Significance Statement
Many kidney transplant patients in INTERCOMEX whose biopsy specimens are diagnosed molecularly or histologically as no rejection have donor-specific HLA antibodies (DSAs, 32%). Although the significance of DSA in no rejection has been unclear, we hypothesized that current diagnostic thresholds miss some DSA-positive patients who may have subtle antibody-mediated rejection (ABMR)–related stress, with potential effect on outcomes. To search for subtle ABMR-related gene expression in “no rejection” biopsy samples, we developed a “DSA-probability” classifier (trained on DSA positivity) in microarray results from 1679 biopsy samples that detected ABMR-related transcripts (e.g., NK cell and IFNG-inducible). Many no rejection biopsy samples had mildly increased expression of ABMR-related transcripts, associated with DSA positivity, and these kidneys had increased risk of failure. Thus, mild ABMR-related stress is more common than previously thought.
Keywords: kidney biopsy, transplantation, gene expression, renal transplantation, rejection
Abstract
Background
Donor -specific HLA antibody (DSA) is present in many kidney transplant patients whose biopsies are classified as no rejection (NR). We explored whether in some NR kidneys DSA has subtle effects not currently being recognized.
Methods
We used microarrays to examine the relationship between standard-of-care DSA and rejection-related transcript increases in 1679 kidney transplant indication biopsies in the INTERCOMEX study (ClinicalTrials.gov NCT01299168), focusing on biopsies classified as NR by automatically assigned archetypal clustering. DSA testing results were available for 835 NR biopsies and were positive in 271 (32%).
Results
DSA positivity in NR biopsies was associated with mildly increased expression of antibody-mediated rejection (ABMR)–related transcripts, particularly IFNG-inducible and NK cell transcripts. We developed a machine learning DSA probability (DSAProb) classifier based on transcript expression in biopsies from DSA-positive versus DSA-negative patients, assigning scores using 10-fold cross-validation. This DSAProb classifier was very similar to a previously described “ABMR probability” classifier trained on histologic ABMR in transcript associations and prediction of molecular or histologic ABMR. Plotting the biopsies using Uniform Manifold Approximation and Projection revealed a gradient of increasing molecular ABMR-like transcript expression in NR biopsies, associated with increased DSA (P<2 × 10−16). In biopsies with no molecular or histologic rejection, increased DSAProb or ABMR probability scores were associated with increased risk of kidney failure over 3 years.
Conclusions
Many biopsies currently considered to have no molecular or histologic rejection have mild increases in expression of ABMR-related transcripts, associated with increasing frequency of DSA. Thus, mild molecular ABMR-related pathology is more common than previously realized.
Donor-specific HLA antibody (DSA) testing is standard-of-care in the follow-up of kidney transplant patients, particularly when they are presenting with signs of rejection (e.g., proteinuria, decreased eGFR). Although the diagnosis of antibody-mediated rejection (ABMR) is on the basis of histologic and molecular features, DSA testing is important confirmation.1 Suppression of DSA is also a principal target for treatments that aim to reverse ABMR. The relationship between DSA testing and ABMR is not straightforward1,2: as a predictor of ABMR pathology, DSA has a considerable number of “false positives”—DSA-positive patients with no ABMR, and “false negatives”—DSA-negative patients with ABMR. For example, in 1679 indication biopsy samples collected during the INTERCOMEX study, DSA was only positive in 74% of histologic ABMR i.e., 26% of ABMR was DSA-negative.3 Furthermore, DSA was positive in 32% of biopsy samples with no histologic rejection and in 29% with histologic T cell–mediated rejection (TCMR). DSA is less frequent when ABMR is less active in molecular late-stage ABMR.4 HLA antibody testing has a high degree of variability both in measurements and interpretation between centers,5 and DSA measurements have complex relationships with the ABMR phenotypes of the patients, particularly with the recognition of DSA-negative ABMR.1,2,6 These issues underscore the need to define the associations of DSA positivity. True heterogeneity within ABMR may not be represented in a binary system of rejection versus no rejection (NR),6 and further exploration of the continuum of ABMR-associated features is needed.
Despite these complexities, DSA remains a powerful probe for understanding the phenotype of kidney transplants, with continuing scientific potential for new insights in the INTERCOMEX multicenter study. We previously developed a microarray-based system (MMDx) for diagnosing ABMR and TCMR in kidney transplant biopsy samples using machine-learning classifiers and archetype group assignment.3,4 In this study, we examined microarray data from 1679 biopsy samples in INTERCOMEX for associations between DSA and gene expression in the biopsy specimen, focusing particularly on the biopsy samples currently classified as NR. For most analyses, NR was assigned automatically by archetype group assignment (NR, n=1012), but results were confirmed in biopsy samples assigned NR by histology or by MMDx sign-out. We developed a DSA probability (DSAProb) classifier in all 1679 biopsy samples and used it to search for DSA-associated transcript expression in biopsy samples with no rejection. We hypothesized that the current molecular and histologic thresholds for diagnosing ABMR miss subtle but potentially important ABMR-related expression in some biopsy specimens classified as NR.
Methods
Study Population
We studied 1679 prospectively collected indication kidney transplant biopsy samples. The demographics are shown in Supplemental Table 3, whereas histology and DSA data are given in Supplemental Table 4. Biopsy samples were prospectively obtained with consent from established centers under local IRB-approved protocols (ClinicalTrials.gov NCT01299168). A portion of one core (mean length 3 mm) was immediately stabilized in RNAlater and shipped to the Alberta Transplant Applied Genomics Centre (http://atagc.med.ualberta.ca) at ambient temperature for RNA extraction and processing as previously described.3 Gene expression was measured using Affymetrix PrimeView arrays unless the biopsy sample was inadequate for analysis (e.g., too small or degraded: approximately 4% of all biopsy samples3). A total of 1745 biopsy specimens had sufficient RNA quality to run on microarrays, of which 1679 had complete histologic diagnoses, as previously published.7
The DSA-associated data were reported by the local center according to standard-of-care, as permitted by their IRB-approved protocol. Specifics such as complement binding were not performed as standard-of-care. Titer or MFI was also not available in any standard way. Additionally, it is not recommended to pool MFI from different centers because MFIs are not highly reproducible across centers.5,8
NR Biopsy Sample Subpopulation Selection
This subpopulation of biopsy samples was defined as biopsy samples with NR, i.e., belonging to the previously defined ‘A1–No Rejection’ archetype group4 and used as a comparator to the full n=1679 population where applicable. This selection of biopsy specimens (n=1012) was used to study associations with both DSA status and the DSA classifier score (described below).
In some analyses, findings were confirmed in biopsy samples called NR in the MMDx sign-out comment (given on the basis of an assessment of all results on the MMDx report) and in biopsy specimens called NR by histology as assigned by the local center. These variables were labeled as MMDx NR and histologic NR, respectively, to distinguish from the main, archetype-based NR population.
Principal Component Analysis and Uniform Manifold Approximation and Projection of the Study Population
Principal component analysis (PCA) is a dimensionality reduction technique used in these analyses for data visualization. All rejection inputs (classifiers predicting histologic ABMR and TCMR, plus classifiers predicting ptc-, g-, cg-, t-, and i-histologic features) were developed previously, trained, and tested on class comparisons comparing an abnormal condition with a more normal condition. PCA was therefore on the basis of a 1679 (samples) × 7 (variables) data matrix, using the ‘PCA’ function in the R ‘FactoMineR’ package.9
UMAP was also used to reduce the dimensionality in gene expression in the population into two dimensions for ease of presentation and assessment. UMAP visualization presents an advantage over PCA because unlike PCA it does not compress the NR biopsy samples but rather displays the detail within these biopsy samples allowing individual score gradients to be shown. UMAP analyses were on the basis of the same 1679 (samples) × 7 (variables) data matrix as was used for PCA. UMAP analysis used the ‘umap()’ function from the ‘umap’ package in R.10
Development of a Molecular Classifier for DSAProb
We developed a DSAProb classifier that uses differentially expressed transcripts associated with DSA in biopsy samples. The classifier used an ensemble of 12 different machine-learning algorithms, with the classifier scores expressed as the median of these 12 algorithms as previously described.7 The DSAProb classifier was trained on DSA-positive versus DSA-negative classes as assigned by each center, with missing values excluded in training, but all biopsy samples were assigned a score in the test sets. All scores were from left-out sets in tenfold crossvalidation, i.e., predicted using training set models that had no information concerning the biopsy samples they were predicting. The classifier was made with functions from the R ‘caret’ library.11
The previously described ABMR probability classifier (ABMRpm ) was used in some analyses as a comparator to the DSAProb classifier and was trained on histologic ABMR versus no ABMR using the same techniques.12
Top Transcripts with Increased Expression in a DSA-Positive versus DSA-Negative Class Comparison
Top transcripts were established in a Bayesian t test comparing a selected positive class with a negative class (i.e., DSA-positive versus DSA-negative). Resulting transcript lists were ordered by P value and the top 30 transcripts increased in expression in the positive class (i.e., DSA-positive) were selected for further analysis. All t tests were performed using functions from the ‘limma’ package in R.
Survival Analyses
Survival analyses examined the survival probability within select biopsy sample subpopulations: DSA-positive versus DSA-negative, high versus low DSAProb score, and high versus low ABMRpm score. These analyses were initially done in one randomly selected biopsy sample per patient (n=1153) and then reduced to only those biopsy samples with no molecular rejection only (n=729). Survival analyses were done using the ‘survfit’ function from the ‘survival’ package in R.13 P values were calculated using the ‘survdiff’ function from the same package. Plots were assembled using ‘ggsurvplot’ in the ‘survminer’ package.14
Results
Population and Demographics
We examined DSA associations in the 1679 indication biopsy samples population and in the 1012 archetypal NR samples subpopulation4 (Supplemental Table 4).7 We chose to define NR by archetypes because this provided an automatically assigned objective cutoff. Several findings were confirmed in biopsy specimens that were called NR by MMDx sign-out (MMDx NR) and by local histology (histologic NR).
Biopsy specimens were called DSA-positive if the patient had a positive DSA status reported by the local center at the time of biopsy, using local standard-of-care methods to determine DSA positivity. Of the 1012 biopsy samples classified as NR, 835 had available DSA testing results: 564 were DSA-negative and 271 were DSA-positive (Supplemental Table 4). DSA class information as reported by each participating center is recorded in Supplemental Table 5, with 191 (14%) of all 1679 biopsy samples identified as Class II DSA, 113 (8%) identified as Class I/II, and 74 (5%) identified as Class I.
Distribution of DSA-Positive Status in all 1679 Biopsy Specimens
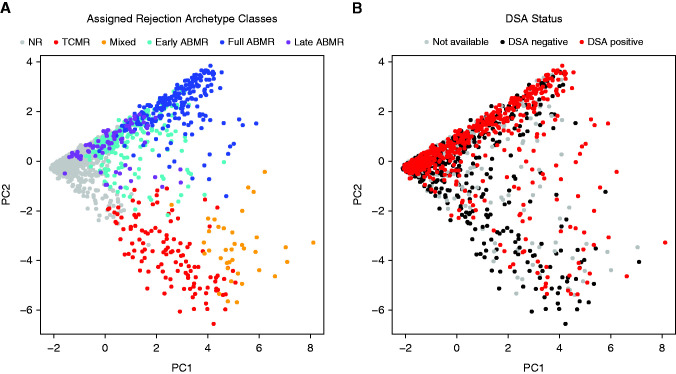
We plotted the distribution of all biopsy samples using PCA, showing the biopsy samples colored by rejection archetype cluster membership (Figure 1A) and by DSA status at time of biopsy (Figure 1B). Biopsy samples were distributed from archetypal NR to rejection in PC1 (left to right, Figure 1A), and from ABMR to TCMR in PC2 (top to bottom, Figure 1A). The distribution in Figure 1B shows that many biopsy specimens with very low probability of molecular rejection (located to the left in PC1) were DSA-positive as recorded by the center.
Figure 1.
Distribution of n=1679 biopsies using principal component analysis (PCA) and colored by archetype groups and DSA status. The main population of 1679 kidney transplant indication biopsy samples colored by (A) previously defined six-class rejection-based archetypal group assignment (NR, TCMR, mixed, early-stage ABMR, fully developed ABMR, and late-stage ABMR), and (B) the known DSA status of each biopsy sample (biopsy samples where the status is unknown are marked in gray). Biopsy specimens are distributed from NR to rejection across PC1 (x axis of [A]), and from ABMR to TCMR across PC2 (y axis of [A] and [B]). Therefore, biopsy samples with low probability of molecular rejection will be located to the left in PC1.
Top Transcripts Associated with DSA Status in All Biopsy Specimens
All top 30 transcripts by P value associated with DSA status in all biopsy specimens (Table 1, n=1679) were also associated with ABMR (e.g., ROBO4, SH2D1B), and many were interferon-γ inducible (e.g., CXCL11, GBP4). Expression of all top transcripts was consistently increased in DSA-positive versus DSA-negative biopsy samples, in DSA-positive NR versus DSA-negative NR biopsy samples, and in DSA-positive ABMR versus DSA-negative ABMR biopsy samples.
Table 1.
Top 30 redundant transcripts associated with known DSA status in a t test in the n=1679 populationa
| Adjusted P Value | Gene Symbol | Gene Name | Transcript Set Annotation | Transcript Expression in All Biopsy Samples n=1679 |
Transcript Expression in NR Biopsy Samples n=1012 |
||
|---|---|---|---|---|---|---|---|
| DSA+ (n=576) |
DSA− (n=818) |
DSA+ (n=271) |
DSA− (n=564) |
||||
| <0.001 | ROBO4 | Roundabout, axon guidance receptor, homolog 4 (Drosophila) | ABMR-RAT | 747b | 590 | 617b | 580 |
| <0.001 | SH2D1B | SH2 domain containing 1B | ABMR-RAT | 18b | 13 | 13b | 12 |
| <0.001 | PLA1A | Phospholipase A1 member A | ABMR-RAT, GRIT | 386b | 268 | 242b | 224 |
| <0.001 | SH2D1B | SH2 domain containing 1B | ABMR-RAT | 32b | 24 | 23b | 22 |
| <0.001 | S1PR5 | Sphingosine-1-phosphate receptor 5 | ABMR-RAT | 23b | 20 | 19b | 19 |
| <0.001 | CX3CL1 | Chemokine (C-X3-C motif) ligand 1 | ABMR-RAT | 641b | 539 | 548b | 510 |
| <0.001 | ROBO4 | Roundabout, axon guidance receptor, homolog 4 (Drosophila) | ABMR-RAT | 510b | 434 | 449b | 433 |
| <0.001 | TRDC | T cell receptor δ constant | ABMR-RAT | 76b | 62 | 64b | 61 |
| <0.001 | LYPD5 | LY6 | ABMR-RAT | 22b | 17 | 16b | 15 |
| <0.001 | COL13A1 | collagen, type XIII, alpha 1 | ABMR-RAT | 45b | 36 | 38b | 37 |
| <0.001 | LYPD5 | LY6 | ABMR-RAT | 17b | 14 | 13b | 13 |
| <0.001 | TRDC | T cell receptor δ constant | ABMR-RAT | 87b | 60 | 55b | 50 |
| <0.001 | TRDC | T cell receptor δ constant | ABMR-RAT | 80b | 49 | 44b | 37 |
| <0.001 | CXCL11 | Chemokine (C-X-C motif) ligand 11 | ABMR-RAT, GRIT | 225b | 99 | 80b | 54 |
| <0.001 | COL13A1 | Collagen, type XIII, alpha 1 | ABMR-RAT | 57b | 50 | 52b | 51 |
| <0.001 | CXCL11 | Chemokine (C-X-C motif) ligand 11 | ABMR-RAT, GRIT | 104b | 46 | 37b | 24 |
| <0.001 | CCL4L1 | Chemokine (C-C motif) ligand 4-like 1 | ABMR-RAT | 56b | 34 | 31b | 27 |
| <0.001 | FGFBP2 | Fibroblast growth factor binding protein 2 | ABMR-RAT | 64b | 46 | 40b | 37 |
| <0.001 | CXCL11 | Chemokine (C-X-C motif) ligand 11 | ABMR-RAT, GRIT | 69b | 31 | 25b | 17 |
| <0.001 | SH2D1B | SH2 domain containing 1B | ABMR-RAT | 36b | 29 | 28b | 27 |
| <0.001 | KLRF1 | Killer cell lectin-like receptor subfamily F, member 1 | ABMR-RAT | 78b | 59 | 56b | 52 |
| <0.001 | CCL4 | Chemokine (C-C motif) ligand 4 | ABMR-RAT | 162b | 95 | 83b | 67 |
| <0.001 | CD160 | CD160 molecule | ABMR-RAT | 40b | 29 | 26b | 25 |
| <0.001 | ROBO4 | Roundabout, axon guidance receptor, homolog 4 (Drosophila) | ABMR-RAT | 153b | 132 | 139b | 136 |
| <0.001 | TRDV3 | T cell receptor δ variable 3 | ABMR-RAT | 21b | 15 | 13b | 12 |
| <0.001 | NCR1 | Natural cytotoxicity triggering receptor 1 | ABMR-RAT | 12b | 10 | 9 | 9 |
| <0.001 | GNLY | Granulysin | ABMR-RAT | 107b | 89 | 84b | 79 |
| <0.001 | GNLY | Granulysin | ABMR-RAT | 96b | 61 | 51b | 45 |
| <0.001 | GNLY | Granulysin | ABMR-RAT | 101b | 67 | 56b | 50 |
| <0.001 | GBP4 | Guanylate binding protein 4 | ABMR-RAT, GRIT | 168b | 112 | 100b | 86 |
ABMR-RAT, ABMR-associated transcripts; GRIT, γ-interferon–inducible transcripts.
Total population of 1679, with 285 biopsy samples with missing DSA status, 1394 included in the analysis.
Highest expression in DSA+ versus DSA− within each category (n=1679 and n=1012).
Mean PBT scores in DSA-positive versus DSA-negative biopsy specimens were compared within archetypally assigned NR, ABMR, and TCMR groups (Supplemental Table 6). PBT scores related to rejection and ABMR (i.e., DSASTs, NKBs, ABMR-RATs, Rej-RATs) were significantly (P<0.05) increased in DSA-positive ABMR and NR biopsy samples compared with DSA-negative biopsy samples in the same groups. In TCMR biopsy samples, only DSASTs were significantly increased in DSA-positive biopsy samples.
Developing a Molecular DSAProb Classifier
We developed an ensemble DSAProb classifier to identify biopsy specimens with increased DSA-associated transcripts on the basis of DSA status in 1679 biopsy specimens. An ensemble of 12 machine learning algorithms used crossvalidation to assign scores to each biopsy sample reflecting the probability of DSA positivity. The median value of the ensemble estimates was used as the final score. The expectation was that the scores would reflect DSA-associated and thus ABMR-related gene expression in the biopsy samples.
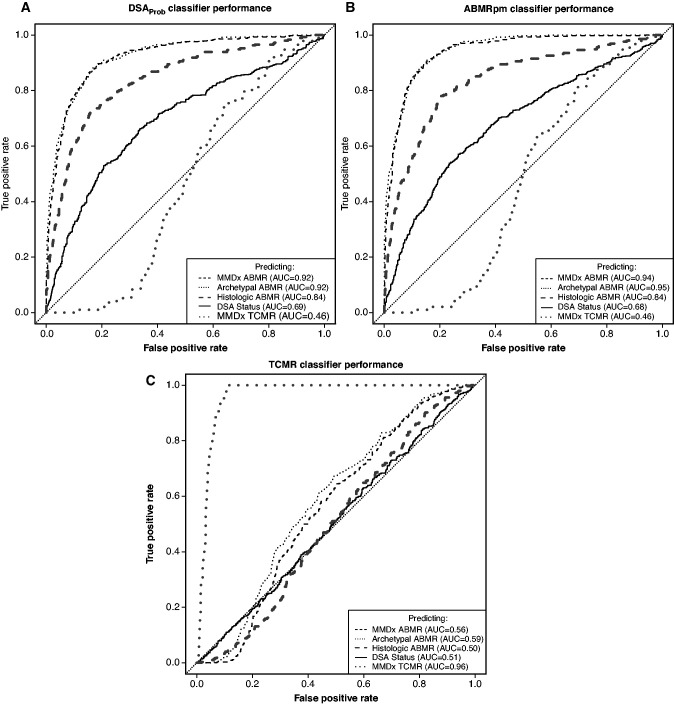
In Figure 2A, DSAProb predicted MMDx ABMR (dashed gray line, AUC=0.92), archetypal ABMR (dotted black line, AUC=0.92), and histologic ABMR (dashed black line, AUC=0.85). It predicted DSA status with a lower AUC (solid black line, AUC=0.69), as expected from the complex relationship of DSA status to biopsy ABMR findings (see Introduction). DSAProb did not predict TCMR (dotted gray line, AUC=0.46). The previously described ABMRpm classifier similarly predicted MMDx, archetypal, and histologic ABMR, and moderately predicted DSA status (AUC=0.94, 0.95, 0.84, and 0.68, respectively), but did not predict TCMR (AUC=0.46, Figure 2B). In contrast, the TCMR classifier predicted molecular TCMR (AUC=0.96) but had no relationship to molecular ABMR, histologic ABMR, or DSA status (AUC range 0.50–0.59, Figure 2C).
Figure 2.
AUCs showing the predictive performance of selected classifiers for ABMR, TCMR, and DSA status. ROC curves demonstrating the predictive performance of the DSAProb classifier score, the ABMRpm classifier score, and the TCMR classifier score for prediction of MMDx ABMR sign-out diagnoses, any of the three ABMR stages described by rejection archetype groups, histologic ABMR as assigned by the local center, DSA status, and MMDx TCMR sign-out diagnoses.
DSAProb classifier scores strongly correlated with those of the previously described ABMRpm classifier. Despite their different training algorithms, both classifiers identified biopsy samples with molecular ABMR-related expression. This relationship was visualized by plotting the DSAProb scores against the ABMRpm scores on logarithmic scales (Spearman correlation coefficient 0.90, P<2 × 10−16, Figure 3). Increasing ABMRpm scores closely followed increasing DSAProb scores, despite the different scaling of the two classifier scores: ABMRpm scores were frequently very low (e.g., below 0.05) whereas DSAProb scores were rarely lower than 0.25 even in NR biopsy samples.
Figure 3.
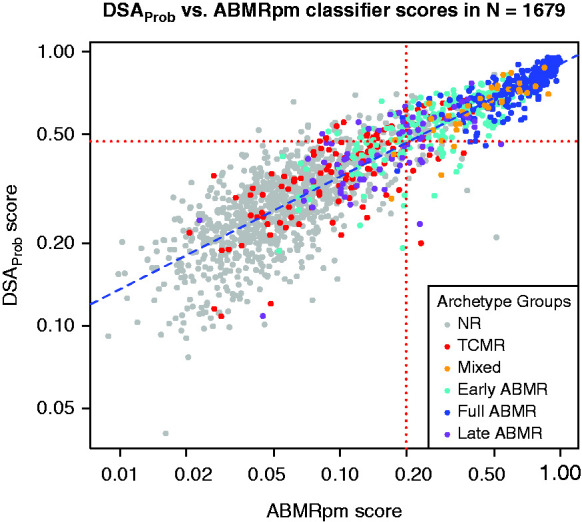
A scatterplot showing the ABMRpm classifier scores versus the DSAProb classifier scores for each biopsy sample in the full (n=1679) population. Biopsy specimens are colored by their assigned rejection-based archetype groups. The blue dotted regression line shows the best fit through the data. Red dotted horizontal and vertical lines show the cutoffs for the ABMRpm classifier score and DSAProb classifier score.
We compared the mean DSAProb and ABMRpm classifier scores with the rejection and TCMR classifier scores in all six rejection archetype groups (Table 2). The mean DSAProb score in NR biopsy samples was 0.30; the mean ABMRpm score was 0.07. DSAProb and ABMRpm scores were high in early-stage and fully developed ABMR and mixed TCMR/ABMR but had lower mean values in late-stage ABMR. DSAProb and ABMRpm scores were slightly elevated in TCMR. DSAProb and ABMRpm scores were both highest in early-stage ABMR, in mixed rejection, and in fully developed ABMR archetype groups, respectively. All mean scores in archetype groups were significantly different when compared with the NR group (P<0.05).
Table 2.
Comparing mean DSAProb, TCMR, ABMRpm, and rejection classifier scores in kidney rejection archetype groupsa (n=1679)
| Classifiers | Six Kidney Rejection Archetype Groups | |||||
|---|---|---|---|---|---|---|
| NR (n=1012) |
TCMR (n=116) |
Mixed (n=37) |
Early ABMR (n=219) |
Full ABMR (n=219) |
Late ABMR (n=76) |
|
| Histology-based classifiers | ||||||
| Rejection probability classifier | 0.12 | 0.64b | 0.90b | 0.56b | 0.77b | 0.35b |
| TCMR probability classifier | 0.03 | 0.40b | 0.57b | 0.06 | 0.05 | 0.04 |
| ABMRpm classifier | 0.07 | 0.14 | 0.46b | 0.35b | 0.68b | 0.19 |
| DSAProb | 0.30 | 0.39 | 0.63b | 0.57b | 0.75b | 0.45 |
All mean classifier scores in TCMR, Mixed, Early ABMR, Full ABMR, and Late ABMR were significantly different from the mean scores in NR; t test P values <0.05.
Rejection archetypes previously published.4
Mean classifier scores that were notably increased compared with the mean in the NR archetype group. These increases are discussed further in the text.
Supplemental Figure 1A illustrates the relationships between DSAProb, ABMRpm, and histologic NR/ABMR diagnostic categories in the 1679 samples population (using the same cutoffs as Figure 3). Supplemental Figure 1B shows the same relationships but with archetypally assigned MMDx diagnostic categories.
Visualizing the Gradient of Increasing DSAProb and ABMRpm Scores
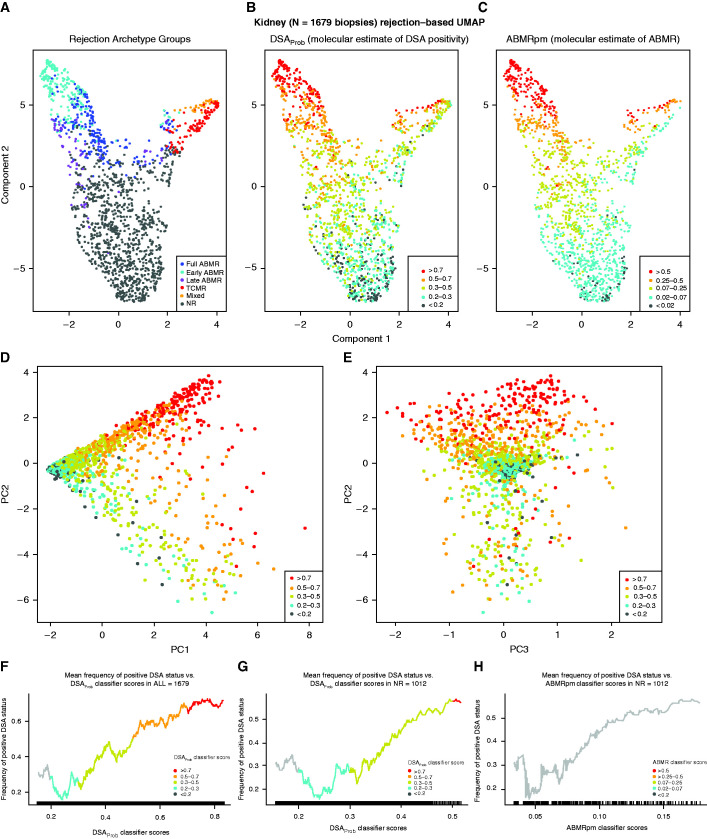
We distributed all biopsy samples on the basis of their transcript expression using UMAP (Figure 4, A–C). Biopsy samples classified as rejection by archetypes were located at the upper region of Component 2, with ABMR on the left of Component 1 and TCMR/mixed rejection on the right (Figure 4A). When biopsy samples were colored by increasing DSAProb score (Figure 4B), the highest scores were in biopsy samples with ABMR, as expected. However, there was also a gradient of increasing DSAProb scores within NR biopsy samples. A similar gradient was seen for ABMRpm scores, which increased over Component 2 including within NR biopsy samples at the bottom of the plot (Figure 4C). The calibration of the cutoffs used for DSAProb and ABMRpm classifiers was different because their respective baseline scores in NR biopsy samples differed, as discussed above.
Figure 4.
Gradients in 1679 biopsies over archetype groups, and DSAProb/ABMRpm classifier scores. All 1679 indication kidney transplant biopsy specimens distributed using UMAP, colored by (A) assigned rejection-based archetypal class, (B) increasing DSAProb classifier score, and (C) increasing ABMRpm score. Biopsy samples with low probability of molecular rejection are located toward the bottom of Component 2 in (A–C). Biopsy samples with rejection are located toward the upper region of Component 2, with ABMR on the left and TCMR on the right of Component 1. This same population was also distributed using PCA. The distribution of PC1 versus PC2 was colored by increasing DSAProb classifier score in (D) and by increasing ABMRpm classifier score in (E). PC1 distributed biopsy samples from NR to rejection (x axis of [D] and [E]), and PC2 separates ABMR from TCMR (y axis of [D] and [E]). Rolling average plots for frequency of percentage positive DSA status (y axis) versus increasing average DSAProb classifier score are shown for all 1679 biopsy samples (F) and in 1012 NR samples (G). (H) The percentage positive DSA versus the rolling average for the ABMRpm classifier in the 1012 NR biopsy samples. Rolling average plots are segmented, and colored on the basis of the same cutoffs for the DSAProb and ABMRpm used in (A–C).
Biopsy samples were also distributed using PCA, with colors designated by the same thresholds used in Figure 4, A–C. DSAProb scores increased with PC1 and PC2 (Figure 4D). Most high DSAProb scores were concentrated in the ABMR region (upper PC2), although a small number were located in the TCMR region of the PCA plot in lower PC2. The ABMRpm classifier scores distributed similarly (Figure 4E).
Relating DSAProb Scores to DSA Status
A rolling average plot showed that as the mean DSAProb score increased, the frequency of DSA positivity also increased in both the entire population (Figure 4F) and NR biopsy samples (Figure 4G), i.e., this classifier was well calibrated. A rolling average of the ABMRpm classifier score showed similar results in the NR biopsy samples (Figure 4H). A slight decrease in the frequency of positive DSA status between 0.2 and 0.3 mean DSAProb score was noted. The DSA-positive status in low-level DSAProb scores is consistent with the baseline of DSA positivity within NR biopsy samples of about 30%.
We examined the DSAProb scores in NR defined in three different ways: by rejection-associated archetype groups (NR), by histology (histologic NR), and by MMDx sign-outs (MMDx NR, Table 3). In all definitions of NR, DSA positivity was associated with mild but statistically significant increases in mean DSAProb scores; i.e., mild increases in ABMR-associated gene expression.
Table 3.
DSA-positive status and DSAProb classifier scores in biopsy specimens called molecular and histologic NR
| Description | Diagnostic Category | Mean DSAProb Score in Groups | P Value | |
|---|---|---|---|---|
| DSA+ | DSA− | |||
| Molecular archetype groups | NR | 0.34 | 0.29 | <0.001 |
| Histology diagnoses | Histologic NR | 0.37 | 0.31 | <0.001 |
| Molecular sign-out diagnosis | MMDx NR | 0.31 | 0.28 | <0.001 |
In total, 1394 biopsy samples had available DSA data, and 285 had no known DSA status at time of biopsy or DSA testing was not done.
We also assessed the relationship between DSAProb scores and DSA positivity in NR biopsy samples by dividing the NR biopsy samples into those with DSAProb scores high (above the median) versus low (below the median, Table 4). NR biopsy specimens with high DSAProb scores were more likely to be from DSA-positive patients (49% versus 27%, P=1.2 × 10−9).
Table 4.
DSA-positive/negative versus DSAProb classifier score above/below mediana in DSA-tested kidney biopsy specimens with no rejection (n=835)
| DSAProb Classifier | DSA Status | Row Total | |
|---|---|---|---|
| DSA-Negative | DSA-Positive (% of row) |
||
| <Median | 454 | 164 (27%) | 618 |
| >Median | 110 | 107 (49%) | 217 |
| Column total | 564 | 271 (32%) | 835 |
Chi-squared test P value <0.001.
Median of DSAProb classifier calculated in the main n=1679 population.
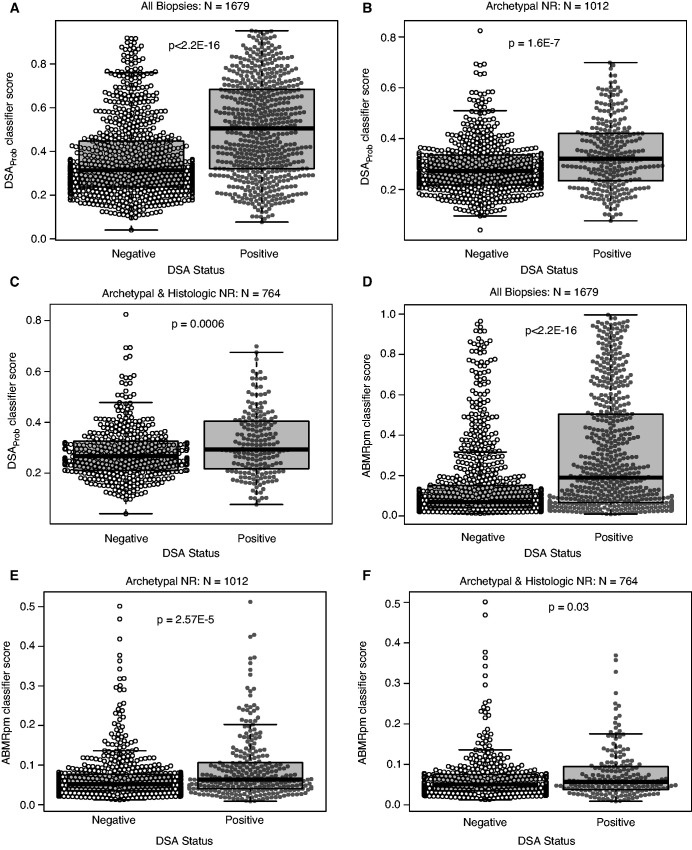
We compared the distribution of DSAProb and ABMRpm scores in DSA-positive versus DSA-negative biopsy samples in the full biopsy sample population (n=1679), the NR biopsy sample population (n=1012), and the population that was both archetypal NR and histologic NR (n=764). In all populations, DSAProb scores were significantly different between DSA-positive and DSA-negative biopsy sample groups (P<0.05, Figure 5), although the differences were smaller with the smaller NR groups, as expected.
Figure 5.
Mean DSAProb and ABMRpm classifier scores differ between DSA positive and DSA negative biopsies. Beeswarms and boxplots showing the (A–C) DSAProb and (D–F) ABMRpm classifier scores divided by DSA status (DSA-positive versus DSA-negative). The center line of the box shows the median score in each group, whereas the box shows the data between the first and third quartiles of the scores (interquartile range or IQR). Whiskers mark 1.5×IQR. DSAProb classifier scores are shown divided into DSA-positive and DSA-negative groups in (A) all 1679 biopsy samples, (B) 1012 biopsy samples with no molecular rejection (NR), and (C) 764 biopsy samples with no molecular or histologic rejection. ABMRpm scores divided into DSA-positive and DSA-negative groups are shown in (D) all 1679 biopsy samples, (E) 1012 NR biopsy samples, and (F) 764 biopsy samples with no molecular or histologic rejection. All P values are calculated from Welch’s two-sample t tests comparing the DSA-positive and DSA-negative groups.
Expression in NR Biopsy Specimens of the Top Genes Associated with the DSAProb and ABMRpm Classifiers
The top 20 genes associated with DSA status and with ABMR diagnoses (i.e., used by the DSAProb and ABMRpm classifiers, respectively) were largely shared between the two classifiers despite their different training algorithms. Of 19,438 unique genes represented on the array, 13 genes were ranked in the top 20 for both classifiers (Table 5). The correlations and P values for the genes associated with both the DSAProb and ABMRpm classifiers were then calculated in NR biopsy specimens (Table 5). All 20 transcripts were significantly correlated with the DSAProb score and ABMRpm classifier score.
Table 5.
Top 20 genes associated with ABMRpm and DSAProb in n=1679 kidney biopsy specimens, correlation and P value calculated in n=1012 NR biopsy specimens
| ABMRpm Classifier | DSAProb Classifier | ||||||
|---|---|---|---|---|---|---|---|
| Symbol | Gene | ABMRpm Classifier Correlation | ABMRpm Classifier P Value | Symbol | Gene | DSAProb Correlation | DSAProb P Value |
| GNLYa | Granulysina | 0.47a | <0.001a | ROBO4a | Roundabout, axon guidance receptor, homolog 4a | 0.70a | <0.001a |
| PLA1Aa | Phospholipase A1 member Aa | 0.45a | <0.001a | GBP4a | Guanylate binding protein 4a | 0.56a | <0.001a |
| ROBO4a | Roundabout, axon guidance receptor, homolog 4a | 0.65a | <0.001a | PLA1Aa | Phospholipase A1 member Aa | 0.33a | <0.001a |
| SH2D1Ba | SH2 domain containing 1Ba | 0.47a | <0.001a | SH2D1Ba | SH2 domain containing 1Ba | 0.40a | <0.001a |
| FGFBP2a | Fibroblast growth factor binding protein 2a | 0.47a | <0.001a | CXCL11a | Chemokine (C-X-C motif) ligand 11a | 0.48a | <0.001a |
| GBP4a | Guanylate binding protein 4a | 0.54a | <0.001a | CCL4a | Chemokine (C-C motif) ligand 4a | 0.45a | <0.001a |
| LYPD5 | LY6 | 0.48 | <0.001 | WARSa | Tryptophanyl-tRNA synthetasea | 0.44a | <0.001a |
| CCL4a | Chemokine (C-C motif) ligand 4a | 0.49a | <0.001a | FGFBP2a | Fibroblast growth factor binding protein 2a | 0.37a | <0.001a |
| PRF1 | Perforin 1 (pore forming protein) | 0.43 | <0.001 | APOL3a | Apolipoprotein L, 3a | 0.51a | <0.001a |
| WARSa | Tryptophanyl-tRNA synthetasea | 0.46a | <0.001a | CX3CL1 | Chemokine (C-X3-C motif) ligand 1 | 0.45 | <0.001 |
| APOL3a | Apolipoprotein L, 3a | 0.54a | <0.001a | TRDCa | T cell receptor δ constanta | 0.41a | <0.001a |
| CXCL11a | Chemokine (C-X-C motif) ligand 11a | 0.43a | <0.001a | PRF1 | Perforin 1 (pore forming protein) | 0.36 | <0.001 |
| ICAM2 | Intercellular adhesion molecule 2 | 0.50 | <0.001 | CD160a | CD160 moleculea | 0.43a | <0.001a |
| TRDCa | T cell receptor δ constanta | 0.47a | <0.001a | IDO1a | Indoleamine 2,3-dioxygenase 1a | 0.41a | <0.001a |
| KLRD1 | Killer cell lectin-like receptor subfamily D, member 1 | 0.40 | <0.001 | CDH5 | Cadherin 5, type 2 (vascular endothelium) | 0.49 | <0.001 |
| CD160a | CD160 moleculea | 0.45a | <0.001a | GNLYa | Granulysina | 0.30a | <0.001a |
| IDO1a | Indoleamine 2,3-dioxygenase 1a | 0.41a | <0.001a | CXCL10 | Chemokine (C-X-C motif) ligand 10 | 0.43 | <0.001 |
| S1PR5 | Sphingosine-1-phosphate receptor 5 | 0.37 | <0.001 | GBP1 | Guanylate binding protein 1, interferon-inducible | 0.44 | <0.001 |
| GZMB | Granzyme B | 0.43 | <0.001 | CXCL9 | Chemokine (C-X-C motif) ligand 9 | 0.41 | <0.001 |
| GIMAP6 | GTPase, IMAP family member 6 | 0.48 | <0.001 | HLA-F | Major histocompatibility complex, class I, F | 0.50 | <0.001 |
The transcript was shared between the top 20 lists in the class comparison used by the ABMRpm classifier and in the class comparison used by the DSAProb classifier.
Relationships between 3-Year Graft Survival, DSA, the DSAProb Score, and the ABMRpm Score
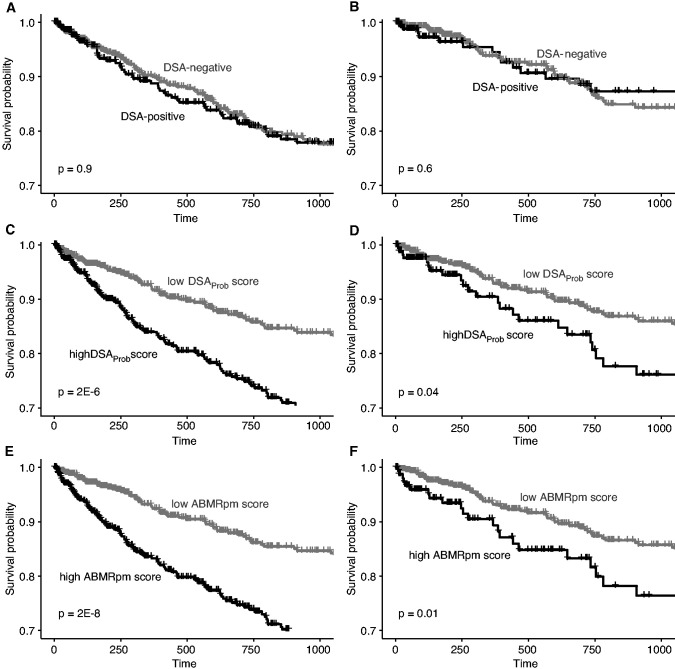
We previously reported15 that in 1679 kidney transplants having indication biopsies, the presence of ABMR in the biopsy sample is associated with decreased survival over 3 years post-transplant but DSA status per se is not. We now compared the survival of kidneys in the NR cohort with that of kidneys in the entire population of 1679, dividing the populations by DSA status, by DSAProb score, or by ABMRpm score (Figure 6).
Figure 6.
Three year graft survival compared between DSA positive and DSA negative biopsies, DSAProb high and DSAProb low biopsies, and ABMRpm high versus ABMRpm low biopsies. Horizontal ticks mark censoring events by 3 years postbiopsy. The survival curves for DSA-positive and DSA-negative groups are shown in (A) all 1679 biopsy specimens and in (B) 1012 NR biopsy specimens. Survival curves are also shown for high versus low DSAProb classifier score in (C) all 1679 biopsy samples and in (D) 1012 NR biopsy samples. Finally, survival curves are shown for high versus low ABMRpm classifier score in (E) all 1679 biopsy samples and in (F) 1012 NR biopsy samples.
DSA in the population of 1679 (Figure 6A) was not associated with decreased survival, as was the case in NR biopsy samples (Figure 6B). In contrast, a higher DSAProb score was associated with significantly decreased 3-year graft survival in the whole cohort of 1679 (Figure 6C), and also in NR biopsy samples (Figure 6D). The findings were similar for high ABMRpm scores (Figure 6, E and F).
Discussion
We examined the possibility that circulating DSA was inducing low-grade tissue damage below the thresholds currently established for rejection in some patients. In all 1679 indication biopsy specimens, DSA was positive in 32% (271 of 835) of archetypally assigned NR biopsies with known DSA status. In this set of 835 NR biopsy samples, many of those from DSA-positive patients had mildly increased expression of ABMR-associated transcripts. We developed a machine-learning classifier trained on DSA-positive versus -negative biopsy samples (DSAProb) and found that it was similar to the previously described ABMRpm classifier trained on histologic ABMR. Both the DSAProb classifier and the ABMRpm classifier increased steadily from NR DSA-negative biopsy samples to NR DSA-positive biopsy samples, and finally to biopsy specimens with diagnosed ABMR. The top transcripts correlating with scores for both classifiers were very similar (13 of 20 shared) and reflected previously described ABMR transcript associations: IFNG-inducible, NK cell, and endothelial transcripts.16 Higher DSAProb and ABMRpm classifier scores in NR biopsy samples were associated with decreased graft survival at 3 years post-transplant, probably due to an increase in ABMR-related parenchymal injury, as previously reported.15 We conclude that in DSA-positive patients, many biopsy samples classified as archetypal NR, or both archetypal NR and histologic NR, demonstrate mildly increased ABMR-associated transcript expression related to increased risk for future graft failure.
Training on transcript expression in DSA-positive biopsy samples and biopsy samples with histologic ABMR produces similar classifiers, indicating that both of these conventional features guide the machine learning to similar molecular features. The DSAProb classifier was derived from gene expression in DSA-positive versus DSA-negative indication biopsy samples, and was not intended to estimate actual DSA status, given the frequency of DSA-positive biopsy samples with no rejection and DSA-negative biopsy samples with ABMR. The point of the DSA classifier is to have a new independent estimate of molecular ABMR-related changes, which can now be added to our ensembles of rejection estimates.7 Subsequent analyses of post-1679 biopsy samples and renal transplant biopsy samples collected in other studies have revealed the same predictive performance as estimated by the DSAProb prediction of DSA status AUC (this AUC is also expected given the number of DSA-positive NR and DSA-negative ABMR cases). DSA is a complex variable in terms of strength (titer), IgG subclass, complement binding, HLA locus specificity, and de novo versus preexisting status, and these features will affect its probability of being pathogenic. These details are often not explored due to cost and limitations in available technology. As measured by SOC, i.e., Luminex beads at each center, there is considerable interlaboratory variation.17 (We are currently analyzing DSA details in an ongoing study to account for the inherent differences in SOC technologies among the centers. At minimum, we have found results showing that most of the DSA-positive patients have class II DSA, with or without class I, but MFI and other quantitation methods cannot be pooled.) Because DSA MFI is not validated for comparison across centers or tissue typing laboratories, a DSA yes/no verdict had to be used for this study, which suffers from this lack of standardization especially at lower MFIs. Future studies should attempt to address this limitation by improving the standard-of-care DSA assessment.
Possible explanations for DSA-negative ABMR include the presence of HLA antibodies not detected by the current diagnostic platforms (e.g., Luminex),18 non-HLA antibodies, autoantibodies, or other unidentified microcirculation stressors that mimic the effects of antibody such as missing self-recognition by NK cells.19,20 The same mechanisms that operate in diagnosed ABMR are probably also operating subtly in DSA-positive NR biopsy samples.
The significance of positive DSA in patients whose biopsy sample has been interpreted as “NR” by current thresholds must now be reinterpreted in light of our present findings. DSA-associated ABMR-like expression in NR biopsy samples is often but not always associated with DSA positivity, and it is still likely that some reported positive DSA is truly nonpathogenic. Some DSA does not activate mechanisms such as complement, which requires IgG hexamer formation.21 This may also apply to activation of Fc receptors, which may also require multimerization.22 Some reported DSAs are reactions with denatured proteins on the Luminex beads that are not relevant to reactivity against native HLA antigens. It will be important to develop improved DSA detection technologies that define non-HLA antibodies, identify new epitopes, discard denatured epitopes, and classify antibody isotypes and FcR interactions to better reflect the pathogenic potential of the specific DSA detected.
The present results indicate that some DSAs in biopsy specimens currently classified NR are acting to alter the renal parenchyma, presumably the microcirculation. This produces low-level ABMR-like transcript expression, e.g., increased expression of interferon-γ-inducible genes, endothelial gene ROBO4, and CCL11.23 In other words, very low-grade ABMR-related mechanisms are operating in many kidneys below the clinical or molecular thresholds for an ABMR diagnosis, and this stress increases the risk for future graft failure. The natural history of these cases will need to be defined, e.g., whether they eventually develop typical ABMR histologic and molecular features or simply progress to nephron loss (atrophy-fibrosis).
Although DSA in biopsy specimens is not significantly associated with TCMR, DSAProb and ABMR molecular classifiers are both associated with slightly higher TCMR classifier scores, and this is not demonstrably related to DSA status in these cases. We are currently studying this effect in detail within the TCMR biopsy samples. DSA positivity does not induce TCMR-selective features but the presence of TCMR does seem to elevate the scores for ABMRpm and DSAProb classifiers mildly, either due to molecular sharing between TCMR and ABMR, or subtle ABMR (probably DSA-negative) accompanying the TCMR. Some molecular features of TCMR (e.g., IFNG effects and CCL4) are shared between TCMR and ABMR,24 and the effector T cells in TCMR share many molecules with the activated NK cells in ABMR.25,26
Increased expression of ABMR-related genes in many biopsy samples considered to have no rejection impels us to reconsider how we classify diseases, and how to establish the thresholds for positivity. ABMR was recognized by the association of microcirculation inflammation, DSA, and dysfunction,27 and later by the addition of C4d staining.28 Subsequent experience showed that the disease was more extensive than this, including C4d-negative ABMR29,30 and DSA-negative ABMR.4,31 Molecular testing was able to define cases without using DSA or C4d, which permitted mapping of the cases that lacked these features, while calibrating against the lesions and clinical syndromes. In contrast, histologic TCMR was always defined by the relationship of histologic lesions to a clinical syndrome distinct from the ABMR microcirculation syndrome27 but was forced to acknowledge a relatively large number of “borderline” cases. New approaches can be applied to histology to create a more probabilistic assessment.32 The present data show that an increase in ABMR-like transcript expression occurs at a level below current diagnostic thresholds, and is associated with increased risk for graft loss. Given the limited efficacy of treatment for ABMR, the challenge now is to establish how to use such probabilistic information to guide management.
The results of this study show how a DSAProb classifier can be used as an estimate of ABMR and to help predict outcomes. We have previously discussed the advantages of ensembles in terms of accuracy,7 and we look for opportunities for new independent estimates to improve on the system as a whole. Our results reveal subtle ABMR-related gene expression that is associated with outcomes even in biopsy samples with no molecular or histologic rejection, using both the DSAProb and ABMR classifiers. It will be important to include both DSAProb and ABMRpm classifier scores in assessment of graft risk and probability of ABMR going forward, in effect triangulating on the true ABMR-related changes. Moreover, DSAProb classifiers will be useful to assess ABMR-related changes in areas such as lung and liver transplantation where histologic ABMR is too poorly developed to permit the development of reliable ABMR-trained classifiers.
Disclosures
G.A. Böhmig reports Consultancy Agreements with Vitaeris Inc, Vancouver, Canada; Research Funding from CareDx and Vitaeris Inc.; Honoraria from Astellas, Fresenius Medical Care, OneLambda, and Sandoz; and Scientific Advisor or Membership with CSL Behring and Vitaeris Inc. J. Bromberg reports Consultancy Agreements with Eurofins; Research Funding from Angion, Astellas, CareDx, Natera, Novartis, and Quark; and Scientific Advisor or Membership with the NIH and Transplantation. G. Einecke reports Honoraria from Biotest AG, CareDx, Chiesi GmbH, and Novartis AG; Scientific Advisor or Membership with Biotest AG, CareDx, Chiesi GmbH, and Novartis AG; and Other Interests/Relationships as Committee member in the German Society for Immunogenetics and the German Society of Transplantation (DTG). G. Gupta reports Consultancy Agreements with CareDx; Research Funding from Gilead Pharmaceuticals; Honoraria from Alexion, CareDx, Mallinckrodt, Natera, and Veloxis; Scientific Advisor or Membership with Frontiers of Medicine; Speakers Bureau with Alexion, CareDx, Mallinckrodt, and Veloxis; and Other Interests/Relationships with AST KOP Executive Committee, AST Transplant Nephrology Fellowship Accreditation Committee, and the National Kidney Foundation Virginia. P.F. Halloran holds shares in Transcriptome Sciences Inc., a University of Alberta research company with an interest in molecular diagnostics; has given lectures for Thermo Fisher; and is a consultant for CSL Behring. P.F. Halloran also reports Honoraria from Astellas; Scientific Advisor or Membership as Chief Executive Officer of Transcriptome Sciences Inc.; Editor-in-chief emeritus of the American Journal of Transplantation; and Speakers Bureau from Astellas and One Lambda. P.F. Halloran held a Canada Research Chair in Transplant Immunology until 2008 and currently holds the Muttart Chair in Clinical Immunology. K.S. Madill-Thomsen reports current employment with Transcriptome Sciences Inc., a University of Alberta Research Company. L.G. Hidalgo reports Scientific Advisor or Membership a as member of the American Society of Histocompatibility and Immunogenetics Board of Directors. O. Viklicky reports Honoraria from Astellas, Chiesi, Fresenius; and Scientific Advisor or Membership with Bayer. A. Perkowska-Ptasinska reports Research Funding from Travere; and Honoraria from Chiesi, Roche, and Travere. All remaining authors have nothing to disclose.
Funding
This research has been principally supported by grants from Genome Canada, Canada Foundation for Innovation, the University of Alberta Hospital Foundation, the Alberta Ministry of Innovation and Advanced Education and Technology, the Mendez National Institute of Transplantation Foundation, and the Industrial Research Assistance Program. Partial support was also provided by funding from a licensing agreement with the One Lambda Division of Thermo Fisher.
Supplementary Material
Acknowledgments
We thank our valued clinicians in the INTERCOMEX study group who partnered with us for this study by contributing biopsy specimens and feedback (Michael Picton, Timm Heinbokel, Harold Yang, Seth Narins, Carmen Lefaucheur, Alexandre Loupy, Marek Myslak, Bertram Kasiske, Arthur Matas, and Arjang Djamali).
Katelynn S. Madill-Thomsen was responsible for manuscript writing/reviewing and data analysis and interpretation. Georg A. Böhmig, Jonathan Bromberg, Gunilla Einecke, Farsad Eskandary, Gaurav Gupta, Luis G. Hidalgo, Marek Myslak, Ondrej Viklicky, and Agnieszka Perkowska-Ptasinska performed biopsy sample collection and reviewed the manuscript. Philip F. Halloran was the principal investigator, and was responsible for manuscript writing/reviewing and data interpretation.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
Contributor Information
Collaborators: Roslyn Mannon, Daniel Serón, Joana Sellarés, Enver Akalin, Declan de Freitas, Michael Picton, Jonathan Bromberg, Matt Weir, Klemens Budde, Timm Heinbokel, Gunilla Einecke, Harold Yang, Seth Narins, Milagros Samaniego-Picota, Carmen Lefaucheur, Alexandre Loupy, Marek Myslak, Agnieszka Perkowska-Ptasinska, Adam Bingaman, Daniel Brennan, Andrew Malone, Bertram Kasiske, Philip F Halloran, Arthur Matas, Arjang Djamali, Georg Böhmig, Farsad Eskandary, and Gaurav Gupta
Data Sharing Statement
CEL files are available on the Gene Expression Omnibus website (GSE124203).
Supplemental Material
This article contains the following supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2021040433/-/DCSupplemental.
Supplemental Table 1. Participating Centers.
Supplemental Table 2. List of abbreviations and their definitions used in the manuscript.
Supplemental Table 3. Demographics and clinical features of the 1679 biopsy cohort and 1012 No Rejection only biopsy cohort.
Supplemental Table 4. Histologic diagnoses and DSA status in the kidney 1679 cohort, and in the 1012 no rejection cohort (n, % of total).
Supplemental Table 5. DSA detail in full population (1394 tested of 1679 total) and in NR biopsies (835 tested of 1012 total).
Supplemental Table 6. Mean PBT scores in DSA+ versus DSA− biopsies within archetypally assigned NR, ABMR, and TCMR groups.
Supplemental Figure 1. Venn diagram showing relationships between A) histologically and B) molecularly defined NR (blue) and ABMR (purple), the DSAprob score >0.47 (orange), and the ABMRpm score >0.2 (green).
References
- 1.Haas M, Loupy A, Lefaucheur C, Roufosse C, Glotz D, Seron D, et al. : The Banff 2017 Kidney Meeting Report: revised diagnostic criteria for chronic active T cell-mediated rejection, antibody-mediated rejection, and prospects for integrative endpoints for next-generation clinical trials. Am J Transplant 18: 293–307, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Senev A, Coemans M, Lerut E, Van Sandt V, Daniëls L, Kuypers D, et al. : Histological picture of antibody-mediated rejection without donor-specific anti-HLA antibodies: clinical presentation and implications for outcome. Am J Transplant 19: 763–780, 2019 [DOI] [PubMed] [Google Scholar]
- 3.Halloran PF, Reeve J, Akalin E, Aubert O, Bohmig GA, Brennan D, et al. : Real time central assessment of kidney transplant indication biopsies by microarrays: The INTERCOMEX Study. Am J Transplant 17: 2851–2862, 2017 [DOI] [PubMed] [Google Scholar]
- 4.Reeve J, Böhmig GA, Eskandary F, Einecke G, Lefaucheur C, Loupy A, et al. ; MMDx-Kidney study group : Assessing rejection-related disease in kidney transplant biopsies based on archetypal analysis of molecular phenotypes. JCI Insight 2: e94197, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reed EF, Rao P, Zhang Z, Gebel H, Bray RA, Guleria I, et al. : Comprehensive assessment and standardization of solid phase multiplex-bead arrays for the detection of antibodies to HLA. Am J Transplant 13: 1859–1870, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Callemeyn J, Ameye H, Lerut E, Senev A, Coemans M, Van Loon E, et al. : Revisiting the changes in the Banff classification for antibody-mediated rejection after kidney transplantation. Am J Transplant 21: 2413-2423, 2021 [DOI] [PubMed] [Google Scholar]
- 7.Reeve J, Böhmig GA, Eskandary F, Einecke G, Gupta G, Madill-Thomsen K, et al. ; INTERCOMEX MMDx-Kidney Study Group : Generating automated kidney transplant biopsy reports combining molecular measurements with ensembles of machine learning classifiers. Am J Transplant 19: 2719–2731, 2019 [DOI] [PubMed] [Google Scholar]
- 8.Halloran K, Parkes MD, Chang J, Famulski K, Reeve J, Hachem R, et al. : Molecular detection of rejection-like changes in proximal bronchial mucosal lung transplant biopsies: initial findings of the INTERLUNG study. J Heart Lung Transplant 37: S80–S81, 2018 [Google Scholar]
- 9.Lê S, Josse J, Husson F: FactoMineR: AnRPackage for multivariate analysis. J Stat Softw 25: 18, 2008 [Google Scholar]
- 10.Konopka T: umap: Uniform Manifold Approximation and Projection. R package version 0240, 2019. Available at: https://cran.r-project.org/package=umap. Accessed February 18, 2021
- 11.Kuhn M: caret: Classification and Regression Training. R package version 6.0-81 ed., 2018. Available at: https://CRAN.R-project.org/package=caret. Accessed February 18, 2021
- 12.Sellarés J, Reeve J, Loupy A, Mengel M, Sis B, Skene A, et al. : Molecular diagnosis of antibody-mediated rejection in human kidney transplants. Am J Transplant 13: 971–983, 2013 [DOI] [PubMed] [Google Scholar]
- 13.Therneau T: A package for survival analysis in R. R package version 32-7, 2020. Available at: https://CRAN.R-project.org/package=survival. Accessed February 18, 2021
- 14.Kassambara A, Kosinski M, Biecek P: survminer: drawing survival curves using 'ggplot2'. R package version 048, 2020. Available at https://CRAN.R-project.org/package=survminer. Accessed February 18, 2021
- 15.Einecke G, Reeve J, Gupta G, Böhmig GA, Eskandary F, Bromberg JS, et al. ; INTERCOMEX Investigators : Factors associated with kidney graft survival in pure antibody-mediated rejection at the time of indication biopsy: Importance of parenchymal injury but not disease activity. Am J Transplant 21: 1391–1401, 2021 [DOI] [PubMed] [Google Scholar]
- 16.Halloran PF, Venner JM, Madill-Thomsen KS, Einecke G, Parkes MD, Hidalgo LG, et al. : Review: The transcripts associated with organ allograft rejection. Am J Transplant 18: 785–795, 2018 [DOI] [PubMed] [Google Scholar]
- 17.Konvalinka A, Tinckam K: Utility of HLA antibody testing in kidney transplantation. J Am Soc Nephrol 26: 1489–1502, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tait BD: Detection of HLA antibodies in organ transplant recipients—Triumphs and challenges of the solid phase bead assay. Front Immunol 7: 570, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Koenig A, Mezaache S, Callemeyn J, Barba T, Mathias V, Sicard A, et al. : Missing self-induced activation of NK cells combines with non-complement-fixing donor-specific antibodies to accelerate kidney transplant loss in chronic antibody-mediated rejection. J Am Soc Nephrol 32: 479–494, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Koenig A, Chen CC, Marçais A, Barba T, Mathias V, Sicard A, et al. : Missing self triggers NK cell-mediated chronic vascular rejection of solid organ transplants. Nat Commun 10: 5350, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Diebolder CA, Beurskens FJ, de Jong RN, Koning RI, Strumane K, Lindorfer MA, et al. : Complement is activated by IgG hexamers assembled at the cell surface. Science 343: 1260–1263, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang Y, Boesen CC, Radaev S, Brooks AG, Fridman WH, Sautes-Fridman C, et al. : Crystal structure of the extracellular domain of a human FcγRIII. Immunity 13: 387–395, 2000 [DOI] [PubMed] [Google Scholar]
- 23.Halloran PF, Venner JM, Famulski KS: Comprehensive analysis of transcript changes associated with allograft rejection: Combining universal and selective features. Am J Transplant 17: 1754–1769, 2017 [DOI] [PubMed] [Google Scholar]
- 24.Halloran PF, Famulski K, Reeve J: The molecular phenotypes of rejection in kidney transplant biopsies. Curr Opin Organ Transplant 20: 359–367, 2015 [DOI] [PubMed] [Google Scholar]
- 25.Hidalgo LG, Einecke G, Allanach K, Halloran PF: The transcriptome of human cytotoxic T cells: similarities and disparities among allostimulated CD4+ CTL, CD8+ CTL and NK cells. Am J Transplant 8: 627–636, 2008 [DOI] [PubMed] [Google Scholar]
- 26.Parkes MD, Halloran PF, Hidalgo LG: Mechanistic sharing between NK cells in ABMR and effector T cells in TCMR. Am J Transplant 18: 63–73, 2018 [DOI] [PubMed] [Google Scholar]
- 27.Halloran PF, Wadgymar A, Ritchie S, Falk J, Solez K, Srinivasa NS: The significance of the anti-class I antibody response. I. Clinical and pathologic features of anti-class I-mediated rejection. Transplantation 49: 85–91, 1990 [DOI] [PubMed] [Google Scholar]
- 28.Feucht HE, Felber E, Gokel MJ, Hillebrand G, Nattermann U, Brockmeyer C, et al. : Vascular deposition of complement-split products in kidney allografts with cell-mediated rejection. Clin Exp Immunol 86: 464–470, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sis B, Reeve J, Einecke G, Bunnag S, Hidalgo L, Mengel M, et al. : Endothelial stress accelerates graft loss in kidneys with transplant glomerulopathy despite lack of C4D staining. Am J Transplant 9: 349–349, 2009 [Google Scholar]
- 30.Einecke G, Sis B, Reeve J, Mengel M, Campbell PM, Hidalgo LG, et al. : Antibody-mediated microcirculation injury is the major cause of late kidney transplant failure. Am J Transplant 9: 2520–2531, 2009 [DOI] [PubMed] [Google Scholar]
- 31.Callemeyn J, Lerut E, de Loor H, Arijs I, Thaunat O, Koenig A, et al. : Transcriptional changes in kidney allografts with histology of antibody-mediated rejection without anti-HLA donor-specific antibodies. J Am Soc Nephrol 31: 2168–2183, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vaulet T, Divard G, Thaunat O, Lerut E, Senev A, Aubert O, et al. : Data-driven derivation and validation of novel phenotypes for acute kidney transplant rejection using semi-supervised clustering. J Am Soc Nephrol 32: 1084–1096, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.