Significance Statement
This study demonstrates for the first time a role of ferroptosis in ADPKD. We show the Pkd1 mutation makes renal epithelial cells prone to ferroptosis through the dysregulation of iron and lipid metabolism. It also suggests the main form of regulated cell death in ADPKD kidneys is ferroptotic but not apoptotic, which helps clarify the controversy over the role of apoptosis in ADPKD. In addition, we found that induction of ferroptosis by erastin promotes cyst growth in Pkd1RC/RC mice, whereas inhibition of ferroptosis by Fer-1 delays cyst growth in rapidly and slowly progressive ADPKD mouse models. These observations suggest management of ferroptosis may be a novel strategy for the treatment of this disease.
Keywords: ferroptosis, iron metabolism, lipid peroxidation, 4HNE, cell proliferation
Abstract
Background
Autosomal dominant polycystic kidney disease (ADPKD), the most common inherited kidney disease, is regulated by different forms of cell death, including apoptosis and autophagy. However, the role in ADPKD of ferroptosis, a recently discovered form of cell death mediated by iron and lipid metabolism, remains elusive.
Methods
To determine a pathophysiologic role of ferroptosis in ADPKD, we investigated whether the absence of Pkd1 (encoding polycystin-1) affected the expression of key factors involved in the process of ferroptosis, using Western blot and qRT-PCR analysis in Pkd1 mutant renal cells and tissues. We also examined whether treatment with erastin, a ferroptosis inducer, and ferrostain-1, a ferroptosis inhibitor, affected cyst growth in Pkd1 mutant mouse models.
Results
We found that kidney cells and tissues lacking Pkd1 exhibit extensive metabolic abnormalities, including reduced expression of the system Xc− amino acid antiporter (critical for import of cystine), of iron exporter (ferroportin), and of GPX4 (a key and negative regulator of ferroptosis). The abnormalities also include increased expression of iron importers (TfR1, DMT1) and HO-1, which in turn result in high iron levels, low GSH and GPX4 activity, increased lipid peroxidation, and propensity to ferroptosis. We further found that erastin increased, and ferrostatin-1 inhibited ferroptotic cell death and proliferation of Pkd1-deficient cells in kidneys from Pkd1 mutant mice. A lipid peroxidation product increased in Pkd1-deficient cells, 4HNE, promoted the proliferation of survived Pkd1 mutant cells via activation of Akt, S6, Stat3, and Rb during the ferroptotic process, contributing to cyst growth.
Conclusion
These findings indicate that ferroptosis contributes to ADPKD progression and management of ferroptosis may be a novel strategy for ADPKD treatment.
Autosomal dominant polycystic kidney disease (ADPKD), the most common inherited renal cystic disease, is caused by mutations of PKD1 (encoding polycystin-1) or PKD2 (encoding polycystin-2).1,2 Although there is widespread consensus on the central role of excessive cell proliferation in ADPKD, the contribution of regulated cell death to cyst growth and loss of functional parenchyma has been controversial. Many different forms of regulated cell death have been characterized in recent years. Apoptosis and, more recently, autophagy-dependent cell death, have received attention in studies of ADPKD.3,4 Apoptotic cell death of the cyst-lining and noncystic tubular epithelial cells was identified as a prominent feature of kidneys from patients with ADPKD and autosomal recessive polycystic kidney disease, and from congenital polycystic kidney (cpk) and pcy mice in 1995, and later confirmed in jck mice and PCK rats.5–8 On the basis of these and later studies,9,10 it was proposed that apoptosis was required for cyst cavitation and cyst growth. More recent studies, however, have shown that apoptosis is not prominent in Pkd1 mutant mice and that induction of apoptosis delays cyst growth in Pkd1 and Pkd2 mouse models.11–14 The inability to differentiate different forms of regulated cell death may have contributed to the controversy surrounding the role of apoptosis in ADPKD.
Ferroptosis is a form of regulated cell death that results from accumulation of redox-active iron and iron-dependent lipid peroxidation when GSH-dependent peroxidase 4 (GPX4), a central component of a complex lipid peroxide repair network, is compromised.15 In addition to GPX4, iron regulatory proteins and heme oxygenase-1 (HO-1) regulate ferroptosis.16 Ferroptosis has been found to be associated with pathologic conditions such as neurodegenerative diseases, cancer, ischemia/reperfusion injury, and AKI.17 Its role in ADPKD has not been studied.
In this study, we investigated the role and mechanisms of ferroptosis in ADPKD and addressed the following fundamental questions: (1) Is cell death in Pkd1 mutant kidneys apoptotic or ferroptotic? (2) Do Pkd1 mutations affect iron metabolism in renal epithelial cells and, if so, how? (3) Does iron-dependent lipid peroxidation promote epithelial cell proliferation in Pkd1 mutant kidneys, and if so, how? And (4) does inhibition of ferroptosis delay disease progression in Pkd1 mutant kidneys? Our findings provide new insights into the role and mechanisms of ferroptosis in ADPKD and highlight a potential therapeutic strategy for its treatment.
Methods
Cell Culture and Reagents
Pkd1 wild-type and Pkd1-null MEK cells, derived from the collecting ducts of kidneys of wild-type and Pkd1-null mice and sorted by the collecting duct marker dolichos biflorus agglutinin, were maintained as previously described. PH2 and PN24 cells (provided by S. Somlo through the George M. O’Brien Kidney Center, Yale University, New Haven, Connecticut, USA) were cultured as described previously.18 Erastin and ferrostain-1 (Fer-1) were purchased from Cayman Chemical and each was dissolved in DMSO (Sigma-Aldrich) at a stock solution of 10 mg/ml. The 4-hydroxynonenal (4-HNE) was purchased from Cayman Chemical and dissolved in ethanol (Sigma-Aldrich) at a stock solution of 5 mM. L-carnosine was purchased from Cayman Chemical and dissolved in double-distilled H20 at a stock solution of 5 mM.
The antibodies used for Western blot analysis included anti-GPX4 (12056), purchased from Abcam, anti-S6 (2217), anti-AKT (9272), anti-caspase-3 (9662), and anti-activation transcription factor 3 (ATF3) (2032), purchased from Cell Signaling Technology, and the phosphorylated antibodies for STAT3-Y705 (9131), S6-S235/236 (2211), AKT-S473 (9271), and Rb-S780 (9307), also purchased from Cell Signaling Technology. Anti-STAT3 (sc-482) was purchased from Santa Cruz Biotechnology Inc. Anti-4HNE (3249) was purchased from R&D Systems. Anti-actin antibody (A5316) was purchased from Sigma-Aldrich. The secondary antibodies, including donkey anti-rabbit IgG–horseradish peroxidase (sc-2313), and goat anti-mouse IgG–horseradish peroxidase (sc-2005) were purchased from Santa Cruz Biotechnology Inc.
Mouse Strains and Treatments
Pkd1RC/RC mice with a 129S6/SvEvTac (129) background, which developed stable and severe cystic disease compared with Pkd1RC/RC mice with a 57BL/6J (B6) and ALB/cJ (BC) background,19 were used to test the effect of erastin and Fer-1 on cyst progression. Pkd1RC/RC received daily intraperitoneal injections of erastin or Fer-1 (20 mg/kg and 4 mg/kg respectively, dissolved in DMSO, with a final DMSO concentration of 10% [v/v] in PBS) from 1 to 3 months. We collected blood and kidney samples 24 hours after the last dose from 3-month-old erastin-treated (n=8), Fer-1–treated (n=8), and vehicle-treated (n=8) mice for histopathologic and biochemical analysis.
Pkd1foxl/flox:Pkhd1-Cre mice received daily intraperitoneal injections of Fer-1 (4 mg/kg, dissolved in DMSO, with a final DMSO concentration of 10% [v/v] in PBS) from postnatal day 7 (PN7) to PN20, and kidneys from five male and five female mice per group were harvested and analyzed at PN21. We collected blood samples 24 hours after the last dose and harvested kidneys from Fer-1– and vehicle-treated mice for histopathologic and biochemical analysis. Pkd2−/− mouse kidneys were provided by the tissue bank of the Mayo Clinic Translational PKD Center.
Normal Kidneys and Kidneys from Patients with ADPKD
Human kidney samples were provided by the tissue bank of the Mayo Clinic Translational PKD Center. The protocol for the collection of human kidneys has been approved by the Institutional Review Board.
Western Blot Analysis
Cell pellets were collected and resuspended in lysis buffer (20 mM Tris-HCl, pH 7.4, 150 mM NaCl, 10% glycerol, 1% Triton X-100, 1 mM Na3VO4, 25 mM β-glycerolphosphate, 0.1 mM PMSF, Roche complete protease inhibitor set, and Sigma-Aldrich phosphatase inhibitor set). The resuspended cell pellet was vortexed for 20 seconds then incubated on ice for 30 minutes and centrifuged at 20,000 g for 30 minutes. The supernatants were collected for Western blot analysis.
Quantitative RT-PCR
Total RNA was extracted using the RNeasy Plus Mini Kit (QIAGEN). Total RNA (1 μg) was used for RT reactions in a 20 μl reaction to synthesize cDNA using an iScript cDNA Synthesis Kit (Bio-Rad). RNA expression profiles were analyzed by real-time PCR using iTaq SYBR Green Supermix with ROX (Bio-Rad) in an iCycler iQ Real-Time PCR Detection System. The complete reactions were subjected to the following program of thermal cycling: 40 cycles of 10 seconds at 95°C and 20 seconds at 60°C. A melting curve was run after the PCR cycles, followed by a cooling step. Each sample was run in triplicate in each experiment, and each experiment was repeated three times. Expression levels of target genes were normalized to the expression level of actin. All primers used are listed in Supplemental Table 1.
BODIPY Staining
Intracelluar accumulation of lipid peroxidation was determined by BODIPY 581/591 11C (Thermo Fisher Scientific) staining coupled with flow cytometry, as previously described.20
Intracellular Iron Level Assay
Intracellular total iron content was determined using an iron assay kit from Sigma-Aldrich (Cas MAK025) and analyzed according to the manufacturer’s protocol. In brief, cells were seeded in six-well plates and treated with the indicated compounds for the duration specified in the text. Cells were collected and rapidly homogenized in iron assay buffer and centrifuged at 16,000 g for 10 minutes at 4°C. An iron reducer was added to cell lysates to reduce ferric iron (Fe3+) to ferrous iron (Fe2+) and incubated for 30 minutes at room temperature in the dark. The iron reaction produced a colored complex and absorbance was measured at 593 nm.
Measurement of Tissue and Cellular GSH Content
Intracellular GSH content was determined using an GSH colorimetric detection kit from Invitrogen (Cas ELAGSHC). For examining the levels of cellular GSH, cells were seeded in six-well plates and treated with the indicated compounds for the duration specified in the text. Cells were then trypsinized and GSH levels were analyzed according to the manufacturer’s protocol. In brief, cell pellets were resuspended in ice-cold 5% aqueous 5-sulfo-salicylic acid dihydrate (Sigma-Aldrich S2130) at 1–40×106 cells/ml and vortexed vigorously to lyse cells. For examining the levels of GSH in kidney tissues, tissues were weighed and homogenized. Each of 10 mg sample in 250 µl cold 5% aqueous 5-sulfo-salicylic acid dihydrate was analyzed to determine GSH levels according to the manufacturer’s protocol. Absorbance was assessed at 405 nm.
MTT Assays
To measure cell death and IC50, we used an MTT-based kit (Sigma-Aldrich), according to the manufacturer’s instructions.
Measurement of Cyst Area
The cyst volume was quantified in whole kidney after hematoxylin and eosin staining using Image-Pro Plus v5 software (Media Cybernetics). The cyst area was calculated as (cyst area/total area)×100%. Three sections from both kidneys were analyzed for each mouse.
Quantitative BUN Determination
Serum samples were first diluted five-fold in distilled water before assay. Next, 5 μl water (blank), 5 μl standard (50 mg/dl), and 5 μl samples were transferred in triplicate into wells of a clear-bottom 96-well plate. We added 200 μl of working reagent, tapping lightly to mix, and incubated the samples for 20 minutes at room temperature. OD was read at 520 nm.
Measurement of Serum Nonheme Iron
Blood samples were collected and centrifuged for 10 minutes at 3000 rpm to obtain serum. Serum nonheme iron was measured using the Iron/TIBC Reagent Set (I7506–60; Pointe Scientific) in accordance with the manufacturer’s instructions.21
Measurement of Tissue Nonheme Iron
Tissue nonheme iron levels were measured using the chromogen method.22 Tissues were weighed and digested in nonheme iron (NHI) acid (10% TCA in 3 M HCl) for 48 hours at 67°C. Equal volumes of sample or iron standard (500 µg/dl) were incubated for 10 minutes at room temperature in 200 µl bathophenanthroline (BAT) buffer (0.2% thioglycolic acid and 0.02% disodium-4,7-diphenyl-1,10-phenanthroline disulfonate in 50% saturated NaAc solution). Samples were read at 535 nm, and unknowns were calculated using a standard curve. The results are presented in micrograms of iron per gram of wet tissue.
Heme Measurement
Serum heme levels were measured using the QuantiChrom Heme Assay Kit (DIHM-250, BioAssay Systems) in accordance with the manufacturer’s instructions.23 Samples were read at 400 nm, and unknowns were calculated by comparing the value with a standard curve of known heme concentrations.
Histology and Immunofluorescence Staining
Paraffin-embedded sections (4 μm) were subjected to hematoxylin and eosin staining and immunofluorescence staining. Kidney sections were counterstained by hematoxylin. For cleaved caspase-3 staining, a rabbit anti-cleaved caspase-3 antibody (9661; Cell Signaling Technology) and Alexa Fluor 488 anti-rabbit IgG secondary antibody were used. For Ki67 staining, a rabbit anti-Ki67 antibody (ab15580; Abcam) and Alexa Fluor 488 anti-rabbit IgG secondary antibody were used. For 4HNE staining, a mouse anti-4HNE antibody (3249; R&D Systems) and Alexa Fluor 555 anti-mouse IgG secondary antibody were used. Images were analyzed using a Nikon Eclipse 80i microscope.
Terminal Deoxynucleotidyl Transferase-Mediated dUTP Nick End-Labeling Assay
We performed the terminal deoxynucleotidyl transferase-mediated dUTP nick end-labeling (TUNEL) assay for erastin-treated and Fer-1 treated kidneys according to the manufacturer’s protocols (In Situ Death Detection Kit; Roche). ProLong Gold Antifade reagent with DAPI (Invitrogen) was used. Immunofluorescence images were obtained with a Nikon Eclipse 80i microscope.
RNA Interference
The RNA oligonucleotides that specifically targeted mouse ATF3 were purchased from Santa Cruz Biotechnology Inc, and were transfected with Dharma-FECT siRNA transfection reagent (Dharmacon). After siRNA transfection for 24 hours, cells were harvested and analyzed by Western blotting and quantitative RT-PCR (qRT-PCR).
Chromatin Immunoprecipitation Assay
The chromatin immunoprecipitation assay was performed according to the described protocol.12 Chromatin DNA was subjected to immunoprecipitation with anti-ATF3 or normal rabbit IgG, and then washed, after which the DNA-protein crosslinks were reversed. The recovered DNA was analyzed by PCR for the binding of ATF3 at the mouse GPX4 promoter.
Cell Cycle Analysis
A total of 150,000 cells were seeded in six-well plates. After the cells were allowed to recover for 16–24 hours, they were starved with serum-free medium for 24 hours. The cells were then left untreated or treated with 4HNE for 24 hours. To pellet and wash the cells, 1 ml PBS was added, the cells were centrifuged at 500 g for 5 minutes, and the supernatant was aspirated. To suspend the cells, 1 ml of cold 70% ethanol was added drop by drop into the cell pellet. The cells were then incubated at −20°C overnight. After that, the cells were washed twice in 1× PBS to remove the ethanol, then centrifuged for 5 minutes at 1000 g, and the supernatant was aspirated. For propidium iodide staining, the cells were suspended in 0.5 ml propidium iodide buffer (containing RNase and Triton X-100) for 10–15 minutes at room temperature. The cells were kept away from light at 4°C before further analysis on the flow cytometer, which took place within 1 hour. FACS data were analyzed with FlowJo software 10.7.
Three-Dimensional Spheroid Model for Mouse IMCD3 Cells
Mouse IMCD3 cells were seeded in Matrigel.24 In brief, mouse IMCD3 cells were treated with trypsin for 3–5 minutes and resuspended in prewarmed (37°C) medium in a concentration of 1,00,000 cells/ml. Next, 100 μl of cell suspension was gently mixed with 100 μl of growth factor–depleted Matrigel (BD Biosciences) in a 1.5 ml microcentrifuge tube. Then the cell-Matrigel mixture was transferred to each well of a 24-well plate. After polymerization for 30 minutes at 37°C, warmed complete culture medium was dripped over the matrix until the well was just covered. The cells were cultured at 37°C with 5% CO2. In general, the cells formed spheroids with cleared lumens in 2–3 days. Erastin and L-carnosine were added to the medium on day 1, and the medium was changed every 24 hours. Ten random visual fields per well were photographed with a Nikon Eclipse 80i microscope. Cyst diameters of all captured cysts were measured with ImageJ V. 1.48 (US National Institutes of Health, Bethesda, MD; http://imagej.nih.gov/ij/).
Statistics
All data are presented as mean±SEM. All statistical analyses were performed using SPSS Statistics 22 software. P values were calculated by two-tailed unpaired Student’s t test, and a P value <0.05 was considered significant.
Results
The Expression of Ferroptosis-Related Genes is Dysregulated in Pkd1 Mutant Renal Epithelial Cells and Tissues
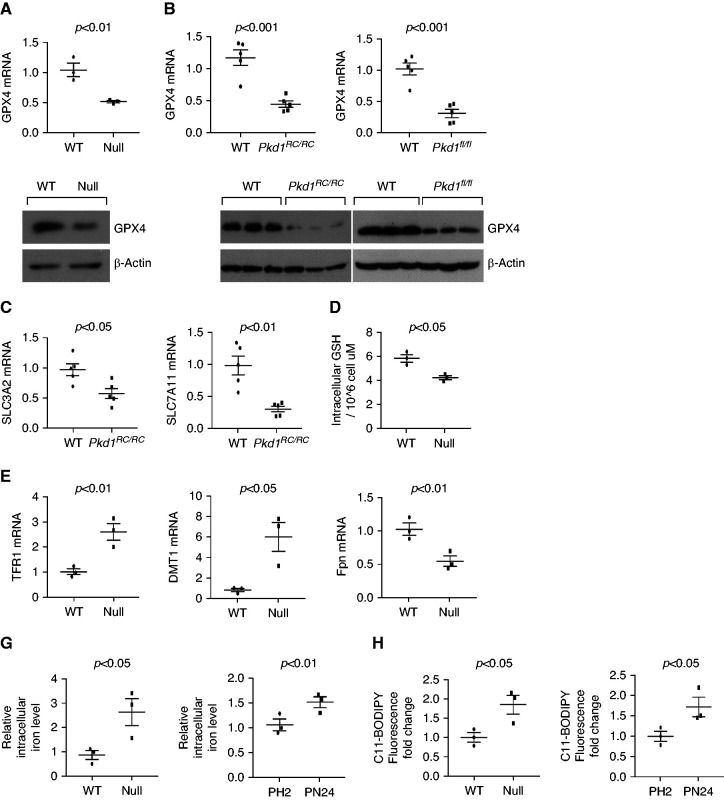
To understand a role of ferroptosis in ADPKD, we first found that the expression of GPX4, a key and negative regulator of ferroptotic cell death,25 was decreased in Pkd1−/− MEK cells (Figure 1A) and in kidneys from Pkd1RC/RC mice and Pkd1flox/flox:Pkhd1-Cre mice (Figure 1B) compared with wild-type MEK cells and with wild-type age-matched kidneys, as examined with qRT-PCR and Western blot analysis. Similarly, qRT-PCR analysis showed that expression of SLC7A11 and of SLC3A2 was lower in kidneys of Pkd1RC/RC mice compared with wild-type age-matched kidneys (Figure 1C), which was associated with lower kidney and cellular levels of GSH, essential for GPX4 enzyme activity (Figure 1D and Supplemental Figure 1A). In addition, qRT-PCR analysis showed that expression of Tfrc (encoding transferrin receptor 1, or TfR1) and of Dmt1 (encoding divalent metal transporter 1, or DMT1), involved in iron import, was upregulated, whereas expression of Fpn (encoding ferroportin), the only known iron exporter,16 was downregulated in Pkd1−/− renal epithelial cells (Figure 1E, Supplemental Figure 1B) and Pkd1RC/RC kidneys (Figure 1F) compared with controls. Dysregulation of GPX4, SLC7A11/SLC3A2, and iron transport genes (Tfrc, Dmt1 and Fpn) was also observed in human ADPKD kidneys (Supplemental Figure 1, C–E) and Pkd2−/− kidneys (Supplemental Figure 2) compared with controls. Importantly, dysregulation of iron transport genes was associated with higher iron levels in Pkd1−/− renal epithelial cells (Figure 1G). Furthermore, lipid peroxidation was increased in Pkd1−/− MEK (Figure 1H, left panel) and PN24 cells (Figure 1H, right panel) compared with wild-type MEK cells and Pkd1+/− PH2 cells, as examined by 11C-BODIPY staining and quantified by flow cytometry analysis. Collectively, these results suggest that Pkd1 null renal epithelial cells are likely to have an increased propensity to ferroptosis.
Figure 1.
The expression of ferroptosis related genes was dysregulated in Pkd1 mutant renal epithelial cells and tissues. (A) qRT-PCR and Western blot analysis of GPX4 levels in Pkd1 wild-type (WT) and Pkd1−/− MEK cells. n=3 independent experiments. (B) qRT-PCR and Western blot analysis of GPX4 levels in kidneys collected from 3-month-old WT (n=5) and Pkd1RC/RC mice (n=5) and in kidneys collected from 21 days old WT (n=5) and Pkd1flox/flox:Pkhd1-Cre mice (n=5). (C) qRT-PCR analysis of SLC3A2 and SLC7A11 mRNA levels in kidneys collected from 3-month-old WT (n=5) and Pkd1RC/RC mice (n=5). (D) Intracellular GSH levels were measured in Pkd1 WT and Pkd1−/− MEK cells using a colorimetric assay kit (Methods). (E and F) qRT-PCR analysis of TFR1, DMT1, and Fpn mRNA levels in Pkd1 WT and null MEK cells (E) and kidneys collected from 3-month-old WT (n=5) and Pkd1RC/RC mice (n=5) (F). (G) Total intracellular iron levels were measured in Pkd1 WT and null MEK cells (left panel) and PH2 and PN24 cells (right panel) by an iron assay kit (Methods). n=3 independent experiments. (H) Lipid peroxidation was measured with 11C-BODIPY staining in Pkd1 WT and null MEK cells (left panel) and PH2 and PN24 cells (right panel) and was quantified with flow cytometry analysis. n=3 independent experiments. Statistical data are presented as the mean±SEM.
Depletion of Pkd1 Increases the Sensitivity of Cystic Renal Epithelial Cells to Erastin, a Ferroptosis Inducer
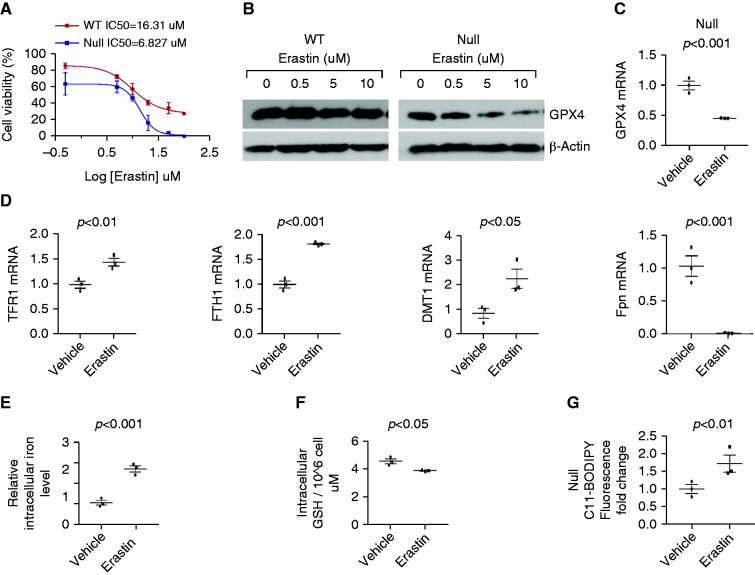
To ascertain whether Pkd1 mutant renal epithelial cells are more prone to ferroptosis, we treated wild-type and Pkd1−/− MEK cells and Pkd1+/− PH2 cells and Pkd1−/− PN24 cells with the ferroptosis inducer erastin. Pkd1−/− MEK cells and PN24 cells were more sensitive to erastin treatment compared with wild-type MEK and Pkd1+/− PH2 cells; the half-maximal effective concentration (EC50) of erastin was 6.827 μM for Pkd1−/− MEK cells compared with 16.31 μM for Pkd1+/+ MEK cells (Figure 2A), and 8.56 μM for PN24 cells compared with 32.86 μM for Pkd1+/− PH2 cells (Supplemental Figure 3A). Treatment with erastin strikingly decreased the expression of GPX4 protein and mRNA in Pkd1−/− MEK and PN24 cells in a dose-dependent manner, although having only a slight effect in Pkd1+/+ MEK cells (Figure 2, B and C) and Pkd1+/− PH2 cells (Supplemental Figure 3, B and C). It also increased the expression of TfR1, ferritin heavy chain 1 (Fth1), and Dmt1, but decreased the expression of Fpn in Pkd1−/− MEK (Figure 2D) and PN24 cells (Supplemental Figure 3D) compared with controls, leading to higher intracellular iron levels in Pkd1−/− MEK (Figure 2E) and PN24 cells (Supplemental Figure 3E). In addition, treatment with erastin decreased the cellular levels of GSH (Figure 2F, Supplemental Figure 3F) in Pkd1 mutant renal epithelial cells compared with control cells; erastin treatment also increased lipid peroxidation in Pkd1−/− MEK (Figure 2G) and PN24 cells (Supplemental Figure 3G) compared with controls, as examined with 11C-BODIPY staining and quantified with flow cytometry analysis. Collectively, these results strongly support that depletion of Pkd1 increases the propensity of renal epithelial cells to erastin induced ferroptosis.
Figure 2.
Depletion of Pkd1 increases the sensitivity of Pkd1 mutant renal epithelial cells to erastin. (A) The IC50 of erastin in Pkd1 WT and Pkd1 null MEK cells. n=3 independent experiments. (B) Western blot analysis of GPX4 expression in Pkd1 WT and Pkd1 null MEK cells treated with different concentrations of erastin for 24 hours. (C) qRT-PCR analysis of GPX4 mRNA levels in Pkd1 null MEK cells treated with erastin (10 μM) and vehicle for 24 hours. n=3 independent experiments. (D) qRT-PCR analysis of mRNA levels of TFR1, FTH1, DMT1, and Fpn in Pkd1 null MEK cells treated with erastin (10 μM) and vehicle for 24 hours. n=3 independent experiments. (E) Intracellular iron levels were measured by an iron assay kit in Pkd1 null MEK cells treated with erastin (10 μM) and vehicle for 24 hours. n=3 independent experiments. (F) Intracellular GSH levels were measured by a colorimetric assay kit in Pkd1 null MEK cells treated with erastin (10 μM) and vehicle for 24 hours. n=3 independent experiments. (G) Lipid peroxidation were measured with 11C-BODIPY staining in Pkd1 null MEK cells treated with erastin (10 μM) and vehicle and was quantified with flow cytometry in those cells. n=3 independent experiments. Statistical data are presented as the mean±SEM.
Treatment with Erastin Induces Death of Epithelial Cells Lining Renal Cysts and Promotes Cyst Growth in Pkd1RC/RC Mouse Kidneys
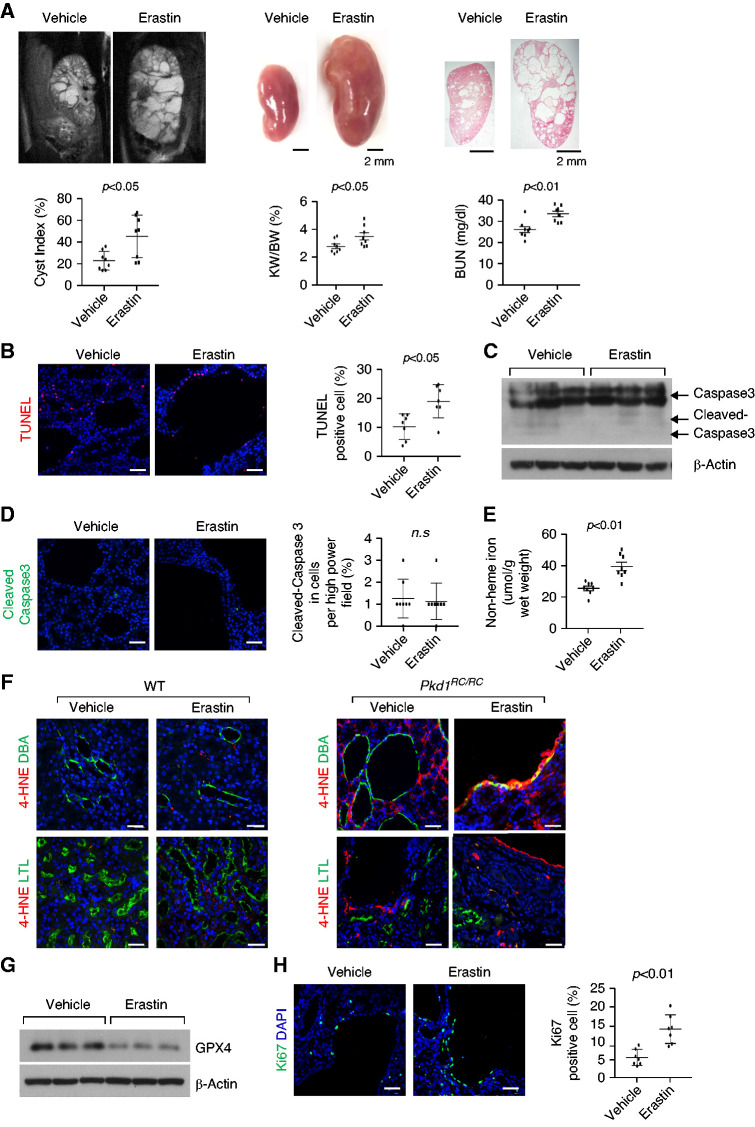
To investigate the role of ferroptosis in vivo, we treated Pkd1RC/RC mice with erastin (20 mg/kg) by intraperitoneal injection. The treatment with erastin promoted disease progression (Figure 3A), reflected by higher cyst index, kidney weight/body weight (KW/BW) ratios and BUN levels compared with controls treated with vehicle only (n=8 in each group). The treatment with erastin also increased death of cyst-lining epithelial cells, measured by the TUNEL assay (Figure 3B). To distinguish between apoptotic and ferroptotic cell death, we examined the levels of caspase-3 and cleaved caspase-3, an apoptotic marker,26 and the levels of tissue nonheme iron, GSH and GPX4, and lipid peroxidation. The levels of caspase-3 and cleaved caspase-3 examined by Western blot and immunostaining (Figure 3, C and D) were not affected, whereas nonheme iron (Figure 3E) and lipid peroxidation examined with 4HNE staining (Figure 3F) were higher and GSH (Supplemental Figure 4A) and GPX4 protein levels were lower (Figure 3G) in the kidneys of Pkd1RC/RC mice treated with erastin compared with controls. These results strongly suggest that treatment with erastin induced ferroptotic cell death but not apoptotic cell death in Pkd1RC/RC kidneys. The treatment with erastin also increased proliferation of cyst-lining epithelial cells, measured by Ki67 staining (Figure 3H), and the phosphorylation and activation of Akt, S6, Stat3, and Rb in Pkd1RC/RC kidneys (Supplemental Figure 4B). The treatment with erastin did not induce cyst formation in Pkd1RC/+ mice (Supplemental Figure 5), suggesting that erastin-induced ferroptosis was able to promote cystogenesis when the level of polycystin-1 expression was reduced below a critical threshold.
Figure 3.
Treatment with erastin induces cyst-lining epithelial cell death and promotes cyst growth in Pkd1RC/RC mice kidneys. (A) Representative images of kidneys from Pkd1RC/RC mice treated with erastin or vehicle from 1 to 3 months and percent cystic area relative to total kidney section area was significantly increased in kidneys from 3 months old Pkd1RC/RC mice treated with erastin versus vehicle. Data reflect all sections quantified for each condition (n=8 in erastin-treated group and n=8 in vehicle-treated group). Scale bars, 2 mm. Treatment with erastin increased KW/BW ratios and BUN levels in Pkd1RC/RC mice compared with those in vehicle-treated control mice. (B) Erastin treatment induced cyst-lining epithelial cell death in kidneys from Pkd1RC/RC mice as detected by TUNEL assay. Scale bars, 50 μm. (C) The expression of cleaved caspase-3 in kidneys from Pkd1RC/RC mice treated with erastin or vehicle analyzed by Western blot analysis. (D) Representative images of immunostaining with cleaved caspase-3 antibody in kidney sections from 3-month-old WT and Pkd1RC/RC mice treated with erastin or vehicle. (E) The levels of nonheme irons in kidneys from Pkd1RC/RC mice treated with erastin (n=8) or vehicle (n=8). (F) Representative images of immunostaining with 4HNE antibody in kidney sections from 3-month- old WT and Pkd1RC/RC mice treated with erastin or vehicle. (G) Western blot analysis of GPX4 expression in kidneys from Pkd1RC/RC mice treated with erastin or vehicle. (H) Representative images of Ki67 staining indicated that cell proliferation was increased in Pkd1RC/RC kidneys treated with erastin versus that in Pkd1RC/RC kidneys treated with vehicle. Scale bars, 50 μm. Statistical data are presented as the mean±SEM.
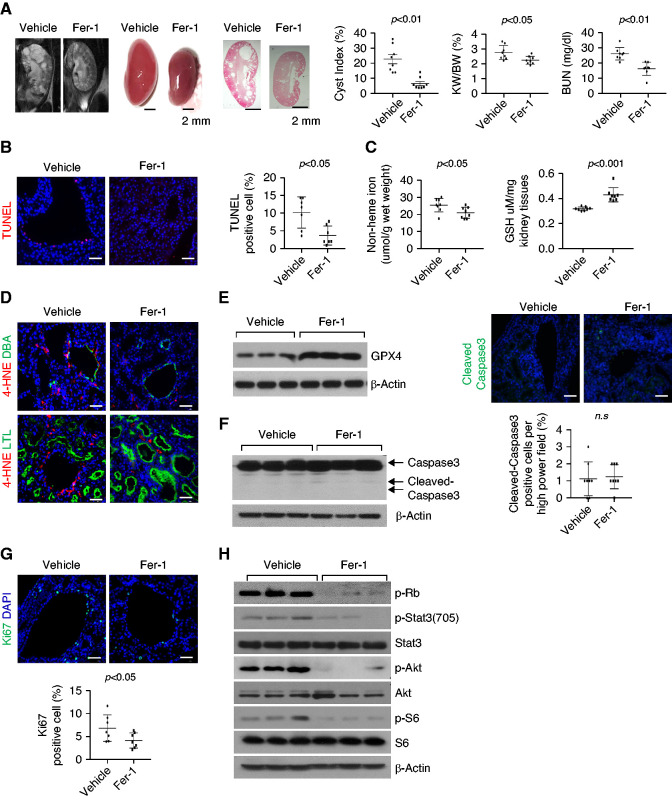
Treatment with Fer-1, a Ferroptosis Inhibitor, Delays Cyst Growth in Pkd1 Mutant Mouse Models
To test whether the inhibition of ferroptosis was able to inhibit cyst growth in vivo, we first treated Pkd1flox/flox:Pkhd1-Cre mice, a rapidly progressive disease model, with ferroptosis inhibitor, Fer-1. The administration of Fer-1 (4 mg/kg) delayed disease progression as reflected by lower cyst index, KW/BW ratios, and BUN levels (Figure 4A) compared with vehicle-treated controls (n=5 in each group). The kidneys of the Fer-1–treated mice compared with the vehicle-treated controls had lower levels of cyst-lining epithelial cell death, measured by the TUNEL assay (Figure 4B), and of nonheme iron and lipid peroxidation, higher levels GSH and GPX4 protein (Figure 4, C–E), and similar levels of caspase-3 and cleaved caspase-3 (Figure 4F). The treatment with Fer-1 also decreased proliferation of cyst-lining epithelial cells, measured by Ki67 staining (Figure 4G) and the phosphorylation of Akt, S6, Stat3, and Rb, measured by Western blot analysis (Figure 4H).
Figure 4.
Treatment with Fer-1 delays cyst growth in Pkd1flox/flox:Pkhd1-Cre mouse kidneys. (A) Representative images of kidneys from Pkd1flox/flox:Pkhd1-Cre mice treated with Fer-1 or vehicle from PN7 to PN21 and percent cystic area relative to total kidney section area was significantly decreased in kidneys from PN21 old Pkd1flox/flox:Pkhd1-Cre mice treated with Fer-1 compared with that in kidneys from aged matched mice treated with vehicle. Data reflect all sections quantified for each condition (n=5 in Fer-1-treated group and n=5 in vehicle-treated group). Scale bars, 2 mm. Treatment with Fer-1 decreased KW/BW ratios and BUN levels in Pkd1flox/flox:Pkhd1-Cre mice compared with those in vehicle-treated control mice. (B) Fer-1 treatment decreased cyst-lining epithelial cell death in kidneys from Pkd1flox/flox:Pkhd1-Cre mice as detected by TUNEL assay. Scale bars, 50 μm. (C) The levels of nonheme irons (left panel) and GSH (right panel) in kidneys from Pkd1flox/flox:Pkhd1-Cre mice treated with Fer-1 (n=5) or vehicle (n=5). (D) Representative images of immunostaining with 4HNE antibody in kidney sections from Pkd1flox/flox:Pkhd1-Cre mice treated with Fer-1 and vehicle. Scale bars, 50 μm. (E) Western blot analysis of GPX4 expression in kidneys from Pkd1flox/flox:Pkhd1-Cre mice treated with Fer-1 or vehicle. (F) The expression of cleaved caspase-3 in kidneys from Pkd1flox/flox:Pkhd1-Cre mice treated with Fer-1 or vehicle analyzed by Western blot analysis and immunostaining. Scale bars, 50 μm. (G) Representative images of Ki67 staining indicated that cell proliferation was decreased in Pkd1flox/flox:Pkhd1-Cre kidneys treated with Fer-1 compared with that in Pkd1flox/flox:Pkhd1-Cre kidneys treated with vehicle. Scale bars, 50 μm. The percentage of Ki67-positive nuclei in cyst-lining epithelial cells was quantified from an average of 1000 nuclei per mouse kidney section. (H) Western blot analysis of the expression and phosphorylation of Rb, STAT3, Akt, and S6 in kidneys from Pkd1flox/flox:Pkhd1-Cre mice treated with Fer-1 or vehicle. Statistical data are presented as the mean±SEM.
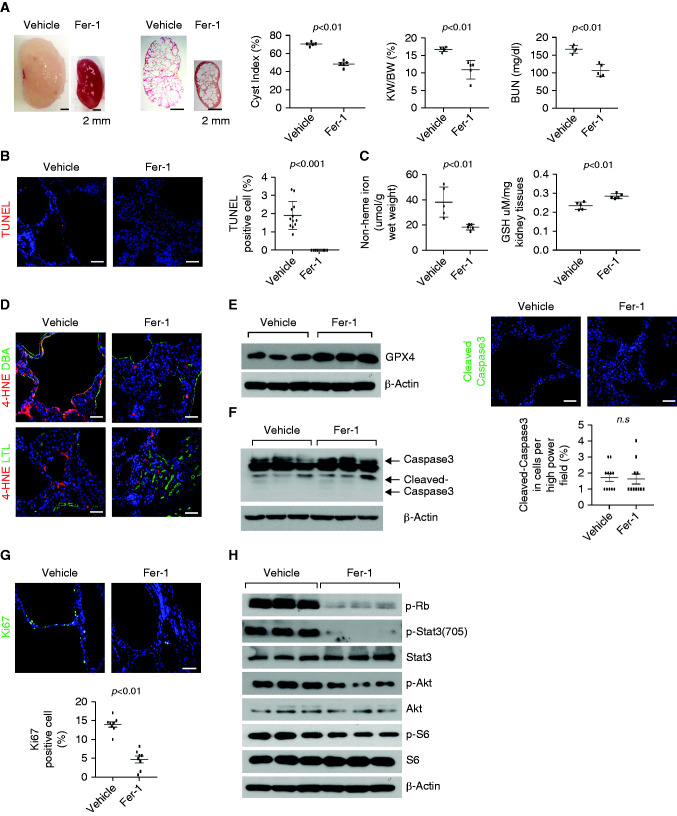
To determine whether Fer-1 would also be effective in a slowly progressive model of ADPKD that resembles more closely the clinical course of ADPKD, we treated Pkd1RC/RC mice. The treatment with Fer-1 significantly delayed disease progression as reflected by the lower cyst index, KW/BW ratios, and BUN levels, compared with vehicle-treated controls (n=8 in each group) (Figure 5A). The kidneys of the Fer-1–treated mice had lower levels of death of cyst-lining epithelial cells, measured by the TUNEL assay (Figure 5B), nonheme iron (Figure 5C, left panel), and lipid peroxidation (Figure 5D), higher levels of GSH content (Figure 5C, right panel) and GPX4 protein (Figure 5E), and similar levels of caspase-3 and cleaved caspase-3 compared with the vehicle-treated controls (Figure 5F). The treatment with Fer-1 also decreased proliferation of cyst-lining epithelial cells, measured by Ki67 staining (Figure 5G), and the phosphorylation of Akt, S6, Stat3, and Rb, measured by Western blot analysis (Figure 5H). These results indicate that treatment with Fer-1 was able to inhibit ferroptosis and delay progression in rapidly progressive and milder models of ADPKD.
Figure 5.
Treatment with Fer-1 delays cyst growth in Pkd1RC/RC mouse kidneys. (A) Representative images of kidneys from Pkd1RC/RC mice treated with Fer-1 or vehicle from 1 to 3 months and percent cystic area relative to total kidney section area was significantly decreased in kidneys from 3-month-old Pkd1RC/RC mice treated with Fer-1 compared with that in kidneys from aged-matched mice treated with vehicle. Data reflect all sections quantified for each condition (n=8 in Fer-1–treated group and n=8 in vehicle-treated group). Scale bars, 2 mm. Treatment with Fer-1 decreased KW/BW ratios and BUN levels in Pkd1RC/RC mice compared with those in vehicle-treated control mice. (B) Fer-1 treatment decreased cyst-lining epithelial cell death in kidneys from Pkd1RC/RC mice as detected by TUNEL assay. Scale bars, 50 μm. (C) The levels of nonheme irons (left panel) and GSH content (right panel) in kidneys from Pkd1RC/RC mice treated with Fer-1 (n=8) or vehicle (n=8). (D) Representative images of immunostaining with 4HNE antibody in kidney sections from Pkd1RC/RC mice treated with Fer-1 and vehicle. Scale bars, 50 μm. (E) Western blot analysis of GPX4 expression in kidneys from Pkd1RC/RC mice treated with Fer-1 or vehicle. (F) The expression of cleaved caspase-3 in kidneys from Pkd1RC/RC mice treated with Fer-1 or vehicle analyzed by Western blot analysis and immunostaining. (G) Representative images of Ki67 staining indicated that cell proliferation was decreased in Pkd1RC/RC kidneys treated with Fer-1 compared with that in kidneys from Pkd1RC/RC mice treated with vehicle. Scale bars, 50 μm. The percentage of Ki67-positive nuclei in cyst-lining epithelial cells was quantified from an average of 1000 nuclei per mouse kidney section. (H) Western blot analysis of the expression and phosphorylation of Rb, STAT3, Akt, and S6 in kidneys from Pkd1RC/RC mice treated with Fer-1 or vehicle. Statistical data are presented as the mean±SEM.
Treatment with Erastin or Fer-1 Results in the Alteration of Mitochondrial Morphology in Pkd1 Mutant Kidneys
Emerging evidence indicates that ferroptotic cells exhibit a reduction or vanishing of mitochondrial cristae and ruptures of the outer mitochondrial membrane,27,28 which can be used to distinguish ferroptotic cells from other forms of cell death. Using transmission electron microscopy, we observed decreased cristae and outer membrane ruptures consistent with ferroptosis in kidneys of Pkd1RC/RC mice, but not in wild-type kidneys (Supplemental Figure 6). Treatment with erastin resulted in a more severe mitochondrial shrinking or vanishing of cristae and mitochondrial outer membrane ruptures, consistent with greater ferroptotic cell death (Supplemental Figure 6). Conversely, treatment with Fer-1 resulted in the normalization of mitochondrial cristae and mitochondrial outer membranes in cyst-lining epithelial cells of Pkd1RC/RC mice (Supplemental Figure 6). These results, together with similar levels of caspase-3 and cleaved caspase-3 and dysregulation of GPX4 and iron levels, further support that the cell death observed in kidneys of Pkd1RC/RC mice represents ferroptotic cell death.
Renal and Systemic Iron Metabolism Mediated by Multiple Iron Regulatory Proteins and HO-1 Plays a Key Role in Triggering Ferroptosis in Pkd1 Mutant Kidneys
Because of the central role of iron metabolism in ferroptosis,29,30 we found that treatment with erastin further increased the expression of TfR1, Fth1, and Dmt1 but decreased the expression of Fpn in Pkd1 mutant kidneys (Supplemental Figure 7A). In contrast, treatment with Fer-1 had the opposite effects in Pkd1 mutant renal epithelial cells (Supplemental Figure 7, B and C) and tissues (Supplemental Figure 7, D and E).
More than 95% of functional iron in the human body is in the form of heme, and its release is regulated by HO. We found that the expression of HO-1 was markedly increased in Pkd1 mutant renal epithelial cells and tissues (Supplemental Figure 8, A and B). Treatment with erastin further increased HO-1 mRNA (Supplemental Figure 8C), and treatment with Fer-1 decreased HO-1 mRNA (Supplemental Figure 8D) in Pkd1−/− MEK and PN24 cells. In addition, treatment with erastin increased HO-1 mRNA in Pkd1RC/RC kidneys (Figure 5E) and serum nonheme iron in Pkd1RC/RC mice (Supplemental Figure 8F), whereas treatment with Fer-1 had the opposite effect in Pkd1RC/RC and Pkd1flox/flox:Pkhd1-Cre kidneys (Supplemental Figure 8, G and H). These results suggest that HO-1–mediated release of iron from heme also facilitates the increase of intracellular iron in Pkd1 mutant renal epithelial cells and iron-dependent ferroptosis in ADPKD kidneys.
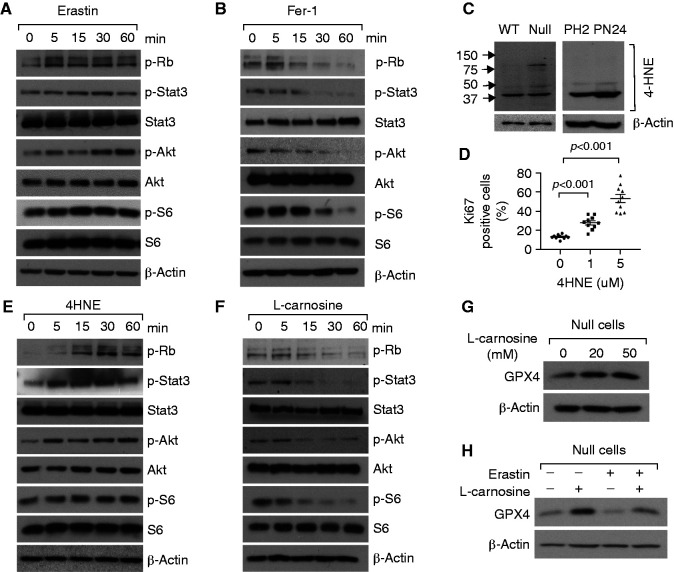
Pkd1 Mutant Renal Epithelial Cell Proliferation Is Regulated by 4-HNE Produced During Ferroptotic Process
Because induction and inhibition of ferroptosis respectively increase or decrease survived cyst-lining epithelial cell proliferation in Pkd1 mutant kidneys, we investigated the underlying mechanism(s). Treatment with erastin increased and treatment with Fer-1 decreased the phosphorylation of Akt, S6, Stat3, and Rb in Pkd1−/− MEK cells (Figure 6, A and B) and PN24 cells (Supplemental Figure 9, A and B). A major α, β-unsaturated hydroxyalkenal, 4-HNE, is a secondary product and a marker of lipid peroxidation that forms protein adducts affecting cell signaling and proliferation.31 We found that the levels of 4-HNE protein adduction were increased in Pkd1−/− MEK and PN24 cells compared with those in control cells (Figure 6C). Treatment with 4-HNE increased the percentage of Ki67-positive Pkd1−/− MEK (Figure 6D) and PN24 cells (Supplemental Figure 9C) in a dose-dependent manner, supporting a direct role of 4-HNE in promoting the proliferation of Pkd1 mutant renal epithelial cells. Treatment with 4-HNE also increased the S-phase entry and the phosphorylation of S6, Akt, and Stat3 in Pkd1 null−/− MEK (Supplemental Figure 9D and Figure 6E) and PN24 cells (Supplemental Figure 9, E and F).
Figure 6.
Pkd1 mutant renal epithelial cell proliferation was regulated by 4-HNE produced during ferroptotic process. (A and B) Western blot analysis of the expression and phosphorylation of Rb, STAT3, Akt, and S6 in Pkd1 null MEK cells treated with erastin (10 μM) (A) or Fer-1 (1 μM) (B) at indicated time points. (C) Western blot analysis of the expression of 4HNE in Pkd1 WT and null MEK cells (left panel) and PH2, PN24 cells (right panel). (D) Ki67 staining indicated that cell proliferation was increased in Pkd1 null MEK cells treated with 4HNE compared with those cells treated with vehicle. The percentage of Ki67-positive nuclei of Pkd1 null MEK cells was quantified from an average of 1000 nuclei per field. Statistical data are presented as the mean±SEM. (E) Western blot analysis of the expression and phosphorylation of Rb, STAT3, Akt, and S6 in Pkd1 null MEK treated with 4HNE (5 μM) at indicated time points. (F) Western blot analysis of the expression and phosphorylation of Rb, STAT3, Akt, and S6 in Pkd1 null MEK cells treated with L-carnosine at indicated time points. (G and H) Western blot analysis of the expression of GPX4 in Pkd1 null MEK cells treated with L-carnosine at indicated concentrations (G) and treated with erastin with or without L-carnosine (H).
To further support a role of 4HNE in promoting the proliferation of Pkd1 mutant renal epithelial cells and cyst growth, we examined the effect of L-carnosine, a selective inhibitor of 4-HNE adducts.32 L-carnosine markedly decreased the phosphorylation of Akt, Rb, S6, and Stat3 in Pkd1−/− MEK cells (Figure 6F) and PN24 cells (Supplemental Figure 10A), increased the expression of GPX4 in Pkd1 null MEK cells (Figure 6G) and PN24 cells (Supplemental Figure 10B), and reversed the downregulation of GPX4 (Figure 7H, Supplemental Figure 10C), decreasing lipid peroxidation (Supplemental Figure 10D) when these cells were treated with erastin. Treatment with L-carnosine also decreased cyst growth in a three-dimensional spheroid model of mouse IMCD3 cells treated with erastin (Supplemental Figure 10, E and F). These results suggest 4-HNE acts as a signal molecule to promote Pkd1 mutant renal epithelial cell proliferation, and that there may be a feedforward loop in which lipid peroxidation–4HNE production–GPX4 downregulation–lipid peroxidation promotes cystogenesis. Treatment with L-carnosine may block this loop and be a potential therapeutic strategy in ADPKD.
Figure 7.
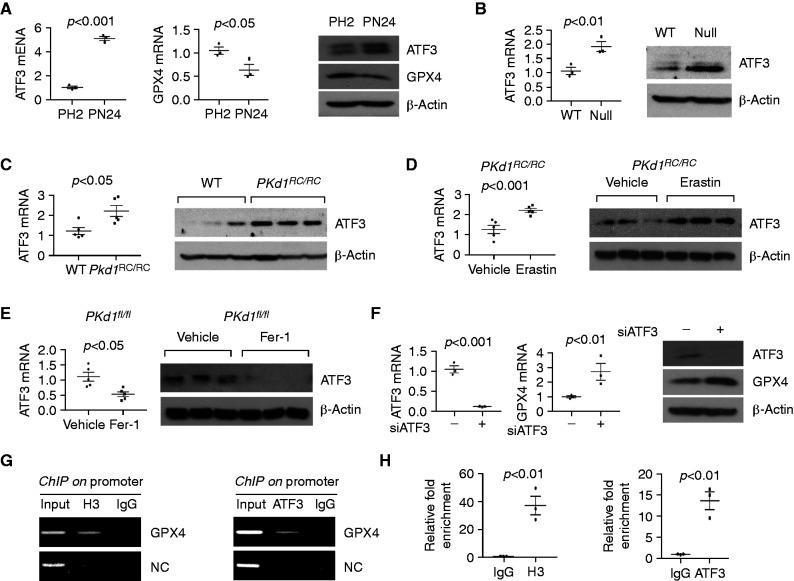
The expression of GPX4 was regulated by ATF3 in Pkd1 mutant renal epithelial cells. (A) qRT-PCR and Western blot analysis of ATF3 and GPX4 mRNA levels in PH2 and PN24 cells. n=3 independent experiments. (B) qRT-PCR and Western blot analysis of ATF3 and GPX4 mRNA levels in Pkd1 WT and Pkd1 null MEK cells. n=3 independent experiments. (C) qRT-PCR and Western blot analysis of the expression of ATF3 in kidneys from 3-month-old WT (n=5) and Pkd1RC/RC mice (n=5). (D) qRT-PCR and Western blot analysis of the expression of ATF3 in kidneys from Pkd1RC/RC mice treated with vehicle (n=5) or erastin (n=5). (E) qRT-PCR and Western blot analysis of the expression of ATF3 in kidneys from Pkd1flox/flox:Pkhd1-Cre mice treated with vehicle (n=5) or Fer-1 (n=5). (F) qRT-PCR and Western blot analysis of the expression of ATF3 and GPX4 in PN24 cells transfected with ATF3 siRNA and control siRNA. n=3 independent experiments. (G and H) chromatin immunoprecipitation (ChIP) (G) and ChIP-qPCR (H) analysis indicated that ATF3 bound to the promoter of GPX4 in PN24 cells. Histone H3 was used as a positive control. Normal rabbit IgG was used as a negative control. Statistical data are presented as the mean±SEM.
The GPX4 Expression Is Regulated by ATF3 in Pkd1 Mutant Renal Epithelial Cells
To understand the mechanism by which Pkd1 regulates the expression of GPX4 in renal epithelial cells, we used a report that ATF3 expression was increased during erastin-induced ferroptosis and that ATF3 repressed SLC7A11 expression via binding to two adjacent motifs (5′-TGATGCAA-3′) in its promoter.33 A conserved ATF3 binding motif, 5′-TGATGCAA-3′, was identified in the GPX4 promoter (Supplemental Figure 11A). We found the expression of ATF3 was upregulated in Pkd1−/− PN24 cells and Pkd1−/− MEK cells compared with Pkd1+/− PH2 cells and wild-type MEK cells, by qRT-PCR and Western blot (Figure 7, A and B); this also occurred in kidneys from Pkd1RC/RC mice compared with wild-type age-matched kidneys (Figure 7C), and in human ADPKD kidneys compared with normal kidneys (Supplemental Figure 11, B and C). Treatment with erastin further increased the levels of ATF3 mRNA and protein in kidneys from Pkd1RC/RC mice (Figure 7D), whereas Fer-1 had the opposite effect in kidneys of Pkd1flox/flox:Pkhd1-Cre mice (Figure 7E). Knockdown of ATF3 with siATF3 increased the expression of GPX4 (Figure 7F) and SLC7A11 (Supplemental Figure 11D) in PN24 cells. The binding of ATF3 to the promoter of GPX4 was confirmed in PN24 cells with a chromatin immunoprecipitation assay (Figure 7, G and H).
One possible mechanism to explain how Pkd1 mutation leads to ATF3 changes is that cytokines such as TNF-α, secreted by infiltrating immune cells, may induce the expression of ATF3 in Pkd1 mutant kidneys, as has been reported in vascular endothelial cells.34 Because TNF-α can stimulate its own expression in Pkd1 mutant renal epithelial cells,35 its secretion by cystic renal epithelial cells may increases its accumulation in cyst fluids to induce the expression of ATF3 in cyst-lining epithelial cells. In support of this hypothesis, we found that treatment with TNF-α increased the expression of ATF3 mRNA and protein but decreased the levels of GPX4 in mouse IMCD3 cells (Supplemental Figure 11, E and F). In sum, these results suggest ATF3 plays a dual role in repressing GPX4 expression and activity in Pkd1 mutant renal epithelial cells.
Discussion
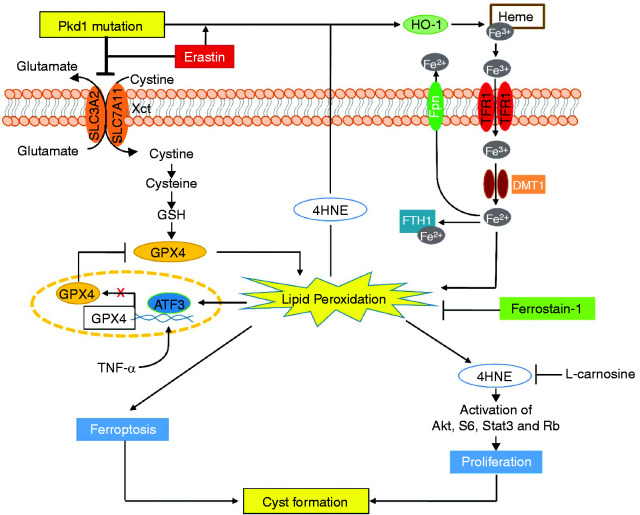
This study shows the role and mechanisms of ferroptosis in the pathogenesis of ADPKD and highlights novel potential therapeutic strategies for the treatment of this disease. The main observations from this study (Figure 8) are the following: (1) low levels of cell death in kidneys from Pkd1 mutant mouse models are mainly ferroptotic, not apoptotic27,28; (2) Pkd1 null cells and kidney tissues from Pkd1 mouse models exhibit GSH expression of the system Xc− amino acid antiporter critical for import of cystine (SLC7A11 and SLC3A2), iron exporter (ferroportin), and GPX4, and increased expression of iron importers (TfR1, DMT1) and HO-1; these in turn result in high iron levels, low GSH and GPX4 activity, increased lipid peroxidation, and propensity to ferroptotic cell death; (3) erastin increased and Fer-1 inhibited ferroptotic cell death and disease progression in Pkd1 mutant mice; and (4) 4HNE, a lipid peroxidation product increased in Pkd1 null cells, promoted the proliferation of Pkd1 mutant cells via activation of Akt, S6, Stat3, and Rb. Collectively, these observations suggest that ferroptosis is an important and therapeutically targetable contributor to ADPKD progression.
Figure 8.
Working model of ferroptosis in regulation of cyst growth in ADPKD. A schematic diagram depicting ferroptosis associated pathways and processes in Pkd1 mutant renal epithelial cells and kidneys. Pkd1 mutation or erastin treatment (1) decreased system Xc− and resulted in the reduction of cystine uptake and the depletion of GSH, leading to inactivate GPX4 enzymatic activity and then increase lipid peroxidation, and (2) increased the expression of multiple iron regulatory proteins (IRPs), including TFR1, FTH1, DMT1, Fpn, and HO-1 and resulted in the increase of the import of HO-1–mediated nonheme iron and the storage of iron in Pkd1 mutant renal epithelial cells to increase lipid peroxidation, both of these processes leading to ferroptosis. The excess lipid peroxidation also stimulated the expression of ATF3, which repressed the expression of SLC7A11 and GPX4, forming a feedback loop of lipid peroxidation–ATF3–SLC7A11/GPX4–lipid peroxidation, and increased the production of 4HNE-protein adducts. Upregulation of 4HNE (1) promotes Pkd1 mutant cell proliferation via activation of Akt, S6, Stat3, and Rb, and (2) upregulates the expression of HO-1, which regulates the release of heme iron and results in its import and storage in Pkd1 mutant renal epithelial cells to promote ferroptosis. All these 4HNE-mediated processes can be inhibited by L-carnosine. In addition, treatment with Fer-1, a ferroptosis inhibitor, delays cyst growth in early stage and long-lasting Pkd1 animal models, which highlights a novel therapeutic strategy for ADPKD treatment.
In the past, apoptosis has been considered the main form of regulated cell death in PKD. Studies showing the essential role of apoptosis for cyst cavitation in three-dimensional cultures of MDCK cells and the induction of epithelial cell proliferation and cyst formation by the knockout of the gene encoding antiapoptotic Bcl-2 suggested a direct role of apoptosis in cyst development and growth.5–8 However, the mechanism of cyst formation in Bcl-2–deficient mice is different from that in Pkd1 mutant mice.36 Furthermore, recent studies have shown that TUNEL-positive cells are not widespread in Pkd1 mutant kidneys and that induction of apoptosis delays cyst growth in Pkd1 and Pkd2 mouse models treated with Smac-mimatic,11 epigenetic,12 and MIF inhibitors,13 and in Pkd2 mouse models treated with anti–miRNA-17 oligonucleotides.14 In addition, induction of autophagy also delays cyst growth in Pkd1 mutant mice and zebrafish with Pkd1a and Pkd2 mutations.37,38 These studies demonstrate that induction of cell death, such as apoptosis or autophagy, plays a beneficial role in ADPKD. In our consideration of another form of regulated cell death, ferroptosis, our initial hypothesis was that induction of ferroptosis may delay cyst growth in Pkd1 mutant kidneys, similar to the induction of cystic renal epithelial cell apoptosis by Smac-mimatic. In fact, we did find that Pkd1 mutant cells are more susceptible to ferroptosis inducer, erastin, with GSH viability in cell culture assays and increased TUNEL staining in treated cystic kidneys (Figures 2 and 4). However, the in vivo results showed the inducer of ferroptosis appears to worsen cystic disease, completely contrary to our initial hypothesis. These results suggest ferroptosis and apoptosis may play a different role in cyst progression.
The cell death detected with the TUNEL assay, regardless of whether it is apoptotic or ferroptotic, is not dominant in aggressive and mild Pkd1 mutant kidneys, suggesting that cystic renal epithelial cell proliferation is be the main force to promote cyst growth in those kidneys. Under conditions of oxidative stress and enhanced lipid peroxidation, the formation of 4HNE and its protein adducts may serve as a secondary messenger to regulate numerous cell processes, such as cell proliferation, transformation, or cell death.39 The increased formation of 4HNE-protein adducts, which has been showed in dolichos biflorus agglutinin–positive distal tubules/collecting ducts in human ADPKD kidneys,40 suggests that 4HNE may participate in regulation of cystic renal epithelial cell proliferation in Pkd1 mutant kidneys. Thus, treatment with the ferroptosis inducer erastin to induce ferroptosis may promote cyst growth via two mechanisms. First, erastin-induced ferroptotic cell death may contribute to cyst enlargement when dead cells are removed from the epithelium lining renal cysts—as Edelstein and colleagues have proposed, removing apoptotic cells may promote cyst enlargement.41 Second, the byproducts such as 4HNE that are produced during ferroptosis process may also increase proliferation of the surviving cystic renal epithelial cells, which should further promote cyst growth in erastin-treated Pkd1 mutant kidneys.
Erastin was initially discovered as a small-molecule compound that selectively killed tumor cells expressing small T antigens and RASV12 and was later widely investigated as an inducer of ferroptosis.15,42 In addition to mediating ferroptosis through the cystine-glutamate transport receptor system Xc−, erastin may also regulate ferroptosis through targeting the voltage-dependent anion channel43 and p53.44 Fer-1 is an arylalkylamine and an active radical-trapping antioxidant, which traps peroxyl radicals generated by ferrous iron from lipid hydroperoxides to inhibit lipid peroxidation and prevent damage to membrane lipids and, thereby, inhibit cell death. No other target proteins or pathways that can be altered by Fer-1 have been reported.
Iron is an essential element and cofactor of a plethora of metabolic enzymes that participate in oxidation-reduction reactions to regulate numerous fundamental biologic functions and processes. Abnormalities in iron homeostasis are associated with many diseases,45 but no previous studies had evaluated the role of iron metabolism in the pathogenesis of ADPKD. Iron homeostasis is regulated via multiple mechanisms and the coordination of multiple iron regulatory proteins, including TFR1, FTL1, FTH1, and DMT1, at the systemic and cellular levels46 to provide adequate iron for essential biologic processes while limiting the toxicity of iron excess. Because most iron in the body is incorporated into proteins as a component of heme, the release of iron from heme by HO, a rate-limiting enzyme in the catabolism of heme, should play a key role in regulating the intracellular heme levels.47 Three isoforms of HO have been identified so far: HO-1, HO-2, and HO-3. HO-1 is expressed in relatively low amounts in all tissues, whereas HO-2 is constitutive and mainly expressed in the brain and testes. HO-3 is reported to be derived from a processed pseudogene.48 Overactivation of HO-1, however, can result in iron accumulation, depletion of GSH, lipid peroxidation, and ferroptosis.49 In this study, we identified two mechanisms that are responsible for the abnormal iron metabolism in Pkd1 mutant renal epithelial cells and tissues: (1) the dysregulation of the enzymes that regulated iron metabolism, including Fpn, TFR1, and DMT1, resulting in increased importing and storage of iron in Pkd1 mutant renal epithelial cells, and (2) the upregulation of HO-1 in Pkd1 mutant renal epithelial cells and tissues, which treatment with 4HNE can further increase.
In conclusion, this is the first study to demonstrate a role of ferroptosis in ADPKD. Our observations suggest that management of ferroptosis may be a novel strategy for the treatment of this disease. Given that ferroptosis can also be rescued with DFOM (an iron chelator), U0126 (a MEK inhibitor), and vitamin E (an antioxidant),50,51 the potential effectiveness of other ferroptosis inhibitors and their combination with other ADPKD treatment drugs merits further exploration.
Disclosures
V. Torres reports having consultancy agreements with Acceleron Pharma, Blueprint Medicines, Mironid, Otsuka Pharmaceuticals, Palladio, Reata, and Sanofi; reports receiving research funding from Blueprint Medicines, Mironid, Otsuka Pharmaceuticals, Palladio Biosciences, Reata, Sanofi Genzyme (all preclinical trial, preclinical research or clinical trials); reports receiving honoraria from Otsuka Pharmaceuticals (to the institution); and reports being a scientific advisor or member of with Mironid, Otsuka Pharmaceuticals, and Sanofi. All remaining authors have nothing to disclose.
Funding
This work was supported by the National Institutes of Health grants R01 DK084097 and R01 DK126662, the PKD Foundation research grant (231G18a) (to X. Li), the National Institutes of Health grant R01 DK44863 and the National Institute of Diabetes and Digestive and Kidney Diseases Polycystic Kidney Disease Center grant P30 DK090728 (to V.E. Torres) and P30 DK106912.
Supplementary Material
Acknowledgments
We are grateful to Dr. S. Somlo for providing cell lines PH2 and PN24 through the George M O’Brien Kidney Center at Yale University (NIH P30 DK079310).
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
Supplemental Material
This article contains the following supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2021040460/-/DCSupplemental.
Supplemental Table 1. The primers used for quantitative real time PCR and ChIP-PCR.
Supplemental Figure 1. The expression of ferroptosis related genes was dysregulated in Pkd1 mutant renal epithelial cells and human ADPKD kidneys.
Supplemental Figure 2. The expression of ferroptosis related genes was dysregulated in Pkd2 mutant kidneys.
Supplemental Figure 3. Depletion of Pkd1 increases the sensitivity of cystic renal epithelial cells to erastin.
Supplemental Figure 4. Treatment with erastin decrease GSH levels but increased cyst-lining epithelial cell proliferation in Pkd1RC/RC kidneys.
Supplemental Figure 5. Treatment with erastin did not promote cyst growth in Pkd1RC/+ mice kidneys.
Supplemental Figure 6. Mitochondrial morphological and function alteration in Pkd1 mutant kidneys treated with erastin or Fer-1.
Supplemental Figure 7. Iron metabolism associated genes were dysregulated in Pkd1 mutation renal epithelial cells and tissues treated with erastin or Fer-1.
Supplemental Figure 8. Renal and systemic iron metabolism mediated by IRPs and HO-1 in Pkd1 mutant kidneys.
Supplemental Figure 9. Pkd1 mutant renal epithelial cell proliferation was regulated by 4-HNE produced during ferroptotic process.
Supplemental Figure 10. Pharmacological inhibition of 4HNE decreased the activation of PKD associated signaling pathways and cyst growth in 3D spheroid from mouse IMCD3 cells.
Supplemental Figure 11. The expression of GPX4 was regulated by ATF3 in Pkd1 mutant renal epithelial cells.
References
- 1.Bergmann C, Guay-Woodford LM, Harris PC, Horie S, Peters DJM, Torres VE: Polycystic kidney disease. Nat Rev Dis Primers 4: 50, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhang L, Li LX, Zhou JX, Harris PC, Calvet JP, Li X: RNA helicase p68 inhibits the transcription and post-transcription of Pkd1 in ADPKD. Theranostics 10: 8281–8297, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhou JXLX: Apoptosis in polycystic kidney disease: From pathogenesis to treatment. In: Li X, Polycystic Kidney Disease [Internet]. Brisbane (AU): Codon Publications; 2015 Nov. Chapter 9 [PubMed] [Google Scholar]
- 4.Nowak KL, Edelstein CL: Apoptosis and autophagy in polycystic kidney disease (PKD). Cell Signal 68: 109518, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Smith LA, Bukanov NO, Husson H, Russo RJ, Barry TC, Taylor AL, et al. : Development of polycystic kidney disease in juvenile cystic kidney mice: Insights into pathogenesis, ciliary abnormalities, and common features with human disease. JASN 17: 2821–2831, 2006 [DOI] [PubMed] [Google Scholar]
- 6.Lager DJ, Qian Q, Bengal RJ, Ishibashi M, Torres VE: The pck rat: A new model that resembles human autosomal dominant polycystic kidney and liver disease. Kidney Int 59: 126–136, 2001 [DOI] [PubMed] [Google Scholar]
- 7.Lin HH, Yang TP, Jiang ST, Yang HY, Tang MJ: Bcl-2 overexpression prevents apoptosis-induced Madin-Darby canine kidney simple epithelial cyst formation. Kidney Int 55: 168–178, 1999 [DOI] [PubMed] [Google Scholar]
- 8.Yu W, Kong T, Beaudry S, Tran M, Negoro H, Yanamadala V, et al. : Polycystin-1 protein level determines activity of the Galpha12/JNK apoptosis pathway. J Biol Chem 285: 10243–10251, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tao Y, Kim J, Faubel S, Wu JC, Falk SA, Schrier RW, et al. : Caspase inhibition reduces tubular apoptosis and proliferation and slows disease progression in polycystic kidney disease. Proc Natl Acad Sci U S A 102: 6954–6959, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Veis DJ, Sorenson CM, Shutter JR, Korsmeyer SJ: Bcl-2-deficient mice demonstrate fulminant lymphoid apoptosis, polycystic kidneys, and hypopigmented hair. Cell 75: 229–240, 1993 [DOI] [PubMed] [Google Scholar]
- 11.Fan LX, Zhou X, Sweeney WE, Jr, Wallace DP, Avner ED, Grantham JJ, et al. : Smac-mimetic-induced epithelial cell death reduces the growth of renal cysts. JASN 24: 2010–2022, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li LX, Fan LX, Zhou JX, Grantham JJ, Calvet JP, Sage J, et al. : Lysine methyltransferase SMYD2 promotes cyst growth in autosomal dominant polycystic kidney disease. J Clin Invest 127: 2751–2764, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen L, Zhou X, Fan LX, Yao Y, Swenson-Fields KI, Gadjeva M, et al. : Macrophage migration inhibitory factor promotes cyst growth in polycystic kidney disease. J Clin Invest 125: 2399–2412, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sun H, Li QW, Lv XY, Ai JZ, Yang QT, Duan JJ, et al. : MicroRNA-17 post-transcriptionally regulates polycystic kidney disease-2 gene and promotes cell proliferation. Mol Biol Rep 37: 2951–2958, 2010 [DOI] [PubMed] [Google Scholar]
- 15.Dixon SJ, Lemberg KM, Lamprecht MR, Skouta R, Zaitsev EM, Gleason CE, et al. : Ferroptosis: An iron-dependent form of nonapoptotic cell death. Cell 149: 1060–1072, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bogdan AR, Miyazawa M, Hashimoto K, Tsuji Y: Regulators of iron homeostasis: New players in metabolism, cell death, and disease. Trends Biochem Sci 41: 274–286, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hu Z, Zhang H, Yang SK, Wu X, He D, Cao K, et al. : Emerging role of ferroptosis in acute kidney injury. Oxid Med Cell Longev 2019: 1–8, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shibazaki S, Yu Z, Nishio S, Tian X, Thomson RB, Mitobe M, et al. : Cyst formation and activation of the extracellular regulated kinase pathway after kidney specific inactivation of Pkd1. Hum Mol Genet 17: 1505–1516, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Arroyo J, Escobar-Zarate D, Wells HH, Constans MM, Thao K, Smith JM, et al. : The genetic background significantly bs the severity of kidney cystic disease in the Pkd1RC/RC mouse model of autosomal dominant polycystic kidney disease. Kidney Int 99: 1392–1407, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Qiu B, Simon MC: BODIPY 493/503 staining of neutral lipid droplets for microscopy and quantification by flow cytometry. Bio Protoc 6: e1912, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang Z, Zhang F, An P, Guo X, Shen Y, Tao Y, et al. : Ferroportin1 deficiency in mouse macrophages impairs iron homeostasis and inflammatory responses. Blood 118: 1912–1922, 2011 [DOI] [PubMed] [Google Scholar]
- 22.Zhang F, Tao Y, Zhang Z, Guo X, An P, Shen Y, et al. : Metalloreductase Steap3 coordinates the regulation of iron homeostasis and inflammatory responses. Haematologica 97: 1826–1835, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pamplona A, Ferreira A, Balla J, Jeney V, Balla G, Epiphanio S, et al. : Heme oxygenase-1 and carbon monoxide suppress the pathogenesis of experimental cerebral malaria. Nat Med 13: 703–710, 2007 [DOI] [PubMed] [Google Scholar]
- 24.Giles RH, Ajzenberg H, Jackson PK: 3D spheroid model of mIMCD3 cells for studying ciliopathies and renal epithelial disorders. Nat Protoc 9: 2725–2731, 2014 [DOI] [PubMed] [Google Scholar]
- 25.Imai H, Matsuoka M, Kumagai T, Sakamoto T, Koumura T: Lipid peroxidation-dependent cell death regulated by GPx4 and ferroptosis. Curr Top Microbiol Immunol 403: 143–170, 2017 [DOI] [PubMed] [Google Scholar]
- 26.Dolka I, Król M, Sapierzyński R: Evaluation of apoptosis-associated protein (Bcl-2, Bax, cleaved caspase-3 and p53) expression in canine mammary tumors: An immunohistochemical and prognostic study. Res Vet Sci 105: 124–133, 2016 [DOI] [PubMed] [Google Scholar]
- 27.Neitemeier S, Jelinek A, Laino V, Hoffmann L, Eisenbach I, Eying R, et al. : BID links ferroptosis to mitochondrial cell death pathways. Redox Biol 12: 558–570, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xie Y, Hou W, Song X, Yu Y, Huang J, Sun X, et al. : Ferroptosis: Process and function. Cell Death Differ 23: 369–379, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cao JY, Dixon SJ: Mechanisms of ferroptosis. Cell Mol Life Sci 73: 2195–2209, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fanzani A, Poli M: Iron, oxidative damage and ferroptosis in rhabdomyosarcoma. IJMS 18: 1718, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gasparovic AC, Milkovic L, Sunjic SB, Zarkovic N: Cancer growth regulation by 4-hydroxynonenal. Free Radic Biol Med 111: 226–234, 2017 [DOI] [PubMed] [Google Scholar]
- 32.Aldini G, Carini M, Yeum KJ, Vistoli G: Novel molecular approaches for improving enzymatic and nonenzymatic detoxification of 4-hydroxynonenal: Toward the discovery of a novel class of bioactive compounds. Free Radic Biol Med 69: 145–156, 2014 [DOI] [PubMed] [Google Scholar]
- 33.Wang L, Liu Y, Du T, Yang H, Lei L, Guo M, et al. : ATF3 promotes erastin-induced ferroptosis by suppressing system Xc. Cell Death Differ 27: 662–675, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Inoue K, Zama T, Kamimoto T, Aoki R, Ikeda Y, Kimura H, et al. : TNFalpha-induced ATF3 expression is bidirectionally regulated by the JNK and ERK pathways in vascular endothelial cells. Genes Cells 9: 59–70, 2004 [DOI] [PubMed] [Google Scholar]
- 35.Li X, Magenheimer BS, Xia S, Johnson T, Wallace DP, Calvet JP, et al. : A tumor necrosis factor-alpha-mediated pathway promoting autosomal dominant polycystic kidney disease. Nat Med 14: 863–868, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hughes P, Robati M, Lu W, Zhou J, Strasser A, Bouillet P: Loss of PKD1 and loss of Bcl-2 elicit polycystic kidney disease through distinct mechanisms. Cell Death Differ 13: 1123–1127, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhu P, Sieben CJ, Xu X, Harris PC, Lin X: Autophagy activators suppress cystogenesis in an autosomal dominant polycystic kidney disease model. Hum Mol Genet 26: 158–172, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chang MY, Ma TL, Hung CC, Tian YC, Chen YC, Yang CW, et al. : Metformin inhibits cyst formation in a zebrafish model of polycystin-2 deficiency. Sci Rep 7: 7161, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Csala M, Kardon T, Legeza B, Lizák B, Mandl J, Margittai É, et al. : On the role of 4-hydroxynonenal in health and disease. Biochim Biophys Acta 1852: 826–838, 2015 [DOI] [PubMed] [Google Scholar]
- 40.Schreiber R, Buchholz B, Kraus A, Schley G, Scholz J, Ousingsawat J, et al. : Lipid peroxidation drives renal cyst growth in vitro through activation of TMEM16A. JASN 30: 228–242, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Holditch SJ, Brown CN, Atwood DJ, Lombardi AM, Nguyen KN, Toll HW, et al. : A study of sirolimus and mTOR kinase inhibitor in a hypomorphic Pkd1 mouse model of autosomal dominant polycystic kidney disease. Am J Physiol Renal Physiol 317: F187–F196, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yang WS, Stockwell BR: Synthetic lethal screening identifies compounds activating iron-dependent, nonapoptotic cell death in oncogenic-RAS-harboring cancer cells. Chem Biol 15: 234–245, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yagoda N, von Rechenberg M, Zaganjor E, Bauer AJ, Yang WS, Fridman DJ, et al. : RAS-RAF-MEK-dependent oxidative cell death involving voltage-dependent anion channels. Nature 447: 865–868, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chu B, Kon N, Chen D, Li T, Liu T, Jiang L, et al. : ALOX12 is required for p53-mediated tumour suppression through a distinct ferroptosis pathway. Nat Cell Biol 21: 579–591, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cronin SJF, Woolf CJ, Weiss G, Penninger JM: The role of iron regulation in immunometabolism and immune-related disease. Front Mol Biosci 6: 116, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pantopoulos K, Porwal SK, Tartakoff A, Devireddy L: Mechanisms of mammalian iron homeostasis. Biochemistry 51: 5705–5724, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Araujo JA, Zhang M, Yin F: Heme oxygenase-1, oxidation, inflammation, and atherosclerosis. Front Pharmacol 3: 119, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hayashi S, Omata Y, Sakamoto H, Higashimoto Y, Hara T, Sagara Y, et al. : Characterization of rat heme oxygenase-3 gene. Implication of processed pseudogenes derived from heme oxygenase-2 gene. Gene 336: 241–250, 2004 [DOI] [PubMed] [Google Scholar]
- 49.Fang X, Wang H, Han D, Xie E, Yang X, Wei J, et al. : Ferroptosis as a target for protection against cardiomyopathy. Proc Natl Acad Sci U S A 116: 2672–2680, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yang WS, Stockwell BR: Ferroptosis: Death by lipid peroxidation. Trends Cell Biol 26: 165–176, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Khanna S, Roy S, Ryu H, Bahadduri P, Swaan PW, Ratan RR, et al. : Molecular basis of vitamin E action: Tocotrienol modulates 12-lipoxygenase, a key mediator of glutamate-induced neurodegeneration. J Biol Chem 278: 43508–43515, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.