Significance Statement
Patients who survive coronavirus disease 2019 (COVID-19) are at higher risk of post-acute sequelae involving pulmonary and several extrapulmonary organ systems—generally referred to as long COVID. However, a detailed assessment of kidney outcomes in long COVID is not yet available. Here we show that, beyond the acute phase of illness, 30-day survivors of COVID-19 exhibited higher risks of AKI, eGFR decline, ESKD, major adverse kidney events (MAKE), and steeper longitudinal decline in eGFR. The risks of kidney outcomes increased according to the severity of the acute infection (categorized by care setting into non-hospitalized, hospitalized, and admitted to intensive care). The findings provide insight into the long-term consequences of COVID-19 on kidney outcomes and suggest that post-acute COVID-19 care should include attention to kidney function and disease.
Keywords: ESRD, ESKD, acute kidney injury, post-acute sequelae of SARS-CoV-2 infection, PASC, post-acute COVID, long COVID, eGFR decline, kidney function, COVID-19
Abstract
Background
COVID-19 is associated with increased risk of post-acute sequelae involving pulmonary and extrapulmonary organ systems—referred to as long COVID. However, a detailed assessment of kidney outcomes in long COVID is not yet available.
Methods
We built a cohort of 1,726,683 US Veterans identified from March 1, 2020 to March 15, 2021, including 89,216 patients who were 30-day survivors of COVID-19 and 1,637,467 non-infected controls. We examined risks of AKI, eGFR decline, ESKD, and major adverse kidney events (MAKE). MAKE was defined as eGFR decline ≥50%, ESKD, or all-cause mortality. We used inverse probability–weighted survival regression, adjusting for predefined demographic and health characteristics, and algorithmically selected high-dimensional covariates, including diagnoses, medications, and laboratory tests. Linear mixed models characterized intra-individual eGFR trajectory.
Results
Beyond the acute illness, 30-day survivors of COVID-19 exhibited a higher risk of AKI (aHR, 1.94; 95% CI, 1.86 to 2.04), eGFR decline ≥30% (aHR, 1.25; 95% CI, 1.14 to 1.37), eGFR decline ≥40% (aHR, 1.44; 95% CI, 1.37 to 1.51), eGFR decline ≥50% (aHR, 1.62; 95% CI, 1.51 to 1.74), ESKD (aHR, 2.96; 95% CI, 2.49 to 3.51), and MAKE (aHR, 1.66; 95% CI, 1.58 to 1.74). Increase in risks of post-acute kidney outcomes was graded according to the severity of the acute infection (whether patients were non-hospitalized, hospitalized, or admitted to intensive care). Compared with non-infected controls, 30-day survivors of COVID-19 exhibited excess eGFR decline (95% CI) of −3.26 (−3.58 to −2.94), −5.20 (−6.24 to −4.16), and −7.69 (−8.27 to −7.12) ml/min per 1.73 m2 per year, respectively, in non-hospitalized, hospitalized, and those admitted to intensive care during the acute phase of COVID-19 infection.
Conclusions
Patients who survived COVID-19 exhibited increased risk of kidney outcomes in the post-acute phase of the disease. Post-acute COVID-19 care should include attention to kidney disease.
Coronavirus disease 2019 (COVID-19) is associated with substantial short-term (acute) morbidity and mortality.1 Evidence suggests that, beyond the acute illness, patients who survive COVID-19 may experience post-acute sequelae—also referred to in the lay vernacular as “long COVID”— which can involve pulmonary and broad extrapulmonary organ system manifestations, including the kidneys.2 However, a detailed in-depth assessment of kidney outcomes in the post-acute phase of COVID-19 infection is not yet available. A better understanding of post-acute COVID-19 kidney outcomes would inform development of care strategies to improve the health and well-being of people with long COVID.
Here, we leverage the breadth and depth of the US Department of Veterans Affairs (VA) national healthcare databases to build a cohort of 89,216 US Veterans who survived the first 30 days of COVID-19 infection and 1,637,467 non-infected controls and followed them longitudinally to provide an in-depth, detailed characterization of the risks (and associated burdens) of post-acute kidney outcomes in the overall cohort, and according to severity of the acute infection (that is, whether patients were non-hospitalized, hospitalized, or admitted to intensive care).
Methods
Cohort
Among users of the Department of Veterans Health Administration (VHA) healthcare system, we identified 203,476 US Veterans who had a record of a laboratory-confirmed COVID-19 test between March 1, 2020 and March 15, 2021 (Supplemental Figure 1); of these, 191,958 veterans had a recorded encounter with the VHA in 2019. From this group, we selected 181,384 individuals who were alive 30 days after testing positive (did not succumb to death during the acute phase of the infection). The date of testing positive was set as time zero (T0). For a comparison group, we identified 5,808,018 users of the VHA who had a record of an encounter with the VHA in 2019; 5,606,309 of whom were alive as of March 1, 2020, and 5,414,351 of whom did not have a positive COVID-19 test between March 1, 2020 and March 15, 2021. We randomly assigned a T0 to the participants in the control group by matching them with a participant who had COVID-19 at a 25:1 rate, resulting in 4,534,600 control-group participants, of which 4,397,509 were alive 30 days after their T0. Those with a record of ESKD before or in the 30 days after T0 were excluded from both groups, and then we finally selected those with a recorded serum creatinine measurement after 30 days from T0 (1,637,467 controls and 89,216 participants with COVID-19), resulting in a final analytic cohort of 1,726,683. Those with a recorded positive COVID-19 test were further defined as being non-hospitalized, hospitalized, and being admitted to the intensive care unit (ICU) by record of inpatient care or admittance to the ICU during the 30 days after T0 (the acute phase of the illness).
Data Sources
This study used data collected during the routine delivery of care from the US Department of VA VHA. Demographic and clinical data were obtained from the Corporate Data Warehouse.3–10 The VA COVID-19 Shared Data Resource (CSDR)11 provided information on veterans who were positive for COVID-19. The CSDR providers’ information collated by the VA’s National Surveillance Tool, which collects near real-time data on cases of COVID-19 from laboratory results and clinical notes (which are examined via natural language processing and subsequent human review). The Area Deprivation Index—a composite contextual measure of poverty, housing quality, employment, and education12,13—supplied a measure of the level of socioeconomic disadvantage at participants’ residential locations.
Outcomes
Outcomes were examined in the period of follow-up from 30 days after T0 up to April 30, 2021, censoring at death or ESKD where applicable. AKI was defined as having an inpatient serum creatinine measurement 30 days after T0 that was 0.3 mg/dl or 50% greater than baseline, where baseline was assessed as the average of all values in the 2 years before T0. We also assessed outcomes of eGFR decline of ≥30%, ≥40%, and ≥50% from baseline. Baseline eGFR was defined by the eGFR measurement most proximal to but before T0, including measures up to 2 years before T0. If outpatient values were available, these were used first, otherwise inpatient values were used (<1%). In the case of missing baseline kidney function values (1.8% and 3.2% in COVID-19 and VHA user groups, respectively), values were imputed on the basis of demographics and baseline health characteristics. An ESKD outcome was defined at the date of first record of receipt of chronic outpatient dialysis or kidney transplant. Major adverse kidney events (MAKE) were defined as a composite of eGFR decline of ≥50%, ESKD, or all-cause mortality. A composite of any kidney disease outcome defined as AKI, eGFR decline of ≥30%, or ESKD was additionally examined. Finally, we examined the rate of change in eGFR during the follow-up period using all outpatient and inpatient values 30 days after T0. All eGFR were calculated using the Chronic Kidney Disease Epidemiology Collaboration equation.14
Covariates
Covariates included a set of 29 predefined potential confounders of the association between COVID-19 and adverse kidney outcomes.1,2,15 Demographic, behavioral, and contextual characteristics included age, Area Deprivation Index, race, sex, and smoking status. Health characteristics included a participant’s baseline eGFR; systolic and diastolic BP; body mass index; and history of cancer, cardiovascular disease, cerebrovascular disease, chronic lung disease, dementia, diabetes mellitus type 2, HIV, and peripheral artery disease. Systolic and diastolic BP were defined as the average of all corresponding measures in the year before T0. Medication history included angiotensin-converting enzyme inhibitors/angiotensin II receptor blockers, antibiotics, antivirals, aspirin, β-blockers, chemotherapeutic agents, diuretics, immunosuppressants, nonsteroidal anti-inflammatory drugs, and proton pump inhibitors.16–19 Clinical comorbidities and medication usage were assessed in the year before T0. We additionally adjusted for record of residence at a long-term care facility and the number of eGFR measurements in the year before T0 as a measure of intensity of interaction with the healthcare system. All continuous covariates were adjusted for as restricted cubic splines with knots at the fifth, 33rd, 66th, and 95th percentiles. Missing baseline body mass index (0.4%) and BP (1%) were imputed.
In addition to these predefined covariates, and to further enhance adjustment of models, we also included a set of 100 variables selected by a high-dimensional variable selection algorithm from several data domains, including diagnoses, pharmacy records, and laboratory tests.1,2,9,20 In brief, from data domains of diagnoses, medication prescriptions, and laboratory tests, all available variables that occurred at least ten times in each group (a total of 834) were examined for differences between the COVID-19 and VHA users group by assessment of unadjusted relative risk. From these, we selected the top 100 variables with the strongest association with group membership for inclusion in adjustment with the predefined covariates. All covariates were assessed in the year before T0.
Statistical Analyses
Cohort participants’ characteristics, overall and by COVID-19 status, are reported as means (SDs), medians (interquartile ranges), or frequencies (percentages), where appropriate.
Unadjusted outcome rates are presented. Differences in the risk of outcomes were assessed by application of inverse probability weighting to cause specific Cox proportional hazard models. Propensity scores were estimated using logistic regression, which were then used to construct weights stabilized by unadjusted group membership probability. Truncation was not applied after examination of mean and SD of weights. Balance was examined by standardized mean differences in the predefined covariates and 100 selected high-dimensional covariates before and after weighting. In addition to this, we examined balance in the 734 high-dimensional covariates not selected as a means of testing for residual differences in these covariates that were not included in the propensity score model, where lack of balance may have suggested that our analytic algorithm did not address potential measured confounders. A standardized mean difference <0.15 was taken as evidence of balance between the COVID-19 and VHA users group.21,22 We estimated the excess burden per 1000 persons of the outcomes associated with COVID-19 at 6 months after T0, where excess burden was estimated by computing the difference between the average estimated survival probability from the weighted Cox model in those with COVID-19 and the VHA user group. Baseline survival probability was estimated using the Breslow method.23 To examine the effect of underlying severity of the acute phase of the illness (non-hospitalized, hospitalized, and admitted to the ICU), analyses were repeated in the comparisons with VHA users, using a similar analytic design.24
We additionally conducted an analysis examining the risks and burdens of AKI, ESKD, and MAKE by the occurrence of AKI during the acute phase of COVID-19 (first 30 days after a positive test). The COVID-19 groups examined included those during the acute phase that were non-hospitalized, hospitalized with no evidence of AKI, and hospitalized with evidence of an AKI. Evidence of an AKI was assessed as an inpatient serum creatinine of 0.3 mg/dl or 50% higher than the baseline serum creatinine. Propensity scores and outcome definitions were revised to incorporate changes in baseline eGFR and serum creatinine through the acute phase of the illness, using the most recently available measure through the 30-day period after a positive test.
We finally examined differences in the intra-individual trajectory of eGFR, starting from 30 days after T0, by severity of the acute infection using linear mixed models. Analyses were conducted in those who had at least two measurements of eGFR during this follow-up period to enhance characterization of intra-individual eGFR change. Trajectories were compared with that of the VHA user group (control). Models were weighted by the stabilized inverse probability of group membership, as previously described. Individual-level random intercepts were included. Differences in the trajectory of eGFR were examined by an interaction between the COVID-19 group and time; differences in the linear slope of eGFR are presented. We additionally examined potential non-linear changes, including a quadratic time term (identified by improvement in Akaike Information Criterion). Differences in the trajectory of eGFR, as compared with the control group, starting from day 30 over the course of a year are plotted with 95% CIs obtained through bootstrap. Differences in trajectories by AKI status during the acute phase of the illness were also assessed. Estimates of all risks, excess burdens, and eGFR trajectories were additionally generated in weighted models that only incorporated the predefined covariates in the modeling of the propensity score.
To examine the robustness of results to study-design specifications, we examined a set of positive and negative outcome controls.25 We examined the association of COVID-19 with positive outcome controls of all-cause mortality and hospitalization, where, on the basis of prior evidence, we would expect to see an association.2,26 Positive outcome controls may be used to detect the presence of latent biases that may result in the absence of associations where one would be expected. We also examined the association of COVID-19 status with negative outcome controls, including being fitted with or having an adjustment of casts or bandages and atopic dermatitis. Negative outcome controls may be used to detect the presence of latent biases that result in spurious associations where none would be expected.
Statistical tests were two sided, where a 95% CI that did not contain unity or a P value <0.05 was considered evidence of an association. Imputation was done using fully conditional specification. Analyses were conducted using SAS Enterprise Guide version 8.2 (SAS Institute, Cary, NC), and results were visualized using R version 4.0.4.27 This study was approved by the Institutional Review Board of the Department of VA St Louis Health Care System (Saint Louis, MO).
Results
There were 1,726,683 US veterans in the cohort overall; 89,216 (5.2%) and 1,637,467 (94.8%) were in the COVID-19 and VHA users (control) group, respectively (Table 1). Median (interquartile range) follow-up time was 164 (127–268) days in those with COVID-19, and 172 (133–282) days in the VHA user group (Table 1). Compared with VHA users (control group), those with COVID-19 were more likely to be younger; of Black race; living in long-term care; had a higher comorbidity burden, including higher rates of chronic lung disease, diabetes, and cardiovascular disease; and had higher rates of being prescribed medications, including proton pump inhibitors and nonsteroidal anti-inflammatory drugs (Table 1). Median time to outcomes of AKI, eGFR decline ≥30%, eGFR decline ≥40%, eGFR decline ≥50%, ESKD, and MAKE by COVID-19 status and intensity of care are provided in Supplemental Table 1.
Table 1.
Demographic and health characteristics of the overall cohort and by COVID-19 status at baseline
| Characteristics | Overall | COVID-19 | VHA Users | |
|---|---|---|---|---|
| n (%) | 1,726,683 | 89,216 (5.2) | 1,637,467 (94.8) | |
| Median follow-up (IQR) | 172 (133–281) | 164 (127–268) | 172 (133–282) | |
| Age (yr), median (IQR) | 68.5 (56.8–74.3) | 65.5 (53.7–73.3) | 68.7 (57.0–74.3) | |
| Race, n (%) | ||||
| White | 1,267,091 (73.4) | 60,508 (67.8) | 1,206,583 (73.7) | |
| Black | 329,937 (19.1) | 21,934 (24.6) | 308,003 (18.8) | |
| Other | 129,655 (7.5) | 6774 (7.6) | 122,881 (7.5) | |
| Male sex, n (%) | 1,575,385 (91.2) | 80,399 (90.1) | 1,494,986 (91.3) | |
| ADI, median (IQR)a | 55.0 (44.2–64.1) | 54.6 (44.5–63.2) | 55.0 (44.2–64.1) | |
| Smoking status, n (%) | ||||
| Never smoked | 915,783 (53.0) | 51,356 (57.6) | 864,427 (52.8) | |
| Former smoker | 413,037 (23.9) | 22,607 (25.3) | 390,430 (23.8) | |
| Current smoker | 397,863 (23.0) | 15,253 (17.1) | 382,610 (23.4) | |
| Long-term care, n (%) | 15,961 (0.9) | 2982 (3.3) | 12,979 (0.8) | |
| Clinical characteristics | ||||
| eGFR (ml/min per 1.73 m2) (n=1,672,359) | ||||
| Median (IQR) | 76.9 (61.8–90.2) | 77.9 (62.5–91.7) | 76.9 (61.7–90.2) | |
| >90, n (%) | 425,597 (25.5) | 24,394 (27.9) | 401,203 (25.3) | |
| 60–90, n (%) | 870,283 (52.0) | 44,182 (50.5) | 826,101 (52.1) | |
| 45–60, n (%) | 232,074 (14.6) | 12,147 (13.9) | 232,074 (14.6) | |
| 30–45, n (%) | 101,026 (6.0) | 5332 (6.1) | 95,694 (6.0) | |
| <30, n (%) | 31,232 (1.9) | 1513 (1.7) | 29,719 (1.9) | |
| Serum creatinine (mg/dl), mean (%) (n=1,672,359) | 1.11 (0.4) | 1.11 (0.3) | 1.11 (0.4) | |
| BMI category, n (%) (n=1,719,839) | ||||
| Underweight/normal weight | 302,216 (17.6) | 11,485 (12.9) | 290,731 (17.8) | |
| Overweight | 598,159 (34.8) | 27,470 (30.9) | 570,689 (40.0) | |
| Obese | 819,464 (47.7) | 50,027 (56.2) | 769,437 (47.2) | |
| Systolic BP (mm Hg), mean (SD) (n=1,709,598) | 133.2 (13.2) | 133.3 (12.5) | 133.2 (13.2) | |
| Diastolic BP (mm Hg), mean (SD) (n=1,709,598) | 77.3 (7.9) | 78.0 (7.7) | 77.3 (8.0) | |
| No. eGFR measurements in the 2 yr prior to T0, median (IQR) | 2 (1–3) | 2 (1–4) | 2 (1–3) | |
| Cancer, n (%) | 164,810 (9.5) | 9487 (10.6) | 155,323 (9.5) | |
| Cardiovascular disease, n (%) | 310,297 (18.0) | 18,154 (20.4) | 292,143 (17.8) | |
| Cerebrovascular disease, n (%) | 101,276 (5.9) | 6397 (7.2) | 94,879 (5.8) | |
| Chronic lung disease, n (%) | 261,646 (15.2) | 15,530 (17.4) | 246,116 (15.0) | |
| Dementia, n (%) | 38,853 (2.3) | 3366 (3.8) | 35,487 (2.2) | |
| Diabetes mellitus type 2, n (%) | 581,080 (33.7) | 35,120 (39.4) | 545,960 (33.3) | |
| HIV, n (%) | 11,928 (0.7) | 871 (1.0) | 11,057 (0.7) | |
| Peripheral artery disease, n (%) | 25,358 (1.5) | 1654 (1.9) | 23,704 (1.5) | |
| Medications, n (%) | ||||
| ACEi/ARB | 682,651 (39.5) | 38,337 (43.0) | 644,314 (39.4) | |
| Antibiotics | 109,706 (6.4) | 8169 (9.2) | 101,537 (6.2) | |
| Antivirals | 51,059 (3.0) | 3629 (4.1) | 47,430 (2.9) | |
| Aspirin | 257,708 (14.9) | 16475 (18.5) | 241,233 (14.7) | |
| β-Blockers | 519,867 (30.1) | 29,506 (33.1) | 490,361 (30.0) | |
| Chemotherapeutic agents | 19,144 (1.1) | 1151 (1.3) | 17,993 (1.1) | |
| Diuretics | 244,845 (14.2) | 14,364 (16.1) | 230,481 (14.1) | |
| Immunosuppressants | 21,488 (1.2) | 1322 (1.5) | 20,166 (1.2) | |
| NSAIDs | 546,424 (31.7) | 35,616 (39.9) | 510,808 (31.2) | |
| PPI | 495,026 (28.7) | 30,583 (34.3) | 46,443 (28.4) | |
IQR, interquartile range; ADI, Area Deprivation Index; BMI, body mass index; ACEi/ARB, angiotensin-converting enzyme inhibitors/angiotensin II receptor blockers; NSAIDs, nonsteroidal anti-inflammatory drugs; PPI, proton pump inhibitor; HIV, human immunodeficiency virus.
ADI is a measure of socioeconomic disadvantage, with a range from low to high disadvantage of 0–100.
Risks and Burdens of Post-acute COVID-19 Kidney Outcomes
Assessment of covariate balance after application of inverse probability weighting suggested that, in the overall cohort, predefined covariates, high-dimensional covariates selected by our algorithm, and those not selected were balanced (Supplemental Figure 2 and Table 2).
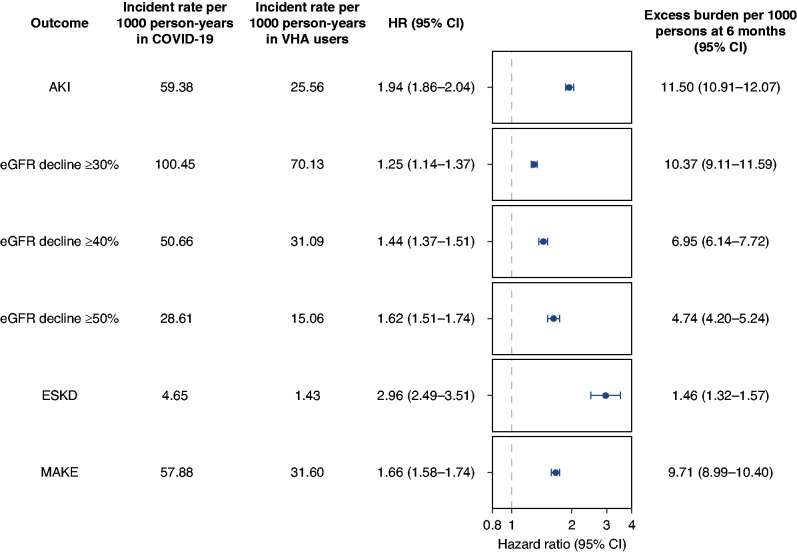
After adjustment for baseline characteristics, beyond the acute illness and compared with VHA users, 30-day survivors of COVID-19 exhibited a higher risk of AKI (adjusted hazard ratio [aHR], 1.94; 95% CI, 1.86 to 2.04), eGFR decline ≥30% (aHR, 1.25; 95% CI, 1.14 to 1.37), eGFR decline ≥40% (aHR, 1.44; 95% CI, 1.37 to 1.51), eGFR decline ≥50% (aHR, 1.62; 95% CI, 1.51 to 1.74), ESKD (aHR, 2.96; 95% CI, 2.49 to 3.51), and MAKE (aHR, 1.66; 95% CI 1.58 to 1.74) (Figure 1). Thirty-day survivors of COVID-19 were additionally at higher risk of having any kidney disease, defined as a composite of AKI, eGFR ≥30%, or ESKD (aHR, 1.35; 95% CI, 1.30 to 1.39) (Supplemental Table 3).
Figure 1.
Risk and excess burden of post-acute COVID-19 kidney outcomes at 6 months. Participants who had COVID-19 were compared with users of the VHA healthcare system who had no record of a positive COVID-19 test (control group). Outcomes were ascertained starting from 30 days after the participant’s positive COVID-19 test through end of follow-up. Unadjusted incident rates in the COVID-19 and VHA users groups per 1000 person-years, HRs, and excess burden per 1000 persons at 6 months are provided. HRs and corresponding 95% CIs are plotted. MAKE was defined as a composite of eGFR decline ≥50%, ESKD, or all-cause mortality. All models were adjusted for a set of 29 predefined variables and 100 variables selected by a high-dimensional variable selection algorithm.
Among 30-day survivors of COVID-19, and beyond the first 30 days of illness, excess burden of several kidney outcomes was evident in the post-acute phase of COVID-19, including AKI (11.50 per 1000 persons at 6 months; 95% CI, 10.91 to 12.07), eGFR decline ≥30% (10.37 per 1000 persons at 6 months; 95% CI, 9.11 to 11.59), eGFR decline ≥40% (6.95 per 1000 persons at 6 months; 95% CI, 6.14 to 7.72), eGFR decline ≥50% (4.74 per 1000 persons at 6 months; 95% CI, 4.20 to 5.24), ESKD (1.46 per 1000 persons at 6 months; 1.32 to 1.57), and MAKE (9.71 per 1000 persons at 6 months; 95% CI, 8.99 to 10.40) (Figure 1). Excess burden of any kidney disease was 13.44 per 1000 persons at 6 months (95% CI, 12.14 to 14.69) (Supplemental Table 3). Results were consistent in models only adjusting for the predefined covariates (Supplemental Table 4).
Post-acute COVID-19 Kidney Outcomes by Severity of the Acute Infection
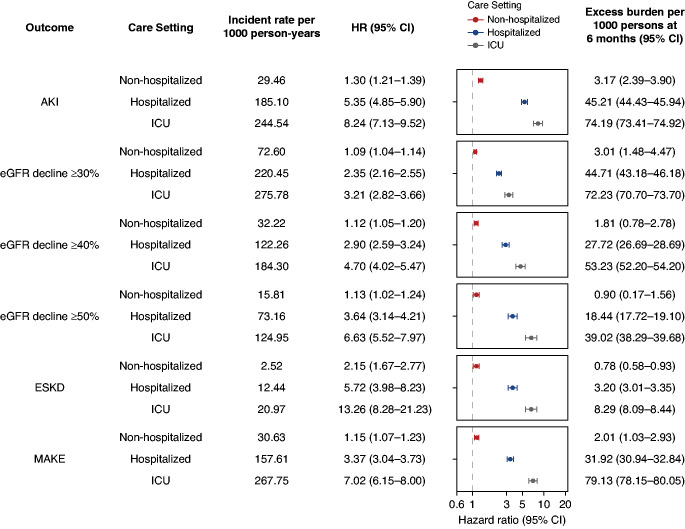
We then further examined the risks and burdens of post-acute kidney outcomes by the severity of disease during the acute phase of the infection (non-hospitalized, hospitalized, and admitted to intensive care). Among those with COVID-19, there were 72,694 (81.5%) that were non-hospitalized, 12,376 (13.9%) that were hospitalized, and 4146 (4.7%) that were admitted to the ICU during the acute phase of the COVID-19 infection. Frequency and incident rate of outcomes are presented in Supplemental Table 5. Assessment of covariate balance after application of weights suggested covariates were well balanced (Supplemental Figure 3 and Supplemental Tables 6–11). Compared with VHA users (control group), the risks and burdens of postacute COVID-19 kidney outcomes increased according to the severity of the acute infection among those with COVID-19 (Figure 2). Pairwise comparisons between all four mutually exclusive groups are provided in Supplemental Tables 12–13. Results were consistent in models only adjusting for the predefined covariates (Supplemental Table 14).
Figure 2.
Risk and excess burden of postacute COVID-19 kidney outcomes at 6 months in mutually exclusive cohorts of veterans with non-hospitalized COVID-19, those hospitalized with COVID-19, and those admitted to intensive care with COVID-19 during the first 30 days (acute phase) of the infection. Participants who had COVID-19 were compared with users of the VHA healthcare system who had no record of a positive COVID-19 test (control group). Outcomes were ascertained starting from 30 days after the participant’s positive COVID-19 test through end of follow-up. Unadjusted incident rates per 1000 person-years, HRs, and excess burden per 1000 persons at 6 months are provided for each COVID-19 group (non-hospitalized, hospitalized, and those admitted to intensive care during the acute phase of the infection). HRs and corresponding 95% CIs are plotted. MAKE was defined as a composite of eGFR decline ≥50%, ESKD, or all-cause mortality. All models were adjusted for a set of 29 predefined variables and 100 variables selected by a high-dimensional variable selection algorithm.
Post-acute COVID-19 Kidney Outcomes by Occurrence of AKI during the Acute Phase
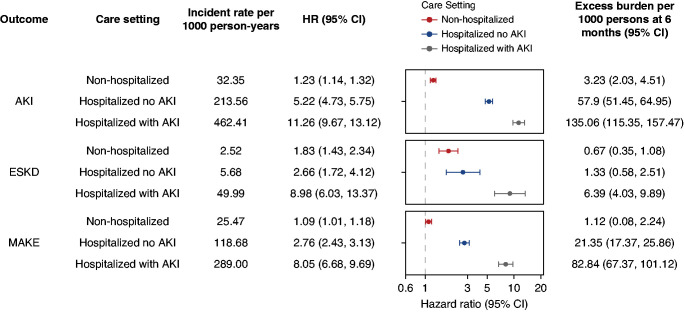
In consideration of changes in kidney function that may have occurred during the acute phase of the illness, we assessed the risks and burden of post-acute kidney outcomes (AKI, ESKD, and MAKE) in those who had COVID-19 and were non-hospitalized, those who were hospitalized but did not have an AKI, and those who were hospitalized and had an AKI during the acute phase of the infection. Frequency of outcomes and incident rates are presented in Supplemental Table 15. Assessment of covariate balance after application of weighting suggested that covariates were well balanced (Supplemental Figure 4 and Supplemental Tables 16–21). Compared with VHA users (control group), a gradient was evident in that risks (and associated burdens) increased across the three examined COVID-19 groups from nonhospitalized individuals to those who were hospitalized with no evidence of an AKI, and risk was highest in people who were hospitalized and had an AKI during the acute phase of the COVID-19 infection (Figure 3, Supplemental Table 22). Compared with those who were hospitalized and did not have an AKI during the acute phase, the risk of postacute kidney outcomes (AKI, ESKD, and MAKE) was higher in those who were hospitalized and had an AKI during the acute phase of the infection (Supplemental Table 22). Results were consistent in models only adjusting for the predefined covariates (Supplemental Table 23).
Figure 3.
and excess burden of post-acute COVID-19 kidney outcomes at 6 months in mutually exclusive cohorts of Veterans with non-hospitalized COVID-19, those hospitalized with COVID-19 with no evidence of an AKI, and those hospitalized with COVID-19 with an AKI during the first 30 days (acute phase) of the infection. Participants who had COVID-19 were compared with users of the VHA healthcare system who had no record of a positive COVID-19 test (control group). Outcomes were ascertained starting from 30 days after the participant’s positive COVID-19 test through end of follow-up. Unadjusted incident rates per 1000 person-years, HRs, and excess burden per 1000 persons at 6 months are provided for each COVID-19 group (non-hospitalized, hospitalized without an AKI, and hospitalized with an AKI during the acute phase of the infection). HRs and corresponding 95% CIs are plotted. MAKE was defined as a composite of eGFR decline ≥50%, ESKD, or all-cause mortality. All models were adjusted for a set of 29 predefined variables and 100 variables selected by a high-dimensional variable selection algorithm.
Post-acute COVID-19 eGFR Trajectories
We built linear mixed models to characterize post-acute COVID-19 eGFR trajectories of 30-day survivors of COVID-19 who had at least two measurements of serum creatinine during follow-up (n=373,151). Adjusted analyses of intra-individual change in eGFR suggested that, compared with VHA users (control group with eGFR slope of −0.49 [95% CI, −0.57 to −0.42] ml/min per 1.73 m2 per year), COVID-19 was associated with an excess eGFR decline of −3.26 (95% CI, −3.58 to −2.94) ml/min per 1.73 m2 per year in those who were non-hospitalized, −5.20 (95% CI, −6.24 to −4.16) ml/min per 1.73m2 per year in those who were hospitalized, and −7.69 (95% CI, −8.27 to −7.12) ml/min per 1.73 m2 per year in those who were admitted to intensive care during the acute phase of COVID-19 infection. Non-linear trajectories suggested that, as follow-up progressed, the rate of excess decline in eGFR attenuated (Figure 4). Additional examination of eGFR trajectories by AKI status during the acute phase suggested a steeper decline in eGFR in those who had an AKI during the acute phase of the illness (Figure 5). Adjusted analyses of intra-individual change in eGFR by AKI status, incorporating changes in kidney function during the acute phase of the illness, suggested that, compared with the VHA users, COVID-19 was associated with an excess eGFR decline of −3.30 (95% CI, −3.62 to −2.99) ml/min per 1.73 m2 per year in those non-hospitalized, −5.27 (95% CI, −5.86 to −4.68) ml/min per 1.73 m2 per year in those hospitalized without an AKI, and −8.41 (95% CI, −9.72 to −7.10) ml/min per 1.73 m2 per year in those hospitalized with an AKI. Results were consistent in models only adjusting for the predefined covariates (Supplemental Figures 5 and 6).
Figure 4.
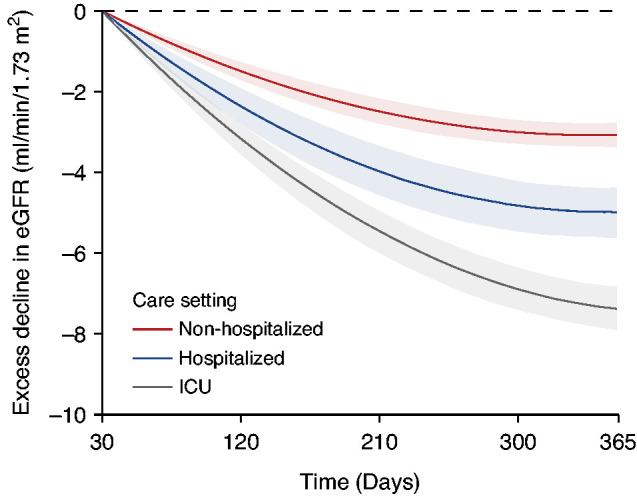
Excess decline in eGFR in post-acute COVID-19 by care setting of the acute phase of the illness. Differences in the trajectory of eGFR by day of follow-up compared with users of the VHA healthcare system with no record of a positive COVID-19 test (control group), estimated after adjustment for baseline characteristics. Changes are estimated starting from 30 days after a COVID-19 positive test. Bands represent the 95% CI.
Figure 5.
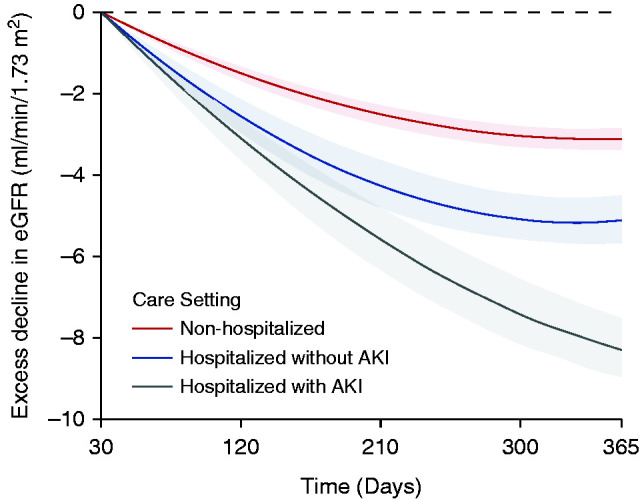
Excess decline in eGFR in postacute COVID-19 by AKI status during the acute phase of the illness. Differences in the trajectory of eGFR by day of follow-up compared with users of the VHA healthcare system with no record of a positive COVID-19 test (control group), estimated after adjustment for baseline characteristics. Changes are estimated starting from 30 days after a COVID-19 positive test. Bands represent the 95% CI.
Positive and Negative Outcome Controls
To test for potential presence of latent biases, we conducted analyses of positive and negative outcome controls, where, on the basis of prior evidence, one would expect to observe an association (positive controls) or the absence of an association (negative controls). Analyses suggested an association of COVID-19 with an increased risk and excess burden of positive outcome controls, including all-cause mortality (HR, 1.76; 95% CI, 1.66 to 1.87) and hospitalization (HR, 1.77; 95% CI, 1.72 to 1.81) after 30 days after testing positive for COVID-19 (Supplemental Table 24). No evidence of an association was observed with negative outcome controls, including fitting or adjustment of casts and bandages (HR, 0.97; 95% CI, 0.89 to 1.06) and atopic dermatitis (HR, 0.99; 95% CI, 0.83 to 1.18).
Discussion
In this work, we characterize post-acute kidney outcomes in a cohort consisting of 89,216 individuals who were 30-day survivors of COVID-19. The results show that, beyond the first 30 days of infection, those who survived COVID-19 exhibited increased risk (and burden) of AKI, eGFR decline, ESKD, and MAKE. The risks (and burdens) of kidney outcomes increased according to the severity of the acute infection. Although AKI during the acute phase contributed to the increased risk of post-acute kidney outcomes, our analyses also suggest that increased risk of post-acute kidney outcomes was evident even among those who did not experience AKI in the acute phase. Examination of intra-individual longitudinal change in eGFR suggested that individuals who survived COVID-19 experienced greater loss of eGFR than non-infected controls, and that eGFR loss was more profound as the severity of the acute COVID-19 infection increased. Taken together, these results suggest that, beyond the acute phase of COVID-19 infection, people with COVID-19 experience higher risk of adverse kidney outcomes. Post-acute care of people with COVID-19 should involve attention and care for acute and chronic kidney disease.
The implications of our findings are clear. Given the large number of people infected with COVID-19 (>43 million people in the United States, and >234 million globally), and given that estimates by the World Health Organization suggest that around 10% of people infected with COVID-19 may experience post-acute sequelae, the numbers of people with long COVID-19 in need of post–COVID-19 care will likely be staggering and will present substantial strain on already overwhelmed health systems.28 Governments and health systems around the world are establishing post-acute COVID-19 clinics to attend to the needs of people with postacute COVID-19 sequelae. The optimal composition of those clinics is not yet clear. The higher risks of adverse kidney outcomes reported in this study highlights the need for integration of kidney care as a component of the multidisciplinary post-acute COVID-19 care. Our estimates of the burden of kidney sequelae may also be useful to inform capacity planning.
Although our analyses suggest that AKI during the acute phase contributes to the increased risk of post-acute kidney outcomes (in that the risk of post-acute kidney outcomes was higher in those hospitalized with an AKI than those hospitalized without an AKI during the acute phase of the infection), it is also evident that the risk was increased in those who did not experience an AKI during the acute phase. Furthermore, our analyses of risks and burdens of post-acute kidney outcomes by care setting of the acute infection highlight two key messages: (1) the risk and associated burden of post-acute kidney outcomes was evident even among individuals whose acute disease was not severe enough to necessitate hospitalization (this will likely have broad implications because this group represents the majority of people with COVID-19), and (2) the risk and associated burden increased across the severity spectrum of the acute COVID-19 infection (from non-hospitalized individuals, to hospitalized individuals, to those admitted to intensive care).
The mechanism or mechanisms of increased risk of AKI, eGFR decline, ESKD, and MAKE in the post-acute phase of COVID-19 infection are not clear. Although initial observations suggested that severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) may have kidney tropism, more recent evidence does not endorse the earlier assessment.29 Other potential explanations include dysregulated immune response or autoimmunity, persistent inflammation, disturbances in endothelial function and the coagulation system, and disturbances in the autonomic nervous system. Mechanisms related to changes in the broader economic and social conditions in the context of the global pandemic that may have differentially affected people with COVID-19 may be also at play.30–36 A deeper understanding of the mechanistic and epidemiologic drivers of the post-acute kidney sequelae of SARS-CoV-2 infection (and, more broadly, the entire spectrum of postacute sequelae of SARS-CoV-2) is urgently needed to help inform care strategies.
This study has several strengths. To build our cohort, we capitalized on the breadth and depth of the electronic health databases of the US Department of Veterans Affairs, which operates the largest nationally integrated healthcare delivery system in the United States. We broadened our covariate-specification approach to include a set of 29 predefined variables selected on the basis of prior evidence and 100 algorithmically selected variables from several high-dimensional data domains, including diagnostic codes, prescription records, and laboratory test results. We evaluated several kidney outcomes, including AKI, eGFR decline, the terminal end point of ESKD, and assessed intra-individual longitudinal changes in eGFR. Our outcomes (for AKI, eGFR decline, and longitudinal eGFR changes) were defined on the basis of laboratory values rather than relying on International Classification of Diseases codes. We tested for potential presence of spurious biases by applying positive and negative outcome controls. We not only provided estimates of risks on the ratio scale (HRs), but also reported estimates of excess burden per 1000 persons due to COVID-19 on the absolute scale; this measure additionally reflects the contribution of baseline risk and provides a useful estimate of potential harm and would be more easily understood by a broader public than relative risk (e.g., HR).
This study has several limitations. The demographic and health characteristics of our VA cohort (older White males) may limit generalizability of the findings. Although we adjusted (through weighting) for both predefined and algorithmically selected high-dimensional covariates, and although covariate balance assessment suggested small standardized mean differences even in the covariates that were not directly included in the propensity score models, residual confounding may not be completely ruled out. Our datasets did not include individual data on urine measures for incorporation in AKI definitions. Although we provide estimates of risk and excess burden by intensity of care during the acute phase of the disease (nonhospitalized, hospitalized, and admitted to intensive care), our analyses did not adjust for other markers of severity within these categories. Finally, as the pandemic continues to evolve, as the effect of vaccinations and new variants (e.g., delta variant) is realized, as long-term follow-up of survivors of COVID-19 extends, and as treatment strategies of the acute disease improve, it is possible that the epidemiology of post-acute COVID-19 kidney outcomes will change as time progresses.
In sum, we show that 30-day survivors of COVID-19 exhibited higher risk of AKI, eGFR decline, ESKD, and MAKE than those not infected by COVID-19. Greater longitudinal eGFR loss was observed in those who survived COVID-19 (compared with noninfected controls). The risk of adverse kidney outcomes increased according to the severity of the acute infection as proxied by the care setting (nonhospitalized, hospitalized, and admitted to intensive care). The totality of the evidence suggests substantial risk of kidney outcomes in people with COVID-19 and highlights the need to integrate a kidney care component in post-acute COVID-19 care pathways.
Disclosures
Z. Al-Aly reports receiving research funding from the Institute for Public Health, US Department of VA, and Washington University in Saint Louis. All remaining authors have nothing to disclose.
Funding
This research was funded by the US Department of VA (to Z. Al-Aly) and two American Society of Nephrology and KidneyCure predoctoral fellowship awards (to B. Bowe and Y. Xie).
Supplementary Material
Acknowledgments
This study used data from the VA COVID-19 Shared Data Resource.
The contents of this article do not represent the views of the US Department of VA or the US Government.
The funders of this study had no role in study design, collection, analysis, and interpretation of data; writing the report; and in the decision to submit the report for publication.
B. Bowe and Z. Al-Aly contributed to the development of the study concept and design; B. Bowe and Y. Xie contributed to data acquisition; B. Bowe, Y. Xie, and Z. Al-Aly contributed to data analysis and interpretation; B. Bowe and Y. Xie contributed to statistical analysis; B. Bowe and Z. Al-Aly drafted the manuscript; critical revision of the manuscript was contributed to by B. Bowe, Y. Xie, E. Xu, and Z. Al-Aly; Administrative, technical, and material support was provided by Z. Al-Aly; Z. Al-Aly contributed supervision and mentorship; and all authors approved the final version of the report. Each author contributed important intellectual content during manuscript drafting or revision and accepts accountability for the overall work by ensuring that questions pertaining to the accuracy or integrity of any portion of the work are appropriately investigated and resolved. Z. Al-Aly takes responsibility that this study has been reported honestly, accurately, and transparently; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned have been explained.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
Data Sharing Statement
All data are available by request from the US Department of VA.
Supplemental Material
This article contains the following supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2021060734/-/DCSupplemental.
Supplemental Table 1. Median time to outcomes in the VHA user groups, those with COVID-19, and those with COVID-19 that were non-hospitalized, hospitalized, and admitted to the ICU.
Supplemental Table 2. Characteristics and standardized mean differences of predefined covariates between COVID-19 and VHA user groups before and after weighting.
Supplemental Table 3. Risk and excess burden of post-acute COVID-19 adverse kidney events in models adjusted for only predefined covariates.
Supplemental Table 4. Characteristics and standardized mean differences of predefined covariates by COVID-19 non-hospitalized and VHA user groups before and after weighting.
Supplemental Table 5. Characteristics and standardized mean differences of predefined covariates by COVID-19 hospitalized and VHA user groups before and after weighting.
Supplemental Table 6. Characteristics and standardized mean differences of predefined covariates by COVID-19 admitted to the ICU and VHA user groups before and after weighting.
Supplemental Table 7. Characteristics and standardized mean differences of predefined covariates by COVID-19 non-hospitalized and COVID-19 hospitalized groups before and after weighting.
Supplemental Table 8. Characteristics and standardized mean differences of predefined covariates by COVID-19 non-hospitalized and COVID-19 admitted to the ICU groups before and after weighting.
Supplemental Table 9. Characteristics and standardized mean differences of predefined covariates by COVID-19 hospitalized and COVID-19 admitted to the ICU groups before and after weighting.
Supplemental Table 10. Pairwise comparison among COVID-19 positive individuals of excess burden of PASC kidney disease by severity of the acute COVID-19 infection.
Supplemental Table 11. Pairwise comparison among COVID-19 positive individuals of excess burden of PASC kidney disease by severity of the acute COVID-19 infection adjusting for predefined covariates only.
Supplemental Table 12. Characteristics and standardized mean differences of predefined covariates by COVID-19 non-hospitalized and VHA user groups before and after weighting in analyses of risks by AKI status during the acute COVID-19 infection.
Supplemental Table 13. Characteristics and standardized mean differences of predefined covariates by COVID-19 hospitalized with no AKI and VHA user groups before and after weighting in analyses of risks by AKI status during the acute COVID-19 infection..
Supplemental Table 14. Characteristics and standardized mean differences of predefined covariates by COVID-19 hospitalized with no AKI and VHA user groups before and after weighting in analyses of risks by AKI status during the acute COVID-19 infection.
Supplemental Table 15. Characteristics and standardized mean differences of predefined covariates in COVID-19 non-hospitalized and COVID-19 hospitalized with no AKI groups before and after weighting in analyses of risks by AKI status during the acute COVID-19 infection.
Supplemental Table 16. Characteristics and standardized mean differences of predefined covariates in COVID-19 non-hospitalized and COVID-19 hospitalized with an AKI groups before and after weighting in analyses of risks by AKI status during the acute COVID-19 infection.
Supplemental Table 17. Characteristics and standardized mean differences of predefined covariates in COVID-19 hospitalized with no AKI and COVID-19 hospitalized with an AKI groups before and after weighting in analyses of risks by AKI status during the acute COVID-19 infection.
Supplemental Table 18. Pairwise comparisons of risk and excess burden of adverse risks by AKI status during the acute COVID-19 infection.
Supplemental Table 19. Pairwise comparisons of risk and excess burden of adverse kidney outcomes to VHA users risks by AKI status during the acute COVID-19 infection adjusting only for predefined covariates.
Supplemental Table 20. Outcome controls for the comparison of COVID-19 positive Veterans vs. VHA users.
Supplemental Figure 1. Cohort flow chart.
Supplemental Figure 2. Covariate balance in the comparison of COVID-19 positive veterans with VHA users AQ20.
Supplemental Figure 3. Covariate balance in the comparison of COVID-19 positive veterans by severity of the acute infection with non-hospitalized VHA users.
Supplemental Figure 4. Covariate balance in the comparison of COVID-19 positive veterans by severity AKI status during the acute phase of the illness.
Supplemental Figure 5. Excess decline in eGFR in post-acute COVID-19 adjusting for predefined covariates.
Supplemental Figure 6. Excess decline in eGFR in post-acute COVID-19 by AKI status during the acute phase of the illness adjusting for only predefined covariates.
References
- 1.Xie Y, Bowe B, Maddukuri G, Al-Aly Z: Comparative evaluation of clinical manifestations and risk of death in patients admitted to hospital with covid-19 and seasonal influenza: Cohort study. BMJ 371: m4677, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Al-Aly Z, Xie Y, Bowe B: High-dimensional characterization of post-acute sequelae of COVID-19. Nature 594: 259–264, 2021 [DOI] [PubMed] [Google Scholar]
- 3.Bowe B, Xie Y, Xian H, Lian M, Al-Aly Z: Geographic variation and US county characteristics associated with rapid kidney function decline. Kidney Int Rep 2: 5–17, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xie Y, Bowe B, Li T, Xian H, Yan Y, Al-Aly Z: Higher blood urea nitrogen is associated with increased risk of incident diabetes mellitus. Kidney Int 93: 741–752, 2018 [DOI] [PubMed] [Google Scholar]
- 5.Bowe B, Xie Y, Yan Y, Al-Aly Z: Burden of cause-specific mortality associated with PM2.5 air pollution in the United States. JAMA Netw Open 2: e1915834, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xie Y, Bowe B, Yan Y, Xian H, Li T, Al-Aly Z: Estimates of all cause mortality and cause specific mortality associated with proton pump inhibitors among US veterans: Cohort study. BMJ 365: l1580, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bowe B, Xie Y, Xian H, Li T, Al-Aly Z: Association between monocyte count and risk of incident CKD and progression to ESRD. CJASN 12: 603–613, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Al-Aly Z, Maddukuri G, Xie Y: Proton pump inhibitors and the kidney: Implications of current evidence for clinical practice and when and how to deprescribe. Am J Kidney Dis 75: 497–507, 2020 [DOI] [PubMed] [Google Scholar]
- 9.Xie Y, Bowe B, Gibson AK, McGill JB, Yan Y, Maddukuri G, et al. : Comparative effectiveness of the sodium-glucose cotransporter 2 inhibitor empagliflozin versus other antihyperglycemics on risk of major adverse kidney events. Diabetes Care 43: 2785–2795, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xie Y, Bowe B, Gibson AK, McGill JB, Maddukuri G, Al-Aly Z: Comparative effectiveness of sodium-glucose cotransporter 2 inhibitors vs sulfonylureas in patients with type 2 diabetes. JAMA Intern Med 181: 1043–1053, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Department of Veterans Affairs : COVID-19: Shared data resource. Available at https://vhacdwdwhweb100.vha.med.va.gov/phenotype/index.php/COVID-19:Shared_Data_Resource#Acknowledgements_COVID-19_Shared_Data_Resource. Accessed May 1, 2020
- 12.Kind AJ, Jencks S, Brock J, Yu M, Bartels C, Ehlenbach W, et al. : Neighborhood socioeconomic disadvantage and 30 day rehospitalizations: An analysis of Medicare data. Ann Intern Med 161: 765, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kind AJH, Buckingham WR: Making neighborhood-disadvantage metrics accessible — The neighborhood atlas. N Engl J Med 378: 2456–2458, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Levey AS, Stevens LA: Estimating GFR using the CKD epidemiology collaboration (CKD-EPI) creatinine equation: More accurate GFR estimates, lower CKD prevalence estimates, and better risk predictions. Am J Kidney Dis 55: 622–627, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bowe B, Cai M, Xie Y, Gibson AK, Maddukuri G, Al-Aly Z: Acute kidney injury in a national cohort of hospitalized US veterans with COVID-19. Clin J Am Soc Nephrol 16: 14–25, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chertow GM, Lazarus JM, Christiansen CL, Cook EF, Hammermeister KE, Grover F, et al. : Preoperative renal risk stratification. Circulation 95: 878–884, 1997 [DOI] [PubMed] [Google Scholar]
- 17.Klepser DG, Collier DS, Cochran GL: Proton pump inhibitors and acute kidney injury: A nested case-control study. BMC Nephrol 14: 150, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Leonard CE, Freeman CP, Newcomb CW, Reese PP, Herlim M, Bilker WB, et al. : Proton pump inhibitors and traditional nonsteroidal anti-inflammatory drugs and the risk of acute interstitial nephritis and acute kidney injury. Pharmacoepidemiol Drug Saf 21: 1155–1172, 2012 [DOI] [PubMed] [Google Scholar]
- 19.Okusa MD: The inflammatory cascade in acute ischemic renal failure. Nephron 90: 133–138, 2002 [DOI] [PubMed] [Google Scholar]
- 20.Schneeweiss S, Rassen JA, Glynn RJ, Avorn J, Mogun H, Brookhart MA: High-dimensional propensity score adjustment in studies of treatment effects using health care claims data. Epidemiology 20: 512–522, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Austin PC: Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat Med 28: 3083–3107, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rubin DB: Using propensity scores to help design observational studies: Application to the tobacco litigation. Health Serv Outcomes Res Methodol 2: 169–188, 2001 [Google Scholar]
- 23.Breslow NE: Discussion of professor Cox’s paper. J R Stat Soc B 34: 216–217, 1972 [Google Scholar]
- 24.Xie Y, Bowe B, Gibson AK, McGill JB, Maddukuri G, Yan Y, et al. : Comparative effectiveness of SGLT2 inhibitors, GLP-1 receptor agonists, DPP-4 inhibitors, and sulfonylureas on risk of kidney outcomes: Emulation of a target trial using health care databases. Diabetes Care 43: 2859–2869, 2020 [DOI] [PubMed] [Google Scholar]
- 25.Lipsitch M, Tchetgen Tchetgen E, Cohen T: Negative controls: A tool for detecting confounding and bias in observational studies. Epidemiology 21: 383–388, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Daugherty SE, Guo Y, Heath K, Dasmarinas MC, Jubilo KG, Samranvedhya J, et al. : SARS-CoV-2 infection and risk of clinical sequelae during the post-acute phase: A retrospective cohort study. BMJ 373: n1098, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wickham H: ggplot2: Elegant Graphics for Data Analysis, New York, Springer, 2016 [Google Scholar]
- 28.The Center for Systems Science and Engineering (CSSE) at Johns Hopkins University : The Johns Hopkins Coronavirus Resource Center, COVID-19 Dashboard. 2021. Available at: https://coronavirus.jhu.edu/map.html. Accessed October 4, 2021
- 29.Delorey TM, Ziegler CGK, Heimberg G, Normand R, Yang Y, Segerstolpe Å, et al. : COVID-19 tissue atlases reveal SARS-CoV-2 pathology and cellular targets. Nature 595: 107–113, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Figueroa JD, Brennan PM, Theodoratou E, Poon MTC, Purshouse K, Din FVN, et al. : Distinguishing between direct and indirect consequences of COVID-19. BMJ 369: m2377, 2020 [DOI] [PubMed] [Google Scholar]
- 31.Townsend E: COVID-19 policies in the UK and consequences for mental health. Lancet Psychiatry 7: 1014–1015, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Knipe D, Evans H, Marchant A, Gunnell D, John A: Mapping population mental health concerns related to COVID-19 and the consequences of physical distancing: A Google trends analysis. Wellcome Open Res 5: 82, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Raker EJ, Zacher M, Lowe SR: Lessons from Hurricane Katrina for predicting the indirect health consequences of the COVID-19 pandemic. Proc Natl Acad Sci U S A 117: 12595–12597, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mahase E: Covid-19: Mental health consequences of pandemic need urgent research, paper advises. BMJ 369: m1515, 2020 [DOI] [PubMed] [Google Scholar]
- 35.Xie Y, Bowe B, Yan Y, Cai M, Al-Aly Z: County-level contextual characteristics and disparities in life expectancy. Mayo Clin Proc 96: 92–104, 2021 [DOI] [PubMed] [Google Scholar]
- 36.Bowe B, Xie Y, Gibson AK, Cai M, van Donkelaar A, Martin RV, et al. : Ambient fine particulate matter air pollution and the risk of hospitalization among COVID-19 positive individuals: Cohort study. Environ Int 154: 106564, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.