Significance Statement
Obesity’s association with diabetes, hypertension, and possibly kidney disease has prompted concerns that these effects might be magnified after kidney donation in the donor’s remaining kidney. Half of US transplant centers exclude donation from kidney donor candidates who are obese. This comparison of mortality, kidney failure, proteinuria, diabetes, and hypertension in 6822 nonobese donors and 1761 obese donors showed that obesity in kidney donors, as in the general population, is associated with increased risk of developing diabetes, hypertension, and proteinuria. Mortality was similar between obese and nonobese donors. Absolute risk of ESKD was 0.5% in nonobese donors, 0.7% in obese donors, and 0.9% in very obese donors (body mass index <30 kg/m2, ≥30 kg/m2, or >35 kg/m2, respectively). Judicious acceptance of obese but otherwise healthy donor candidates should be considered.
Keywords: obesity, kidney donation, living donation, glomerular filtration rate, hypertension, outcomes
Abstract
Background
Obesity is associated with the two archetypal kidney disease risk factors: hypertension and diabetes. Concerns that the effects of diabetes and hypertension in obese kidney donors might be magnified in their remaining kidney have led to the exclusion of many obese candidates from kidney donation.
Methods
We compared mortality, diabetes, hypertension, proteinuria, reduced eGFR and its trajectory, and the development of kidney failure in 8583 kidney donors, according to body mass index (BMI). The study included 6822 individuals with a BMI of <30 kg/m2, 1338 with a BMI of 30–34.9 kg/m2, and 423 with a BMI of ≥35 kg/m2. We used Cox regression models, adjusting for baseline covariates only, and models adjusting for postdonation diabetes, hypertension, and kidney failure as time-varying covariates.
Results
Obese donors were more likely than nonobese donors to develop diabetes, hypertension, and proteinuria. The increase in eGFR in obese versus nonobese donors was significantly higher in the first 10 years (3.5 ml/min per 1.73m2 per year versus 2.4 ml/min per 1.73m2 per year; P<0.001), but comparable thereafter. At a mean±SD follow-up of 19.3±10.3 years after donation, 31 (0.5%) nonobese and 12 (0.7%) obese donors developed ESKD. Of the 12 patients with ESKD in obese donors, 10 occurred in 1445 White donors who were related to the recipient (0.9%). Risk of death in obese donors was not significantly increased compared with nonobese donors.
Conclusions
Obesity in kidney donors, as in nondonors, is associated with increased risk of developing diabetes and hypertension. The absolute risk of ESKD is small and the risk of death is comparable to that of nonobese donors.
Obesity has been identified as a risk factor for mortality, diabetes mellitus (DM), and is possibly a risk factor for kidney disease.1–4 Concerns about the association of obesity with adverse health outcomes have resulted in a restricted access of overweight kidney donor candidates to donation. In fact, a survey of US transplant centers has indicated that many do not consider candidates with body mass index (BMI) >30 kg/m2.5 Although the latter survey was carried out in 2007, there has been no major change in mean BMI in US donors between 2001 and 2016 but the proportion of mildly obese (BMI 30–34.9 kg/m2) donors has increased from 17% in 2001 to 20.6% in 2016.6 More contemporary evidence also revealed that 30% of European transplant centers do not accept obese donors.7 The Kidney Disease Improving Global Outcomes Clinical Practice Guidelines on the Evaluation and Care of Living Kidney Donors state that there is limited evidence from the donor population to make recommendations for donor acceptance on the basis of BMI alone among obese donor candidates.8 Previous important studies have reported increased mortality and ESKD in obese donors.9,10 ESKD after donation is rare, so studying more common events such as eGFR change and proteinuria development, which are not captured by registry data, would be of added value. In this study, we report on mortality, eGFR trajectory, ESKD, and other health outcomes in 8583 kidney donors who donated between 1963 and 2007 and were contacted in 2010–2012. Studying the effect of being overweight or obese in kidney donors offers two unique opportunities. First, it provides a population that is typically healthy and therefore well suited to study the association between obesity and kidney outcomes with minimal confounding. Second, such studies will possibly enhance the quality of informed consent process for obese donor candidates.
Materials and Methods
Study Population
We used the publicly available dataset from the Renal and Lung Living Donor Evaluation (RELIVE) Study, which was a National Institute of Allergy and Infectious Diseases–sponsored study. RELIVE evaluated outcomes of 8922 kidney donors from the University of Minnesota, the Mayo Clinic-Rochester, and the University of Alabama, Birmingham. Donation took place between 1963 and 2007. Donors’ medical records were abstracted for baseline demographic information, anthropometric measurements, prior or current diagnosis or treatment for hypertension and hyperlipidemia, laboratory data, family history of hypertension, diabetes, kidney disease, and cardiovascular disease (CVD), as previously described.11 Race/ethnicity were categorized as non-Hispanic White, non-Hispanic Black, Hispanic, non-Hispanic Asian, other, and unknown. BP readings were collected at multiple time points during the donor evaluation, and the average of the three lowest readings was used as baseline. Donors were contacted by mail in 2010–2012, requesting participation in the RELIVE study. If no response was received, a follow-up letter and at least two phone calls were made. In addition, a fee-based internet service was used to locate donors and update their addresses and phone numbers. Donors were asked if they developed diabetes, hypertension, kidney disease, CVD, cancer, or other conditions and were asked to respond to quality-of-life surveys. Participating centers contributed data elements collected over the years for more complete ascertainment of postdonation events. Collectively, postdonation data came from donor surveys, participating centers’ own records, review of medical records provided by the donors themselves, and from the recipients. This study was exempt from Institutional Review Board approval because it used deidentified data, available at www.import.org/shared/study/SDY289.
Outcome
The exposure of interest was BMI at donation. Donors were stratified by baseline BMI: nonobese <30 kg/m2, 30–34.9 kg/m2 (mild obesity [World Health Organization, WHO, Class I]) and ≥35 kg/m2 (severe obesity [WHO Class II and III]). Postdonation DM was defined as fasting plasma glucose ≥126 mg/dl, requirement for insulin or oral hypoglycemic agents, evidence of retinopathy or nephropathy, or self-report. Postdonation hypertension was defined as self-reported use of antihypertensive medications or BP ≥140/90 mmHg. CVD was defined as a diagnosis of myocardial infarction, heart failure, stroke, or the need for coronary or peripheral arterial interventions. Hyperlipidemia was defined as having one or more of the followings: self-report, receiving treatment, total cholesterol >200 mg/dl, LDL >130 mg/dl, triglycerides >150 mg/d, or HDL <40 mg/dl. Proteinuria was defined by the presence of one or more of the following: urine dipstick protein ≥2+, urine protein/osmolality ratio >0.42, urine random protein >15 mg/dl, or 24-hour protein >300 mg/day. The Chronic Kidney Disease Epidemiology Collaboration equation was used to estimate the GFR.13 ESKD was defined by the need for dialysis, or being listed for, or receiving a kidney transplant. Ascertainment of ESKD was from centers’ records, donors themselves or their recipients.
Statistical Analysis
Baseline characteristics are reported as frequencies and proportions for categorical variables and median and interquartile range (IQR) for continuous ones. Differences between donor BMI groups are compared using Pearson’s chi-squared or Fisher's exact tests for categorical variables and the Kruskal–Wallis test for continuous variables. Multiple imputation by chained equations was used to impute missing data for blood pressure at evaluation (0.3% missing), fasting plasma glucose (5.4% missing), serum creatinine (0.4% missing), relationship to recipient (0.31% missing), smoking (2.3% missing), and hyperlipidemia diagnosis (0.7% missing).
Cox regression modeling was conducted to estimate the adjusted hazard ratio (aHR) for CVD, death, death-censored diabetes, hypertension, proteinuria, eGFR <60 ml/min per 1.73m2, eGFR <45 ml/min per 1.73m2, eGFR <30 ml/min per 1.73m2, ESKD, and a composite of eGFR <30 ml/min per 1.73m2 or ESKD. The latter was chosen because ESKD is rare after donation and no formal linkage to the Organ Procurement and Transplantation Network can be made from the public dataset. The selection of variables for the Cox proportional hazard models were conducted using the least absolute shrinkage and selection operator (Lasso method) with the crossvalidation selection option and by the clinical importance of the identified variable.14,15 The models were adjusted for age, sex, ethnicity, baseline creatinine, BMI, relationship to recipient, smoking, systolic and diastolic blood pressure, family history of kidney disease, hyperlipidemia, and fasting plasma glucose. The models for ESKD were adjusted for age, ethnicity, BMI, relationship to the recipient, and postdonation diabetes and hypertension as time-varying covariates. The model for eGFR <30 ml/min per 1.73m2 or ESKD was adjusted for BMI, relationship to the recipient and postdonation diabetes and hypertension as time-varying covariates. Mortality outcome was adjusted for postdonation kidney failure and outcomes other than death were censored for death. Additional analyses with BMI as a continuous variable were also performed. Cumulative incidence for outcomes other than death was estimated using the competing risk method described by Fine and Gray.16
The trend of eGFR over time is depicted by the cubic spline plots. Difference in eGFR slope between obese and nonobese donors was compared using a generalized linear mixed model. The coefficient (slope) and 95% confidence interval (95% CI), which represents mean change over time, was reported for each group. The generalized linear mixed model used eGFR as the dependent variable and BMI category as the independent variable and used a random intercept plus random slope model and unstructured covariance option. The cubic splines were also used to depict the distribution of adjusted hazard ratio of evaluated study outcomes across BMI range. All analyses were performed on Stata version 16.1 (StataCorp LLC, College Station, TX, USA). A P value of <0.05 was considered statistically significant.
Results
General Characteristics of RELIVE Study Donors
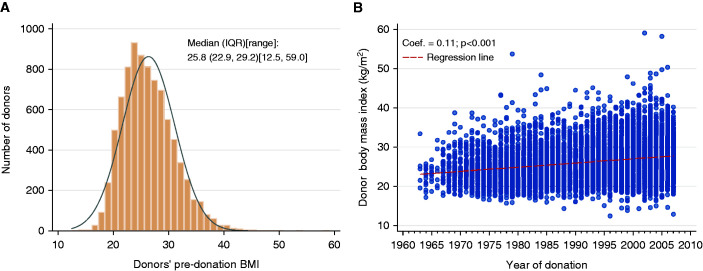
Baseline information on demographic and laboratory variables were available on 91.2%–100% of the cohort, but baseline total cholesterol and triglycerides were available in only 50% of the cohort (Supplemental Table 1). Of the 8922 kidney donors, 8583 (96%) had available baseline BMI and known vital status (Figure 1). Median age of the entire cohort was 39 years, 43.8% were male, 85.1% were non-Hispanic White, and 9.1% were non-Hispanic Black (Table 1). The majority (80.2%) donated to a family member, the median BMI was 25.8kg/m2, and median eGFR was 88 ml/min per 1.73 m2.
Figure 1.
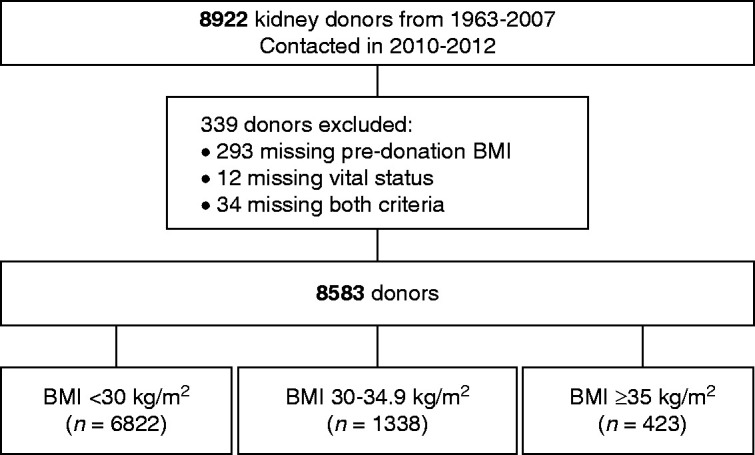
Study participants.
Table 1.
Baseline characteristics
| Characteristics | All Donors | BMI <30 kg/m2 | BMI 30–34.9 kg/m2 | BMI ≥35 kg/m2 | P Value |
|---|---|---|---|---|---|
| (n=8583) | (n=6822) | (n=1338) | (n=423) | ||
| Age (years) | 39 (31, 48) | 39 (30, 48) | 41 (33, 49) | 38 (32, 46) | <0.001 |
| Male | 3756 (43.8) | 2988 (43.8) | 626 (46.8) | 142 (33.6) | <0.001 |
| Race/ethnicity | <0.001 | ||||
| Non-Hispanic White | 7300 (85.1) | 5855 (85.8) | 1117 (83.5) | 328 (77.5) | |
| Non-Hispanic Black | 784 (9.1) | 564 (8.3) | 153 (11.4) | 67 (15.8) | |
| Hispanic | 162 (1.9) | 119 (1.7) | 33 (2.5) | 10 (2.4) | |
| Non-Hispanic Asian | 78 (0.9) | 72 (1.1) | 4 (0.3) | 2 (0.5) | |
| Other | 114 (1.3) | 87 (1.3) | 19 (1.4) | 8 (1.9) | |
| Unknown | 145 (1.7) | 125 (1.8) | 12 (0.9) | 8 (1.9) | |
| Related to recipient | 6868 (80.2) | 5511 (81.0) | 1018 (76.4) | 339 (80.3) | <0.001 |
| 1st degree relative with hypertension | 3230 (41.2) | 2491 (40.0) | 558 (45.3) | 181 (46.8) | <0.001 |
| 1st degree relative with diabetes | 3132 (39.1) | 2478 (39.0) | 465 (37.2) | 189 (47.8) | <0.001 |
| 1st degree relative with kidney disease | 5879 (71.5) | 4749 (72.6) | 848 (66.3) | 282 (69.8) | <0.001 |
| 1st degree relative with heart disease | 2318 (29.6) | 1783 (28.7) | 396 (32.2) | 139 (36.1) | <0.001 |
| BMI (kg/m2) | 25.8 (22.9, 29.2) | 24.6 (22.3, 27.0) | 31.8 (30.8, 33.0) | 37.0 (35.8, 39.0) | <0.001 |
| Systolic BP (mmHg) | 121 (113, 130) | 120 (112, 129) | 125 (118, 133) | 127 (120, 135) | <0.001 |
| Diastolic BP (mmHg) | 74 (68, 80) | 73 (68, 79) | 75 (70, 81) | 76 (70, 81) | <0.001 |
| Smoking | 4063 (48.5) | 3307 (50) | 591 (45.3) | 165 (39.6) | <0.001 |
| Total cholesterol (mg/dl) | 193 (169, 218) | 191 (167, 216) | 200 (178, 225) | 196 (173, 221) | <0.001 |
| LDL (mg/dl) | 115 (94, 137) | 113 (92, 136) | 121 (100, 142) | 116 (97, 140) | <0.001 |
| HDL (mg/dl) | 51 (42, 62) | 52 (43, 64) | 46 (39, 56) | 45 (38, 54) | <0.001 |
| Triglyceride (mg/dl) | 104 (75, 148) | 97 (71, 138) | 128 (90, 191) | 141 (106, 204) | <0.001 |
| Fasting glucose (mg/dl) | 92 (85, 99) | 91 (85, 99) | 95 (89, 102) | 95 (88, 103) | <0.001 |
| Serum creatinine (mg/dl) | 0.9 (0.8, 1.1) | 0.9 (0.8, 1.1) | 1 (0.8, 1.1) | 0.9 (0.8, 1.1) | <0.001 |
| eGFR (ml/min per 1.73m2) | 88 (76, 101) | 89 (77, 102) | 86 (75, 99) | 88 (74, 103) | <0.001 |
| Donation to end of follow-up (year) | 14 (8, 25) | 15 (9, 27) | 11 (7, 18) | 11 (8, 17) | <0.001 |
Values are in frequency and % for categorical variables and median (IQR) for continuous variables.
There were 6822 (73%) donors with BMI <30 kg/m2, median BMI 24.6 kg/m2, and 1761 (27%) with BMI ≥30 kg/m2 (median BMI 32.5 kg/m2). Of the 1761 obese donors, 76% had a BMI 30–34.9 and 24% had a BMI ≥35 kg/m2. There was minimal increase in mean BMI over the study period, Figure 2. Obese donors were a year older, less likely to be White or related to the recipient, had higher systolic and diastolic BP, fasting plasma glucose, total cholesterol, LDL, and triglycerides (Table 1). Comparison between the 1338 mildly obese donors (BMI 30–34.9 kg/m2, WHO Class I) with the 423 donors who are very obese (BMI >35 kg/m2, WHO Class II and III) revealed that very obese donors were on average 3 years younger, less likely to be men, more likely to have a first-degree relative with diabetes, less likely to be smokers, and had a higher baseline triglyceride level (141 versus 128 mg/dl).
Figure 2.
Baseline BMI and trend over study period. (A) BMI at donation. (B) BMI by year of donation. Distribution of the predonation BMI were reported by the median and IQR. Distribution of the BMI by year of donation was presented by a scatter plot with regression line. The regression coefficient and 95% CI were reported.
Completeness of outcome capturing was variable but available for 90%–100% of donors (Supplemental Table 2). In 2010–2012, 418 (4.8%) donors were deceased, 1085 (12.7%) had CVD, 2298 (29.8%) had hypertension, 566 (6.5%) had DM, and 43 (0.5%) developed ESKD (Table 2). Postdonation weights were available in 1085 out of 8583 donors and 51.6% gained weight after donation. The numbers of donors who gained weight in the three BMI categories were 459 out of 868 (52.9%), 77 out of 167 (46.1%), and 24 out of 50 (48%), respectively, P=0.24. The median weight gain after a median of 6.2 years (IQR, 5.3–7.3) years from donation in three groups was 2.4 kg, 2.8 kg, and 5.5 kg, respectively.
Table 2.
Proportions of donors with study outcomes at last follow-up
| Outcome (Events/donors With Data) | BMI Category | ||
|---|---|---|---|
| <30 kg/m2 | 30–34.9 kg/m2 | ≥35 kg/m2 | |
| n (%) | n (%) | n (%) | |
| Death (n=418/n=8583) | 363 (5.3) | 43 (3.2) | 12 (2.8) |
| CVD (n=1085/n=8569) | 865 (12.7) | 162 (12.2) | 58 (13.7) |
| Diabetes (n=566/n=7879) | 424 (6.8) | 98 (8.0) | 44 (11.1) |
| Hypertension (n=2298/n=7696) | 1773 (28.5) | 396 (35.4) | 129 (37.4) |
| Proteinuria (n=1073/n=7687) | 790 (13.0) | 201 (16.6) | 82 (21.4) |
| eGFR <60 (n=4655/n=8374) | 3598 (54.0) | 824 (63.1) | 233 (56.8) |
| eGFR <45 (n=1011/n=8374) | 750 (11.3) | 207 (15.9) | 54 (13.2) |
| eGFR <30 (n=59/n=8374) | 42 (0.6) | 13 (1.0) | 4 (1.0) |
| ESKD (n=43/n=8016) | 31 (0.5) | 8 (0.6) | 4 (1.0) |
| eGFR<30 or ESKD (n=85/n=8552) | 60 (0.9) | 18 (1.4) | 7 (1.7) |
n, number of outcome events; N, total number of donors with ascertained outcome status. This table presents all donors who had outcomes at study end in 2010–2012 (prevalent patients, i.e., also includes donors with a pre-existing condition).
Postdonation Diabetes, Hypertension, and CVD
After a median follow-up of 14.0 (IQR, 8–25) years, obese donors were more likely to develop diabetes; aHR, 2.37; (95% CI, 1.79 to 3.14); P<0.001. Fasting plasma glucose, systolic BP, and development of postdonation hypertension were also associated with increased risk of DM (Table 3). Older age, male, higher systolic and diastolic blood pressure, a higher fasting plasma glucose, non- Hispanic White ethnicity, and hyperlipidemia were associated with a higher risk of hypertension (Table 3). Donors with BMI ≥30–34.9 kg/m2 were 50% more likely to develop hypertension; aHR 1.53 (95% CI, 1.27 to 1.85), P<0.001 as were those with BMI ≥35 kg/m2, aHR, 1.50 (95% CI, 1.33 to 1.70), P<0.001 (Table 3). Results from models with and without adjustment for postdonation events yielded similar results. CVD was reported in 12.6% of donors (12.7% in nonobese donors and 12.5% in those with BMI ≥30 kg/m2) 11.9±11 years after donation. Older age, male, higher systolic and diastolic BP, hyperlipidemia, smoking, and development of postdonation diabetes were associated with increased risk of CVD. Donors with BMI ≥35 kg/m2 were also more likely to develop CVD; aHR, 1.80 (95% CI, 1.24 to 2.61), P=0.002.
Table 3.
Multivariable risk of diabetes and hypertension: Cox regression
| Variable | Model 1 | Model 2 | ||
|---|---|---|---|---|
| aHR (95% CI) | P Value | aHR (95% CI) | P Value | |
| Diabetes | n=6974 | n=7011 | ||
| Age (years) | 1.01 (1.00, 1.02) | 0.14 | 1.01 (1.00, 1.02) | 0.08 |
| Male | 1.06 (0.77, 1.47) | 0.72 | 1.07 (0.78, 1.47) | 0.68 |
| Not non-Hispanic White | 1.19 (0.78, 1.83) | 0.42 | 1.17 (0.77, 1.78) | 0.46 |
| Related to the recipient | 0.78 (0.49, 1.24) | 0.29 | 0.81 (0.52, 1.29) | 0.38 |
| BMI (kg/m2) | ||||
| <30 | (reference) | |||
| 30–34.9 | 2.01 (1.45, 2.78) | <0.001 | 2.07 (1.49, 2.86) | <0.001 |
| ≥35 | 3.51 (2.28, 5.42) | <0.001 | 3.52 (2.28, 5.43) | <0.001 |
| Systolic BP (mmHg) | 1.01 (1.00, 1.03) | 0.07 | 1.02 (1.00, 1.03) | 0.02 |
| Diastolic BP (mmHg) | 0.99 (0.97, 1.01) | 0.38 | 0.99 (0.97, 1.02) | 0.53 |
| Fasting glucose (mg/dl) | 1.03 (1.02, 1.05) | <0.001 | 1.03 (1.02, 1.05) | <0.001 |
| Serum creatinine (mg/dl) | 0.82 (0.35, 1.93) | 0.65 | 0.77 (0.33, 1.76) | 0.53 |
| Smoking | 1.20 (0.93, 1.53) | 0.16 | 1.23 (0.96, 1.57) | 0.10 |
| Hyperlipidemia | 1.16 (0.88, 1.54) | 0.30 | 1.16 (0.88, 1.54) | 0.30 |
| Postdonation hypertension | 1.03 (1.01, 1.05) | 0.01 | – | – |
| Postdonation ESKD | 1.01 (0.97, 1.04) | 0.75 | – | – |
| Hypertension | n=6328 | n=6924 | ||
| Age (years) | 1.02 (1.01, 1.02) | <0.001 | 1.02 (1.01, 1.02) | <0.001 |
| Male | 1.17 (1.04, 1.31) | 0.01 | 1.18 (1.06, 1.33) | 0.004 |
| Not non-Hispanic White | 1.69 (1.47, 1.95) | <0.001 | 1.67 (1.46, 1.92) | <0.001 |
| Related to the recipient | 0.91 (0.80, 1.04) | 0.16 | 0.91 (0.80, 1.04) | 0.16 |
| BMI (kg/m2) | ||||
| <30 | (reference) | (reference) | ||
| 30–34.9 | 1.50 (1.32, 1.70) | <0.001 | 1.50 (1.33, 1.70) | <0.001 |
| ≥35 | 1.55 (1.29, 1.87) | <0.001 | 1.53 (1.27, 1.85) | <0.001 |
| Systolic BP (mmHg) | 1.05 (1.04, 1.05) | <0.001 | 1.05 (1.04, 1.05) | <0.001 |
| Diastolic BP (mmHg) | 0.99 (0.98, 1.00) | 0.02 | 0.99 (0.98, 1.00) | 0.02 |
| Fasting glucose (mg/dl) | 1.01 (1.01, 1.01) | <0.001 | 1.01 (1.01, 1.02) | <0.001 |
| Serum creatinine (mg/dl) | 0.93 (0.69, 1.27) | 0.66 | 0.97 (0.72, 1.30) | 0.82 |
| Smoking | 0.99 (0.90, 1.09) | 0.79 | 0.99 (0.91, 1.09) | 0.89 |
| Hyperlipidemia | 0.90 (0.81, 0.99) | 0.04 | 0.92 (0.83, 1.02) | 0.12 |
| Post-donation diabetes | 1.01 (1.00, 1.02) | 0.09 | – | – |
| Post-donation ESKD | 1.03 (1.00, 1.06) | 0.08 | – | – |
Model 1: Adjusted for postdonation diabetes, hypertension, and ESKD as time-dependent covariates; Model 2: Not adjusted for postdonation diabetes and hypertension.
Proteinuria and eGFR Outcomes
Proteinuria was reported in 13% of nonobese donors and 17.8% of obese donors 8±10.2 years after donation. Older age, hyperlipidemia, higher systolic BP, baseline creatinine, and development of postdonation diabetes and ESKD were associated with proteinuria. Being related to the recipient and diastolic blood pressure were associated with decreased risk of proteinuria. BMI 30–34.9 kg/m2 and ≥35 kg/m2 were associated with an increased risk of proteinuria; aHR, 1.56 (95% CI, 1.32 to 1.85), P<0.001 and aHR, 2.04 (95% CI, 1.63 to 2.56), P<0.001, respectively (Table 4). In addition, donors with BMI ≥35 kg/m2 were 31% more likely than donors with BMI 30–34 kg/m2 to develop proteinuria. BMI 30–34.9 kg/m2 was associated with a higher risk of eGFR <60 ml/min per 1.73m2, eGFR <45 ml/min per 1.73m2, and a higher risk of eGFR <30 ml/min per 1.73m2, but BMI >35kg/m2 was not associated with a further increase in these risks (Table 5).
Table 4.
Multivariable risk of proteinuria: Cox regression
| Variable | Model 1 (n=7029) | Model 2 (n=7068) | ||
|---|---|---|---|---|
| aHR (95% CI) | P Value | aHR (95% CI) | P Value | |
| Age (years) | 1.01 (1.01, 1.02) | <0.001 | 1.01 (1.01, 1.02) | <0.001 |
| Male | 0.87 (0.75, 1.02) | 0.09 | 0.88 (0.76, 1.03) | 0.11 |
| Not non-Hispanic White | 1.18 (0.95, 1.45) | 0.13 | 1.18 (0.95, 1.45) | 0.13 |
| Related to the recipient | 0.53 (0.45, 0.62) | <0.001 | 0.54 (0.46, 0.63) | <0.001 |
| BMI (kg/m2) | ||||
| <30 | (reference) | (reference) | ||
| 30–34.9 | 1.56 (1.32, 1.85) | <0.001 | 1.62 (1.37, 1.90) | <0.001 |
| ≥35 | 2.04 (1.63, 2.56) | <0.001 | 2.06 (1.65, 2.58) | <0.001 |
| Systolic BP (mmHg) | 1.01 (1.00, 1.02) | 0.002 | 1.01 (1.01, 1.02) | 0.001 |
| Diastolic BP (mmHg) | 0.99 (0.97, 1.00) | 0.01 | 0.98 (0.97, 1.00) | 0.004 |
| Fasting glucose (mg/dl) | 1.00 (1.00, 1.01) | 0.21 | 1.00 (1.00, 1.01) | 0.07 |
| Serum creatinine (mg/dl) | 3.59 (2.52, 5.13) | <0.001 | 3.50 (2.46, 4.99) | <0.001 |
| Smoking | 0.96 (0.85, 1.09) | 0.55 | 0.98 (0.86, 1.11) | 0.74 |
| Hyperlipidemia | 1.61 (1.41, 1.85) | <0.001 | 1.64 (1.43, 1.88) | <0.001 |
| Postdonation diabetes | 1.02 (1.01, 1.03) | 0.001 | – | – |
| Postdonation hypertension | 1.01 (0.99, 1.02) | 0.35 | – | – |
Model 1: Adjusted for postdonation diabetes and hypertension as time-dependent covariates; Model 2: Not adjusted for postdonation diabetes and hypertension.
Table 5.
Multivariable risk of eGFR <60, eGFR <45, and eGFR <30 ml/min per 1.73m2: Cox regression
| Variable | Model 1 (n=7089) | Model 2 (n=7684) | ||
|---|---|---|---|---|
| aHR (95% CI) | P Value | aHR (95% CI) | P Value | |
| eGFR<60 ml/min per 1.73m2 | ||||
| Related to the recipient | 0.68 (0.64, 0.73) | <0.001 | 0.70 (0.65, 0.74) | <0.001 |
| BMI (kg/m2) | ||||
| <30 | (reference) | (reference) | ||
| 30–34.9 | 1.31 (1.21, 1.41) | <0.001 | 1.29 (1.20, 1.39) | <0.001 |
| ≥35 | 1.13 (0.99, 1.29) | 0.07 | 1.12 (0.98, 1.27) | 0.09 |
| Systolic BP (mmHg) | 1.01 (1.01, 1.01) | <0.001 | 1.01 (1.01, 1.01) | <0.001 |
| Fasting glucose (mg/dl) | 1.01 (1.01, 1.01) | <0.001 | 1.01 (1.01, 1.01) | <0.001 |
| Postdonation diabetes | 0.99 (0.98, 1.00) | 0.13 | – | – |
| Postdonation hypertension | 0.99 (0.99, 1.00) | 0.09 | – | – |
| eGFR<45 ml/min per 1.73m2 | ||||
| Related to the recipient | 0.67 (0.56, 0.79) | <0.001 | 0.70 (0.59, 0.82) | <0.001 |
| BMI (kg/m2) | ||||
| <30 | (reference) | (reference) | ||
| 30–34.9 | 1.56 (1.32, 1.84) | <0.001 | 1.54 (1.31, 1.81) | <0.001 |
| ≥35 | 1.28 (0.97, 1.70) | 0.09 | 1.22 (0.93, 1.61) | 0.16 |
| Systolic BP (mmHg) | 1.02 (1.01, 1.03) | <0.001 | 1.02 (1.02, 1.03) | <0.001 |
| Fasting glucose (mg/dl) | 1.01 (1.01, 1.02) | <0.001 | 1.01 (1.01, 1.02) | <0.001 |
| Postdonation diabetes | 1.00 (0.99, 1.01) | 0.97 | – | – |
| Postdonation hypertension | 1.01 (1.00, 1.03) | 0.13 | – | – |
| eGFR<30 ml/min per 1.73m2 | ||||
| Related to the recipient | 3.12 (0.41, 23.53) | 0.27 | 3.34 (0.45, 24.87) | 0.24 |
| BMI (kg/m2) | ||||
| <30 | (reference) | (reference) | ||
| 30–34.9 | 2.61 (1.28, 5.30) | 0.01 | 2.51 (1.24, 5.08) | 0.01 |
| ≥35 | 2.48 (0.87, 7.07) | 0.09 | 2.24 (0.79, 6.36) | 0.13 |
| Systolic BP (mmHg) | 1.03 (1.00, 1.05) | 0.04 | 0.00 (0.00, 0.00) | |
| Fasting glucose (mg/dl) | 1.02 (1.00, 1.04) | 0.03 | 1.04 (1.01, 1.06) | 0.002 |
| Postdonation diabetes | 1.02 (0.99, 1.05) | 0.24 | 1.02 (1.00, 1.04) | 0.01 |
| Postdonation hypertension | 1.03 (0.99, 1.08) | 0.18 | – | – |
Model 1: Adjusted for postdonation diabetes and hypertension as time-dependent covariates; Model 2: Not adjusted for postdonation diabetes and hypertension.
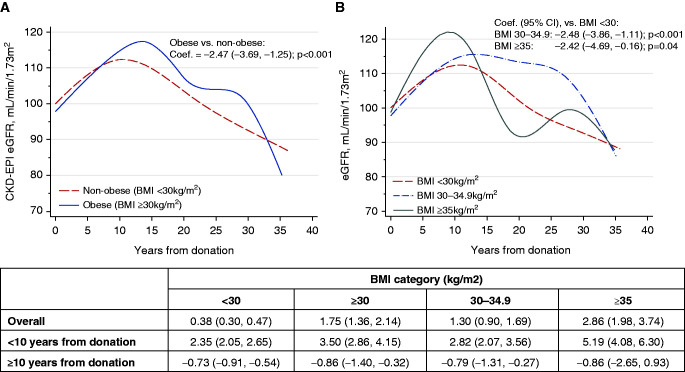
Serial creatinine measurements were available in 70% of donors (3.1±2.8 measurements/donor) allowing comparison of eGFR slopes (Supplemental Table 3). The overall change in eGFR was 0.38 ml/min per 1.73m2 per year (95% CI, 0.30 to 0.05) in nonobese donors versus 1.75 ml/min per 1.73m2 per year (95% CI, 1.36 to 2.14) in obese donors, P<0.001. eGFR rose more sharply in obese donors in the first decade after donation; 3.5 ml/min per 1.73m2 per year versus 2.4 ml/min per 1.73m2 per year, P<0.001. Afterward, both obese and nonobese donors experienced a comparable eGFR decline; 0.73 ml/min per 1.73m2 per year in nonobese donors versus 0.86 ml/min per 1.73m2 per year in obese donors, P=0.76 (Figure 3). Donors with BMI ≥35 kg/m2 had the highest overall annual eGFR change at 2.86 ml/min per 1.73m2 per year and also a more pronounced rise in eGFR in the first 10 years after donation at 5.19 ml/min per 1.73m2 per year. Beyond 10 years from donation, rate of eGFR change was similar in all BMI categories. The median % reduction in eGFR from donation to most recent available creatinine measurement was 14%, 16%, and 16%, P=0.05 in the three BMI categories, respectively.
Figure 3.
Trajectory of eGFR (ml/min per 1.73m2) over time. (A) BMI <30 versus 30 kg/m2. (B) BMI <30, 30–34.9, >35 kg/m2. The trend of eGFR over time is depicted by the cubic spline plots. Difference in eGFR slope between obese and nonobese donors was compared using a generalized linear mixed model. The coefficient (slope) and 95% CI, which represents mean change over time, was reported for each group. The generalized linear mixed model used eGFR as the dependent variable and BMI category as the independent variable, and used a random intercept plus random slope model and unstructured covariance option. The cubic splines were also used to depict the distribution of adjusted hazard ratio of evaluated study outcomes across BMI range. Because the eGFR slope appeared to change direction after 10 years, a subanalysis using the piecewise generalized linear model for years 0–10 and years 10–30 were conducted.
ESKD
In total, 43 out of 8016 donors with available data developed ESKD 19.3±10.3 years after donation; 31 out of 6370 (0.5%) nonobese donors and 12 out of 1646 (0.7%) obese donors. Of the 12 patients with ESKD in obese donors, eight occurred in the 1338 (0.7%) mildly obese donors, and four in the 425 (0.9%) severely obese donors. Obese and nonobese donors who developed ESKD were highly comparable on baseline laboratory and demographic characteristics with one exception; obese donors had a lower baseline eGFR (82 ml/min per 1.73m2 in those with BMI 30–34.9 kg/m2, 73 ml/min per 1.73m2 in those with BMI >33 kg/m2 compared with 92 ml/min per 1.73m2 in nonobese donors), P=0.14 (Table 6). Of the 12 obese donors who developed ESKD, six are male, 11 are white, ten of whom are related to their recipients, and seven smoked. Only one patient with ESKD was seen among the 220 obese Black donors. The risk of ESKD was higher in non-White donors; aHR, 4.06 (95% CI, 1.84 to 8.98), P=0.001, donors with BMI 30–34.9 kg/m2; aHR, 2.30 (95% CI, 1.03 to 5.14), BMI >35 kg/m2; aHR, 4.29 (95% CI, 1.39 to 13.2), P=0.01 (Table 7). The risk of ESKD was, however, not increased in donors with BMI ≥35 kg/m2 compared with donors with BMI 30–34.9 kg/m2; aHR, 1.85 (95% CI, 0.42 to 8.13), P=0.42. The composite of eGFR <30 ml/min per 1.73 m2 or ESKD developed in 85 donors out of 8552 donors with available data: 60 out of 6799 (1%) nonobese donors and 25 out of 1753 (1.4%) obese donors 16.7±13.2 years after donation. Postdonation hypertension and obesity were associated with increased risk of this outcome (Table 7). Obese and very obese donors were more likely to develop ESKD or eGFR <30 ml/min per 1.73m2 compared with donors without obesity; aHR, 2.22 (95% CI, 1.19 to 4.15), P=0.01 and aHR, 2.72 (95% CI, 1.16 to 6.36), P=0.02, respectively.
Table 6.
Baseline characteristics of donors who developed ESKD
| Characteristics | All donors with ESKD (n=43) | BMI <30 kg/m2 (n=31) | BMI 30.0-34.9 kg/m2 (n=8) | BMI ≥35 kg/m2 (n=4) | P Value |
|---|---|---|---|---|---|
| Age (yrs) | 41 (30, 50) | 41 (27, 43) | 47 (40, 52) | 47 (34, 51) | 0.22 |
| Male | 22 (51.2) | 16 (51.6) | 5 (62.5) | 1 (25.0) | 0.55 |
| Race/ethnicity | 1.00 | ||||
| Non-Hispanic White | 34 (79.1) | 23 (74.2) | 7 (87.5) | 4 (100.0) | |
| Non-Hispanic Black | 5 (11.6) | 4 (12.9) | 1 (12.5) | 0 (0.0) | |
| Hispanic | 1 (2.3) | 1 (3.2) | 0 (0.0) | 0 (0.0) | |
| Non-Hispanic Asian | 1 (2.3) | 1 (3.2) | 0 (0.0) | 0 (0.0) | |
| Other | 2 (4.7) | 2 (6.5) | 0 (0.0) | 0 (0.0) | |
| Related to recipient | 40 (93.0) | 30 (96.8) | 6 (75.0) | 4 (100.0) | 0.10 |
| 1st degree relative with hypertension | 22 (56.4) | 16 (57.1) | 4 (57.1) | 2 (50.0) | 1.00 |
| 1st degree relative with diabetes | 14 (35.0) | 10 (35.7) | 2 (25.0) | 2 (50.0) | 0.67 |
| 1st degree relative with kidney disease | 33 (76.7) | 26 (83.9) | 5 (62.5) | 2 (50.0) | 0.18 |
| 1st degree relative with heart disease | 14 (36.8) | 12 (44.4) | 1 (14.3) | 1 (25.0) | 0.43 |
| BMI (kg/m2) | 27.8 (24.1, 30.7) | 25 (23.6, 28) | 31.5 (30.9, 32.8) | 36.3 (35.8, 39.0) | <0.001 |
| Systolic BP (mmHg) | 123 (114, 130) | 120 (113, 129) | 124 (119, 130) | 130 (126, 133) | 0.14 |
| Diastolic BP (mmHg) | 78 (71, 82) | 77 (71, 80) | 78 (70, 83) | 84 (81, 85) | 0.09 |
| Smoking | 25 (64.1) | 18 (62.1) | 4 (66.7) | 3 (75) | 1.00 |
| Total cholesterol (mg/dl) | 205 (175, 215) | 201 (174, 208) | 232 (211, 252) | 171 (171, 171) | 0.06 |
| LDL (mg/dl) | 91 (47, 161) | 69 (47, 91) | 161 (161, 161) | – | 0.22 |
| HDL (mg/dl) | 47 (38, 56) | 44 (35, 53) | 50 (41, 59) | – | 0.44 |
| Triglyceride (mg/dl) | 140 (90, 195) | 172 (105, 281) | 137 (101, 165) | 85 (56, 114) | 0.27 |
| Fasting glucose (mg/dl) | 92 (84, 104) | 92 (82, 103) | 94 (84, 113) | 90 (68, 96) | 0.51 |
| Serum creatinine (mg/dl) | 1.0 (0.8, 1.1) | 1.0 (0.8, 1.1) | 1.1 (1.0, 1.2) | 1.0 (0.8, 1.1) | 0.22 |
| eGFR (ml/min per 1.73m2) | 83 (76, 104) | 92 (79, 107) | 82 (71, 85) | 73 (67, 99) | 0.14 |
| Donation to end of follow-up (year) | 34 (21, 39) | 36 (20, 39) | 26 (20, 30) | 30 (26, 37) | 0.11 |
Values are in frequency and % for categorical variables and median (IQR) for continuous variables.
Table 7.
Multivariable risk of ESKD or eGFR <30 ml/min/1.73m2 or ESKD: Cox regression
| Variable | Model 1 | Model 2 | ||
|---|---|---|---|---|
| aHR (95% CI) | P Value | aHR (95% CI) | P Value | |
| ESKD | n=7787 | n=7993 | ||
| Age (yrs) | 1.02 (0.99, 1.05) | 0.23 | 1.03 (1.01, 1.06) | 0.01 |
| Not non-Hispanic White | 5.00 (2.00, 12.49) | 0.001 | 4.06 (1.84, 8.98) | 0.001 |
| Related to the recipient | 0.39 (0.09, 1.74) | 0.22 | 0.47 (0.11, 1.97) | 0.30 |
| BMI ≥30 (kg/m2) | ||||
| <30 | (reference) | (reference) | ||
| 30–34.9 | 1.90 (0.77, 4.64) | 0.16 | 2.30 (1.03, 5.14) | 0.04 |
| ≥35 | 3.21 (0.87, 11.81) | 0.08 | 4.29 (1.39, 13.20) | 0.01 |
| Postdonation diabetes | 1.03 (1.00, 1.07) | 0.05 | – | – |
| Postdonation hypertension | 1.04 (0.98, 1.09) | 0.19 | – | – |
| eGFR<30 ml/min/1.73m2 or ESKD | n=7846 | n=8528 | ||
| Related to the recipient | 1.21 (0.40, 3.68) | 0.73 | 1.62 (0.55, 4.79) | 0.38 |
| BMI ≥30 (kg/m2) | ||||
| <30 | (reference) | (reference) | ||
| 30–34.9 | 2.22 (1.19, 4.15) | 0.01 | 2.16 (1.18, 3.93) | 0.01 |
| ≥35 | 2.72 (1.16, 6.36) | 0.02 | 2.70 (1.21, 6.05) | 0.02 |
| Systolic BP (mmHg) | 1.02 (1.00, 1.04) | 0.11 | 1.03 (1.01, 1.05) | 0.002 |
| Fasting glucose (mg/dl) | 1.02 (1.00, 1.03) | 0.09 | 1.02 (1.00, 1.04) | 0.02 |
| Postdonation diabetes | 1.02 (1.00, 1.05) | 0.08 | – | – |
| Postdonation hypertension | 1.05 (1.00, 1.10) | 0.04 | – | – |
Model 1: Adjusted for postdonation diabetes and hypertension as time-dependent covariates; Model 2: Not adjusted for postdonation diabetes and hypertension.
Mortality
In total, 418 (4.2%) donors died 20.8±10.2 years after donation: 363 (5.3%) nonobese donors and 55 (3.2%) obese donors. Cause of death is shown in Supplemental Table 4. In the Cox regression model that adjusted for baseline characteristics and three postdonation time-varying covariates (diabetes, hypertension, and ESKD), we found that older age, male, non-White ethnicity, smoking, hyperlipidemia, postdonation hypertension to be associated with increased risk of death (Table 8). Neither mild nor severe obesity, however, were associated with increased risk of death; aHR, 0.84 (95% CI, 0.57 to 1.23), P=0.37 in mildly obese donors and 1.54 (95% CI, 0.81 to 2.19), P=0.19 in severely obese donors (Table 8). In a second model addressing risk of death and all possible permutations of obesity at baseline (yes versus no) and development of postdonation ESKD (yes versus no) as covariates with the absence of obesity at baseline and no development of postdonation ESKD as a reference group, we observed similar findings: older age, male, non-White ethnicity, smoking, hyperlipidemia, and postdonation hypertension were associated with increased risk of death. Obese donors, whether they developed postdonation ESKD or not, were not at increased risk of death.
Table 8.
Multivariable risk of mortality: Cox regression
| Variable | Model 1 | Model 2 | ||
|---|---|---|---|---|
| aHR (95% CI) | P Value | aHR (95% CI) | P Value | |
| Adjusted for the time-varying postdonation ESKD | (n=7187) | (n=7846) | ||
| Age (yrs) | 1.12 (1.10, 1.14) | <0.001 | 1.09 (1.08, 1.10) | <0.001 |
| Male | 1.90 (1.38, 2.62) | <0.001 | 1.87 (1.45, 2.42) | <0.001 |
| Not non-Hispanic White | 1.87 (1.14, 3.06) | 0.01 | 1.65 (1.11, 2.46) | 0.01 |
| Related to the recipient | 0.83 (0.41, 1.68) | 0.61 | 0.89 (0.51, 1.56) | 0.69 |
| BMI (kg/m2) | ||||
| <30 | (reference) | (reference) | ||
| 30–34.9 | 1.04 (0.65, 1.67) | 0.86 | 0.84 (0.57, 1.23) | 0.37 |
| ≥35 | 1.64 (0.74, 3.65) | 0.23 | 1.54 (0.81, 2.94) | 0.19 |
| Systolic BP (mmHg) | 1.01 (0.99, 1.02) | 0.31 | 1.00 (0.99, 1.01) | 0.67 |
| Diastolic BP (mmHg) | 1.01 (0.99, 1.04) | 0.36 | 1.00 (0.98, 1.02) | 0.92 |
| Fasting glucose (mg/dl) | 1.01 (1.00, 1.01) | 0.23 | 1.00 (1.00, 1.01) | 0.23 |
| Serum creatinine (mg/dl) | 0.20 (0.08, 0.49) | <0.001 | 0.30 (0.14, 0.63) | 0.001 |
| Smoking | 1.90 (1.41, 2.55) | <0.001 | 1.96 (1.57, 2.44) | <0.001 |
| Hyperlipidemia | 1.25 (0.92, 1.70) | 0.15 | 1.26 (1.01, 1.57) | 0.04 |
| Postdonation diabetes | 1.00 (0.98, 1.02) | 0.92 | – | – |
| Postdonation hypertension | 0.97 (0.96, 0.98) | <0.001 | – | – |
| Postdonation ESKD | 1.01 (0.98, 1.05) | 0.49 | – | – |
| Adjusted for obesity/ESKD status | ||||
| Age (yrs) | 1.12 (1.10, 1.14) | <0.001 | – | – |
| Male | 1.88 (1.36, 2.60) | <0.001 | – | – |
| Not non-Hispanic White | 1.89 (1.15, 3.11) | 0.01 | – | – |
| Related to the recipient | 0.84 (0.42, 1.69) | 0.62 | – | – |
| Systolic BP (mmHg) | 1.01 (0.99, 1.02) | 0.28 | – | – |
| Diastolic BP (mmHg) | 1.01 (0.99, 1.04) | 0.35 | – | – |
| Fasting glucose (mg/dl) | 1.01 (1.00, 1.01) | 0.27 | – | – |
| Serum creatinine (mg/dl) | 0.20 (0.08, 0.48) | <0.001 | – | – |
| Smoking | 1.91 (1.42, 2.57) | <0.001 | – | – |
| Hyperlipidemia | 1.27 (0.93, 1.74) | 0.13 | – | – |
| Postdonation diabetes | 1.00 (0.98, 1.02) | 0.89 | – | – |
| Postdonation hypertension | 0.97 (0.96, 0.98) | <0.001 | – | – |
| Obesity/ESKD subgroup | – | – | ||
| Nonobese/no ESKD postdonation | (reference) | – | – | |
| Obese/no ESKD postdonation | 1.00 (0.98, 1.02) | 0.84 | – | – |
| Nonobese/ESKD postdonation | 1.01 (0.98, 1.04) | 0.59 | – | – |
| Obese/ESKD postdonation | 1.05 (0.92, 1.20) | 0.47 | – | – |
Model 1: Adjusted for postdonation diabetes and hypertension as time-dependent covariates; Model 2: Not adjusted for postdonation diabetes and hypertension.
Overall Risk of Study Outcomes
The overall multivariable risk of DM, hypertension, CVD, reduced eGFR, proteinuria, and ESKD were increased in obese donors (Table 9). Severely obese donors were more likely to develop diabetes and proteinuria than mildly obese donors but had comparable risk of CVD, hypertension, reduced eGFR, and ESKD. The differences in the mortality among the BMI categories were not statistically significant in the multivariable analysis (Figure 4). Table 9 also provides a summary of the multivariable risk of the different outcomes using BMI as a continuous variable. With the exception of death, each unit increase in BMI was associated with an increased risk of all studied outcomes. Supplemental Tables 5–9 provide the detailed results of all study outcomes utilizing BMI as a continuous variable. The evaluation of the proportional assumption indicated that except for the mortality, age did not violate the proportional assumption in the models for other outcomes. The Cox model for mortality with age as time-dependent covariate indicated the HR for would increase 2% for each incremental year increase. However, the inclusion of age as a time-dependent covariate did not affect the final findings.
Table 9.
Overall multivariable risk of death, CVD, eGFR outcomes and ESKD
| Outcome | BMI 30–34.9 Versus BMI <30 | BMI≥35 Versus BMI <30 | ||||||
|---|---|---|---|---|---|---|---|---|
| Model 1 | Model 2 | Model 1 | Model 2 | |||||
| aHR (95% CI) | P Value | aHR (95% CI) | P Value | aHR (95% CI) | P Value | aHR (95% CI) | P Value | |
| Categorical BMI | ||||||||
| Death (n=418/n=8583) | 1.04 (0.65, 1.67) | 0.86 | 0.84 (0.57, 1.23) | 0.37 | 1.64 (0.74, 3.65) | 0.23 | 1.54 (0.81, 2.94) | 0.19 |
| CVD (n=1085/n=8569) | 1.22 (0.93, 1.60) | 0.15 | 1.32 (1.09, 1.59) | 0.004 | 1.80 (1.24, 2.61) | 0.002 | 1.51 (1.12, 2.03) | 0.01 |
| Diabetes (n=566/n=7879) | 2.01 (1.45, 2.78) | <0.001 | 2.07 (1.49, 2.86) | <0.001 | 3.51 (2.28, 5.42) | <0.001 | 3.52 (2.28, 5.43) | <0.001 |
| Hypertension (n=2298/n=7696) | 1.50 (1.32, 1.70) | <0.001 | 1.50 (1.33, 1.70) | <0.001 | 1.55 (1.29, 1.87) | <0.001 | 1.53 (1.27, 1.85) | <0.001 |
| Proteinuria (n=1073/n=7687) | 1.56 (1.32, 1.85) | <0.001 | 1.62 (1.37, 1.90) | <0.001 | 2.04 (1.63, 2.56) | <0.001 | 2.06 (1.65, 2.58) | <0.001 |
| eGFR <60 (n=4655/n=8374) | 1.31 (1.21, 1.41) | <0.001 | 1.29 (1.20, 1.39) | <0.001 | 1.13 (0.99, 1.29) | 0.07 | 1.12 (0.98, 1.27) | 0.09 |
| eGFR <45 (n=1011/n=8374) | 1.56 (1.32, 1.84) | <0.001 | 1.54 (1.31, 1.81) | <0.001 | 1.28 (0.97, 1.70) | 0.09 | 1.22 (0.93, 1.61) | 0.16 |
| eGFR <30 (n=59/n=8374) | 2.61 (1.28, 5.30) | 0.01 | 2.51 (1.24, 5.08) | 0.01 | 2.48 (0.87, 7.07) | 0.09 | 2.24 (0.79, 6.36) | 0.13 |
| ESKD (n=43/n=8016) | 1.90 (0.77, 4.64) | 0.16 | 2.30 (1.03, 5.14) | 0.04 | 3.21 (0.87, 11.81) | 0.08 | 4.29 (1.39, 13.20) | 0.01 |
| Composite of eGFR<30 or ESKD (n=85/n=8552) | 2.22 (1.19, 4.15) | 0.01 | 2.16 (1.18, 3.93) | 0.01 | 2.72 (1.16, 6.36) | 0.02 | 2.70 (1.21, 6.05) | 0.02 |
| Model 1 | Model 2 | |||
|---|---|---|---|---|
| aHR (95% CI) | P Value | aHR (95% CI) | P Value | |
| Continuous BMI | ||||
| Death (n=418/n=8583) | 1.02 (0.98, 1.07) | 0.28 | 1.01 (0.98, 1.04) | 0.65 |
| CVD (n=1085/n=8569) | 1.03 (1.01, 1.06) | 0.002 | 1.03 (1.01, 1.05) | <0.001 |
| Diabetes (n=566/n=7879) | 1.11 (1.08, 1.13) | <0.001 | 1.11 (1.09, 1.13) | <0.001 |
| Hypertension (n=2298/n=7696) | 1.05 (1.04, 1.06) | <0.001 | 1.05 (1.04, 1.06) | <0.001 |
| Proteinuria (n=1073/n=7687) | 1.05 (1.04, 1.07) | <0.001 | 1.05 (1.04, 1.07) | <0.001 |
| eGFR <60 (n=4655/n=8374) | 1.03 (1.02, 1.04) | <0.001 | 1.03 (1.02, 1.04) | <0.001 |
| eGFR <45 (n=1011/n=8374) | 1.05 (1.03, 1.06) | <0.001 | 1.05 (1.03, 1.06) | <0.001 |
| eGFR <30 (n=59/n=8374) | 1.07 (1.01, 1.13) | 0.02 | 1.08 (1.02, 1.14) | 0.01 |
| ESKD (n=43/n=8016) | 1.08 (1.02, 1.15) | 0.01 | 1.12 (1.07, 1.18) | <0.001 |
| Composite of eGFR<30 or ESKD (n=85/n=8552) | 1.08 (1.03, 1.13) | 0.003 | 1.09 (1.04, 1.14) | <0.001 |
n, number of outcome events; N, total number of donors with ascertained outcome status; Model 1: Adjusted for postdonation diabetes, hypertension, and ESKD as time-dependent covariate; Model 2: Not adjusted for postdonation diabetes, hypertension and ESKD.
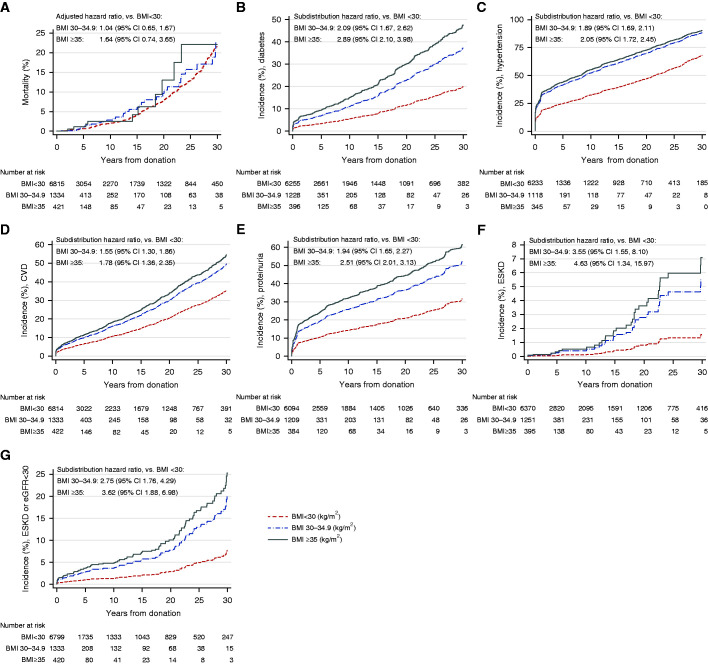
Figure 4.
Cumulative incidence of major outcomes. (A) Mortality. (B) Diabetes. (C) Hypertension. (D) CVD. (E) Proteinuria. (F) ESKD. (G) Composite of ESKD or eGFR<30 ml/min per 1.72m2. Kaplan–Meier curve was used for mortality; aHR for mortality obtained from the multivariable Cox proportional hazard model. Adjusted competing risk curves were used for outcomes other than mortality with the subdistribution hazard ratio obtained from the multivariable subdistribution hazard models (Fine and Gray method).
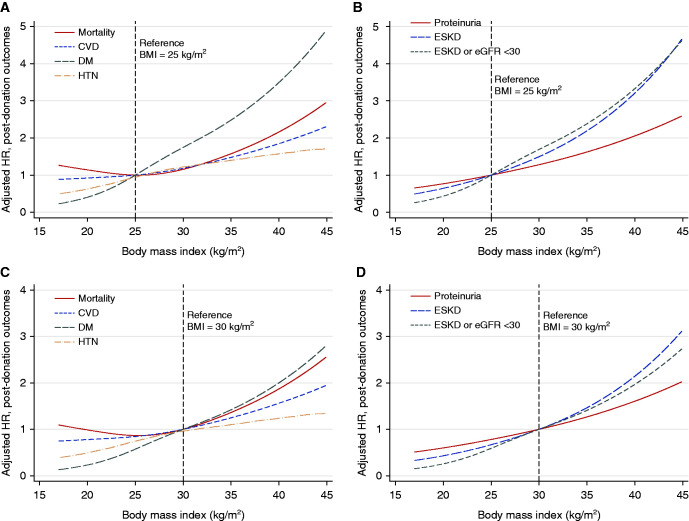
The overall hazard ratio of mortality, diabetes, CVD, hypertension, proteinuria, eGFR <30 ml/min per 1.73 m2 or ESKD, and ESKD at different levels of BMI as depicted in Figure 5. We used BMI of 25 kg/m2 because it represents ideal body weight and BMI of 30 kg/m2 as it represents the obesity diagnostic threshold. A higher BMI was associated with a graded increase in the risk of all outcomes studied.
Figure 5.
Cubic spline plot for aHR, by BMI. (A) Nonrenal outcomes, BMI = 25 kg/m2 as the reference. (B) Renal outcomes, BMI = 25 kg/m2 as the reference. (C) Nonrenal outcomes, BMI = 30 kg/m2 as the reference. (D) Renal outcomes, BMI = 30 kg/m2 as the reference. aHRs were obtained from the multivariable Cox regression models for individual outcomes.
Discussion
These results affirm the known associations between obesity and the development of diabetes, hypertension, and proteinuria observed in the general population. Obese donors incurred a 0.2% higher risk of kidney failure compared with nonobese donors. The risk of death, although higher in obese donors, was not statistically significant.
Kidney donors are highly selected individuals who are screened very carefully for subtle kidney and cardiac disease, do not have diabetes, and very few have hypertension. Therefore, obese donors may represent what has been referred to as metabolically healthy obese individuals.17 In a meta-analysis specifically addressing whether healthy overweight or obesity is associated with all-cause mortality, Kramer et al. demonstrated that metabolically healthy obese individuals were 20.4% more likely to die or have a fatal or nonfatal cardiovascular event.18 Of note, two of the four studies with the largest statistical weight and with >10 years of follow-up in this meta-analysis demonstrated no excess mortality.19,20 Importantly, smoking was not accounted for in any of the studies included in this meta-analysis. In kidney donors, Locke et al. demonstrated a 32% higher risk of death.10 In this important study, baseline BMI was unfortunately missing in 41,177 of the 119,769 (34.4%) donors and no adjustments were made for important covariates that are associated with mortality such as hyperlipidemia, family history of CVD, the development of postdonation diabetes, and hypertension. Minimal missing baseline data and more comprehensive adjustments in this current analysis may explain the discrepancy with the results from the larger US donor population. Certainly, differences in sample size and therefore event rate are also important. Of importance, the seminal work by Locke et al. showed an absolute risk of mortality of 3.0% in obese donors versus 2.89% in nonobese donors at 20 years; rates that are highly comparable to what we report here.10
The absolute risk of ESKD was small but increased in obese donors. The absolute risk was 0.9% in those with BMI ≥35 kg/m2, 0.7% in those with BMI 30–34.9 kg/m2 compared with 0.5% in those with BMI <30 kg/m2. Studies in the general population have generally yielded similar magnitude of association between obesity and ESKD. Grams et al. demonstrated a 16% increase in risk of ESKD for those with BMI ≥30 kg/m2 in their analysis of almost 5 million participants in seven contemporary cohorts at low risk for kidney disease who were followed for 4–16 years.21 Specific to kidney donors, Locke et al. demonstrated that although obese donors were 86% more likely to develop ESKD, the absolute risk was extremely small; 0.93% in obese donors versus 0.39% in nonobese donors 20 years after donation9; highly comparable to the risk reported here.
Obese donors had a brisker increase in eGFR in the first decade after donation. Many have voiced concerns about the possible additive hyperfiltration reported with obesity and hyperfiltration engendered by nephrectomy. Hyperfiltration after the latter, however, is due to an increase in the glomerular surface area, rather than an increase in intraglomerular pressure seen with obesity.22,23 Proteinuria occurred more frequently in obese donors who also develop more diabetes and hypertension and whether this is reflective of “hyperfiltration injury” cannot be entirely ruled out. The occurrence of eGFR <30 ml/min per 1.73m2 and ESKD occurred almost exclusively in related donors, which points more to the major importance of family history of kidney disease to kidney failure after donation, akin to the known familial clustering of kidney failure in nondonor populations.24–26
The strong association observed between obesity and diabetes is perhaps the most voiced argument against accepting obese donor candidates, because diabetes is the most common cause of kidney failure. In our previous studies of kidney donors who developed diabetes after donation, we observed no excess risk for kidney failure but the diabetic renal syndrome takes many years to develop and therefore factoring the age of the obese donor candidate in the decision making is critically important. In addition, the analysis by Grams et al. suggests those with a GFR of 60 ml/min per 1.73m2 are six times more likely to develop ESKD, and therefore a kidney donor who develops diabetes may experience a similarly increased risk considering that kidney donors GFR after donation is in that range.21 Although preliminary evidence suggests donors with impaired glucose tolerance, and even diabetes, at the time of donation have an overall risk of ESKD that is comparable to what is observed in patients with diabetes with full complement of nephrons, the length of follow-up of these studies is too short to draw strong recommendations and more research is needed.27,28
The concern about obese donor candidates’ higher likelihood of developing hypertension has also been suggested as another rationale for declining obese donor candidates. Roughly, a third of kidney donors develop hypertension after donation and the overall risk of ESKD in older (>50 years) hypertensive donors is <1%.29,30 We recently studied the outcomes of 904 hypertensive kidney donors from the RELIVE study and found these donors have comparable long-term outcomes to donors without hypertension at donation.12 Continued careful selection of hypertensive donors and long-term follow-up should be always emphasized.
We have previously studied 940 mainly White kidney donors who had serial pre- and postdonation BMI measurements and were followed for 22.3 (15.4–35.8) years, and found similar results to what we report here; obesity was associated with diabetes and hypertension development but not death or ESKD.31 Moreover, only 12% of donors with predonation BMI ≥30 kg/m2 were able to lose the weight requested by the transplant team and those who were asked to lose weight had the largest weight gain during follow-up. The current analysis also confirms that most donors do gain weight after donation. Certainly, the clear benefit of maintaining a healthy weight and weight loss on diabetes and hypertension development should be emphasized.32,33
Another factor that needs to be considered regarding the candidacy of obese donors is rate of surgical complications. Heimbach et al. demonstrated that donor nephrectomy took 19 minutes longer in donors with BMI ≥35 kg/m2 compared with donors with BMI <25 kg/m2.34 Reese et al. have shown that readmission rate, reoperation, conversion to open nephrectomy, vascular, and other complications are similar to those encountered in donors with BMI <30 kg/m2.35
The strengths of this study include minimal missing baseline data and ascertainment of studied outcomes in over 90% of the cohort. We were able to account for postdonation events such as hypertension, diabetes, profile eGFR trajectory, and proteinuria. This study has limitations. Although ethnically diverse, 85% of donors are White compared with roughly 70% in the larger US kidney donor population. ESKD is rare after donation and no formal linkage was made to ascertain all patients. Therefore, this analysis is underpowered in addressing this particular outcome. We certainly hope that all studies addressing kidney failure after donation always suffer from low statistical power. Reporting on more common outcomes such as proteinuria, eGFR, and its trajectory remedy some of the issue of low power, albeit it only partly. The RELIVE study spanned almost 50 years of live kidney donations and many changes have occurred over this long period, which we tried to account for by adjustment for year of donation. Many data elements came from the donors themselves, which makes this analysis susceptible to recall bias. The concordance of self-reported treated hypertension and diabetes are, however, excellent.36,37 Only 1085 out of 8583 donors had serial weight measurements available and length of follow-up was longer for nonobese donors. Certainly, the variability of the three centers’ practices in selection of donors cannot be analyzed in a granular manner.
In all, these data suggest that obesity, akin to what is observed in the general population, is associated with increased risk of hypertension and diabetes. The absolute risk of ESKD in mainly White obese kidney donors is small and the risk of mortality does not appear to be increased. Declining obese donors and delaying nephrectomy for months until weight loss is achieved should perhaps be reconsidered, because the absolute risk is small and many obese donors who lose weight to meet the transplant center requirement will gain the weight back. Moreover, many of these candidates might be willing to take this incremental risk in exchange for the huge survival benefit to their intended recipient, not to mention the possible improvement in their own quality of life. Waiting for donor candidates to lose weight to bring their absolute risk of ESKD down to the 0.5% risk seen in nonobese donors should be weighed against the 10%–20% annual adjusted mortality for those with kidney failure. Most importantly, if these candidates were to be considered more often, they should be counseled about maintaining a healthy weight. Having reduced GFR by virtue of nephrectomy may indeed put them at a higher risk for kidney failure, if faced with the development of diabetes or hypertension, which is particularly important for younger donor candidates.
Disclosures
A. Matas reports receiving research funding from Alexion, CareDX, CSL Behring, Shire, and Veloxis; and reports being a scientific advisor to or member of Jazz. H. E. Adrogue reports having an ownership interest in Dialyspa Dialysis Unit Houston Texas; and Speakers Bureau from AstraZeneca and Care DX. H.N. Ibrahim reports having consultancy agreements with Novartis; reports receiving research funding from the National Institutes of Health; reports receiving honoraria from Relypsa; and reports being a scientific advisor to or member of Exosome Diagnostics. All remaining authors have nothing to disclose.
Funding
None.
Supplementary Material
Acknowledgments
Dr. Hassan Ibrahim conceived of the idea and design and contributed to the analysis; Drs. Horacio Adrogue, Sean Hebert, Arthur Matas, Dina Murad, and Nurse Practitioner Hana Nguyen contributed to study design; Drs. Ed Graviss and Duc Nguyen conducted the analysis; and all authors contributed to manuscript preparation. We thank Allison Fox Chase for her expert administrative support. This work was presented as an oral presentation at the Annual American Transplant Congress, 2021.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
See related editorial “Understanding Risks and Our Responsibility to Living Donors,” on pages 2691–2693.
Supplemental Material
This article contains the following supplemental material online at https://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2021040548/-/DCSupplemental.
Supplemental Table 1. Availability of baseline variables.
Supplemental Table 2. Availability of outcome variables.
Supplemental Table 3. Number of donors with serial postdonation creatinine values.
Supplemental Table 4. Primary cause of death.
Supplemental Table 5. Multivariable risk for diabetes and hypertension.
Supplemental Table 6. Multivariable risk for proteinuria.
Supplemental Table 7. Multivariable risk for eGFR <60, eGFR <45, and eGFR <30 ml/min per 1.73m2.
Supplemental Table 8. Multivariable risk for ESKD and eGFR <30 ml/min per 1.73m2 or ESKD.
Supplemental Table 9. Multivariable risk for mortality.
References
- 1.Abdullah A, Amin FA, Hanum F, Stoelwinder J, Tanamas S, Wolf R, et al. : Estimating the risk of type-2 diabetes using obese-years in a contemporary population of the Framingham Study. Glob Health Action 9: 30421, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang Y, Chen X, Song Y, Caballero B, Cheskin LJ: Association between obesity and kidney disease: A systematic review and meta-analysis. Kidney Int 73: 19–33, 2008 [DOI] [PubMed] [Google Scholar]
- 3.Afshin A, Forouzanfar MH, Reitsma MB, Sur P, Estep K, Lee A, et al. ; GBD 2015 Obesity Collaborators : Health effects of overweight and obesity in 195 countries over 25 years. N Engl J Med 377: 13–27, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aune D, Sen A, Prasad M, Norat T, Janszky I, Tonstad S, et al. : BMI and all cause mortality: Systematic review and non-linear dose-response meta-analysis of 230 cohort studies with 3.74 million deaths among 30.3 million participants. BMJ 353: i2156, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mandelbrot DA, Pavlakis M, Danovitch GM, Johnson SR, Karp SJ, Khwaja K, et al. : The medical evaluation of living kidney donors: A survey of US transplant centers. Am J Transplant 7: 2333–2343, 2007 [DOI] [PubMed] [Google Scholar]
- 6.Naik AS, Cibrik DM, Sakhuja A, Samaniego M, Lu Y, Shahinian V, et al. : Temporal trends, center-level variation, and the impact of prevalent state obesity rates on acceptance of obese living kidney donors. Am J Transplant 18: 642–649, 2018 [DOI] [PubMed] [Google Scholar]
- 7.Lafranca JA, Spoon EQW, van de Wetering J, Ijzermans J, Dor F: Attitudes among transplant professionals regarding shifting paradigms in eligibility criteria for live kidney donation. PLoS One 12: e0181846, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kidney Disease: Improving Global Outcomes (KDIGO) : 2017 Living Donor Guideline. 2017. Available at: https://kdigo.org/guidelines/living-kidney-donor/. Accessed June 2, 2021 [Google Scholar]
- 9.Locke JE, Reed RD, Massie A, MacLennan PA, Sawinski D, Kumar V, et al. : Obesity increases the risk of end-stage renal disease among living kidney donors. Kidney Int 91: 699–703, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Locke JE, Reed RD, Massie AB, MacLennan PA, Sawinski D, Kumar V, et al. : Obesity and long-term mortality risk among living kidney donors. Surgery 166: 205–208, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Taler SJ, Messersmith EE, Leichtman AB, Gillespie BW, Kew CE, Stegall MD, et al. ; RELIVE Study Group : Demographic, metabolic, and blood pressure characteristics of living kidney donors spanning five decades. Am J Transplant 13: 390–398, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ibrahim H, Hebert SA, Murad DN, et al. : Outcomes of hypertensive kidney donors using current and past hypertension definitions, KI Reports, 6, 1242–1253, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, et al. ; CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) : A new equation to estimate glomerular filtration rate. Ann Intern Med 150: 604–612, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stata Lasso. Stata Reference Manual. Vol Release 16, College Station, TX, StataCorp, Stata Press, 2019 [Google Scholar]
- 15.Hastie T, Tibshirani RWM: Statistical Learning with Sparsity: The Lasso and Generalizations, Boca Raton, FL, Chapman & Hall/CRC, 2015 [Google Scholar]
- 16.Fine JP, Gray RJ: A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc 94: 496–509, 1999 [Google Scholar]
- 17.Wildman RP, Muntner P, Reynolds K, McGinn AP, Rajpathak S, Wylie-Rosett J, et al. : The obese without cardiometabolic risk factor clustering and the normal weight with cardiometabolic risk factor clustering: Prevalence and correlates of 2 phenotypes among the US population (NHANES 1999-2004). Arch Intern Med 168: 1617–1624, 2008 [DOI] [PubMed] [Google Scholar]
- 18.Kramer CZ, Zinman B, Retnakaran R: Are metabolically healthy overweight and obesity benign conditions?: A systematic review and meta-analysis. Ann Intern Med 159: 758–769, 2013 [DOI] [PubMed] [Google Scholar]
- 19.Song Y, Manson JE, Meigs JB, Ridker PM, Buring JE, Liu S: Comparison of usefulness of body mass index versus metabolic risk factors in predicting 10-year risk of cardiovascular events in women. Am J Cardiol 100: 1654–1658, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ogorodnikova AD, Kim M, McGinn AP, Muntner P, Khan U, Wildman RP: Incident cardiovascular disease events in metabolically benign obese individuals. Obesity (Silver Spring) 20: 651–659, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grams ME, Garg AX, Lentine KL: Kidney-failure risk projection for the living kidney-donor candidate. N Engl J Med 374: 2094–2095, 2016 [DOI] [PubMed] [Google Scholar]
- 22.Blantz RC, Steiner RW: Benign hyperfiltration after living kidney donation. J Clin Invest 125: 972–974, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lenihan CR, Busque S, Derby G, Blouch K, Myers BD, Tan JC: Longitudinal study of living kidney donor glomerular dynamics after nephrectomy. J Clin Invest 125: 1311–1318, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Satko SG, Sedor JR, Iyengar SK, Freedman BI: Familial clustering of chronic kidney disease. Semin Dial 20: 229–236, 2007 [DOI] [PubMed] [Google Scholar]
- 25.Zhang J, Thio CHL, Gansevoort RT, Snieder H: Familial aggregation of CKD and heritability of kidney biomarkers in the general population: The Lifelines Cohort Study. Am J Kidney Dis 77: 869–878, 2021 [DOI] [PubMed] [Google Scholar]
- 26.Skrunes R, Svarstad E, Reisæter AV, Vikse BE: Familial clustering of ESRD in the Norwegian population. Clin J Am Soc Nephrol 9: 1692–1700, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Okamoto M, Suzuki T, Fujiki M, Nobori S, Ushigome H, Sakamoto S, et al. : The consequences for live kidney donors with preexisting glucose intolerance without diabetic complication: Analysis at a single Japanese center. Transplantation 89: 1391–1395, 2010 [DOI] [PubMed] [Google Scholar]
- 28.Hebert SA, Murad DN, Nguyen DT, Graviss EA, Adrogue HE, Matas A, et al. : Outcomes of kidney donors with impaired fasting glucose [published online ahead of print February 5, 2021]. Transplantation 2021 [DOI] [PubMed] [Google Scholar]
- 29.Sanchez OA, Ferrara LK, Rein S, Berglund D, Matas AJ, Ibrahim HN: Hypertension after kidney donation: Incidence, predictors, and correlates. Am J Transplant 18: 2534–2543, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Al Ammary F, Luo X, Muzaale AD, Massie AB, Crews DC, Waldram MM, et al. : Risk of ESKD in older live kidney donors with hypertension. Clin J Am Soc Nephrol 14: 1048–1055, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Issa N, Sánchez OA, Kukla A, Riad SM, Berglund DM, Ibrahim HN, et al. : Weight gain after kidney donation: Association with increased risks of type 2 diabetes and hypertension. Clin Transplant 32: e13360, 2018 [DOI] [PubMed] [Google Scholar]
- 32.Hamman RF, Wing RR, Edelstein SL, Lachin JM, Bray GA, Delahanty L, et al. : Effect of weight loss with lifestyle intervention on risk of diabetes. Diabetes Care 29: 2102–2107, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moore LL, Visioni AJ, Qureshi MM, Bradlee ML, Ellison RC, D’Agostino R: Weight loss in overweight adults and the long-term risk of hypertension: The Framingham study. Arch Intern Med 165: 1298–1303, 2005 [DOI] [PubMed] [Google Scholar]
- 34.Heimbach JK, Taler SJ, Prieto M, Cosio FG, Textor SC, Kudva YC, et al. : Obesity in living kidney donors: Clinical characteristics and outcomes in the era of laparoscopic donor nephrectomy. Am J Transplant 5: 1057–1064, 2005 [DOI] [PubMed] [Google Scholar]
- 35.Reese PP, Feldman HI, Asch DA, Thomasson A, Shults J, Bloom RD: Short-term outcomes for obese live kidney donors and their recipients. Transplantation 88: 662–671, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Skinner KM, Miller DR, Lincoln E, Lee A, Kazis LE: Concordance between respondent self-reports and medical records for chronic conditions: Experience from the Veterans Health Study. J Ambul Care Manage 28: 102–110, 2005 [DOI] [PubMed] [Google Scholar]
- 37.Tisnado DM, Adams JL, Liu H, Damberg CL, Chen WP, Hu FA, et al. : What is the concordance between the medical record and patient self-report as data sources for ambulatory care? Med Care 44: 132–140, 2006 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.