Significance Statement
CKD is defined by both functional changes (such as in eGFR and proteinuria) and renal histologic alterations. Although kidney function is acutely regulated, histologic changes such as interstitial fibrosis, tubular atrophy, and glomerulosclerosis could represent chronic damage, thus might provide additional information about disease severity. In an analysis of 859 kidney tissue samples, the authors found that the relationship between histologic changes and eGFR is not linear. At CKD stages 3–5, eGFR correlates with interstitial fibrosis/tubular atrophy and glomerulosclerosis reasonably well, whereas at earlier disease stages, eGFR poorly estimates histologic damage. Patients with diabetes, hypertension, or Black race had more severe histologic damage at the same eGFR. The inclusion of glomerulosclerosis significantly improved the kidney function decline estimation.
Keywords: chronic kidney disease, glomerular filtration rate, histopathology, fibrosis, glomerulosclerosis
Abstract
Background
Patients with diabetic or hypertensive kidney disease rarely undergo kidney biopsy because nephrologists commonly believe that biopsy-related risk outweighs the potential benefits of obtaining histologic information to guide clinical decisions. Although kidney function is acutely regulated, histologic changes such as interstitial fibrosis, tubular atrophy, and glomerulosclerosis may represent chronic kidney damage, and thus might provide additional information about disease severity. However, whether histologic analysis provides information complementary to clinically used kidney function measurements, such as eGFR and proteinuria, is unclear.
Methods
We performed a standardized semiquantitative histologic analysis of 859 nephrectomies obtained from individuals with or without diabetes mellitus or hypertension and varying degrees of kidney dysfunction. Changes in glomeruli, tubules, interstitium, and the vasculature were scored using 17 descriptive parameters in a standardized manner. We used multivariable linear and logistic regression analyses and unbiased, hierarchical clustering to assess associations between histologic alterations and clinical variables.
Results
At CKD stages 3–5, eGFR correlates reasonably well with the degree of glomerulosclerosis and interstitial fibrosis and tubular atrophy (IFTA). In patients with CKD stages 1–2, the degree of histologic damage was highly variable and eGFR poorly estimated the degree of damage. Individuals with diabetes mellitus, hypertension, or Black race had significantly more glomerulosclerosis and IFTA, at the same eGFR level. Inclusion of glomerulosclerosis improved the kidney function decline estimation, even at early disease stages.
Conclusions
Histologic analysis is an important complementary method for kidney disease evaluation, especially at early disease stages. Some individuals present with relatively severe structural damage despite preserved eGFR.
CKD is defined by functional changes, reduction of eGFR, proteinuria, or by structural kidney damage (glomerulosclerosis [GS] and interstitial fibrosis [IF] and tubule atrophy [TA]).1 Diabetes mellitus (DM) and hypertension (HTN) are responsible for most (70%) CKD. Histopathological characterization of diabetic kidney disease dates back more than six decades.2 More recently, the diagnosis of diabetic and hypertensive CKD is established by clinical parameters, such as eGFR and proteinuria.
Kidney biopsies in DM and HTN are usually restricted for patients with atypical presentations because nephrologists commonly believe the risk of the kidney biopsy outweighs the potential benefit of obtaining histologic information for clinical decision making.3 Research biopsy samples have only been obtained in the Pima Indian population4 or in a small subgroup of patients with type 1 diabetes;5 however, the generalizability of these findings is unclear, given small study sizes, ethnic differences, and that most patients in our clinics have type 2 not type1 diabetes. Recent studies have almost exclusively relied on clinically indicated biopsies that are biased toward patients with unusual presentations and advanced disease stages.6 These studies indicate the dominance of chronic changes, such as GS, IF and tubular atrophy (IFTA), and vascular sclerosis.7–9 In single-center collections of clinically obtained kidney biopsies that were heavily biased toward patients with glomerular diseases, GS and IFTA correlated with eGFR.10–12
Given the observed correlation between eGFR and fibrosis, it remains unclear whether histologic alterations represent an independent domain of disease severity from eGFR.10–18 In several scenarios, the eGFR is a poor estimator of kidney damage and prognosis. Acute changes in eGFR make it difficult to evaluate disease severity in deceased kidney donors, or after the initiation of treatment with inhibitors of the angiotensin converting enzyme inhibitor, angiotensin receptor blocker, or sodium glucose cotransporter inhibitor. It is also challenging to estimate kidney damage in subjects with extreme age, extreme body size (obesity and cachexia), and severe liver disease. A histologic damage–based CKD staging system might be useful in these scenarios for risk stratification.
Baseline kidney function strongly predicts CKD progression at CKD stages 3–5.19 In contrast, kidney function is a poor predictor of progression in subjects with eGFR ≥60 ml/min per 1.73 m2.2 Sethi and colleagues proposed that histologic damage could be a better predictor of kidney function decline than eGFR,13 because it represents chronic irreversible events.14 The role of fibrosis in kidney function decline prediction has not been formally tested because most histologic studies do not adjust for baseline kidney function, which is a key determinant of progression, and/or analyze samples with advanced CKD. Two recent reports indicated that inclusion of GS and/or IFTA can improve prediction of kidney function decline even after adjusting for baseline kidney function when applied to clinically indicated biopsy samples, which again were significantly biased toward patients with glomerular disease.12,17 Multiple other studies did not replicate this observation.11,18
In this study we analyzed the precision of eGFR and other clinical parameters in estimating histologic abnormalities in subjects with and without DM and HTN, and varying degrees of CKD, with the aim of clarifying the potential role of histologic information in clinical assessment of diabetic and hypertensive CKD in an unbiased collection of tissue samples. In addition, we also examined the role of histologic damage in predicting kidney function decline.
Methods
Study Population
This study consisted of a cross-sectional evaluation of 1007 human subjects undergoing clinically indicated nephrectomies for renal neoplasia across six academic medical centers. Demographic, clinical information and laboratory data were obtained from medical records by an “honest broker,” as established by Cooperative Human Tissue Network. The study protocol was approved by the institutional review board of the University of Pennsylvania and no informed consent was obtained because the study was considered exempt.
Subjects were included in the analysis if they were ≥18 years old, had serum creatinine measurement at time of enrollment, and had a tumor-free renal cortex. eGFR values were calculated using the CKD Epidemiology Collaboration equation20 on the basis of serum creatinine, age, sex, and self-reported or clinician-determined race, as obtained from medical charts. The final sample size was 859 and Supplemental Figure 1 shows the study flow chart. For a subset of samples, we were able to obtain longitudinal kidney function measurements. The longitudinal cohort included 280 subjects with ≥3 months follow-up, of which 167 had complete clinical information after nephrectomy. On average, we collected three kidney function measurements (serum creatinine) over a period of at least 1 year, spanning pre- and postnephrectomy.
Sample Procurement and Histopathological Analysis
Deidentified human kidney tissues were collected from the non-neoplastic portion of surgical nephrectomies at least 2 cm away from the cancer margin. Kidney samples were formalin fixed, paraffin embedded, and stained with periodic acid–Schiff. Samples were scored in an unbiased manner by a specialized renal pathologist. On average, we scored 129 (mean SD 123) glomeruli per sample. More than 93% of samples had >10 glomeruli, an important advantage of using nephrectomy samples, compared with the limited number of glomeruli present in biopsy specimens.
In total, 17 light microscopy (LM) parameters were selected for semiquantitative scoring to describe the tubulointerstitium, glomeruli, and renal vasculature. Definitions of these LM descriptors are detailed Supplemental Table 1, and are adapted from the Neptune digital pathology protocol,21 the Oxford classification of IgA nephropathy,22 and the Renal Pathology Society classification of diabetic nephropathy.23 Glomerular descriptors included global GS, segmental sclerosis, Kimmelstiel–Wilson (KW) nodules, glomerular wall–thickening, pericapsular fibrosis, glomerular hypoperfusion, mesangial matrix, and mesangial cellularity. Vascular descriptors included arteriolar hyalinosis and arterial intimal fibrosis. Tubulointerstitial descriptors included acute tubular injury,24 TA, tubular reabsorption droplets, IF, interstitial eosinophils, lymphocytes, and plasma cells.
Statistics
Statistical analyses were performed using RStudio v3.6.2 (R Development Core Team, Vienna, Austria) and STATA version 16.0 (Statacorp LP, College Station, TX). Descriptive statistics (i.e., mean, median, and proportion) were used to describe clinical and demographic characteristics across all subjects. Continuous variables were compared using Kruskal–Wallis test for non-normally distributed variables and ANOVA for normally distributed variables. Categorical and binary variables were compared using chi-squared test. Spearman correlations coefficients (rho) were estimated eGFR and histopathological variables.
We analyzed the following covariates at the time of nephrectomy: age, sex, presence of DM, presence of HTN, race, body mass index (BMI), BP, serum albumin, and urine dipstick. Race/ethnicity was reported as Black (e.g., African American), White (e.g., Caucasian), Asian, Hispanic, multiracial, and other, and was recategorized as Black and non-Black, as used in the CKD Epidemiology Collaboration equation. Variables with >20% missingness were not included into the primary regression model but were assessed secondarily for effect.
Continuous histopathologic variables including IFTA and GS, were each log transformed (with constant, i.e., 1, added to account for scores of zero) for the linear regression analyses. The relationship between histopathologic variables and eGFR was modeled as a polynomial function of eGFR (e.g., eGFR2 and eGFR3) and adjusted for age, sex, race, BMI, diabetes, and/or HTN status. To obtain adjusted means for log-transformed variables on the original scale, we exponentiated the model predictions and subtracted 1 (given constant of 1 added to samples before log transformation), and then used smearing adjustment to decrease bias introduced by the retransformation.25 Stepwise regression with a combination of forward and backward selection was used to guide variable selection26 in the construction of primary regression models for histopathologic dependent variables. Independent variables tested were age, sex, race, eGFR, BMI, diabetes, and HTN. Several variables were binarized, using the following criteria: any segmental sclerosis, >1+ glomerular hypoperfusion, >1+ mesangial cellularity, >1+ mesangial matrix, >1+ glomerular wall thickening, >1+ pericapsular fibrosis, any acute tubular injury, any tubular reabsorption, >1+ interstitial lymphocytic infiltrate, any interstitial plasmocytic infiltrate, any interstitial eosinophils, >1+ intimal fibrosis, and >1+ arterial hyalinosis. Logistic regression was used to test binary outcomes. The significance level for removal from the model was set at ≥0.2 and significance level for addition to the model was set at <0.05. Robust standard errors were used and an alpha level <0.05 was considered statistically significant. Model fitness was determined by Akaike Information Criterion and likelihood ratio testing.
Longitudinal eGFR was modeled using linear mixed modeling in which subject-specific eGFR slopes were estimated by regressing repeated eGFR values on the time variable (random effects), which was added to the population-average model (fixed-effects model) with the overall model fit estimated via maximum likelihood.27
To identify unbiased subgroups on the basis of LM descriptors, we used hierarchical clustering methods on scaled data.28 The average silhouette method was used to determine the optimal number of clusters with data shown as a cluster dendrogram.29 Radar plots were used to show differences between clusters in terms of LM. To compare the continuous and categorical clinical, laboratory, and pathologic parameters between groups, Kruskal–Wallis and chi-squared tests were used, respectively.
Results
Clinical and Histologic Characteristics of Study Participants
Kidney tissue samples were obtained from the non-neoplastic portion of tumor nephrectomies at six academic medical centers. Supplemental Figure 1 shows the inclusion and exclusion criteria leading to our final sample size of 859, with available histopathological and clinical information (Table 1). The mean subject age was 61 (SD±13) years, 64% were male, and 20% were of Black race. In total, 35% of subjects had a history of DM and 69% had a history of HTN. Mean BP was 136/77 mm Hg in the cohort. The median BMI was 29 kg/m2. The mean eGFR was 65 (SD±26.7) ml/min per 1.73 m2 and 55% of subjects had no detectable proteinuria by dipstick analysis. As expected, some of the clinical variables showed relatedness. Kidney function (eGFR) correlated with age, HTN, systolic BP, BMI, and proteinuria. Diabetic subjects were older (median age 66 versus 60 years, P<0.001), had a significantly lower median eGFR (65 versus 69 ml/min per 1.73 m2, P<0.001) and the majority had HTN (88.2% versus 59.4%, P<0.001) (data not shown).
Table 1.
Clinical and histopathological characteristics of subjects in our multi-institutional kidney tissue biobank
| Clinical and Histopathological Characteristics (n=859) | Result |
|---|---|
| eGFR, mean (SD), ml/min per 1.73 m2 | 65 (26.7) |
| Age, mean (SD), yr | 61 (13) |
| DM, n (%) | 304 (35.4) |
| HTN, n (%) | 595 (69) |
| Sex, M, n (%) | 548 (63.8) |
| Race, Black, n (%) | 176 (20.4) |
| BMI, median (IQR), kg/m2 | 29.4 (25.7–34.2) |
| Systolic BP, mean (SD), mm Hg | 136 (20) |
| Diastolic BP, mean (SD), mm Hg | 77 (12) |
| Serum albumin, median (IQR), g/dl | 4.05 (3.7–4.4) |
| Dipstick proteinuria (%) | |
| Negative | 54.9 |
| Trace | 9.2 |
| 30 mg/dl | 14.9 |
| 100 mg/dl | 12.5 |
| 300 mg/dl | 1.3 |
| >1000 mg/dl | 7.6 |
| Tubulointerstitial | |
| Acute tubular injury, median (IQR) (%) | 0 (0–2) |
| TA, median (IQR) (%) | 5 (2–10) |
| Tubular reabsorption 0–3 (%) | |
| 0 | 81.4 |
| 1 | 16.1 |
| 2 | 2.1 |
| 3 | 0.5 |
| Interstitial fibrosis, median (IQR) (%) | 5 (2–15) |
| Interstitial eosinophils 0–3 (%) | |
| 0 | 78.8 |
| 1 | 19.6 |
| 2 | 1.4 |
| 3 | 0.2 |
| Interstitial lymphocytic infiltrates 0–3 (%) | |
| 0 | 25.7 |
| 1 | 48.3 |
| 2 | 19.7 |
| 3 | 6.3 |
| Interstitial plasmocytic infiltrates 0–3 (%) | |
| 0 | 65.4 |
| 1 | 28.3 |
| 2 | 5.4 |
| 3 | 0.9 |
| Glomerular | |
| Segmental sclerosis, median (IQR) (range) (%) | 0 (0) (0–29) |
| GS, median (IQR) (%) | 6.7 (2.7–16.1) |
| Wall thickening 0–3 (%) | |
| 0 | 88.9 |
| 1 | 7.2 |
| 2 | 2.9 |
| 3 | 1 |
| Hypoperfused 0–3 (%) | |
| 0 | 33.7 |
| 1 | 53.7 |
| 2 | 9.8 |
| 3 | 2.8 |
| Mesangial matrix 0–3 (%) | |
| 0 | 78.2 |
| 1 | 13.2 |
| 2 | 4.4 |
| 3 | 4.2 |
| Mesangial cellularity 0–3 (%) | |
| 0 | 83.1 |
| 1 | 9.4 |
| 2 | 4.3 |
| 3 | 3.1 |
| KW nodules present (%) | 3.5 |
| Pericapsular fibrosis 0–3 (%) | |
| 0 | 43.7 |
| 1 | 41.7 |
| 2 | 14.5 |
| 3 | 0.1 |
| Vascular | |
| Arteriolar hyalinosis 0–3 (%) | |
| 0 | 67.3 |
| 1 | 23.6 |
| 2 | 6.3 |
| 3 | 2.8 |
| Intimal fibrosis 0–3 (%) | |
| 0 | 13.7 |
| 1 | 41.2 |
| 2 | 32.4 |
| 3 | 12.7 |
IQR, interquartile range; M, male.
Histopathological abnormalities were scored semiquantitatively for 17 descriptors on formalin-fixed, paraffin-embedded, and periodic acid–Schiff-stained slides. The median (interquartile range) of combined IFTA was 5% (2%–15%). Glomerular analysis showed a median of 6.7% (2.7%–16.1%) global GS. In our collection of approximately 1000 samples, we identified 170 samples with focal GS, defined as the presence of segmental sclerosis involving <50% of total glomeruli. Assessment of blood vessels indicated that 45.1% of subjects had at least moderate (2+) intimal fibrosis.
As expected, histologic alteration reflecting chronic damage showed important relatedness. The correlation between the tubulointerstitial changes, such as interstitial fibrosis and tubular atrophy, was the highest at 0.94 (Spearman’s rho). The correlation between tubular and glomerular abnormalities was also strong, at 0.66 (Spearman’s rho) between IF and GS. The correlation between vascular changes (intimal fibrosis) and GS and IF were lower (rho 0.44, and 0.48, respectively). Acute injury parameters, such as tubule injury and reabsorption droplets, did not correlate with chronic changes.
Modeling Histologic Abnormalities Using Clinical and Demographic Variables
To understand the extent clinical variables can estimate the observed histologic abnormalities in kidney biopsies, we modeled histologic abnormalities as dependent variables using stepwise linear regression models. Only samples with complete clinical and demographic information were included in the analyses (n=750, Supplemental Table 2).
Several clinical parameters correlated with glomerular, tubulointerstitial, and vascular histological abnormalities (Table 2). We were able to estimate GS on the basis of DM and HTN status, Black race, age, and eGFR. Mesangial matrix expansion, mesangial cellularity, and pericapsular fibrosis showed associations with eGFR, DM, and female sex, and/or Black race. KW nodules showed association with eGFR and DM status. Tubulointerstitial alterations such as IFTA and lymphocytic infiltrate were best modeled by Black race, presence of DM, HTN, eGFR, and BMI. Vascular changes, including intimal fibrosis, showed association with eGFR, age, HTN, and BMI. In general, there was a significant overlap between HTN- and DM-associated lesions except KW nodules, which were highly specific for DM. Overall, DM showed better association with glomerular lesions, whereas HTN also correlated with vascular lesions. Notably, BMI improved models describing vascular, glomerular, and/or tubular abnormalities, and age improved the estimation of GS and intimal fibrosis, indicating the important role of these clinical variables.
Table 2.
The relationship between histologic alterations and clinical and demographic characteristics
| Dependent Variables | F | Adjusted r2 | Probability>F | Independent Variable | Beta | Robust SE | 95% Confidence Interval | P Value | |
|---|---|---|---|---|---|---|---|---|---|
| GS (%) | 54.37 | 0.29 | <0.001 | ||||||
| Glomerular | |||||||||
| DM | 0.306 | 0.079 | 0.151 | 0.462 | <0.001 | ||||
| HTN | 0.122 | 0.083 | −0.042 | 0.286 | 0.14 | ||||
| eGFR | −0.018 | 0.002 | −0.021 | −0.014 | <0.001 | ||||
| Age | 0.010 | 0.003 | 0.004 | 0.017 | <0.01 | ||||
| BMI | −0.007 | 0.005 | −0.017 | 0.003 | 0.09 | ||||
| Wald Chi2 | Pseudo r2 | Prob > chi2 | |||||||
| KW nodules (yes/no) | 24.67 | 0.21 | 0.001 | ||||||
| DM | 1.945 | 0.505 | 0.955 | 2.934 | <0.001 | ||||
| eGFR | −0.037 | 0.497 | −3.507 | −1.558 | <0.001 | ||||
| Mesangial cellularity (yes/no) | 68.4 | 0.22 | <0.001 | ||||||
| DM | 1.693 | 0.341 | 1.025 | 2.361 | <0.001 | ||||
| Female | 0.412 | 0.309 | −0.193 | 1.017 | 0.18 | ||||
| eGFR | −0.033 | 0.006 | −0.045 | −0.021 | <0.001 | ||||
| HTN | 0.743 | 0.552 | −0.340 | 1.830 | 0.18 | ||||
| Mesangial matrix (yes/no) | 69.64 | 0.24 | <0.001 | ||||||
| DM | 2.030 | 0.340 | 1.368 | 2.700 | <0.001 | ||||
| Female | 0.403 | 0.292 | −0.169 | 0.975 | 0.17 | ||||
| eGFR | −0.033 | 0.005 | −0.044 | −0.022 | <0.001 | ||||
| Black race | 0.528 | 0.340 | −0.138 | 1.200 | 0.12 | ||||
| Glomerular wall thickening (yes/no) | 29.88 | 0.24 | <0.001 | ||||||
| DM | 1.460 | 0.442 | 0.592 | 2.324 | <0.01 | ||||
| eGFR | −0.050 | 0.010 | −0.069 | −0.031 | <0.001 | ||||
| Pericapsular fibrosis (yes/no) | 83.25 | 0.18 | <0.001 | ||||||
| Black race | 0.745 | 0.252 | 0.015 | 1.238 | <0.01 | ||||
| DM | 0.606 | 0.234 | 0.148 | 1.064 | <0.01 | ||||
| BMI | −0.023 | 0.018 | −0.058 | 0.012 | 0.20 | ||||
| eGFR | −0.037 | 0.005 | −0.046 | −0.028 | <0.001 | ||||
| Tubulointerstitium | |||||||||
| TA (%) | 58.32 | 0.30 | <0.001 | ||||||
| DM | 0.314 | 0.079 | 0.159 | 0.469 | <0.001 | ||||
| Black race | 0.274 | 0.093 | 0.092 | 0.456 | <0.01 | ||||
| HTN | 0.197 | 0.085 | 0.029 | 0.364 | 0.02 | ||||
| eGFR | −0.021 | 0.001 | −0.024 | −0.018 | <0.001 | ||||
| BMI | −0.012 | 0.005 | −0.021 | −0.002 | 0.02 | ||||
| Interstitial fibrosis (%) | 59.15 | 0.30 | <0.001 | ||||||
| Black race | 0.285 | 0.088 | 0.112 | 0.458 | <0.01 | ||||
| DM | 0.230 | 0.078 | 0.077 | 0.383 | <0.01 | ||||
| HTN | 0.237 | 0.084 | 0.073 | 0.402 | <0.01 | ||||
| eGFR | −0.020 | 0.001 | −0.023 | −0.017 | <0.001 | ||||
| BMI | −0.013 | 0.005 | −0.023 | −0.004 | <0.01 | ||||
| Wald Chi2 | Pseudo r2 | Prob > chi2 | |||||||
| Interstitial lymphocytic infiltrate (yes/no) | 113.68 | 0.16 | <0.001 | ||||||
| DM | 0.615 | 0.200 | 0.223 | 1.001 | <0.01 | ||||
| HTN | 0.419 | 0.243 | −0.057 | 0.894 | 0.09 | ||||
| Black race | 0.301 | 0.216 | −0.122 | 0.723 | 0.16 | ||||
| eGFR | −0.033 | 0.004 | −0.041 | −0.026 | <0.001 | ||||
| BMI | −0.027 | 0.015 | −0.054 | 0.001 | 0.06 | ||||
| Interstitial plasma cell infiltrate (yes/no) | 98.34 | 0.13 | <0.001 | ||||||
| HTN | 0.433 | 0.207 | 0.028 | 0.838 | 0.04 | ||||
| DM | 0.422 | 0.181 | 0.066 | 0.778 | 0.02 | ||||
| eGFR | −0.030 | 0.004 | −0.037 | −0.023 | <0.001 | ||||
| BMI | −0.023 | 0.013 | −0.048 | 0.002 | 0.07 | ||||
| Vessels | |||||||||
| Intimal fibrosis (yes/no) | 107.97 | 0.12 | <0.001 | ||||||
| HTN | 0.720 | 0.187 | 0.354 | 1.085 | <0.001 | ||||
| Age | 0.039 | 0.007 | 0.026 | 0.053 | <0.001 | ||||
| eGFR | −0.016 | 0.003 | −0.022 | −0.009 | <0.001 | ||||
| BMI | −0.015 | 0.011 | −0.037 | 0.007 | 0.19 | ||||
| Arterial hyalinosis (yes/no) | 31.12 | 0.11 | <0.001 | ||||||
| HTN | 0.731 | −0.030 | 1.629 | 0.458 | 0.11 | ||||
| DM | 0.655 | 0.093 | 1.217 | 0.287 | 0.02 | ||||
| eGFR | −0.019 | −0.030 | −0.008 | 0.006 | <0.001 | ||||
Models are organized by dependent variables, that is, glomerular, tubulointerstitial, or vascular abnormality. Linear regression was used for continuous variables (GS, IF, and TA after log transformation) and logistic regression for binary variables. Prob, probability.
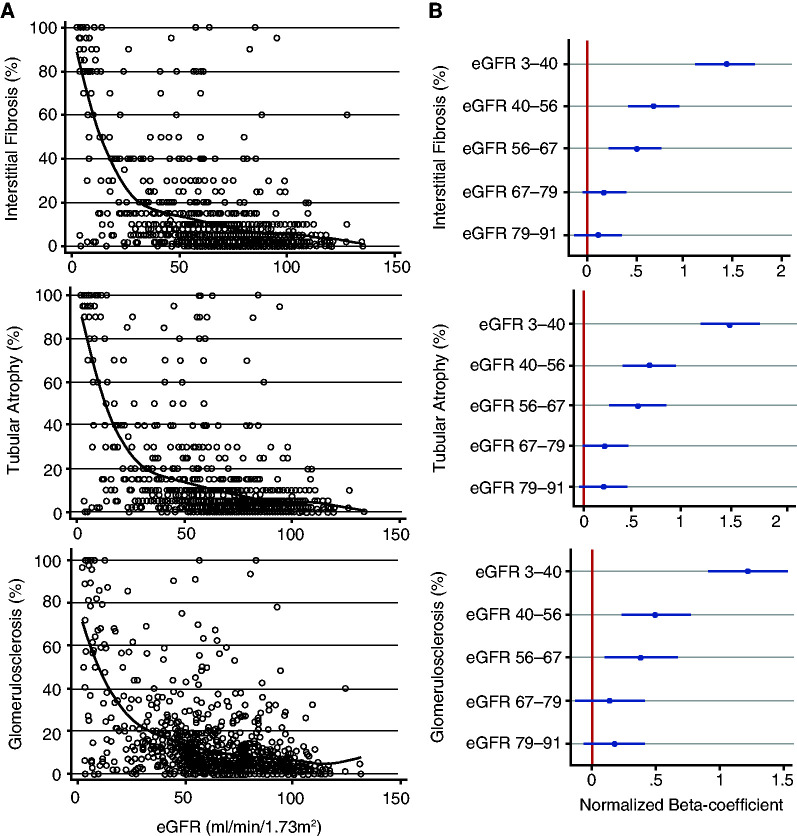
Although we observed a relationship between GS, IFTA (histologic damage), and kidney function, we noted that clinical variables only explained a small fraction of the variance seen by histologic analysis. We hypothesized that this could be explained by a potential nonlinear relationship. Therefore, we generated scatterplots depicting the relationship between eGFR and IFTA and GS (Figure 1A). Local linear polynomial smoothing line highlighted the nonlinear relationship between histologic changes and eGFR.
Figure 1.
IF, TA, and GS have a nonlinear relationship with eGFR. (A) Local two-degree polynomial smoothing for IF, TA, and GS (black line) overlay on scatterplots of IF, TA, or GS versus eGFR (open circles). (B) Beta-coefficients (with 95% confidence intervals [95% CI]) of six eGFR quantiles in regression models normalized to the highest eGFR sextile (eGFR 91–135 ml/min per 1.73 m2). Models were adjusted for age, sex, race, diabetes, HTN, BMI, and systolic BP.
We next created eGFR sextiles of 143 subjects per group to assess the relationship of GS and IFTA at different eGFR levels (Supplemental Table 3). Figure 1B shows beta-coefficient plots normalized to the highest eGFR quantile (eGFR>91 ml/min per 1.73 m2). At eGFR levels below 67 ml/min per 1.73 m2, we observed a stronger association between eGFR and IF, TA and GS than for samples with for eGFR>67 ml/min per 1.73 m2. Indeed, the Spearman correlation between IF, TA, and eGFR was 0.41 for samples eGFR<60 ml/min per 1.73 m2 versus 0.17 for those above. The Spearman correlation between GS and eGFR was 0.37 versus 0.12 at CKD stages 3–5 versus 1–2, and between eGFR and intimal fibrosis it was 0.26 versus 0.09, respectively.
In summary, our results indicate that kidney function estimates histologic damage much better in samples with more advanced kidney disease and fairly poorly in early disease (eGFR≥60 ml/min per 1.73 m2).
How Much IFTA and GS Is Expected at Defined eGFR Levels?
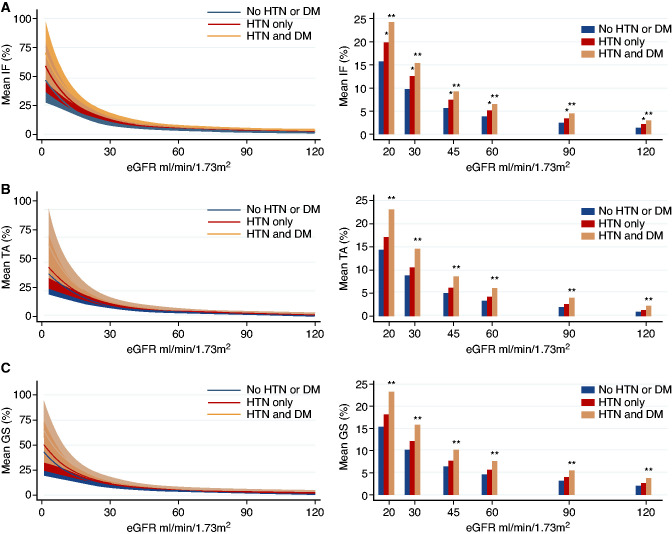
Next, we aimed to define the degree of histologic damage that characterizes each CKD stage using polynomial linear regression, which could model the observed nonlinear relationship. We generated estimates for IFTA and GS for any given eGFR using a polynomial linear regression model, adjusting for covariates previously showing significant effect, namely, age, sex, race, BMI, presence of DM, and/or HTN. Figure 2 shows the relationship between IFTA, GS, and eGFR stratified by DM and HTN (Supplemental Table 4, A–C). We found that subjects with the same eGFR, but with DM and HTN had significantly greater IFTA and GS. For example, eGFR of 60 ml/min per 1.73 m2 was associated with 6.5% IFTA versus <4% IFTA (P<0.001) for subjects with or without DM and HTN, respectively. Similarly, we estimated significantly greater GS in the presence of DM and HTN than absence of DM and HTN (7.5% versus 4.6%, P<0.001). Although DM alone was associated with significantly greater GS at a given eGFR than absence of HTN/DM, most samples with DM also had HTN, we therefore could not untangle the DM and HTN interaction.
Figure 2.
The relationship of histologic alterations and kidney function stratified by HTN and diabetes. (A–C) Adjusted means for percent IF, TA, and GS stratified by absence of HTN and DM (n=193, navy lines±shaded 95% CI and navy bars), HTN alone (n=290, light blue lines±shaded 95% CI and light blue bars), and presence of HTN and DM (n=238, orange lines±shaded 95% CI shaded and orange bars). Percentages of IF, TA, and GS modeled using linear regression as a cubic function of eGFR3 adjusted for age, sex, race, HTN, diabetes, and BMI. Asterisk denotes P<0.05 compared with the “No HTN or DM” group, **P<0.01, * <0.05.
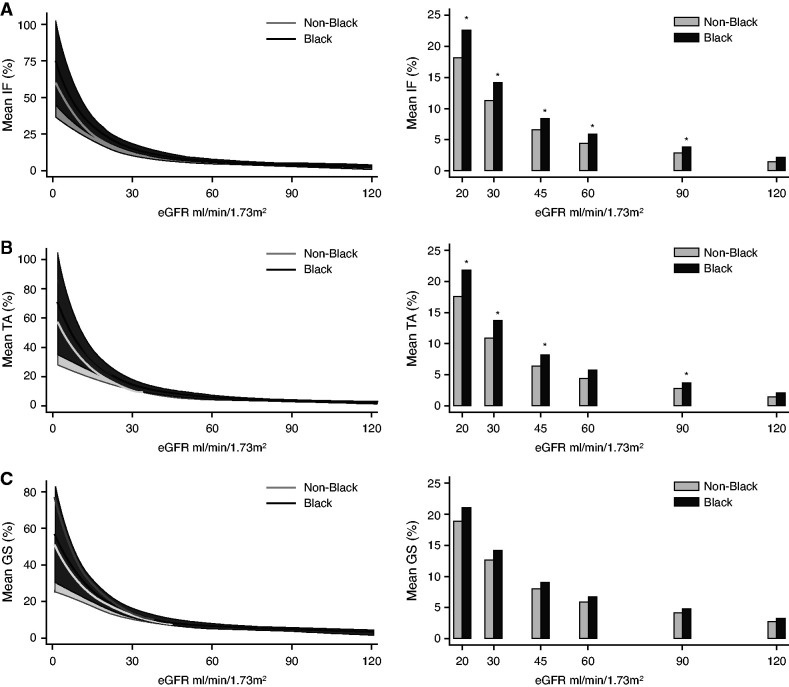
Our preliminary analysis also indicated an association between race and IFTA, therefore we next stratified samples by race (Figure 3). We found that compared with non-Black race, Black race alone was associated with more severe IFTA (P<0.05) but not GS, especially at eGFR values ≤30 ml/min per 1.73 m2 (Supplemental Table 5, A–C). In our study, Black subjects were younger, had lower prevalence of DM, but had significantly higher systolic and diastolic BP and proteinuria on urine dipstick at the time of nephrectomy (Supplemental Table 6). Re-estimation of the eGFR without using the race coefficient showed no statistically significant differences in the degree of IFTA in subjects with Black race compared with non-Black race (Supplemental Table 7). Hence, the differences observed between Black and non-Black participants were eliminated by the removal of race from the eGFR equation.
Figure 3.
The relationship of histologic alterations and kidney function is altered by race. (A–C) Adjusted means for percent IF, TA, and GS stratified by non-Black race (n=590, gray lines±shaded 95% CI and light gray bars) and Black race (n=160, black lines±shaded 95% CI and black bars). Percentages of IF, TA, and GS modeled using linear regression as a cubic function of eGFR3 adjusted for age, sex, race, HTN, diabetes, and BMI. *P<0.05 compared with the non-Black race group.
In summary, we developed estimates for the degree of IFTA and GS for any given eGFR and CKD stage. We found that for the observed eGFR, DM, HTN and Black race were associated with more severe GS and IFTA.
Histology-Based Unbiased Clustering Analysis Highlights Heterogeneity in Structure at Early Disease Stages
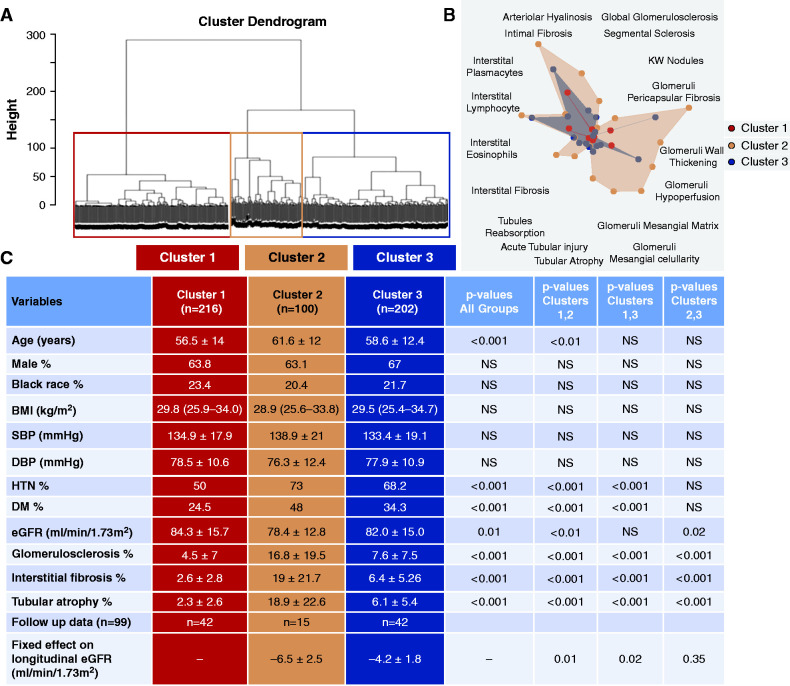
To better understand structural heterogeneity in samples with an eGFR≥60 ml/min per 1.73 m2 (Supplemental Table 8) we performed unbiased cluster analysis, on the basis of histologic descriptors. Our unbiased hierarchical clustering identified three distinct clusters (Figure 4). One group (cluster 2) had a notably greater degree of IFTA, 19% versus 3% (cluster 1) or 6% (cluster 3), which was associated with a lower eGFR (78 versus 84 and 82 ml/min per 1.73 m2).
Figure 4.
Histopathology-based clustering of subjects with eGFR≥60 ml/min per 1.73 m2. (A) Hierarchical clustering dendrogram shows three distinct clusters. (B) Radar plot of histopathologic characteristics in cluster 1 (red), cluster 2 (orange), and cluster 3 (blue). (C) Clinical and select histopathologic characteristics of histopathology-based clusters. Clusters with follow-up data are indicated, and associations with longitudinal eGFR are assessed using linear mixed model. Complete fixed effects used in the model include: cluster, age, sex, race (as Black or non-Black), presence of diabetes or HTN, BMI (kg/m2), nephrectomy status (total versus partial), tumor size (cm), and length of follow-up (years). Random effects include subject-specific eGFR slopes obtained by regressing an average of three eGFR values on the time variable. SBP, systolic BP; DBP, diastolic BP.
Clusters 1 and 3, however, showed no significant differences in eGFR (84±16 and 82±15 ml/min per 1.73 m2, P>0.05). Despite no clear differences in kidney function, cluster 3 had significantly greater IF, 6.4 versus 2.6%, and GS, 7.6 versus 4.5% (P<0.001) than cluster 1 samples. We observed no differences in age, sex, race, BMI, or BP. We noted the increased incidence of DM and HTN in cluster 3 with more severe IFTA and GS. Using linear mixed modeling, we also assessed whether histopathology-based clusters were associated with eGFR decline over time. Cluster 2, which had significantly lower eGFR and higher severity of IFTA and GS, was also associated with eGFR decline (P=0.01). Importantly, we found that cluster 3 was also associated with significantly faster eGFR decline over time compared with cluster 1 (P=0.02, Figure 4C and Supplemental Table 9), despite no significant difference in baseline eGFR.
In summary, our unbiased histology-based clustering indicates that some samples, especially those with DM and HTN, show severe IFTA and GS on histologic analysis, despite preserved eGFR.
Histologic Alterations Improve Kidney Function Decline Estimation
Next, we aimed to understand whether histologic abnormalities such as IFTA and GS provides complementary information to eGFR and improve kidney function decline estimation in our cohort.
We analyzed the relationship between renal structural findings and the rate of decline of eGFR in a cohort of participants with a minimum of 3 months of follow-up data (Table 3). Our longitudinal cohort had a baseline eGFR of 66 (SD±24) ml/min per 1.73 m2 and a mean follow-up eGFR of 54 (SD±25) ml/min per 1.73 m2 after a median follow-up time of 3 years (interquartile range, 1.1–5.6 years).
Table 3.
Clinical and histopathological variables associated with kidney function decline: Clinical and histopathologic characteristics of longitudinal cohort
| Clinical and Pathologic Characteristics of Follow-up Data (n=280) | Result |
|---|---|
| eGFR, mean (SD), ml/min per 1.73 m2 | 65.7 (24.2) |
| Follow up eGFR, mean (SD), ml/min per 1.73 m2 | 53.9 (24.8) |
| Age, mean (SD), yr | 62.8 (12) |
| DM, n (%) | 107 (38.2) |
| HTN n (%) | 206 (73.6) |
| Sex, M, n (%) | 168 (60) |
| Race, Black, n (%) | 56 (20) |
| BMI, mean (SD), kg/m2 | 31.5 (7.5) |
| Systolic BP, mean (SD), mm Hg | 137 (22) |
| Diastolic BP, mean (SD), mm Hg | 77 (12) |
| Follow up eGFR slope, median (IQR) ml/min per 1.73 m2/yr | −4.8 (−12.2 to −1.3) |
| Percent eGFR slope change/yr, median (IQR) | −6.7 (−17.4 to −2.0) |
| Partial/total nephrectomy (%) | 29.2/70.8 |
| Tumor size, mean (SD) cm | 6.2 (3.5) |
| Tubulointerstitial | |
| Acute tubular injury, median (IQR) (%) | 0 (0–2) |
| TA, median (IQR) (%) | 5 (2–10) |
| Tubular reabsorption 0–3 (%) | |
| 0 | 81.1 |
| 1 | 15.7 |
| 2 | 2.5 |
| 3 | 0.7 |
| Interstitial fibrosis, median (IQR) (%) | 5 (2–10) |
| Interstitial eosinophils 0–3 (%) | 81.1/17.9/1.1 |
| 0 | 81.1 |
| 1 | 17.9 |
| 2 | 1.1 |
| 3 | 0 |
| Interstitial lymphocytic infiltrates 0–3 (%) | |
| 0 | 27.1 |
| 1 | 53.2 |
| 2 | 16.1 |
| 3 | 3.6 |
| Interstitial plasma cell infiltrates 0–3 (%) | |
| 0 | 69.6 |
| 1 | 26.1 |
| 2 | 3.9 |
| 3 | 0.4 |
| Glomerular | |
| Segmental sclerosis, median (IQR) (range) (%) | 0 (0) |
| GS, median (IQR) (%) | 5.8 (2.8–12.7) |
| Wall thickening 0–3 (%) | |
| 0 | 91.4 |
| 1 | 5 |
| 2 | 3.2 |
| 3 | 0.4 |
| Hypoperfused 0–3 (%) | |
| 0 | 34.3 |
| 1 | 56.4 |
| 2 | 8.6 |
| 3 | 0.7 |
| Mesangial matrix 0–3 (%) | |
| 0 | 77.7 |
| 1 | 13.3 |
| 2 | 4.5 |
| 3 | 4.4 |
| Mesangial cellularity 0–3 (%) | |
| 0 | 82.5 |
| 1 | 11.8 |
| 2 | 3.2 |
| 3 | 2.5 |
| KW nodules present (%) | 2.9 |
| Pericapsular fibrosis 0–3 (%) | |
| 0 | 42.1 |
| 1 | 46.8 |
| 2 | 11.1 |
| 3 | 0 |
| Vascular | |
| Arteriolar hyalinosis 0–3 (%) | |
| 0 | 69.6 |
| 1 | 24.6 |
| 2 | 3.9 |
| 3 | 1.8 |
| Intimal fibrosis 0–3 (%) | |
| 0 | 12.5 |
| 1 | 45.7 |
| 2 | 35.7 |
| 3 | 6.1 |
IQR, interquartile range; M, male.
To determine whether histopathological variables improve the precision of the rate of kidney function decline, we used linear mixed models (Supplemental Table 10). We found that GS, age, baseline eGFR, and radical (versus partial) nephrectomy predicted longitudinal eGFR (Table 4). Inclusion of GS into the model significantly improved the model fitness (likelihood ratio test <0.01 and Akaike Information Criterion 3898 versus 3913).
Table 4.
Clinical and histopathological variables associated with kidney function decline: Variables (fixed effects) associated with eGFR decline in linear mixed model
| Longitudinal eGFR (n=167) | Coefficient | SE | z | P>|z| | 95% Confidence Interval | |
|---|---|---|---|---|---|---|
| Age (per 1 year older) | −0.25 | 0.06 | −4.21 | <0.001 | −0.36 | −0.13 |
| eGFR baseline (per 1 ml/min per 1.73 m2) | 0.74 | 0.03 | 25.18 | <0.001 | 0.68 | 0.80 |
| GS (per 1% difference) | −0.25 | 0.06 | −4.35 | <0.001 | −0.36 | −0.14 |
| Radical nephrectomy (yes/no) | −4.32 | 1.40 | −3.10 | <0.01 | −7.05 | −1.58 |
| Tumor size (per 1 cm difference) | −0.01 | 0.20 | −0.03 | 0.98 | −0.39 | 0.38 |
| Sex, F (yes/no) | −1.68 | 1.34 | −1.26 | 0.21 | −4.30 | 0.94 |
| Race, Black (yes/no) | −2.64 | 1.96 | −1.35 | 0.18 | −6.49 | 1.20 |
| BMI (per 1 kg/m2) | −0.04 | 0.08 | 0.47 | 0.66 | −0.20 | 0.13 |
| Diabetes (yes/no) | 0.59 | 1.26 | 0.52 | 0.64 | −1.87 | 3.06 |
| HTN (yes/no) | −0.43 | 1.48 | −0.29 | 0.77 | −3.33 | 2.48 |
| Length of follow-up time (per 1 year difference) | −0.02 | 0.12 | −0.14 | 0.89 | −0.26 | 0.22 |
Variables meeting significance (P<0.05) shown in bold. Coefficients represent fixed effects obtained in linear mixed model using subject-specific eGFR slopes obtained by regressing repeated eGFR values on the time variable (random effects). F, female.
Clinical parameters are poor estimates of kidney function decline in patients with eGFR ≥60 ml/min per 1.73 m2,30,31 therefore we reanalyzed this subgroup in our cohort. We found that even in subjects with eGFR≥60 ml/min per 1.73 m2, GS significantly improved modeling eGFR changes over time (P<0.01, Table 5).
Table 5.
Clinical and histopathological variables associated with kidney function decline: Variables (fixed effects) associated with eGFR decline in linear mixed model in subjects with eGFR≥60 at time of nephrectomy
| Longitudinal eGFR, if Baseline eGFR>60 (n=99) | Coefficient | SE | z | P>|z| | 95% Confidence Interval | |
|---|---|---|---|---|---|---|
| Age (per 1 year older) | −0.25 | 0.09 | −2.88 | <0.01 | −0.41 | −0.08 |
| eGFR baseline (per 1 ml/min per 1.73 m2) | 0.75 | 0.06 | 12.45 | <0.001 | 0.63 | 0.87 |
| GS (per 1% difference) | −0.28 | 0.09 | −2.94 | <0.01 | −0.46 | -0.09 |
| Radical nephrectomy (yes/no) | −6.19 | 1.89 | −3.27 | <0.01 | −9.90 | −2.48 |
| Tumor size (per 1 cm) | 0.38 | 0.26 | 1.47 | 0.14 | −0.13 | 0.89 |
| Sex, F (yes/no) | −2.25 | 1.84 | −1.22 | 0.22 | −5.85 | 1.35 |
| Race, Black (yes/no) | −2.88 | 2.70 | −1.07 | 0.29 | −8.17 | 2.40 |
| BMI (per 1 kg/m2) | 0.05 | 0.11 | 0.43 | 0.67 | −0.17 | 0.26 |
| Diabetes (yes/no) | 0.65 | 1.75 | 0.37 | 0.71 | −2.78 | 4.08 |
| HTN (yes/no) | −0.40 | 1.89 | −0.21 | 0.83 | −4.10 | 3.30 |
| Length of follow up time (per 1 year difference) | −0.04 | 0.16 | −0.25 | 0.80 | −0.36 | 0.28 |
F, female.
Overall, we found that baseline eGFR is an important predictors of kidney function decline, however the inclusion of histologic variables (GS) improved kidney function decline estimation. The degree of GS improved kidney function decline estimation even in subjects with eGFR≥60 ml/min per 1.73 m2.
Discussion
In this study, we examined the relationship between functional and structural changes observed in patients with DM, HTN, and varying degree of kidney dysfunction. Given the small but significant risk associated with a kidney biopsy, it is important to understand whether clinical parameters can estimate histologic alterations or histologic alterations provide complementary information. We found that eGFR and clinical parameters estimate IFTA and GS reasonably well in patients with more advanced CKD (stage 3–5). We found that eGFR underestimates the degree of GS and IFTA in patients with DM, HTN, and Black race. Our analysis emphasizes that eGFR alone is an imperfect measure of histologic changes in early CKD stages, given some subjects showed fairly significant damage despite preserved eGFR. Importantly, we found that histologic damage assessment improves kidney function decline estimation, even in subjects with relatively preserved kidney function.
Previous studies assessing the correlation between kidney function and histologic abnormalities have analyzed clinically indicated biopsies with mixed glomerular diseases,10–12,32 and relied on histologic information from clinical charts. These studies could be biased by practitioners performing a biopsy when suspecting a specific glomerular disease. In addition, histologic reading of biopsies by different pathologists shows modest correlation. In this study, we developed a large, multicenter biobank of human kidney tissue samples derived from nephrectomies that included samples with a wide distribution of age, sex, eGFR, race, high prevalence of HTN (69%), and DM (35%) and a subset of follow-up data over 3 years. Our collection is highly valuable because little information is available for subjects with HTN and DM and preserved kidney function. We were able score relatively large kidney specimens in a standardized manner.
We found a nonlinear relationship between histopathologic variables and eGFR. In our dataset, the relationship between eGFR and IFTA and GS was weak, at eGFR≥60 ml/min per 1.73 m2. Previous studies assessing the relationship between renal structure and function were biased toward samples with advanced CKD, where the relationship between IFTA and GS is much stronger. There are several potential implications of the observed nonlinear relationship. First, eGFR as a functional measurement shows important, sometimes, rapid variability (e.g., volume-depleted state). Fibrosis may not be a limiting or key determinant of kidney function variability at high eGFR values, but seems an important component for more advanced disease. Another important limitation is that creatinine-based eGFR estimations are less accurate at high eGFR ranges, and we cannot exclude the role of such imprecision in kidney function estimation. Second, our analyses emphasize that the presence of DM and HTN markedly alters the relationship between eGFR and fibrosis, such that the degree of IFTA and GS was approximately 7%–15% in subjects with DM and HTN versus <10% without DM/HTN at CKD stage 3 (eGFR 30–59 ml/min per 1.73 m2). The simplest interpretation is that underlying hyperfiltration plays a role in determining eGFR in patients with DM.2 It is important to note that two of the most commonly used drug classes, inhibitors of angiotensin converting enzyme inhibitor, angiotensin receptor blocker, or sodium glucose cotransporter inhibitors, initially reduce the eGFR,33 which is interpreted as an important renoprotective mechanism of these commonly used drugs.
We observed a significantly greater degree of IFTA in the Black population at most eGFR levels, compared with the non-Black population. Interestingly, the Black population in our study was younger but without significant differences in the diagnosis of DM or HTN. BP at the time of nephrectomy were significantly higher in Black individuals compared with non Black individuals. The difference between Black and non-Black participants was eliminated by removing race from the eGFR equation. Our data are relevant to the controversy surrounding the use of race in estimations of kidney disease.34,35
Our study has several limitations. Most importantly, because samples were obtained from nephrectomies, we cannot exclude the role of tumor-associated sclerosis or immune cell infiltration. Even if the cause of IFTA and GS was potentially tumor associated, it would not invalidate our results because our study was not aimed at understanding the cause of IFTA and GS, simply its association with eGFR. The relative percentages of IFTA and GS are internally consistent, but absolute estimations will need external validation. In addition, we relied on electronic medical records to provide information on disease status and laboratory values. Consequently, we were only able to evaluate longitudinal follow-up data in a subset of patients and could not control for differences in AKI incidence or medication use. Another important limitation is the dearth of information on glycemic control and albuminuria, an important diagnostic component of CKD estimation, but often not analyzed in the clinical setting. Our study is also limited by lack of genetic data, including APOL1 genotype information.36
In the two-fold definition of CKD, as both a functional and structural entity, this study emphasizes the importance of the presently underappreciated structural component. The study underscores that subjects could have marked histologic damage, despite the preserved eGFR, especially those with DM, HTN, and Black race. Given the important role of fibrosis in predicting kidney function decline, our results indicate the importance of obtaining kidney biopsies on these patients.
In summary, we present a comprehensive analysis defining the relationship between kidney function and structural abnormalities in subjects with and without DM and HTN at different eGFRs, including subjects with preserved kidney function. We observed that the relationship between structural damage and kidney function is weak at earlier stages of CKD but strengthens at later CKD stages. Our study highlights that in the setting of DM, HTN, or Black race, the degree of structural damage is more severe than estimation on the basis of eGFR would indicate. Our results suggest that histologic analysis is an important complementary method for kidney disease evaluation, especially at early disease stages, in patients with DM, HTN, and Black race, because some subjects present with relatively severe structural damage despite preserved eGFR.
Disclosures
A. Havasi reports having consultancy agreements with Janssen. J. Hill reports current employment with, and receiving research funding from, Boehringer Ingelheim Pharmaceuticals Inc. K. Susztak reports consultancy agreements with AstraZeneca, Bayer, Jnana Therapeutics, Maze, and Pfizer; reports having an ownership interest in Jnana; reports receiving research funding from Bayer, Boehringer Ingelheim, Calico, Gilead, GlaxoSmithKline, Lilly, Maze, Merck, Novo Nordisk, Novartis, and Regeneron; reports receiving honoraria from AstraZeneca, Bayer, Maze, and Jnana Therapeutics; and reports being a scientific advisor or member via Editorial boards of Cell Metabolism, eBioMedicine, Kidney International, Journal of American Society of Nephrology, Journal of Clinical Investigation, Jnana, and Med; and is on the scientific advisory board of Jnana Therapeutics. M. B. Palmer reports being a scientific advisor or member of the Editorial Board of the American Journal of Kidney Disease. All remaining authors have nothing to disclose.
Funding
This work was supported by the National Institutes of Health (NIH) National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) grants R01 DK076077, R01 DK087635, and R01 DK105821 to Dr. Susztak’s laboratory. Funding supporting research to G. Quinn is provided by the NIDDK training grant T32 DK007006-46. Additional funding was provided by the NIH National Center for Advancing Translational Sciences under award number UL1TR001878. Work performed in the Susztak laboratory is also supported by Gilead Sciences, Regeneron, Novo Nordisk, GlaxoSmithKline, Boehringer Ingelheim, and Bayer.
Supplementary Material
Acknowledgments
The authors wish to thank Dr. Jordana B. Cohen for her invaluable input. Drs. A. Abedini, M. Palmer, G. Quinn, and K. Susztak designed the study and drafted the paper; Drs. A. Abedini, H. Liu, Z. Ma, and G. Quinn prepared and organized study datasets; Drs. A. Abedini, A. Cucchiara, H. Liu, M. Palmer, G. Quinn, and K. Susztak analyzed the data; Drs. A. Abedini, M. Palmer, and G. Quinn made the figures; Dr. A. Cucchiara reviewed statistical methods and analysis; all authors revised and approved the final version of the manuscript. The content of this study is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. The funders have no influence on the design or analyses run in this study.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
SUPPLEMENTAL MATERIAL
This article contains the following supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2021010044/-/DCSupplemental.
Supplemental Table 1. Histopathological variables and definitions.
Supplemental Table 2. Clinical and histopathological characteristics of samples with complete data used in the regression analyses.
Supplemental Table 3. Clinical and select histopathological variables by eGFR sextiles.
Supplemental Tables 4A–C. The degree histopathological damage stratified by presence or absence of HTN and diabetes.
Supplemental Table 5A–C. The degree histopathological damage stratified and by race (Black versus non-Black).
Supplemental Table 6A and B. Clinical and histopathological characteristics of subjects according to race.
Supplemental Table 7A and B. The degree IF and TA stratified by race (Black versus non-Black) without use of race coefficient in estimation of GFR.
Supplemental Table 8A and B. Clinical and histopathological characteristics of samples with estimated glomerular filtration rate ≥60 and <60 ml/min per 1.73 m2.
Supplemental Table 9. Predictors of eGFR decline using linear mixed model of longitudinal eGFR in subjects with eGFR≥60 at time of nephrectomy including unbiased histopathology-based clusters.
Supplemental Table 10. Clinical and histopathological characteristics of follow-up samples with complete data used in linear mixed model analyses.
Supplemental Figure 1. Study flow chart and inclusion/exclusion criteria.
References
- 1.Levey AS, Eckardt K-U, Dorman NM, Christiansen SL, Cheung M, Jadoul M, et al. : Nomenclature for kidney function and disease: Report of a Kidney Disease: Improving Global Outcomes (KDIGO) Consensus Conference. Kidney Int 97: 1117–1129, 2020. 32409237 [DOI] [PubMed] [Google Scholar]
- 2.Reidy K, Kang HM, Hostetter T, Susztak K: Molecular mechanisms of diabetic kidney disease. J Clin Invest 124: 2333–2340, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Townsend RR, Guarnieri P, Argyropoulos C, Blady S, Boustany-Kari CM, Devalaraja-Narashimha K, et al. ; TRIDENT Study Investigators : Rationale and design of the transformative research in diabetic nephropathy (TRIDENT) study. Kidney Int 97: 10–13, 2020 [DOI] [PubMed] [Google Scholar]
- 4.Pagtalunan ME, Miller PL, Jumping-Eagle S, Nelson RG, Myers BD, Rennke HG, et al. : Podocyte loss and progressive glomerular injury in type II diabetes. J Clin Invest 99: 342–348, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mauer M, Zinman B, Gardiner R, Suissa S, Sinaiko A, Strand T, et al. : Renal and retinal effects of enalapril and losartan in type 1 diabetes. N Engl J Med 361: 40–51, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Palmer MB, Abedini A, Jackson C, Blady S, Chatterjee S, Sullivan KM, et al. : The role of glomerular epithelial injury in kidney function decline in patients with diabetic kidney disease in the TRIDENT cohort. Kidney Int Rep 6: 1066–1080, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nangaku M: Chronic hypoxia and tubulointerstitial injury: A final common pathway to end-stage renal failure. J Am Soc Nephrol 17: 17–25, 2006 [DOI] [PubMed] [Google Scholar]
- 8.Nath KA: Tubulointerstitial changes as a major determinant in the progression of renal damage. Am J Kidney Dis 20: 1–17, 1992 [DOI] [PubMed] [Google Scholar]
- 9.Tangri N, Ferguson TW, Wiebe C, Eng F, Nash M, Astor BC, et al. : Validation of the kidney failure risk equation in kidney transplant recipients. Can J Kidney Health Dis 7: 2054358120922627, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eadon MT, Schwantes-An T-H, Phillips CL, Roberts AR, Greene CV, Hallab A, et al. : Kidney histopathology and prediction of kidney failure: A retrospective cohort study. Am J Kidney Dis 76: 350–360, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Menn-Josephy H, Lee CS, Nolin A, Christov M, Rybin DV, Weinberg JM, et al. : Renal interstitial fibrosis: An imperfect predictor of kidney disease progression in some patient cohorts. Am J Nephrol 44: 289–299, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Srivastava A, Palsson R, Kaze AD, Chen ME, Palacios P, Sabbisetti V, et al. : The prognostic value of histopathologic lesions in native kidney biopsy specimens: Results from the Boston Kidney Biopsy Cohort Study. J Am Soc Nephrol 29: 2213–2224, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sethi S, D’Agati VD, Nast CC, Fogo AB, De Vriese AS, Markowitz GS, et al. : A proposal for standardized grading of chronic changes in native kidney biopsy specimens. Kidney Int 91: 787–789, 2017 [DOI] [PubMed] [Google Scholar]
- 14.Beckerman P, Qiu C, Park J, Ledo N, Ko Y-A, Park AD, et al. : Human kidney tubule-specific gene expression based dissection of chronic kidney disease traits. EBioMedicine 24: 267–276, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Amdur RL, Feldman HI, Gupta J, Yang W, Kanetsky P, Shlipak M, et al. ; CRIC Study Investigators : Inflammation and progression of CKD: The CRIC study. Clin J Am Soc Nephrol 11: 1546–1556, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hannan M, Ansari S, Meza N, Anderson AH, Srivastava A, Waikar S, et al. : Risk factors for CKD progression: Overview of findings from the CRIC study. Clin J Am Soc Nephrol 16: 648–659: 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yamanouchi M, Hoshino J, Ubara Y, Takaichi K, Kinowaki K, Fujii T, et al. : Clinicopathological predictors for progression of chronic kidney disease in nephrosclerosis: A biopsy-based cohort study. Nephrol Dial Transplant 34: 1182–1188, 2019 [DOI] [PubMed] [Google Scholar]
- 18.Neuwirt H, Perco P, Kainz A, Mühlberger I, Leierer J, Braniff S-J, et al. : A 3-biomarker-panel predicts renal outcome in patients with proteinuric renal diseases. BMC Med Genomics 7: 75, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grams ME, Sang Y, Ballew SH, Carrero JJ, Djurdjev O, Heerspink HJL, et al. : Predicting timing of clinical outcomes in patients with chronic kidney disease and severely decreased glomerular filtration rate. Kidney Int 93: 1442–1451, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, et al. ; CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) : A new equation to estimate glomerular filtration rate. Ann Intern Med 150: 604–612, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barisoni L, Troost JP, Nast C, Bagnasco S, Avila-Casado C, Hodgin J, et al. : Reproducibility of the NEPTUNE descriptor-based scoring system on whole-slide images and histologic and ultrastructural digital images. Mod Pathol 29: 671–684, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Roberts ISD, Cook HT, Troyanov S, Alpers CE, Amore A, Barratt J, et al. ; Working Group of the International IgA Nephropathy Network and the Renal Pathology Society : The Oxford classification of IgA nephropathy: Pathology definitions, correlations, and reproducibility. Kidney Int 76: 546–556, 2009 [DOI] [PubMed] [Google Scholar]
- 23.Tervaert TWC, Mooyaart AL, Amann K, Cohen AH, Cook HT, Drachenberg CB, et al. ; Renal Pathology Society : Pathologic classification of diabetic nephropathy. J Am Soc Nephrol 21: 556–563, 2010 [DOI] [PubMed] [Google Scholar]
- 24.Kudose S, Hoshi M, Jain S, Gaut JP: Renal histopathologic findings associated with severity of clinical acute kidney injury. Am J Surg Pathol 42: 625–635, 2018 [DOI] [PubMed] [Google Scholar]
- 25.Duan N: Smearing estimate: A nonparametric retransformation method. J Am Stat Assoc 78: 605–610, 1983 [Google Scholar]
- 26.Murtaugh PA: Performance of several variable-selection methods applied to real ecological data. Ecol Lett 12: 1061–1068, 2009 [DOI] [PubMed] [Google Scholar]
- 27.Shou H, Hsu JY, Xie D, Yang W, Roy J, Anderson AH, et al. ; Chronic Renal Insufficiency Cohort (CRIC) Study Investigators : Analytic considerations for repeated measures of eGFR in cohort studies of CKD. Clin J Am Soc Nephrol 12: 1357–1365, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kimes PK, Liu Y, Neil Hayes D, Marron JS: Statistical significance for hierarchical clustering. Biometrics 73: 811–821, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lengyel A, Botta-Dukát Z: Silhouette width using generalized mean-A flexible method for assessing clustering efficiency. Ecol Evol 9: 13231–13243, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Coresh J, Turin TC, Matsushita K, Sang Y, Ballew SH, Appel LJ, et al. : Decline in estimated glomerular filtration rate and subsequent risk of end-stage renal disease and mortality. JAMA 311: 2518–2531, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tangri N, Stevens LA, Griffith J, Tighiouart H, Djurdjev O, Naimark D, et al. : A predictive model for progression of chronic kidney disease to kidney failure. JAMA 305: 1553–1559, 2011 [DOI] [PubMed] [Google Scholar]
- 32.Li P, Gupta S, Mothi SS, Rennke HG, Leaf DE, Waikar SS, et al. : Histopathologic correlates of kidney function: Insights from nephrectomy specimens. Am J Kidney Dis 77: 336–345: 2020 [DOI] [PubMed] [Google Scholar]
- 33.Breyer MD, Susztak K: The next generation of therapeutics for chronic kidney disease. Nat Rev Drug Discov 15: 568–588, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Eneanya ND, Yang W, Reese PP: Reconsidering the consequences of using race to estimate kidney function. JAMA 322: 113–114, 2019 [DOI] [PubMed] [Google Scholar]
- 35.Vyas DA, Eisenstein LG, Jones DS: Hidden in plain sight—Reconsidering the use of race correction in clinical algorithms. N Engl J Med 383: 874–882, 2020 [DOI] [PubMed] [Google Scholar]
- 36.Bajaj A, Ihegword A, Qiu C, Small AM, Wei W-Q, Bastarache L, et al. : Phenome-wide association analysis suggests the APOL1 linked disease spectrum primarily drives kidney-specific pathways. Kidney Int 97: 1032–1041, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.