as continuous and dichotomized variables predicted increase in SIBDQ at baseline. At 6 months, the SIBDQ was negatively correlated with HADS-total, HADS-A, HADS-D, as continuous and dichotomized variables at baseline. This effect was more evident in association with HADS-D score at baseline with low SIBDQ score at 6 months (Beta Coefficient −0.492 [95% CI −2.35 to −0.822]; P < 0.001). However, increased SF12 at baseline also predicted an increase in SIBDQ at 6 months (Table 2).
A logistic regression model was performed to assess the association between HADS and disease activity on dichotomized SIBDQ. The SIBDQ scores were dichotomized into low and high SIBDQ score results based on 50% percentile. This SIBDQ cut-off was approximately 52. The strongest predictor for low SIBDQ was HADS-D score and this was independent of disease severity (OR 0.702 [95% CI 0.527 to 0.934]). The 6-month HADS anxiety and depression scores were greater in patients with a low SIBDQ score at 6 months compared to patients with a high SIBDQ score at 6 months (low SIBDQ mean HADS-A 9.62 ± 3.91 versus high SIBDQ mean HADS-A 5.10 ± 3.99; low SIBDQ mean HADS-D 6.48 ± 4.01 versus high SIBDQ mean HADS-D 2.07 ± 2.60; P < 0.001; Table 4). Moreover, there was a negative moderate correlation between HADS total at 6 months and SIBDQ at 6 months (r = −0.750; P < 0.001; Figure 1).
Table 4.
Difference in HADS anxiety and HADS depression scores between patients with low SIBDQ score versus high SIBDQ score at 6 months
| SIBDQ Score 6 months | |||
|---|---|---|---|
| 6 months | Low score Mean (SD) (n = 29) |
High score Mean (SD) (n = 29) |
P value |
| Age (in years) | 41.0 (16.5) | 38.6 (16.7) | 0.576 |
| HADS Anxiety Score | 9.62 (3.91) | 5.10 (3.99) | <0.001 |
| HADS Depression Score | 6.48 (4.01) | 2.07 (2.60) | <0.001 |
| CRP | 6.29 (8.83) | 8.59 (14.8) | 0.480 |
| CD HBI | 22 4.77 (4.72) |
23 2.09 (2.86) |
0.0250 |
| UC PMS | 1 2.00 |
5 1.00 (1.73) |
0.626 |
CD, Crohn’s disease; CRP, C-reactive protein; HADS, Hospital Anxiety and Depression Scale; HBI, Harvey-Bradshaw Index; PMS, Partial Mayo Score; SIBDQ, Short Inflammatory Bowel Disease Questionnaire; UC, ulcerative colitis.
Discussion
This study aimed to assess whether anxiety and depression scores could predict quality of life in IBD patients through SIBDQ scores using a multivariate linear regression model. We demonstrated that a low SIBDQ score at baseline predicted decreased SIBDQ at 6 months, while increased HADS scores were associated with lower SIBDQ scores at baseline. We also showed that anxiety and depression are common among IBD patients and are associated with a poor HRQoL. In fact, the presence of anxiety and depression are more effective predictors of HRQoL than IBD activity. These findings are particularly intriguing as healthcare providers may be more inclined to focus on disease activity and physical manifestations of the disease and provide less dedicated attention to studying the psychological state of patients.
Patients with IBD are commonly diagnosed between the ages of 15 and 30 years, encompassing adolescence and early adulthood (1). This represents a dynamic period of time, where individuals typically focus on building relationships, establishing careers and striving toward personal and financial independence (14). Any disease that affects the mental state of individuals may also subsequently interfere with careers and relationships at a critical period in life which could have long-lasting consequences (15).
Bidirectional communication across the gut-brain axis has been well characterized for its role in contributing to numerous neuropsychiatric disorders and alterations in mood (16,17). Intestinal inflammation, as seen in poorly controlled IBD, impairs mucosal barrier function and increases systemic exposure to luminal antigens. This induces a systemic inflammatory response which impairs the blood–brain barrier function and contributes to neuroinflammation and increases anxiety and depression behaviours (16,17). Our findings provide clinical evidence to support the impact of disease activity on psychological well-being, as patients who presented with normal anxiety scores and active disease at baseline were more likely to develop abnormal anxiety scores at the 6-month follow-up. The reciprocal was also true where patients with quiescent disease at baseline but abnormal anxiety scores had higher rates of clinical exacerbation of their IBD. Furthermore, the presence of anxiety and depression predicted a decreased quality of life in our IBD patients.
Our study was not only able to support the correlation between mental health and HRQoL, but also showed that mental health is a predictor of quality of life. Brooks et al. (18) studied risk factors for psychological morbidity in young adults and demonstrated that depression and anxiety are more prevalent in IBD populations compared to the general population and that the severity of anxiety and depression is positively correlated with disease activity. Clinical depression and anxiety do not cause IBD, but there is evidence to indicate that they can lead to exacerbation of the disease (18). Graff et al. (19) suggested that depression and anxiety could be related to IBD treatments. Furthermore, depression may contribute to lower quality of life in depressed individuals compared to nondepressed individuals (19). Our results supported findings from other studies (20) which showed that depression may be have a stronger effect on HRQoL compared to disease activity alone using the Beck Depression Inventory-II as a predictor of SIBDQ scores.
Distressed psychological state and poor HRQoL may lead to poor adherence to medication, further contributing to poor disease control and relapse of disease activity (5). Our study found a correlation between anxiety scores in patients and HRoQL, while a previous quantitative review showed a correlation between depression and noncompliance to treatment (21). Jackson et al. (5) found a percentage of non-adherence in IBD patients due to psychosocial variables, including distress, quality of life, social support and personality. The psychological diagnosis in these patients was highly correlated with non-adherence to medication (5). Overall, psychological distress and quality of life exhibit the highest impact on non-adherence among the psychosocial general causes (16,22). It will be important for future studies to further explore the impact of disease activity with mood disorders in IBD patients on HRQoL.
The findings presented in this paper were limited by a small sample size, which resulted in relatively wide confidence intervals in assessing the association between anxiety, depression and disease-related, disease-specific quality of life. Moreover, 2 out of 10 questions in the SIBDQ questionnaire and 2 out of 8 questions in the HADS questionnaire could have had overlapping interpretations. Furthermore, at the time of the study, fecal calprotectin testing was not widely available, which limited disease activity assessment to CRP, a nonspecific biomarker of inflammation (23).
In conclusion, physicians need to look beyond the physical manifestations of disease activity and evaluate the psychological status of their patients in order to improve IBD care and overall patient quality of life. Future work should investigate the role of early psychological assessments and interventions in preventing the exacerbation of IBD disease activity and improving patient quality of life.
Funding
This work was supported by the Hamilton Health Sciences 2013 Clinical Health Professional Investigator Grant [Grant number HPI-12009]. M.I.P.S. received an Innovation grant from Crohn’s Colitis Canada. Authors had full access to all of the data (including statistical reports and tables) in the study.
Author Contributions
Y.F.: Substantial contributions to the conception and design of study; acquisition and analysis of data; drafting the work; final approval of the manuscript to be published; agreement to be accountable for all aspects of the work. J.P.: Substantial contributions to the conception and design of study; acquisition and analysis of data; drafting the work; final approval of the manuscript to be published; agreement to be accountable for all aspects of the work. D.A.: Substantial contributions to the conception and design of study; acquisition of data; review and final approval of the manuscript to be published; agreement to be accountable for all aspects of the work. S.H.: Substantial contributions to the conception and design of study; acquisition of data; review and final approval of the manuscript to be published; agreement to be accountable for all aspects of the work. J.M.: Substantial contributions to the conception and design of study; acquisition of data; review and final approval of the manuscript to be published; agreement to be accountable for all aspects of the work. F.T.: Substantial contributions to the conception and design of study; acquisition of data; final approval of the manuscript to be published; agreement to be accountable for all aspects of the work. I.P.: Substantial contributions to the conception and design of study; acquisition of data; review and final approval of the manuscript to be published; agreement to be accountable for all aspects of the work. P.M.: Substantial contributions to the conception and design of study; acquisition and analysis of data; final approval of the manuscript to be published; agreement to be accountable for all aspects of the work. U.C.: Substantial contributions to the conception and design of trial; acquisition and analysis of trial data; final approval of the manuscript to be published; agreement to be accountable for all aspects of the work.
Conflict of Interest
The authors wish to declare no conflicts of interest regarding the publication of this article.
Supplementary Material
References
- 1. Hou JK, Kramer JR, Richardson P, et al. The incidence and prevalence of inflammatory bowel disease among U.S. veterans: A national cohort study. Inflamm Bowel Dis 2013;19(5):1059–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Engelmann G, Erhard D, Petersen M, et al. Health-related quality of life in adolescents with inflammatory bowel disease depends on disease activity and psychiatric comorbidity. Child Psychiatry Hum Dev 2015;46(2):300–7. [DOI] [PubMed] [Google Scholar]
- 3. Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand 1983;67(6):361–70. [DOI] [PubMed] [Google Scholar]
- 4. Mikocka-walus A, Pittet V. Symptoms of depression and anxiety are independently associated with clinical recurrence of inflammatory bowel disease. Clin Gastroenterol Hepatol 2016;14(6):829–35. [DOI] [PubMed] [Google Scholar]
- 5. Jackson CA, Clatworthy J, Robinson A, et al. Factors associated with non-adherence to oral medication for inflammatory bowel disease: A systematic review. Am J Gastroenterol 2010;105(3):525–39. [DOI] [PubMed] [Google Scholar]
- 6. Carabotti M, Scirocco A, Maselli MA, et al. The gut-brain axis: Interactions between enteric microbiota, central and enteric nervous systems. Ann Gastroenterol 2015;28(2):203–9. [PMC free article] [PubMed] [Google Scholar]
- 7. Guthrie E, Jackson J, Shaffer J, et al. Psychological disorder and severity of inflammatory bowel disease predict health-related quality of life in ulcerative colitis and Crohn’s disease. Am J Gastroenterol 2002;97(8):1994–9. [DOI] [PubMed] [Google Scholar]
- 8. Limesurvey GmbH. LimeSurvey: An Open Source Survey Tool. Hamburg, Germany: LimeSurvey GmbH. http://www.limesurvey.org. [Google Scholar]
- 9. Hart DL. Test-retest reliability of an abbreviated self-report overall health status measure. J Orthop Sports Phys Ther 2003;33(12):734–44. [DOI] [PubMed] [Google Scholar]
- 10. Irvine EJ, Zhou Q, Thompson AK. The Short Inflammatory Bowel Disease Questionnaire: A quality of life instrument for community physicians managing inflammatory bowel disease. CCRPT Investigators. Canadian Crohn’s Relapse Prevention Trial. Am J Gastroenterol 1996;91(8):1571–8. [PubMed] [Google Scholar]
- 11. Ware JE, Kosinski M, Keller SD. A 12-item short-form health survey: Construction of scales and preliminary tests of reliability and validity. Med Care 2017;34:220–33. [DOI] [PubMed] [Google Scholar]
- 12. Lewis JD, Chuai S, Nessel L, et al. Use of the noninvasive components of the Mayo score to assess clinical response in ulcerative colitis. Inflamm Bowel Dis 2008;14(12):1660–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. IBM Corp. IBM SPSS Statistics for Windows, Version 23.0. Armonk, NY: IBM Corp, 2015. [Google Scholar]
- 14. Fuller-Thomson E, Lateef R, Sulman J. Robust association between inflammatory bowel disease and generalized anxiety disorder: Findings from a nationally representative Canadian study. Inflamm Bowel Dis 2015;21(10):2341–8. [DOI] [PubMed] [Google Scholar]
- 15. Farrokhyar F, Marshall JK, Easterbrook B, et al. Functional gastrointestinal disorders and mood disorders in patients with inactive inflammatory bowel disease: Prevalence and impact on health. Inflamm Bowel Dis 2006;12(1):38–46. [DOI] [PubMed] [Google Scholar]
- 16. Abautret-Daly Á, Dempsey E, Parra-Blanco A, et al. Gut-brain actions underlying comorbid anxiety and depression associated with inflammatory bowel disease. Acta Neuropsychiatr 2018;30(5):275–96. [DOI] [PubMed] [Google Scholar]
- 17. Gracie DJ, Guthrie EA, Hamlin PJ, et al. Bi-directionality of brain-gut interactions in patients with inflammatory bowel disease. Gastroenterology 2018;154(6):1635–1646.e3. [DOI] [PubMed] [Google Scholar]
- 18. Brooks AJ, Rowse G, Ryder A, et al. Systematic review: Psychological morbidity in young people with inflammatory bowel disease—risk factors and impacts. Aliment Pharmacol Ther 2016;44(1):3–15. [DOI] [PubMed] [Google Scholar]
- 19. Graff LA, Walker JR, Bernstein CN. Depression and anxiety in inflammatory bowel disease: A review of comorbidity and management. Inflamm Bowel Dis 2009;15(7):1105–18. [DOI] [PubMed] [Google Scholar]
- 20. Zhang CK, Hewett J, Hemming J, et al. The influence of depression on quality of life in patients with inflammatory bowel disease. Inflamm Bowel Dis 2013;19(8):1732–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. DiMatteo MR, Lepper HS, Croghan TW. Depression is a risk factor for noncompliance with medical treatment meta-analysis of the effects of anxiety and depression on patient adherence. Arch Intern Med 2000;160:2101–7. [DOI] [PubMed] [Google Scholar]
- 22. Peyrin-biroulet L. Adherence to anti-TNF therapy in inflammatory bowel diseases : A systematic review. Inflamm Bowel Dis 2013;19:1528–33. [DOI] [PubMed] [Google Scholar]
- 23. Kochhar G, Lashner B. Utility of biomarkers in the management of inflammatory bowel disease. Curr Treat Options Gastroenterol 2017;15(1):105–15. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.