Coronavirus disease 2019 (COVID-19) is associated with a poor prognosis in patients on dialysis (DP), and strong efforts have been made to protect this vulnerable group by vaccination against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Fortunately, first results demonstrate a mostly efficient vaccination response against the wild-type strain (that first appeared in Wuhan) after two doses, albeit with lower antibody titers as compared with healthy controls.1–3 However, the new SARS-CoV-2 variants of concern (VOC) B.1.1.7 (α) and B.1.351 (β) are raising concerns due to their potential to escape the vaccination responses, and this can aggravate the problem of lower responsiveness in DP. In fact, data on vaccination breakthrough identified B.1.1.7 as a strain that can cause severe COVID-19 in fully vaccinated patients. First data on humoral vaccination response toward SARS-CoV-2 VOCs for healthy individuals show that sera from vaccinated individuals have a high potential to neutralize B.1.1.7, whereas the capacity to neutralize B.1.351 is markedly reduced.4 However, data about the protective capacity of currently available vaccines against VOC in the dialysis population are still missing.
Consequently, we analyzed the humoral immune response in 34 patients on hemodialysis, of whom 30 were additionally characterized for their cellular immune response a minimum of 14 days after the second dose of BNT162b2 mRNA vaccination (Supplemental Table 1). Healthy young individuals (n=14) served as positive controls for humoral responses. SARS-CoV-2 spike-protein wild-type (S-WT) IgG was assessed with ELISA (Euroimmun). Neutralizing antibodies were analyzed by cytopathic effects of S-WT, B.1.1.7, and B.1.351 whole viruses on Vero E6 lines in presence of patient sera. T cells reactive against S-WT, B.1.1.7, and B.1.351 strains were assessed by multiparameter flow cytometry. A detailed description of materials and methods can be found in Supplemental Material.
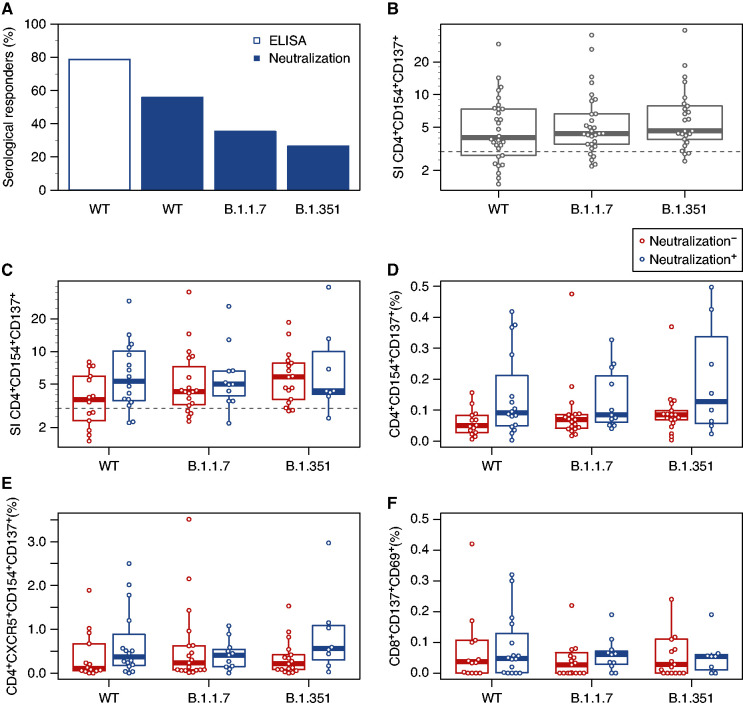
Humoral response as defined by detection of IgG specific for SARS-CoV-2 S-WT was observed in 79% of analyzed DP, whereas 100% of healthy young donors demonstrated IgG detection. Neutralizing antibody levels are highly predictive of protection against symptomatic SARS-CoV-2 infection.5 The capacity of sera of vaccinated individuals to neutralize S-WT was observed in only 56% of DP but 100% of the healthy controls (Figure 1A, Supplemental Figures 1 and 2). All patients negative for IgG ELISA also lacked neutralizing activity. Interestingly, seven DP (21%) were positive for SARS-CoV-2 IgG but had no neutralizing antibodies, suggesting that screening with simple IgG ELISA might underestimate the frequency of nonresponders among DP. Considering neutralization of VOC strains, the response was even lower, with 35% and 26% of patients having serum-neutralizing antibodies against B.1.1.7 and B.1.351, respectively (Figure 1A). In contrast, sera of healthy donors were neutralizing in 93% (B.1.1.7) and 86% (B.1.351).
Figure 1.
Fully vaccinated patients on hemodialysis show humoral and cellular immunity toward SARS-CoV-2 wild-type (WT), α-, and β-strains. Patients on hemodialysis (DP, n=34) were included at least 2 weeks after complete vaccination with BioNTech/Pfizer vaccine BNT162b2. (A) Anti–SARS-CoV-2 spike-protein binding antibodies assessed by ELISA (n=33 DP) and neutralizing antibodies assessed with WT virus hCoV-19, VOC B.1.1.7 (α), or VOC B.1351 (β; n=34 DPs). (B) Ratio of activation markers CD154 and CD137 in CD4+ CD3+ T cells in specifically stimulated samples and negative control (stimulation index [SI]; n=30 WT; n=30 B.1.1.7; n=25 B.1.351). Values above three are considered positive. (C) CD4+ CD3+ T cell activation SI compared between humoral responders (neutralization positive) and nonresponders (neutralization negative) as defined by neutralizing antibody in patient sera (n=16 WT, n=11 B.1.1.7, and n=8 B.1.351 for responders; n=14 WT, n=19 B.1.1.7, and n=17 B.1.3.5.1 for nonresponders). (D) Frequency of activated (CD154+ and CD137+) CD4+ CD3+ T cells among humoral responders and nonresponders. (E) Frequency of activated (CD154+ and CD137+) follicular helper-like (CXCR5+ CD4+ CD3+) T cells among humoral responders and nonresponders. (F) Frequency of activated (CD69+ and CD137+) CD8+ CD3+ T cells among humoral responders and non-responders. Two data points are above the axis limit. Box plots depict the median, first quartile, and third quartile of a variable; the maximum length of the whiskers corresponds to 1.5 times the interquartile range. Comparisons were performed only between samples stimulated with the same strain. Differences between two groups were analyzed using the unpaired, two-tailed Mann–Whitney U test. P values of P<0.05 cannot be reported.
Although neutralizing antibodies are commonly assumed to be essential to prevent infections, T cell immunity is known to combat infection, consequently protecting from severe COVID-19 and death. Despite the relatively low neutralizing capacity against both VOC, substantial CD4+ T cell responses against S-WT, B.1.1.7, and B.1.351, defined as stimulation index above three,6 could be detected in 73%, 83%, and 88% of DPs, respectively (Figure 1B, Supplemental Figure 3). Of interest, 57%, 79%, and 88% of patients with failed humoral immunity were able to mount T cells directed against S-WT, B.1.1.7, and B.1.351, respectively (Figure 1C). Accordingly, the frequencies of S protein–reactive CD4+ T cells were lower in humoral non-responders than responders (Figure 1D). Detectable cells were functionally active as demonstrated by simultaneous production of several effector cytokines, such as IFNγ, TNFα, and IL-2 (Supplemental Figures 4 and 5). They were predominantly of effector memory type, regardless of the VOC, suggesting rapid reactivity after re-encounter with the respective antigen (Supplemental Figure 4 and 6). As expected given the lack of neutralizing antibodies, SARS-CoV-2–reactive circulating follicular helper T cells, characterized by CXCR5 expression, were lower in humoral nonresponders (Figure 1E). CD8+ T cells showed low response to the vaccination in all patients (Figure 1F), consistent with previous reports on vaccination responses.7 Age did not influence humoral and cellular vaccination responses in our cohort (Supplemental Figure 7, Supplemental Table 1).
In conclusion, impaired humoral immunity directed against VOCs B.1.1.7 and B.1.357 and S-WT was observed in DPs fully vaccinated with BNT162b2. However, the majority of humoral non-responders were able to generate T cell responses directed against S-WT and both VOC, which might reduce the risk of severe or even fatal courses of COVID-19. Thus, the results of this work suggest that mounted cellular response could be sufficient to protect against severe COVID-19, despite lower humoral immunity in most DP.
Disclosures
T.H. Westhoff reports research grants and/or speakers’ honoraria and/or advisory boards’ honoraria from Amgen, Astellas, AstraZeneca, Bayer, Bristol-Myers Squibb, Daichii-Sankyo, Hexal, MSD, Novartis, Otsuka, Pfizer, and Sanofi Aventis, and other interests/relationships with the German Society of Nephrology, the German Society of Hypertension, and the German Transplant Association. All remaining authors have nothing to disclose.
Funding
This work was supported by Bundesministerium für Bildung und Forschung grant NoChro (13GW0338B) e:KID (01ZX1612A), Bundesministerium für Wirtschaft und Energie grant EpiCov (ZIM, KK5029902AJ0), and Stiftung Mercator grant RIMUR (St-2018-0014).
Supplementary Material
Acknowledgments
We are grateful to all patients participating in the study. We thank Thorsten Wolff, Jessica Schulz, and Christian Mache from the Robert Koch Institute (Fachbereich 17) and Martin Beer from the Friedrich Loeffler Institute for providing B.1.1.7 and B.1.351 isolates.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
Supplemental Material
This article contains the following supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2021050672/-/DCSupplemental.
Supplemental Table 1. Patient characteristics.
Supplemental Figure 1. Venn diagrams for patients on hemodialysis with surrogate markers of immunity at least 2 weeks after two doses of SARS-CoV-2 vaccination BNTb162b.
Supplemental Figure 2. Humoral immunity of healthy donors at least 2 weeks after two doses of SARS-CoV-2 vaccination BNTb162b.
Supplemental Figure 3. Representative gating strategy for the analysis of activated CD4+, CD4+ CXCR5+, and CD8+ T cells.
Supplemental Figure 4. Representative gating strategy for the analysis of cytokines IL-2, TNFα, and IFNγ producing activated CD4+ T cells.
Supplemental Figure 5. Frequency of cytokine-producing cells among activated (CD154 and CD137+) CD4+ CD3+ T cells in humoral responders and nonresponders.
Supplemental Figure 6. Frequency of memory phenotypes among activated (CD154 and CD137+) CD4+ CD3+ T cells in humoral responders and nonresponders.
Supplemental Figure 7. Influence of age on SARS-CoV-2–reactive T cell responses.
Supplementary Material. Methods.
References
- 1.Ikizler TA, Coates PT, Rovin BH, Ronco P: Immune response to SARS-CoV-2 infection and vaccination in patients receiving kidney replacement therapy. Kidney Int 99: 1275–1279, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Attias P, Sakhi H, Rieu P, Soorkia A, Assayag D, Bouhroum S, et al. : Antibody response to the BNT162b2 vaccine in maintenance hemodialysis patients. Kidney Int 99: 1490–1492, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yanay NB, Freiman S, Shapira M, Wishahi S, Hamze M, Elhaj M, et al. : Experience with SARS-CoV-2 BNT162b2 mRNA vaccine in dialysis patients. Kidney Int 99: 1496–1498, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang P, Nair MS, Liu L, Iketani S, Luo Y, Guo Y, et al. : Antibody resistance of SARS-CoV-2 variants B.1.351 and B.1.1.7. Nature 593: 130–135, 2021 [DOI] [PubMed] [Google Scholar]
- 5.Khoury DS, Cromer D, Reynaldi A, Schlub TE, Wheatley AK, Juno JA, et al. : Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat Med 27: 1205–1211, 2021 [DOI] [PubMed] [Google Scholar]
- 6.Thieme CJ, Anft M, Paniskaki K, Blazquez-Navarro A, Doevelaar A, Seibert FS, et al. : Robust T cell response toward spike, membrane, and nucleocapsid SARS-CoV-2 proteins is not associated with recovery in critical COVID-19 patients. Cell Rep Med 1: 100092, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Geers D, Shamier MC, Bogers S, den Hartog G, Gommers L, Nieuwkoop NN, et al. : SARS-CoV-2 variants of concern partially escape humoral but not T-cell responses in COVID-19 convalescent donors and vaccinees. Sci Immunol 6: eabj1750, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.