Significance Statement
In this retrospective observational evaluation of SARS-CoV-2 mRNA vaccine seroresponse defined by levels of Ig-G against the receptor-binding domain of the S1 subunit of SARS-CoV-2 spike antigen ≥2 U/L in patients receiving maintenance dialysis, 165 out of 186 (88.7%) were responsive (with 70% at maximum titer) ≥14 days after completing the second dose. This early study suggests that, despite the possibly reduced immunogenicity among patients on dialysis, the short-term incidence of development of antispike antibody is good, giving hope that most of these patients who are vulnerable, once immunized, will be protected from COVID-19. Longer-term evaluation is needed to determine the durability of this protection, the role of repeating vaccine series in initial nonresponders, and the role of booster doses among responders.
Keywords: dialysis, end-stage kidney disease, chronic kidney disease, vaccine, SARS-CoV-2, COVID-19, immune deficiency, clinical immunology, end-stage kidney disease
Abstract
Background
Patients receiving maintenance dialysis represent a high-risk, immune-compromised population with 15%–25% COVID-19 mortality rate who were unrepresented in clinical trials of mRNA vaccines.
Methods
All patients receiving maintenance dialysis who received two doses of SARS-CoV-2 mRNA vaccines with antibody test results drawn ≥14 days after the second dose, as documented in the electronic health record through March 18, 2021, were included. Response was on the basis of levels of Ig-G against the receptor binding domain of the S1 subunit of SARS-CoV-2 spike-antigen (seropositive ≥2 U/L) using an FDA-approved semiquantitative chemiluminescent assay (ADVIA Centaur XP/XPT COV2G).
Results
Among 186 patients on dialysis from 30 clinics in eight states tested 23±8 days after receiving two vaccine doses, there were 165 (88.7%) responders with 70% at maximum titer. There was no significant difference between BNT162b2/Pfizer (148 out of 168, 88.1%) and mRNA-1273/Moderna (17 out of 18, 94.4%), P=0.42. All 38 patients with COVID-19 history were responders, with 97% at maximum titer. Among patients without COVID-19, 127 out of 148 (85.8%) were responders, comparable between BNT162b2/Pfizer (113 out of 133) and mRNA-1273/Moderna (14 out of 15) vaccines (85.0% versus 93.3%, P=0.38).
Conclusions
Most patients receiving maintenance dialysis responded after two doses of BNT162b2/Pfizer or mRNA-1273/Moderna vaccine, suggesting the short-term development of antispike antibody is good, giving hope that most of these patients who are vulnerable, once immunized, will be protected from COVID-19. Longer-term evaluation is needed to determine antibody titer durability and if booster dose(s) are warranted. Further research to evaluate the approach to patients without a serologic response is needed, including benefits of additional dose(s) or administration of alternate options.
Nearly 550,000 people in the United States have ESKD treated with maintenance dialysis and prevalence estimates approach 3 million worldwide.1,2 These patients, particularly those receiving hemodialysis, comprise a population of vulnerable individuals who are limited in their ability to physically distance, have a high incidence of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection, and have coronavirus disease 2019 (COVID-19)–associated mortality of 15%–25%,3–5 making effective vaccination a priority. Despite this heightened risk and prior reports of diminished response to vaccines against viruses such as hepatitis-B and influenza,6–8 the immune response to SARS-CoV-2 vaccination among patients on dialysis is unknown due to insufficient trial data. Accordingly, we evaluated data from multiple Dialysis Clinic, Inc. (DCI) dialysis clinics in the United States that assessed antibody response after administration of SARS-CoV-2 mRNA vaccines.
Methods
Study Setting
DCI implemented a standard clinical protocol for systematic measurement of SARS-CoV-2 antispike protein Ig-G (spike-Ab-IgG) that physicians could activate for vaccination in the outpatient dialysis clinic, similar to that for hepatitis B postvaccination testing.6 Because access to the SARS-CoV-2 mRNA vaccines for patients on dialysis varied among and within states, the vaccine was administered at long-term care facilities, hospitals, locally designated vaccination centers and pharmacies, and in some instances, at the dialysis facility. Treating physicians or medical directors ordered postvaccination spike-Ab-IgG titers on the basis of patient accessibility and clinical interest. All vaccinations were verified and recorded in the DCI electronic health record (EHR). This interim report includes a retrospective evaluation of all patients receiving two doses of either BNT162b2/Pfizer or mRNA-1273/Moderna vaccine per the manufacturers’ recommendations across 30 DCI clinics in eight states, with spike-Ab-IgG as ordered by 46 nephrologists, measured ≥14 days after the second dose, documented as of March 18, 2021. This study was reviewed and approved by the Western Copernicus Group Institutional Review Board Work Order 1–1456342–1.
SARS-CoV-2 Spike-Ab Assay
DCI measured spike-Ab-IgG against the receptor-binding domain of the S1 subunit of SARS-CoV-2 spike antigen using US Food and Drug Administration–approved chemiluminescent assay (ADVIA Centaur XP/XPT COV2G).9,10 This semiquantitative assay has a range between 0 and ≥20 U/L, with a weakly reactive level at 1 U/L and a DCI laboratory (lab) positive threshold of ≥2 U/L. The assay system could undergo further autodilution beyond 20 U/L but the DCI lab implemented the version that stops at 20 U/L. The S1 receptor–binding domain antibodies are relevant to vaccines incorporating this immune-dominant region to elicit neutralizing (and therefore likely protective) antibodies in vaccinated individuals.11 In primary analyses, we defined responders to the vaccine as those whose postvaccination titers were ≥2 U/L and nonresponders had titers <2 U/L. As a sensitivity analysis, we calculated response rates including weakly reactive titers of ≥1 U/L.
EHR
On the basis of contemporary practices, SARS-CoV-2 infection status was assessed via nasopharyngeal, nasal, or oropharyngeal swabs sent locally for RT-PCR testing when indicated. All patients were screened before every treatment with a questionnaire for recent exposure or symptoms, and were queried about interim testing for SARS-CoV-2, or a new diagnosis of COVID-19 for them or a close contact. Furthermore, staff obtained positive test results from external sources, whether a patient was assessed in the dialysis clinic, at a testing center, or at a hospital. New COVID-19 diagnoses are tracked weekly and discrepancies in the DCI-EHR are followed up by corporate nurses for accurate documentation. Vaccination dates, spike-Ab-IgG results, demographic and clinical data including age, sex, race/ethnicity, body mass index, dialysis parameters (i.e., vintage, modality, access, adequacy), albumin, number and types of comorbidities, use of immunomodulatory agents, history of transplantation or COVID-19 diagnosis, and other vaccines administered or hospitalization within 14 days of vaccination were obtained. This retrospective evaluation was performed under Western Institutional Review Board exemption (1–1013341–1).
Statistical Evaluation
Comparisons between responders and nonresponders utilized chi-square or ANOVA for categorical variables and t test/Kruskal–Wallis/Mann–Whitney test for normal/non-normally distributed continuous variables. Statistical analyses were performed using SAS Software (version 9.4; Cary, NC).
Results
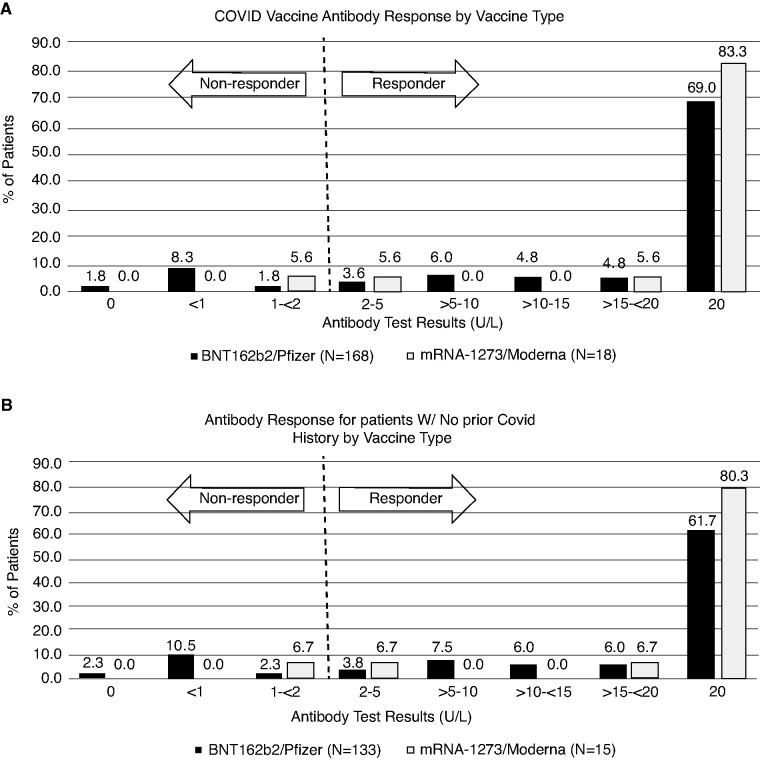
Antibody tests were ordered as part of routine monthly blood draws by 46 nephrologists in the 30 DCI clinics. A total of 395 patients on maintenance dialysis under their care were eligible, with two doses of vaccine administered by March 3, 2021. Among them, 360 patients (91%) had spike-Ab-IgG test ordered, although only 186 (47%) had results ≥14 days after their second vaccine dose available at the time of analysis on March 18, 2021. Among these 186 patients on dialysis, mean age was 68±12 years, with 47% women, 21% of Black race, 26% residents in long-term care facilities, and 97% undergoing in-center hemodialysis (Table 1). Spike-Ab-IgG was positive (≥2 U/L) in 165 out of 186 (88.7%) without significant difference between BNT162b2/Pfizer (148 out of 168) and mRNA-1273/Moderna (17 out of 18) vaccines at 88.1% versus 94.4%, P=0.42. Characteristics of recipients categorized by vaccine manufacturer are shown in Supplemental Table 1. The distribution of titer levels drawn over 23±8 days after the second dose is shown in Figure 1A. Nonresponders had mean spike-Ab-IgG titer of 0.4±0.2 versus 18.2±4.3 among responders (P<0.001). If we consider weakly reactive spike-Ab-IgG levels ≥1 U/L as responders, four patients are added, raising the overall responder rate to 90.8% (169 out of 186).
Table 1.
Characteristics of patients on maintenance dialysis with data on vaccine response (antibody ≥2 U/L)
| Demographics | All Patients (n=186) |
Prior COVID-19 (n=38) |
Responders (n=127) |
Nonresponders (n=21) |
|---|---|---|---|---|
| BNT162b2 (Pfizer) | 168 (90.3) | 35 (92.1) | 113 (89.0) | 20 (95.2) |
| mRNA-1273 (Moderna) | 18 (9.7) | 3 (7.9) | 14 (11.0) | 1 (4.8) |
| Age (yr) | 67.9±12.2 | 65.2±13.7 | 68.4±11.9 | 70.0±10.7 |
| <55 | 29 (15.6) | 10 (26.3) | 16 (12.6) | 3 (14.3) |
| 55–64 | 34 (18.3) | 7 (18.4) | 25 (19.7) | 2 (9.5) |
| 65–74 | 71 (38.2) | 13 (34.2) | 49 (38.6) | 9 (42.9) |
| 75+ | 52 (28.0) | 8 (21.1) | 37 (29.1) | 7 (33.3) |
| Women | 88 (47.3) | 16 (42.1) | 57 (44.8) | 15 (71.4) a |
| Race | ||||
| White | 73 (39.3) | 12 (31.6) | 50 (39.4) | 11 (52.4) |
| Black | 39 (21.0) | 12 (31.6) | 24 (18.9) | 3 (14.3) |
| Native American | 33 (17.7) | 7 (18.4) | 24 (18.9) | 2 (9.5) |
| Asian/Pacific Islander | 19 (10.2) | 3 (7.9) | 15 (11.8) | 1 (4.8) |
| Other/unknown | 22 (11.8) | 4 (10.5) | 14 (11.0) | 4 (19.1) |
| Hispanic | 15 (8.1) | 4 (10.5) | 10 (7.9) | 1 (4.8) |
| Vintage (mo) | 58.1±54.3 | 65.2±57.2 | 59.2±53.5 | 38.7±52.2 a |
| Body mass index (kg/m2) | 28.7±7.1 | 28.8±6.9 | 28.6±6.9 | 29.2±8.8 |
| Long-term care facility resident | 48 (25.8) | 26 (68.4) | 15 (11.8) | 7 (33.3) a |
| Modality | ||||
| In-center hemodialysis | 180 (96.8) | 38 (100.0) | 122 (96.0) | 20 (95.2) |
| Peritoneal dialysis | 5 (2.7) | 0 (0.0) | 4 (3.2) | 1 (4.8) |
| Home hemodialysis | 1 (0.5) | 0 (0.0) | 1 (0.8) | 0 (0.0) |
| Adequate dialysis doseb | 174 (93.6) | 37 (97.4) | 118 (92.1) | 19 (90.5) |
| Serum albumin (g/dl) | 3.8±0.4 | 3.7±0.4 | 3.9±0.4 | 3.5±0.6 c |
| Other vaccines within 14 d | 20 (10.8) | 5 (13.2) | 10 (7.9) | 5 (23.8) a |
| Pneumococcal | 4 (2.2) | 1 (2.6) | 2 (1.6) | 1 (4.8) |
| Hepatitis B | 17 (9.1) | 5 (13.2) | 8 (6.3) | 4 (19.1) a |
| Potential immunosuppression | 36 (19.4) | 4 (10.5) | 25 (19.7) | 7 (33.3) |
| Immune-modulating meds | 29 (15.6) | 3 (7.9) | 20 (15.8) | 6 (28.6) |
| Prior transplant | 11 (5.9) | 1 (2.6) | 7 (5.5) | 3 (14.3) |
| Immunodeficiency disorder | 10 (5.4) | 0 (0.0) | 6 (4.7) | 4 (19.1) a |
| Hospitalization within 14 d | 34 (18.3) | 9 (23.7) | 15 (11.8) | 10 (47.6) c |
| Disabilityd | 17 (9.1) | 8 (21.1) | 9 (7.1) | 0 (0.0) |
| Tobacco use | 22 (11.8) | 4 (10.5) | 17 (13.4) | 1 (4.8) |
| Alcohol use disorder | 14 (7.5) | 5 (13.2) | 9 (7.1) | 0 (0.0) |
| Substance use disorder | 8 (4.3) | 1 (2.6) | 5 (3.9) | 2 (9.5) |
| No. of comorbidities | 3.2±1.9 | 3.3±1.6 | 3.1±1.8 | 4.0±2.0 c |
| Comorbid conditions | ||||
| Diabetes mellitus | 129 (69.4) | 32 (84.2) | 81 (63.8) | 16 (76.2) |
| Hypertension | 152 (81.7) | 32 (81.6) | 103 (81.1) | 18 (85.7) |
| Congestive heart failure | 37 (19.9) | 4 (10.5) | 23 (18.1) | 10 (47.6) c |
| COPD | 34 (18.3) | 9 (2.7) | 21 (16.5) | 4 (19.1) |
| Stroke/cerebrovascular disorder | 23 (12.4) | 4 (10.5) | 15 (11.8) | 4 (19.1) |
| Peripheral vascular disease | 25 (13.4) | 3 (7.9) | 19 (15.0) | 3 (14.3) |
| Thyroid disorder | 36 (19.4) | 8 (21.1) | 22(17.3) | 6 (28.6) |
| History of cancer | 18 (9.7) | 3 (7.9) | 11 (8.7) | 4 (19.1) |
Values are presented as mean±SD or n (%). P values compare responders to nonresponders among those without a history of COVID-19. Bold values indicate statistically significant differences between parameters. COPD, chronic obstructive pulmonary disease.
P<0.05.
Adequate dialysis defined by spKt/V≥1.2 for hemodialysis or weekly Kt/V≥1.7 for peritoneal dialysis.
P<0.01.
Amputee, wheelchair use, or inability to perform activities of daily living.
Figure 1.
Distribution of anti-spike protein IgG titers for patients who are SARS-CoV-2 mRNA vaccinated on maintenance dialysis drawn ≥14 days after the second dose. (A) All patients (n=186); (B) subset of patients without COVID-19 history (n=148).
All 38 patients (20%) with prior COVID-19 diagnosis at the time of antibody draw were considered responders, irrespective of vaccine type, with 37 out of 38 at maximum limit of the assay. A single patient had an antibody titer drawn 8 days before the first vaccine administration and the spike-Ab-IgG was maximal at 20 U/L (drawn approximately 234 days after initial diagnosis of COVID-19); the spike-Ab-IgG titer drawn 22 days after the second vaccine dose was also maximal at 20 U/L. An additional two patients with known prior COVID-19 diagnosis (made 286 and 295 days before the first dose) had antibody titer drawn 5 days after their initial dose of vaccine. One had spike-Ab-IgG titer of 3.61 U/L and the other 20 U/L, but both had maximal titers after the second dose (Supplemental Table 2). COVID-19 diagnosis occurred between 2 and 316 days before completing vaccination in 36 out of 38 patients. The remaining two patients were long-term care facility residents each exposed to a roommate, and were tested while asymptomatic at 1 and 5 days after their second vaccine dose, respectively (with spike-Ab-IgG titers obtained 20 and 23 days after vaccination, respectively). Neither was hospitalized.
Among patients without known COVID-19, 127 out of 148 (85.8%) were responders with no significant difference between BNT162b2/Pfizer (113 out of 133) and mRNA-1273/Moderna (14 out of 15) vaccines (85.0% versus 93.3%, P=0.38). Antibodies were drawn 24±8 days after the second dose and the distribution is shown in Figure 1B. If we consider weakly reactive spike-Ab-IgG levels ≥1 as responders, four patients are added, raising overall responder rate to 88.5% (131 out of 148). Proportionally, fewer responders were women (57 of 72) versus (70 of 76) men (79.2% versus 92.1%, P=0.03). Results of univariate analysis are indicated in Table 1. Nonresponders were newer to dialysis and more likely to have other vaccines administered or be hospitalized within 14 days of SARS-CoV-2 vaccination, have potential immunosuppressed state related to immune-deficiency disorders and congestive heart failure. A summary of the clinical characteristics of vaccine nonresponders is provided in Table 2.
Table 2.
Detailed summary of characteristics of SARS-CoV-2 mRNA vaccine nonresponders (n=21)
| Age | Sex | Race | Ethnicity | Notable Comorbid Conditions | Prescribed Medications Affecting Immune Function | Days from Vaccine Second Dose to Ab Test | Clinical Event within 14 d of COVID Vaccination | |
|---|---|---|---|---|---|---|---|---|
| Hospitalization | Other Vaccine | |||||||
| 40s | F | Black | Unknown | DM, CHF, hepatitis B, PVD, active bacterial infection | None | 14 | Yes | None |
| 50s | F | White | Non-Hispanic | Thyrotoxicosis, bipolar disorder, depression | None | 35 | None | None |
| 50s | F | White | Non-Hispanic | HIV, bipolar disorder, illicit drug use, cervical CA history | Lamivudine, dolutegravir, ritonavir, darunavir | 19 | None | None |
| 50s | F | White | Non-Hispanic | DM, CHF, hypothyroid | None | 39 | None | None |
| 60s | F | Unknown | Unknown | DM, CHF, Lyme disease, depression, osteoarthritis, gout, uterine CA history | None | 32 | None | None |
| 60s | F | Unknown | Non-Hispanic | DM | None | 19 | Yes | None |
| 60s | F | White | Unknown | A fib, CHF, breast CA history | None | 20 | Yes | None |
| 60s | F | Native American | Non-Hispanic | DM | None | 27 | Yes | Heplisav |
| 60s | F | Native American | Non-Hispanic | DM, seizure disorder, CVA, PVD, depression, calciphylaxis | None | 41 | None | None |
| 70s | F | Black | Non-Hispanic | SLE, prior transplant, SVC syndrome | Hydroxychloroquine, prednisone | 17 | None | None |
| 70s | F | White | Non-Hispanic | DM, gout, osteoarthritis, prior transplant | Prednisone, tacrolimus | 19 | None | None |
| 70s | F | Asian | Non-Hispanic | CHF, SLE, hepatitis B, osteoarthritis, colon CA history | Prednisone, mycophenolate, hydroxychloroquine, entecavir | 19 | None | None |
| 70s | M | Unknown | Unknown | DM, GERD, prior transplant | Prednisone, tacrolimus, mycophenolate, thymoglobulina | 17 | Yes | Prevnar |
| 70sb | M | White | Non-Hispanic | DM, A fib, depression | None | 14 | None | None |
| 70s | F | White | Unknown | DM, depression, COPD | None | 38 | Yes | None |
| 70s | M | White | Non-Hispanic | DM, hypothyroid, gout, neuropathy, Melanoma CA history, | None | 25 | Yes | Heplisav |
| 70sc | M | Black | Non-Hispanic | DM, CHF, DVT, BPH, GERD, gout | None | 24 | Yes | Heplisav |
| 80s | F | White | Non-Hispanic | DM, CHF, CAD, hypothyroid, gout, depression | None | 39 | Yes | None |
| 80s | F | White | Hispanic | DM, A fib, CHF, hypothyroid | None | 41 | Yes | None |
| 80s | M | White | Non-Hispanic | DM, A fib, CHF, COPD, gout | Hydroxychloroquine | 21 | Yes | None |
| 80s | M | Unknown | Non-Hispanic | DM, A fib, CHF, hypothyroid, COPD | None | 23 | Yes | None |
F, female; DM, diabetes mellitus; CHF, congestive heart failure; PVD, peripheral vascular disease; CA, cancer; A fib, atrial fibrillation; CVA, cerebro-vascular accident; M, male; SLE, systemic lupus erythromatosus; GERD, gastroesophageal reflux disease; BPH, benign prostatic hypertrophy; CAD, coronary artery disease; COPD, chronic obstructive pulmonary disease.
Communicated by nephrologist.
Received Moderna vaccine, all others received Pfizer vaccine.
Peritoneal dialysis modality, all others utilized hemodialysis.
Discussion
Among 186 patients on maintenance dialysis in the United States, 88.8% had spike-Ab-IgG >2 U/L, measured ≥14 days after two doses of SARS-CoV-2 mRNA vaccine. This is only slightly lower than the 100% response reported from phase 2 clinical trials utilized for these vaccines’ emergency use authorization11,12; notably these trials did not include patients receiving dialysis. Such a lower responder rate was not unexpected, given the widespread immune dysfunction observed in patients who were dialysis dependent and prescription medications that may affect immune response. These immune alterations included skewed Th1/Th2 responses, impaired function of professional antigen-presenting cells, and susceptibility of B cells to apoptosis.13 The combined deficits in T cell, antigen-presenting cells, and B cell functions render patients who are dialysis dependent less likely to seroconvert and to maintain protective titers over time compared with individuals without kidney disease,14 a finding previously observed with hepatitis B and H1N1 influenza vaccines.7,8
Factors associated with nonresponse in univariate analyses of our cohort include female sex, younger vintage, residing in a long-term care facility, potential immunosuppression from immune deficiency disorders, heart failure, covaccination with hepatitis B vaccine, and hospitalization during the perivaccination period, although the number of nonresponders is small, precluding multivariable analyses. A recent meta-analysis suggests women receiving dialysis respond to vaccines equally to men, although differences in seroconversion rates by sex were noted in some hepatitis B series.7 However, we caution that some of these associations, including those for women, may not be sustained as we accumulate more data. Of note, potential immunosuppressed states and acute hospitalization during the vaccine series have face validity to affect immune response, particularly in the setting of hypoalbuminemia and uremic inflammation.7,13,15
Considering the devastating effect of COVID-19 on patients receiving maintenance dialysis compared with the potentially dramatic benefit of vaccination on severity of illness,3–5 it is imperative to explore strategies to monitor and maximize vaccine response. Such knowledge may be useful for patient education and dialogue to reinforce preventive measures. Strategies to improve response rates to vaccines may include additional doses or higher vaccine doses. Monitoring longitudinal spike-Ab-IgG could inform potential use of booster doses, similar to algorithms used for hepatitis B vaccination. Such tracking is a component of the quality program at DCI and will be critical in informing the development of alternative vaccination regimens.
A strength of the current report is inclusion of a real-world diverse dialysis population. Although limited by small sample size, the data appear sufficient to emphasize that antibody response should be expected for the majority of patients on maintenance dialysis. However, the small sample size limits our ability to conduct multivariable analyses and limits the power to detect significant differences between vaccine types. Our sample tended to be older, with a high proportion of long-term care facility residents and individuals of Native American and Asian/Pacific Islander ancestry, and thus may not be entirely generalizable to the US population. The physician orders linking the spike-antibody testing within the scheduled routine monthly blood draws contributed to a substantial number of test results being unavailable for the current analysis. Monthly blood draws were not timed to vaccination completion (or when the clinic became aware that the vaccination was completed).
Systemic biases potentially influencing sampling may include initial vaccination performed disproportionately in long-term care facilities where generally older patients on dialysis with more comorbidities resided and state-specific variability in prioritizing patients on dialysis for vaccination. Additionally, although physicians who ordered antibody tests generally measured titers on the basis of vaccination access, it is possible that they exerted some influence over which patients received the vaccine early on, particularly when some states allowed community-dwelling elderly patients to be vaccinated. Furthermore, DCI serves some areas where Native American tribes were devastated and were targeted for early vaccination. Lastly, it is not clear what if any role vaccine hesitancy played during this early period. Nevertheless, although our cohort may not mirror that of the US dialysis population, the direction of these biases may have led to sampling more vulnerable patients in whom vaccination could provide the greatest benefit and who may have had the greater risk for potential immune compromise, which further emphasizes the high rate of responders that we observed postvaccination. Of note, our study was also limited by the absence of baseline serum spike antibody titers. Although we separated the patients with prior COVID-19 infections whom we noted were all classified as responders, there may still be some unaccounted asymptomatic COVID-19 infections in the remainder of responders. Future studies could also evaluate T cell responses given that specific clinical implications of serology tests including the spike-Ab-IgG we utilized, need further elucidation.
In conclusion, the vast majority of patients on maintenance dialysis responded with spike-Ab-IgG titers to a complete series of both SARS-CoV-2 mRNA BNT162b2 and mRNA-1273 vaccines. Early evidence indicated that vaccinated patients on dialysis with prior COVID-19 develop robust antibody responses. Our findings support an equitable and aggressive vaccination strategy for all eligible patients on maintenance dialysis regardless of age, sex, race, ethnicity, disability, or prior COVID-19. Finally, this early study suggests that, despite the possibly impaired immune response of patients receiving maintenance dialysis to the mRNA SARS-CoV-2 vaccines, the short-term incidence of development of antispike antibodies appears to be good, giving hope that most of these vulnerable patients, once immunized, will be protected from COVID-19. Longer-term evaluation is needed to determine the durability of this protection, the role of repeating vaccine series in initial nonresponders, and the role of booster dose(s) among responders.
Disclosures
A.I. Chin reports being a scientific advisor to or membership of the National Quality Forum, renal committee; and reports other interests/relationships as Medical Director of a dialysis clinic owned by DCI. A.I. Chin, C.P. Argyropoulos, D.E. Weiner, and L.H. Salman are medical directors of DCI facilities. C.P. Argyropoulos reports having consultancy agreements with Alkahest and Momenta Pharma; reports receiving research funding from DCI and the University of Pennsylvania; reports being a scientific advisor or member of Baxter Healthcare, Bayer, and the Health Services Advisory Group; and reports having other interests/relationships in Akebia (Principal Investigator [PI]) in two phase 3 trials of an investigational product for the correction and maintenance of anemia in patients with nondialysis-dependent CKD and one phase 3 study of the same agent in dialysis, AbbVie Sub-I in a phase 3 study of an experimental agent in diabetic nephropathy, DCI as Medical Director, Outpatient Dialysis Unit in Cuba and New Mexico, and Dialysis Outcomes and Practice Patterns Study PI for CKD-Dialysis Outcomes and Practice Patterns Study. D.E. Weiner reports receiving funding paid to his institution for his role as Medical Director of Clinical Research for DCI; reports consultancy agreements via the Medical Advisory Boards for Akebia (2020, 2021), Cara Therapeutics (2020), Janssen Biopharmaceuticals (2019), and Tricida (2019); reports receiving honoraria for Akebia, paid to DCI; reports receiving research funding paid to AstraZeneca (site PI, DAPA-CKD Trial, capitated on the basis of recruitment, completed 2020), DCI (including local site PI for multiple clinical trials contracted through DCI, including trials sponsored by Ardelyx, ongoing, and Cara Therapeutics, completed), Goldfinch Bio (site PI, capitated on the basis of recruitment, ongoing), and Janssen Biopharmaceuticals (site PI, CREDENCE Trial, capitated on the basis of recruitment, completed 2019); reports receiving honoraria from the National Kidney Foundation (NKF) for an editorial position at Kidney Medicine and American Journal of Kidney Diseases, Elsevier for royalties from the NKF's Primer on Kidney Diseases; and reports being a scientific advisor or member as Co-Editor-in-Chief of NKF Primer on Kidney Diseases 8th Edition, Editor-in-Chief of Kidney Medicine, Medical Director of Clinical Research at DCI, member of the ASN Quality and Policy Committees and ASN representative to Kidney Care Partners, Scientific Advisory Board of the NKF; and reports other interests/relationships as Chair of the adjudications committee for Evaluation of Effect of TRC101 on Progression of Chronic Kidney Disease in Subjects With Metabolic Acidosis Trial (George Institute, CRO, sponsored by Tricida), Member of Data Monitoring Committee, “Feasibility of Hemodialysis with GARNET? in Chronic Hemodialysis Patients with a Bloodstream Infection” trial (Avania CRO). D.S. Johnson reports being a scientific advisor to or member of Alive Hospice and American Association of Kidney Patients. D.S. Johnson, E. Lacson, H.J. Manley, and G. Aweh are employees of DCI. L.H. Salman reports receiving research funding from Albany Medical Center, Roach funds, Transonics Inc.; reports patents and inventions for the use of 4-methylumbelliferone in diabetic kidney disease; and reports having other interests/relationships with American Society of Diagnostic and Interventional Nephrology, the American Society of Nephrology, the Data Safety Monitoring Board of Phraxis, and the Renal Physician Association. The remaining author has nothing to disclose.
Funding
This work was supported by the National Institute of Diabetes and Digestive and Kidney Diseases grant T32-DK007777 (to C. Hsu).
Supplementary Material
Acknowledgments
We are grateful for the assistance from the DCI Medical Directors, Clinic Managers, and staff caring for our patients throughout the pandemic who worked to have our patients vaccinated against SARS-CoV-2 and tirelessly documented the information in the EHR. We further thank the nephrologists who have implemented DCI clinical and quality improvement protocols and care recommendations, including the SARS-CoV-2 seroresponse testing algorithm. We also thank Beth Kammer from DCI Laboratories, Vlad Ladik, and Brian Tinger from DCI IT Analytics Department, Karen Majchrzak with DCI Research and the many Senior/Area Operations Directors who supported this quality improvement project. D. Johnson and E. Lacson designed the study and managed logistics; C. Argyropoulos, A. Chin, E. Lacson, L. Salman, and D. Weiner worked on study implementation; G. Aweh and H. J. Manley created the analytical files; G. Aweh, E. Lacson, and H. J. Manley analyzed the data; G. Aweh and E. Lacson made the figures; C. Argyropoulos, E. Lacson, H. Manley, and D. Weiner drafted the paper; all authors recommended edits and approved the final version of the manuscript.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
Supplemental Material
This article contains the following supplemental material online at https://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2021040432/-/DCSupplemental.
Supplemental Table 1. Characteristics of maintenance dialysis patients by vaccine received.
Supplemental Table 2. Three patients with COVID-19 history and prior antibody results.
References
- 1.GBD Chronic Kidney Disease Collaboration : Global, regional, and national burden of chronic kidney disease, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 395: 709–733, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.United States Renal Data System : 2020 USRDS Annual Data Report: Epidemiology of Kidney Disease in the United States, Bethesda, MD, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, 2020. Available at: https://adr.usrds.org/2020/end-stage-renal-disease/1-incidence-prevalence-patient-characteristics-and-treatment-modalities. Accessed September 29, 2021 [Google Scholar]
- 3.Hsu CM, Weiner DE, Aweh G, Miskulin DC, Manley HJ, Stewart C, et al. : COVID-19 among US dialysis patients: Risk factors and outcomes from a national dialysis provider. Am J Kidney Dis 77: 748–756.e1, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sim JJ, Huang CW, Selevan DC, Chung J, Rutkowski MP, Zhou H: COVID-19 and survival in maintenance dialysis. Kidney Med 3: 132–135, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Couchoud C, Bayer F, Ayav C, Béchade C, Brunet P, Chantrel F, et al. ; French REIN registry : Low incidence of SARS-CoV-2, risk factors of mortality and the course of illness in the French national cohort of dialysis patients. Kidney Int 98: 1519–1529, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chi C, Patel P, Pilishvilli T, Moore M, Murphy T, Strikas R: Guidelines for vaccinating kidney dialysis patients and patients with chronic kidney disease. 2012. Available at: https://www.cdc.gov/dialysis/pdfs/vaccinating_dialysis_patients_and_patients_dec2012.pdf. Accessed March 26, 2021
- 7.Udomkarnjananun S, Takkavatakarn K, Praditpornsilpa K, Nader C, Eiam-Ong S, Jaber BL, et al. : Hepatitis B virus vaccine immune response and mortality in dialysis patients: A meta-analysis. J Nephrol 33: 343–354, 2020 [DOI] [PubMed] [Google Scholar]
- 8.Crespo M, Collado S, Mir M, Cao H, Barbosa F, Serra C, et al. : Efficacy of influenza A H1N1/2009 vaccine in hemodialysis and kidney transplant patients. Clin J Am Soc Nephrol 6: 2208–2214, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Seimens Healthineers: SARS-CoV-2 IgG Assays . 2021. Available at: https://www.siemens-healthineers.com/en-us/laboratory-diagnostics/assays-by-diseases-conditions/infectious-disease-assays/sars-cov-2-igg-assay. Accessed March 22, 2021
- 10.United Stated Food and Drug Administration : In Vitro Diagnostics EUAs. 2021. Available at: https://www.fda.gov/medical-devices/coronavirus-disease-2019-covid-19-emergency-use-authorizations-medical-devices/in-vitro-diagnostics-euas. Accessed March 22, 2021
- 11.Mulligan MJ, Lyke KE, Kitchin N, Absalon J, Gurtman A, Lockhart S, et al. : Phase I/II study of COVID-19 RNA vaccine BNT162b1 in adults. Nature 586: 589–593, 2020 [DOI] [PubMed] [Google Scholar]
- 12.Chu L, McPhee R, Huang W, Bennett H, Pajon R, Nestorova B, et al. ; mRNA-1273 Study Group : A preliminary report of a randomized controlled phase 2 trial of the safety and immunogenicity of mRNA-1273 SARS-CoV-2 vaccine. Vaccine 39: 2791–2799, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kato S, Chmielewski M, Honda H, Pecoits-Filho R, Matsuo S, Yuzawa Y, et al. : Aspects of immune dysfunction in end-stage renal disease. Clin J Am Soc Nephrol 3: 1526–1533, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dinits-Pensy M, Forrest GN, Cross AS, Hise MK: The use of vaccines in adult patients with renal disease. Am J Kidney Dis 46: 997–1011, 2005 [DOI] [PubMed] [Google Scholar]
- 15.Bergström J, Lindholm B, Lacson E Jr., Owen W Jr., Lowrie EG, Glassock RJ, et al. : What are the causes and consequences of the chronic inflammatory state in chronic dialysis patients? Semin Dial 13: 163–175, 2000 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.