Significance Statement
How ESKD-related care changed during the initial phases of the coronavirus disease 2019 pandemic is unknown. Using United States Renal Data System data, we compared ESKD-related care in the first half of 2020 with historical trends. The initial height of the pandemic saw a 25% drop in documented ESKD incidence (most strikingly in the oldest individuals), pre-emptive kidney transplantation halved, mean eGFR at dialysis initiation decreased, odds of initiation with peritoneal dialysis (versus hemodialysis) increased by nearly 25%, and odds of starting hemodialysis with a catheter increased by 30%. These are all major changes in the care of patients with incident ESKD. At the initial height of the pandemic, the weekly number of patients with documented incident ESKD fell to a level not observed since 2011.
Keywords: COVID-19, dialysis, ESKD, transplantation, USRDS
Abstract
Background
The COVID-19 pandemic caused major disruptions to care for patients with advanced CKD.
Methods
We investigated the incidence of documented ESKD, ESKD treatment modalities, changes in eGFR at dialysis initiation, and use of incident central venous catheters (CVCs) by epidemiologic week during the first half of 2020 compared with 2017–2019 historical trends, using Centers for Medicare and Medicaid Services data. We used Poisson and logistic regression for analyses of incidence and binary outcomes, respectively.
Results
Incidence of documented ESKD dropped dramatically in 2020 compared with the expected incidence, particularly during epidemiologic weeks 15–18 (April, incidence rate ratio [IRR], 0.75; 95% CI, 0.73 to 0.78). The decrease was most pronounced for individuals aged ≥75 years (IRR, 0.69; 95% CI, 0.66 to 0.73). Pre-emptive kidney transplantation decreased markedly during weeks 15–18 (IRR, 0.56; 95% CI, 0.46 to 0.67). Mean eGFR at dialysis initiation decreased by 0.33 ml/min per 1.73 m2 in weeks 19–22; non-Hispanic Black patients exhibited the largest decrease, at 0.61 ml/min per 1.73 m2. The odds of initiating dialysis with eGFR <10 ml/min per 1.73 m2 were highest during weeks 19–22 (May, OR, 1.14; 95% CI, 1.05 to 1.17), corresponding to an absolute increase of 2.9%. The odds of initiating peritoneal dialysis (versus hemodialysis) were 24% higher (OR, 1.24; 95% CI, 1.14 to 1.34) in weeks 11–14, an absolute increase of 2.3%. Initiation with a CVC increased by 3.3% (OR, 1.30; 95% CI, 1.20 to 1.41).
Conclusions
During the first wave of the COVID-19 pandemic, the number of patients starting treatment for ESKD fell to a level not observed since 2011. Changes in documented ESKD incidence and other aspects of ESKD-related care may reflect differential access to care early in the pandemic.
The coronavirus disease 2019 (COVID-19) pandemic has caused major disruptions to healthcare delivery and affected patients across all of medicine,1–4 including patients with kidney disease.5 In patients with ESKD, the pandemic dramatically affected rates and patterns of hospitalization and mortality.6,7 During the initial phases of the pandemic in the United States, the rate of death increased by 17% among patients receiving dialysis compared with prepandemic historical rates.7 At the same time, there was a 13% reduction in new initiations of treatment for ESKD that occurred between the first and second quarters of 2020,8 perhaps due to a decrease in nonemergent in-person physician encounters and a resultant postponement of dialysis initiation.
The decision to initiate RRT, particularly dialysis, and the course of transition from CKD to ESKD are critical issues. This is particularly true in the case of maintenance dialysis, given that dialysis is life changing for patients and that aspects of dialysis initiation, such as modality selection, likely influence outcomes.9–11 It is possible that initiation of treatment was delayed or that disruption of preparatory activities, such as modality education or vascular access placement, may have led to a shift toward in-center hemodialysis (HD) with a central venous catheter (CVC) as initial access. Conversely, a desire to avoid perceived risks inherent in in-center HD could have triggered a shift toward peritoneal dialysis (PD).
Using data from the United States Renal Data System (USRDS), a near-universal registry of ESKD in the United States, we investigated the incidence of known, or documented, ESKD, initiation of HD and PD, and use of pre-emptive kidney transplantation (KT) during the first half of 2020, relative to projections based on trends derived from previous years. Additionally, we determined changes in mean eGFR upon initiation of dialysis and, among patients initiating HD, use of CVCs. Further, given the disproportional burden of COVID-19 among racial and ethnic minority groups and elders, we investigated whether any observed changes may have varied by strata of age and race/ethnicity. We hypothesized that the decrease in initiation of PD would be greater than that for HD, use of CVCs upon HD initiation would increase, and patients would be diagnosed with ESKD at lower levels of eGFR, relative to the period before the pandemic. We also hypothesized that many of these findings would be more pronounced in older, compared with younger, individuals transitioning to ESKD, and in members of racial and ethnic minority groups compared with non-Hispanic White individuals.
Methods
We analyzed an extract of the Centers for Medicare and Medicaid Services (CMS) Renal Management Information System (REMIS), encompassing data available at the end of the third quarter of 2020. REMIS enumerates the ESKD population and includes submissions of form CMS-2728 (“ESRD Medical Evidence Report”), dialysis facility admission and discharge records from the Consolidated Renal Operations in a Web-Enabled Network, and KT records from the Organ Procurement and Transplantation Network. Due to lags in form submission, data are essentially complete through the second quarter of 2020.
We assessed the incidence of documented ESKD during epidemiologic weeks 3–26 of 2020 (January 12 to June 27) and, for comparison, during corresponding weeks of 2017–2019. An epidemiologic week, which begins on a Sunday and ends on the next Saturday, is a widely used construct in infectious disease surveillance and is used by the Centers for Disease Control and Prevention to facilitate year-over-year comparisons. During each week, we identified all adult patients (aged ≥18 years) with newly diagnosed ESKD (initiation of maintenance dialysis or receipt of a pre-emptive KT). For every patient, we identified age on the date of ESKD diagnosis, race and ethnicity (non-Hispanic White, non-Hispanic Black, Hispanic, Asian, other), and sex. In the subset of patients with a CMS-2728 form (>97%), we also identified the presence of diabetes mellitus and heart failure. For each patient in the subset, we estimated the probability of death during the first year of ESKD, using a Cox regression model of death in adult patients with newly diagnosed ESKD in 2017–2019. Each patient was followed from the date of ESKD diagnosis to the earliest of death, the date 1 year after the date of ESKD diagnosis, or December 31, 2019. The model included age, race/ethnicity, sex, diabetes mellitus, and heart failure. This model facilitated an evaluation of the apparent risk among patients with newly diagnosed ESKD in 2020 versus 2017–2019.
In each epidemiologic week, we tallied the number of patients with newly diagnosed ESKD, overall and by RRT modality (dialysis, pre-emptive KT); tallied the number of patients initiating dialysis, stratified by modality (HD, PD); measured mean eGFR at dialysis initiation; and tabulated utilization of a CVC at HD initiation. HD included both in-facility and home HD (although <0.5% performed HD in the home setting). GFR was estimated by the Chronic Kidney Disease Epidemiology Collaboration equation.12
For analyses of the incidence of documented ESKD and dialysis initiation, we fit Poisson regression models. In each epidemiologic week (from week 3–26) of each year (from 2017 to 2020), we aggregated patients with the same combination of age, race/ethnicity, and sex, with age as an integer value. This combination formed the observational unit in the model. Thus, the dependent variable in each model was the number of patients in the combination, whereas the offset variable was the natural logarithm of the number of residents in the combination, according to US Census Bureau estimates of the population in January of the corresponding year. The model included a slope effect for calendar year, but only during 2017–2019; a categoric effect for 2020 versus 2017–2019; a categoric effect for interval of epidemiologic weeks; an interaction of the aforementioned categoric effects; and adjustment for age, race/ethnicity, and sex. The parameterization of time facilitated estimation of adjusted, interval-specific “effects” of 2020 versus the forecast of 2020 on incidence, had secular trends during 2017–2019 continued. In plain language, these effects were incidence rate ratios (IRRs) that quantified the ratio of observed to forecasted numbers of patients with incident ESKD in 2020. To estimate IRRs within demographic subgroups, we refit regression models with additional interactions of time with age and time with race/ethnicity; in the former, age was categorized as 18–44, 45–64, 65–74, and ≥75 years.
For eGFR at dialysis initiation, we fit a normal linear regression model. Each patient who initiated dialysis during epidemiologic weeks 3–26 of 2017–2020 was included in the analysis. The model included the same set of effects and adjustments as was specified in models of incidence, and adjustments for diabetes mellitus and heart failure. The model was refit with interactions of time with age, time with race/ethnicity, and time with the crossclassification of diabetes mellitus and heart failure. We subsequently categorized eGFR as <10 or ≥10 ml/min per 1.73 m2 and fit two models of eGFR <10 ml/min per 1.73 m2: a linear probability regression model (LPRM) and a logistic regression model. The LPRM was estimated by a two-step process. First, we fit a logistic regression model of the outcome and, with the fitted model, estimated the SD of the outcome in each patient. Second, we fit a normal linear regression model of the outcome, with each patient weighted by the inverse of the SD. Ultimately, the LPRM provided estimates of the absolute effect of 2020 (a putative proxy for the COVID-19 pandemic), relative to 2017–2019, on the probability of dialysis initiation with eGFR <10 ml/min per 1.73 m2, whereas the logistic regression model provided estimates of the relative effect of 2020, compared with 2017–2019, on the odds of the outcome. We repeated this estimation routine for two other binary outcomes: (1) PD (versus HD) among patients who initiated dialysis, and (2) a CVC (versus an arteriovenous fistula or graft) in the subset of patients who initiated HD.
Data were accessed under the auspices of an agreement with CMS, and research was approved by the institutional review board at the Hennepin Healthcare Research Institute. All analyses were executed in SAS, version 9.4 (Cary, NC).
Results
Patient Characteristics
Characteristics of the population transitioning to ESKD in 2020, compared with 2017–2019, are shown in Table 1. Because a US national emergency was declared on March 13, 2020 (during epidemiologic week 11), we divided 2020 (and, analogously, 2017–2019) into prepandemic and pandemic periods (epidemiologic weeks 3–10 and 11–26, respectively). Although there were few, if any, substantive differences in patient characteristics between epidemiologic weeks 3–10 and 11–26 for 2017–2019, differences in receipt of a pre-emptive kidney transplant, eGFR at dialysis initiation, dialysis initiation with PD, and CVC use were slightly more pronounced for 2020; this was also the case for the mean estimated probability of death during the first year of ESKD, which is shown in Supplemental Figure 1.
Table 1.
Patient characteristics in epidemiologic weeks 3–26 of 2017–2020, among adult patients with newly diagnosed ESKD
| Characteristics | 2017–2019 | 2020 | ||
|---|---|---|---|---|
| Weeks 3–10 | Weeks 11–26 | Weeks 3–10 | Weeks 11–26 | |
| Mean weekly ESKD incidence (N) | 2676 | 2542 | 2694 | 2203 |
| Age (yr), % | ||||
| 18–44 | 11.7 | 11.9 | 12.3 | 12.6 |
| 45–64 | 38.3 | 38.0 | 38.2 | 39.0 |
| 65–74 | 27.1 | 27.1 | 26.6 | 26.7 |
| ≥75 | 22.9 | 23.0 | 23.0 | 21.7 |
| Race/ethnicity, % | ||||
| Non-Hispanic White | 52.8 | 52.8 | 48.0 | 48.7 |
| Non-Hispanic Black | 24.8 | 24.8 | 26.4 | 27.3 |
| Hispanic | 15.4 | 15.6 | 17.6 | 16.5 |
| Asian | 4.8 | 4.8 | 5.5 | 5.1 |
| Other/unknown | 2.2 | 2.1 | 2.5 | 2.3 |
| Sex, % | ||||
| Female | 41.2 | 41.8 | 40.9 | 41.4 |
| Male | 58.8 | 58.2 | 59.1 | 58.7 |
| Comorbid conditions, %a | ||||
| DM− and HF− | 28.0 | 28.8 | 27.9 | 29.0 |
| DM− and HF+ | 7.7 | 7.6 | 7.4 | 7.3 |
| DM+ and HF− | 43.4 | 43.2 | 43.9 | 44.3 |
| DM+ and HF+ | 20.9 | 20.4 | 20.8 | 19.5 |
| Mean estimated probability of death during the first year of ESKD, %a | 16.2 | 16.2 | 15.9 | 15.5 |
| RRT, % | ||||
| Dialysis | 97.6 | 97.4 | 96.9 | 97.4 |
| Preemptive KT | 2.4 | 2.6 | 3.2 | 2.6 |
| eGFR (ml/min per 1.73 m2), mean (SD)b | 10.11 (4.58) | 10.08 (4.53) | 10.03 (4.41) | 9.81 (4.41) |
| eGFR <10 ml/min per 1.73 m2, %b | 55.0 | 55.3 | 55.3 | 57.6 |
| PD utilization at dialysis initiation, %b | 10.2 | 11.3 | 12.1 | 14.1 |
| Catheter utilization at HD initiation, %c | 81.1 | 80.7 | 82.2 | 83.7 |
DM, diabetes mellitus; HF, heart failure.
Among all patients with a submission of form CMS-2728.
Among patients with a submission of form CMS-2728 and who initiated dialysis.
Among patients with a submission of form CMS-2728 and who initiated HD.
Initiation of Treatment for ESKD
In 2017–2019, the mean weekly number of patients initiating treatment for ESKD was 2714 during weeks 3–6, 2639 during weeks 7–10, and 2555 during weeks 11–26. The mean weekly number of patients initiating treatment for ESKD was comparable during weeks 3–10 of 2020, but was sharply lower during weeks 11–26, with only 2203 persons per week (Figure 1 and Supplemental Tables 1–4).
Figure 1.
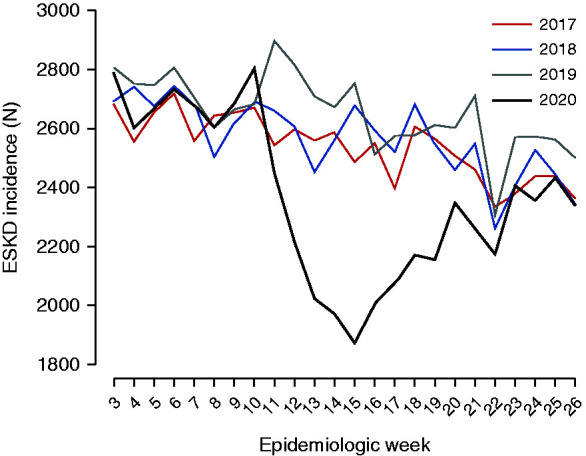
Weekly counts of documented ESKD incidence during weeks 3–26 of 2017–2020.
Adjusted IRRs of documented ESKD in 2020, versus the forecast for 2020 had secular trends in 2017–2019 continued, are displayed Figure 2. Documented ESKD incidence declined in weeks 11–14 (April), with a 25% decrease at the nadir (IRR, 0.75; 95% CI, 0.73 to 0.78) during weeks 15–18 (May); incidence then rebounded to approach prepandemic levels during weeks 23–26 (June, IRR, 0.93; 95% CI, 0.90 to 0.95).
Figure 2.
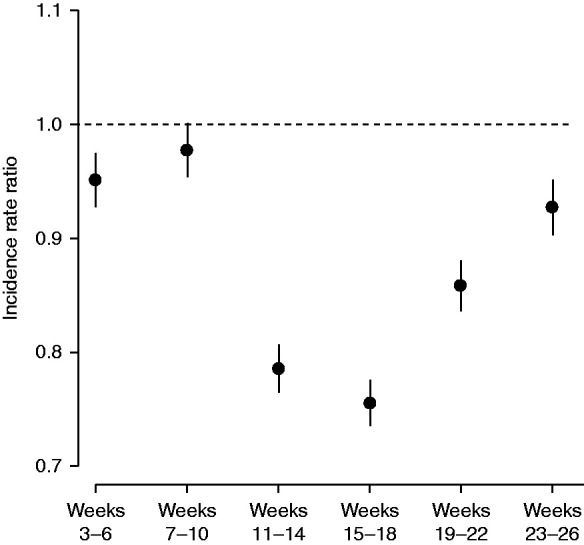
Adjusted, interval-specific IRRs of documented ESKD during 2020 versus the forecast of 2020, had the secular trend of 2017–2019 continued.
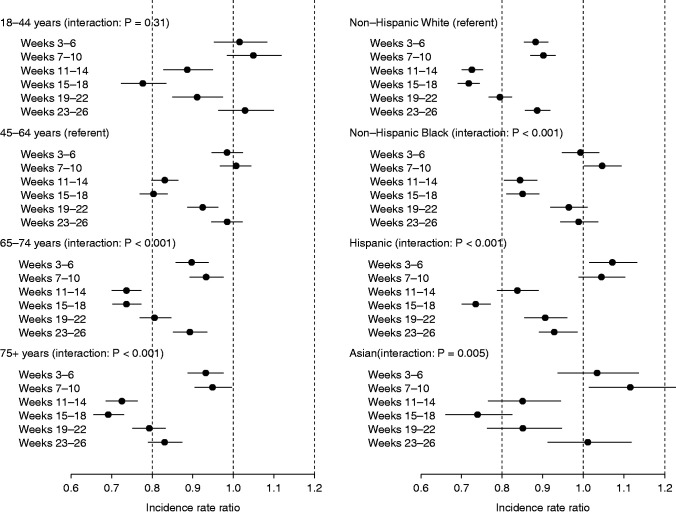
IRRs of the transition to ESKD over this period are shown by strata of age and race in Figure 3, A and B. Regarding age, the documented ESKD incidence decreased most in 2020 during weeks 15–18 among older individuals: IRRs of 0.80 (95% CI, 0.77 to 0.84) for individuals aged 45–64 years, 0.73 (95% CI, 0.70 to 0.77) for those aged 65–74 years, and 0.69 (95% CI, 0.66 to 0.73) for those aged ≥75 years. Furthermore, whereas IRRs were not significantly different from 1.00 for individuals aged <65 years in weeks 23–26 (June), IRRs remained significantly less than unity for those aged ≥65 years in this period. Overall, compared with individuals aged 45–64 years (the referent group), IRRs decreased significantly more during weeks 11–26 for individuals aged 65–74 years (P<0.001) and ≥75 years (P<0.001). In terms of race and ethnicity, during weeks 15–18 (April), the reduction in rates of documented incident ESKD was least for non-Hispanic Black individuals (IRR, 0.85; 95% CI, 0.81 to 0.89) compared with those who were non-Hispanic White (IRR, 0.72; 95% CI, 0.69 to 0.74), Hispanic (IRR, 0.73; 95% CI, 0.69 to 0.78), and Asian (IRR, 0.74; 95% CI, 0.66 to 0.82). By weeks 23–26 (June), IRRs remained significantly <1.00 in non-Hispanic White and Hispanic patients, at 0.89 (95% CI, 0.86 to 0.92) and 0.93 (95% CI, 0.88 to 0.98), respectively, but IRR had returned to a prepandemic level in non-Hispanic Black patients. Overall, the IRRs for documented incident ESKD among non-Hispanic Black patients decreased significantly less (P<0.001) during weeks 11–26 than for those who were non-Hispanic White.
Figure 3.
Adjusted, interval-specific IRRs of documented ESKD during 2020 versus the forecast of 2020, had the secular trend of 2017–2019 continued, in subgroups defined by age (left panel) and race/ethnicity (right panel).
Dialysis initiation (comprising >97% of all patients transitioning to ESKD) revealed a similar pattern. Compared with an IRR of 0.95 (95% CI, 0.92 to 0.97) during weeks 3–6, the IRR for dialysis initiation during weeks 15–18 was 0.76 (95% CI, 0.74 to 0.78). Patterns by age and race/ethnicity groups were similar to those of new ESKD overall (data not shown).
Adjusted IRRs for HD initiation, PD initiation, and pre-emptive KT are shown in Figure 4. Incidence of HD initiation fell in weeks 11–14 (IRR, 0.75; 95% CI, 0.73 to 0.77) and reached a nadir in weeks 15–18 (IRR, 0.72; 95% CI, 0.70 to 0.74). Subsequently, incidence of HD initiation partly rebounded. In contrast, PD initiation was relatively unchanged in 2020 compared with projections on the basis of data from 2017 to 2019 through week 14 (IRRs approximately 1.0) but declined in weeks 15–18 (IRR, 0.85; 95% CI, 0.79 to 0.92) and weeks 19–22 (IRR, 0.83; 95% CI, 0.77 to 0.89), before recovering in weeks 23–26 (IRR, 0.95; 95% CI, 0.89 to 1.03). Pre-emptive KT, for which IRRs were substantially greater than one in weeks 3–10, decreased markedly during weeks 15–18 (IRR, 0.56; 95% CI, 0.46 to 0.67) but increased thereafter (IRR, 0.90; 95% CI, 0.78 to 1.03) during weeks 23–26.
Figure 4.
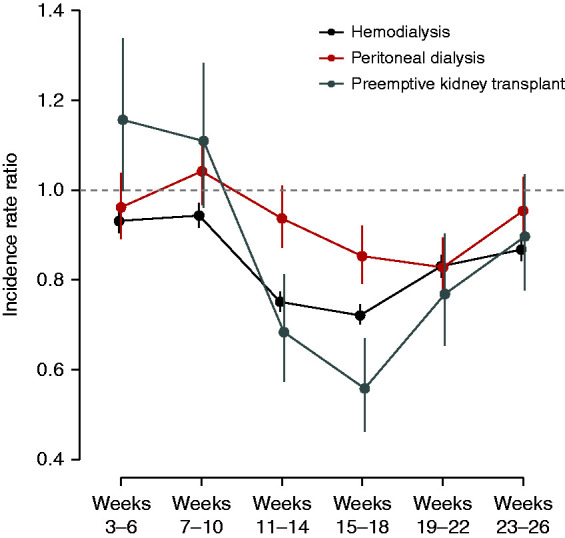
Adjusted, interval specific IRRs of HD, PD, and preemptive KT during 2020 versus the forecast of 2020, had the secular trend of 2017–2019 continued.
Characteristics at Dialysis Initiation: eGFR, Dialysis Modality, and Catheter Use
Adjusted differences in mean eGFR at dialysis initiation between 2020, versus the forecast for 2020 had secular trends in 2017–2019 continued, are displayed in Figure 5. The mean eGFR at ESKD onset decreased in 2020 compared with prior years. Mean eGFR was not significantly different from the forecast in weeks 3–14, but was 0.19 (95% CI, 0.07–0.31), 0.33 (95% CI, 0.22–0.45), and 0.28 (95% CI, 0.17–0.40) ml/min per 1.73 m2 lower in weeks 15–18, 19–22, and 23–26, respectively.
Figure 5.
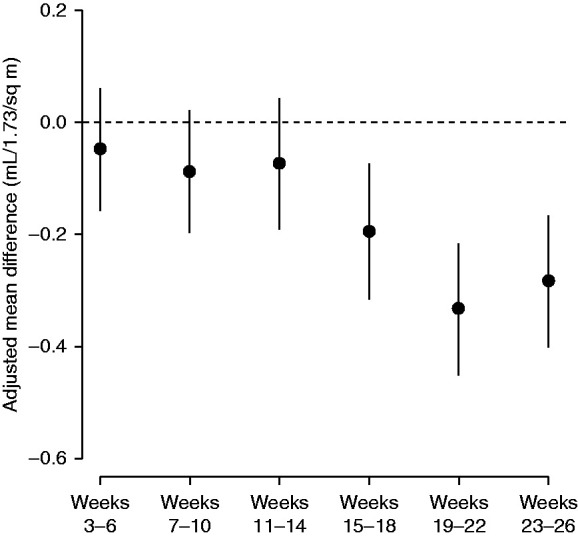
Adjusted, interval-specific differences in mean eGFR during 2020 versus the forecast of 2020, had the secular trend of 2017–2019 continued.
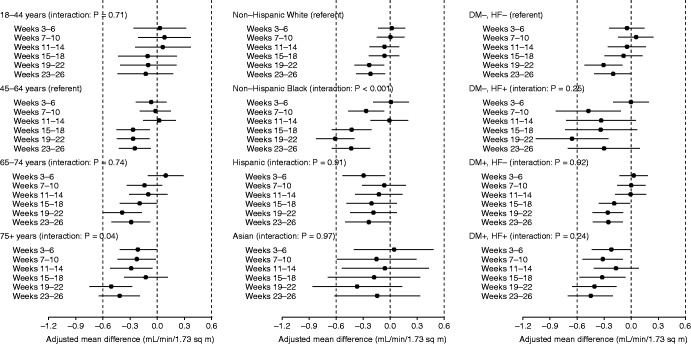
The reduction in mean eGFR at dialysis initiation during the weeks 19–22 was more pronounced among older age groups. For example, by weeks 23–26, mean eGFRs at initiation were 0.13, 0.25, 0.29, and 0.42 ml/min per 1.73 m2 lower in 2020 in patients aged 18–44, 45–64, and 65–74, and ≥75 years, respectively (Figure 6A). Overall, the pattern of eGFR decrease was significantly greater (P=0.04) in individuals aged ≥75 years compared with those aged 45–64 years (referent group), with the decrease in eGFR in the latter occurring primarily late in the period examined (i.e., weeks 19–26). Regarding race/ethnicity, non-Hispanic Black patients exhibited the largest decreases in mean eGFR in 2020, with adjusted differences of −0.42, −0.61, and −0.43 ml/min per 1.73 m2 in weeks 15–18, 19–22, and 23–26, respectively (Figure 6B), P<0.001 for non-Hispanic Black compared with non-Hispanic White patients. Overall, the pattern of eGFR at initiation did not differ significantly by the presence or absence of heart failure or diabetes (Figure 6C).
Figure 6.
Adjusted, interval-specific differences in mean eGFR during 2020 versus the forecast of 2020, had the secular trend of 2017–2019 continued, in subgroups defined by age (left panel) and race/ethnicity (right panel).
The absolute effect (shown as a percentage) and relative effect (shown as an odds ratio [OR]) for initiating dialysis with an eGFR <10 ml/min per 1.73m2, initiating dialysis using PD, and initiating HD with a CVC, are shown in Table 2. Despite significant decreases in mean eGFR, the absolute percentage of patients who initiated dialysis with an eGFR of <10 ml/min per 1.73 m2 was only 2.9 (95% CI, 1.6 to 4.2) points higher by weeks 19–22, and 2.3 (95% CI, 1.0 to 3.6) points higher by weeks 23–26 (Table 2). The adjusted OR of initiating dialysis with an eGFR of <10 ml/min per 1.73m2 in 2020 compared with 2017–2019 were greatest during weeks 19–22 (OR, 1.14; 95% CI, 1.05 to 1.17).
Table 2.
Adjusted, interval-specific effects of 2020 versus the forecast of 2020 on the incidence of eGFR <10 ml/min per 1.73 m2 and PD utilization among all patients who initiated dialysis and the incidence of catheter utilization among all patients who initiated hemodialysis
| Weeks | Absolute Effect, Difference (95% CI)a | Relative Effect, OR (95% CI)b |
|---|---|---|
| Percentage of dialysis patients with eGFR <10 ml/min per 1.73 m2 | ||
| Weeks 3–6 | 0.01 (−1.20 to 1.22) | 1.00 (0.95 to 1.05) |
| Weeks 7–10 | 0.48 (−0.73 to 1.70) | 1.02 (0.97 to 1.08) |
| Weeks 11–14 | 1.14 (−0.16 to 2.40) | 1.05 (0.99 to 1.11) |
| Weeks 15–18 | 1.74 (0.41 to 3.07) | 1.08 (1.02 to 1.15) |
| Weeks 19–22 | 2.86 (1.57 to 4.16) | 1.14 (1.07 to 1.20) |
| Weeks 23–36 | 2.31 (1.03 to 3.59) | 1.11 (1.05 to 1.17) |
| Percentage of dialysis patients performing PD | ||
| Weeks 3–6 | 0.40 (−0.35 to 1.15) | 1.05 (0.96 to 1.13) |
| Weeks 7–10 | 0.95 (0.18 to 1.72) | 1.11 (1.03 to 1.20) |
| Weeks 11–14 | 2.33 (1.50 to 3.17) | 1.24 (1.14 to 1.34) |
| Weeks 15–18 | 1.79 (0.93 to 2.66) | 1.17 (1.07 to 1.27) |
| Weeks 19–22 | 0.03 (−0.79 to 0.86) | 1.00 (0.92 to 1.09) |
| Weeks 23–36 | 1.13 (0.30 to 1.96) | 1.09 (1.01 to 1.18) |
| Percentage of hemodialysis patients with a CVC | ||
| Weeks 3–6 | −1.06 (−2.08 to −0.04) | 0.94 (0.87 to 1.00) |
| Weeks 7–10 | −0.55 (−1.57 to 0.47) | 0.97 (0.91 to 1.04) |
| Weeks 11–14 | 0.84 (−0.25 to 1.92) | 1.07 (0.99 to 1.15) |
| Weeks 15–18 | 3.34 (2.25 to 4.44) | 1.30 (1.20 to 1.41) |
| Weeks 19–22 | 1.05 (−0.04 to 2.14) | 1.08 (1.00 to 1.17) |
| Weeks 23–36 | 0.04 (−1.05 to 1.13) | 1.00 (0.93 to 1.08) |
“Forecast” of 2020 based on the secular trends of 2017–2019 continuing into 2020.
Percentage differences estimated from LPRMs.
ORs estimated from logistic regression models.
Although initiation of both HD and PD declined in the first half of 2020, initiation of PD declined less. Therefore, the percentage of patients who initiated dialysis with PD increased. Specifically, the absolute percentage of patients starting PD was 2.3 (95% CI, 1.5–3.2) points higher in weeks 11–14 and 1.8 (95% CI, 0.9–2.7) points higher in weeks 15–18. This corresponds to adjusted ORs of PD (versus HD) initiation of 1.24 (95% CI, 1.14 to 1.34) and 1.17 (95% CI, 1.07 to 1.27), respectively. The relative increase in PD utilization became less prominent thereafter. The percentage of patients who initiated HD with a catheter briefly increased after the onset of the pandemic. Specifically, in weeks 15–18, this percentage increased by 3.3 (95% CI, 2.3–4.4) points, relative to the forecast of 2020; this corresponds to an adjusted OR of catheter utilization (versus arteriovenous access utilization) of 1.30 (95% CI, 1.20 to 1.41). The percentage of patients initiating HD with a CVC had decreased to prepandemic levels by weeks 23–26.
Point estimates and 95% CIs for data displayed in Figures 2–6 are shown in Supplemental Tables 5–9.
The mean estimated probability of first-year death among incident ESKD patients was 16.2% in 2017-2019, 15.9% in weeks 3-10 of 2020, and 15.5% in weeks 11-26 of 2020 (Supplemental Figure 1).
Discussion
To attempt to quantify the association of the COVID-19 pandemic with changes in documented ESKD incidence and ESKD-related care, we compared outcomes observed in 2020 with those that would have been expected on the basis of 2017–2019 secular trends. We found that documented ESKD incidence decreased by 25% during weeks 15–18, a decrease most pronounced in older patients. The rate of initiation of HD fell relatively more than the rate of initiation of PD. Mean eGFR at dialysis initiation decreased—a finding most pronounced in non-Hispanic Black individuals. Additionally, use of CVCs for HD increased. These alterations in ESKD-related care will likely have profound ongoing implications for patients with kidney disease and the broader healthcare system.
The abrupt decline in documented ESKD incidence is unprecedented: the approximately 2200 persons per week known to reach ESKD during the initial height of the pandemic has not been observed since 2011. There are several possibilities for this historical decrease. The first is that patients with advanced CKD who were approaching the need for RRT died before a diagnosis of ESKD. Potential deaths could have been the result of COVID-19 infection directly or of untreated medical conditions—either ESKD itself or associated comorbidity (e.g., cardiovascular or pulmonary disease). This finding is plausible because we found that individuals with incident ESKD in 2020 had a very slightly lower 1-year probability of death compared with individuals with incident ESKD in earlier years; this suggests that the former may represent a healthy “survivor cohort” from among individuals with advanced (predialysis) CKD. CKD stages 4 and 5 have been identified as leading risk factors for hospitalization in patients with COVID-19 infection,13 lending plausibility to the hypothesis that deaths in patients with advanced CKD due to COVID-19 infection reduced the pool of individuals at risk of developing diagnosed ESKD in the short term. Decreases in nonemergent outpatient clinic encounters, accompanied by reciprocal increases in telephone and video encounters14,15—during which direct physical examination and point-of-service laboratory assessment is not possible—could have led to an increase in pre-ESKD deaths due to unrecognized or untreated declines in health status. If this was indeed the case, documented ESKD incidence will likely return to prepandemic levels, and a compensatory “rebound” in ESKD is unlikely to be forthcoming.16
A second possibility is that diagnosis of ESKD and initiation of maintenance dialysis were delayed or deferred. This could have occurred in several ways. A decline in non–COVID-19–related admissions nationwide17 may have reduced opportunities to diagnose advanced CKD, or to recognize that preexisting CKD was progressing. Furthermore, fewer nonemergent procedures may have reduced rates of AKI among individuals with advanced CKD. Alternatively, the transition to RRT (most commonly dialysis, but also pre-emptive KT) may have been delayed due to a combination of changes in healthcare delivery processes in hospitals, clinical judgment by nephrologists, and individual patient choices, a finding supported by the decline in mean eGFR at dialysis initiation.
In the case of dialysis initiation, adequate preparation may have been compromised, at least initially: placement of PD catheters and permanent HD accesses were likely reduced due to lack of consensus about the “essential” nature of such surgeries early in the pandemic, although by epidemiologic week 13, CMS had clarified that access-related surgeries were considered essential procedures.18 In the case of PD, opportunities for in-person training were likely curtailed due to the need for clinics to practice physical distancing and, possibly, quarantining of dialysis training staff with COVID-19 infections or exposures. In the case of transplantation, odds of a pre-emptive KT were nearly halved during weeks 15–18, a result of transplant programs cancelling or postponing nonemergent surgeries. In contrast to the hypothesis that the decrease in documented ESKD incidence was the result of deaths in patients with advanced CKD before ESKD onset, a delay or deferral in dialysis initiation or pre-emptive KT will likely result in a rebound in ESKD incidence.
The findings we report have critical implications for patients, nephrologists and other providers, public health officials and policy planners, and payers. The first implication concerns the decrease in mean eGFR at dialysis initiation. Although a 2%–3% increase in the percentage of patients initiating dialysis at an eGFR <10 ml/min per 1.73 m2 may appear to be small, the increase occurred rapidly and was similar in magnitude to a shift during the 3 years after publication of the Initiating Dialysis Early and Late study in the middle of 2010.19 Nephrologists may have deliberately chosen to delay dialysis initiation as the pandemic unfolded. Whether this benefited or harmed patients is uncertain. Available evidence suggests initiating HD at a higher eGFR (e.g., ≥10 ml/min per 1.73 m2) is not associated with a survival advantage.19 The pandemic may serve as a sort of “natural experiment” about the implications of initiating dialysis at a lower eGFR, although the observed decline was modest. A lack of discernible adverse outcomes in patients initiating dialysis at a lower eGFR might support broader efforts to defer dialysis initiation when possible. Alternatively, the lower mean eGFR at dialysis initiation may also reflect unrecognized decline as outpatient encounters were disrupted; if so, this would likely result in an increase in emergent dialysis starts, typically with HD (rather than a home-based therapy) and with a CVC. Future study is critical for determining the implications of this decline in mean eGFR at dialysis initiation.
The broader implications of a relative decrease in initiation of HD, compared with PD, must also be carefully considered. Although both new HD and PD starts declined during the pandemic, HD starts decreased more; as a result, odds of initiating PD increased. This relative increase in PD, noted by others,20 does not necessarily indicate that the pandemic was “beneficial” for efforts to increase the use of home-based modalities. Because patients who plan to initiate PD are generally healthier than those who start HD and have higher health literacy, it is possible that patients who initiated PD navigated the transition to dialysis better during the pandemic. In contrast, the emphasis on physical distancing may have cast PD as a therapy preferable to in-center HD among nephrologists and/or a subset of patients undecided between modalities. Whether the pandemic will result in a permanent increase in the proportion of patients with incident ESKD initiating with PD is an important question. In addition, the relative increase in PD has key implications for emerging regulatory and payment models. Using 2020, a period unprecedented in the history of dialysis, to establish benchmarks for home dialysis utilization in 2021 and beyond may be inappropriate and unrealistic. Payment models, including ESRD Treatment Choices and Kidney Care Choices,21 must thoughtfully account for the atypical reasons that underlie the relative increase in use of PD in 2020, because relative PD utilization in incident patients may fall as ESKD incidence returns to normal—or even supranormal—levels as the pandemic continues to unfold.
The incidence of documented ESKD decreased most in the oldest individuals and least in non-Hispanic Black individuals. For example, at its nadir, the relative incidence of documented ESKD in 2020 (versus expected) was 20% lower in individuals aged 45–64 years, compared with what would have been predicted based on historical norms, but was >30% lower in individuals aged ≥75 years. It is possible that the pandemic and its societal consequences—such as social isolation, decreased ability to travel freely, individuals’ concerns over contact with the public, and reduced access to nonemergency care—may have disproportionately affected the decision to initiate dialysis among the oldest patients. In terms of race/ethnicity, the magnitude of the relative reduction in documented ESKD at its nadir was approximately half as great in non-Hispanic Black individuals than in members of other racial and ethnic groups. At the same time, however, the decline in mean eGFR at dialysis initiation was most pronounced in non-Hispanic Black patients. Although a decrease in eGFR at dialysis initiation may not actually be harmful to patients—indeed, it may be beneficial if patients are receiving appropriate predialysis care—this finding could reflect a scenario in which declines in eGFR were relatively more likely to accelerate due to disruptions in medical care or to remain unnoticed or underappreciated among non-Hispanic Black individuals compared with others. Reduced access to in-person care and increased reliance on technology-requiring video encounters may possibly have resulted in a relatively greater number of emergent dialysis starts among non-Hispanic Black patients compared with others, although the precise clinical circumstances surrounding dialysis initiation during the pandemic will require further detailed study before conclusions can be drawn.
This study has several limitations. Outcome data were slightly incomplete, given that epidemiologic week 26 ended on June 27, 2020, and REMIS data used for this study were extracted on September 30, 2020. However, approximately 97% of patients with incident ESKD had Medical Evidence Reports available for analysis. We did not have access to predialysis records (e.g., billing claims), so we cannot characterize the clinical course of patients who progressed to require RRT; the death rate may well have increased in patients with CKD stages 4 and 5, resulting in individuals never having progressed to ESKD. Practice changes caused by the pandemic and its geographic spread were highly variable across the United States; as such, our findings can be considered only broadly representative of clinical events that transpired within the United States. Additionally, societal changes were underway during the period, including changes to the Affordable Care Act, changes in Medicaid eligibility on a state-by-state basis, and the introduction of the Advancing American Kidney Health Initiative; these changes may have affected dialysis preparedness, or the likelihood of use of one dialysis modality over another, in ways we cannot quantify. The growth in home-based dialysis modalities, particularly PD, may well have been particularly accentuated in 2020 had the pandemic not struck, leading to uncertainty about the forecast of PD utilization in 2020. Finally, use of the Medical Evidence Report for documentation of eGFR at dialysis initiation is imperfect, and likely does not account for variation in eGFR at the time of dialysis initiation; this is especially important when dialysis initiation occurs in the setting of AKI, such as AKI due to COVID-19 infection.
In conclusion, we found that documented ESKD incidence decreased substantially during the first wave of the pandemic, especially in older patients. HD initiation fell relatively more than PD. Mean eGFR at dialysis initiation decreased modestly, a finding most pronounced in non-Hispanic Black individuals. Use of CVCs upon HD initiation increased. Whether and how these trends continued to unfold as the pandemic progressed and whether they are associated with longer-term outcomes should be studied.
Disclosures
D.T. Gilbertson reports receiving research funding from Acadia, Amgen, AstraZeneca, DaVita, Genentech, Gilead, Health Resources and Services Administration, Merck, National Institutes of Health (NIH), and OPKO Renal; having a consulting relationship and agreements with Amgen, providing general input in epidemiologic and biostatistical research. K.L. Johansen reports participating in the steering committee of Anemia Studies in Chronic Kidney Disease (ASCEND), a clinical trial program evaluating the efficacy and safety of the prolyl hydroxylase inhibitor daprodustat supported by GlaxoSmithKline. Because K.L. Johansen is an associate editor for JASN, she was not involved in the peer review process for this manuscript. A guest editor oversaw the peer review and decision-making process for this manuscript. E.D. Weinhandl reports having a consulting relationship with Fresenius Medical Care North America, providing input in epidemiologic research about home dialysis; being employed by Fresenius Medical Care North America and NxStage Medical (after the acquisition of NxStage Medical) between March 2016 and April 2020; having consultancy agreements with Fresenius Medical Care North America and Outset Medical; serving on the advisory board of Home Dialyzors United, on the board of directors of the Medical Education Institute, and on the National Quality Forum Scientific Methods Panel. J.B. Wetmore reports receiving research funding from Amgen, AstraZeneca, Bristol Myers Squibb (BMS)/Pfizer, Genentech, Merck, NIH (National Institute of Diabetes and Digestive and Kidney Diseases [NIDDK]), and OPKO Health; receiving honoraria from Aurinia, BMS–Pfizer Alliance (for advisory board activities), and Reata; participating in advisory boards for Aurinia Pharmaceuticals, OPKO Health, and Reata Pharmaceuticals; having consultancy agreements via ad hoc consulting for BMS–Pfizer Alliance; serving as a scientific advisor for, or member of, BMS–Pfizer Alliance; and receiving honoraria for academic continuing medical education from Healio and the Nephrology Self-Assessment Program. All remaining authors have nothing to disclose.
Funding
The data reported here have been supplied by the USRDS, which is funded by the NIDDK through contract N01 75N94019C00006.
Supplementary Material
Acknowledgments
This study was completed by the USRDS Coordinating Center, as part of its contract with the NIDDK. The originally submitted manuscript was approved by NIDDK contracting officer representative, Dr. Kevin Abbott. The NIDDK had no role in study design, data collection, analysis, or interpretation, or writing of the report. The USRDS Coordinating Center is located at the Chronic Disease Research Group, a division of the Hennepin Healthcare Research Institute in Minneapolis, Minnesota.
The interpretation and reporting of these data are the responsibility of the author(s) and in no way should be seen as an official policy or interpretation of the US Government.
D.T. Gilbertson, K.L. Johansen, J. Liu, E.D. Weinhandl, and J.B. Wetmore designed the study; Y. Peng acquired and processed the data; E.D. Weinhandl analyzed the data; E.D. Weinhandl and J.B. Wetmore drafted the article; K.L. Johansen, J. Liu, E.D. Weinhandl, and J.B. Wetmore revised the article; and all authors approved the final version of the article.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
Supplemental Material
This article contains the following supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2021040579/-/DCSupplemental.
Supplemental Table 1. Weekly counts of ESKD incidence during weeks 3–26 of 2017–2020.
Supplemental Table 2. Weekly counts of incident ESKD patients who initiated hemodialysis during weeks 3–26 of 2017–2020.
Supplemental Table 3. Weekly counts of incident ESKD patients who initiated peritoneal dialysis during weeks 3–26 of 2017–2020.
Supplemental Table 4. Weekly counts of incident ESKD patients who received a preemptive kidney transplant during weeks 3–26 of 2017–2020.
Supplemental Table 5. Adjusted, interval-specific incidence rate ratios of ESKD during 2020 versus the forecast of 2020, had the secular trend of 2017–2019 continued.
Supplemental Table 6. Adjusted, interval-specific incidence rate ratios of ESKD during 2020 versus the forecast of 2020, had the secular trend of 2017–2019 continued, in subgroups defined by age and race/ethnicity.
Supplemental Table 7. Adjusted, interval-specific incidence rate ratios of hemodialysis, peritoneal dialysis, and preemptive kidney transplantation during 2020 versus the forecast of 2020, had the secular trend of 2017–2019 continued.
Supplemental Table 8. Adjusted, interval-specific differences in mean eGFR during 2020 versus the forecast of 2020, had the secular trend of 2017–2019 continued.
Supplemental Table 9. Adjusted, interval-specific differences in mean eGFR during 2020 versus the forecast of 2020, had the secular trend of 2017–2019 continued, in subgroups defined by age, race/ethnicity, and comorbidity.
Supplemental Figure 1. Distributions of the estimated probability of death during the first year of ESKD, among adult patients with newly diagnosed ESKD in epidemiologic weeks 3–26 of 2017–2020 and a submission of form CMS-2728.
References
- 1.Hartnett KP, Kite-Powell A, DeVies J, Coletta MA, Boehmer TK, Adjemian J, et al. ; National Syndromic Surveillance Program Community of Practice : Impact of the COVID-19 pandemic on emergency department visits - United States, January 1, 2019-May 30, 2020. MMWR Morb Mortal Wkly Rep 69: 699–704, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shioda K, Weinberger DM, Mori M: Navigating through health care data disrupted by the COVID-19 pandemic. JAMA Intern Med 180: 1569–1570, 2020 [DOI] [PubMed] [Google Scholar]
- 3.Anderson KE, McGinty EE, Presskreischer R, Barry CL: Reports of forgone medical care among US adults during the initial phase of the COVID-19 pandemic. JAMA Netw Open 4: e2034882, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kumar J, Kumar P: COVID-19 pandemic and health-care disruptions: Count the most vulnerable. Lancet Glob Health 9: e722–e723, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Winkelmayer WC, Khairallah P, Charytan DM: Nephrology and COVID-19. JAMA 324: 1137–1138, 2020 [DOI] [PubMed] [Google Scholar]
- 6.Johansen KL, Chertow GM, Foley RN, Gilbertson DT, Herzog CA, Ishani A, et al. : US Renal Data System 2020 Annual Data Report: Epidemiology of kidney disease in the United States. Am J Kidney Dis 77[Suppl 1]: A7–A8, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weinhandl ED, Wetmore JB, Peng Y, Liu J, Gilbertson DT, Johansen KL: Initial effects of COVID-19 on patients with ESKD. J Am Soc Nephrol 32: 1444–1453, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.United States Renal Data System : ESRD quarterly update. 2020. Available at: https://www.usrds.org/esrd-quarterly-update/. Accessed April 18, 2021
- 9.McDonald SP, Marshall MR, Johnson DW, Polkinghorne KR: Relationship between dialysis modality and mortality. J Am Soc Nephrol 20: 155–163, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weinhandl ED, Foley RN, Gilbertson DT, Arneson TJ, Snyder JJ, Collins AJ: Propensity-matched mortality comparison of incident hemodialysis and peritoneal dialysis patients. J Am Soc Nephrol 21: 499–506, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Molnar MZ, Mehrotra R, Duong U, Bunnapradist S, Lukowsky LR, Krishnan M, et al. : Dialysis modality and outcomes in kidney transplant recipients. Clin J Am Soc Nephrol 7: 332–341, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, et al. ; CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) : A new equation to estimate glomerular filtration rate. Ann Intern Med 150: 604–612, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Oetjens MT, Luo JZ, Chang A, Leader JB, Hartzel DN, Moore BS, et al. : Electronic health record analysis identifies kidney disease as the leading risk factor for hospitalization in confirmed COVID-19 patients. PLoS One 15: e0242182, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Koonin LM, Hoots B, Tsang CA, Leroy Z, Farris K, Jolly T, et al. : Trends in the use of telehealth during the emergence of the COVID-19 pandemic - United States, January-March 2020. MMWR Morb Mortal Wkly Rep 69: 1595–1599, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baum A, Kaboli PJ, Schwartz MD: Reduced in-person and increased telehealth outpatient visits during the COVID-19 pandemic. Ann Intern Med 174: 129–131, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nugent J, Aklilu A, Yamamoto Y, Simonov M, Li F, Biswas A, et al. : Assessment of acute kidney injury and longitudinal kidney function after hospital discharge among patients with and without COVID-19. JAMA Netw Open 4: e211095, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Birkmeyer JD, Barnato A, Birkmeyer N, Bessler R, Skinner J: The impact of the COVID-19 pandemic on hospital admissions in the United States. Health Aff (Millwood) 39: 2010–2017, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Neumann ME: CMS prioritizes dialysis access procedures, offers regulatory relief for nephrologists amid COVID-19. Nephrol News Issues, 2020. Available at: https://www.healio.com/news/nephrology/20200327/cms-prioritizes-dialysis-access-procedures-offers-regulatory-relief-for-nephrologists-amid-covid19. Accessed April 2, 2021
- 19.Cooper BA, Branley P, Bulfone L, Collins JF, Craig JC, Fraenkel MB, et al. ; IDEAL Study : A randomized, controlled trial of early versus late initiation of dialysis. N Engl J Med 363: 609–619, 2010 [DOI] [PubMed] [Google Scholar]
- 20.Brown EA, Perl J: Increasing peritoneal dialysis use in response to the COVID-19 pandemic: Will it go viral? J Am Soc Nephrol 31: 1928–1930, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Centers for Medicare and Medicaid Services : Medicare Program; specialty care models to improve quality of care and reduce expenditures. 42 CFR 512. Fed Regist 85: 61114–61381, 2020 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.