Abstract
Background:
Stem cell based therapy has been encouraged as an attractive method in regenerative medicine. Poor survival and maintenance of the cells transferred into the damaged tissue are broadly accepted as serious barriers to enhancing the efficacy of regenerative medicine. For this reason, some antioxidants such as L-carnitine (LC) are used as a favorite strategy to improve cell survival and retention properties.
Aims:
This study aims to evaluate the effect of LC on the expression of CD34 marker and its effect on apoptosis and SUZ12 gene expression.
Methods:
Rat bone marrow mono-nuclear cells (rBMNCs) were isolated. Then, CD34+ hematopoietic stem cells (HSCs) were enriched using the magnetic activated cell sorting (MACS) method. The cells were treated with 0.2 and 0.4 mM LC. Gene and protein expression levels of the CD34 were then measured by real-time PCR and flow cytometry, respectively. The percentage of apoptosis and SUZ12 gene expression were measured using the Annexin V/PI method and real-time PCR, respectively.
Results:
The results showed that in the experimental group, of the CD34+ HSCs treated with 0.2 mM LC, gene and protein expressions of CD34 increased by 1.7 fold and 0.49%, respectively. At the concentration of 0.4 mM, the early cell apoptosis increased by 25.9% (P<0.05). Also, in the concentration of 0.2 and 0.4 mM LC, the SUZ12 gene expression increased by 1.10 and 1.75 folds compared to the control group (P<0.05 and P<0.01), respectively.
Conclusion:
The results of this study could be used to improve chronic myeloid leukemia (CML) as a multidirectional therapeutic strategy.
Key Words: Apoptosis, CD34, Hematopoietic stem cells, L-carnitine, SUZ12
Introduction
As a powerful antioxidant, L-carnitine (LC) has many biological effects such as increasing the activities of antioxidant enzymes, decreasing population doubling time and aging, improving lifespan of mesenchymal stem cells (MSCs), metabolism of fatty acids, and decreasing lipid peroxidation (Mobarak et al., 2017 ▶). Bone marrow contains a number of cell surface antigens for identification, isolation and enumeration of hematopoietic stem cells (HSCs) and progenitors called transmembrane CD34 glycoprotein with a molecular weight of 110 kDa (Attar, 2014 ▶). This marker is expressed in the vascular endothelium of 30% of patients with acute leukemia. CD34, as a specific selection marker, belongs to the sialomucin family and plays an important role in the adhesion of progenitors to bone marrow stroma via L-selectin, with the ability to produce and differentiate all blood cells including immune cells (de Fabritiis et al., 1993 ▶; Lin et al., 2020 ▶). Contrary to the abundance of CD34 markers in progenitor cells, this marker is rarely seen in peripheral blood (Nielsen et al., 2009 ▶). For this reason, in order to isolate the marker-containing cells from peripheral blood, progenitor stem cells must be stimulated to enter the peripheral blood (Walz et al., 2006 ▶).
Apoptosis is a type of precise, natural, and physiological cell death in the body that is genetically encoded and causes cell destruction during which structural changes and disintegration of cellular DNA occurs (Ghose et al., 2020 ▶). It is an energy dependent process and some anticancer therapies are based on activating this pathway. It occurs during differentiation, cell count regulation, embryonic and organ development, tissue homeostasis and defense against pathogens and immune regulation (Obeng, 2021 ▶). Caspase enzymes are cysteine proteases that play a major role in initiating and performing apoptosis. By activating caspases, a number of key enzymes, including polymerase, are involved in repairing damaged DNA, and the structural proteins of the cell are broken down, leading to cell death (Kesavardhana et al., 2020 ▶).
Polycomb group (PcG) proteins contain PcG proteins such as enhancer of zeste homolog 2 (EZH2), suppressor of zeste 12 protein homolog (SUZ12), and embryonic ectoderm development (EED) (Lee et al., 2015 ▶; Penfornis et al., 2018 ▶). Deregulation of PcG proteins has been reported in several cancer types (Bracken et al., 2003 ▶). SUZ12 is essential for polycomb repressive complex 2 (PRC2), and its inactivation results in early lethality of mouse embryos. SUZ12 is often up-regulated in liver, breast, and colon tumors (Pizzatti et al., 2010 ▶). The role of signaling pathways such as E2F/Rb and Wnt/β-catenin in the regulation of SUZ12 in solid tumors has been previously indicated. For the first time, Pizzatti et al. (2010) ▶ found that SUZ12 is over-expressed in the bone marrow of patients with CML-blastic phase due to the activation of a non-canonical Wnt signaling pathway (Pizzatti et al., 2010 ▶). Pasini et al. (2007) ▶ showed that SUZ12 is essential for the proper differentiation of embryonic stem cells (ESCs), most likely by directly controlling specific gene expressions during cellular commitment (Pasini et al., 2007 ▶). In another study, it was pointed out that complete loss of SUZ12 resulted in failure of hematopoiesis and loss of HSCs maintenance (Lee et al., 2015 ▶). Also, it was indicated that because PRC2 is a potential target for cancer therapy, the significant consequences of modest changes in PRC2 activity, as well as the cell and developmental stage-specific effects, will need to be carefully considered in any therapeutic context (Lee et al., 2015 ▶).
This study aims to evaluate the effect of LC on the CD34 gene and protein expression, apoptosis and SUZ12 gene expression on CD34+ HSCs as a therapeutic approach in CML.
Materials and Methods
Reagents
All culture plates and reagents related to cell culture, if not otherwise specified, were purchased from SPL Life Sciences Co., Ltd. (South Korea) and Gibco Co. (UK), respectively.
Bone marrow mononuclear cells isolation and CD34 + HSCs enrichment
Ethical consent was applied by an ethics committee at Tabriz University of Medical Sciences, Tabriz, Iran (Ethic Code No.: IR.TBZMED.VCR.REC.1396.849) according to guidelines from Helsinki-Ethical Principles for medical research and experiments on animals. 3 male Rattus norvegicus were purchased and euthanized using ketamine/xylazin as previously reported by Fathi et al. (2020c) ▶. In brief, bone marrow contents from the tibia and femur were collected by flushing with a syringe needle containing washing buffer (PBS supplemented with 5% FBS). Bone marrow contents were washed with washing buffer and were layered over the same volume of Ficoll-Paque (Innotrain, Germany). Thereafter, the middle phase, containing the mononuclear cells (MNCs) layer was transferred to a new 15-ml falcon tube and o-incubated with 100 μL of CD34+ micro beads (Miltenyi Biotech) for 30 min. Re-suspended cells were then passed through the magnetic-activated cell sorting (MACS) column (Miltenyi Biotec) and enriched CD34+ cells were retrieved by flushing the column (Fathi et al., 2020b ▶).
Purity assessment of CD34 + HSCs
Purity assessment of the enriched cells was performed by flow cytometry as previously explained by Fathi et al. (2020b). In brief, approximately 20 × 104 enriched CD34+ HSCs were incubated by 5 µL of FITC-conjugated antibody CD34 (Santacruz, Lifespan BioSciences, USA) (1 µg/106 cells) and were then subjected to the flow cytometry instrument. The output data were processed using FlowJo software version X.0.7.
Quantitative real-time PCR
In this study, the cells were divided into three groups: group I as the control group (CD34+ HSCs without any LC treatment) and group II and III as experimental groups (CD34+ HSCs with 0.2 and 0.4 mM LC treatment). The suitable concentration of LC was previously investigated by Farahzadi et al. (2016) ▶. They reported that LC increased cell proliferation at 0.2, and 0.4 concentrations at 48 h. Accordingly, 48 h was used in this study as well. In another study reported by Mobarak et al. (2017) ▶, it was shown that 0.2 mM LC increased cell proliferation. For this reason, two concentrations, 0.2 and 0.4 mM LC, were chosen for the present study (Farahzadi et al., 2016 ▶). For this purpose, LC was dissolved in PBS and prepared as 1 M concentration. In the following, LC was added to the wells at final concentrations of 0.2 and 0.4 mM for up to 48 h at 37°C in 5% CO2. At the end of the treatment period, CD34+ HSCs from the three control and experimental groups were collected. Total RNA was extracted and cDNA was synthesized. The mRNA expressions of target genes included CD34, SUZ12 and β-actin. Fluorescence data was calculated in relation to b-actin CT values by the 2-ΔΔCT method. Primers (Table 1) were designed using Oligo 7 v.7.52 software (Adibkia et al., 2021 ▶; Heidari et al., 2021 ▶).
Table 1.
Primer sequences used for the real-time PCR assays
| No. | Gene | Primer pair sequence | Product length (bp) |
|---|---|---|---|
| XM_039087645.1 | SUZ12 | GCTGTTCAGAGTAACTCGTCC CAAACACTGTCATTTGTGCAAC |
158 |
| Nm_001107202.2 | CD34 | CAGAACTTTCCAGCAAACTCC ACTCCCGAGGTAACCAATGC |
145 |
Flow cytometry analysis of CD34 cell surface marker assessment
After treatment with LC, the CD34+ HSCs from control and experimental groups were collected and subjected to flow cytometry to evaluate the CD34 cell surface marker. In brief, approximately 50 × 104 CD34+ HSCs were incubated with an appropriate amount of FITC-conjugated antibody CD34 (Santacruz, Lifespan BioSciences, USA) (1 µg/106 cells) in washing buffer for 30 min on ice. After washing the cells, FACS instrument was used to quantify the fluorescence intensity, and the data were analyzed with a FlowJo software (version 6.2) (Montazersaheb et al., 2020 ▶; Fathi et al., 2021 ▶).
Flow cytometry detection of apoptosis by Annexin V/PI assay
As mentioned above, the CD34+ HSCs from control and experimental groups were trypsinized, washed twice with PBS, re-suspended in the binding buffer (Ref No.: 00-0055-56, ebioscience) and kept for 20 min. Then, CD34+ HSCs were incubated with the binding buffer containing 5 μL of FITC-conjugated Annexin V (Ref. No.: 11-8005-74, ebioscience) for 15 min. Next, cells were washed with the binding buffer and exposed to PI solution in 100 μL binding buffer. Flow cytometry was performed by FACSCalibur (BD Bioscience), and the data were analyzed using FlowJo software ver. X.0.7 (Montazersaheb et al., 2019 ▶; Fathi et al., 2021 ▶).
Statistical analysis
The results were analyzed using the Graph Pad Prism version 6.01 software program. T-tests and one-way ANOVA were used to determine any significant differences among the groups and flow-cytometry was analyzed by FlowJo software. Statistical significance was determined to be P<0.05. All experimental procedures were repeated three times.
Results
Identification of CD34 + HSCs
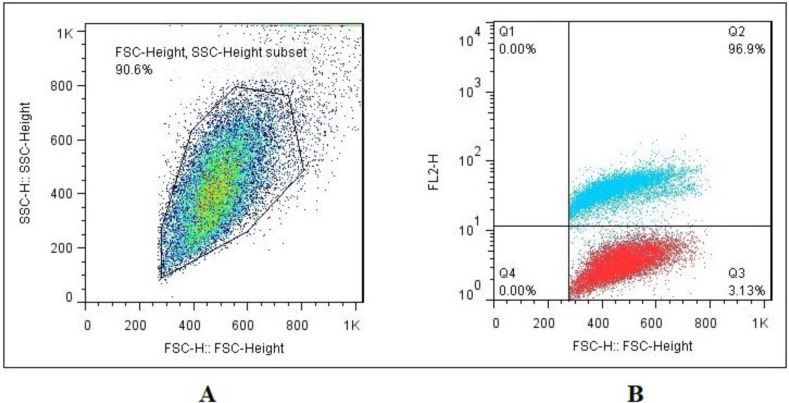
As shown in Figs. 1A and B, flow cytometry analysis indicated that enriched bone marrow-derived CD34+ cells by MACS had high levels of expression of CD34 (96.9%). In other words, the enriched cells had a high level of CD34 expression.
Fig. 1.
Characterization of enriched bone marrow-derived CD34+-expressing cells by flow cytometry. (A) A total population of cells for CD34 evaluation, and (B) Flow cytometry showed that 96.9% of cells were positive for CD34
LC caused to change the CD34 and SUZ12 gene expression
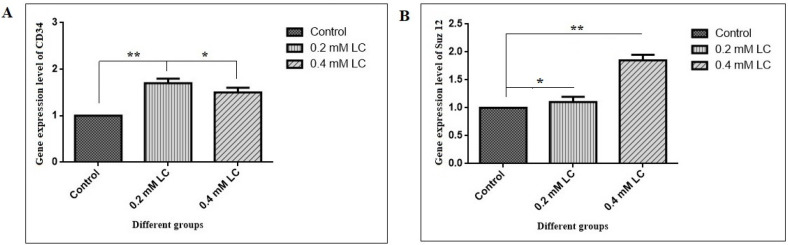
To evaluate the effect of LC on CD34 and SUZ12 gene expressions of CD34+ HSCs, the mRNA was examined by real-time PCR. As shown in Fig. 2A, the mRNA expression level of CD34 in the presence of 0.2 and 0.4 mM LC significantly increased by about 1.7 and 1.5 folds, respectively (P<0.01 and P<0.05). In addition, a significant increase in the mRNA expression level of SUZ12 was shown by about 1.10 and 1.75 folds in the presence of 0.2 and 0.4 mM LC, respectively (P<0.05 and P<0.01) (Fig. 2B).
Fig. 2.
Relative (A) CD34 and (B) SUZ12 mRNA expression levels of CD34+ hematopoietic stem cells in the presence of 0.2 and 0.4 mM LC for 48 h of incubation. CD34+ HSCs were cultured for 48 h in the presence of 0.2 and 0.4 mM LC. Total RNA was then extracted from cultured CD34+ cells in group I as control group (CD34+ HSCs without any LC treatment) and group II and III as experimental groups (CD34+ HSCs with 0.2 and 0.4 mM LC treatment) as described in the methods section and subjected to real-time PCR assay, mean±SEM, n=3, *P<0.05, and **P<0.01. The Y-axis shows the fold of the mRNA expression level
LC caused to change the CD34 protein expression of CD34 + HSCs
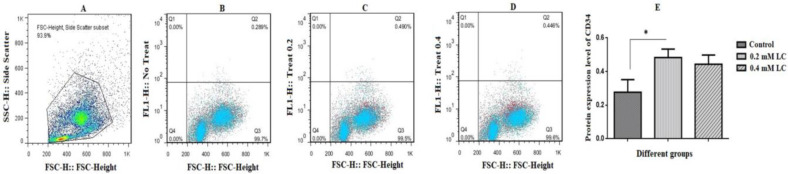
At the end of culture time in the absence and presence of LC, the CD34+ HSCs were analyzed for expressions of CD34 cell surface markers as shown in Figs. 3A-E. The results revealed that the expression of CD34 surface marker increased and decreased in the presence of 0.2 and 0.4 mM LC, respectively. This change in cell surface marker expression was only significant at 0.2 mM LC (P<0.05).
Fig. 3.
CD34 cell surface marker protein expression levels of CD34+ hematopoietic stem cells in the presence of 0.2 and 0.4 mM LC for 48 h of incubation. At the end of treatment period, CD34+ HSCs were collected in both control (CD34+ HSCs without any LC treatment) and experimental groups (CD34+ HSCs with 0.2 and 0.4 mM LC treatment). (A) Cell population, (B) CD34 marker assessment in the control group, (C) CD34 marker assessment in the presence of 0.2 mM LC, (D) CD34 marker assessment in the presence of 0.4 mM LC. The quantification was shown in part E. As shown in part C, in the presence of 0.2 mM LC, the expression of CD34 significantly increased by about 1.75 folds in the experimental group compared to the control group, mean±SEM, n=3, and *P<0.05
Investigation of apoptosis percentage by Annexin V/PI assay
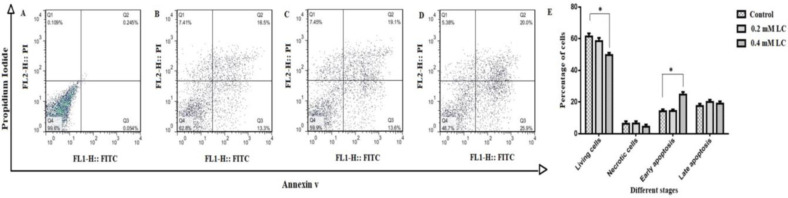
As we know, early apoptotic cells are Annexin+ and PI- and late apoptotic or necrotic cells are positive to both Annexin+ and PI+. In order to assess the effect of LC on apoptosis, CD34+ HSCs was treated with 0.2 and 0.4 mM for 48 h. Figs. 4A-E show the contour diagrams of Annexin V and PI stained CD34+ HSCs using flow cytometry after 48 h of treatment with LC. As illustrated in Figs. 4A-E, in the presence of 0.4 mM LC, about 25.9% of the CD34+ HSCs were in early apoptosis (Annexin+, PI-), which was 1.94 times higher than that of the control group (P<0.05).
Fig. 4.
Flow cytometry analysis of CD34+ hematopoietic stem cells treated with LC with a combination of Annexin V-FITC, propidium iodide (PI). A shift from bottom-right quadrant panel (early apoptosis) to top-right quadrant panel (late apoptosis) and top-left quadrant panel (necrosis) was observed. (A) Unstained cells, (B) Control group (CD34+ HSCs without any LC treatment), (C) Experimental group in the presence of 0.2 mM LC, (D) Experimental group in the presence of 0.4 mM LC, and (E) Statistical analysis
Discussion
Reactive oxygen species (ROS) is one of the most important free radicals, which is generated through various pathways and plays an important role in the progression of leukemia (Thomas et al., 2013 ▶). One of the important challenges in the treatment of leukemia is the predominance of cells resistant to common chemical compounds that increase the ineffectiveness of anticancer drugs (Wang et al., 2011 ▶; Wu et al., 2011 ▶). In leukemia patients, the response to treatment is positive at first but the cells increase their effect resistance so that the chemotherapy is not effective (Druker et al., 2006 ▶). Therefore, the use of new therapies such as cell-based therapy and anti-cancer drugs is considered necessary and important (Fathi et al., 2019 ▶). In addition, poor survival, aging and maintenance of transplanted cells are broadly accepted as serious barriers against the efficacy of regenerative therapy (Fathi et al., 2020a ▶). The use of external factors such as antioxidants to improve cell survival and retention properties is a favorite strategy for researchers, therefore finding effective and suitable agents such as vitamins and antioxidants to increase the viability of these cells could help promote their function in clinical applications (Farahzadi et al., 2016 ▶). Recently, many studies have been conducted to investigate the antioxidant effects of LC and its role in cell maintenance, differentiation and inhibition of aging, which proves the important function of this substance in the body (Mobarak et al., 2017 ▶). Several studies were conducted on the role of LC in promoting cell viability and maintenance (Fathi et al., 2020a ▶). Hematopoiesis is a process that often takes place in the bone marrow, during whose various stages progenitor cells and eventually blood cells are produced. CD34 is one of the important cell markers that undergoes changes in the expression of cell surface in hematopoiesis and cell differentiation (Attar, 2014 ▶). Civin (1984) ▶ first found in 1981 that 1 to 4% of bone marrow cells with the potential of self-renewal capability express a type of cell surface marker that was later called CD34. Primary hematopoietic cells that lose self-renewal properties begin to express CD34 markers on their surface. This occurs in the transformation of LT-HSC (long term-HSC) into the common myeloid and lymphoid progenitor cells, which also have a higher ability to differentiate. Therefore, primary hematopoietic cells can be called CD34+ and CD38- cells (Genovese et al., 2014 ▶). HSCs, as pluripotent and self-renewal cells, contain CD34- and CD133+ that begin to express CD34 instead of CD133 by differentiating towards mature cells. Expression of this marker continues from long-term (LT)-HSC to blast cell lines and is not re-expressed again (Attar, 2014 ▶). Therefore, the differentiation of cells towards the lower and more distinguished cell lines increases the expression of CD34 cell surface markers in HSCs. Although the structure of the CD34 cell surface marker is not fully understood, its role in preventing or stimulating cell adhesion, proliferation and cell differentiation has been demonstrated (Nielsen et al., 2009 ▶; Scherberich et al., 2013 ▶). Production and differentiation of HSCs are strongly influenced by a group of hematopoietic cytokines (Montazersaheb et al., 2021 ▶). Each cytokine can have a variety of effects on the cells including cell preservation, proliferation, differentiation and growth (Metcalf, 2008 ▶). For example, cytokine interleukin (IL)-6, which aligns with granulocyte-macrophage colony-stimulating factor (GM-CSF), increases differentiation in myeloid cells (Tie et al., 2019 ▶). Few studies have been performed on the effect of LC on cytokine production. In one study reported by Kouttab et al. (1993) ▶, it was shown that LC increases production of IL-6, IL-1α, IL-1β, and tumor necrosis factor (TNF)-α. In the section of the present study which investigated the effect of LC on the expression of CD34 cell surface markers, an increase in the expression of this marker was observed. Based on previous studies and considering the role of LC in increasing the secretion of cytokines and consequently increasing cell differentiation, a significant relationship can be found between the effect of LC on rBMNCs and increased differentiation of pluripotent stem cells towards mature cells followed by increased expression of CD34 cell surface marker expressions. Induction of differentiation and prevention of cell self-renewals is one of the most important principles in the treatment of leukemia (Gambelli et al., 2012 ▶; Yang et al., 2020 ▶). Induction of apoptosis is one of the strategies of preventing the development of leukemia. Various factors are able to cause apoptosis in cells, each of which uses a specific pathway. For example Fas (CD95/Apo-1) antigen is a type 1 cell membrane protein and a part of the TNF/nerve growth factor (NGF) receptor superfamily which, by binding to its own ligand (Fas ligand (FasL), is from the TNF family) or reacting with the anti-Fas/IgM monoclonal antibody, sends apoptotic signals into the cell, creating a cascade that eventually causes cell death (Fouqué et al., 2015 ▶; Levoin et al., 2020 ▶). Fas and TNFR-1, in addition to the extra-cytoplasmic part, are very similar in the cytoplasmic part in that both are necessary for the ligands to bind and transmit death signals (Fouqué et al., 2015 ▶).
In the present study, which partially examined the effect of LC on the apoptosis of rBMNCs, these cells were treated with concentrations of 0.2 and 0.4 mM LC. One limitation of the study was that different concentrations of LC were not used. Flow cytometry analysis showed a significant increase in apoptosis in the treated groups compared to the control. In a study by Jiang et al. (2016), it was found that the rate of apoptosis in hepatocyte cancer cells increased in the LC treated group compared to the healthy group. These changes in the occurrence of apoptosis have been reported due to increased levels of TNF-α, Fas, and caspase-8, which are involved in the external pathway of apoptosis, as well as decreased levels of the BCL-2 protein. Thus, LC increases apoptosis in hepatocyte cancer cells by decreasing BCL-2 as an anti-apoptotic protein and increasing the regulation of FasL (Fan et al., 2009 ▶). In another study on the effect of LC on apoptosis in myocardial and skeletal muscle cells, TNF-α decreases and apoptosis were observed (Vescovo et al., 2002 ▶). Therefore, it can be concluded that LC has different effects on the rate of apoptosis in different cells. This can be attributed to metabolic differences in cancerous and normal cells. Moreover, Vescovo et al. (2002) ▶ reported that free LC levels in cancer cells were lower compared to normal cells (Vescovo et al., 2002 ▶). In another study reported by Yazdanpanah et al. (1997) ▶, it was shown that the ratio of free carnitine to its esters in cancer patients differed from that of normal cells. The researchers attributed this to metabolic disorders caused by carnitine-related cancers.
According to one study, increases in the production of TNF-α are completely dependent on the dose of LC. Given that TNF-α is one of the important factors in causing apoptosis in cells, LC can be introduced as one of the inducers of apoptosis (Durazzo et al., 2020 ▶). In another study, Mutomba et al. (2000) ▶ investigated the effect of carnitine and palmitoyl carnitine on caspase activity and apoptosis rate, and obtained interesting results. In this study on Jurket cells (T-lymphocytes), apoptosis was induced by the Anti-Fas antibody. In the presence of LC, the rate of apoptosis in normal cells in a dose-dependent manner increased apoptosis, however, in apoptosis-induced cells, LC reduced apoptosis. In contrast, in the presence of Anti-Fas antibody, palmitoyl carnitine increased apoptosis. The study also found that LC and palmitoyl carnitine had an adverse effect on caspase activity (Vardiyan et al., 2020 ▶).
According to these studies as well as the results of the present study, it can be concluded that LC has a dual effect on the incidence of apoptosis. These effects vary depending on the cell type and whether the cell is cancerous or normal. In addition to activating apoptosis signaling pathways, LC increased apoptosis in all cancer cells, a finding that can be attributed to an increase in the energy available to cells through fatty acid oxidation (Fan et al., 2009 ▶).
In another part of the present study, the effect of LC on the expression of SUZ12 gene as a polycomb protein was investigated. At the concentration of 0.4 mM LC, gene expression was almost doubled compared to the non-treated control. Polycomb group proteins are a group of proteins that regulate epigenetic changes in chromatin cells. They are essential for embryonic cell development and stem cell regeneration, and play a key role in establishing and maintaining the pattern of gene expression during development (Kanno et al., 2008 ▶). Leukemia progression is a process in which advanced genetic abnormalities occur, including the deletion or mutation of repressive genes, amplification of oncogenes and chromosomal abnormalities (Baylin et al., 2006 ▶). Epigenetic changes that do not affect the original DNA sequence can also alter gene expression and cause cancer (Lu et al., 2020 ▶).
In conclusion, this research indicates that 0.2 and 0.4 mM LC could promote the gene expression of SUZ12 as well as the protein expression of CD34 cell surface marker CD34+ HSCs. LC could also, effect CD34+ HSCs through the induction of apoptosis. Since the 0.4 mM LC caused early apoptosis and decreased the percentage of living cells, 0.2 mM LC is recommended for further investigations.
Conflict of interest
The authors declare that they have no conflict of interest.
Acknowledgements
This research was completed for partial fulfillment of the requirements for the degree of Doctorate of Veterinary Medicine (DVM) (with the registration certificate to the tracking number: 2513310). The project was supported by the University of Tabriz, Tabriz, Iran. The authors wish to thank staff of the specialization clinic in Faculty of Veterinary Medicine, University of Tabriz, Tabriz, Iran.
References
- Adibkia K, Ehsani A, Jodaei A, Fathi E, Farahzadi R, Barzegar-Jalali M. Silver nanoparticles induce the cardiomyogenic differentiation of bone marrow derived mesenchymal stem cells via telomere length extension. Beilstein. J. Nanotechnol. 2021;12:786–797. doi: 10.3762/bjnano.12.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attar A. Changes in the cell surface markers during normal hematopoiesis: a guide to cell isolation. G. J. Hematol. Blood. Trans. 2014;1:20–28. [Google Scholar]
- Baylin SB, Ohm JE. Epigenetic gene silencing in cancer-a mechanism for early oncogenic pathway addiction? Nat. Rev. Cancer. 2006;6:107–116. doi: 10.1038/nrc1799. [DOI] [PubMed] [Google Scholar]
- Bracken AP, Pasini D, Capra M, Prosperini E, Colli E, Helin K. EZH2 is downstream of the pRB-E2F pathway, essential for proliferation and amplified in cancer. EMBO. J. 2003;22:5323–5335. doi: 10.1093/emboj/cdg542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Civin C. A hematopoietic progenitor cell surface antigen defined by a monoclonal antibody raised against KG-Ia cells. J. Immunol. 1984;133:157–165. [PubMed] [Google Scholar]
- de Fabritiis P, Dowding C, Bungey J, Chase A, Angus G, Szydlo R, Goldman JM. Phenotypic characterization of normal and CML CD34-positive cells: only the most primitive CML progenitors include Ph-neg cells. Leuk. Lymphoma. 1993;11:51–61. doi: 10.3109/10428199309054730. [DOI] [PubMed] [Google Scholar]
- Druker BJ, Guilhot F, O’Brien SG, Gathmann I, Kantarjian H, Gattermann N, Deininger MW, Silver RT, Goldman JM, Stone RM, Cervantes F, Hochhaus A. Five-year follow-up of patients receiving imatinib for chronic myeloid leukemia. N. Engl. J. Med. 2006;355:2408–2417. doi: 10.1056/NEJMoa062867. [DOI] [PubMed] [Google Scholar]
- Durazzo A, Lucarini M, Nazhand A, Souto SB, Silva AM, Severino P, Souto EB, Santini A. The nutraceutical value of carnitine and its use in dietary supplements. Molecules. 2020;25:2127–2148. doi: 10.3390/molecules25092127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan JP, Kim HS, Han GD. Induction of apoptosis by L-carnitine through regulation of two main pathways in Hepa1c1c 7 cells. Amino Acids. 2009;36:365–372. doi: 10.1007/s00726-008-0093-y. [DOI] [PubMed] [Google Scholar]
- Farahzadi R, Mesbah-Namin SA, Zarghami N, Fathi E. L-carnitine effectively induces hTERT gene expression of human adipose tissue-derived mesenchymal stem cells obtained from the aged subjects. Int. J. Stem. Cells. 2016;9:107–114. doi: 10.15283/ijsc.2016.9.1.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fathi E, Farahzadi R, Javanmardi S, Vietor I. L-carnitine extends the telomere length of the cardiac differentiated CD117+-expressing stem cells. Tissue Cell. 2020a;67:1–9. doi: 10.1016/j.tice.2020.101429. [DOI] [PubMed] [Google Scholar]
- Fathi E, Farahzadi R, Valipour B. Alginate/gelatin encapsulation promotes NK cells differentiation potential of bone marrow resident C-kit+ hematopoietic stem cells. Int. J. Biol. Macromol. 2021;177:317–327. doi: 10.1016/j.ijbiomac.2021.02.131. [DOI] [PubMed] [Google Scholar]
- Fathi E, Farahzadi R, Vietor I, Javanmardi S. Cardiac differentiation of bone-marrow-resident c-kit+ stem cells by L-carnitine increases through secretion of VEGF, IL6, IGF-1, and TGF-β as clinical agents in cardiac regeneration. J. Biosci. 2020b;45:1–11. [PubMed] [Google Scholar]
- Fathi E, Sanaat Z, Farahzadi R. Mesenchymal stem cells in acute myeloid leukemia: a focus on mechanisms involved and therapeutic concepts. Blood Res. 2019;54:165–174. doi: 10.5045/br.2019.54.3.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fathi E, Valipour B, Farahzadi R. Targeting the proliferation inhibition of chronic myeloid leukemia cells by bone marrow derived-mesenchymal stem cells via ERK pathway as a therapeutic strategy. Acta Med. Iran. 2020c;58:199–206. [Google Scholar]
- Fathi E, Vietor I. Mesenchymal stem cells promote caspase expression in Molt-4 leukemia cells via GSK-3α/and ERK1/2 signaling pathways as a therapeutic strategy. Curr. Gene. Ther. 2021;21:81–88. doi: 10.2174/1566523220666201005111126. [DOI] [PubMed] [Google Scholar]
- Fouqué A, Legembre P. The CD95/CD95L signaling pathway: a role in carcinogenesis. Cancer Immunol. 2015;1846:143–160. [Google Scholar]
- Gambelli F, Sasdelli F, Manini I, Gambarana C, Oliveri G, Miracco C, Sorrentino V. Identification of cancer stem cells from human glioblastomas: growth and differentiation capabilities and CD133/prominin-1 expression. Cell. Biol. Int. 2012;36:29–38. doi: 10.1042/CBI20110013. [DOI] [PubMed] [Google Scholar]
- Genovese P, Schiroli G, Escobar G, Di Tomaso T, Firrito C, Calabria A, Moi D, Mazzieri R, Bonini C, Holmes MC, Gregory PD, Burg M, Genter B, Montini E, Lombardo A, Naldini L. Targeted genome editing in human repopulating haematopoietic stem cells. Nature. 2014:235–240. doi: 10.1038/nature13420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghose P, Shaham S. Cell death in animal development. Development. 2020;147:1–12. doi: 10.1242/dev.191882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidari HR, Fathi E, Montazersaheb S, Mamandi A, Farahzadi R, Zalavi S, Nozad Charoudeh H. Mesenchymal stem cells cause telomere length reduction of Molt-4 cells via caspase-3, BAD and P53 apoptotic athway. Int. J. Mol. Cell. Med. 2021;10:113–122. doi: 10.22088/IJMCM.BUMS.10.2.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanno R, Janakiraman H, Kanno M. THIS ARTICLE HAS BEEN RETRACTED Epigenetic regulator polycomb group protein complexes control cell fate and cancer. Cancer Sci. 2008;99:1077–1084. doi: 10.1111/j.1349-7006.2008.00797.x. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Kesavardhana S, Malireddi RS, Kanneganti TD. Caspases in cell death, inflammation, and pyroptosis. Annu. Rev. Immunol. 2020;38:567–595. doi: 10.1146/annurev-immunol-073119-095439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kouttab NM, De Simone C. Modulation of cytokine production by carnitine. Mediators. Inflamm. 1993;2:25–28. doi: 10.1155/S0962935193000717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SC, Miller S, Hyland C, Kauppi M, Lebois M, Di Rago L, Metcalf D, Kinkel SA, Josefsson EC, Blewitt ME, Majewski IJ, Alexander WS. Polycomb repressive complex 2 component Suz12 is required for hematopoietic stem cell function and lymphopoiesis. Blood. 2015;126:167–175. doi: 10.1182/blood-2014-12-615898. [DOI] [PubMed] [Google Scholar]
- Levoin N, Jean M, Legembre P. CD95 structure, aggregation and cell signaling. Front. Cell. Dev. Biol. 2020;8:1–13. doi: 10.3389/fcell.2020.00314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin HD, Fong CY, Biswas A, Bongso A. Allogeneic human umbilical cord Wharton’s jelly stem cells increase several-fold the expansion of human cord blood CD34+ cells both in vitro and in vivo. Stem. Cell. Res. Ther. 2020;11:1–18. doi: 10.1186/s13287-020-02048-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y, Chan YT, Tan HY, Li S, Wang N, Feng Y. Epigenetic regulation in human cancer: the potential role of epi-drug in cancer therapy. Mol. Cancer. 2020;19:1–16. doi: 10.1186/s12943-020-01197-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malaguarnera M, Risino C, Gargante MP, Oreste G, Barone G, Tomasello AV, Costanzo M, Cannizzaro MA. Decrease of serum carnitine levels in patients with or without gastrointestinal cancer cachexia. World J. Gastroenterol. 2006;12:4541–4545. doi: 10.3748/wjg.v12.i28.4541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metcalf D. Hematopoietic cytokines. Blood. 2008;111:485–491. doi: 10.1182/blood-2007-03-079681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mobarak H, Fathi E, Farahzadi R, Zarghami N, Javanmardi S. L-carnitine significantly decreased aging of rat adipose tissue-derived mesenchymal stem cells. Vet. Res. Commun. 2017;41:41–47. doi: 10.1007/s11259-016-9670-9. [DOI] [PubMed] [Google Scholar]
- Montazersaheb S, Avci ÇB, Bagca BG, Ay NPO, Tarhriz V, Nielsen PE, Charoudeh HN, Hejazi MS. Targeting TdT gene expression in Molt-4 cells by PNA-octaarginine conjugates. Int. J. Biol. Macromol. 2020;164:4583–4590. doi: 10.1016/j.ijbiomac.2020.09.081. [DOI] [PubMed] [Google Scholar]
- Montazersaheb S, Fathi E, Farahzadi R. Cytokines and signaling pathways involved in differentiation potential of hematopoietic stem cells towards natural killer cells. Tissue Cell. 2021;70:1–8. doi: 10.1016/j.tice.2021.101501. [DOI] [PubMed] [Google Scholar]
- Montazersaheb S, Kazemi M, Nabat E, Nielsen PE, Hejazi MS. Downregulation of TdT expression through splicing modulation by antisense peptide nucleic acid (PNA) Curr. Pharm. Biotechnol. 2019;20:168–178. doi: 10.2174/1389201020666190206202650. [DOI] [PubMed] [Google Scholar]
- Mutomba MC, Yuan H, Konyavko M, Adachi S, Yokoyama CB, Esser V, McGarry JD, Babior BM, Gottlieb RA. Regulation of the activity of caspases by L-carnitine and palmitoylcarnitine. FEBS. Lett. 2000;28:19–25. doi: 10.1016/s0014-5793(00)01817-2. [DOI] [PubMed] [Google Scholar]
- Nielsen JS, McNagny KM. CD34 is a key regulator of hematopoietic stem cell trafficking to bone marrow and mast cell progenitor trafficking in the periphery. Microcircirculation. 2009;16:487–496. doi: 10.1080/10739680902941737. [DOI] [PubMed] [Google Scholar]
- Obeng E. Apoptosis (programmed cell death) and its signals-A review. Braz. J. Biol. 2021;81:1133–1143. doi: 10.1590/1519-6984.228437. [DOI] [PubMed] [Google Scholar]
- Pasini D, Bracken AP, Hansen JB, Capillo M, Helin K. The polycomb group protein Suz12 is required for embryonic stem cell differentiation. Mol. Cell. Biol. 2007;27:3769–3779. doi: 10.1128/MCB.01432-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penfornis P, Fernandes JD, Pochampally RR. Polycomb group protein Suz12 is regulated by a novel miRNA-like small RNA. Sci. Rep. 2018;8:1–14. doi: 10.1038/s41598-018-19989-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzatti L, Binato R, Cofre J, Gomes BE, Dobbin J, Haussmann ME, D’Azambuja D, Bouzas LF, Abdelhay E. SUZ12 is a candidate target of the non-canonical WNT pathway in the progression of chronic myeloid leukemia. Genes Chromosomes Cancer. 2010;49:107–118. doi: 10.1002/gcc.20722. [DOI] [PubMed] [Google Scholar]
- Scherberich A, Di Di Maggio N, McNagny KM. A familiar stranger: CD34 expression and putative functions in SVF cells of adipose tissue. World J. Stem. Cells. 2013;5:1–8. doi: 10.4252/wjsc.v5.i1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas N, Zachariah SM. Pharmacological activities of chromene derivatives: an overview. Asian J. Pharm. Clin. Res. 2013;6:11–15. [Google Scholar]
- Tie R, Li H, Cai S, Liang Z, Shan W, Wang B, Tan Y, Zheng W, Huang H. Interleukin-6 signaling regulates hematopoietic stem cell emergence. Exp. Mol. Med. 2019;51:1–12. doi: 10.1038/s12276-019-0320-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vardiyan R, Ezati D, Anvari M, Ghasemi N, Talebi A. Effect of L-carnitine on the expression of the apoptotic genes Bcl-2 and Bax. Clin. Exp. Reprod. Med. 2020;47:155–160. doi: 10.5653/cerm.2019.03440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vescovo G, Ravara B, Gobbo V, Sandri M, Angelini A, Della Barbera M, Dona M, Peluso G, Calvani M, Mosconi L, Libera LD. L-Carnitine: a potential treatment for blocking apoptosis and preventing skeletal muscle myopathy in heart failure. Am. J. Physiol. Cell. Physiol. 2002;283:C802–C810. doi: 10.1152/ajpcell.00046.2002. [DOI] [PubMed] [Google Scholar]
- Walz C, Sattler M. Novel targeted therapies to overcome imatinib mesylate resistance in chronic myeloidleukemia (CML) Crit. Rev. Oncol. Hematol. 2006;57:145–164. doi: 10.1016/j.critrevonc.2005.06.007. [DOI] [PubMed] [Google Scholar]
- Wang L, Liu Y, Li W, Jiang X, Ji Y, Wu X, Xu L, Qiu Y, Zhao K, Wei T, Li Y, Zhao Y, Chen C. Selective targeting of gold nanorods at the mitochondria of cancer cells: implications for cancer therapy. Nano. Lett. 2011;11:772–780. doi: 10.1021/nl103992v. [DOI] [PubMed] [Google Scholar]
- Wu YN, Yang LX, Shi XY, Li IC, Biazik JM, Ratinac KR, Chen DH, Thordarson P, Shieh DB, Braet F. The selective growth inhibition of oral cancer by iron core-gold shell nanoparticles through mitochondria-mediated autophagy. Biomaterials. 2011;32:4565–4573. doi: 10.1016/j.biomaterials.2011.03.006. [DOI] [PubMed] [Google Scholar]
- Yang L, Shi P, Zhao G, Xu J, Peng W, Zhang J, Zhang G, Wang X, Dong Z, Chen F, Cui H. Targeting cancer stem cell pathways for cancer therapy. Signal Transduct. Target. Ther. 2020;5:1–35. doi: 10.1038/s41392-020-0110-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yazdanpanah M, Luo X, Lau R, Greenberg M, Fisher LJ, Lehotay DC. Cytotoxic aldehydes as possible markers for childhood cancer. Free. Radic. Biol. Med. 1997;23:870–878. doi: 10.1016/s0891-5849(97)00070-1. [DOI] [PubMed] [Google Scholar]
- Zoghbi HY, Beaudet AL. Epigenetics and human disease. Cold. Spring Harb. Perspect. Biol. 2016;8:1–28. doi: 10.1101/cshperspect.a019497. [DOI] [PMC free article] [PubMed] [Google Scholar]