Significance Statement
MicroRNA-192-5p (miR-192-5p) is upregulated in the glomeruli and urine of patients with idiopathic membranous glomerulonephritis (iMGN). It derives from glomerular endothelial cells, is packed into exosomes, and decreases podocyte nephronectin (NPNT) in the glomerular basement membrane (GBM) by paracrine signaling. Patients with iMGN have reduced glomerular NPNT expression. Whole-body knockdown of npnt in zebrafish and podocyte-specific knockout of Npnt in mice damage the GBM, increasing lucidity of the lamina rara interna, which admits high molecular weight proteins. Reduced NPNT leading to GBM leakiness might be an important part of iMGN pathophysiology, initiating podocyte antigen presentation, and admitting autoantibodies into the subepithelial space. NPNT might be a prognostic parameter and noninvasive marker for iMGN. Monitoring and targeting this miR could be a promising diagnostic and therapeutic approach for iMGN.
Keywords: glomerular disease, membranous nephropathy, glomerular filtration barrier, podocyte, glomerular basement membrane, microRNAs, glomerular endothelial cells, nephronectin
Visual Abstract
Abstract
Background
Autoantibodies binding to podocyte antigens cause idiopathic membranous glomerulonephritis (iMGN). However, it remains elusive how autoantibodies reach the subepithelial space because the glomerular filtration barrier (GFB) is size selective and almost impermeable for antibodies.
Methods
Kidney biopsies from patients with iMGN, cell culture, zebrafish, and mouse models were used to investigate the role of nephronectin (NPNT) regulating microRNAs (miRs) for the GFB.
Results
Glomerular endothelial cell (GEC)-derived miR-192-5p and podocyte-derived miR-378a-3p are upregulated in urine and glomeruli of patients with iMGN, whereas glomerular NPNT is reduced. Overexpression of miR-192-5p and morpholino-mediated npnt knockdown induced edema, proteinuria, and podocyte effacement similar to podocyte-derived miR-378a-3p in zebrafish. Structural changes of the glomerular basement membrane (GBM) with increased lucidity, splitting, and lamellation, especially of the lamina rara interna, similar to ultrastructural findings seen in advanced stages of iMGN, were found. IgG-size nanoparticles accumulated in lucidity areas of the lamina rara interna and lamina densa of the GBM in npnt-knockdown zebrafish models. Loss of slit diaphragm proteins and severe structural impairment of the GBM were further confirmed in podocyte-specific Npnt knockout mice. GECs downregulate podocyte NPNT by transfer of miR-192-5p–containing exosomes in a paracrine manner.
Conclusions
Podocyte NPNT is important for proper glomerular filter function and GBM structure and is regulated by GEC-derived miR-192-5p and podocyte-derived miR-378a-3p. We hypothesize that loss of NPNT in the GBM is an important part of the initial pathophysiology of iMGN and enables autoantigenicity of podocyte antigens and subepithelial immune complex deposition in iMGN.
The glomerular filtration barrier (GFB) is composed of specialized glomerular endothelial cells (GEC), the glomerular basement membrane (GBM), and podocytes.
Beside podocyte slit diaphragms, the GBM is believed to be the most important barrier component with size- and charge-selective filtration functions.1,2 Molecules larger than albumin are impaired from traversing the GBM.3 Primary idiopathic membranous glomerulonephritis (iMGN) is caused by autoantibodies binding to podocyte antigens.4–6 The most frequent autoantibody detectable in MGN is the antiphospholipase A2 receptor antibody (PLA2R-ab). However, it remains unknown how PLA2R is exposed as cryptogenic antigen and how autoantibodies reach the subepithelial space in MGN, because the GFB is normally almost completely impermeable for antibodies and podocytes remain inaccessible to antigen-presenting cells if endothelial cells and the GBMs are not damaged.
Furthermore, how autoantibodies are produced at disease onset remains unclear. PLA2R is weakly expressed in podocytes under normal conditions and is not accessible to circulating T cells, which activate antigen-specific B cells.7
Patients who are PLA2R-ab negative and do not have any evidence for a secondary cause of the disease most likely develop other podocyte autoantibodies, some of which have been recently described, and others that remain to be discovered.6,8–10
During glomerulogenesis, the GBM arises from the fusion of separate basement membranes synthesized by the immature epithelial podocytes and GECs.11 The glomerular GBM is composed of laminin-521 (α5β2γ1), collagen α3α4α5(IV), nidogen, and agrin.12 Additionally, other extracellular matrix proteins, such as fibronectin, usherin, and nephronectin (NPNT) have been identified.13–15 Moreover, mesangial cells organize the glomerular capillaries by adhering to laminin in the GBM.16,17 Functional and physiologic analyses of the GFB revealed the importance of the GBM as a size- and charge-selective filtration barrier.1 Molecules larger than albumin are impaired from traversing the GBM. As suggested by Smithies,18 the GBM behaves like a concentrated gel with size-selective properties. Miner and colleague1 proposed that, even in primary podocyte disease, the GBM is the critical component of the GFB because podocyte cytoskeleton and slit diaphragm proteins are connected to cell surface receptors that link to the GBM. Thus, damage in podocytes leads to rearrangements of GBM composition and thereby increased permselectivity. Disruption of the GBM is a feature seen in different glomerular diseases, such as MGN, Alport disease, Pierson syndrome, and diabetic nephropathy, usually presenting with heavy proteinuria in later stages.19–24
NPNT is another extracellular protein important for interaction of the mesangium with the GBM.25 Our previous publication indicates that NPNT might also be important for proper podocyte function and GBM structure.26 Matrix proteins such as NPNT can be regulated by microRNAs (miRs). MiRs are short noncoding RNAs that inhibit translation of mRNAs. Aberrant miRs have been found in various pathologic conditions, including kidney diseases.27 We previously showed that podocyte-derived miR-378a-3p down regulates npnt.26
We hypothesize that miRs regulating GBM composition might increase GBM permeability and enable autoantibody binding to podocytes in MGN. In addition to podocyte-derived miR-378a-3p, we now identified GEC-derived miR-192-5p upregulated in urine and glomeruli from patients with iMGN. Both miRs target NPNT, which was downregulated in patients with PLA2R-ab+ MGN. Loss of NPNT by either podocyte-derived miR-378a-3p or GEC-derived miR-192-5p caused structural and functional impairments of the GBM with lamellation, splicing and widening of the lamina rara interna, and loss of circulating plasma proteins in zebrafish. Serial block-face scanning electron microscopy (SBF-SEM) allowed three-dimensional reconstruction of the altered GBM in npnt knockdown zebrafish. Injections of nanoparticles in different sizes into the circulation of zebrafish visualized a size-dependent penetration of particles through the glomerular filter in miR-378a-3p– or miR-192-5p–induced npnt knockdown zebrafish. Functional and ultrastructural changes of the GBM could be confirmed in podocyte-specific npnt knockout mice.
With the help of coculture experiments, we observed that GECs decrease podocyte NPNT by paracrine signaling through miR-192-5p–containing exosomes. Taken together, our results indicate miRNAs are important cofactors in the pathogenesis of iMGN.
Methods
Cell Culture
Immortalized human podocytes were proliferated under permissive conditions at 33°C. When cultivated at 37°C, the SV40 T-antigen was inactivated for cell differentiation. The culture medium for human podocytes was RPMI 1640 (Gibco), with 10% FCS (PAN-Biotech), 1% penicillin/streptomycin, and 0.1% insulin-transferrin-selenium (ThermoFisher Scientific). Human GEC (Clonetech) were cultivated in endothelial cell media (VascuLife EnGS-MV Medium, LifeLine Cell Technology). This medium was supplemented with 5 ng/ml rhEGF, 1 µg/ml hydrocortisone hemisuccinate, 10 mM l-glutamine, 0.2% rhEnGS, 50 µg/ml ascorbic acid, 0.75 U/ml heparin sulfate, 5% FBS, 30 mg/ml gentamycin, and 15 µg/ml amphotericin B (all supplements, LifeLine Cell Technology). Cells were stimulated with 5 ng/ml TGF-β for 48 hours (Supplemental Methods).
Quantitative PCR
Purification of total RNAs including miRs from cells, microvesicles, and urine samples was done with miRNeasy Kit (Qiagen) according to the manufacturer’s protocol. For miR PCR, we used TaqMan MicroRNA Assays from Life Technologies, according to the manufacturer’s protocols. Program for reverse transcription was 3 minutes 16°C, 30 minutes 42°C, and 5 minutes 85°C. Real-time PCR parameters were 10 minutes at 95°C and 40 cycles of 15 seconds at 95°C, after 1 minute at 60°C.
For mRNA targets, we used sybr green–based real-time PCR with the following protocol: 1 minute at 95°C, followed by 35 cycles of 10 seconds at 95°C, 10 seconds at 60°C, and 10 seconds at 72°C, followed by 5 seconds at 95°C and 1 minute at 65°C. Individual samples were run in triplicate.
miR Transfection
We used the mirVana miRNA mimic has–miR-192-5p (Life Technologies, Carlsbad) and mirVana miRNA mimic negative control 1 (miR-CTRL mimic, Life Technologies, Carlsbad) for cell culture experiments in human podocytes. MirVana miRNA mimic negative control 1 is a random sequence miRNA mimic molecule that has been extensively tested in human cell lines and tissues and validated to not produce identifiable effects on known miRNA function. Then 7-day differentiated cultured human podocytes were transfected with 100 nM has–miR-192-5p mimic/miR-CTRL for 4 hours using Lipofectamin and Opti-MEM Medium (ThermoFisher Scientific) according to manufacturer’s protocol.
For visualizing miR-192, we used an Alexa555-tagged miR-192 (MirVanaTM, custom miRNA mimic, ThermoFisher Scientific). Transfection was carried out as described above at a concentration of 200 nM.
Western Blot
Next, 20 μg of podocyte cell lysate was resolved in 10% SDS-PAGE and transferred to a nitrocellulose membrane. Detection of protein bands was performed using horseradish peroxidase–labeled secondary antibodies and visualized using enhanced chemoluminescence reagents. The primary antibodies were anti-NPNT (POEM) sc393033, Santa Cruz, anti-GAPDH sc3223, Santa Cruz.
In Vivo Studies in Zebrafish
Zebrafish were mated at 28.5°C. Larvae grew in standard E3 solution. Injection of miR-CTRL (25 µM, mirVana miRNA mimic, negative control; Life Technologies, Carlsbad, CA), miR-378a-3p mimic (25 µM, mirVana miRNA mimic, has-378; Life Technologies, Carlsbad, CA), and miR-192-5p mimic (5 µM, mirVana miRNA mimic, hsa-192; Life Technologies Carlsbad, CA) and CTRL-MO (5′CCTCTTACCTCAGTTACAATTTATA3′) and npnt-MO (5′TGTGAAACGGC AGACGGAACTCACT3′) into zebrafish eggs was done at cell stages one to four, as previously described.26 For measurement of glomerular filter integrity, transgenic zebrafish that express a GFP-tagged vitamin D–binding protein (Tg[l-fabp:DBP:eGFP] fish) were used. Maximum fluorescence intensities of grayscale images of the pupils of the fish were measured using Image J (Version 1.48; Wayne Rasband National Institutes of Health) and reported in relative units of brightness. The Mount Desert Island Biologic Laboratory animal care committee approved the animal protocol (Institutional Animal Care and Use Committee protocol 0804).
Transmission Electron Microscopy of Zebrafish and Mice Glomeruli
Zebrafish larvae and mice kidneys were fixed in solution D and embedded in EPON according to the manufacturer’s protocol (recipe/protocol from EMS, Hatfield, PA). Semithin (300 nm) and ultrathin (90 nm) sectioning was performed with a microtome (Reichert Austria Ultracut) and transferred to copper slit grids (EMS). Grids were stained with uranyl acetate (2%) for 30 minutes, then lead citrate for 15 minutes with three washing steps in between.
SBF-SEM of Zebrafish Larvae
Zebrafish tissue was fixed, stained, and resin embedded. Details of the adapted reduced osmium tetroxide–thiocarbohydrazide–osmium tetroxide protocol were already described in Beike et al.28 For three-dimensional analysis by SBF-SEM, the embedded sample was mounted with conductive epoxy glue (Chemtronics, CircuitWorks, Kennesaw) on an aluminum specimen pin (Gatan, Pleasanton, CA), trimmed with glass knives, and sputter coated with a 20 nm gold layer. Next, 850 (knockdown) sections and 1500 (control) sections were cut with 80 nm section thickness in a Zeiss Merlin VP Compact SEM (Carl Zeiss AG, Oberkochen, Germany) equipped with a Gatan 3View2XP system (Gatan Inc., Pleasanton, CA) and the block-face was imaged with 8–10 nm pixel size in the variable pressure mode at 10–30 Pa. Fiji software was used for image processing.29 Segmentation of the images was performed manually using the IMOD software (https://bio3d.colorado.edu/imod/). Videos were generated with Microscopy Image Browser.30
Immunofluorescence Staining
For paraffin tissue sections, mouse kidneys or zebrafish were fixed in 2% PFA for 1 day, dehydrated in ascending concentrations of ethanol in PBS (25%, 50%, 70%, and 100%) and transferred in xylene (100%) for 5 minutes before embedding in paraffin (60°C) overnight. With a rotational microtome (Leica SM 2000 R) sections of 5 μm were cut, incubated in ethanol (100%, 70%, 50%, and 25%) and stained with HOECHST. The following primary antibodies were used: mouse anti-NPNT (1:50, AF4298, R&D), guinea pig polyclonal antinephrin (1:50, Progen Biotechnik), rabbit anti-NPNS2 (1:50, 20384–1-AP, ThermoFisher Scientific). Secondary antibodies were Alexa Fluor 488 goat anti–anti-rabbit (Life Technology) and Alexa Fluor 647 goat anti-mouse antibodies (Life Technology) and Alexa Fluor 647 goat anti-guinea pig (ab150187).
In Situ Hybridization
For miR-192-5p and miR378a-3p in situ hybridization the miRCury LNA ISH Optimization Kit (FFPE, Qiagen) was used according to the protocol provided by the manufacturer. Probes were used for U6 snRNA, scrambled probe, miR-192-5p, and miR-378a-3p on FFPE tissue sections of human kidneys.
Injection of Nanoparticles in Zebrafish Larvae
Generation of sodium thiosulfate (NaSCN) oligo cluster was performed by Smithies et al. and Lawrence et al.31,32 as described before. After a delay of 5 seconds, NaSCN reduces gold chloride in alkali solution and forms an oligo cluster with a permeation coefficient close to albumin and, after a delay of 45 seconds, an oligo cluster with permeation characteristics of IgG.31,32 NaSCN oligo clusters were injected in the cardinal vein of zebrafish at 120 hours post fertilization (hpf). Then 4.6 µl of particles diluted 1:2 in injection buffer were injected. Zebrafish were harvested and fixed in solution D 5 minutes after injection. Nanoparticles detected in GEC, GBM, or podocytes were counted.
Npnt fl/fl; Nphs2 cre+ Mutants
Npnt fl/fl; Nphs2cre+ mutants were generated by crossing Npnt fl/fl mice with Nphs2 cre+ mice. Npnt fl/fl mice were purchased from Mutant Mouse Resource and Research Center. Exon1 of the Npnt gene of these mice is flanked by loxP sites. Mice were genotyped by standard PCR. Approval for mouse experiments was given from Niedersächsischen Landesamt für Verbraucherschutz und Landesmittelsicherheit (Z 2017/40), Regierung von Unterfranken (55.2.2-2532-2-1155), and Sachgebiet Tierschutz at Friedrich-Alexander Universität Erlangen-Nürnberg (TS-2/2019).
Microvesicle Isolation
GECs were cultivated in the presence or absence of 5 ng/ml TGF-β in endothelial cell media supplemented with 10% FCS (MV media), which has been depleted of microvesicles by ultracentrifugation. After 48 hours of stimulation, the supernatant was collected and serially centrifuged as previously published.33 Briefly, centrifugation steps were 5 minutes at 300 × g, 20 minutes at 1200 × g, 30 minutes at 10.000 × g, and 129 minutes at 102.000 × g in an Optima XPN-100 Ultracentrifuge (Beckman Coulter) with a fixed-angle rotor (Type 70 Ti, Beckman Coulter) in thick wall polycarbonate tubes (Beckman Coulter). Pelleted microvesicles were either resuspended in QIAzol Lysis Reagent (Qiagen) for TaqMan-based miR analysis or in MV-podocyte media and added to human podocytes in culture. Resuspended microvesicles from the GEC supernatant were added to 7–10-day differentiated human podocytes, and cultured for a further 24–48 hours.
Microvesicle Staining
Isolated microvesicles were stained with the PKH67 Green Fluorescent Cell Linker Kit (Sigma Aldrich) according to the manufacturer’s protocol. The washing step was carried out at 102.000 × g for 2 hours at 4°C.
GEC Electroporation
To transfect a CD63-pHluorin reporter plasmid, which was a kind gift from Frederic J. Verweij, Institut National de la Santé et de la Recherche Médicale, Paris, and published before,34 or a GFP-expressing plasmid (pE-GFP-N1, addgene), cells were prepared by washing with PBS and Opti-MEM Medium (ThermoFisher Scientific). Then 10 µg of plasmid per 1–2.5 million cells were electroporated at 280 V, Discharge Interval 50, 1=1MSEC (Progenetor 11, Hoefer). Cells were seeded and cultivated at 37°C. In the case of subsequent transfection with miR-192–Alexa555, electroporated cells were allowed to adhere for at least 24 hours.
Coculture of Human Immortalized Podocytes and Human GECs
Before coculture, human immortalized podocytes, differentiated for 10–14 days at 37°C, were labeled with 10 µM eBioscience Cell Proliferation Dye eFluor 450 (Invitrogen) according to the manufacturer’s instruction. Blue fluorescent human immortalized podocytes and GFP-labeled human GECs, transfected with miR or treated with lipofectamine only, were cocultured for 48 hours using the hanging droplet method.35 Podocyte media and endothelial cell media was mixed 1:2 and 4000 podocytes and 1600 GECs per droplet were used. The coculture was either tracked for 48 hours with an automated imaging machine (Acquifer) or incubated at 37°C for 48 hours to be imaged with a confocal microscope. For this, the coculture was fixed, after washing with PBS, with histofix (Roth) for 15 minutes.
Human Urine Samples
Urine samples of patients with MGN have been obtained from the German Hamburger MGN registry. Informed consent was declared by each patient.
Statistical Analysis
For comparison of mean values between two groups, an unpaired t test was used. ANOVA was applied for comparison of data from more than two groups. Statistical significance was evaluated using GraphPad Prism. The experimental findings were considered statistically significant if P<0.05. All photomicrographs were made at similar intensity and background.
We quantified NPNT and miR-378a-3p/miR-192-5p positive areas in the glomeruli using Image J software and reported the results as mean NPNT intensity per glomerular section and positive dots per glomerular section.
Results
Glomerular miR-192-5p Is Upregulated, whereas NPNT Expression is Downregulated in Patients with iMGN
We have previously published results from a miR screening including 754 different miRs in pooled urinary samples from patients with different glomerular diseases and healthy controls published previously,36 and we identified the upregulation of miR-378a-3p and miR-192-5p in iMGN (Supplemental Figure 1).
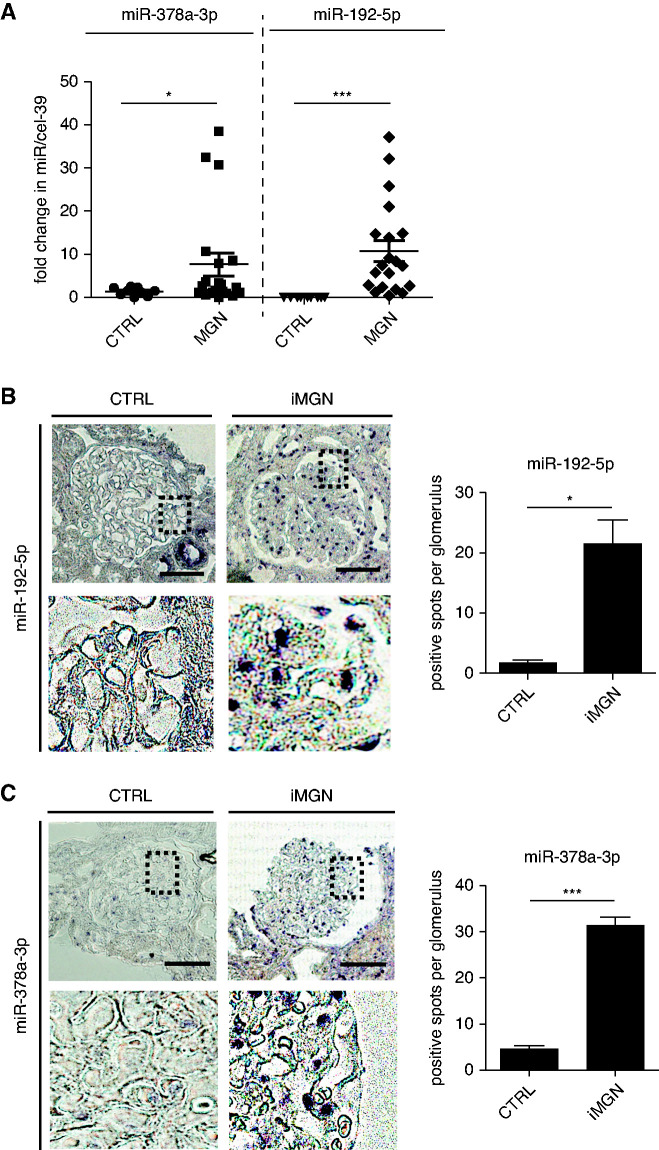
In this study, we confirmed the upregulation of miR-378a-3p and miR-192-5p in an independent cohort of patients with iMGN (Figure 1A). The most frequent autoantibodies in iMGN are anti-PLA2R-ab. Because patients who are PLA2R-ab negative, who do not have any evidence for secondary MGN, most likely have other podocyte autoantibodies, such as anti-THSD7A-ab, anti-NEP-ab, anti-NELL-1-ab, and anti-EXT1/2-ab,6,8–10,37 we did not differentiate between PLA2R-ab positive and negative iMGN. No correlation between urinary miR-378a-3p/miR-192-5p with urinary creatinine was observed, indicating that elevated miR levels in iMGN urine were not due to more concentrated urine. There also was no correlation of these urinary miRs with PLA2R-ab titer and both miRs were elevated independent from antibody status (Supplemental Figure 1B). In situ hybridization revealed an increased expression of miR-192-5p in glomeruli of patients with iMGN with predominant expression in GECs (Figure 1B). We could further confirm an upregulation of our previously published miR-378a-3p with predominant podocyte location of this miR in glomeruli of patients with iMGN comparted to control in an independent cohort of patients (Figure 1C, Supplemental Figure 2). MiR-192-5p has three different binding sides in the 3′UTR region of NPNT mRNA at position 175–195, 1184–1203, and 2522–2545 (Supplemental Figure 3, A and B).
Figure 1.
Glomerular miR-192-5p is upregulated in patients with iMGN. (A) Quantitative PCR (qPCR) for miR-378a-3p and miR-192-5p in 10 healthy controls (CTRL) and 20 patients with iMGN. Data were normalized to spiked-in cel-miR39. *P<0.05, **P<0.01. (B) Left: In situ hybridization for miR-192-5p on a representative kidney biopsy from patients with iMGN and CTRL. Scale bar=50 µm. Right: Quantification of relative glomerular miR-192-5p expression of kidney biopsies from patients with iMGN compared with CTRL. *P<0.05. n=10 patients and three controls, three glomeruli of each were analyzed. (C) Left: In situ hybridization for miR-378a-3p on a representative kidney biopsy from patients with iMGN and CTRL. Scale bar=50 µm. Right: Quantification of relative glomerular miR-378a-3p expression of kidney biopsies from patients with iMGN compared with CTRL. *P<0.05. n=10 patients and three controls, three glomeruli of each were analyzed.
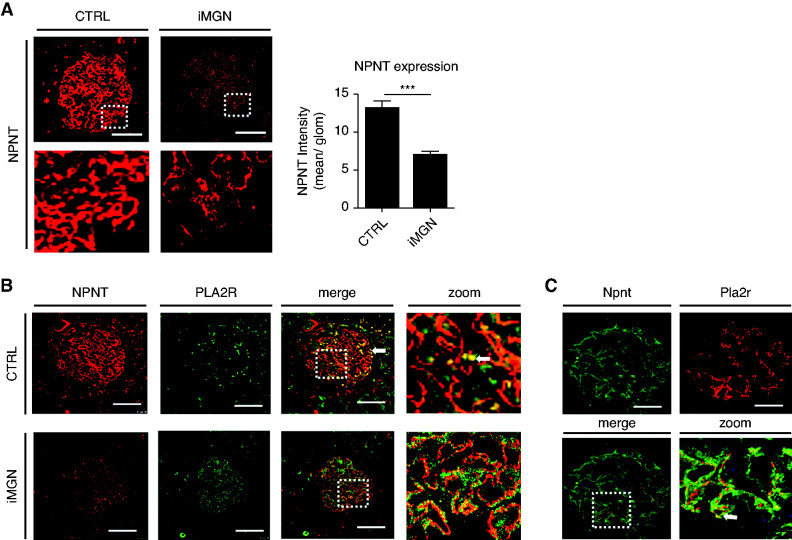
To investigate NPNT expression in iMGN, we stained the same biopsies with increased expression of miR-192-5p and miR-378a-3p for NPNT. Glomerular NPNT expression was reduced in iMGN compared with control (Figure 2A). Interestingly, we could detect a colocalization of NPNT expression with PLA2R in glomeruli of patients with iMGN (Figure 2B) and in transgenic Pla2r mice (a gift from Gunther Zahner, Hamburg) (Figure 2C). These mice express the Pla2r specifically on podocytes and a pathology comparable to MGN is induced by the injection of antiPla2r antibodies.38
Figure 2.
NPNT expression is downregulated in patients with MGN. (A) Left: Immunofluorescence staining for NPNT on a representative kidney biopsy from CTRL and patients with iMGN. Scale bar=50 µm. Right: Quantification of glomerular NPNT staining intensity from CTRL and kidney biopsies from patients with MGN. ***P<0.001. n=39 glomeruli of ten patients and three controls were analyzed. (B) Immunofluorescence staining for NPNT and PLA2R on a representative kidney biopsy from a patient with iMGN and CTRL showing colocalization. Arrowhead points to artificial staining of erythrocytes in CTRL. Scale bar=20 µm. (C) Immunofluorescence staining for Npnt and Pla2R on transgenic Pla2R overexpressing mice showing colocalization within the glomerulus. Scale bar=20 µm.
GEC-derived miR-192-5p and Podocyte-derived miR-378a-3p Regulate Expression of Podocytic NPNT
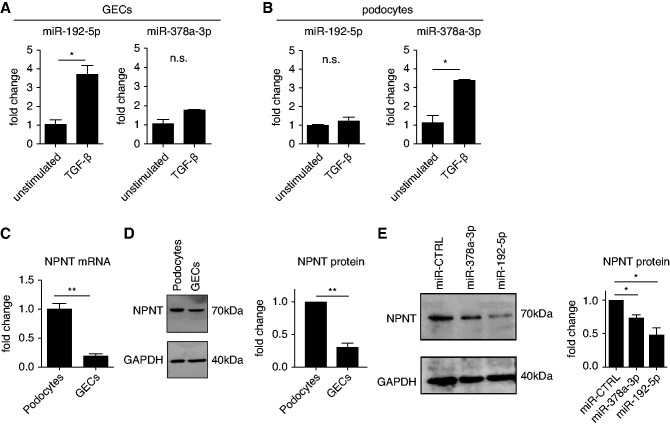
Because miR-378a-3p and miR-192-5p were increased and NPNT expression was decreased in iMGN, we investigated the miR-dependent regulation of NPNT in GEC in culture. Stimulation of human GECs with TGF-β increased miR-192-5p expression, whereas miR-378a-3p expression was unchanged (Figure 3A). In contrast, stimulation of cultured human podocytes with TGF-β increased miR-378a-3p expression, whereas miR-192-5p expression was unchanged (Figure 3B). Thus, we identified miR-192-5p as a GEC-derived and miR-378a-3p as a podocyte-derived miR. Compared with podocytes, GECs express significantly less NPNT mRNA and NPNT protein (Figure 3, C and D). Transfection of human podocytes with either a miR-378a-3p mimic or a miR-192-5p mimic decreased NPNT expression on protein level (Figure 3E), confirming that both miRs regulate podocytic NPNT expression in vitro.
Figure 3.
Glomerular endothelial cell miR-192-5p and podocyte miR-378a-3p regulate podocyte NPNT. (A) qPCR for miR-192-5p and miR-378a-3p expression normalized to U6 RNA in unstimulated and 48 hours TGF-β stimulated human GECs. Change in miR expression is given as fold change compared with the unstimulated condition. *P<0.05; n.s., nonsignificant. (B) qPCR for miR-192-5p and miR-378a-3p expression normalized to U6 RNA in unstimulated and 48-hour TGF-β–stimulated human podocytes. Change in miR expression is given as fold change compared with the unstimulated condition. *P<0.05. (C) qPCR for NPNT mRNA normalized by HPRT mRNA in cultured human podocytes and cultured human GECs. Difference in NPNT mRNA expression is given as fold change compared with podocytes. n=4; **P<0.01. (D) Left: Western blot analysis of NPNT in cultured human podocytes and cultured human GECs. Right: Difference in NPNT protein expression normalized by GAPDH is given as fold change compared with podocytes. n=3; **P<0.01. (E) Left: Western blot analysis of NPNT in cultured human podocytes after transfection with a miR-CTRL mimic, a miR-378a-3p mimic, and a miR-192-5p mimic. Right: Quantification of Western blot bands normalized by GAPDH. Results are given as fold change compared with unstimulated cells. n=4; * P<0.05.
miR-192–5p Overexpression Induces Proteinuria with Increased Lucidity and Widening of the Lamina Rara Interna of the GBM in Zebrafish
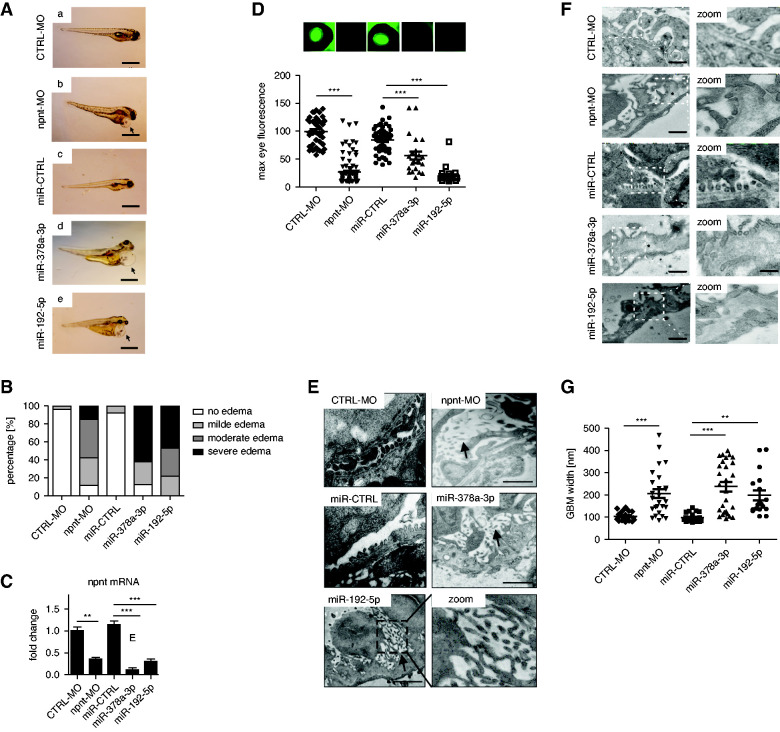
Next, we wanted to investigate the role of miR-192-5p on functional and ultrastructural changes of the GFB in vivo by using the zebrafish model. For direct comparison of both npnt-regulating miRs, we show additional data on the previously published miR-378a-3p phenotypes next to miR-192-5p overexpression and morpholino (MO)-induced npnt knockdown in the following experiments. Overexpression of miR-192-5p in zebrafish by injection of a specific miR-mimic in cell-stage one to four larvae caused pericardial and yolk sack edema, similar to the phenotype induced by npnt knockdown with a npnt-MO or miR-378a-3p overexpression described earlier (Figure 4, A and B).26 We confirmed that npnt-MO injection and miR-192-5p overexpression reduced npnt mRNA in whole zebrafish larvae (Figure 4C). Semithin sectioning of the zebrafish confirmed the edema phenotype of miR-192-5p–, miR-378a-3p–, and npnt-MO–injected zebrafish (Supplemental Figure 4, A’–E’). Next to the general edema, widening of the glomerular capillary loops were detected in MO- or miR-induced npnt knockdown animals (Supplemental Figure 4, A’’–E’’). Moreover, loss of the slit diaphragm protein Nephrin occurred in npnt-MO–, miR-378a-3p–, and miR-192-5p–injected zebrafish larvae (data not shown). To quantify the loss of plasma proteins, we used our established transgenic fluorescent zebrafish line (Tg[l-fabp:DBP:eGFP]) expressing a vitamin D–binding protein fused with eGFP.39,40 We compared loss of green fluorescent plasma proteins measured in the retinal plexus of the fish after miR mimic and MO injection indicating proteinuria. miR-192-5p mimic and npnt-MO caused significant loss of circulating proteins compared with control that was similar to the results observed with miR-378a-3p overexpression (Figure 4D).26
Figure 4.
miR- and MO-induced knockdown of npnt induces edema, loss of circulating plasma protein, GBM pathology, and loss of podocyte markers in zebrafish. (A) Phenotype pictures of zebrafish larvae at 120 hpf. Zebrafish were injected with (a) CTRL-MO, (b) npnt-MO, (c) miR-CTRL mimic, (d) miR-378a-3p mimic, and (e) miR-192-5p mimic at cell stages one to four. Scale bar = 500 µm. Arrowheads in (b)/(d)/(e) illustrate pericardial edema. (B) Quantification of phenotypic changes by grading each larva at 96 hpf in terms of severity of pericardial effusion and yolk sac edema. Numbers are given in percentage. (C) Fold change in npnt mRNA expression in zebrafish larvae at 120 hpf in CTRL-MO–, npnt-MO–, miR-CTRL–, miR-378a-3p mimic–, and miR-192-5p mimic–injected zebrafish larvae. *P<0.05, **P<0.01, *** P<0.001. (D) Representative pictures of the zebrafish eye and quantification of loss of circulating high molecular weight proteins by measuring maximum eye fluorescence in the retinal vessel plexus of Tg(l-fabp:DBP:eGFP) zebrafish at 120 hpf. Zebrafish were injected with CTRL-MO or npnt-MO, miR-CTRL mimic, miR-378a-3p mimic, and miR-192-5p mimic at cell stages one to four. ***P<0.001; n=298. (E) TEM of zebrafish pronephroi at 120 hpf shows microvillus formation of podocytes and podocyte protrusion in the GBM after npnt-MO, miR-378a-3p, or miR-192-5p mimic injection (black arrows). Zebrafish were injected with MOs or miR mimics at cell stages one to four. Scale = 500 nm. (F) TEM pictures of the zebrafish GFB. Zebrafish larvae were injected with CTRL-MO, npnt-MO, miR-CTRL mimic, miR-378a-3p mimic, and miR-192-5p mimic. All zebrafish had an age of 120 hpf at the time of fixation. Scale bar = 500 nm. Stars illustrate widening of the GBM in npnt-MO–, miR-378a-3p mimic–, and miR-192-5p mimic–injected zebrafish. (G) Statistical analysis of GBM thickness in CTRL-MO–, npnt-MO–, miR-CTRL mimic–, miR-378a-3p mimic–, and miR-192-5p mimic–injected zebrafish larvae. ***P<0.001, **P<0.01. n=10. TEM, transmission electron microscopy.
In the search for the structural correlate for proteinuria, we found podocyte damage with podocyte effacement and formation of long apical microvilli, which was described before41 in npnt-MO– and miR-192-5p mimic–injected zebrafish, comparable with miR-378a-3p–mimic overexpression (Figure 4E, arrows). Next to podocyte damage, widening of the lamina rara interna and lamina densa of the GBM due to increased lucidity, splicing and lamellation was evident in npnt-MO–, miR-192-5p mimic–, and miR-378a-3p–injected zebrafish (Figure 4F). These structural changes of the GBM were very similar to splicing and lamellation seen in advanced stages of human iMGN (Supplemental Figure 5). In contrast, podocyte foot processes, GBM, and fenestrated glomerular endothelium were well preserved in miR-CTRL– and CTRL-MO–injected zebrafish. We quantified GBM morphologic changes by measuring the GBM thickness. Mean GBM thickness of CTRL-MO–injected zebrafish was 104 nm (78–144 nm), whereas that of npnt-MO–injected zebrafish was 205 nm (85–468 nm). The mean GBM width of miR-378a-3p mimic–injected zebrafish was 237 nm (94–398 nm) and that of miR-192-5p–injected zebrafish was 198 nm (104–404 nm), which was significantly more than in CTRL-miR with mean GBM thickness of 98 nm (79–142 nm) (Figure 4G).
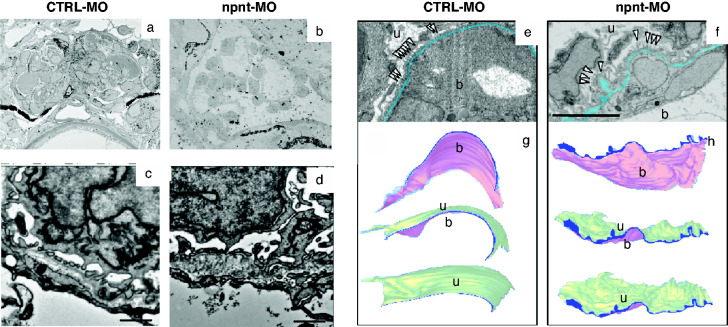
To determine whether the detected changes are focal or generalized, we analyzed pronephroi of control and npnt-MO injected zebrafish by SBF-SEM (Figure 5, A–D). This method enabled us to perform a precise three-dimensional visualization of the GBM throughout the whole glomerulus. Three-dimensional reconstruction allowed to judge the extent of ultrastructural alterations in the z-direction and could rule out that GBM pathology in npnt knockdown zebrafish was an artifact due to the cutting angle. Morphologic abnormalities were found throughout the glomerulus (Figure 5, E–H, Supplemental Videos 1 and 2). Using volume electron microscopy, we were able to evaluate the topography of the GBM over a range of several micrometers, illustrating its wrinkled appearance due to the irregular thickness of the GBM in the npnt-knockdown zebrafish. In control zebrafish, the GBM was normal and appeared in a thin layer that was regular on the blood and on the urinary side (Figure 5, G and H).
Figure 5.
Three-dimensional reconstruction of the GBM. (A) and (B) TEM of the whole pronephros of (A) CTRL-MO– and (B) npnt-MO–injected zebrafish larvae used for SEM analysis. Representative TEM images of SBF-SEM of (C) CTRL-MO– and (D) npnt-MO–injected zebrafish from SBF-SEM samples. Scale bar = 1 µm. (E) and (F) SBF-SEM of the GFB of CTRL-MO (E), and npnt-MO (F) used for three-dimensional reconstruction. The GBM is labeled in blue. Scale bar = 5 µm. (G) and (H) Three-dimensional reconstruction of the GFB of (G) CTRL-MO– and (H) npnt-MO–injected zebrafish demonstrating the GBM from the urinary side (u) and from the blood side (b). The GBM is labeled in blue. Analysis was performed at 120 hpf. White arrowheads demarcate podocytes.
Loss of npnt in the GBM Allows Penetration of Albumin- and IgG-Sized Nanoparticles through the GFB
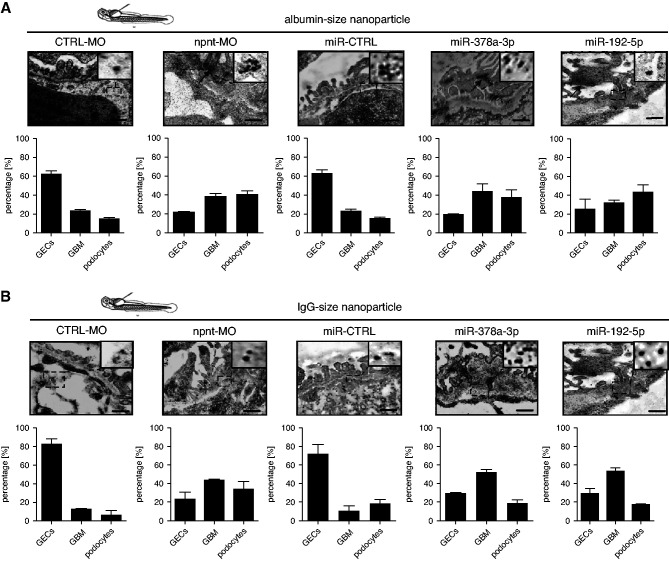
We hypothesized that loss of npnt could not only lead to proteinuria, but also have a pathophysiological relevance for the penetrance of autoantibodies through the GBM. To prove that the GFB was on the side of plasma protein loss after npnt knockdown, we injected nanoparticles of different sizes in the zebrafish circulation, and analyzed their location in the glomerular filter with the help of electron microscopy. These nanoparticles were a gift from Marlon Lawrence and were previously published.32 Nanoparticles comparable with a size of albumin passed the GFB only in low concentrations in control zebrafish. In miR-378a-3p mimic–, miR-192-5p mimic–, and npnt-MO–injected zebrafish, albumin-sized nanoparticles crossed the GFB in high amounts and partially accumulated in podocytes (Figure 6A).
Figure 6.
Penetration of nanoparticles through the GFB reveal functional impairments after MO– and miR–induced npnt knockdown. (A) Upper panel: TEM pictures of GFB of zebrafish larvae at 120 hpf. Zebrafish were injected with a CTRL-MO or npnt-MO (100 µM), miR-CTRL mimic, miR-378a-3p mimic, and miR-192-5p mimic. After 120 hpf 4.6 µl of NaSCN gold nanoparticles with the size of albumin were injected in the cardinal vein of the fish. Zebrafish were fixed 10 minutes later for embedding. The insert on the upper right of each picture gives a higher magnification to better visualize the particles. Black arrows show accumulation of particles in podocytes. Scale bar=250 nm. Lower panel: Quantification of albumin size nanoparticles in GECs, GBM, and podocytes in CTRL–, NPNT-MO–, miR-CTRL–, miR-378a-3p–, miR-192–5p–injected zebrafish. Calculation is given in percentage of particles quantified over 2 µm of GFB. (B) Upper panel: TEM pictures of GFB of zebrafish larvae at 120 hpf. Zebrafish were injected with a CTRL-MO or npnt-MO (100 µM), miR-CTRL mimic, miR-378a-3p mimic, and miR-192-5p mimic. After 120 hpf, 4.6 µl of NaSCN gold nanoparticles with the size of IgG were injected in the cardinal vein of the fish. Zebrafish were fixed 10 minutes later for embedding. The insert on the upper right of each picture gives a higher magnification to better visualize the particles. Scale bar=250 nm. Lower panel: Quantification of IgG size nanoparticles in GECs, GBM, and podocytes in CTRL–, NPNT-MO–, miR-CTRL–, miR-378a-3p–, miR-192-5p–injected zebrafish. Calculation is given in percentage of particles quantified over 2 µm of GFB.
IgG-sized nanoparticles accumulated at the endothelial side of the GBM in control fish (Figure 6B). In miR-378a-3p mimic–, miR-192-5p mimic–, and npnt-MO–injected zebrafish, IgG-sized nanoparticles passed the GFB, especially in areas where the GBM was affected (Figure 6B). This indicates an increased permeability of the GFB allowing a transglomerular passage of macromolecules up to the size of IgG in the npnt knockdown situation.
Podocyte-specific Npnt Knockout Mice Develop Proteinuria and Widening of the Lamina Rara Interna of the GBM
In contrast to the zebrafish experiments that caused a whole body npnt knockdown, conditional gene targeting using Cre recombinase provides a tool to achieve gene deletion precisely in defined cells and at specific times of development. This was of special importance because full knockout of Npnt causes kidney agenesis or hypoplasia.42,43
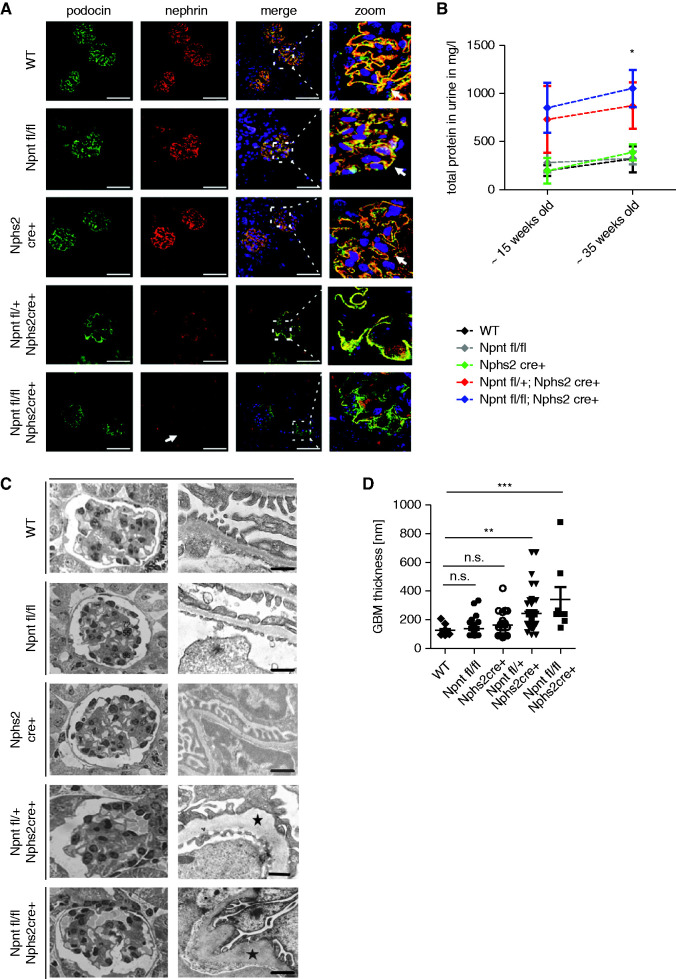
On breeding Npnt floxed (Npnt fl/fl) mice with mice transgenic for Cre recombinase under the control of a nphs2 promoter, Npnt was excised by Cre recombinase only in podocytes. Because we were aware of reports showing that Cre expression can cause toxicity, cell-specific injury, and pathologic remodeling of the GBM, even in the absence of a floxed allele44 we designed our experiments to include a control group that expressed the Cre transgene alone (Nphs2cre+), and floxed Npnt genes (Npnt fl/fl) alone. Hematoxylin and eosin staining of kidney tissue sections showed no significant abnormalities at 14 and 35 weeks of age (Supplemental Figure 6A). Podocyte Npnt staining was reduced in Npnt fl/+; Nphs2 cre+ mice and not detectable in Npnt fl/fl; Nphs2 cre+ mice (Supplemental Figure 6B). Immunofluorescence staining of podocin and nephrin revealed colocalization of both proteins in control mice but significant loss of Nephrin in Npnt fl/+; Nphs2 cre+ mice. Nephrin loss was aggravated in Npnt fl/fl; Nphs2 cre+ mice (Figure 7A). Interestingly, podocyte-specific npnt mutants did not develop significant proteinuria until 30 weeks of age. Only at 35 weeks of age, proteinuria became significant (Figure 7B). Serum creatinine levels were not significantly different from control in podocyte-specific Npnt knockout mice (Supplemental Figure 7A).
Figure 7.
Podocyte-specific Npnt knockout mice show altered expression of podocyte markers and structural impairments of the GBM. (A) Immunofluorescence staining for nephrin and podocin in WT mice, Npnt fl/fl mice, Nphs2 cre+ mice, Npnt fl/+; Nphs2 cre+ mice and Npnt fl/fl; Nphs2 cre+ mice. Nuclei were stained with Hoechst. The merged pictures and higher magnifications of merged pictures are given to illustrate colocalization of podocin and nephrin (white arrows in zoom picture). In Npnt fl/fl; Nphs2 cre+ mice nephrin expression is reduced (white arrow). Scale bar=50 µm. (B) Proteinuria in mg/L in WT, Npnt fl/fl, Nphs2 Cre+, Npnt fl/+; Nphs2 cre+ and Npnt fl/fl; Nphs2 cre+ mice at approximately 15 and approximately 35 weeks of age. *P<0.05. (C) Semithin sections of glomeruli and TEM picture of the ultrafiltration barrier of 15-week-old mice. WT, Npnt fl/fl; Nphs2 cre+, Npnt fl/+; Nphs2 cre+; and Npnt fl/fl; Nphs2 cre+ mice are shown as indicated. Black star illustrates GBM pathology. Scale bar=500 nm. (D) Statistical analysis of GBM width of 15 weeks old WT, Npnt fl/fl, Nphs2 cre+, Npnt fl/+; Nphs2 cre+ and Npnt fl/fl; Nphs2 cre+ mice. *P<0.05, **P<0.01, ***P<0.001. WT, wild type.
On an ultrastructural level, the GFB of Npnt fl/fl mice and Nphs2 cre+ mice were comparable to wild-type mice. In contrast Npnt fl/+; Nphs2 cre+ mice and Npnt fl/fl; Nphs2 cre+ mice showed rarefication of cell number in capillary loops and areas of widened GBM with severe structural changes and increased GBM lucidity and partial podocyte effacement (Figure 7, C and D). These experiments show that specific loss of podocyte Npnt rather than systemic Npnt loss induces the glomerular phenotype. To investigate the hypothesis that loss of Npnt in the GBM allows the presentation of podocyte antigens to immune cells and induces autoantibody production, cell lysate of murine podocytes was electrophoresed in a 10% polyacrylamide gel under nonreducing conditions and transferred to a nitrocellulose membrane. Sera of Npnt f/fl; Nphs2 cre+ mice and sera of control mice were used as primary antibody at a dilution of 1:100. Antimouse IgG was used as secondary antibody. The serum of Npnt fl/fl; Nphs2cre+ mice showed more immunoreactivity toward podocyte proteins because we detected more bands of higher molecular weight on western blot. This was a first hint for potential autoimmunity against podocyte antigens in our Npnt knockout mouse, which needs further validation (Supplemental Figure 7B).
miR-192-5p Travels from GECs to Podocytes via Exosomes Causing Decrease in NPNT Expression
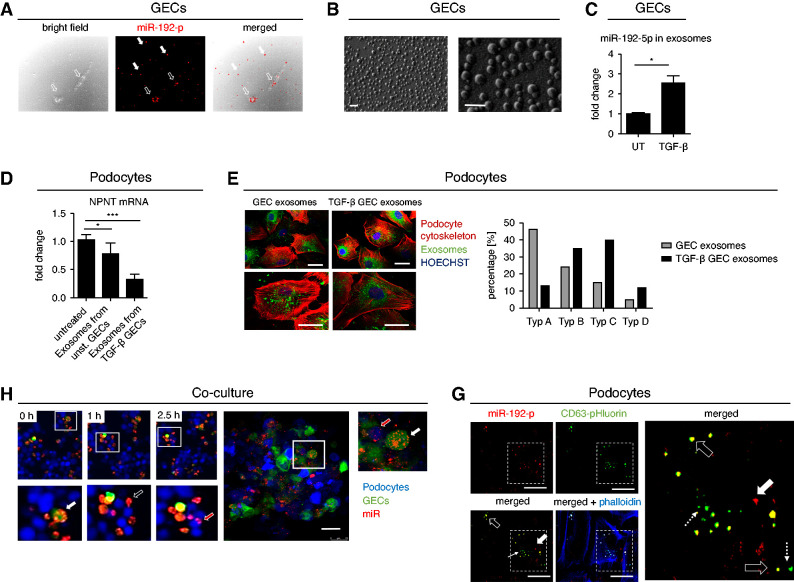
Podocytes and GECs both express miR-192-5p, although GECs are the predominate cell type (Figure 3, A and B). To unravel transport mechanisms of paracrine miR communication we transfected GECs with a red fluorescent miR-192-5p mimic and could show that miR-192-5p was secreted (Figure 8A).
Figure 8.
GEC-derived exosomes carry miR-192-5p that is taken up by podocytes causing a decrease in NPNT expression. (A) Secretion of Alexa555-tagged miR-192-5p mimic (red) from GECs. White arrows show miR-192-5p outside the cells, black arrow heads show miR-192-5p inside the GECs. Scale bar = 50 µm. (B) Surface electron microscopy image of GEC-derived exosomes secreted in the cell culture supernatant. Scale bar 20 nm. (C) qPCR for miR-192–5p expression normalized to U6 RNA in secreted exosomes derived from unstimulated and TGF-β-stimulated GECs. Change in miR expression is given as fold change compared with the unstimulated condition. n=4; *P<0.05. (D) qPCR for NPNT mRNA in cultured human podocytes treated with exosomes derived from untreated and TGF-β–treated GECs. n=3, ***P<0.001. (E) Treatment of cultured human podocytes with exosomes (labeled in green) derived from unstimulated and TGF-β–stimulated GECs. Exosomes derived from TGF-β–stimulated GECs caused cytoskeletal rearrangements (labeled in red). Scale bar 25 µm. (F) Left: Still frames of a time-lapse of three-dimensional coculture of GFP-labeled human GECs (green) with podocytes that were in vivo labeled with cell proliferation dye eFluor 450 (blue). GECs were transfected with Alexa555-tagged miR-192-5p mimic (red) before coculture. Higher magnifications in the lower row illustrate miR-192-5p in GECs (white arrow), miR-192-5p outside the cells (black arrow) and miR-192-5p inside podocytes (red arrow). Right: Confocal fluorescent microscopy picture of the same coculture after 24 hours to show higher resolution. Red fluorescent miR-192-5p is located inside the green fluorescent GECs (white arrow) and inside the blue fluorescent podocytes (red arrow). Scale bar = 50 µm. (G) Endocytosis of miR-192-5p containing GEC-derived exosomes in cultured human podocytes. CD63-pHluorin plasmid was electroporated into GECs before transfection with Alexa555-tagged miR-192-5p. Exosomes were isolated from GECs and incubated with human podocytes. Black arrows show overlap of red fluorescent miR-192-5p and green fluorescent exosomes (miR-192-5p containing exosomes), dotted white arrows show exosomes without miR-192-5p and thick arrow shows naked miR-192-5p. Scale bar = 50 µm.
We isolated exosomes from the supernatant of untreated and TGF-β–treated GECs (Figure 8B). miR-192-5p expression was not only increased in cell lysates of GECs but was also elevated in exosomes derived from GECs treated with TGF-β compared with untreated cells (Figure 8C). Furthermore, podocyte treatment with exosomes derived from TGF-β–stimulated GECs decreased NPNT mRNA (Figure 8D). Thus, we hypothesize that GEC-derived miR-192-5p is transferred to podocytes via exosomes, leading to decreased NPNT expression. We could show that GEC-derived exosomes, which were fluorescently labeled after isolation from the cell culture supernatant, were taken up by podocytes (Figure 8E). Exosomes derived from TGF-β–stimulated GECs caused cytoskeletal rearrangement and loss of stress fibers in podocytes as a hint for podocyte damage (Figure 8E).
Next, we transfected green-fluorescent GECs with a red fluorescent miR-192-5p mimic and cocultured the GECs with podocytes labeled with a blue fluorescent in vivo dye three dimensionally. In time-lapse experiments, we could live track the release of miR-192-5p from GECs and the uptake of miR-192-5p in podocytes in this coculture (Figure 8F, Supplemental Video 3). However, these experiments were not able to differentiate between exosome-mediated miR transfer and naked miR transfer. Therefore, we used a pH-dependent green fluorescent CD63 plasmid (pCMV-CD63-pHluorin plasmid) published before by Verweij et al.34 In this plasmid, a pH-sensitive optical reporter (CD63-pHluorin) is quenched when facing the acidic lumen of the MVB. On fusion with the plasma membrane at the time of exocytosis, low luminal pH is immediately neutralized, resulting in a sudden increase in green fluorescent intensity. By electroporation of this plasmid into GECs and transfecting the cells with red fluorescent miR-192-5p mimic, we could track endocytosis of these miR-containing exosomes in podocytes (Figure 8G).
Discussion
GBM proteins include collagen IV, laminin, heparan sulfate proteoglycan, nidogen, and other matrix proteins, such as NPNT. Disturbance in different GBM proteins has been reported in different glomerular diseases. Mutations affecting the laminin β2 gene cause either Pierson syndrome or congenital nephrotic syndrome type 5.45 Alport syndrome caused by mutations in collagen IVα3, collagen IVα4, or collagen IVα5 genes presents with hematuria and can lead to ESKD. Typically, the GBM in Alport syndrome is markedly thickened with a basket-like structure.46,47
Although there has been a lot of discussion about the structural correlate for the barrier, the GBM (beside podocyte slit diaphragms) is still believed to be the most important barrier component. Functional and physiologic analyses of the GFB using various tracers revealed the importance of the GBM as a size- and charge-selective filtration barrier.1,2 Molecules larger than albumin were restricted to the inside of the glomerular capillary loops and were impaired from traversing the GBM.3 Lawrence et al.32 showed that gold nanoparticles penetrate the lamina densa of the GBM in a size-dependent manner. Nanoparticles comparable in size with IgG dimers did not permeate it. They also showed the slit diaphragm is essential for capillary structure, but may not directly determine glomerular size selectivity. Even in primary podocyte disease, the GBM might be the critical component of the GFB as podocyte structures are connected to the GBM.1 GBM abnormalities and GEC damage rather than podocyte effacement were found as prognostic markers in glomerular disease.48 iMGN is caused by autoantibodies binding to podocyte antigens.4–6 However, it remains elusive how autoantibodies reach the subepithelial space if the GFB is almost not permeable for antibodies under normal conditions.49 It has been suggested before that other cells expressing PLA2R1/THSD7A, for example in the lung, may become damaged and release extracellular vesicles, leading to the onset of autoimmune activity and the development of iMGN. A significant correlation not only between various inflammatory lungs diseases and air pollution but also between iMGN and the concentration of respirable particulate matter 2.5 has been discovered.50 In contrast, air pollution also triggers the release of miRs. For example, 54 circulating miRs were found to be dose- and pollutant species–dependently associated with particulate matter 10, particulate matter 2.5, black carbon, ultrafine particles, and NO2.51 Hou et al.52 also detected particulate matter effects on inflammation-related genes targeted by miR-192-5p. Thus, air pollution might trigger the release of miR-192-5p from endothelial cells. Narrowing of glomerular capillaries, loss of fenestra of endothelial cells, and an increased number of endothelial cells in capillary lumens could be shown in iMGN, indicting GEC injury.53 We could detect an upregulation of miR-192-5p in stressed GECs. In this study, we hypothesize that an miR-mediated change in GBM protein composition might enable autoantibodies to reach the subpodocyte space in MGN. We demonstrated that the loss of NPNT is also associated with a changed expression of PLA2R and an increase and possible redistribution of PLA2R expression as it is observed in iMGN. This could also be an important step to expose PLA2R to autoantibodies. Analyzing miR-screening data from patients with different glomerular diseases showed miR-378a-3p and miR-192-5p upregulated in urine from patients with iMGN.36 We confirmed the upregulation of podocyte-derived miR-378a-3p and GEC derived miR-192-5p in an independent cohort of patients with iMGN. miR-192-5p and miR-378a-3p were further upregulated in glomeruli of patients with iMGN and both miRs target NPNT. In line with our findings, miR-192-5p was found to be elevated in blood and urine in patients with MGN in a previously published study.54
To discover the functional role of miR-192-5p in vivo, we used our zebrafish model and directly compared the results with our previous published miR-378a-3p and npnt-knockout model. Overexpression of miR-192-5p caused edema, loss of plasma proteins, and severe alterations of the GBM with splicing and increased lucidity leading to a thickened appearance of the GBM that resembled the phenotype of iMGN and that of our previously published model of miR-378a-3p and npnt-MO.26 Normal human GBM thickness has been estimated in several reports. Osawa et al.55 showed that the average thickness of normal GBM is 315 nm. In other reports, the mean GBM thickness was reported to be 370±50 nm in male adults and 320±50 nm in female adults.56 Randles et al.57 reported that the GBM of mice is much thinner, with a mean thickness of around 100 nm in 16–18 week of wild-type mice. As far as we know, the GBM thickness of zebrafish larvae has not been reported before. Control zebrafish had a GBM thickness comparable with that of adult mice, with a mean width of approximately 100 nm. In contrast, npnt-MO–, miR-378a-3p–, and miR-192-5p–injected zebrafish larvae developed significant thickening of the GBM. However, conventional transmission electron microscopy cannot elucidate the three-dimensional relationships between glomerular cells and the extracellular matrix. A novel innovative technology that enables the investigation of the three-dimensional ultrastructure of specimens is SBF-SEM. In this study, the specimen is automatically cut by an ultramicrotome and images are observed by backscattered electrons.58,59 We analyzed pronephroi of control and npnt knockdown zebrafish by SBF-SEM. This type of microscopy enabled us to evaluate the topography of the GBM over a range of several micrometers. Three-dimensional analysis and reconstruction revealed that widening of the GBM was found throughout the glomerulus in npnt knockout zebrafish. SBF-SEM further allowed us to illustrate its wrinkled appearance, especially on the urinary side in npnt knockdown zebrafish. To our knowledge, this is the first report of SBF-SEM of zebrafish pronephroi.
To investigate filter properties of the GFB, we injected differently sized nanoparticles in our zebrafish npnt knockout models. Lawrence et al.32 already demonstrated the importance of the GBM in size selection with the help of nanoparticles in mouse models of Pierson syndrome and Alport syndrome. In other reports, nanoparticles with a size <10 nm were found to transverse the glomerular endothelial fenestration, whereas those >130 nm did not pass through the endothelial cell pores.60
In our study, we compared the permeability of the GFB of npnt knockdown zebrafish and miR-378a-3p–/miR-192-5p–overexpressing zebrafish to control, and could show an increased leakiness of IgG-size nanoparticles in npnt knockdown and miR overexpressing zebrafish. In miR-378a-3p mimic–, miR-192–5p mimic–, and npnt-MO–injected zebrafish IgG-sized nanoparticles were distributed through the thickened GBM and penetrated effaced podocytes. Particles tended to accumulate in thickened areas within the GBM. Similarly, ferritin particles were present within intracellular vesicles and lysosomes after injection in Alport mice.61
MO- and miR-based experiments in zebrafish caused a rather unspecific npnt knockdown in all cells of the body. Full knockout of Npnt caused kidney agenesis or hypoplasia.42,43 Furthermore, Patra et al.62 reported the role of npnt in heart development. Therefore, renal and cardiac contribution to our described phenotype had to be distinguished. We used the Cre-loxP system to generate podocyte-specific npnt knockout mice. Balkawade et al.44 reported that Cre expression can cause toxicity, cell-specific injury, and pathologic remodeling of the GBM even in the absence of a floxed allele. Thus, appropriate controls for potential Cre-mediated toxicity in conditional genes are needed when working with Cre-loxP system. We used Nphs2 cre+ and Npnt fl/fl mice as controls. Nphs2 cre+ and Npnt fl/fl mice developed regular GBM and podocytes. Npnt fl/+; Nphs2cre+ mice and Npnt fl/fl; Nphs2 cre+ mice showed areas of disturbed GBM with widening of the lamina densa. Interestingly, structural abnormalities in Npnt fl/+; Nphs2 cre+ mice and Npnt fl/fl; Nphs2 cre+ mice preceded proteinuria. Several studies have shown that GBM thickening and mesangial expansion initially develop without albuminuria.63–65 Furthermore, GBM alterations may begin as early as 2 years after the onset of diabetes, before albuminuria.19–21,66 Similarly, podocyte effacement can occur in the absence of proteinuria.67 In addition to the GBM pathology, reduced nephrin expression was seen in podocyte-specific Npnt knockout mice. Loss of nephrin was reported to occur independent of podocyte loss as an early event in proteinuric kidney diseases.68 Furthermore, we detected first hints for the induction of autoimmunity toward podocyte antigens in podocyte-specific Npnt knockout mice, suggesting that loss of Npnt in the GBM allows the presentation of podocyte antigens to immune cells. However, we were not able to detect subepithelial immune complexes in our model. Thus, these finding need further investigation in aged mice.
Taken together, our podocyte-specific Npnt knockout mouse model underlines the cell-specific importance of podocyte Npnt for proper GBM structure and glomerular filter functions.
To unravel transport mechanisms of the paracrine miR communication, we investigated the role of exosomes. Exosomes are a type of small extracellular vesicles that are formed as intraluminal vesicles in late endosomal organelles called multivesicular bodies and secreted after fusion with the plasma membrane, and thereby deliver specific cellular cargo to recipient cells in diverse contexts.69 In the kidney, increasing evidence supports that exosomes are signaling vesicles for intraglomerular, tubular-glomerular, and tubule-interstitial communication.70 It was also described before that miRs were packaged into protective exosomes in a different context.71 We could show that exosomes secreted by TGF-β–treated GECs contained increased miR-192-5p, which decreased podocyte NPNT and caused cytoskeleton rearrangements.
To prove the hypothesis of exosome-mediated paracrine signaling of miR-192-5p, we performed time-lapse experiments and tracked the release of miR-192-5p from GECs and the uptake of miR-192-5p in podocytes in coculture experiments. To unravel the mechanism of this paracrine signaling, we used a pCMV-CD63-pHluorin plasmid-labeling exosomes after pH change on release from cells.34 By electroporation of this plasmid into GECs and transfecting the cells with red fluorescent miR-192-5p mimic, we could track exocytosis of miR-192-5p–containing exosomes in GECs and endocytosis of these exosomes in podocytes. Thus, GEC-derived miR-192-5p downregulated podocyte NPNT via exosome-dependent paracrine signaling. Similar to our results, mRNA transport from GECs to podocytes has been described previously.37
We are aware that our model cannot reflect the complexity of GBM changes of human disease, but our data support the hypothesis that GBM components can adapt very rapidly and induce changes of GBM architecture, leading to increased permeability of the GFB and potentially autoimmunity.
Paracrine miR signaling mediated by exosome transport is an interesting mechanism for glomerular communication in physiology and disease. GEC-derived miRs targeting GBM proteins might play a role in iMGN by increasing GBM permeability, allowing autoantibodies to reach the subpodocyte space.
Disclosures
K. Amann reports receiving honoraria from Bayer Co. and Novartis. M. Schiffer reports consultancy agreements with Walden Biosc.; reports receiving honoraria from Astellas, Chiesi, GlaxoSmithKline, and Novartis; and reports being a scientific advisor to or member of Kidney International. All remaining authors have nothing to disclose.
Funding
This work was supported by the Deutsche Forschungsgemeinschaft (MU 3797/1-1, SCHI 587/11-1). This work was also partially funded by Jochen-Kalden-Förderprogramm of IZKF, Friedrich Alexander University of Erlangen, Else Kröner-Fresenius-Stiftung and the Eva Luise und Horst Köhler Stiftung – Project No: 2019_KollegSE.04 and Research Center On Rare Kidney Diseases (RECORD), University Hospital Erlangen.
Supplementary Material
Acknowledgments
We thank Marlon Lawrence for providing NaSCN oligo clusters in the size of albumin and IgG for this study. For excellent technical assistance with SBF-SEM sample preparation, we acknowledge Susanne Fassbender. We also thank Prof. Dr. Christian Mühlfeld for fruitful discussions and expert opinion. Urine from patients with iMGN was obtained from the German Hamburger MGN registry. Prof. Dr. K. Amann reviewed the manuscript and gave expert opinion on electron microscopy data; Prof. Dr. C. Daniel provided human histology slides and electron microscopy pictures of patients with iMGN; M. Gröner performed cardinal vein injections of nanoparticles in zebrafish larvae; Dr. J. Hegermann and Dr. C. Wrede performed transmission electron microscopy/SBF-SEM and three-dimensional reconstruction of zebrafish pronephros; Prof. Dr. J. Müller-Deile wrote the manuscript, prepared all figures, performed zebrafish experiments, in situ hybridization, and transmission electron microscopy of zebrafish and mice; A. Ohs performed immunofluorescence in mice and helped with zebrafish and mouse experiments; V. Rose performed coculture experiments with GECs and podocytes; Dr. N. Sopel performed cell cultures, zebrafish, and mouse experiments; Prof. Dr. M. Schiffer reviewed the manuscript; and Prof. Dr. G. Zahner provided kidney tissue samples from Pla2R-antibody induced MGN mouse model.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
Supplemental Material
This article contains the following supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2020121699/-/DCSupplemental.
Supplemental Figure 1. Urinary miR-378a-3p and miR-192-5p is upregulated in patients with iMGN independent from urine concentration and PLA2R titer.
Supplemental Figure 2. Positive and negative controls for miR in situ hybridization and immunofluorescence staining.
Supplemental Figure 3. miR-378a-3p-NPNT and miR-192-5p-NPNT binding sites.
Supplemental Figure 4. Cross-section at the pronephros level and TEM pictures of the zebrafish pronephros.
Supplemental Figure 5. Ultrastructural changes of the GFB in human iMGN.
Supplemental Figure 6. Histology of podocyte-specific Npnt knockout mice.
Supplemental Figure 7. Serum of Npnt fl/fl; Nphs2cre+ mice show immunoreactivity toward podocyte proteins.
Supplemental Video 1. Three-dimensional reconstruction of wild type zebrafish larvae with the help of SBF-SEM.
Supplemental Video 2. Three-dimensional reconstruction of npnt mophant zebrafish with the help of SBF-SEM.
Supplemental Video 3. Live track of the release of miR-192-5p from GECs and the uptake of miR-192-5p in podocytes.
References
- 1.Suh JH, Miner JH: The glomerular basement membrane as a barrier to albumin. Nat Rev Nephrol 9: 470–477, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Farquhar MG, Wissig SL, Palade GE: Glomerular permeability. I. Ferritin transfer across the normal glomerular capillary wall. J Exp Med 113: 47–66, 1961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brenner BM, Hostetter TH, Humes HD: Molecular basis of proteinuria of glomerular origin. N Engl J Med 298: 826–833, 1978 [DOI] [PubMed] [Google Scholar]
- 4.Beck LH Jr, Bonegio RG, Lambeau G, Beck DM, Powell DW, Cummins TD, et al. : M-type phospholipase A2 receptor as target antigen in idiopathic membranous nephropathy. N Engl J Med 361: 11–21, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tomas NM, Beck LH, Meyer-Schwesinger C: Thrombospondin type-1 domain-containing 7A in idiopathic membranous nephropathy. N Engl J Med 371: 2277–2287, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beck LH Jr: PLA2R and THSD7A: Disparate paths to the same disease? J Am Soc Nephrol 28: 2579–2589, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Debiec H, Ronco P: Immune response against autoantigen PLA2R is not gambling: Implications for pathophysiology, prognosis, and therapy. J Am Soc Nephrol 27: 1275–1277, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bobart SA, Tehranian S, Sethi S, Alexander MP, Nasr SH, Moura Marta C, et al. : A target antigen-based approach to the classification of membranous nephropathy. Mayo Clin Proc 96: 577–591, 2021 [DOI] [PubMed] [Google Scholar]
- 9.Sethi S, Madden BJ, Debiec H, Charlesworth MC, Gross L, Ravindran A, et al. : Exostosin 1/exostosin 2-associated membranous nephropathy. J Am Soc Nephrol 30: 1123–1136, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alsharhan L, Beck LH Jr: Membranous nephropathy: Core curriculum 2021. Am J Kidney Dis 77: 440–453, 2021 [DOI] [PubMed] [Google Scholar]
- 11.Abrahamson DR: Origin of the glomerular basement membrane visualized after in vivo labeling of laminin in newborn rat kidneys. J Cell Biol 100: 1988–2000, 1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miner JH: Glomerular basement membrane composition and the filtration barrier. Pediatr Nephrol 26: 1413–1417, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yurchenco PD: Basement membranes: Cell scaffoldings and signaling platforms. Cold Spring Harb Perspect Biol 3: a004911, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miner JH: The glomerular basement membrane. Exp Cell Res 318: 973–978, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lennon R, Byron A, Humphries JD, Randles MJ, Carisey A, Murphy S, et al. : Global analysis reveals the complexity of the human glomerular extracellular matrix. J Am Soc Nephrol 25: 939–951, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sakai T, Kriz W: The structural relationship between mesangial cells and basement membrane of the renal glomerulus. Anat Embryol (Berl) 176: 373–386, 1987 [DOI] [PubMed] [Google Scholar]
- 17.Kikkawa Y, Virtanen I, Miner JH: Mesangial cells organize the glomerular capillaries by adhering to the G domain of laminin alpha5 in the glomerular basement membrane. J Cell Biol 161: 187–196, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Smithies O: Why the kidney glomerulus does not clog: A gel permeation/diffusion hypothesis of renal function. Proc Natl Acad Sci U S A 100: 4108–4113, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marshall CB: Rethinking glomerular basement membrane thickening in diabetic nephropathy: Adaptive or pathogenic? Am J Physiol Renal Physiol 311: F831–F843, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Osterby R, Gundersen HJ: Glomerular size and structure in diabetes mellitus. I. Early abnormalities. Diabetologia 11: 225–229, 1975 [DOI] [PubMed] [Google Scholar]
- 21.Caramori ML, Parks A, Mauer M: Renal lesions predict progression of diabetic nephropathy in type 1 diabetes. J Am Soc Nephrol 24: 1175–1181, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Haramoto T, Makino H, Ikeda S, Wieslander J, Ota Z: Ultrastructural localization of the three major basement membrane components–Type IV collagen, heparan sulfate proteoglycan and laminin–In human membranous glomerulonephritis. Am J Nephrol 14: 30–36, 1994 [DOI] [PubMed] [Google Scholar]
- 23.Zhang YZ, Lee HS: Quantitative changes in the glomerular basement membrane components in human membranous nephropathy. J Pathol 183: 8–15, 1997 [DOI] [PubMed] [Google Scholar]
- 24.Funk SD, Lin MH, Miner JH: Alport syndrome and Pierson syndrome: Diseases of the glomerular basement membrane. Matrix Biol 71–72: 250–261, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zimmerman SE, Hiremath C, Tsunezumi J, Yang Z, Finney B, Marciano DK: Nephronectin regulates mesangial cell adhesion and behavior in glomeruli. J Am Soc Nephrol 29: 1128–1140, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Müller-Deile J, Dannenberg J, Schroder P, Lin MH, Miner JH, Chen R, et al. : Podocytes regulate the glomerular basement membrane protein nephronectin by means of miR-378a-3p in glomerular diseases. Kidney Int 92: 836–849, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ledeganck KJ, Gielis EM, Abramowicz D, Stenvinkel P, Shiels PG, Van Craenenbroeck AH: MicroRNAs in AKI and kidney transplantation. Clin J Am Soc Nephrol 14: 454–468, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Beike L, Wrede C, Hegermann J, Lopez-Rodriguez E, Kloth C, Gauldie J, et al. : Surfactant dysfunction and alveolar collapse are linked with fibrotic septal wall remodeling in the TGF-β1-induced mouse model of pulmonary fibrosis. Lab Invest 99: 830–852, 2019 [DOI] [PubMed] [Google Scholar]
- 29.Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, et al. : Fiji: An open-source platform for biological-image analysis. Nat Methods 9: 676–682, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Belevich I, Joensuu M, Kumar D, Vihinen H, Jokitalo E: Microscopy image browser: A platform for segmentation and analysis of multidimensional datasets. PLoS Biol 14: e1002340, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Smithies O, Lawrence M, Testen A, Horne LP, Wilder J, Altenburg M, et al. : Stable oligomeric clusters of gold nanoparticles: Preparation, size distribution, derivatization, and physical and biological properties. Langmuir 30: 13394–13404, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lawrence MG, Altenburg MK, Sanford R, Willett JD, Bleasdale B, Ballou B, et al. : Permeation of macromolecules into the renal glomerular basement membrane and capture by the tubules. Proc Natl Acad Sci U S A 114: 2958–2963, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang Y, Liu D, Chen X, Li J, Li L, Bian Z, et al. : Secreted monocytic miR-150 enhances targeted endothelial cell migration. Mol Cell 39: 133–144, 2010 [DOI] [PubMed] [Google Scholar]
- 34.Verweij FJ, Bebelman MP, Jimenez CR, Garcia-Vallejo JJ, Janssen H, Neefjes J, et al. : Quantifying exosome secretion from single cells reveals a modulatory role for GPCR signaling. J Cell Biol 217: 1129–1142, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Foty R: A simple hanging drop cell culture protocol for generation of 3D spheroids. J Vis Exp 51: 2720, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Müller-Deile J, Dannenberg J, Liu P, Lorenzen J, Nyström J, Thum T, et al. : Identification of cell and disease specific microRNAs in glomerular pathologies. J Cell Mol Med 23: 3927–3939, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wu X, Gao Y, Xu L, Dang W, Yan H, Zou D: Exosomes from high glucose-treated glomerular endothelial cells trigger the epithelial-mesenchymal transition and dysfunction of podocytes. Sci Rep 7: 9371, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Meyer-Schwesinger C, Tomas NM, Dehde S, Seifert L, Hermans-Borgmeyer I, Wiech T, et al. : A novel mouse model of phospholipase A2 receptor 1-associated membranous nephropathy mimics podocyte injury in patients. Kidney Int 97: 913–919, 2020 [DOI] [PubMed] [Google Scholar]
- 39.Hanke N, King BL, Vaske B, Haller H, Schiffer M: A fluorescence-based assay for proteinuria screening in larval zebrafish (danio rerio). Zebrafish 12: 372–376, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hanke N, Staggs L, Schroder P, Litteral J, Fleig S, Kaufeld J, et al. : “Zebrafishing” for novel genes relevant to the glomerular filtration barrier. BioMed Res Int 2013: 658270, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wrede C, Hegermann J, Mühlfeld C: Novel cell contact between podocyte microprojections and parietal epithelial cells analyzed by volume electron microscopy. Am J Physiol Renal Physiol 318: F1246–F1251, 2020 [DOI] [PubMed] [Google Scholar]
- 42.Linton JM, Martin GR, Reichardt LF: The ECM protein nephronectin promotes kidney development via integrin alpha8beta1-mediated stimulation of Gdnf expression. Development 134: 2501–2509, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dai L, Li J, Xie L, Wang W, Lu Y, Xie M, et al. : A bi-allelic frameshift mutation in NPNT causes bilateral renal agenesis in humans. J Am Soc Nephrol 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Balkawade RS, Chen C, Crowley MR, Crossman DK, Clapp WL, Verlander JW, et al. : Podocyte-specific expression of Cre recombinase promotes glomerular basement membrane thickening. Am J Physiol Renal Physiol 316: F1026–F1040, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hasselbacher K, Wiggins RC, Matejas V, Hinkes BG, Mucha B, Hoskins BE, et al. : Recessive missense mutations in LAMB2 expand the clinical spectrum of LAMB2-associated disorders. Kidney Int 70: 1008–1012, 2006 [DOI] [PubMed] [Google Scholar]
- 46.Hudson BG, Tryggvason K, Sundaramoorthy M, Neilson EG: Alport’s syndrome, Goodpasture’s syndrome, and type IV collagen. N Engl J Med 348: 2543–2556, 2003 [DOI] [PubMed] [Google Scholar]
- 47.Kashtan CE: Alport syndrome and thin glomerular basement membrane disease. J Am Soc Nephrol 9: 1736–1750, 1998 [DOI] [PubMed] [Google Scholar]
- 48.Royal V, Zee J, Liu Q, Avila-Casado C, Smith AR, Liu G, et al. : Ultrastructural characterization of proteinuric patients predicts clinical outcomes. J Am Soc Nephrol 31: 841–854, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liu W, Gao C, Dai H, Zheng Y, Dong Z, Gao Y, et al. : Immunological pathogenesis of membranous nephropathy: Focus on PLA2R1 and its role. Front Immunol 10: 1809, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Xu X, Wang G, Chen N, Lu T, Nie S, Xu G, et al. : Long-term exposure to air pollution and increased risk of membranous nephropathy in China. Am Soc Nephrol 27: 3739–3746, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Krauskopf J, Caiment F, van Veldhoven K, Chadeau-Hyam M, Sinharay R, Chung KF, et al. : The human circulating miRNome reflects multiple organ disease risks in association with short-term exposure to traffic-related air pollution. Environ Int 113: 26–34, 2018 [DOI] [PubMed] [Google Scholar]
- 52.Hou L, Barupal J, Zhang W, Zheng Y, Liu L, Zhang X, et al. : Particulate air pollution exposure and expression of viral and human MicroRNAs in blood: The Beijing truck driver air pollution study. Environ Health Perspect 124: 344–350, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Morita M, Mii A, Shimizu A, Yasuda F, Shoji J, Masuda Y, et al. : Glomerular endothelial cell injury and focal segmental glomerulosclerosis lesion in idiopathic membranous nephropathy. PLoS One 10: e0116700, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhou G, Zhang X, Wang W, Zhang W, Wang H, Xin G: Both peripheral blood and urinary miR-195-5p, miR-192-3p, miR-328-5p and their target genes PPM1A, RAB1A and BRSK1 may be potential biomarkers for membranous nephropathy. Med Sci Monit 25: 1903–1916, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Osawa G, Kimmelstiel P, Seiling V: Thickness of glomerular basement membranes. Am J Clin Pathol 45: 7–20, 1966 [DOI] [PubMed] [Google Scholar]
- 56.Steffes MW, Barbosa J, Basgen JM, Sutherland DE, Najarian JS, Mauer SM: Quantitative glomerular morphology of the normal human kidney. Lab Invest 49: 82–86, 1983 [PubMed] [Google Scholar]
- 57.Randles MJ, Collinson S, Starborg T, Mironov A, Krendel M, Königshausen E, et al. : Three-dimensional electron microscopy reveals the evolution of glomerular barrier injury. Sci Rep 6: 35068, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Takaki T, Ohno N, Saitoh S, Nagai M, Joh K: Podocyte penetration of the glomerular basement membrane to contact on the mesangial cell at the lesion of mesangial interposition in lupus nephritis: A three-dimensional analysis by serial block-face scanning electron microscopy. Clin Exp Nephrol 23: 773–781, 2019 [DOI] [PubMed] [Google Scholar]
- 59.Arkill KP, Qvortrup K, Starborg T, Mantell JM, Knupp C, Michel CC, et al. : Resolution of the three dimensional structure of components of the glomerular filtration barrier. BMC Nephrol 15: 24, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Scindia YM, Deshmukh US, Bagavant H: Mesangial pathology in glomerular disease: Targets for therapeutic intervention. Adv Drug Deliv Rev 62: 1337–1343, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Abrahamson DR, Isom K, Roach E, Stroganova L, Zelenchuk A, Miner JH, et al. : Laminin compensation in collagen alpha3(IV) knockout (Alport) glomeruli contributes to permeability defects. J Am Soc Nephrol 18: 2465–2472, 2007 [DOI] [PubMed] [Google Scholar]
- 62.Patra C, Diehl F, Ferrazzi F, van Amerongen MJ, Novoyatleva T, Schaefer L, et al. : Nephronectin regulates atrioventricular canal differentiation via Bmp4-Has2 signaling in zebrafish. Development 138: 4499–4509, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Fioretto P, Steffes MW, Mauer M: Glomerular structure in nonproteinuric IDDM patients with various levels of albuminuria. Diabetes 43: 1358–1364, 1994 [DOI] [PubMed] [Google Scholar]
- 64.Lane PH, Steffes MW, Mauer SM: Glomerular structure in IDDM women with low glomerular filtration rate and normal urinary albumin excretion. Diabetes 41: 581–586, 1992 [DOI] [PubMed] [Google Scholar]
- 65.Caramori ML, Kim Y, Huang C, Fish AJ, Rich SS, Miller ME, et al. : Cellular basis of diabetic nephropathy: 1. Study design and renal structural-functional relationships in patients with long-standing type 1 diabetes. Diabetes 51: 506–513, 2002 [DOI] [PubMed] [Google Scholar]
- 66.Drummond K, Mauer M; International Diabetic Nephropathy Study Group : The early natural history of nephropathy in type 1 diabetes: II. Early renal structural changes in type 1 diabetes. Diabetes 51: 1580–1587, 2002 [DOI] [PubMed] [Google Scholar]
- 67.Lahdenkari AT, Lounatmaa K, Patrakka J, Holmberg C, Wartiovaara J, Kestilä M, et al. : Podocytes are firmly attached to glomerular basement membrane in kidneys with heavy proteinuria. J Am Soc Nephrol 15: 2611–2618, 2004 [DOI] [PubMed] [Google Scholar]
- 68.Verma R, Venkatareddy M, Kalinowski A, Li T, Kukla J, Mollin A, et al. : Nephrin is necessary for podocyte recovery following injury in an adult mature glomerulus. PLoS One 13: e0198013, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.van Niel G, D’Angelo G, Raposo G: Shedding light on the cell biology of extracellular vesicles. Nat Rev Mol Cell Biol 19: 213–228, 2018 [DOI] [PubMed] [Google Scholar]
- 70.Lv LL, Feng Y, Tang TT, Liu BC: New insight into the role of extracellular vesicles in kidney disease. J Cell Mol Med 23: 731–739, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Fleshner M, Crane CR: Exosomes, DAMPs and miRNA: Features of stress physiology and immune homeostasis. Trends Immunol 38: 768–776, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.