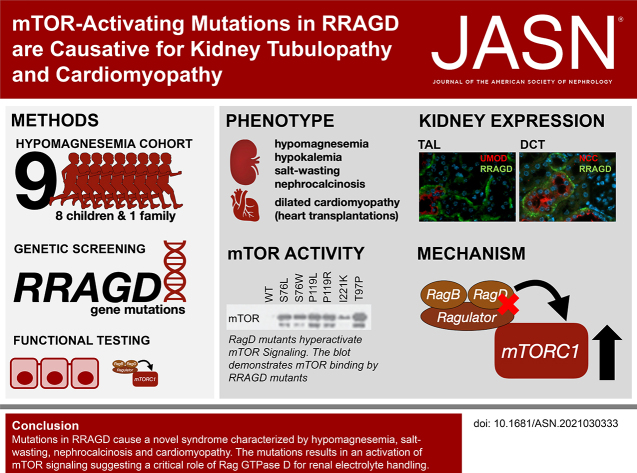
Significance Statement
Although advances in genetic techniques have resulted in the identification of rare hereditary disorders of renal magnesium and salt handling, some patients with tubulopathy lack a genetic diagnosis. In a cohort of patients with profound hypomagnesemia, renal salt wasting, nephrocalcinosis, and dilated cardiomyopathy, the authors performed whole-exome and -genome sequencing and identified heterozygous variants in RRAGD, which encodes a small Rag guanosine triphosphatase (GTPase). Subsequent functional analyses in vitro showed that the identified variants induce a constitutive activation of mechanistic target of rapamycin (mTOR) signaling in vitro. These findings not only establish a novel monogenic disorder of the kidney tubule, but demonstrate the essential role of mTOR signaling for distal tubular electrolyte handling and cardiac function.
Keywords: hypomagnesemia, Bartter syndrome, genetic renal disease, magnesium, kidney stones, TRPM6, nephrocalcinosis, hypokalemia, mTOR, rag complex, salt wasting
Visual Abstract
Abstract
Background
Over the last decade, advances in genetic techniques have resulted in the identification of rare hereditary disorders of renal magnesium and salt handling. Nevertheless, approximately 20% of all patients with tubulopathy lack a genetic diagnosis.
Methods
We performed whole-exome and -genome sequencing of a patient cohort with a novel, inherited, salt-losing tubulopathy; hypomagnesemia; and dilated cardiomyopathy. We also conducted subsequent in vitro functional analyses of identified variants of RRAGD, a gene that encodes a small Rag guanosine triphosphatase (GTPase).
Results
In eight children from unrelated families with a tubulopathy characterized by hypomagnesemia, hypokalemia, salt wasting, and nephrocalcinosis, we identified heterozygous missense variants in RRAGD that mostly occurred de novo. Six of these patients also had dilated cardiomyopathy and three underwent heart transplantation. We identified a heterozygous variant in RRAGD that segregated with the phenotype in eight members of a large family with similar kidney manifestations. The GTPase RagD, encoded by RRAGD, plays a role in mediating amino acid signaling to the mechanistic target of rapamycin complex 1 (mTORC1). RagD expression along the mammalian nephron included the thick ascending limb and the distal convoluted tubule. The identified RRAGD variants were shown to induce a constitutive activation of mTOR signaling in vitro.
Conclusions
Our findings establish a novel disease, which we call autosomal dominant kidney hypomagnesemia (ADKH-RRAGD), that combines an electrolyte-losing tubulopathy and dilated cardiomyopathy. The condition is caused by variants in the RRAGD gene, which encodes Rag GTPase D; these variants lead to an activation of mTOR signaling, suggesting a critical role of Rag GTPase D for renal electrolyte handling and cardiac function.
The kidney tubule balances fluid, electrolyte, and mineral homeostasis. The genetic deciphering of hereditary disturbances of electrolyte reabsorption by the tubule, collectively known as tubulopathies, has greatly improved the understanding of electrolyte transport along the nephron.1,2 Over the last decade, we and others have identified critical components of epithelial magnesium ion (Mg2+) reabsorption pathways in the thick ascending limb of the loop of Henle (TAL) and the distal convoluted tubule (DCT).2,3 In the TAL, 50%–70% of the filtered Mg2+ is reabsorbed paracellularly via the claudin16–19 complex.4 This paracellular Mg2+ transport is dependent on the transcellular salt reabsorption and negatively regulated by the basolateral calcium-sensing receptor.3,5 In the DCT, Mg2+ reabsorption is mediated by transient receptor potential melastatin type 6 and 7 (TRPM6/TRPM7) channels.6–12
In our cohort of patients with hypomagnesemia, we noted a subset of patients sharing a tubulopathy characterized by (1) renal salt wasting, (2) profound hypomagnesemia, and (3) nephrocalcinosis, combined with a dilated cardiomypathy (DCM). Because DCM had not been described in patients with known hereditary forms of hypomagnesemia,2 we hypothesized that these individuals were affected by a yet-undefined disease phenotype. By next-generation sequencing, we identified Ras-related GTP binding D (RRAGD) variants in eight unrelated individuals, and in eight members of a large family with symptomatic hypomagnesemia.
Methods
Subjects
We initially studied a cohort of six individuals with a phenotype combining DCM and a complex renal tubular disorder, including hypomagnesemia (median serum magnesium level of 0.42 mmol/L). Our second cohort consisted of three additional families in which the index patients presented with an isolated tubulopathy. Clinical and biologic data at the time of disease manifestation were collected retrospectively from medical charts. We re-evaluated all patients and obtained follow-up biochemical data. The detailed clinical history of each individual is provided in the Supplemental Appendix 2. All genetic studies were approved by the local ethics committees in Paris, Münster, and Liège, and informed consent was obtained from all subjects and/or their parents.
Serum magnesium, potassium, and chloride levels of individuals with RRAGD mutations were statistically compared with nonaffected family members. Samples were tested for normal distribution using the Kolmogorov–Smirnov test. For statistical analyses, a one-sided t test in case of normal distribution and a one-sided Mann–Whitney U test in case of non-normal distribution were used.
Sequencing
Patients F1.1–F4.1 were subjected to whole-exome sequencing (WES; for details see Supplemental Appendix 1). Exome data were analyzed for shared genes with variants under all modes of inheritance. After discovery of heterozygous RRAGD variants in these four patients, two additional patients with DCM and renal tubulopathy were analyzed by conventional Sanger sequencing (F5.1 and F6.1). The analyses were then expanded to 25 patients with a similar renal tubular disorder, but without a known cardiac phenotype, which revealed two additional patients (F7.1 and F8.1).13
Due to the lack of genetic material, a RRAGD mutation could not be confirmed in the affected father of individual F4.1 who died from DCM at 41 years of age. Of note, an older sister of individual F7.1 died in infancy from acute cardiac failure during a pneumonia episode and the autopsy revealed DCM. Careful genetic re-evaluation revealed mosaicism (17%) in leukocytes of the healthy mother for the respective RRAGD mutation (see Supplemental Appendix 2).
We analyzed family F9, which had multiple members affected by isolated renal tubular disease, independently under the assumption of an autosomal dominant disease by whole-genome sequencing (WGS). For this purpose, DNA samples of members III.1, III.6, and IV.2 of family 9 were subjected to WGS on the Illumina X Ten platform (Hartwig Medical Foundation, Amsterdam, The Netherlands). Data analysis was performed by mapping paired end reads (2 × 100 bp) from the NextSeq instrument against the hg19 human reference genome using the Burrows–Wheeler Aligner with recommended standard settings. Filtering steps excluded common single nucleotide polymorphisms (>1%), introns, untranslated regions, synonymous variants, and low-coverage regions (less than five reads). Overlaps of the remaining variants were generated using VCFMiner to select genes that were present in F1.1–F4.1, which resulted in the identification of one remaining gene, RRAGD.14 An extensive description of the sequencing procedure has been provided in the Supplemental Appendix 1.
Later, two additional patient cohorts were examined: (1) a cohort with isolated tubulopathy and suspected diagnosis of Gitelman syndrome (n=58) who had been subjected to WES; and (2) a cohort of patients with dilated cardiomyopathy (n=74), in whom mutations in known cardiomyopathy genes had previously been excluded by targeted exome sequencing of 174 cardiovascular genes, including 49 cardiomyopathy genes, as previously described.17
Cell Lysates and Immunoprecipitation
Human embryonic kidney (HEK293T) cells were transfected with Flag-S6K1, RagA, and RagD mutants, as described previously.15 To determine the response of mechanistic target of rapamycin complex 1 (mTORC1) to amino acid stimulation or starvation under the control of Rag mutants, 2 million HEK293T cells were plated onto a 10-cm dish. Twenty-four hours later, the cells were transfected with 2 ng of S6K1 and 100 ng of each Rag wild-type (wt) or mutant construct. Thirty-six hours later, amino acid stimulation or starvation was performed for 0.5–1 hour. Cells were rinsed once with ice-cold PBS and lysed with Triton X-100 lysis buffer (40 mM sodium-HEPES, pH 7.4; 5 mM magnesium chloride; 100 mM ATP; 10 mM tetrasodium pyrophosphate; 10 mM sodium β-glycerol phosphate; 1% vol/vol Triton X-100; and one tablet of protease inhibitor cocktail per 25 ml of buffer). The lysates were cleared by centrifugation at 15,000 rpm at 4°C in a microcentrifuge for 10 minutes. For immunoprecipitations, the FLAG M2 beads were pre-equilibrated in Triton X-100 lysis buffer. Thirty microliters of a 50/50 slurry of the FLAG M2 affinity beads were then added to cleared lysates and incubated at 4°C for 2 hours. After immunoprecipitation, the beads were washed one time with Triton X-100 lysis buffer and three times with Triton X-100 lysis buffer supplemented with 500 mM sodium chloride. Immunoprecipitated proteins were denatured by the addition of 50 µl of 2.5× SDS buffer, resolved by SDS-PAGE, and analyzed by immunoblotting.
Sample Preparation for Interactome Analysis
FlpIn inner medullary collecting duct cells stably expressing green fluorescent protein (GFP)–GFP, GFP-Rragd-wt, or GFP-Rragd-Ser75Leu fusion proteins were generated as described previously.16 Lysates were cleared by centrifugation, followed by incubation with GFP µMACS magnetic beads (Miltenyi) for 1 hour. Then, lysates were loaded on a µMACS column and precipitated proteins were GSH, alkylated, and on-column digestion was performed overnight, as previously described.16 Eluates were acidified the next day using 1%–2% formic acid and stage-tip cleanup was performed, as previously described, and samples were dried using a vacuum centrifuge. Peptides were resuspended in 0.1% formic acid and were separated using a 1-hour gradient on an nLC coupled to a Q Exactive Plus tandem mass spectrometer (Thermo Scientific) or an LTQ orbitrap XL mass spectrometer. Experimental details are provided in the Supplemental Appendix 1.
Expression Analysis
Total RNA was extracted from the tissues of C57BL/6 mice and isolated DCT cells of parvalbumin-GFP mice using TRIzol Reagent (Thermo Fisher Scientific, Waltham, MA) according to the manufacturer’s protocol. To remove genomic DNA, the isolated RNA was subsequently subjected to DNase (Promega, Madison, WI) treatment. RNA was reverse transcribed using Moloney murine leukemia virus reverse transcription (Thermo Fisher Scientific) for 1 hour at 37°C. Rragd (forward, CACCTGAGCTTTTACCTGA; reverse, TCAGCAGATTCTCCAGCGTC) gene expression levels were quantified by SYBR Green (Bio-Rad) on a CFX96 Real-Time PCR Detection System (Bio-Rad) and normalized for glyceraldehyde 3-phosphate dehydrogenase (forward, TAACATCAAATGGGGTGAGG; reverse, GGTTCACACCCATCACAAAC) expression levels.
Immunohistochemistry was performed on kidney tissues of C57BL/6 mice using primary antibodies against RagD, aquaporin-2 (AQP2), sodium chloride cotransporter (NCC), and uromodulin (UMOD), as described previously.17 Paraffin-embedded kidney sections were subjected to deparaffinization and rehydration, followed by permeabilization in 0.3% (vol/vol) PBS–Triton X-100 (Sigma). Sections were incubated overnight at 4°C with the primary antibodies sheep anti-NCC (1:400, sheep S965B; Medical Research Council Protein Phosphorylation and Ubiquitylation Unit, Dundee, United Kingdom), sheep anti–Tamm Horsfall (1:200, MBS220487; Bio-Trend), and guinea pig anti-AQP2 (1:100; kindly provided by Dr. Peter Deen) and subsequently incubated overnight at 4°C with the primary antibody rabbit anti-RRAGD (1:2000, NBP2-32106; Novus Biologicals). Experimental details are provided in the Supplemental Appendix 1.
Results
Patients
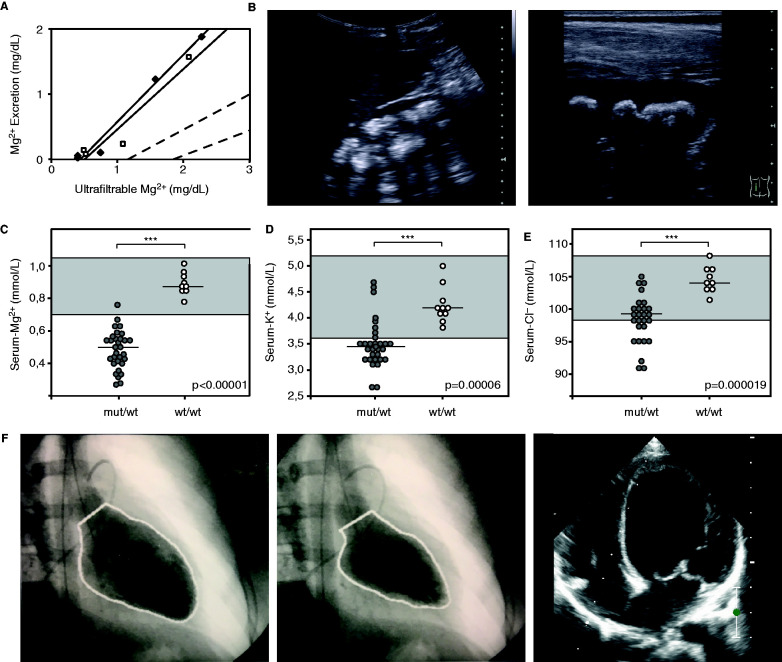
We initially identified eight unrelated individuals with hypomagnesemia and a complex renal tubulopathy phenotype (Table 1), accompanied by muscle spasms and seizures in three individuals. Inappropriately high fractional excretion rates of magnesium indicated renal magnesium wasting (Table 1). In individual F2.1, a magnesium loading test was performed, which disclosed a decreased renal tubular threshold for magnesium reabsorption (Figure 1A). Additional renal findings included polyuria, hypokalemia, hypochloremia, a tendency toward metabolic alkalosis, hypercalciuria, and nephrocalcinosis, pointing to a defect in the TAL (Figure 1, B–E, Table 1). Of note, the prenatal course of individuals F3.1 and F4.1 was complicated by polyhydramnios, as observed in antenatal Bartter syndrome.18 All family members of these probands were clinically unaffected. In six patients (F1–F6), the tubulopathy was accompanied by early and severe DCM. Indeed, three patients (F1.1, F3.1, and F5.1) initially presented in infancy with clinical signs of cardiac insufficiency, including feeding difficulties, vomiting, failure to thrive, sweating, tachycardia, and tachypnea (detailed clinical descriptions are provided in the Supplemental Appendix 2). DCM resulted in heart failure in individuals F1.1–F3.1. Therefore, they received a heart transplantation (Figure 1F). Whereas echocardiography demonstrated a preserved cardiac function at adolescent age in patient F4.1, patients F5.1–F7.1 had an impaired but stable left ventricular function under heart failure medication. Of the patients with DCM, solely patient F3.1 received a loop diuretic before the diagnosis of her tubulopathy, making a contribution of diuretic treatment to the development of medullary nephrocalcinosis, which was observed in the majority of patients, unlikely.
Table 1.
Clinical and laboratory characteristics of patients with RRAGD mutations
| Individuals | F1.1 | F2.1 | F3.1 | F4.1 | F5.1 | F6.1 | F7.1 | F8.1 | F9.1 | Family 9 Median (n=8) |
|---|---|---|---|---|---|---|---|---|---|---|
| Origin | US | France | France | Belgium | Germany | Belgium | Germany | Turkey | Belgium | Belgium |
| Sex | M | F | F | M | F | M | F | M | M | 4F/4M |
| Age at manifestation | 3 yr | 3 yr | 6 mo | 5 yr | 7 mo | 14 yr | 2 yr | 6 yr | 20 yr | 2.5–24 yr |
| Initial findings | ||||||||||
| Dilated cardiomyopathy (age at diagnosis) | Yes (3 yr) | Yes (12 yr) | Yes (6 mo) | No | Yes (7 mo) | Yes (14 yr) | Yes (4 yr) | No | No | No |
| Fractional shortening (%; N>25) | 7 | 8 | 15 | 31 | 15 | — | 18 | — | — | — |
| Ejection fraction (%; N=55–70) | 15 | 31 | — | 61 | 16 | 50 | — | — | — | — |
| Left ventricular end-diastolic diameter (mm)a | 53 (>P99.9)d |
55 (>P99.9)d |
33 (P99.7)d |
34 (P17.1)d |
42 (>P99.9)d |
— | 46 (>P99.9)d |
— | — | — |
| Hypomagnesemia-related symptoms | No | Tetany | Cerebral seizure | Tetany, muscle cramps | No | No | No | Cerebral seizure | Tetany, muscle cramps | 7/8 |
| Nephrocalcinosis | Yes | Yes | Yes | No | Yes | No | Yes | Yes | No | 1/8 |
| Polyuriab | No | No | Yes | No | Yes | No | Yes | Yes | Yes | 2/8 |
| Metabolic alkalosis | No | ? | Yes | Yes | No | No | No | Yes | Yes | 4/8 |
| Initial laboratory findings | ||||||||||
| S-Na (mmol/L; N=138–145) | 135 | — | 137 | 137 | 141 | 136 | 142 | 141 | 140 | 139 |
| S-K (mmol/L; N=3.5–5.0) | 4.7 | 3.2 | 3.5 | 3,4 | 3.5 | 3.5 | 3.9 | 2.7 | 3.5 | 3.3 |
| S-Cl (mmol/L; N=98–106) | 98 | — | 95 | — | 100 | 95 | 105 | 91 | 95 | 98,5 |
| S-Ca (mmol/L; N=2.20–2.70) | 2.05 | 1.78 | 2.20 | 2.2 | 2.11 | 2.37 | 2.50 | 1.8 | 2.41 | 2.36 |
| S-Mg (mmol/L; N=0.60–0.95) | 0.50 | 0.27 | 0.40 | 0.43 | 0.40 | 0.42 | 0.54 | 0.26 | 0.54 | 0.49 |
| S-PO4 (mmol/L; N=1.20–1.80) | 1.56 | — | 1.44 | 1.59 | 1.50 | — | 1.53 | 1.7 | 0.87 | 1.00 |
| S-creatinine (mg/dl) | 0.51 | 0.70 | 0.24 | 0.23 | 0.21 | 0.75 | 0.30 | 0.47 | 0.74 | 0.51 |
| S-HCO3 (mmol/L; N=22–29) | — | — | 24 | 29.3 | — | — | 28.5 | 29.1 | — | — |
| iPTH (pg/ml; N=9–65) | 33 | — | 17 | 29 | 37 | — | 91 | 108 | 21 | 18 |
| FE-Na (%; N<2) | 1.0 | — | 0.34 | 0,4 | 0.8 | — | 0.9 | 0.34 | 0.4 | 0.40 |
| FE-K (%; N=4–16) | 13 | — | 27 | 6,3 | 24 | — | 23 | 14 | 7.5 | 6.2 |
| Ca-Crea ratio (mol/mol; N<0.9) | 0.67 | 1.8 | 1.24c | 0.12 | 1.46c | — | 2.27 | 1.38 | 0.25 | — |
| FE-Mg (%; N< 4) | 47 | 13.2 | 7.0 | 4.3 | 16 | — | 13.2 | 13.8 | 3.7 | — |
| Follow-up findings | ||||||||||
| Age at last follow-up (yr) | 6 | 20 | 14 | 10 | 35 | 5 | 19 | 40 | 2.5–61 | |
| Heart transplant (age) | Yes (3.3 yr) | Yes (25 yr) | Yes (15 yr) | No | No | No | No | No | No | No |
| Fractional shortening (%; N>25) | d.n.a. | d.n.a. | d.n.a. | 33 | 22 | 14 | 28 | 38 | — | — |
| Ejection fraction (%; N=55–70) | d.n.a. | d.n.a. | d.n.a. | 62 | 41 | 45 | 50 | 67 | 78 | 60–78 |
| Left ventricular end-diastolic diameter (mm)a | d.n.a | d.n.a. | d.n.a. | 37 (P1.8)d |
42 (P77.3)d |
49 | 43 (>P99.9)d |
48 | — | — |
| Most recent laboratory findings | ||||||||||
| S-Na (mmol/L; N=135–145) | 138 | — | 140 | 140 | 139 | 140 | 142 | 141 | 140 | 140 |
| S-K (mmol/L; N=3.5–5.0) | 4.6 | — | 3.7 | 3,3 | 3.3 | 4.6 | 3.2 | 3.5 | 3.5 | 3.4 |
| S-Cl (mmol/L; N=98–107) | 104 | 100 | 100 | 103 | 104 | 95 | 97 | 98 | ||
| S-Ca (mmol/L; N=2.20–2.65) | 2.57 | — | 2.34 | 2,41 | 2.50 | 2.42 | 2.40 | 2.27 | 2.3 | 2.4 |
| S-Mg (mmol/L; N=0.7–1.1) | 0.58 | — | 0.43 | 0,55 | 0.50 | 0.62 | 0.66 | 0.53 | 0.46 | 0.48 |
| S-creatinine (mg/dl) | 0.47 | — | 0.64 | 0,52 | 0.60 | 1.18 | 0.40 | 0.69 | 0.67 | 0.57 |
| S-HCO3 (mmol/L; N=22–26) | 23.0 | — | 28.0 | 25.0 | 27.0 | 24.1 | 24.2 | 29.9 | 35.0 | 29.4 |
| iPTH (pg/ml; N=10–65) | 94 | — | 54 | / | 34 | 17.4 | 60 | 41 | 18 | 17 |
| FE-Na (%; N<2) | 1.0 | — | 1.00 | 0.34 | 1.04 | 0.67 | 0.9 | 0.79 | 0.77 | 0.57 |
| FE-K (%; N=4–16) | 15.5 | — | 11.0 | 33.7 | 18.9 | 13.8 | 34 | 18.2 | 12.1 | 10.7 |
| FE-Cl (%) | 2.71 | — | — | — | 1.52 | — | — | — | — | — |
| Ca-Crea ratio (mol/mol; N<0.9) | 0.34 | — | 0.09 | 0.22 | 0.25 | 1.02 | 1.14 | 0.08 | 0.37 | — |
| FE-Mg (%; N<4) | 26.0 | — | 6.3 | 17.5 | 12.8 | 3.3 | 9.4 | 14.6 | 5.9 | 5.2 |
| Therapy | ||||||||||
| Magnesium supplementation | Yes | ? | Yes | Yes | Yes | Yes | Yes | Yes | Yes | 6/8 |
| Potassium supplementation | No | ? | Yes | Yes | Yes | No | Yes | Yes | No | 3/8 |
| Heart failure medication | Yes | ? | Yes | No | Yes | Yes | Yes | No | No | 0/8 |
| Genetic findings (RRAGD mutations) | ||||||||||
| Nucleotide level | c.227C>T | c.356C>G | c.662T>A | c.227C>T | c.356C>G | c.227C>T | c.356C>T | c.227C>G | c.289A>C | c.289A>C |
| Protein level | p.Ser76Leu | p.Pro119Arg | p.Ile221Lys | p.Ser76Leu | p.Pro119Arg | p.Ser76Leu | p.Pro119Leu | p.Ser76Trp | p.Thr97Pro | p.Thr97Pro |
| Inheritance | De novo | De novo | De novo | Dominant? | De novo | De novo | Maternal mosaicism | De novo | Dominant | Dominant |
US, United States; M, male; F, female; ?, unknown; S, serum; Na, sodium; K, potassium; Cl, chloride; Mg, magnesium; PO4, phosphate; HCO3, bicarbonate; iPTH, intact parathyroid hormone; FE, fractional excretion; Ca-crea ratio, calcium-creatinine ratio; d.n.a., does not apply.
Calculations according to Pettersen et al.57
>2 L/m2 body surface area per day.
Molar Ca-crea ratio N <2.2 for infants.
P = percentile of reference weight and height adjusted reference values.
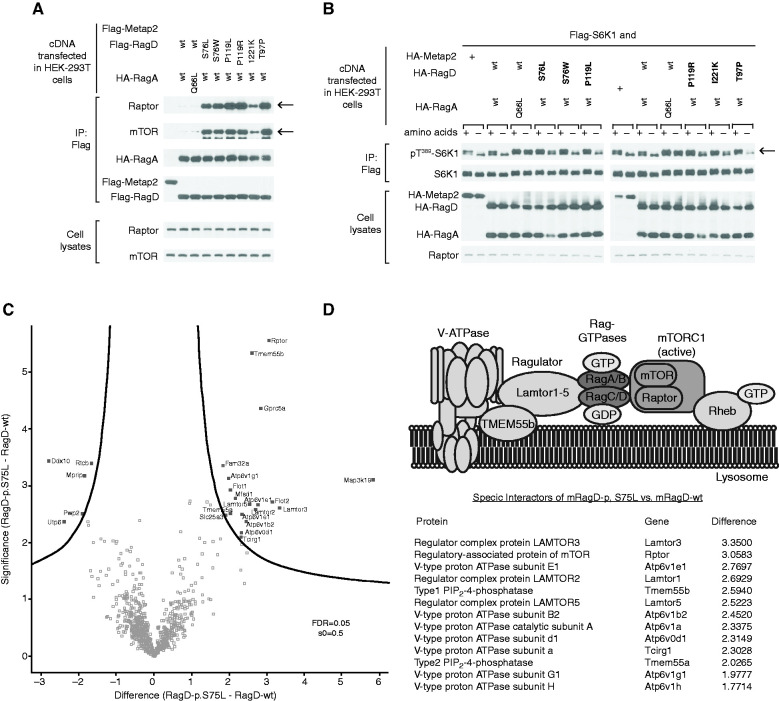
Figure 1.
Patients have dilated cardiomyopathy and renal tubulopathy. (A) Magnesium loading test in individual F2.1. Urinary magnesium excretion is plotted against the ultrafiltrable fraction of serum magnesium before and during parenteral infusion of magnesium at increasing concentrations (two separate tests, indicated by open and closed boxes). The dashed lines represent the normal range. (B) Renal ultrasound of individual F7.1 showing severe medullary nephrocalcinosis (grade III according to Hoyer).56 (C–E) Comparison of serum magnesium, potassium, and chloride levels of individuals with RRAGD mutations (shaded circles) and of unaffected family members (open circles) (initial as well as follow-up values included). Horizontal bars represent medians; reference ranges for serum electrolytes are indicated by gray boxes. Apart from significant hypomagnesemia, individuals with RRAGD mutations exhibited significantly lower serum potassium and serum chloride levels, reflecting renal salt wasting. ***P<0.001, one-sided t test (magnesium, chloride) and Mann–Whitney U test (potassium). (F) Left ventriculography (performed at the age of 12 years) of individual F2.1 who presented with cardiomyopathy. Pictures show left ventricular volumes during diastole (left) and systole (middle), demonstrating a reduced ejection fraction of 40% (normal, 55%–70%). Initial echocardiogram (right) of individual F7.1 performed after genetic diagnosis (apical four-chamber view) showing the dilated left ventricle. Cl−, chloride ion; K+, potassium ion; mut, mutant.
Two patients (F7.1 and F8.1) clinically presented in infancy and early childhood with severe hypomagnesemia and signs of renal salt wasting. Patient F8.1 still displays an unremarkable cardiac function at adult age. In contrast, the initial cardiac evaluation of patient F7.1 after genetic diagnosis revealed DCM and a significantly impaired cardiac function requiring heart failure treatment.
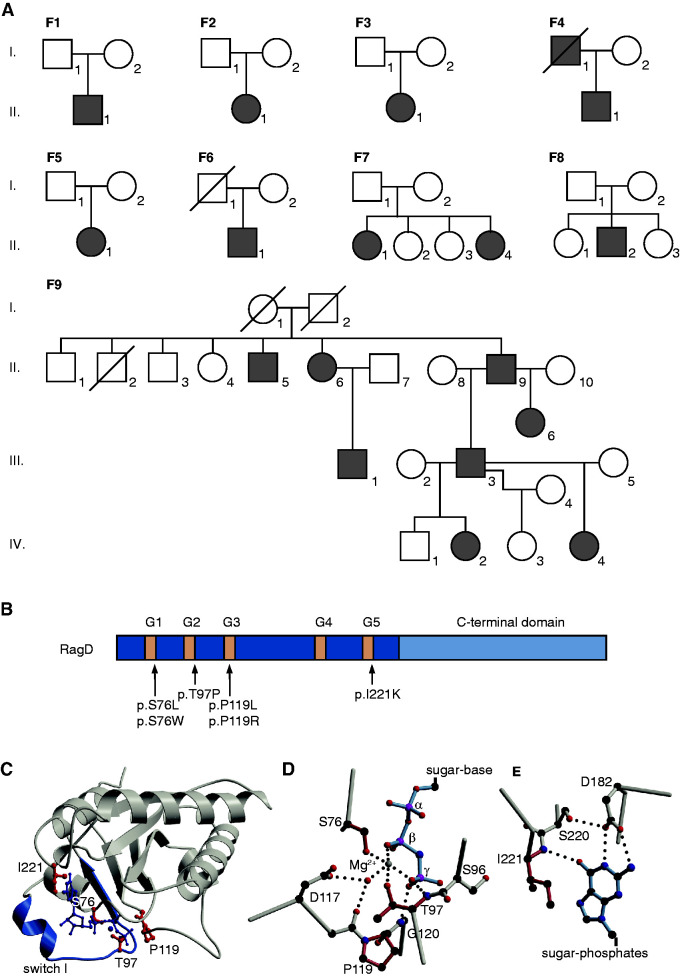
In parallel to these eight unrelated patients, we analyzed a large family (F9; Figure 2A) with eight affected members presenting with profound and symptomatic hypomagnesemia resistant to oral magnesium supplements (pedigree and clinical details in Figure 2 and Supplemental Table 1, respectively). The index patient presented in early adulthood with muscle cramps and tetany. Affected individuals of family F9 exhibited hypokalemia, metabolic alkalosis, and an activation of the renin-angiotensin-aldosterone system (RAAS) together with normal or low BP, suggesting the diagnosis of Gitelman syndrome (Figure 1C, Table 1). None of these individuals showed cardiac abnormalities.
Figure 2.
Mutations in the RagD protein are located in the GTP-binding sites. (A) Pedigrees of all families included in this study. Filled symbols represent affected individuals. Squares indicate male family members and circles represent female family members. (B) Domain organization of RagD with GTP-binding motifs in dark blue (G1–G5). Mutations are indicated with an arrow. (C) Crystal structure of RagD in complex with a GTP analogue (GppNHp; Protein Data Bank entry 2q3f). Mutated residues are shown in red. GppNHp and the coordinated Mg2+ are depicted in dark blue. (D and E) Detailed view of the (D) phosphate moiety of the nucleotide and (E) the nucleotide base. Affected residues are colored in light red. Dotted lines indicate hydrogen bonds.
To characterize the renal salt wasting phenotype in more detail, serum potassium and chloride levels were compared between affected individuals (n=16) and available nonaffected family members (n=8) (Figure 1, D and E). These analyses demonstrated significantly lower values in affected individuals that, together with metabolic alkalosis and RAAS activation, was highly indicative of renal salt wasting.
Identification of RRAGD Mutations
After exclusion of mutations in known genes for hypomagnesemia and salt wasting, we performed WES in individuals F1.1–F4.1, which revealed RRAGD as a single common mutated gene (Figure 2B). Subsequently, additional heterozygous RRAGD mutations were discovered in patients F5.1 and F6.1, and in patients F7.1 and F8.1 with isolated tubulopathy. Sequencing of unaffected parents demonstrated that the identified RRAGD mutations mostly occurred de novo. Due to the lack of genetic material, a RRAGD mutation could not be confirmed in the affected father of patient F4.1, who died from DCM at 41 years of age. Of note, an older sister of patient F7.1 died in infancy from acute cardiac failure during a pneumonia episode and the autopsy revealed DCM. Careful genetic re-evaluation revealed mosaicism (17%) in leukocytes of the healthy mother for the respective RRAGD mutation (see Supplemental Appendix 2). Family F9, with multiple affected family members, was initially analyzed separately under the assumption of a dominant trait. After exclusion of known genes for hypomagnesemia, WGS revealed a heterozygous RRAGD mutation (p.Thr97Pro) that segregated with the disease. Retrospectively, two-point linkage was performed resulting in a suggestive LOD score of 2.41 as evidence for linkage between the mutation and the disease phenotype. The reanalysis and screening of two additional cohorts with either suspected Gitelman syndrome (n=58) or isolated DCM did not identify additional pathogenic RRAGD variants.
RRAGD encodes a small Rag guanosine triphosphatase (GTPase), which is a member of the Ras family of GTP-binding proteins.19 All RagD mutations affect highly conserved amino acid residues in GTP-binding domains conserved in small GTPases (G domains, G1–G5, Figure 2, B–E, Supplemental Figure 1).20 Ser76 is part of the G1 motif, also known as P-loop; Thr97 constitutes the G2 motif; and Pro119 resides in the G3 motif (Figure 2, B–E). These motifs are involved in the interaction with the phosphate moiety of the nucleotide. Ile221 resides in a sequence stretch corresponding to the G5 motif. The variants are expected to display low nucleotide affinity and fast nucleotide exchange. This may interfere with RagD-mediated signaling in different ways (please find a detailed description in the Supplemental Appendix 3). The mutations result in amino acid residues that are sterically more demanding and, at least in some cases, are incompatible with the fold observed in a crystal structure of human RagD (Protein Data Bank entry 2q3f), where switch I is folded over the nucleotide. Such a closed conformation is typically found in GTP- or GDP-bound small G proteins.21
RagD Is Expressed in the Distal Tubule of the Kidney
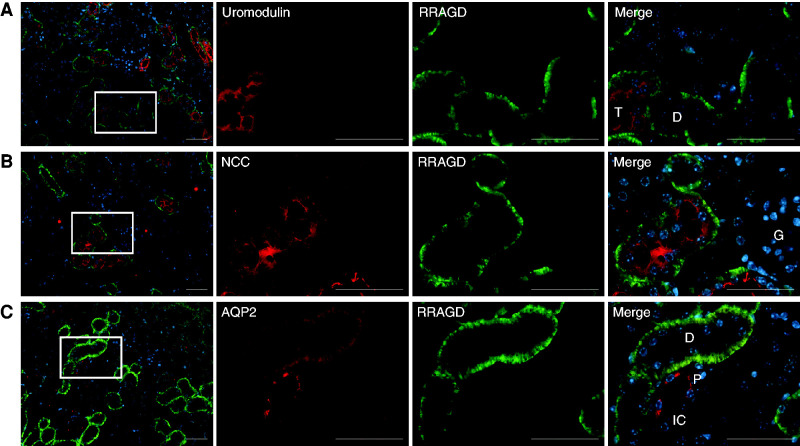
The mRNA expression of mouse Rragd was quantified in tissues of C57BL/6 mice and isolated DCT cells of parvalbumin-GFP mice by quantitative real-time PCR according to previously established methods.22 RagD showed a ubiquitous expression pattern with significant transcript levels in heart and kidney (Supplemental Figure 2A). Using primary antibodies against marker proteins for distal tubular segments—AQP2 for cortical collecting duct, NCC for the DCT, and UMOD for the TAL, as described previously17—RagD was shown to be colocalized with UMOD and NCC in the TAL and DCT nephron segments, respectively (Figure 3). Of note, mRNA expression of RagD was enriched in the DCT compared with total kidney (Supplemental Figure 2B). In contrast, RagD showed no colocalization with AQP2. These expression analyses confirm previous high-throughput quantitative transcriptomic and proteomic profiling data from microdissected rat kidney tubule segments.23,24 Additionally, single-cell RNA analyses of Ransick et al.25 point to a strong expression of Rragd in intercalated cells of the cortical and medullary collecting duct.
Figure 3.
RagD is expressed in the DCT. (A–C) Double immunofluorescence staining of mouse kidney sections for RagD in green and (A) UMOD (red), (B) NCC (red), or (C) AQP2 (red). Bar, 50 µm. D, distal tubule; G, glomerulus; IC, intercalated cell; P, principal cell; T, TAL.
RagD Mutations Activate the mTOR Pathway
All RagD mutants showed increased binding to regulatory associated protein of mTOR (Raptor) and mTOR, the essential components of mTORC1 (Figure 4A), after exogenous expression in HEK293T cells. All human RagD mutants resulted in increased S6K1 phosphorylation in the presence of nutrients, indicative of active mTORC1 signaling. Of note, some mutants increased S6K1 phosphorylation even during starvation (Figure 4B).
Figure 4.
RRAGD mutations increase mTOR signaling in vitro. (A) Immunoblot analysis demonstrating that RagD mutations (bold) increase Raptor and mTOR binding (rows indicated by arrow). Hemagglutinin-RagA (HA-RagA) and FLAG-RagD blots indicate equal (co-)precipitation of the Rag dimer (middle rows). Transfected HEK293T cells contained equal amounts of Raptor and mTOR, as detected in the cell lysate fraction (bottom rows). Metap-2 (indicated by +) used as a negative control for nonspecific flag binding. (B) mTORC1 signaling in response to amino acids and starvation assessed by phosphorylation of its substrate S6 kinase (S6K1) at threonine 389 (Thr389), indicated by an arrow. Compared to wt RagD, all RagD mutants caused an increased S6K1 phosphorylation in the presence of amino acids. The expression of transfected RagA, RagD, and S6K1, and of endogenous Raptor, was confirmed by immunoblotting (bottom rows). Constitutively active RagA-p.Q66L served as a positive control.34 Metap-2 (indicated by +) served as a negative control for nonspecific flag binding. (C) Volcano plot of the interactome of murine RagD-p.S75L versus RagD-wt. The significance (P value) of changes between RagD-p.S75L and RagD-wt (−log10 scale) is plotted against the difference (fold change) between RagD-p.S75L and RagD-wt (log2 scale). Dots beyond the curved line represent proteins with significantly increased abundance (false discovery rate [FDR], 0.05; s0=0.5) in RagD-p.S75L samples (right) or RagD-wt samples (left). (D) Model of the V-ATPase-Ragulator-Rag-GTPase-mTORC1 complex and table listing the identified components from (C) that showed significantly increased binding to RagD-p.S75L in log2 fold change. IP, immunoprecipitation.
To understand the molecular mechanisms of mTORC1 activation at the lysosomal membrane in an independent assay and to evaluate the effect of human RagD-p.Ser76Leu on mTORC1 complexes, we performed an unbiased interactome analysis by label-free quantitative proteomics. For this purpose, FlpIn murine renal tubular epithelial cells expressing either a wt or mutant GFP.RagD-p.Ser75Leu fusion protein were subjected to immunoprecipitation of GFP.RagD and liquid chromatography with tandem mass spectrometry (Figure 4C). Using a hierarchic clustering of protein intensities from the dataset and applying stringent cutoff criteria, we identified 32 proteins that differentially coprecipitated with mRagD-p.Ser75Leu in comparison with wt RagD in seven independent experiments. Interestingly, mutant RagD-p.Ser75Leu showed a significantly increased coprecipitation of multiple proteins of the mTORC1 signaling complex at the lysosomal membrane (Figure 4D). These included the mTORC1 subunit Raptor and different subunits of lysosomal vacuolar-type H+-ATPase (V-ATPase, Atp6v-proteins). Additionally, proteins that tether the Rag GTPase dimer to the lysosomal surface were identified, such as members of the Lamtor protein family (Lamtor3, Lamtor5) and TMEM55B (Figure 4, C and D, Supplemental Figure 3). Of note, no major differences in expression or subcellular localization patterns were observed between the wt and the mutant fusion protein (Supplemental Figure 3).
Discussion
This study describes a previously unrecognized hereditary disease phenotype combining an electrolyte-losing tubulopathy and DCM, which is caused by heterozygous missense variants in the RRAGD gene encoding Rag GTPase D. The identified RRAGD variants lead to increased mTOR signaling. We propose to call this novel inherited disease autosomal dominant kidney hypomagnesemia (ADKH-RRAGD).
The cardinal symptom of individuals with RRAGD mutations is a severe hypomagnesemia, resulting in muscle cramps, tetany, and cerebral seizures. Renal magnesium wasting was associated with hypercalciuria and nephrocalcinosis in a subset of individuals, a phenotype highly reminiscent of familial hypomagnesemia with hypercalciuria and nephrocalcinosis (FHHNC; Online Mendelian Inheritance in Man numbers 248250 and 248190).2,3 In FHHNC, mutations in two tight junction proteins, claudin-16 and claudin-19, cause a defective paracellular reabsorption of calcium and magnesium in the TAL.26–28 Indeed, immunostaining experiments showed RagD expression in the TAL, suggesting RagD variants may impair calcium ion and Mg2+ reabsorption in this tubular segment.
In a subset of patients, a renal phenotype with salt wasting and hypokalemic alkalosis prevailed, resembling Bartter syndrome. The prenatal finding of polyhydramnios in three individuals supported this assumption. Unfortunately, a systematic evaluation of the RAAS in our entire cohort was prevented by early cardiac insufficiency and treatment with diuretics and angiotensin-converting enzyme inhibitors. However, a marked activation of the RAAS was also noted in individual F2.1 years before cardiac manifestation, supporting the suspicion of a mixed FHHNC/salt-wasting phenotype. In contrast, hypercalciuria and nephrocalcinosis lacked in affected members of family F9. This phenotypic variability might be explained by a less pronounced activation of mTOR signaling by the RagD-p.T97P mutant, but could also reflect a primary dysfunction of the DCT with a Gitelman syndrome–like phenotype rather than a TAL defect. Similar observations have been made in Bartter syndrome type III due to CLCNKB defects, in which individuals might present with a phenotype ranging from antenatal Bartter syndrome to Gitelman syndrome.29,30 Future research will be required to elucidate the exact targets of increased mTOR signaling in distal tubular segments.
Besides the tubulopathy, present in all patients, DCM represented a serious clinical symptom leading to early heart failure and resulting in a heart transplantation in a substantial subset of patients. In fact, the impairment of cardiac function and the medical treatment with angiotensin-converting enzyme inhibitors and diuretics might have hampered the diagnosis of the tubulopathy in individual patients. Moreover, calcineurin inhibitor treatment might have aggravated hypomagnesemia in patients after cardiac transplantation.
Interestingly, the phenotype of our cohort is reminiscent of two individuals from a previously described Finnish family with unresolved renal tubular disease and cardiomyopathy.31,32 The clinical picture of these individuals is highly suggestive of ADKH-RRAGD. Unfortunately, the cardiac phenotype resulted in an early fatal issue in this family, which hampers any further genetic investigation.
RRAGD encodes a small Rag guanosine triphosphatase (GTPase), which is an essential component of the nutrient-sensing pathway that activates mTOR signaling.19 mTOR serves as the main nutrient sensor of the cell, coordinating signals from extracellular growth factors and intracellular nutrient availability, such as amino acids.15,33 mTOR forms the catalytic subunit of two distinct protein complexes, known as mTORC1 and mTORC2. Upon amino acid stimulation, Rag GTPases target mTORC1 to the lysosome where its kinase is activated.15 Indeed, our interaction studies demonstrate increased interaction of RagD-S76L to components of the mTOR signaling pathway and the Ragulator complex (Figure 4).
The function of the Rag GTPases depends on the differential binding of guanosine nucleotides.15 Engineered mutations that interfere with nucleotide binding were shown to critically affect Rag signaling and mTORC1 activation.34 The human RRAGD mutations discovered in this study affect residues involved in the binding of guanosine nucleotides (Figure 2, B–D, Supplemental Figure 1). They lead to a constitutively increased activation of mTORC1, as reflected by an increased phosphorylation of its substrate S6K1 and by increased binding to components of the amino acid–sensing, mTORC1-containing complex (Figure 4). Previously, engineered mutations at the identical serine residue in RagC (p.Ser75Leu) and RagD (p.Ser76Leu) were shown to strongly interact with mTORC1, leading to constitutive mTOR pathway activation, irrespective of amino acid availability.15 Of note, when Rag subunits are highly overexpressed, some might become mislocalized and inappropriately encounter and activate mTORC1 in the absence of amino acids. How the individual mutations exactly affect GDP/GTP binding remains to be examined.
In a significant number of patients, RRAGD mutations resulted from de novo mutational events that are thought to originate either from errors in DNA replication or from inefficient repair of spontaneous DNA lesions.35 The mutagenesis rate varies in different areas of the human genome, which is attributed to genome structure, but also to chromatin state and transcription rate. The occurrence of recurrent mutational events at identical positions (i.e., RRAGD p.S76 or p.P119) suggests so-called mutational hotspots. An example are CpG dinucleotide sites facilitating C>T transitions.36 Indeed, the RRAGD c.227C>T variant is located at such a CpG site, which may explain the recurrent mutation at RagD-p.S76.
Data on the role of mTOR signaling in tubular physiology remain scarce. Previous studies mainly focused on the tubular effects of decreased mTORC1 signaling.37,38 Inactivation of mTOR signaling along the renal tubule was shown to lead either to a Fanconi-like syndrome or to a urine-concentrating defect attributed to impaired distal tubular transport processes.39 Remarkably, hypomagnesemia and hypokalemia have been observed in rats after sirolimus treatment that were attributed to GSH expression of NKCC2 (SLC12A1) in the TAL.40 Moreover, inhibition of mTOR signaling also resulted in changes in TRPM6 expression in the DCT.41 Conversely, mTORC1 activation by knockout of Tsc1 in renal tubular cells resulted in cyst formation resembling polycystic kidney disease.42,43 The role of mTORC1 in cyst formation depends on its downstream target S6K1.44 Other mTORC1 targets, including autophagy mutants and 4E-BP1/4E-BP2, do not recapitulate a polycystic kidney phenotype.45,46 Therefore, follow-up studies will be needed to identify the precise roles of RagD and mTORC1 signaling in the distal tubular handling of electrolytes.
Although the molecular pathways downstream of mTOR in the TAL and DCT remain to be determined, mTORC2 has been demonstrated to regulate the aldosterone-sensitive epithelial sodium channel in the collecting duct.47,48 mTORC2 activates serum and glucocorticoid inducible kinase 1 (SGK1), resulting in increased cell surface expression of the epithelial sodium channel. However, it should be noted that the RAG complex specifically activates mTORC1.
The finding of DCM in a subset of our patients also substantiates a critical role of Rag GTPases for cardiac function. Previously, inactivation of Rag GTPases RagA and RagB was shown to result in hypertrophic cardiomyopathy in mice.49 Moreover, a de novo mutation in RagC (p.Ser75Tyr), leading to increased mTOR activity, has been described in an individual with DCM.50 Recent studies in clinical and experimental models of DCM have implicated mTOR hyperactivation as a major cause of DCM.51,52 In line with this, inhibition of mTOR by rapamycin has been demonstrated to successfully improve cardiac function in animal models of DCM.53–55 Of note, not all identified RRAGD mutations overactivated mTORC1 signaling equally. Our experiments indicate that p.Thr97Pro results in less S6K1 activation (Figure 4), which may explain why individuals carrying this mutation do not suffer from DCM even at adult age. A targeted treatment of increased mTOR activity in patients with RRAGD mutations will represent an appealing therapeutic strategy in the future.
Taken together, the discovery of RRAGD mutations establishes a novel hereditary tubulopathy linked to dysregulation of the mTOR pathway. The diagnosis of ADKH-RRAGD should be considered in individuals presenting either with early-onset DCM and/or hypomagnesemia of renal origin. Future research will hopefully help to enlighten the exact role of mTOR for distal tubular transport processes and renal magnesium handling in particular.
Disclosures
B.B. Beck reports receiving honoraria from Alnylam and Oxthera; and serving as a scientific advisor for, or member of, the Oxalosis and Hyperoxaluria Foundation. C. Bergmann reports receiving honoraria from Alexion, Atheneum, Kyowa Kirin, Merck, Otsuka, PTC, and Sanofi; serving on the on the advisory boards for Alexion and PTC, and on the medical and scientific board of the German PKD Foundation; having other interests in/relationships with American Society of Nephrology, Deutsche Gesellschaft für Nephrologie (DGfN), European Renal Association–European Dialysis and Transplant Association (ERA-EDTA), European Society for Paediatric Nephrology, German Pediatric Nephrology Association (GPN), and International Pediatric Nephrology Association, as a member of the kidney community; receiving research funding from Limbach; having ownership interest in Medizinische Genetik Mainz; and being employed by Medizinische Genetik Mainz, Limbach Genetics. A. Braun reports receiving research funding from the Köln Fortune Grant (Faculty of Medicine, University of Cologne). S. Burtey reports receiving honoraria from AstraZeneca, Fresenius Kabi, Gambro, and Nipro. K. Dahan reports serving as president and a member of the advisory committees for the nonprofit patient organizations Association for Information and research on Renal Genetic diseases (AIRG), Familial Adenomatous Polyposis Association (FAPA), and Retina; receiving research funding from Alexion, Alnylam, IPSEN, MSD, Pfizer, Servier, and Sobi; and serving on the board of directors of the Institute for Pathology and Genetics (IPG). P. Houillier reports having consultancy agreements with Amgen, Kyowa Kirin, and Shire/Takeda; receiving research funding from Amgen and Takeda/Shire; having other interests in/relationships with the European Society of Endocrinology via a working group on calcium and bone; serving as a member of the editorial board for JASN; receiving honoraria from Kyowa Kirin and Takeda/Shire; and serving as a scientific advisor for the National Centre of Competence in Research (NCCR) Kidney.CH (Switzerland). F. Jouret reports serving as a scientific advisor for, or member of, the Belgian Society of Nephrology and the French-speaking Society of Transplantation. N.V.A.M. Knoers reports serving as chair of the ERA-EDTA Working Group on Inherited Kidney Diseases, and chair of the Molecular Diagnostics Task Force for the European Rare Kidney Disease Reference Network; receiving honoraria from ErasmusMC, The Netherlands, for Strategy Evaluation Protocol (SEP) evaluation; and serving as a scientific advisor or member of different research advisory committees without any financial compensation. M. Konrad reports having consultancy agreements with Otsuka. M. C. Liebau reports serving on editorial boards for Current Pediatric Reviews, Frontiers in Pediatrics, and Pediatric Nephrology; serving on the council for the European Society for Paediatric Nephrology, on the scientific committee of the German PKD Foundation, on a scientific advisory committee for the PKD Foundation; having consultancy agreements with Otsuka Pharma as a member of an advisory board representing the University Hospital of Cologne; receiving honoraria from Pfizer. J. Oh reports having consultancy agreements with, and receiving honoraria from, Alexion, Alynylam, Amgen, Boehringer Ingelheim, Chiesi, Horizon, Novartis, Recordati, and Sanofi; receiving research funding from Amgen, Chiesi, Novartis, and Sanofi; and serving as a scientific advisor for, or member of, Pediatric Nephrology. D. Sabatini reports serving as a scientific advisory board member of Atavistik Bio Inc., Faeth Therapeutics Inc., Montai Health (f/k/a FL65), Navitor, Toran Pharmaceuticals (f/k/a VL54), and Vescor Therapeutics. K. Shen reports receiving research funding from National Institutes of Health/National Cancer Institute (grant K22CA241362). R. Vargas-Poussou reports serving as a scientific advisor for, or member of, Advicenne. All remaining authors have nothing to disclose.
Funding
This work was supported by the Nederlandse Organisatie voor Wetenschappelijk Onderzoek (Netherlands Organization for Scientific Research) grant NWO VENI 016.186.012, Diabetes Fonds (Dutch Diabetes Research Foundation) grant 2017.81.014, Nierstichting (Dutch Kidney Foundation) grant 15OKG18 (to K.Y. Renkema), and the European Union Seventh Framework Programme EURenOmics project FP7/2007-2013, number 305608. F. Jouret is a Fellow of the Fonds National de la Recherche Scientifique in Belgium. This work was financially supported by the IMAGEN project which is co-funded by the PPP Allowance made available by Health∼Holland, Top Sector Life Sciences & Health, to stimulate public-private partnerships (IMplementation of Advancements in GENetic Kidney Disease, LSHM20009) and the Dutch Kidney Foundation (20OP+018). Additionally, we received support from ZonMW under the frame of EJPRD, the European Joint Programme on Rare Diseases (EJPRD2019-40).
Supplementary Material
Acknowledgments
The authors are grateful to the patients for their participation in this study. We thank Ms. Edith Peters, Ms. Femke Latta, Miss Valentina Carotti, Mr. Ruud Tilleman, Mr. Matthijs Snelders, Ms. Laurence Poma, and Ms. Ruth Herzog for their excellent technical support, and Dr. Tanja Seidel for thoughtful patient care.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
Supplemental Material
This article contains the following supplemental material online at https://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2021030333/-/DCSupplemental.
Supplemental Appendix 1. Complete methods.
Supplemental Appendix 2. Detailed clinical description of affected individuals.
Supplemental Appendix 3. Detailed description of the structural consequences of the mutations.
Supplemental Appendix 4. References.
Supplemental Table 1. Biochemical parameters F9.
Supplemental Figure 1. RagD mutations affect conserved residues.
Supplemental Figure 2. Tissue expression of RagD.
Supplemental Figure 3. Analysis of the RagD interactome.
References
- 1.Downie ML, Lopez Garcia SC, Kleta R, Bockenhauer D: Inherited tubulopathies of the kidney: Insights from genetics. Clin J Am Soc Nephrol 16: 620–630, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Viering DHHM, de Baaij JHF, Walsh SB, Kleta R, Bockenhauer D: Genetic causes of hypomagnesemia, a clinical overview. Pediatr Nephrol 32: 1123–1135, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.de Baaij JHF, Hoenderop JGJ, Bindels RJM: Magnesium in man: Implications for health and disease. Physiol Rev 95: 1–46, 2015 [DOI] [PubMed] [Google Scholar]
- 4.Hou J, Renigunta A, Gomes AS, Hou M, Paul DL, Waldegger S, et al. : Claudin-16 and claudin-19 interaction is required for their assembly into tight junctions and for renal reabsorption of magnesium. Proc Natl Acad Sci USA 106: 15350–15355, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gong Y, Renigunta V, Himmerkus N, Zhang J, Renigunta A, Bleich M, et al. : Claudin-14 regulates renal Ca++ transport in response to CaSR signalling via a novel microRNA pathway. Embo J 31: 1999–2012, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mittermeier L, Demirkhanyan L, Stadlbauer B, Breit A, Recordati C, Hilgendorff A, et al. : TRPM7 is the central gatekeeper of intestinal mineral absorption essential for postnatal survival. Proc Natl Acad Sci USA 116: 4706–4715, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schäffers OJM, Hoenderop JGJ, Bindels RJM, de Baaij JHF: The rise and fall of novel renal magnesium transporters. Am J Physiol Renal Physiol 314: F1027–F1033, 2018 [DOI] [PubMed] [Google Scholar]
- 8.Schlingmann KP, Weber S, Peters M, Niemann Nejsum L, Vitzthum H, Klingel K, et al. : Hypomagnesemia with secondary hypocalcemia is caused by mutations in TRPM6, a new member of the TRPM gene family. Nat Genet 31: 166–170, 2002 [DOI] [PubMed] [Google Scholar]
- 9.de Baaij JH, Blanchard MG, Lavrijsen M, Leipziger J, Bindels RJ, Hoenderop JG: P2X4 receptor regulation of transient receptor potential melastatin type 6 (TRPM6) Mg2+ channels. Pflugers Arch 466: 1941–1952, 2014 [DOI] [PubMed] [Google Scholar]
- 10.Ferioli S, Zierler S, Zaißerer J, Schredelseker J, Gudermann T, Chubanov V: TRPM6 and TRPM7 differentially contribute to the relief of heteromeric TRPM6/7 channels from inhibition by cytosolic Mg2+ and Mg·ATP. Sci Rep 7: 8806, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nair AV, Hocher B, Verkaart S, van Zeeland F, Pfab T, Slowinski T, et al. : Loss of insulin-induced activation of TRPM6 magnesium channels results in impaired glucose tolerance during pregnancy. Proc Natl Acad Sci USA 109: 11324–11329, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thebault S, Alexander RT, Tiel Groenestege WM, Hoenderop JG, Bindels RJ: EGF increases TRPM6 activity and surface expression. J Am Soc Nephrol 20: 78–85, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Altmüller J, Motameny S, Becker C, Thiele H, Chatterjee S, Wollnik B, et al. : A systematic comparison of two new releases of exome sequencing products: The aim of use determines the choice of product. Biol Chem 397: 791–801, 2016 [DOI] [PubMed] [Google Scholar]
- 14.Hart SN, Duffy P, Quest DJ, Hossain A, Meiners MA, Kocher JP: VCF-Miner: GUI-based application for mining variants and annotations stored in VCF files. Brief Bioinform 17: 346–351, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sancak Y, Peterson TR, Shaul YD, Lindquist RA, Thoreen CC, Bar-Peled L, et al. : The rag GTPases bind raptor and mediate amino acid signaling to mTORC1. Science 320: 1496–1501, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dafinger C, Rinschen MM, Borgal L, Ehrenberg C, Basten SG, Franke M, et al. : Targeted deletion of the AAA-ATPase Ruvbl1 in mice disrupts ciliary integrity and causes renal disease and hydrocephalus. Exp Mol Med 50: 1–17, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kurstjens S, Smeets B, Overmars-Bos C, Dijkman HB, den Braanker DJW, de Bel T, et al. : Renal phospholipidosis and impaired magnesium handling in high-fat-diet-fed mice. FASEB J 33: 7192–7201, 2019 [DOI] [PubMed] [Google Scholar]
- 18.Walsh PR, Tse Y, Ashton E, Iancu D, Jenkins L, Bienias M, et al. : Clinical and diagnostic features of bartter and gitelman syndromes. Clin Kidney J 11: 302–309, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Saxton RA, Sabatini DM: mTOR signaling in growth, metabolism, and disease. Cell 168: 960–976, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nicastro R, Sardu A, Panchaud N, De Virgilio C: The architecture of the rag GTPase signaling network. Biomolecules 7: 48, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vetter IR, Wittinghofer A: The guanine nucleotide-binding switch in three dimensions. Science 294: 1299–1304, 2001 [DOI] [PubMed] [Google Scholar]
- 22.de Baaij JHF, Stuiver M, Meij IC, Lainez S, Kopplin K, Venselaar H, et al. : Membrane topology and intracellular processing of cyclin M2 (CNNM2). J Biol Chem 287: 13644–13655, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee JW, Chou CL, Knepper MA: Deep sequencing in microdissected renal tubules identifies nephron segment-specific transcriptomes. J Am Soc Nephrol 26: 2669–2677, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Limbutara K, Chou CL, Knepper MA: Quantitative proteomics of all 14 renal tubule segments in rat. J Am Soc Nephrol 31: 1255–1266, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ransick A, Lindström NO, Liu J, Zhu Q, Guo JJ, Alvarado GF, et al. : Single-cell profiling reveals sex, lineage, and regional diversity in the mouse kidney. Dev Cell 51: 399–413.e7, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Konrad M, Schaller A, Seelow D, Pandey AV, Waldegger S, Lesslauer A, et al. : Mutations in the tight-junction gene claudin 19 (CLDN19) are associated with renal magnesium wasting, renal failure, and severe ocular involvement. Am J Hum Genet 79: 949–957, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Simon DB, Lu Y, Choate KA, Velazquez H, Al-Sabban E, Praga M, et al. : Paracellin-1, a renal tight junction protein required for paracellular Mg2+ resorption. Science 285: 103–106, 1999 [DOI] [PubMed] [Google Scholar]
- 28.Hanssen O, Castermans E, Bovy C, Weekers L, Erpicum P, Dubois B, et al. : Two novel mutations of the CLDN16 gene cause familial hypomagnesaemia with hypercalciuria and nephrocalcinosis. Clin Kidney J 7: 282–285, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jeck N, Konrad M, Peters M, Weber S, Bonzel KE, Seyberth HW: Mutations in the chloride channel gene, CLCNKB, leading to a mixed Bartter-Gitelman phenotype. Pediatr Res 48: 754–758, 2000 [DOI] [PubMed] [Google Scholar]
- 30.Zelikovic I, Szargel R, Hawash A, Labay V, Hatib I, Cohen N, et al. : A novel mutation in the chloride channel gene, CLCNKB, as a cause of Gitelman and Bartter syndromes. Kidney Int 63: 24–32, 2003 [DOI] [PubMed] [Google Scholar]
- 31.Laine J, Jalanko H, Alakulppi N, Holmberg C: A new tubular disorder with hypokalaemic metabolic alkalosis, severe hypermagnesuric hypomagnesaemia, hypercalciuria and cardiomyopathy. Nephrol Dial Transplant 20: 1241–1245, 2005 [DOI] [PubMed] [Google Scholar]
- 32.Runeberg L, Collan Y, Jokinen EJ, Lähdevirta J, Aro A: Hypomagnesemia due to renal disease of unknown etiology. Am J Med 59: 873–881, 1975 [DOI] [PubMed] [Google Scholar]
- 33.Shimobayashi M, Hall MN: Making new contacts: The mTOR network in metabolism and signalling crosstalk. Nat Rev Mol Cell Biol 15: 155–162, 2014 [DOI] [PubMed] [Google Scholar]
- 34.Shen K, Choe A, Sabatini DM: Intersubunit crosstalk in the rag GTPase heterodimer enables mTORC1 to respond rapidly to amino acid availability. Mol Cell 68: 821, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Maki H: Origins of spontaneous mutations: Specificity and directionality of base-substitution, frameshift, and sequence-substitution mutageneses. Annu Rev Genet 36: 279–303, 2002 [DOI] [PubMed] [Google Scholar]
- 36.Acuna-Hidalgo R, Veltman JA, Hoischen A: New insights into the generation and role of de novo mutations in health and disease. Genome Biol 17: 241, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Grahammer F, Haenisch N, Steinhardt F, Sandner L, Roerden M, Arnold F, et al. : mTORC1 maintains renal tubular homeostasis and is essential in response to ischemic stress. Proc Natl Acad Sci USA 111: E2817–E2826, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Grahammer F, Wanner N, Huber TB: mTOR controls kidney epithelia in health and disease. Nephrol Dial Transplant 29: i9–i18, 2014 [DOI] [PubMed] [Google Scholar]
- 39.Grahammer F, Ramakrishnan SK, Rinschen MM, Larionov AA, Syed M, Khatib H, et al. : mTOR regulates endocytosis and nutrient transport in proximal tubular cells. J Am Soc Nephrol 28: 230–241, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.da Silva CA, de Bragança AC, Shimizu MH, Sanches TR, Fortes MA, Giorgi RR, et al. : Rosiglitazone prevents sirolimus-induced hypomagnesemia, hypokalemia, and downregulation of NKCC2 protein expression. Am J Physiol Renal Physiol 297: F916–F922, 2009 [DOI] [PubMed] [Google Scholar]
- 41.Ikari A, Sanada A, Sawada H, Okude C, Tonegawa C, Sugatani J: Decrease in transient receptor potential melastatin 6 mRNA stability caused by rapamycin in renal tubular epithelial cells. Biochim Biophys Acta 1808: 1502–1508, 2011 [DOI] [PubMed] [Google Scholar]
- 42.Centini R, Tsang M, Iwata T, Park H, Delrow J, Margineantu D, et al. : Loss of Fnip1 alters kidney developmental transcriptional program and synergizes with TSC1 loss to promote mTORC1 activation and renal cyst formation. PLoS One 13: e0197973, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhou J, Brugarolas J, Parada LF: Loss of Tsc1, but not Pten, in renal tubular cells causes polycystic kidney disease by activating mTORC1. Hum Mol Genet 18: 4428–4441, 2009 [DOI] [PubMed] [Google Scholar]
- 44.Bonucci M, Kuperwasser N, Barbe S, Koka V, de Villeneuve D, Zhang C, et al. : mTOR and S6K1 drive polycystic kidney by the control of Afadin-dependent oriented cell division. Nat Commun 11: 3200, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Le Bacquer O, Combe K, Patrac V, Ingram B, Combaret L, Dardevet D, et al. : 4E-BP1 and 4E-BP2 double knockout mice are protected from aging-associated sarcopenia. J Cachexia Sarcopenia Muscle 10: 696–709, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Orhon I, Dupont N, Zaidan M, Boitez V, Burtin M, Schmitt A, et al. : Primary-cilium-dependent autophagy controls epithelial cell volume in response to fluid flow. Nat Cell Biol 18: 657–667, 2016 [DOI] [PubMed] [Google Scholar]
- 47.Gleason CE, Frindt G, Cheng CJ, Ng M, Kidwai A, Rashmi P, et al. : mTORC2 regulates renal tubule sodium uptake by promoting ENaC activity. J Clin Invest 125: 117–128, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lu M, Wang J, Jones KT, Ives HE, Feldman ME, Yao LJ, et al. : mTOR complex-2 activates ENaC by phosphorylating SGK1. J Am Soc Nephrol 21: 811–818, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kim YC, Park HW, Sciarretta S, Mo JS, Jewell JL, Russell RC, et al. : Rag GTPases are cardioprotective by regulating lysosomal function. Nat Commun 5: 4241, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Long PA, Zimmermann MT, Kim M, Evans JM, Xu X, Olson TM: De novo RRAGC mutation activates mTORC1 signaling in syndromic fetal dilated cardiomyopathy. Hum Genet 135: 909–917, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Caragnano A, Aleksova A, Bulfoni M, Cervellin C, Rolle IG, Veneziano C, et al. : Autophagy and inflammasome activation in dilated cardiomyopathy. J Clin Med 8: 1519, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jin B, Shi H, Zhu J, Wu B, Geshang Q: Up-regulating autophagy by targeting the mTOR-4EBP1 pathway: A possible mechanism for improving cardiac function in mice with experimental dilated cardiomyopathy. BMC Cardiovasc Disord 20: 56, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Choi JC, Worman HJ: Reactivation of autophagy ameliorates LMNA cardiomyopathy. Autophagy 9: 110–111, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Marin TM, Keith K, Davies B, Conner DA, Guha P, Kalaitzidis D, et al. : Rapamycin reverses hypertrophic cardiomyopathy in a mouse model of LEOPARD syndrome-associated PTPN11 mutation. J Clin Invest 121: 1026–1043, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ramos FJ, Chen SC, Garelick MG, Dai DF, Liao CY, Schreiber KH, et al. : Rapamycin reverses elevated mTORC1 signaling in lamin a/C-deficient mice, rescues cardiac and skeletal muscle function, and extends survival. Sci Transl Med 4: 144ra103, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hoyer PF: Nephrocalcinose. In: Ultraschalldiagnostik in Pädiatrie Und Kinderchirurgie: Lehrbuch und Atlas, edited by Deeg KH, Hofmann V, and Hoyer PF, Stuttgart, Germany, Thieme, 1996, pp 372–374 [Google Scholar]
- 57.Pettersen MD, Du W, Skeens ME, Humes RA: Regression equations for calculation of z scores of cardiac structures in a large cohort of healthy infants, children, and adolescents: An echocardiographic study. J Am Soc Echocardiogr 21: 922–934, 2008 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.