Abstract
Background
Severe or fulminant Clostridioides difficile infection (SFCDI) is associated with significant morbidity and mortality. Emerging evidence suggests fecal microbiota transplant (FMT) may be a promising therapy for SFCDI.
Aim
This systematic review determines the safety and efficacy of FMT in medically refractory SFCDI.
Methods
A systematic search of the literature was conducted using PubMed (1965 to 2020), Web of Science (1900 to 20), EMBASE (1974 to 2020), and Cochrane Review (1945 to 2020). Quality appraisal by NIH Study Quality Assessment tools, and data extraction were performed by two teams of independent researchers. The primary outcome was resolution of SFCDI 4 weeks after the final FMT. Pooled resolution rates were calculated using generalized linear mixed models estimates.
Results
Two hundred and forty patients from 10 studies (8 case series, 1 case–control and 1 randomized study) were included with 209 individual patient-level data. FMT resulted in resolution of SFCDI within 4 weeks in 211/240 individuals for a pooled estimate of 88% (95% confidence interval [CI]: 0.83 to 0.91). The mean number of FMT required was 1.6 for severe and 2.0 for fulminant CDI resolution. The pooled proportional estimates for patients requiring CDI-directed antimicrobials after FMT was 50% (95% CI: 0.06 to 0.94) for severe CDI and 67.0% (95% CI: 0.30 to 0.91) for fulminant CDI. Serious adverse event rates were low.
Conclusion
FMT appears effective in treating SFCDI patients with low adverse events, but requires multiple treatments with a significant proportion of patients requiring additional anti-CDI antibiotics to achieve resolution. The optimal route of FMT delivery remains unknown. The presence of pseudomembranous colitis may guide additional FMT or anti-CDI antibiotic treatment.
Keywords: Clostridioides difficile, Fecal microbiota transplantation
Introduction
Clostridioides difficile infection (CDI) is the most common nosocomial infectious diarrhea (1), resulting in 29,300 deaths yearly in the United States (2). In Canada, surveillance of the 10 provinces showed an incidence rate of 535 per 100,000 hospital admissions in 2011 (3). Up to 8% of patients with CDI develop severe or fulminant infection (SFCDI) (4). The most recent Infectious Diseases Society of America (IDSA) (5) and Society for Healthcare Epidemiology of America (SHEA) (6) guidelines define severe CDI as leukocytosis with a white blood cell count of ≥15,000 cells/mL or a serum creatinine level >1.5 mg/dL (or 132 µmol/L). Fulminant CDI, replacing complicated CDI, is CDI contributing to hypotension or shock, ileus and megacolon (5,6). Complicated CDI was used to denote CDI associated with one of the following: ICU admission, hypotension, fever > 38.5°C, ileus or significant abdominal distention, mental status changes, white blood cell > 35,000 cells/mm3 or < 2000 cells/mm3, serum lactate > 2.2 mmol/L or end organ failure.
The IDSA CDI management guidelines recommend high-dose oral vancomycin 500 mg four times daily, metronidazole 500 mg intravenously every 8 hours and vancomycin 500 mg four times daily per rectum for SFCDI with early surgical consultation (5). Surgical management decreases mortality rates from 80% (6) to 41.3% in SFCDI (7), but it is associated with significant morbidity. Surgery is also contraindicated in patients with frailty or significant comorbidities. Therapeutic options for SFCDI patients not responding to CDI-directed antimicrobials are very limited. Thus, a noninvasive and yet effective therapy is urgently needed for these patients.
Fecal microbiota transplantation (FMT) has been shown to be the most effective treatment in patients with mild to moderate recurrent CDI (8). FMT involves transferring stool from healthy donors to the recipients by various means including colonoscopy, gastroscopy, enema, NG tube or oral capsules (8), with similar efficacy ranging from 87% to 89% (8–10). Furthermore, FMT seems to be safe in immunocompromised individuals (11). Although there are studies evaluating the efficacy of FMT in SFCDI, first described in 2013 (12), the evidence is not as robust. Fischer and colleagues described a protocol consisting of sequential FMTs via colonoscopy with the need for repeat FMT and continued vancomycin guided by clinical response and pseudomembranes at each colonoscopy (13). This protocol resulted in success rates of 100% (10/10) in severe CDI and 89% (17/19) in fulminant CDI in this small retrospective case series (13). Hocquart and colleagues found that early FMT conferred survival advantage in severe CDI (OR, 0.08 [95% CI, 0.016 to 0.34], P = 0.001), but not in nonsevere cases in a retrospective cohort study (14). Ianiro and colleagues demonstrated that a pseudomembrane-driven FMT protocol consisting of multiple FMTs and concomitant vancomycin was significantly more effective than a single FMT followed by vancomycin in treating SFCDI (100% versus 75%; P = 0.01) in a small randomized trial (15). A decline in CDI-related colectomy rates was reported after the introduction of an FMT program (16,17). However, prospective studies of FMT in the treatment of SFCDI continue to be lacking. Additionally, there is significant heterogeneity in how and when FMT is administered or the number of FMTs required. Furthermore, some studies reported continuing vancomycin after FMT while others did not. Therefore, the optimal FMT regimen for SFCDI remains undefined.
Previous systematic reviews involving the use of FMT focused on mild–moderate recurrent CDI; none specifically assessed safety or efficacy of FMT in SFCDI. Moreover, many published studies on FMT for CDI did not independently analyze severe/fulminant disease from mild CDI. The aim of this study is to conduct a systematic review and meta-analysis on existing literature regarding efficacy and other clinically important outcomes of FMT in the treatment of SFCDI.
METHODS
Search Strategy and Inclusion and Exclusion Criteria
This systematic review and meta-analysis was conducted according to the Preferred Reporting Items for Systematic reviews and Meta-analysis (PRISMA) (18). We searched PubMed (1965-Apr. 2020), Web of Science (1900-Apr. 2020), EMBASE (1974-Apr. 2020) and Cochrane Review (1945-Apr. 2020) for English articles regarding outcomes for severe and/or fulminant CDI treated with FMT. Studies were included if the following inclusion criteria were met: (a) published human studies where subjects have symptomatic diarrhea due to CDI; (b) subjects with CDI having received FMT via any delivery method; (c) severe and/or fulminant CDI confirmed by laboratory parameters, radiologic evidence of toxic megacolon, and/or pseudomembranes, hypotension/shock; (d) studies with a minimum score of 4 on the NIH quality assessment. Studies were excluded if they were: (a) evaluating FMT for illnesses other than CDI; (b) conference abstracts without full publications; (c) studies which included mild, moderate, or recurrent CDI with SFCDI where outcomes could not be separated based on disease severity; (d) manuscripts not written in English; (e) review articles, commentaries and editorials lacking original data; (f) subject with follow-up <4 weeks after FMT; (g) subject did not receive either vancomycin or metronidazole prior to FMT; (h) studies published >20 years ago due to inadequate CDI disease severity classification; (i) case reports with NIH score <4 for case series, <7 for case control studies, <8 for randomized controlled studies, or those with a serious risk of bias.
Keywords used in the search included a combination of fecal microbiota transplant, fecal transplant, stool transplant, severe/fulminant C. difficile infection or colitis, pseudomembranous colitis or pseudomembranes (see Supplementary Table 1 for sample of full search strategy).
Study Selection
Electronic searches were supplemented by manual searches of references of review articles and included studies. Study references and citations were exported from databases and into Refworks (ProQuest, Ann Arbour, MI). Full text of each article was reviewed to ensure it contained information on the topic of selection. To identify other potentially eligible publications, the bibliographic section of all articles was also reviewed to screen for other relevant articles (C.Z.S.). After removing duplicates, the title and abstract of each study was screened by a single reviewer (Y.N.S.) to determine eligibility for inclusion. By using inclusion and exclusion criteria, full articles on potentially relevant studies were assessed by two teams of independent reviewers (Y.N.S./K.W./D.K. and D.Y./L.R./S.V.Z.). Data were extracted from each study with a standardized and pretested extraction form. Differences of opinion were resolved through consensus in the research team.
Endpoints and Subgroup Analysis
Our primary outcome of interest is resolution of SFCDI, defined as the absence of CDI 4 weeks after the final FMT treatment. The 4-week time point was chosen because most FMT treatment failure occurred prior to this (19). CDI recurrence typically occurs within 2 to 4 weeks after discontinuing CDI-directed therapy, although the timeline of recurrence in epidemiology studies is 8 weeks (19).
Secondary outcomes included (a) Resolution of severe CDI 4, 8 and 12 weeks after the final FMT treatment calculated at study level and cumulative individual patient level; (b) Resolution of fulminant CDI 4, 8 and 12 weeks after final FMT treatment calculated at study level and cumulative individual patient level; (c) Resolution of SFCDI with additional anti-CDI therapy after final FMT; (d) The number of FMT required to achieve resolution of severe and fulminant CDI, respectively; (e) Duration of anti-CDI antibiotics required post-FMT to achieve resolution of severe and fulminant CDI, respectively; (f) Length of hospital stay following final FMT; (g) Serious adverse events, including: Colectomy rate and CDI-related mortality rate upto 12 weeks after final FMT treatment and FMT-related infectious complications such as bloodstream infections; (h) Minor adverse events including constipation, diarrhea and abdominal pain.
Data Extraction
The data extraction form included name of first author, year of publication, country, study design (including randomized controlled trials, case control, case series), quality assessment, CDI disease severity, antibiotic trigger for CDI, patient baseline characteristics, FMT treatment protocol (including pre- and post-FMT antibiotic regimen), FMT administration, number of FMT required, CDI resolution rate, follow-up duration and adverse events. Authors were contacted where clarification of data was necessary. Fischer and colleagues (20), Ianiro and colleagues (15), and Kelly and colleagues (11) provided individual patient-level data on their studies where patients with mild to fulminant CDI were included.
Quality Assessment and Risk of Bias From Individual Studies
Two teams of investigators independently assessed all studies with ≥4 patients using the National Institute of Health Study Quality Assessment Tools (21) for case series and controlled intervention studies. The score range for case series is 0 to 9 and a score <4 was deemed as poor in quality.(21) The score range for controlled intervention studies is 0 to 14; a score <6 was deemed poor in quality and was excluded from this review. Any disagreements were resolved through consensus. In addition, the risk of bias was determined with the Risk of Bias In Non-randomized Studies of Interventions tool (22) as well as the Risk of Bias tool for randomized control trial (23). The scores ranged from low, moderate, serious, to critical on each domain and overall bias score. Studies with serious risk of bias were excluded.
Statistical Methods
Our primary outcome of interest is resolution of SFCDI at 4 weeks. The studies were meta-analyzed with a pooled estimate of the proportion resolved and its associated 95% confidence interval estimated using a random intercept logistic regression model for all included studies (24). Results were visually presented using forest plots. Heterogeneity across studies was assessed using the Cochran Q test and I2 statistic, where significant heterogeneity across all studies was determined at Q < 0.10 and I2 > 50%. Where possible, patient characteristics were pooled across studies and presented as weighted means, weighted based on the sample size of each study relative to the overall sample size of all included studies. All analyses were done with R 4.0.2 (R Core Team) (25). Survival rates following treatment for SFCDI at 1, 2 and 3 months along with FMT success rates at the same time frames were also calculated from individual patient-level data.
RESULTS
Study Selection and Characteristics
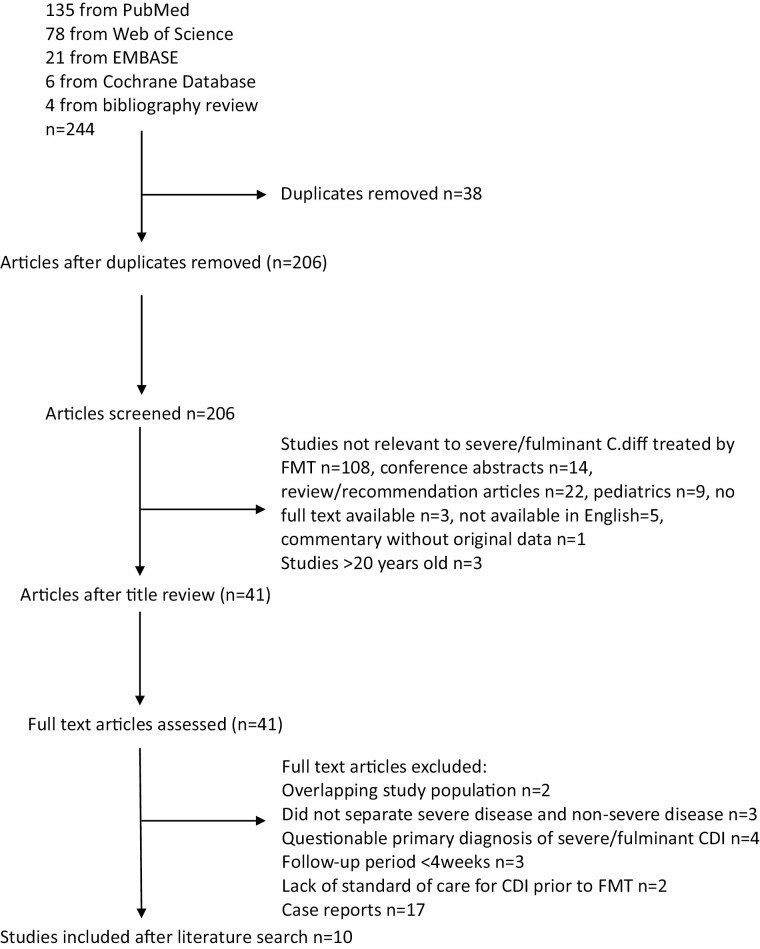
A total of 206 articles were identified of which 10 met our inclusion criteria (Figure 1). Of the included studies, one was a randomized trial (15), one was a case control study (26) and eight were case series (11,12,20,27–31). One study solely investigated severe CDI (29), one study specifically investigated fulminant CDI (27) and eight studies included both severe and fulminant CDI (11,12,15,20,26,27,30,31). Seven studies were conducted in the United States (11,12,20,26,27,29,30), two in Italy (15,31) and one (28) examined cases in both the United States and Australia. Study characteristics of all included studies are given in Table 1. The follow-up duration varied from 8 to 65.7 weeks, and the weighted mean was 27.6 weeks.
Figure 1.
Flowchart outlining the selection strategy during literature search.
Table 1.
Study characteristics
| Type of study | Study | Sample size | Patient age (mean) | CDI severity | F/U duration (weeks) | FMT administration | Amount of fecal material delivered per FMT | Average # of FMT needed to treat | FMT regimen PMC driven (12) | Antibiotic usage* | # of patients achieving overall cure from FMT | Time to discharge after final FMT therapy (Weeks) | NIH Score for Case series or RCTs |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Case Series | Weingarden (11) | 4 | 72.75 | SFCDI | 27 | Lower endoscopy | N/A | 1.6 | No | Physician’s discretion | 2/4 | 3.5 | 7 |
| Gundacker (26) | 8 | 68 | SFCDI | 65.7 | Upper GI + lower endoscopy + enema | 30g | 1.5 | No | Physician’s discretion | 6/8 | unclear | 8 | |
| Alukal (27) | 9 | 67.78 | F | 16 | Upper GI + lower endoscopy + enema | 50g | 1 | No | Physician’s discretion | 8/9 | 20.78 | 8 | |
| Kelly (10) | 11 | 67 | SFCDI | 12 | Upper GI + lower endoscopy | N/A | 1.45 | No | Physician’s discretion | 10/11 | unclear | 8 | |
| Zainah (28) | 14 | 73.4 | S | 14.3 | Upper GI + lower endoscopy | 30-50g | 1.43 | No | Physician’s discretion | 11/14 | unclear | 6 | |
| Aroniadis (29) | 17 | 66 | SFCDI | 45.6 | Upper GI + lower endoscopy + enema | N/A | unclear | No | Physician’s discretion | 15/17 | unclear | 5 | |
| Ianiro (30) | 48 | 74.87 | SFCDI | 63.7 | Lower endoscopy | ≥50g | 2.08 | No | Physician’s discretion | 45/48 | 6.18 | 9 | |
| Fischer (19) | 57 | 72 | SFCDI | 12 | Lower endoscopy | 50-200g | 1.6 | Yes | Part of protocol | 52/57 | 11 | 9 | |
| Case control | Tixier (25) | 16 | 62.9 | SFCDI | 30 | Lower endoscopy | N/A | 1.4 | Yes | Part of protocol | 13/16 | unclear | 8 |
| Randomized Trial | Ianiro (14) | 56 | 74.5 | SFCDI | 8 | Lower endoscopy | ≥50g | 2.25 | Yes | Part of protocol | 49/56 | unclear | 10 |
*More details are given in Supplementary Table 5.
Routes of FMT Delivery and CDI-Directed Antibiotics During and/or After FMT Treatment Regimen
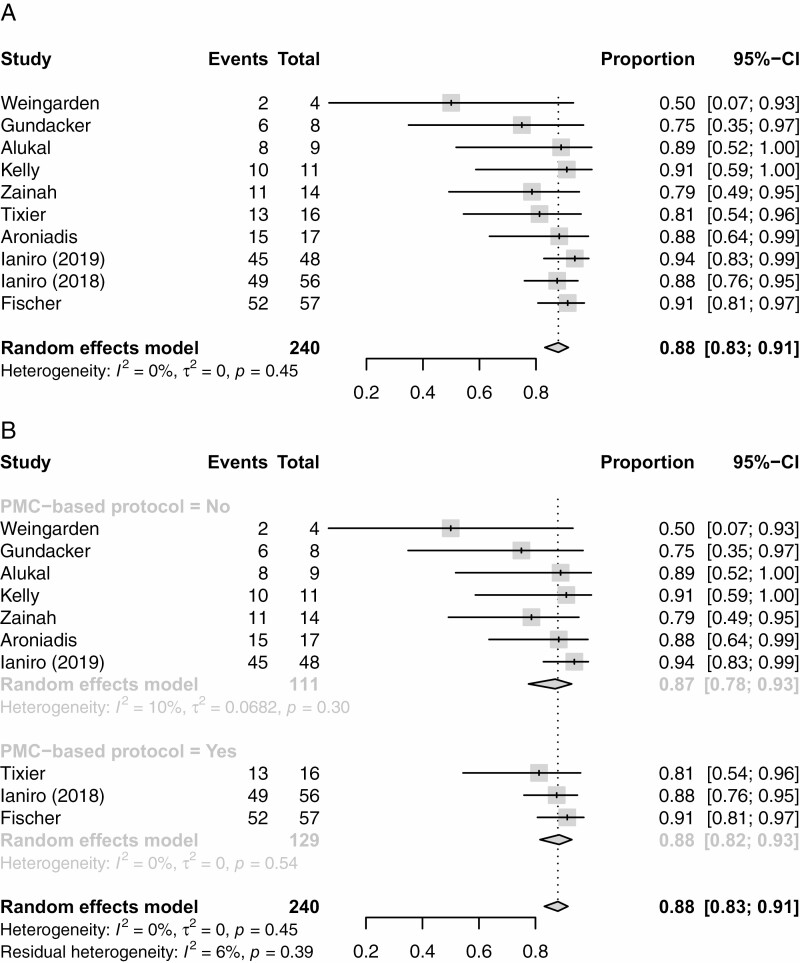
All studies used lower endoscopy to deliver FMT however, 5 of the 10 studies also included upper GI route and enema (11,27–30). Three studies (15,20,26) used a treatment protocol driven by the presence of pseudomembranous colitis (PMC) (13), where FMT continued until the disappearance of PMC. A total of 3 out of 10 studies had further anti-CDI treatment after FMT as part of their protocol (15,20,26), one was offered to every patient (15), and the other two were offered depending on the presence of PMC (20,26). A total of 7 out of 10 studies provided anti-CDI antibiotics post-FMT at the discretion of the clinician (11,12,27–31), 3 studies offered antibiotics for only persistent CDI symptoms (12,27,30), 2 studies had unclear indications for antibiotic usage (11,28) and patients from 2 studies did not receive any antibiotics following final FMT (29,31) due to study design.
Patient Characteristics
The total number of patients included was 240; 81/240 (33.8%) were male and mean age was 71.7 years (weighted). A total of 108/240 (45%) patients had severe and the remainder had fulminant CDI. Most of these included subjects were inpatients, except for two studies, (11,30), which included 79% and 18% outpatients, respectively, at the time of FMT delivery. A total of 8/10 studies included information on major comorbidities of patients, and the mean Charlson Comorbidity Index (CCI) in these patients was 5.2 (weighted). Eight studies provided individual patient-level data (11,12,15,20,27–29,31).
Quality Assessment of Included Studies
Quality assessment of included studies is available in Supplementary Table 2a and b. In addition, the risk of bias for most of the nonrandomized studies were low to moderate and the risk of bias of the randomized control trial had some concern with the reported randomization process (Supplementary Table 3a and b).
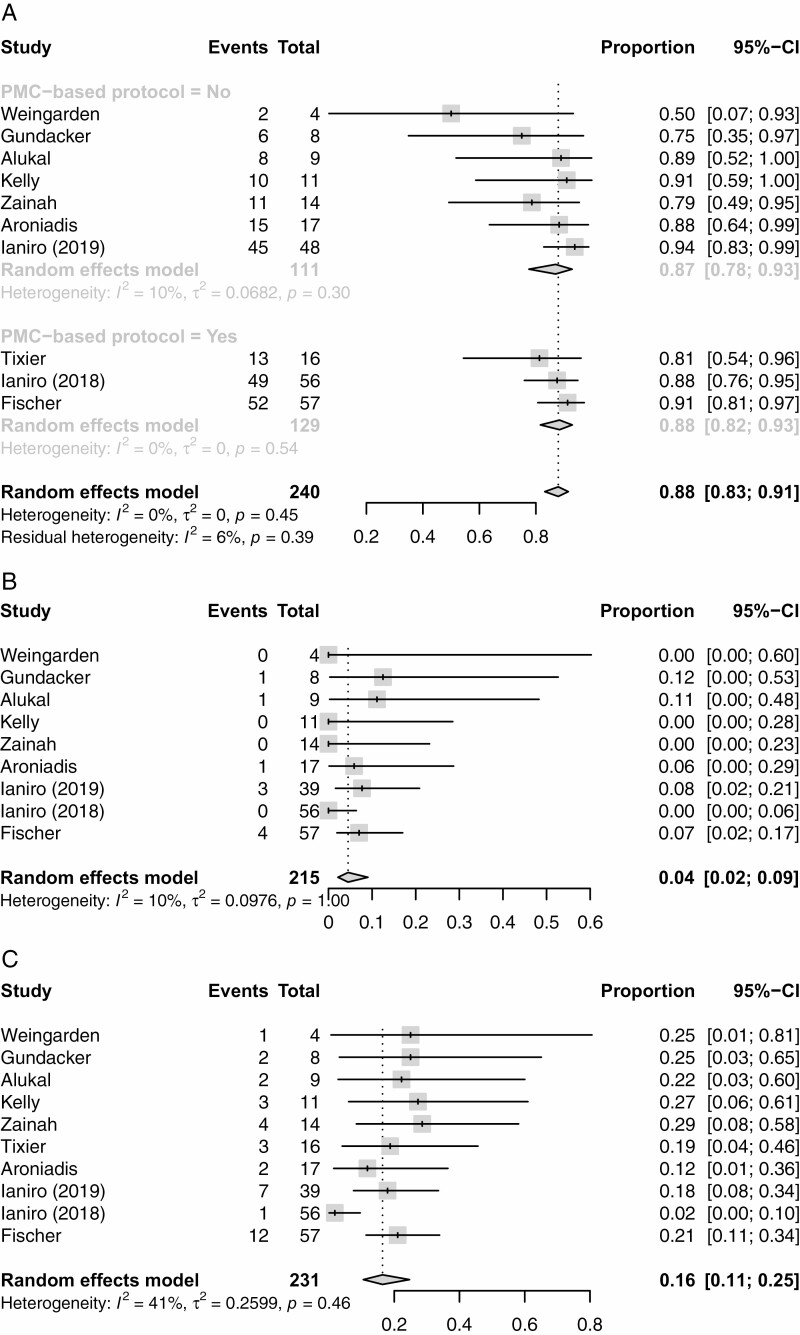
Resolution of SFCDI Following FMT
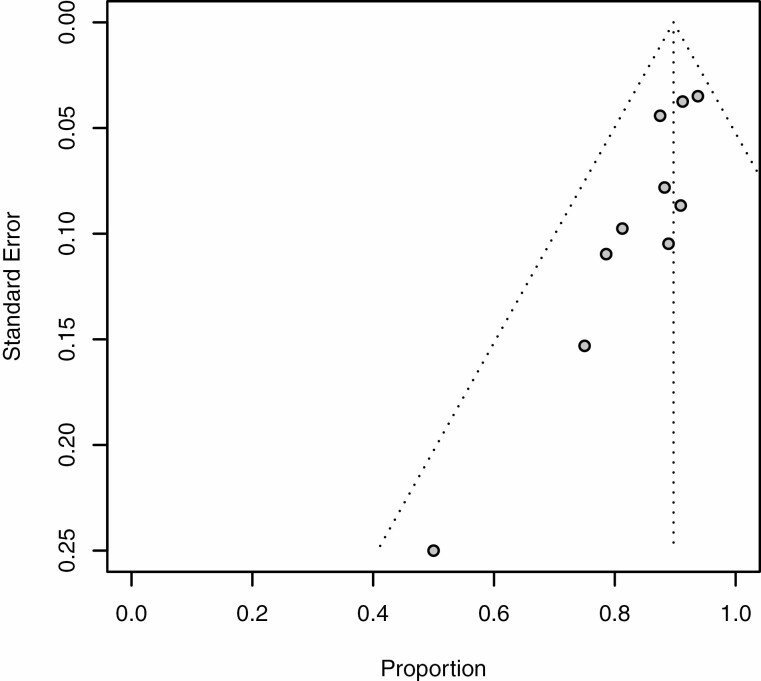
The primary outcome, resolution of SFCDI 4 weeks following FMT treatment, had a pooled estimate of 88% (95% CI 0.83 to 0.91%), and are shown as forest plots in Figure 2A and as a funnel plot in Figure 3.
Figure 2.
(A) Forest plot for the proportion with successful resolution of severe of fulminant CDI4 weeks after treatment. (B) FM treatment success rates 4 weeks after PMC bases protocol versus clinician judgement.
Figure 3.
Funnel plot of total SFCDI resolution rate within 4 weeks in all included studies.
Number of FMT Required to Treat SFCDI and Length of Stay Following Final FMT
The total number of FMT needed to achieve SFCDI resolution ranged from 1 to 6, and the mean number of FMT was 1.65 FMTs (weighted). The length of time to discharge after final FMT ranged from 3.5 to 21 days, and the mean was 9.53 days (weighted).
Number of FMT and Additional Anti-CDI Treatment Following FMT to Achieve Severe and Fulminant CDI Resolution, Respectively
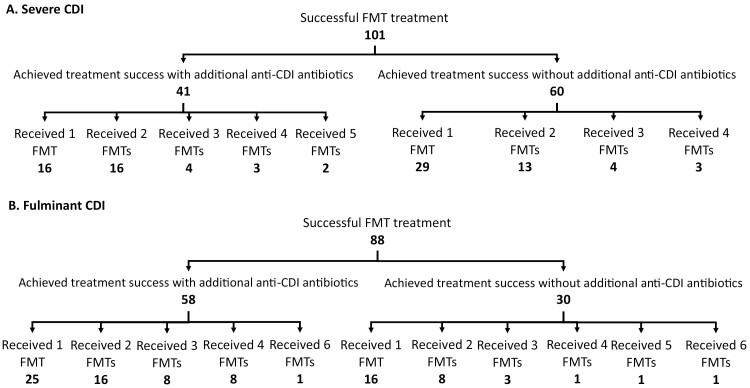
The mean number of FMT needed to achieve resolution of severe CDI was 1.6 and 50% (95% CI 6% to 94%) of patients did not receive additional anti-CDI treatments following the final FMT. The exact details of treatment subgroups can be visualized in Figure 4A. For patients who received additional CDI-directed treatments, the mean duration of antibiotics was 15.7 days. The mean number of FMT needed to achieve resolution of fulminant CDI was 2.0, and 33% (95% CI 9% to 70%) of patients did not receive additional anti-CDI treatments after FMT. The exact details of treatment subgroups can be visualized in Figure 4B. For patients who received additional antibiotics, the mean duration of course was 13.8 days.
Figure 4. (.
A) Flowchart outlining the number of severe CDI patients achieving treatment success with varying treatment protocols. (B) Flowchart outlining the number of fulminant CDI patients achieving treatment success with varying treatment protocols.
Total denominator used here is different from the denominator for individual resolution rates because some patients never achieved CDI resolution with FMT. Patients who did not achieve CDI resolution were excluded from this analysis since we could not ascertain the number of FMT needed to achieve CDI resolution.
Cumulative Survival Rates
For 8 of the 10 included studies with individual patient-level data (11,12,15,20,27–29,31), survival rates following SFCDI at 1, 2 and 3 months were calculated along with FMT success rates at these time frames. This data, along with colectomy rates, and CDI mortality rates are presented in Table 2. This table excludes data from studies that did not provide individual patient-level data. Since some patients were lost over the prespecified follow-up duration of 3 months, these patients were excluded from treatment outcomes and adverse event analyses.
Table 2.
CDI survival rate, FMT treatment success rate and adverse events of interest from studies that provided individual patient-level data
| Fulminant CDI | 95% CI | Severe CDI | 95% CI | |
|---|---|---|---|---|
| Survival rate at 1 month (#alive/#total number of patients) | 88.1% (89/101) | 80.4–93.1% | 94.3% (100/106) | 88.2–97.4% |
| Survival rate at 1 month (#alive/#total number of patients) | 88.0% (88/100) | 80.2–93.0% | 90.5% (95/105) | 83.4–94.7% |
| Survival rate at 1 month (#alive/#total number of patients) | 76.2% (48/63) | 64.4–85.0% | 74.0% (57/77) | 63.3–82.5% |
| FMT success rate at 1 month | 87.1% (88/101) | 79.2–92.3% | 95.3% (101/106) | 89.4–98.0% |
| FMT success rate at 2 months | 87.0% (87/100) | 79.0–92.2% | 92.4% (97/105) | 85.7–96.1% |
| FMT success rate at 3 months | 81.0% (51/63) | 69.6–88.8% | 80.5% (62/77) | 70.3–87.8% |
| Colectomy rate | 3.0% (3/101) | - | 0% (0/106) | - |
| Death due to CDI | 6.9% (7/101) | - | 1.9% (2/106) | - |
Adverse Events
Adverse events are given in Table 3. Serious adverse events included colectomy in 5/240 (2.1%), possibly FMT-related deaths in 2/215 (0.9%) and FMT-related infectious complications in 3/173 (1.7%) patients. FMT-related infectious complications include Klebsiella bacteremia post-FMT (26), ventilator acquired pneumonia 2 days post-FMT (28) and sepsis within 24 hours of FMT (20) (Supplementary Table 4a). The latter two complications resulted in mortality, contributing to FMT-related deaths (Supplementary Table 4b). CDI-related mortality rate from treatment failure is 9/215 (4.2%). Overall colectomy rate 3 months after FMT treatment is presented in Figure 5A. CDI attributed mortality rate 3 months after FMT treatment is presented in Figure 5B. All cause-mortality rates 3 months after FMT treatment are presented in Figure 5C. Minor adverse events included self-limited constipation [40/56 (71.4%)], diarrhea [42/72 (58.3%)] and abdominal pain [3/11 (27.3%)].
Table 3.
Adverse events, including studies without individual patient-level data
| Types of adverse events | # of subjects | |
|---|---|---|
| Mortality | All-cause mortality | 37/231 (13.0%) |
| CDI-related mortality | 9/215 (4.2%) | |
| Possibly related to FMT* | 2/215 (0.9%) | |
| FMT unrelated | 23/215 (10.7%) | |
| Post-hospital discharge | 18/166 (10.8%) | |
| Colectomy rate | 5/240 (2.1%) | |
| Abdominal pain | 3/11 (27.3%) | |
| Constipation | 40/56 (71.4%) | |
| Diarrhea | 42/72 (58.3%) | |
| Infectious complications* | FMT related | 3/173 (1.7%) |
| FMT unrelated | 2/157 (1.3%) |
*For breakdown of infectious complications and deaths possibly related to FMT, please refer to Supplementary Table 3a and b.
Figure 5.
Adverse events. (A) Total rates of colectomy 3 months after FMT treatment in all included studies. (B) Total rates of CDI-related death 3 months after FMT treatment in all included studies. (C) Rates of all-cause death in all included studies.
Discussion
This systematic review analyzed the existing data based on eight case series, one case–control study, and one randomized trial on safety and efficacy in achieving resolution of SFCDI 4 weeks post-FMT. There was significant heterogeneity in terms of included patient population and study methodology (e.g., FMT dose, frequency and administration route, duration of anti-CDI treatment prior to FMT, when and if CDI-directed antibiotics were added to FMT treatment regimens or follow-up duration). We have chosen our primary outcome as the resolution of SFCDI 4 weeks after final FMT since the majority of FMT failure occurs prior to that time point (19). Previous FMT studies for mild to moderate recurrent CDI typically used 8 weeks to determine treatment outcome, although recurrences usually occur within 2 to 4 weeks (8,19). SFCDI patients usually do not have CDI recurrence beyond 4 weeks after successful FMT treatment (19). Therefore, we felt that assessing treatment outcome at 4 weeks is a clinically important and relevant time point. Furthermore, we included 8 and 12 weeks as additional outcomes. We found that 88% of the included patients analyzed had resolution of CDI, a rate similar to mild to moderate recurrent CDI, although the majority of the included 240 patients required at least 2 FMT in quick succession, with most patients receiving treatment by lower endoscopy. The overall serious adverse events, colectomy, CDI mortality and all-cause mortality rates were low, highlighting the safety of FMT even in this group of patients. Some still require CDI-directed therapy after final FMT, suggesting that FMT alone is insufficient, although currently there are no markers to identify these patients.
What remains unknown in treating severe or fulminant CDI is the ideal route of delivery or timing to administer subsequent FMT (8). Fischer proposed a PMC driven protocol where sequential FMT was delivered by colonoscopy 5 to 7 days after the initial colonoscopy; if PMC is present, vancomycin is continued and a repeat FMT by colonoscopy is planned in 5 to 7 days (13). This continues until the resolution of PMC. In contrast, some of the included studies used clinical acumen, patient symptoms and laboratory markers to determine when to administer repeated FMT. This can lead to variable practice and difficulty in developing a treatment algorithm. One may argue that a PMC based protocol can lead to less variation in practice and improve treatment outcome and patient safety, since it takes ‘guessing’ out of the treatment plan due to leaving it to clinicians’ discretion. However, colonoscopy is not only invasive but also costly. It is not known if colonoscopy delivery is the only way to treat these patients.
Although FMT may be highly effective in this patient population, access to FMT may not be equal. A 2021 Environmental Scan survey (32) revealed that FMT is currently offered in seven Canadian jurisdictions (Alberta, British Columbia, Newfoundland and Labrador, Nova Scotia, Ontario, Prince Edward Island and Quebec). The more populated provinces of Alberta, British Columbia, Quebec and Ontario have the most established programs, and most facilities providing FMT are hospitals, specialty clinics (including dedicated FMT clinics), or regional health authorities based in large cities. This means that patients presenting at small centers will have difficulty accessing FMT treatment (32). Future efforts to establish provincial stool banks and distribution channels will alleviate this problem.
This study has several strengths. First, we critically appraised only studies defined as high in quality as per the NIH study quality assessment tool. Second, we were able to obtain individual patient data by contacting respective authors of included studies in order to differentiate treatment outcomes in severe from fulminant CDI, since most studies pooled these patients. There are several limitations of this analysis. The majority of the studies were case series without a control group and therefore prone to biases. Furthermore, there was significant heterogeneity of these case series in terms of how often and how many FMT treatments were given, when and whether CDI-directed antibiotic was given or stopped after FMT, when to discontinue FMT, or how long each patient was being followed. Although all patients had received FMT by lower endoscopy, some patients also received additional FMT by other routes, including gastroscopy and enema. The single randomized trial comparing single versus multiple FMT is at risk of bias since the study was not blinded (15). In some studies FMT was triggered if diarrhea did not improve or resolve by day 5 of CDI-directed antibiotics. One may argue that some of these patients may not require FMT if the antibiotics were given for a longer duration up to 10 days. On the other hand, early FMT may potentially be life saving if these patients were truly antibiotic refractory and delaying FMT may contribute to morbidity and mortality. Given the significant heterogeneity in methodology, the therapeutic benefits of FMT may potentially be over estimated, which is another limitation of this study. One may also argue that the combination of FMT with a narrow spectrum antibiotic such as fidaxomicin may be superior to FMT plus vancomycin, which was commonly used in these studies. It would be interesting to see if combining FMT with fidaxomicin may reduce the number of FMT needed to achieve SFCDI resolution. Additionally, the relatively small number of included studies, significant heterogeneity and small sample size preclude meaningful sub-group analysis. For instance, the use of antibiotics post-FMT varied widely between the studies with one study offering antibiotics to all subject (15), two studies selectively offering antibiotics as part of a PMC-driven protocol (20,26) and seven studies offering antibiotics at the discretion of the clinician (11,12,27–31). The sample size for each sub-group was not large enough for robust sub-group analysis. With the uniqueness of this population and the severity of their illness it would be difficult to conduct a large-scale randomized trial.
In approaching patients with medically refractory SFCDI our systematic review and meta-analysis suggests that FMT is a highly effective and safe therapy. Future studies are needed to determine the most efficacious approach to administer the FMT in this patient population.
Supplementary Material
ACKNOWLEDGEMENTS
We would like to acknowledge the University of Alberta Hospital Foundation for Grant support for this project.
AUTHOR CONTRIBUTIONS
Y.N.S.: Study design, planning, conducting, collection, and interpretation of data and drafting/editing manuscript. L.R.: Conducting, collection, and interpretation of data and drafting/editing manuscript, co-senior author. D.Y.Y.: Conducting, collection, and interpretation of data and drafting/editing manuscript. K.W.: Conducting, collection, and interpretation of data and drafting/editing manuscript. E.M.A.: Conducted statistical analysis and editing manuscript. C.Z.S.: Collection of data and editing the manuscript. M.F.: Collection of data. G.I.: Collection of data and editing the manuscript. C.K.: Collection of data. S.V.Z.: Conducting, collection, and interpretation of data and drafting/editing manuscript. D.K.: Planning, conducting, collection, and interpretation of data and drafting/editing manuscript and co senior author. All the above mentioned authors have approved the final draft submitted.
CONFLICT OF INTEREST
Y.N.S., L.R., D.Y.Y., K.W., E.M., C.Z.S., G.I., S.V.Z., and D.K. have no conflicts of interest. C.K. has research support from Finch Therapeutics for a clinical trial and is an unpaid clinical advisor to OpenBiome.
References
- 1. Lessa FC, Winston LG, McDonald LC; Emerging Infections Program C. difficile Surveillance Team . Burden of Clostridium difficile infection in the United States. N Engl J Med 2015;372(24):2369–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gerding DN, Johnson S. Clostridium difficile infection, including pseudomembranous colitis. In: Jameson JL, Fauci AS, Kasper DL, Hauser SL, Longo DL, Loscalzo J, editors. Harrison’s principles of internal medicine, 20e. New York, NY: McGraw-Hill Education; 2018:818–821 [Google Scholar]
- 3. Pereira JA, McGeer A, Tomovici A, et al. Incidence and economic burden of Clostridioides difficile infection in Ontario: A retrospective population-based study. CMAJ Open 2020;8(1):E16–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Adams SD, Mercer DW. Fulminant Clostridium difficile colitis. Curr Opin Crit Care 2007;13(4):450–5. [DOI] [PubMed] [Google Scholar]
- 5. McDonald LC, Gerding DN, Johnson S, et al. Clinical practice guidelines for Clostridium difficile infection in adults and children: 2017 update by the Infectious Diseases society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA). Clin Infect Dis. 2018;66:e1–e48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Surawicz CM, Brandt LJ, Binion DG, et al. Guidelines for diagnosis, treatment, and prevention of Clostridium difficile infections. Am J Gastroenterol 2013;108(4):478–98; quiz 499. [DOI] [PubMed] [Google Scholar]
- 7. Bhangu A, Nepogodiev D, Gupta A, et al. ; West Midlands Research Collaborative . Systematic review and meta-analysis of outcomes following emergency surgery for Clostridium difficile colitis. Br J Surg 2012;99(11):1501–13. [DOI] [PubMed] [Google Scholar]
- 8. Ianiro G, Maida M, Burisch J, et al. Efficacy of different faecal microbiota transplantation protocols for Clostridium difficile infection: A systematic review and meta-analysis. United European Gastroenterol J 2018;6(8):1232–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kassam Z, Lee CH, Yuan Y, et al. Fecal microbiota transplantation for Clostridium difficile infection: Systematic review and meta-analysis. Am J Gastroenterol 2013;108(4):500–8. [DOI] [PubMed] [Google Scholar]
- 10. Cammarota G, Ianiro G, Gasbarrini A. Fecal microbiota transplantation for the treatment of Clostridium difficile infection: A systematic review. J Clin Gastroenterol 2014;48(8):693–702. [DOI] [PubMed] [Google Scholar]
- 11. Kelly CR, Ihunnah C, Fischer M, et al. Fecal microbiota transplant for treatment of Clostridium difficile infection in immunocompromised patients. Am J Gastroenterol 2014;109(7):1065–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Weingarden AR, Hamilton MJ, Sadowsky MJ, et al. Resolution of severe Clostridium difficile infection following sequential fecal microbiota transplantation. J Clin Gastroenterol 2013;47(8):735–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fischer M, Sipe BW, Rogers NA, et al. Faecal microbiota transplantation plus selected use of vancomycin for severe-complicated Clostridium difficile infection: Description of a protocol with high success rate. Aliment Pharmacol Ther 2015;42(4):470–6. [DOI] [PubMed] [Google Scholar]
- 14. Hocquart M, Lagier JC, Cassir N, et al. Early fecal microbiota transplantation improves survival in severe Clostridium difficile infections. Clin Infect Dis 2018;66(5):645–50. [DOI] [PubMed] [Google Scholar]
- 15. Ianiro G, Masucci L, Quaranta G, et al. Randomised clinical trial: Faecal microbiota transplantation by colonoscopy plus vancomycin for the treatment of severe refractory Clostridium difficile infection-single versus multiple infusions. Aliment Pharmacol Ther 2018;48(2):152–9. [DOI] [PubMed] [Google Scholar]
- 16. Cammarota G, Ianiro G, Magalini S, et al. Decrease in surgery for Clostridium difficile infection after starting a program to transplant fecal microbiota. Ann Intern Med 2015;163(6):487–8. [DOI] [PubMed] [Google Scholar]
- 17. Cheng YW, Phelps E, Nemes S, et al. Fecal microbiota transplant decreases mortality in patients with refractory severe or fulminant Clostridioides difficile infection. Clin Gastroenterol Hepatol 2020;18(10):2234–2243.e1. [DOI] [PubMed] [Google Scholar]
- 18. Moher D, Liberati A, Tetzlaff J, et al. ; PRISMA Group . Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Ann Intern Med 2009;151(4):264–9, W64. [DOI] [PubMed] [Google Scholar]
- 19. Allegretti JR, Allegretti AS, Phelps E, et al. Classifying fecal microbiota transplantation failure: An observational study examining timing and characteristics of fecal microbiota transplantation failures. Clin Gastroenterol Hepatol 2018;16(11):1832–3. [DOI] [PubMed] [Google Scholar]
- 20. Fischer M, Sipe B, Cheng YW, et al. Fecal microbiota transplant in severe and severe-complicated Clostridium difficile: A promising treatment approach. Gut Microbes 2017;8(3):289–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. National Institute of Health. Study Quality Assessment Tools. https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools. [cited May 24, 2020].
- 22. Sterne JA, Hernán MA, Reeves BC, et al. ROBINS-I: A tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016;355:i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sterne JAC, Savović J, Page MJ, et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019;366:l4898. [DOI] [PubMed] [Google Scholar]
- 24. Stijnen T, Hamza TH, Ozdemir P. Random effects meta-analysis of event outcome in the framework of the generalized linear mixed model with applications in sparse data. Stat Med 2010;29(29):3046–67. [DOI] [PubMed] [Google Scholar]
- 25. R Core Team. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2020. https://www.R-project.org/. Accessed on May 27, 2020 [Google Scholar]
- 26. Tixier EN, Verheyen E, Ungaro RC, et al. Faecal microbiota transplant decreases mortality in severe and fulminant Clostridioides difficile infection in critically ill patients. Aliment Pharmacol Ther 2019;50(10):1094–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Gundacker N, Walker J, Rodriguez J, Morrow C. Fecal microbiota transplant in severe/complicated Clostridium difficile infection: A retrospective case series. Infect Dis Clin Pract. 2017;25:264–267 [Google Scholar]
- 28. Alukal J, Dutta SK, Surapaneni BK, et al. Safety and efficacy of fecal microbiota transplant in 9 critically ill patients with severe and complicated Clostridium difficile infection with impending colectomy. J Dig Dis 2019;20(6):301–7. [DOI] [PubMed] [Google Scholar]
- 29. Zainah H, Hassan M, Shiekh-Sroujieh L, et al. Intestinal microbiota transplantation, a simple and effective treatment for severe and refractory Clostridium difficile infection. Dig Dis Sci 2015;60(1):181–5. [DOI] [PubMed] [Google Scholar]
- 30. Aroniadis OC, Brandt LJ, Greenberg A, et al. Long-term follow-up study of fecal microbiota transplantation for severe and/or complicated Clostridium difficile infection: A multicenter experience. J Clin Gastroenterol 2016;50(5):398–402. [DOI] [PubMed] [Google Scholar]
- 31. Ianiro G, Murri R, Sciumè GD, et al. Incidence of bloodstream infections, length of hospital stay, and survival in patients with recurrent Clostridioides difficile infection treated with fecal microbiota transplantation or antibiotics: A prospective cohort study. Ann Intern Med 2019;171(10):695–702. [DOI] [PubMed] [Google Scholar]
- 32. Quay T, Walter M.. Fecal Microbiota Therapy in Canada: An Environmental Scan. Ottawa: CADTH. 2020; Environmental Scan no. 95. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.