Significance Statement
In ANCA-associated vasculitis (AAV), noninvasive biomarkers of active renal inflammation, such as urinary soluble CD163, are needed for early detection of active disease before irreversible end organ damage occurs. Clinical translation requires a diagnostic-grade assay, prospective assessment of its diagnostic utility in AAV flare, and assessment of its utility in proteinuric states. The authors report use of an accredited, diagnostic-grade assay for urinary soluble CD163, derivation of cutoff values, and application of the assay to a prospective cohort of patients with potential renal vasculitis flare. They found that urinary soluble CD163 displays high precision in separating RV flare from flare mimics. They also observed increased false-positive results in the setting of high-grade proteinuria, which they demonstrated can be effectively corrected by normalization to the urine protein value, thereby restoring diagnostic accuracy.
Keywords: ANCA, CD163, biomarker, crescentic glomerulonephritis, macrophage, urine
Visual Abstract
Abstract
Background
Up to 70% of patients with ANCA-associated vasculitis (AAV) develop GN, with 26% progressing to ESKD. Diagnostic-grade and noninvasive tools to detect active renal inflammation are needed. Urinary soluble CD163 (usCD163) is a promising biomarker of active renal vasculitis, but a diagnostic-grade assay, assessment of its utility in prospective diagnosis of renal vasculitis flares, and evaluation of its utility in proteinuric states are needed.
Methods
We assessed a diagnostic-grade usCD163 assay in (1) a real-world cohort of 405 patients with AAV and 121 healthy and 488 non-AAV disease controls; (2) a prospective multicenter study of 84 patients with potential renal vasculitis flare; (3) a longitudinal multicenter cohort of 65 patients with podocytopathy; and (4) a cohort of 29 patients with AAV (with or without proteinuria) and ten controls.
Results
We established a diagnostic reference range, with a cutoff of 250 ng/mmol for active renal vasculitis (area under the curve [AUC], 0.978). Using this cutoff, usCD163 was elevated in renal vasculitis flare (AUC, 0.95) but remained low in flare mimics, such as nonvasculitic AKI. usCD163’s specificity declined in patients with AAV who had nephrotic-range proteinuria and in those with primary podocytopathy, with 62% of patients with nephrotic syndrome displaying a “positive” usCD163. In patients with AAV and significant proteinuria, usCD163 normalization to total urine protein rather than creatinine provided the greatest clinical utility for diagnosing active renal vasculitis.
Conclusions
usCD163 is elevated in renal vasculitis flare and remains low in flare mimics. Nonspecific protein leakage in nephrotic syndrome elevates usCD163 in the absence of glomerular macrophage infiltration, resulting in false-positive results; this can be corrected with urine protein normalization.
ANCA-associated vasculitis (AAV) is a chronic disease with periods of remission and relapse. Kidney involvement is characterized by focal necrotizing GN.1 There is a need for noninvasive methods of detection of active renal inflammation to prevent irreversible end organ damage, including ESKD. Of patients with AAV, 40% experience a relapse within the first 5 years after initial diagnosis,2 and 26% develop ESKD.3 It is estimated that 43% of patients with renal AAV who progress to ESKD do so without clinically detected renal vasculitis (RV) activity.4
The gold standard for diagnosis of kidney involvement in AAV involves performing a biopsy. However, a kidney biopsy is an invasive procedure, and sequential biopsies are not feasible in routine clinical practice.5 Noninvasive clinical tools for detection of active renal inflammation include serum creatinine, hematuria, proteinuria, and red blood cell casts. However, these biomarkers lack sensitivity for detection of early renal structural and functional loss and do not reliably differentiate active vasculitis from flare mimics.6,7
Urine is an ideal biospecimen because it is readily available and biomarkers in urine may reflect local kidney injury. Retrospective studies raise the possibility that urine biomarkers may obviate the need for kidney biopsy.8,9 CD163 functions as a monocyte/macrophage-specific scavenger receptor for hemoglobin-haptoglobin complexes, which is cleaved from the surface of activated macrophages.10 Soluble CD163 (sCD163) is present at relatively high concentration in serum, playing a role in innate defense by reversibly binding bacteria and free hemoglobin.11,12 sCD163 appears in the urine when activated glomerular macrophages are present. sCD163 possesses many ideal biomarker properties with low levels in health, stability at room temperature for up to 7 days, and ease of measurement by ELISA.13 Prior work in retrospective cohorts has shown elevated concentrations of urinary sCD163 (usCD163) in active RV at diagnosis, in subtle RV flare, and in lupus nephritis.10,13–16
To further develop usCD163 as a clinical biomarker, a diagnostic-grade assay is required. A clinical ELISA platform was developed by Euroimmun GMBH. In this study, we validate its use in real-world samples to facilitate clinical accreditation. Few biomarkers of kidney disease have made this transition to clinical practice, with the recent exception of serum anti–phospholipase A2 antibody in membranous GN (MN).17
A clinical concern remains that usCD163 quantification may be a sophisticated measure of proteinuria, reflecting glomerular leak of the protein through the damaged glomerular filtration barrier. The optimal setting to assess the contribution of serum protein leakage is nephrotic syndrome. Primary podocytopathies, such as minimal change disease (MCD) and FSGS, characteristically lack inflammatory infiltrates (including macrophages) on light microscopy, so local sCD163 production should be minimal.18 Leakage of serum sCD163 across the glomerular filtration barrier could occur in this setting due to extensive foot process effacement.15 Protein-normalized usCD163 has been reported in lupus nephritis.15,16 Thus, to fully define the clinical role for usCD163 use as a biomarker of RV, in which glomerular scarring without active inflammation may cause nonspecific glomerular protein leak, we conducted a prospective study to assess its utility using a clinical-grade analytic platform in RV flare and assessed mechanisms for accounting for elevated levels of proteinuria.
Methods
Patient Identification and Recruitment
Cohort 1: Real-World Assessment of a Diagnostic-Grade Assay
To support the use of a clinically validated, diagnostic-grade ELISA, we prospectively used a novel clinical-grade sCD163 assay in a cohort of unselected patients attending the Vasculitis Ireland Network (VINE) service (https://www.tcd.ie/medicine/thkc/vasculitis/), including patients with CKD with varying levels of proteinuria.19 Clinical status was adjudicated after chart review, which was blinded to usCD163 concentration. To establish a reference range, healthy volunteers were recruited at Galway University Hospitals/National University of Ireland Galway, as previously described.20,21 Between March 2016 and December 2018, a cross-sectional study was carried out. Participants were identified through poster advertisement/word of mouth. Each participant completed a comprehensive health questionnaire and underwent a battery of investigations to ensure that participants did not have any medical conditions/recent illnesses that could affect study validity.
Cohort 2: Prospective Diagnosis of RV Flare
To prospectively evaluate the utility of usCD163 in the diagnosis of RV flare, we enrolled patients previously recruited to the Irish Rare Kidney Disease Registry and Biobank (www.tcd.ie/medicine/thkc/research/rare.php) who presented to one of seven VINE centers with a clinical suspicion of RV flare. All enrolled patients met Chapel Hill Consensus Conference classification criteria for small vessel vasculitis.22 All patients provided informed consent, and the study was approved by the local research ethics committee in each hospital.
Cohort 3: Effect of Nephrotic Syndrome on usCD163 Excretion
To investigate the effect of proteinuria on usCD163 concentration in the absence of glomerular macrophage infiltration, we leveraged a cohort of patients with primary podocytopathy (MCD and FSGS) and MN. This cohort was obtained from the Nephrotic Syndrome Study Network (NEPTUNE) program, a multicenter international longitudinal study of primary nephrotic syndrome.23 Recruits provided paired urine samples from active (urine protein >3.5 g/d) and remission (urine protein <0.5 g/d) nephrotic syndrome.
Cohort 4: Assessment of Methods To Correct usCD163 Value in the Setting of Heavy Proteinuria
Patients with AAV and healthy controls were identified from the Irish Rare Kidney Disease Registry and Biobank. Inclusion criteria included (1) active RV (hematuria and proteinuria on urinalysis), (2) remission vasculitis with persistent proteinuria (proteinuria >2+ on urinalysis), (3) remission vasculitis without proteinuria (proteinuria 0 on urinalysis), and (4) healthy controls. All recruits provided synchronous paired urine and serum samples.
Sample Processing
Real-world VINE (cohort 1) and Rare Kidney Disease biobank (cohorts 2 and 4) urine and serum samples were centrifuged at 2000 × g for 10 minutes at 4°C, with storage of the supernatant at −80°C until assay. Serum samples from the healthy controls (cohort 1) were allowed to clot for 1 hour, then centrifuged at 800 × g for 15 minutes, aliquoted, and stored at −80°C. Urine was centrifuged at 2,000 × g for 10 minutes at 4°C, aliquoted, and stored at −80°C. NEPTUNE (cohort 3) samples were collected according to predetermined protocolized procedures. Urine was spun at 1000 × g for 12 minutes and serum samples were allowed to clot for 30 minutes, and then they were centrifuged at 2000 × g for 12 minutes. Both sample types were then frozen at −80°C until shipping on dry ice.
sCD163 Measurement, Establishment of Reference Range, and Normalization Techniques
Two sCD163 assays were used in this study: the assay used in prior work14 and a newly developed diagnostic-grade assay. In all cohorts, usCD163 was initially measured by the capture ELISA used in our prior experimental work (human sCD163 DuoSet, DY1607 ELISA, R&D Systems).10,13–15. This assay was developed for use in serum and has manufacturing steps in its protocol. To facilitate clinical translation of usCD163, we partnered with Euroimmun GMBH to develop a precoated diagnostic-grade sandwich sCD163 ELISA specifically optimized for measurement of usCD163. Batch numbers E190708BA, E181018CD, and E180424BD were used. These assays were performed centrally in a diagnostic laboratory (Clinical Immunology, St. James’s Hospital). As per prior studies of usCD163, we normalized the usCD163 level to the creatinine level, as determined by a modified Jaffe technique. Urine creatinine and protein were measured by Roche Cobas Creatinine plus (05 6612 7) and Total Protein (11877801) modules, respectively. To assess interassay performance, we measured usCD163 measured using both ELISA methods in cohorts 1 and 2. The upper limit of normal in the Euroimmun assay was defined as the 97.5th centile of healthy control values.
Various techniques were used to incorporate data regarding nonspecific urine protein leak:
Normalization of usCD163 concentration to total urine protein (pg/mg).
- Fractional excretion of sCD163 (FE-CD163):
- Albumin/CD163 ratio of serum to urine:
Clinical Assessment
Cohort 1: Real-World Assessment of a Diagnostic-Grade Assay
Vasculitis disease activity was recorded using the Birmingham Vasculitis Activity Score (BVAS). Active RV was defined as a BVAS greater than zero for one or more renal items, including the presence of hematuria by urine dipstick; urine samples were not routinely assessed for the presence of casts or dysmorphic red cells. The presence or absence of active vasculitis was adjudicated without knowledge of the sCD163 concentration.
Cohort 2: Prospective Diagnosis of RV Flare
At the time of potential RV flare, detailed demographic and phenotypic information was collected. A challenge we faced in our study design was in accurately determining a diagnosis of RV flare. There are no consensus criteria for this diagnosis and, in clinical practice, a combination of serum creatinine, urine sediment analysis for hematuria, proteinuria, and red blood cell casts are used. This pragmatic approach has also been taken in clinical trials with BVAS/Wegener’s granulomatosis major renal criteria (which do not require biopsy) used as inclusion criteria.24,25 In this prospective observational study, kidney biopsy was not mandated. Biopsy sample interpretation is limited by the focal nature of histologic findings, with false-negative results in 1%–3% of biopsy specimens and reported sensitivity of 91%.26,27 Final diagnostic category (RV flare or no RV flare) was adjudicated by a committee 6 weeks after the encounter, which was blinded to usCD163 results. This committee reviewed renal major and minor BVAS criteria, trends in serum creatinine, urinary protein, red blood cell casts (if available), subsequent clinical management (response to immunosuppression), and renal biopsy sample (if available). The absence of tissue confirmation of crescentic GN is both a limitation of this study and a strength because it reflects clinical and research practice definitions of active RV. Physicians were asked, at the time of clinical review, to classify the likelihood of vasculitis flare as “possible” or “highly probable.” Clinical information was collected in real time and retrospectively from the clinical encounter before the potential flare visit and from subsequent clinical encounters. Vasculitis disease activity was recorded using the BVAS.
Cohort 3: Effect of Nephrotic Syndrome on usCD163 Excretion
Patients were considered actively nephrotic if their urine protein was >3.5 g/d, and in remission if their urine protein was <0.5 g/d.
Cohort 4: Assessment of Methods To Correct usCD163 Value in the Setting of High-Grade Proteinuria
Vasculitis activity was recorded as per cohort 1.
Statistical Analyses
Clinical data, laboratory data, and ELISA results were analyzed using GraphPad Prism version 9 and R. Biomarker values were non-normally distributed and are thus reported as median and interquartile ranges (IQRs). Kruskal–Wallis, Mann–Whitney U, chi-squared, and unpaired t tests were used to determine the significance of associations for non-normally and normally distributed data. Correlations were measured using the Spearman correlation coefficient. OptimalCutpoints R package was used to determine diagnostic cutoff ranges and to generate receiver-operator characteristic curves.28,29 Two methods were used to determine the most clinically relevant diagnostic cutoffs: the Youden calculation was selected to maximize the sum of sensitivity and specificity and yield the most clinically relevant cutoff values,29,30 and the net reclassification index was calculated as per Pencina et al.31 We developed an interactive web application using Shiny (version 1.6.0) in R (https://thkc.shinyapps.io/usCD163/).32
Results
Baseline Characteristics
We first sought to develop and validate an accredited sCD163 kit that is validated for testing in urine and could be deployed in clinical practice. The characteristics of cohort 1 comprised healthy controls (n=121) and sequential clinic patients with systemic vasculitis (n=274 in remission, n=131 with active RV) and disease controls without AAV (n=488), as described in Supplemental Table 1. Cohort 2 comprised patients enrolled prospectively in our study evaluating RV flare: 121 patients were screened for inclusion and 37 were excluded, leaving 84 patients available for analysis (Supplemental Figure 1). Baseline demographics and clinical characteristics are summarized in Table 1, and clinical characteristics at the time of potential RV flare are outlined in Supplemental Table 2. Those who were subsequently adjudicated as having an RV flare had higher absolute and Δ serum creatinine, and higher levels of proteinuria and hematuria on dipstick. Physician impression of high flare probability was more frequent in those with RV flare, and these recruits were also more likely to undergo renal biopsy. Clinical characteristics of those with false + and − usCD163 concentrations are outlined in Supplemental Table 3. Cohort 3 (NEPTUNE, primary nephrotic syndrome) included 65 patients (MCD, n=20; FSGS, n=23; and MN, n=22) for comparison (Supplemental Table 4). Cohort 4 (AAV and proteinuria) included 39 patients: ten with active RV, ten in remission with residual proteinuria, nine in remission with no proteinuria, and ten healthy controls (clinical characteristics outlined in Supplemental Table 5 and absolute concentrations of usCD163 are outlined in Supplemental Table 6).
Table 1.
Baseline characteristics in cohort 2 derived from clinical parameters from last review before study visit (flare or flare mimic)
| Baseline Characteristics | n=84 | P Value | |
|---|---|---|---|
| Renal Flare (n=31) | No Renal Flare (n=53) | ||
| Female sex, % (n) | 48.4 (15) | 35.8 (19) | 0.26 |
| Age (yr), mean (SD) | 63.1 (16.2) | 60.3 (13.5) | 0.39 |
| Diagnosis, % (n) | 0.96 | ||
| MPA | 54.8 (17) | 54.7 (29) | — |
| GPA | 38.7 (12) | 35.8 (19) | — |
| EGPA | 3.2 (1) | 3.8 (2) | — |
| AAV/AGBM | 3.2 (1) | 5.7 (3) | — |
| Disease duration (yr), median (IQR) | 4.0 ( 1.7–7.8) | 4.1 (1.7–6.5) | 0.81 |
| Prior renal involvement, % (n) | 87.1 (27) | 83.0 (44) | 0.62 |
| Baseline eGFR (ml/min), mean (SD) | 48.2 (27.5) | 54.1 (19.7) | 0.44 |
| Baseline hematuria, median (IQR) | 2+ (0–3+) | 1+ (0–2+) | 0.05 |
| Baseline proteinuria, median (IQR) | 1+ (0–3+) | 0+ (0–2+) | 0.09 |
| Baseline PCR (mg/mmol), median (IQR) | 96 (36–329) | 34 (17–55) | 0.01 |
| Current immunosuppression, % (n) | 0.33 | ||
| None | 54.8 (17) | 41.5 (22) | — |
| Azathioprine | 16.1 (5) | 33.9 (18) | — |
| MMF | 6.4 (2) | 13.2 (7) | — |
| MTX | 3.2 (1) | 3.7 (2) | — |
| Rituximab | 3.2 (1) | 3.7 (2) | — |
| Other | 9.6 (3) | 9.4 (5) | — |
| No data available | 12.9 (4) | 0 (0) | |
| Current corticosteroids (n=83), % (n) | 45.2 (14) | 43.4 (23) | 0.82 |
MPA, microscopic polyangiitis; GPA, granulomatosis with polyangiitis; EGPA, eosinophilic granulomatosis with polyangiitis; AAV/AGBM, overlap syndrome of dual positive ANCA and anti-GBM antibodies; MMF, mycophenolate mofetil; MTX, methotrexate.
Optimization of Clinical-Grade sCD163 ELISA
To use usCD163 as a clinical biomarker, an accredited, validated ELISA platform is required. In collaboration with Euroimmun GMBH, who have generated such a platform, we validated its use in real-world samples to facilitate clinical accreditation (cohort 1). The upper limit of normal, defined as the 97.5th centile in healthy controls, was 1.32 ng/ml or 254.9 ng/mmol (normalized to urine creatinine; Figure 1A). In sequential patients with systemic vasculitis, the 97.5th centile in patients in remission was 3.2 ng/ml or 520.3 ng/mmol. The 2.5th centile in patients with active RV was 0.75 ng/ml or 194.9 ng/mmol. In 22 (18%) healthy control samples, the value obtained was below the lower limit of detection. Median (IQR) non-normalized usCD163 values were 0.29 (0.2–0.4) ng/ml, 0.64 (0.4–1.1) ng/ml and 5.0 (2.6–9.4) ng/ml in healthy controls and those with remission vasculitis and active RV, respectively. The corresponding respective values (IQR) normalized to urine creatinine were 33.7 (19.2–69.4) ng/mmol, 97.8 (51.0–147.6) ng/mmol, and 938.9 (491.8–1469) ng/mmol. The values for the disease control samples are summarized in Supplemental Figure 2. On the basis of these values, we propose an upper limit of normal cutoff for use in clinical practice of 250 ng/mmol (non-normalized, 1.3 ng/mL), which we applied in the prospective study. When distinguishing active RV from remission vasculitis in this real-world cohort, the area under the curve (AUC) of the receiver-operator characteristic curve of non-normalized usCD163 was 0.945 (Figure 1B) and the AUC of normalized usCD163 was 0.978 (Figure 1C). usCD163 values measured in a subgroup of these patients (n=97) using both the Euroimmun and R&D Duoset assay methods were highly correlated (r=0.87; Figure 1D).
Figure 1.
Real-world clinical testing for usCD163 using a clinically accredited ELISA test (cohort 1). (A) Comparison of all usCD163 values in healthy control (HC) and patients with vasculitis in remission and with active RV. Both normalized (to urine creatinine, left axis) and non-normalized (right axis) values are presented. The dotted line reflects the optimal cut point for normalized values (250 ng/mmol) and the solid line reflects the optimal cut point for non-normalized values (1.3 ng/mL). Receiver-operator characteristic curve AUC values for (B) non-normalized and (C) normalized values, comparing active RV and remission vasculitis, are included. (D) Correlation of clinically accredited (Euroimmun) precoated ELISA kit with the R&D Duoset kit (as used in prior work).
usCD163 Is Elevated in RV Flare
We then applied this diagnostic-grade assay to a prospective cohort of patients with a clinical suspicion of renal flare of AAV (cohort 2). Of the 84 patients assessed, 31 (36.9%) were adjudicated (blind to usCD163 values) as suffering from a RV flare (Table 2). The usCD163 values in the 19 patients undergoing kidney biopsy are summarized in Supplemental Figure 3. In those adjudicated as having an RV flare, the median (IQR) usCD163 concentration was 805.8 (439–1705) ng/mmol creatinine, and in those without RV flare the median (IQR) usCD163 concentration was 100.0 (52–174) ng/mmol creatinine (P<0.0001; Figure 2A). The AUC for detection of active RV in this prospective study was 0.95 (P<0.0001; Figure 2B). The optimal diagnostic cutoff was 253 ng/mmol, virtually identical to our previously defined optimal cutoff (250 ng/mmol). Biomarker characteristics in this clinical setting are summarized in Table 3.
Table 2.
Final adjudicated diagnosis and usCD163 values in RV flare and RV flare mimics
| Final Adjudicated Diagnosis | usCD163 (ng/mmol), median (IQR) | N (%) |
|---|---|---|
| Renal flare | 805.8 (439.1–1705) | 31 (36.9) |
| Systemic flare | 88.2 (71.6–197.8) | 13 (15.5) |
| AKIa | 105.4 (48.4–191.0) | 7 (8.3) |
| Sepsis | 141.0 (75.5–232.7) | 11 (13.1) |
| CKD progression | 103.8 (54.7–524.9) | 3 (3.6) |
| Isolated hematuria | 53.0 (24.4–95.8) | 11 (13.1) |
| Other | 122.9 (42.1–201.3) | 8 (9.5) |
Not due to RV.
Figure 2.
Prospective evaluation of usCD163 testing in the clinical setting of possible RV flare (cohort 2). (A) Comparison of normalized usCD163 values in those subsequently adjudicated as having suffered an RV flare versus those that did not have a RV flare. ****P<0.0001, Mann–Whitney test. (B) Receiver-operator characteristic curve for normalized usCD163 comparing RV flare with no RV flare in this prospective cohort. (C) Normalized usCD163 values in patients subsequently evaluated as not having suffered an RV flare. (D) Normalized usCD163 values according to the pretest physician assessment of “possible” or “highly probable” RV flare. In each case, the dotted line represents the upper limit of normal (250 ng/mmol).
Table 3.
Biomarker characteristics in cohort 2 (n=84) compared with adjudication committee diagnosis of RV flare or non-RV flare
| Diagnostic Tool | % (95% CI) | |||||
|---|---|---|---|---|---|---|
| Sensitivity | Specificity | PPV | NPV | FP/FN | AUC (95% CI) | |
| usCD163 >250 ng/mmol | 96.8 (83.3 to 99.9) | 86.8 (74.7 to 94.5) | 81.1 (68.2 to 89.6) | 97.9 (82.1 to 95.8) | 7/1 | 0.950 (0.90 to 0.99) |
| Physician impression of high probability | 90.3% (74.2 to 97.9) | 79.2 (65.9 to 89.2) | 71.8 (56.3 to 92.3) | 93.3 (81.2 to 96.7) | 11/3 | 0.848 (0.771 to 0.924) |
PPV, positive predictive value; NPV, negative predictive value; FP, false positive; FN, false negative.
There was no difference in usCD163 concentrations between diagnostic categories in those adjudicated as non-RV flare, as summarized in Table 2 and Figure 2C. The treating physician thought that RV flare was “possible” in 45 (53.6%, median [IQR] usCD163 of 95.8 [53.4–173.9] ng/mmol) and “highly probable” in 39 (46.4%, median [IQR] usCD163 of 524.9 [271.7–1134] ng/mmol; Figure 2D). Of the “possible” renal flares, 42 (93.3%) were adjudicated as not having a RV flare; the negative predictive value for usCD163 in this setting was 95.2%. Of the “highly probable” renal flares, 11 (28.2%) were adjudicated as not having a renal flare; the positive predictive value of usCD163 in this setting was 84.4%. usCD163 concentration at diagnosis did not predict subsequent development of ESKD (Supplemental Figure 3, B–D).
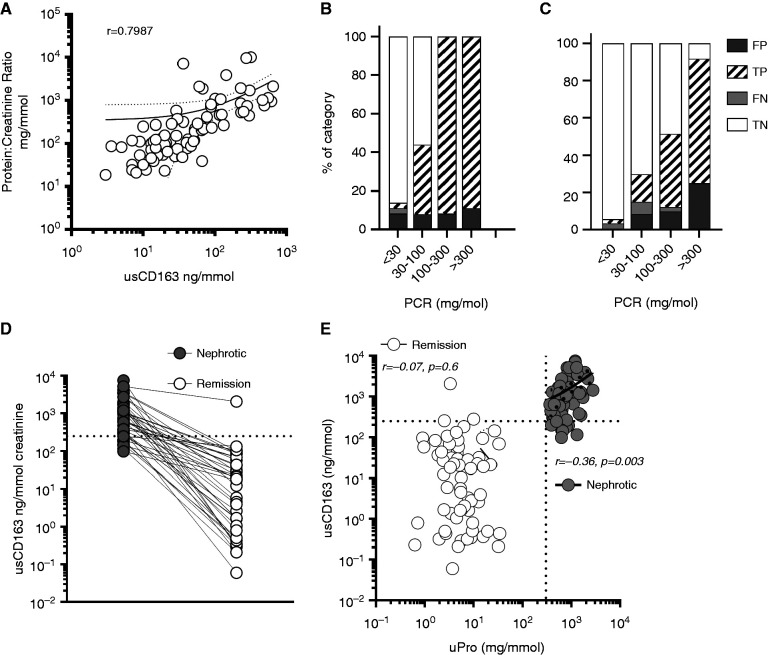
Effect of Proteinuria on usCD163 False-Positive Rate
usCD163 concentration was strongly correlated with urine protein-creatinine ratio (PCR) in this prospective cohort (Figure 3A). We hypothesized that usCD163 concentration reflects glomerular protein leak from the serum. If this hypothesis is true, the fraction of false-positive results would rise as total urine protein rises. To test this, we first quantified the fraction of false positives with increasing urine protein excretion in the prospective RV flare cohort (cohort 2). We found that, in the setting of clinical suspicion for RV flare, the fraction of false positives does not rise with increased proteinuria (Figure 3B). Increasing proteinuria in this restricted setting was almost invariably due to active RV. We then analyzed the fraction of false positives in cohort 1 (a large, mixed, unselected real-world cohort of patients with vasculitis). In this setting, which more accurately reflects widespread use of the test in clinical practice, the fraction of false-positive results did increase with increasing proteinuria (Figure 3C), suggesting that nonspecific protein excretion in nephrotic syndrome elevates usCD163 in the absence of active glomerular macrophage infiltration. To assess the effect of nephrotic-range proteinuria on usCD163 concentration, in the absence of glomerular macrophage infiltration or CD163 mRNA transcription (Supplemental Figure 4A), we next tested paired urine samples from patients with biopsy sample–proven primary podocytopathy using samples from both nephrotic and remission periods. Median (IQR) usCD163 concentration (Figure 3D) in nephrotic syndrome was 654.9 (305–1505) ng/mmol creatinine, and in remission it was 9.1 (1–47) ng/mmol. Similar values were obtained in MN (Supplemental Figure 4B). In active nephrotic syndrome, 56 (86.1%) patients were positive for usCD163, which most likely reflected glomerular leakage of the protein rather than its intraglomerular production. Supporting this hypothesis, urine protein concentration was correlated with usCD163 in active nephrotic syndrome (but not in remission; Figure 3E), and in patients with CKD, where an inflection point at a urine albumin level of 50 mg/L was noted (Supplemental Figure 4C).
Figure 3.
Assessment of the effect of proteinuria on usCD163 estimation. (A) Correlation of usCD163 with urine total protein concentration. (B) Effect of increasing amount of proteinuria on false-positive (FP) rate in cohort 2, a restricted clinical setting of clinical suspicion of RV flare, and in (C) cohort 1, a real-world cohort reflecting the general use of the test in clinical practice. (D) usCD163 values in paired active and remission nephrotic syndrome in patients with biopsy sample–proven podocytopathy. (E) Correlation of usCD163 values in patients with active nephrotic syndrome and in remission. FN, false negative; TN, true negative; TP, true positive
Clinical Application of usCD163 Measurement in the Setting of Proteinuria
To systematically assess methods of proteinuria correction in AAV, we first estimated the FE-CD163 in a group of patients who were in remission with either minimal or heavy proteinuria (cohort 4; Figure 4A). The FE-CD163 value in patients in remission without proteinuria (2.0; IQR, 0.7–63) was comparable with the value in healthy controls (2.6; IQR, 0.3–12.5) and >2-log lower than patients in remission with heavy proteinuria (345.7; IQR, 33–658; P<0.005), confirming glomerular leak of sCD163 in these patients. The log2 FE-CD163 was correlated with log2 urinary PCR (r=0.58, P=0.0001; Figure 4A). We then compared the utility of normalization of usCD163 to total urine protein or urine albumin, and the albumin/sCD163 ratio of serum to urine (which provides as estimate of local glomerular production of sCD163), to the standard approach of normalizing to urine creatinine (Figure 4B). To distinguish patients in remission, but with persistent heavy proteinuria, from patients with active RV, the three approaches were similar, with simple normalization to total urine protein (Figure 4D) slightly outperforming the more complex albumin/sCD163 ratio method (Figure 4E) and albumin normalization (Figure 4F). This suggests that, in patients with heavy proteinuria, normalization of the usCD163 value to total urine protein is the method of greatest clinical utility for noninvasive identification of active RV. The optimal usCD163/protein cutoff ratio in these patients was 2.5 ng/mg. Applying this cutoff value to patients with nephrotic syndrome from cohort 3 led to all patients being reclassified as usCD163 negative (Supplemental Figure 4C), and, when applied to real-world patients from cohort 1 who had AAV and >0.5 g/L total protein, sensitivity was 90% and specificity was 85.7% (Figure 4G).
Figure 4.
Assessment of methods for correction of usCD163 in the setting of heavy proteinuria (cohort 4). (A) Correlation of FE-CD163 with urine PCR (uPCR). Light gray data points reflect healthy controls. (B) FE-CD163 in patients with vasculitis in remission with, and without, heavy proteinuria. Healthy control (HC) shown for comparison. (C) usCD163, normalized to creatinine, in patients with active and remission RV (with and without proteinuria). The c-statistic refers to the area under the respective receiver-operating characteristic curve in attempting to differentiate active RV from both proteinuric and nonproteinuric patients in remission. (D–F) Various mechanisms for normalizing usCD163 in the setting of proteinuria: (D) normalized to total urine protein, (E) normalized using the albumin/sCD163 ratio of serum to urine, and (F) normalized to urine albumin. (G) The total protein-normalized cutoff of 2.5 ng/mg was applied to those cohort 1 patients with AAV and a urine protein value >0.5 g/L. *P<0.05, **P<0.01, ***P<0.001, ****P<0.0001, Kruskal–Wallis test, with Dunn multiple comparisons test. Sens, sensitivity; spec, specificity.
Discussion
One of the greatest challenges in caring for patients with AAV is the early detection of active disease before accrual of irreversible end organ damage. usCD163 is a promising biomarker of crescentic GN with elevated concentrations in active RV and lupus nephritis.10,13,14,16 This is the first study of usCD163 using a diagnostic-grade assay that allows evidence-based translation into clinical practice, the establishment of a real-world reference range, and information on the potential caveats of interpretation in the setting of high-grade proteinuria. We prospectively used usCD163 to distinguish active RV flare from flare mimics in a clinical context, where it performed with sufficient accuracy to potentially reduce the need for kidney biopsy in this setting. We noted in our real-word data analysis that false-positive events occurred when the patient had heavy proteinuria due to leakage of the sCD163 protein across the injured glomerular basement membrane. Systematic comparison of several normalization strategies identified simple normalization to total urine protein as the technique of greatest utility when nephrotic-range proteinuria is present.
The diagnostic-grade sCD163 assay was validated in 487 samples from patients with AAV and 121 healthy controls and compared with the previously used research-grade assay. These two assay results were highly correlated. The reported usCD163 concentrations with the diagnostic-grade assay in active RV were similar to our prior work studying the time of diagnosis and subtle flare.13,14 Derived cutoff values were similar to prior published work with the current proposed cutoff of >250 ng/mmol compared with >300 ng/mmol13 and >143 ng/mmol14 (when used in combination with urine monocyte chemoattractant protein-1). The validation of this clinical-grade ELISA allows clinical translation of usCD163 from a research test to a clinical diagnostic tool.
We demonstrated, in a multicenter prospective study, that usCD163 levels are elevated in RV flare, remain low in clinically relevant flare mimics, and are superior to currently used noninvasive clinical tools. usCD163 had favorable biomarker characteristics for the detection of RV flare, the proposed cutoff value of >250 ng/mmol being associated with a sensitivity, specificity, and AUC of 96.8%, 86.8%, and 0.95, respectively. Importantly, these biomarker metrics differ from previous published work in this field by taking as the comparator (against which to test the ability to identify RV flare) an unselected cohort of patients with vasculitis who displayed a variety of flare mimics, as opposed to a cohort of healthy controls or patients in long-term remission. This directly reflects the proposed use of the test in clinical practice alongside traditional biomarkers. We identified two clinical scenarios that further refine use of the test. First, about half of the study cohort were recruited because the physician felt there was a high chance that the patient was suffering from an RV flare. In this context, usCD163 displayed high positive predictive value (84.4%), suggesting the test was useful for confirming the physician’s assessment. Second, in the other half of the cohort, the physician felt there was a “possible” renal flare (with a lower pretest probability), in which context the negative predictive value was 95.2%, suggesting the test may be useful to “rule out” in this clinical setting. In both scenarios, measurement of usCD163 has the potential to reduce the need for kidney biopsy.
We sought to explore the effect of high-grade proteinuria on potential leakage of serum sCD163 across the glomerular filtration barrier leading to detection of nonlocally produced sCD163 in urine. In healthy glomeruli, usCD163 does not cross the glomerular filtration barrier due to its high mol wt.33 To explore this potential caveat, we studied the fraction of false positives in prospectively recruited patients with potential RV flares (cohort 2). The fraction of false positives did not rise when stratified by proteinuria in this cohort. However, increasing proteinuria in this restricted setting was almost invariably due to active RV. We then analyzed the fraction of false positives in an unselected longitudinal cohort of patients with vasculitis (cohort 1). In this setting, which reflects the use of the test in routine clinical practice, the fraction of false-positive results did increase with increasing proteinuria (PCR >300 mg/mmol; Figure 3C), suggesting nonspecific protein excretion increases usCD163 concentration, even in the absence of glomerular macrophage infiltration. To explore this hypothesis further, the clinical scenario of nephrotic syndrome was selected due to glomerular filtration barrier disruption from foot process effacement with an absence of glomerular macrophage infiltration. usCD163 concentrations were elevated in active nephrotic syndrome, with median values of 655 ng/mmol, which is comparable with median levels of 939 ng/mmol in our active vasculitis population, and exceeds the diagnostic threshold for active vasculitis of >250 ng/mmol. In paired samples from the same patients in remission, we found that usCD163 levels returned to the normal range.
We then explored different methods of correcting for a potential leak of sCD163 across the glomerular basement membrane. The FE-CD163 in patients in remission without proteinuria and healthy controls were comparable but were >2-log higher than in patients in remission with heavy proteinuria, confirming glomerular leak of sCD163 in this setting. Correction for urinary protein and albumin (instead of creatinine normalization) led to attenuation of the increased signal seen in active nephrotic syndrome. We also assessed the effect of fractional excretion relative to paired urine and serum albumin, mirroring techniques used to assess renal fractional excretion of sodium, and quantification of cerebrospinal fluid glucose and protein.34,35 Normalizing usCD163 to total urine protein, albumin, and sCD163 ratio of serum to urine albumin values yielded broadly similar results. However, simple normalization to total urine protein (AUC, 0.91) was marginally better than both albumin normalization (AUC, 0.89) and the more complex albumin/sCD163 ratio equation (0.88). Therefore, we propose that, in high-grade proteinuria, normalization of the usCD163 value to total urine protein is the method of greatest clinical utility for noninvasive identification of active RV, with a potential cutoff of 2.5 ng/mg. We propose applying this technique only in those with total urine protein value >0.5 g/L because normalization against lower protein values causes artificial inflation of the result. Use of these values will need to be validated in larger cohorts. To aid in the translation of our findings to clinical practice, we have developed an online calculator (https://thkc.shinyapps.io/usCD163/) that provides clinical context to urine creatinine and protein-normalized usCD163 concentrations in AAV.
In conclusion, this study has further defined use of usCD163 as a diagnostic tool for detection of RV flare using a diagnostic-grade assay, thereby allowing translation of usCD163 from a research assay at the bench to a clinical-grade test to be used at the bedside. In the setting of high-grade proteinuria, normalization of sCD163 to urine protein rather than creatinine improves diagnostic accuracy. Future research directions include assessment of the utility of serial usCD163 measurement to aid in early identification of subclinical flare; assessment of usCD163 as an early marker of response to, or resistance to, immunotherapy; and in the identification of treatment futility in dialysis-dependent RV. Beyond AAV, the role of usCD163 in lupus has been described, but its utility in other glomerular diseases with crescentic GN requires further investigation. Broader use of usCD163 as a screening test to identify AKI of glomerular origin (perhaps using a point-of-care test) and prompt nephrology referral has the potential to lead to earlier identification of treatment-responsive glomerular diseases.
Disclosures
M.R. Clarkson reports receiving honoraria from AstraZeneca and Sanofi Genzyme. N. Conlon reports receiving honoraria from Novartis and Takeda, and research funding from Takeda. M.D. Griffin reports receiving honoraria from American Society of Nephrology as associate editor, Hebei Medical University China, and National Institutes of Health; serving on the editorial boards for Frontiers in Renal Pharmacology and Transplantation, and as the section editor for Mayo Clinic Proceedings; and receiving research funding from Randox Laboratories. T.P. Griffin reports having consultancy agreements with AstraZeneca and Novo Nordisk; receiving honoraria from AstraZeneca, Novo Nordisk, and Sanofi; receiving a grant from the European Commission (Horizon 2020 Collaborative Health Project NEPHSTROM; grant number 634086); receiving research funding from a Hardiman Scholarship from the College of Medicine, Nursing and Health Science, National University of Ireland, Galway; receiving a bursary from the Irish Endocrine Society/Royal College of Physicians of Ireland; and having collaborated with Randox Teoronta. M. Kretzler reports receiving research funding (via a sponsored research project as principal investigator at University of Michigan) from amfAR, Angion, AstraZeneca, Boehringer-Ingelheim, Certa, Chan Zuckerberg Initiative, Chinook, Elpidera, Gilead, Goldfinch, Ionis, Jansen, JDRF, Lilly, National Institutes of Health (NIH), Novo Nordisk, Regeneron, RenalytixAI, and Travere; having consultancy agreements (as an employee of University of Michigan) with Astellas, Boehringer-Ingelheim, Certa, Janssen, Novo Nordisk, and Poxel; serving on the editorial boards for JASN, Kidney Disease, and Kidney International; and serving on the advisory board for NephCure Kidney International. M.A. Little reports having consultancy agreements with AnaptysBio, Chemocentryx, and LightStone; serving as a scientific advisor for, or member of, ChemoCentryx; having a licensing agreement with Euroimmun GMBH and codeveloping the usCD163 assay with them; and receiving research funding from Mundipharma GMBH and Vifor Pharma. P.V. O’Hara reports receiving honoraria from Amgen, AstraZeneca, and Vifor Pharmaceuticals. All remaining authors have nothing to disclose.
Funding
The project was supported by Science Foundation Ireland award 11/Y/B2093, Vasculitis Foundation award 205178/13967, and Health Research Board grant HRA-POR-2015-1205. S. M. Moran was supported by the Health Research Board award NSAFP-2013-02. M. D. Griffin is supported by Science Foundation Ireland grant 13/RC/2073 (CÚRAM Research Centre). J. Scott and C. Judge supported by supported the Irish Clinical Academic Training Programme, which is cofunded by the Wellcome Trust and the Health Research Board (grant 203930/B/16/Z), the Health Service Executive National Doctors Training and Planning, and the Health and Social Care, Research and Development Division, Northern Ireland. The Nephrotic Syndrome Study Network Consortium (NEPTUNE) is a part of the NIH Rare Disease Clinical Research Network, supported through a collaboration between the NIH Office of Rare Diseases Research, National Center for Advancing Translational Sciences, and the NIDDK (grant U54-DK-083912). Additional funding and/or programmatic support for this project has also been provided by the University of Michigan, NephCure Kidney International.
Supplementary Material
Acknowledgments
We acknowledge the support of the clinicians in VINE (https://www.tcd.ie/medicine/thkc/vasculitis/) and the nephrology community in Ireland, in particular, Eamonn Molloy, Catherine Wall, Peter Lavin, Brenda Griffin, Donal Sexton, Peter Conlon, Conall O’Seaghada, Declan de Freitas, Colm Magee, Yvonne O’Meara, William D. Plant, Joseph A. Eustace, Alan Watson, Susan Murray, Claire Kennedy, Vicky Sandys, Yvelynne Kelly, Dervla Connaughton, Laura Slattery, Adrian Whelan, and Tze Liang Goh. We also acknowledge support of the VINE research nurse team, in particular, Ruth Argue and Claire Foley. We also acknowledge Vincent O’Reilly, Eoin O’Brien, Fionnuala Hickey, Alice Coughlan, Michelle Ryan, and other members of the Trinity Health Kidney Research Group for their support and expertise in assay optimization. We acknowledge the expertise and guidance of Holger J. Møller on usCD163 assay development and interpretation.
S.M. Moran and M.A. Little devised and conducted studies; S.M. Moran, J. Dunne, E. Groarke, and K. McLoughlin conducted usCD163 assays; J. Dunne, E. Groarke, and K. McLoughlin optimized the Euroimmun assay; M. Kretzler and members of the NEPTUNE consortium enrolled patients with nephrotic syndrome; J. Scott, M.A. Little, N. Conlon, and S.M. Moran were members of the adjudication committee; T.P. Griffin and M.D. Griffin recruited healthy and disease controls; S.M. Moran, J. Scott, M.R. Clarkson, N. Conlon, J. Holian, P.V. O’Hara, C. Judge, M.D. Griffin, and M.A. Little recruited study candidates across all sites; and C. Judge developed the online calculator.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
Contributor Information
Collaborators: J. Sedor, K. Dell, M. Schachere, J. Negrey, K. Lemley, E. Lim, T. Srivastava, A. Garrett, C. Sethna, K. Laurent, G. Appel, M. Toledo, L. Greenbaum, C. Wang, C. Kang, S. Adler, J. LaPage, A. Athavale, M. Itteera, M. Atkinson, S. Boynton, F. Fervenza, J. Lieske, M. Hogan, V. Chernitskiy, F. Kaskel, M. Ross, P. Flynn, J. Kopp, J. Blake, H. Trachtman, O. Zhdanova, F. Modersitzki, S. Vento, R. Lafayette, K. Mehta, C. Gadegbeku, S. Quinn-Boyle, M. Hladunewich, H. Reich, P. Ling, M. Romano, A. Fornoni, C. Bidot, M. Kretzler, D. Gipson, A. Williams, J. LaVigne, V. Derebail, K. Gibson, A. Froment, S. Grubbs, L. Holzman, K. Meyers, K. Kallem, J. Lalli, K. Sambandam, Z. Wang, M. Rogers, A. Jefferson, S. Hingorani, K. Tuttle, M. Bray, M. Kelton, A. Cooper, J.J. Lin, Stefanie Baker, M. Kretzler, L. Barisoni, H. Desmond, C. Gadegbeku, B. Gillespie, D. Gipson, L. Holzman, V. Kurtz, L. Mariani, M. Sampson, M. Larkina, J. Zee, S. Li, C. Lienczewski, J. Liu, T. Mainieri, M. Wladkowski, A. Williams, Carmen Avila-Casado, Serena Bagnasco, Joseph Gaut, Stephen Hewitt, Jeff Hodgin, Kevin Lemley, Laura Mariani, Matthew Palmer, Avi Rosenberg, Virginie Royal, David Thomas, Jarcy Zee, Laura Barisoni, and Cynthia Nast
Supplemental Material
This article contains the following supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2021030382/-/DCSupplemental
Supplemental Table 1. Characteristics of cohort 1, used to develop reference ranges for the Irish National Accreditation Board.
Supplemental Table 2. Clinical characteristics of cohort 2 at time of study visit (flare or flare mimic).
Supplemental Table 3. Clinical characteristics of false positive and false negative usCD163 values in cohort 2 (prospective assessment of renal vasculitis flare).
Supplemental Table 4. Characteristics of cohort 3 used to investigate the effect of proteinuria on urine sCD163 excretion
Supplemental Table 5. Characteristics of cohort 4, patients with ANCA vasculitis and disease controls used to investigate the impact of proteinuria on false positive diagnostic rate
Supplemental Table 6. Cohort 4. Median and interquartile range levels of soluble CD163 values in active renal vasculitis, remission vasculitis with proteinuria, remission vasculitis without proteinuria
Supplemental Figure 1. Flow diagram of cohort 2 recruitment from screening to enrollment and subsequent diagnosis
Supplemental Figure 2. Real world usCD163 values in a range of disease controls using the Euroimmun assay.
Supplemental Figure 3. usCD163 in those who underwent kidney biopsy (cohort 2) and relationship to subsequent development of ESKD.
Supplemental Figure 4. Effect of proteinuria on usCD163 values.
References
- 1.Jennette JC, Olson JL, Schwartz MM, Silva FG, Thomas DB: Pauci-immune and antineutrophil cytoplasmic autoantibody–mediated crescentic glomerulonephritis and vasculitis. In: Heptinstall’s Pathology of the Kidney, edited by Olson JL, Jennette JC, Schwartz MM, Silva FG, Philadelphia, Lippincott Williams and Wilkins, 2007, pp 643–674 [Google Scholar]
- 2.Booth AD, Almond MK, Burns A, Ellis P, Gaskin G, Neild GH, et al. ; Pan-Thames Renal Research Group : Outcome of ANCA-associated renal vasculitis: a 5-year retrospective study. Am J Kidney Dis 41: 776–784, 2003 [DOI] [PubMed] [Google Scholar]
- 3.Mohammad AJ, Segelmark M: A population-based study showing better renal prognosis for proteinase 3 antineutrophil cytoplasmic antibody (ANCA)-associated nephritis versus myeloperoxidase ANCA-associated nephritis. J Rheumatol 41: 1366–1373, 2014 [DOI] [PubMed] [Google Scholar]
- 4.Lionaki S, Hogan SL, Jennette CE, Hu Y, Hamra JB, Jennette JC, et al. : The clinical course of ANCA small-vessel vasculitis on chronic dialysis. Kidney Int 76: 644–651, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Poggio ED, McClelland RL, Blank KN, Hansen S, Bansal S, Bomback AS, et al. ; Kidney Precision Medicine Project : Systematic review and meta-analysis of native kidney biopsy complications. Clin J Am Soc Nephrol 15: 1595–1602, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Menn-Josephy H, Lee CS, Nolin A, Christov M, Rybin DV, Weinberg JM, et al. : Renal interstitial fibrosis: an imperfect predictor of kidney disease progression in some patient cohorts. Am J Nephrol 44: 289–299, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rhee RL, Davis JC, Ding L, Fervenza FC, Hoffman GS, Kallenberg CGM, et al. : The utility of urinalysis in determining the risk of renal relapse in ANCA-associated vasculitis. Clin J Am Soc Nephrol 13: 251–257, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Møller HJ, Tesar V, Little MA: Urine sCD163: a window onto glomerular inflammation. Nephrol Dial Transplant 31: 1970–1972, 2016 [DOI] [PubMed] [Google Scholar]
- 9.Weissinger EM, Wittke S, Kaiser T, Haller H, Bartel S, Krebs R, et al. : Proteomic patterns established with capillary electrophoresis and mass spectrometry for diagnostic purposes. Kidney Int 65: 2426–2434, 2004 [DOI] [PubMed] [Google Scholar]
- 10.Dekkema GJ, Abdulahad WH, Bijma T, Moran SM, Ryan L, Little MA, et al. : Urinary and serum soluble CD25 complements urinary soluble CD163 to detect active renal anti-neutrophil cytoplasmic autoantibody-associated vasculitis: A cohort study. Nephrol Dial Transplant 34: 234–242, 2018 [DOI] [PubMed] [Google Scholar]
- 11.Møller HJ, Peterslund NA, Graversen JH, Moestrup SK: Identification of the hemoglobin scavenger receptor/CD163 as a natural soluble protein in plasma. Blood 99: 378–380, 2002 [DOI] [PubMed] [Google Scholar]
- 12.Fabriek BO, van Bruggen R, Deng DM, Ligtenberg AJ, Nazmi K, Schornagel K, et al. : The macrophage scavenger receptor CD163 functions as an innate immune sensor for bacteria. Blood 113: 887–892, 2009 [DOI] [PubMed] [Google Scholar]
- 13.O’Reilly VP, Wong L, Kennedy C, Elliot LA, O’Meachair S, Coughlan AM, et al. : Urinary soluble CD163 in active renal vasculitis. J Am Soc Nephrol 27: 2906–2916, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moran SM, Monach PA, Zgaga L, Cuthbertson D, Carette S, Khalidi NA, et al. ; Vasculitis Clinical Research Consortium : Urinary soluble CD163 and monocyte chemoattractant protein-1 in the identification of subtle renal flare in anti-neutrophil cytoplasmic antibody-associated vasculitis. Nephrol Dial Transplant 35: 283–291, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Endo N, Tsuboi N, Furuhashi K, Shi Y, Du Q, Abe T, et al. : Urinary soluble CD163 level reflects glomerular inflammation in human lupus nephritis. Nephrol Dial Transplant 31: 2023–2033, 2016 [DOI] [PubMed] [Google Scholar]
- 16.Mejia-Vilet JM, Zhang XL, Cruz C, Cano-Verduzco ML, Shapiro JP, Nagaraja HN, et al. : Urinary soluble CD163: a novel noninvasive biomarker of activity for lupus nephritis. J Am Soc Nephrol 31: 1335–1347, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Beck LH Jr, Bonegio RG, Lambeau G, Beck DM, Powell DW, Cummins TD, et al. : M-type phospholipase A2 receptor as target antigen in idiopathic membranous nephropathy. N Engl J Med 361: 11–21, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vivarelli M, Massella L, Ruggiero B, Emma F: Minimal change disease. Clin J Am Soc Nephrol 12: 332–345, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Griffin TP, Islam MN, Wall D, Ferguson J, Griffin DG, Griffin MD, et al. : Plasma dephosphorylated-uncarboxylated Matrix Gla-Protein (dp-ucMGP): Reference intervals in Caucasian adults and diabetic kidney disease biomarker potential. Sci Rep 9: 18452, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hamon SM, Griffin TP, Islam MN, Wall D, Griffin MD, O’Shea PM: Defining reference intervals for a serum growth differentiation factor-15 (GDF-15) assay in a Caucasian population and its potential utility in diabetic kidney disease (DKD). Clin Chem Lab Med 57: 510–520, 2019 [DOI] [PubMed] [Google Scholar]
- 21.Islam MN, Griffin TP, Whiriskey R, Hamon S, Cleary B, Blake L, et al. : Reference intervals for commonly requested biochemical and haematological parameters in a healthy Irish adult Caucasian population [published online ahead of print February 11, 2021]. Ir J Med Sci [DOI] [PubMed] [Google Scholar]
- 22.Jennette JC, Falk RJ, Bacon PA, Basu N, Cid MC, Ferrario F, et al. : 2012 revised International Chapel Hill Consensus Conference Nomenclature of Vasculitides. Arthritis Rheum 65: 1–11, 2013 [DOI] [PubMed] [Google Scholar]
- 23.Gadegbeku CA, Gipson DS, Holzman LB, Ojo AO, Song PX, Barisoni L, et al. : Design of the Nephrotic Syndrome Study Network (NEPTUNE) to evaluate primary glomerular nephropathy by a multidisciplinary approach. Kidney Int 83: 749–756, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Smith RM, Jones RB, Specks U, Bond S, Nodale M, Aljayyousi R, et al. ; RITAZAREM coinvestigators; RITAZAREM co-investigators : Rituximab as therapy to induce remission after relapse in ANCA-associated vasculitis. Ann Rheum Dis 79: 1243–1249, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stone JH, Merkel PA, Spiera R, Seo P, Langford CA, Hoffman GS, et al. ; RAVE-ITN Research Group : Rituximab versus cyclophosphamide for ANCA-associated vasculitis. N Engl J Med 363: 221–232, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Aasarød K, Bostad L, Hammerstrøm J, Jørstad S, Iversen BM: Wegener’s granulomatosis: inflammatory cells and markers of repair and fibrosis in renal biopsies--a clinicopathological study. Scand J Urol Nephrol 35: 401–410, 2001 [DOI] [PubMed] [Google Scholar]
- 27.Hauer HA, Bajema IM, van Houwelingen HC, Ferrario F, Noël LH, Waldherr R, et al. ; European Vasculitis Study Group (EUVAS) : Renal histology in ANCA-associated vasculitis: differences between diagnostic and serologic subgroups. Kidney Int 61: 80–89, 2002 [DOI] [PubMed] [Google Scholar]
- 28.R Core Team : R: A language and environment for statistical computing. Vienna, Austria, R Foundation for Statistical Computing, 2021 [Google Scholar]
- 29.López-Ratón M, Rodríguez-Álvarez MX, Cadarso-Suárez C, Gude-Sampedro F: OptimalCutpoints: An R package for selecting optimal cutpoints in diagnostic tests. J Stat Softw 61: 1–36, 2014 [Google Scholar]
- 30.Youden WJ: Index for rating diagnostic tests. Cancer 3: 32–35, 1950 [DOI] [PubMed] [Google Scholar]
- 31.Pencina MJ, D’Agostino RB Sr, D’Agostino RB Jr, Vasan RS: Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Stat Med 27: 157–172, discussion 207–212, 2008 [DOI] [PubMed] [Google Scholar]
- 32.Ji X, Kattan MW: Tutorial: development of an online risk calculator platform. Ann Transl Med 6: 46, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Møller HJ: Soluble CD163. Scand J Clin Lab Invest 72: 1–13, 2012 [DOI] [PubMed] [Google Scholar]
- 34.Steiner RW: Interpreting the fractional excretion of sodium. Am J Med 77: 699–702, 1984 [DOI] [PubMed] [Google Scholar]
- 35.Deisenhammer F, Bartos A, Egg R, Gilhus NE, Giovannoni G, Rauer S, et al. ; EFNS Task Force : Guidelines on routine cerebrospinal fluid analysis. Report from an EFNS task force. Eur J Neurol 13: 913–922, 2006 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.