Abstract
Background:
Bovine anaplasmosis is an infectious disease with worldwide distribution. It spreads by various routes mainly through tick bites.
Aims:
This study aimed to investigate bovine related Anaplasma spp. in cattle from three northern governorates of Egypt by serological and molecular assays, to evaluate the associated risk factors and to analyze the phylogeny of revealed A. marginale isolates.
Methods:
During 2020, a total of 650 blood samples were collected from asymptomatic cattle in the governorates of Kafr El-Sheikh (n=240), Menofia (n=230), and Al-Gharbia (n=180). Sera samples were examined using the Anaplasma antibody test kit, cELISA v2. Blood genomic DNA of seropositive cattle was then examined by PCRs specific to A. marginale, A. centrale, and A. bovis. Selected positive samples were subjected to nucleotide sequencing. Risk factors (i.e. geographical area, breed, type of production, sex, age, herd size, season, husbandry system, tick infestation, and application of acaricides) were evaluated by logistic regression approach.
Results:
In total, 130 cattle (20%, 95% CI: 17.1–23.3) were recorded seropositive for Anaplasma species. Major risk factors associated with seropositivity were being crossbred, dairy cattle, aged more than 5 years, summer season, herd size of below 300, pasture grazing, tick infestation, and not being subjected to regular treatment with acaricides. By using species-specific PCR, only A. marginale was detected. Nucleotide sequencing showed the occurrence of two different msp4 genotypes.
Conclusion:
This study shows the high prevalence of A. marginale in cattle of Kafr El-Sheikh, Al-Gharbia, and Menofia. However, the connection between Anaplasma species and their tick vectors remains unknown in Egypt and merits further investigations. Since these infections primarily spread through ixodid tick bites, effective ectoparasite control strategies, regular examination of cattle and successful chemoprophylaxis are recommended.
Key Words: Anaplasma, Cattle, Molecular detection, Phylogeny, Seroprevalence
Introduction
Tick-borne diseases (TBDs) are of economic significance to the livestock industry (Jongejan and Uilenberg, 2004 ▶). Globally, 80% of the world’s cattle population are at risk of TBDs with economic losses estimated at US$30 billion per year (Lew-Tabor and Valle, 2016 ▶). One of the most economically important hemoparasitic TBDs of bovine in the world is undoubtedly anaplasmosis (Uilenberg, 1995 ▶) transmitted by several species of hard ticks (Ixodidae) belonging to the genera Ixodes, Haemaphysalis, Amblyomma, Rhipicephalus, Hyalomma, and Dermacentor (Dantas-Torres and Otranto, 2017 ▶).
Anaplasmosis is a tick-borne disease caused by bacteria of the genus Anaplasma, which infects a wide range of wild and domestic animals (Ben Said et al., 2018b ▶, 2019). Although some of the nine recognized and possible Anaplasma species i.e. A. phagocytophilum, A. marginale, A. bovis, A. centrale, A. ovis, A. mesaeterum, A. platys, A. caudatum, A. odocoilei, A. capra, and “Candidatus Anaplasma camelii” exhibit a certain degree of host specificity, some of them (e.g., A. phagocytophilum, and A. platys) may infect more than one animal species, including humans (Dantas-Torres and Otranto, 2017 ▶; Sharifiyazdi et al., 2017 ▶; Sazmand et al., 2019 ▶; Selmi et al., 2019 ▶; Alanazi et al., 2020 ▶; Selmi et al., 2020 ▶). Bovine anaplasmosis is mainly caused by erythrocytes-infecting A. marginale, monocytes-infecting A. bovis (Belkahia et al., 2015 ▶; Ben Said et al., 2018a ▶), and strains genetically related to granulocytes-infecting A. phagocytophilum (A. phagocytophilum-like 1 and 2) (Ben Said et al., 2015 ▶; Ben Said et al., 2017b ▶), and related to platelets-infecting A. platys (A. platys-like) (Zobba et al., 2014 ▶; Battilani et al., 2017 ▶; Ben Said et al., 2017a ▶; Selmi et al., 2019 ▶). However, A. marginale is responsible for almost all outbreaks of clinical bovine anaplasmosis (OIE, 2018 ▶). In cattle, the infection is spread mainly by ixodid ticks, but also by other arthropod vectors such as biting flies, and blood-contaminated objects e.g. needles, ear tags, dehorning and castration equipment. Transplacental transmission may also contribute to the epidemiology of the disease in some regions (Aubry and Geale, 2011 ▶). The disease is widespread in tropical and subtropical regions and is characterized by fever, anemia, weakness, enlarged lymph nodes, abortion, decreased milk production, jaundice, and sometimes death (Kocan et al., 2010 ▶). Cattle that recover from acute infection remain persistently infected carriers for whole life and may act as a source of infection in naïve cattle populations, causing endemic disease stability (Kocan et al., 2015 ▶).
In Egypt, previous studies reported A. marginale infection in cattle, buffaloes, and camels (El-Naga and Barghash, 2016 ▶; Elhariri et al., 2017 ▶; AL-Hosary et al., 2020 ▶; El-Dakhly et al., 2020 ▶; Nasreldin et al., 2020 ▶; Parvizi et al., 2020 ▶). In addition, DNA of A. marginale has been detected in Hyalomma anatolicum, and Rhipicephalus annulatus collected from cattle (Loftis et al., 2006 ▶). However, information on bovine anaplasmosis is still incomplete in some parts of the country. Therefore, the objectives of the present study were as follow:
I) Investigation of the seroprevalence of Anaplasma spp. in cattle from three northern governorates,
II) Evaluation of the associated risk factors,
III) Molecularly detection of A. marginale, A. centrale, and A. bovis by PCR,
IV) Genetically characterizing the positive samples.
Materials and Methods
Study area
Egypt is a transcontinental country that stretches across northeastern Africa and southwestern Asia. It is divided into 27 governorates. The large regions of the Sahara desert, which make up most of Egypt’s territory, are sparsely inhabited. This research was carried out in three northern governorates namely Kafr El-Sheikh (31.1107°N, 30.9388°E), Al-Gharbia (30.8754°N, 31.0335°E), and Menofia (30.5972°N, 30.9876°E) (Fig. 1). These governorates are geographically located between two branches of the Nile River in northern Egypt, and are characterized by hot desert climate with common temperature range of 15 to 35°C. However, high temperatures varying between 35 and 45°C is usually observed between July and August. These governorates have variable average rainfall in the range of 100–200 mm that mainly occurs during the winter months. The study areas are agriculture area and have many exchange pools suitable for the multiplication of arthropod vectors.
Fig. 1.
Map of Egypt indicating governorates where the studied animals are located
Samples collection
The sample size (n=650) was calculated based on the described formula (Thrusfield and Christley, 2018 ▶) with an expected prevalence of 18.5% (Parvizi et al., 2020 ▶), a confidence interval of 95%, and an accuracy of 5%. During four seasons in 2020, jugular vein blood samples were collected from individual cattle using clean sterilized vacuum tubes with and without EDTA for molecular and serological assays. No individual was sampled more than once. The sera were separated by centrifugation at 3500 ×g for 10 min. A clinical examination including measurement of rectal temperature, pulse and respiratory rates was performed on all animals before sampling. The examined cattle were grouped according to breed (Holstein, crossbreed, native), type of production (dairy, beef), gender (male, female), age (<2, 2–5, >5 years old), and herd size (<100, 100–300, >300). Also, for each cattle, the season, the husbandry system (stall feeding, pasture grazing, pasture grazing plus stall feeding), the tick infestation, and the application of acaricides were recorded.
This study was approved by the Ethical Research Committee, Faculty of Veterinary Medicine, Benha University, Egypt.
Serological analysis
All blood sera samples were examined using Anaplasma antibody test kit, cELISA v2 (VMRD, Pullman, Washington, USA) according to the manufacturer’s instructions. This competitive ELISA (cELISA) based on the recombinant major surface protein 5 (rMSP5) is licensed for the detection of antibodies directed against the MSP5 protein of A. marginale, A. centrale, and A. ovis (Dreher et al., 2005 ▶). It has a diagnostic sensitivity of 100% and a specificity of 99.7% (Chung et al., 2014 ▶). The results were expressed as percent inhibition (%I) and calculated as follow:
1 - (OD620 of sample /OD620 of negative control) × 100
The sample was considered positive if %I ≥30.
Genomic DNA extraction and PCR assay
Genomic DNA were extracted from 200 μL aliquots of EDTA-treated blood samples of serologically positive cattle using QIAamp® DNA Mini Kit (Qiagen, Hilden, Germany) according to the manufacturer’s protocol. DNA samples were then examined by conventional PCRs using primers AmargMSP4Fw: 5´-CTG AAG GGG GAG TAA TGG G-3´ and AmargMSP4Rev: 5´-GGT AAT AGC TGC CAG AGA TTC C-3´ for A. marginale (Torina et al., 2012), AC1f: 5´-CTG CTT TTA ATA CTG CAG GAC TA-3´ and AC1r: 5´-ATG CAG CAC CTG TGT GAG GT-3´ for A. centrale (Kawahara et al., 2006 ▶), and AB1f: 5´-CTC GTA GCT TGC TAT GAG AAC-3´ and AB1r: 5´-TCT CCC GGA CTC CAG TCT G-3´ for A. bovis (Kawahara et al., 2006 ▶). All the PCR reactions were performed using the Thermo Scientific™ DreamTaq™ Green PCR Master Mix (2X) (Thermo Scientific, Waltham, US) in a T100™ thermal cycler (BioRad, California, USA). For all reactions, DNA from blood samples positive for the pathogen served as a positive control. The amplified PCR products were separated on a 1.5% agarose gel (UltraPure™ Agarose, Thermo Scientific, Waltham, USA) stained with ethidium bromide and visualized by a UV transilluminator (Gel DocTM XR+, BioRad, California, USA).
DNA sequencing
None of the examined cattle were infected with A. centrale or A. bovis. Two positive amplicons of A. marginale sized ca. 344 bp from 3 years old female cattle from Al-Gharbia governorate and 3.5 years old female cattle from Kafr El-Sheikh governorate were randomly selected, extracted from the gel, and purified with QIAquick Gel Extraction Kit (Qiagen, Hilden, Germany). The amplicons were then sequenced in both directions using BigDyeTM Terminators v3.1 Cycle Sequencing Kit (Applied Biosystems, California, USA) in an ABI-PRISM 3500 automated sequencer (Thermo Scientific, Waltham, US). Sequence reads were analyzed with BioEdit® Sequence Alignment Editor (Hall, 1999 ▶), assembled into consensus sequences and compared to those available in the GenBank® database using the basic local alignment search tool (http://blast.ncbi.nlm.nih.gov/ Blast.cgi).
Phylogenetic analysis
Partial sequences of the msp4 gene isolated from Anaplasma marginale with the size of 301 bp were aligned with the corresponding sequences available from the GenBank® database, using Clustal W (http://www. clustalw.genome.jp). Similarity searches were performed using BLAST (http://blast.ncbi.nlm.nih.gov) (Altschul et al., 1997 ▶). DNAMAN program (ver. 5.2.2; Lynnon Biosoft, Que., Canada) was also used to calculate genetic distances computed by the maximum composite likelihood method (Tamura and Nei, 1993 ▶). Neighbor-Joining trees were built using the same software (Saitou and Nei, 1987 ▶). Statistical support for internal branches was established by bootstrap analysis with 1000 replications (Felsenstein, 1985 ▶).
Statistical analysis
The results were transferred to SPSS software (ver. 24.0, IBM, USA) for further analysis. The Chi-square test was used to compare seropositivity to Anaplasma species and the results were considered significant if P≤0.05. Univariable logistic regression analysis was used to evaluate the association between anaplasmosis seroprevalence and variables of location (Kafr El-Sheikh, Al-Gharbia, Menofia), breed (Holstein, crossbreed, native), type of production (dairy, beef), gender (male, female), age (<2, 2–5, >5 years old), herd size (<100, 100–300, >300), season, husbandry system (stall feeding, grazing, grazing plus stall feeding), tick infestation, and application of acaricides. Variables with a P≤0.05 in the univariable analyses were evaluated with multivariable models to determine the risk factors, odds ratio (OR), and confidence interval (CI) of each significant variable in univariable analyses.
Results
Of 650 cattle tested by cELISA, 130 (20%, 95% CI: 17.1–23.3) were found to be seropositive for targetted Anaplasma spp.. None of the analyzed animals showed typical clinical signs of anaplasmosis e.g. anemia, fever, pale mucous membranes, weakness. Univariate statistical modeling revealed that the risk of seropositivity was significantly associated with breed and age of cattle, production type, husbandry system, herd size, the season of sampling, tick infestation, and not the application of acaricides. However, the geographic region and the gender of cattle did not have a significant role in seropositivity to Anaplasma spp. in this study (Table 1). Multivariate logistic regression analysis of eight risk factors with P≤0.05 in univariable analysis revealed a higher probability of seropositivity in crossbreed cattle, dairy cattle, and animals over the age of 5 years. Furthermore, seropositivity was highest during the summer, in grazing cattle, in animals infested with ticks, and in those not receiving acaricides regularly (Table 2).
Table 1.
Risk factors associated with seroprevalence rates of anaplasmosis in 650 cattle in Egypt according to different variables
| Variables | No. | No of positive animals (%) | 95% CIa | Statistics |
|---|---|---|---|---|
| Governorate | ||||
| Kafr El-Sheikh | 240 | 53 (22.1) | 17.3–27.7 | χ2=1.234 dfb=2 P=0.5 |
| Menofia | 230 | 45 (19.6) | 15–25.2 | |
| Al-Gharbia | 180 | 32 (17.7) | 12.8–24 | |
| Breed | ||||
| Holstein | 200 | 49 (24.5) | 19.1–30.9 | χ2=11.692 df=2 P=0.003 |
| Crossbreed | 350 | 73 (20.9) | 16.9–25.4 | |
| Native | 100 | 8 (8.0) | 4.1–15 | |
| Age | ||||
| <2 years | 150 | 12 (8.0) | 4.6–13.5 | χ2=28.569 df=2 P=0.0001 |
| 2–5 years | 380 | 77 (20.3) | 16.5–24.5 | |
| >5 years | 120 | 41 (34.2) | 26.3–43 | |
| Sex | ||||
| Male | 61 | 8 (13.1) | 6.8–23.8 | χ2=1.955 df=1 P=0.16 |
| Female | 589 | 122 (20.7) | 17.6–24.2 | |
| Season | ||||
| Winter | 120 | 11 (9.2) | 5.2–15.7 | χ2=56.783 df=3 P=0.0001 |
| Spring | 180 | 19 (10.6) | 6.9–15.9 | |
| Summer | 280 | 94 (33.6) | 28.3–39.3 | |
| Autumn | 70 | 6 (8.6) | 3.9–17.5 | |
| Production type | ||||
| Dairy | 500 | 115 (23.0) | 19.5–28.9 | χ2=12.188 df=1 P=0.0001 |
| Beef | 150 | 15 (10) | 6.2–15.8 | |
| Husbandry system | ||||
| Stall feeding | 150 | 15 (10) | 6.2–15.8 | χ2=13.820 df=2 P=0.001 |
| Pasture grazing | 155 | 41 (26.4) | 20.1–33.9 | |
| Pasture grazing plus stall feeding | 345 | 74 (21.4) | 17.5–26.1 | |
| Herd size | ||||
| <100 | 340 | 81 (23.8) | 19.6–28.6 | χ2=6.883 df=2 P=0.03 |
| 100–300 | 170 | 29 (17.1) | 12.2–23.4 | |
| >300 | 140 | 20 (14.3) | 9.5–21 | |
| Tick infestation | ||||
| Yes | 380 | 95 (25.0) | 20.9–29.6 | χ2=14.294 df=1 P=0.0001 |
| No | 270 | 35 (12.9) | 9.5–17.5 | |
| Application of acaricides | ||||
| Every three months | 201 | 21 (10.5) | 6.9–15.5 | χ2=19.531 df=2 P=0.0001 |
| Irregular | 405 | 94 (23.2) | 19.4–27.6 | |
| Not applied | 44 | 15 (34.1) | 21.8–48.8 | |
| Total | 650 | 130 (20) | 17.1–23.3 |
a CI: Confidence interval, and b df: Degree of freedom. Significant variables (P≤0.05)
Table 2.
Multivariant logistic regression analysis of risk factors associated with seroprevalence rate of anaplasmosis in 650 cattle in Egypt according to different variables
| Variable | ßa | SEb | OR3 | 95% CI4 | P-value |
|---|---|---|---|---|---|
| Breed | |||||
| Holstein | 1.317 | 0.404 | 3.7 | 1.9–8.2 | 0.001 |
| Crossbreed | 2.419 | 0.384 | 11.2 | 5.3–23.8 | 0.0001 |
| Native (constant) | – | – | – | – | – |
| Type of production | |||||
| Dairy | 0.989 | 0.292 | 2.7 | 1.5–4.6 | 0.001 |
| Beef (constant) | – | – | – | – | – |
| Age | |||||
| 2–5 | 1.072 | 0.327 | 2.9 | 1.5–5.5 | 0.001 |
| >5 | 1.786 | 0.357 | 5.9 | 2.9–12 | 0.0001 |
| <2 years (constant) | – | – | – | – | – |
| Herd size | |||||
| <100 | 0.334 | 0.240 | 1.4 | 0.8–2.2 | 0.0001 |
| 100–300 | 0.334 | 0.240 | 0.7 | 0.4–1.1 | 0.0001 |
| >300 (constant) | – | – | – | – | – |
| Season | |||||
| Winter | 0.074 | 0.531 | 1.1 | 0.4–3.1 | 0.001 |
| Spring | 0.230 | 0.491 | 1.3 | 0.5–3.3 | 0.0001 |
| Summer | 3.181 | 0.446 | 24.1 | 10–57.6 | 0.0001 |
| Autumn | – | – | – | – | – |
| Grazing system | |||||
| Pasture grazing | 1.175 | 0.327 | 3.2 | 1.7–6.2 | 0.0001 |
| Pasture grazing plus stall feeding | 0.899 | 0.302 | 2.5 | 1.4–4.4 | 0.003 |
| Stall feeding (constant) | – | – | – | – | – |
| Presence of ticks | |||||
| Yes | 0.806 | 0.216 | 2.2 | 1.5–3.4 | 0.0001 |
| No (constant) | – | – | – | – | – |
| Application of acaricides | |||||
| Irregular | 0.952 | 0.259 | 2.6 | 1.6–4.3 | 0.0001 |
| Not applied | 1.489 | 0.393 | 4.4 | 2.1–9.6 | 0.0001 |
| Every three months (constant) | – | – | – | – | – |
a ß: Wald statistic, b SE: Standard error, c CI: Confidence interval, and d OR: Odds ratio. Significant variables (P≤0.05)
PCR examination of blood samples from 130 seropositive cattle using specific primers revealed that all tested animals (100%) were infected with A. marginale. None of the examined cattle were infected with A. centrale or A. bovis.
Anaplasma marginale infections were validated by sequencing of 301 bp msp4 gene from two randomly selected positive cattle samples. Sequences alignment revealed two distinct genotypes differed by one nucleotide (data not shown). The average nucleotide identity was 99.6% among genotypes. Genotypes were 99.0 to 100% homologous in comparisons with previous A. marginale genotypes existing in the GenBank. The new nucleotide sequences generated from AS01 and AS02 A. marginale isolates were deposited in the GenBank® (http://www.ncbi.nlm.nih.gov/) under the accession numbers MZ695054 and MZ695055, respectively. When compared to the A. centrale reference sequence (GenBank accession number AF428090), nucleotide identities were estimated to be 83.4 and 83.1%, respectively.
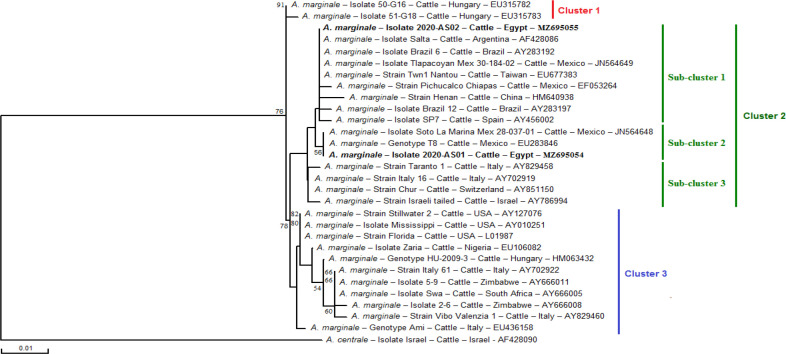
Phylogenetic analysis, based on the alignment of the two genotypes of this study with partial msp4 sequences from the GenBank, originated three main clusters with the robustness of nodes’ rates estimated at 78 and 76% (Fig. 2). The first cluster included two isolates from Hungary. The second cluster contained strains from Latin America (e.g. Mexico, Brazil, and Argentina), Asia (e.g. Taiwan and China), and Southern Europe (e.g. Spain and Italy). The third cluster included isolates mainly from Africa (Nigeria, Zimbabwe, and South Africa), North America (represented exclusively by the USA), and Southern Europe (Italy, Spain). The last included two isolates from Mexico (Fig. 2). Egyptian strains were assigned to the second cluster. In particular, isolate AS01 clustered in a second sub-cluster with two strains from Mexico, and isolate AS02 clustered in the first sub-cluster with 5 strains from Latin America, one strain from Spain, and two others from Asia (Fig. 2).
Fig. 2.
Phylogenetic relationships of Anaplasma marginale isolates of the present study and other A. marginale isolates and strains available in the GenBank based on msp4 partial sequence of 301 bp. The analyses were performed using the Neighbor-Joining method based on the maximum composite likelihood method (Tamura and Nei, 1993) in the DNAMAN program (ver. 5.2.2, Lynnon Biosoft, Que., Canada). Sequences are presented by isolate, genotype or strain name, host species, country of origin, and GenBank® accession number. One A. centrale msp4 partial sequence was added as an out-group. Numbers associated with the nodes represent the percentage of 1000 bootstrap iterations supporting the nodes (only percentages greater than 50% were represented). The sequences of A. marginale newly obtained in the present study are in bold
Discussion
Presented data indicate that cattle populations (i.e. 20%) in three northern governorates of Kafr El-Sheikh, Menofia, and Al-Gharbia are exposed to A. marginale, and A. centrale. As summarized in Table 3, previous studies have reported antibodies against A. marginale in 18.5% to 54.8% of cattle in different regions of Egypt (Fereig et al., 2017 ▶; AL-Hosary et al., 2020 ▶; Parvizi et al., 2020 ▶). Interestingly, the seroprevalence of anaplasmosis was 22.1% and 19.6% in Kafr El-Sheikh, and Menofia, however, in a previous study in 2015–2016, no seropositive cattle were recorded in these governorates (Parvizi et al., 2020 ▶). Since none of the animals showed clinical signs of anaplasmosis, the examined cattle should have been infected subclinically or persistently (Aubry and Geale, 2011 ▶), so unrestricted transport of these animals could contribute to both the spread of anaplasmosis and the increase in strain diversity (Kocan et al., 2015 ▶).
Table 3.
Reports of Anaplasma marginale in cattle (Bos taurus) and their ticks in Egypt
| Host/vector | Governorate/city | No. of examined population | % Prevalence | Method | Year of study | Reference |
|---|---|---|---|---|---|---|
| Cattle | Dakahlia and Damietta | 3310 | 3.5 | Blood smear | 2005–2006 | |
| 3.7 | IFAT | |||||
| Cattle | Dakahlia | 650 | 6.3 | Blood smear | 2005-2007 | |
| Damietta | 4640 | 9.05 | ||||
| Cattle | Qalyoubia | 100 | 60 | Blood smear | 2011 | |
| Cattle | Dakahlia | 164 | 20.1 | cPCR | 2012–2013 | |
| Cattle | Qena | 90 | 28 | ELISA | 2014–2015 | |
| Cattle | 24 governorates | 758 | 18.5 | ELISA | 2015–2016 | |
| Cattle | Beni-Suef | 50 | 4 | cPCRa | 2015–2018 | El-Dakhly et al. (2020) ▶b |
| El-Fayoum | 50 | 16 | ||||
| El-Wadi El-Gadid | 50 | 12 | ||||
| Cattle | Menofia | 92 | 15.2 | cPCR | 2017 | |
| Cattle | New Valley | 31 | 61.3 | Blood smear | 2017–2018 | |
| Cattle | EL-Minia, Assiut, EL-Fayoum, New Valley | 309 | 16.2 | Blood smear | 2018 | |
| 54.8c | ELISA | |||||
| 68.3 | qPCRd | |||||
| 50.2 | RLBe | |||||
| Cattle | Kharga, EL-Fayoum, Assuit | 41 | 92.7 | RLB and cPCR | 2018 | |
| Cattle | Kafr El-Sheikh | 240 | 22.1 | ELISA and cPCR | 2020 | This study |
| Al-Gharbia | 180 | 17.7 | ||||
| Menofia | 230 | 19.6 | ||||
| Hyalomma excavatum | Siwa | NSe | NS | cPCR | 2002–2003 | |
| Rhipicephalus annulatus | Wadi el Natroun | NS | NS | cPCR | 2002–2003 | |
| Hyalomma excavatum | Assuit, El-Fayoum | NS | NS | RLB and cPCR | 2018 | |
| Rhipicephalus annulatus | Assuit, El-Fayoum | NS | NS | RLB and cPCR | 2018 |
a cPCR: Conventional PCR, b Overall prevalence was 10.6%, c 188 cattle were included in the serology, d qPCR: Real-time PCR, e RLB: Reverse line blot, and e NS: Not stated
Compared to other North African countries, the seroprevalence in our study remains higher than that observed in Algeria at 7.4% (Ziam and Benaouf, 2004 ▶). Moreover, our finding was similar to that observed in Morocco with a prevalence rate of 16.5% (Ait Hamou et al., 2012 ▶). In Sudan, anaplasmosis prevalence occurs at much higher levels which are estimated at 37.8 and 38.9% (Salih et al., 2008 ▶; Salih et al., 2009 ▶). However, prevalence rates reported for countries must be taken with caution, since one standardized assay such as sampling procedure was not used in each study, and infection rates may vary even between neighboring farms (Ait Hamou et al., 2012 ▶).
In this study, seropositivity to Anaplasma spp. was more frequent in older cattle and dairy animals that are usually kept longer for production compared to beef cattle that are slaughtered at young ages. Similarly, dairy cattle under 1 year of age have been shown to have the lowest risk of anaplasmosis compared to other age groups i.e. cattle from 1–3, 3–5, and >5 years old (Noaman and Moradi, 2019 ▶). These observations may be explained by the fact that older animals are more exposed to arthropod infestations as they live longer and went through more vector seasons (Ben Said et al., 2018b ▶).
Furthermore, cattle of native breeds had a significantly lower risk of anaplasmosis. In contrast, in Tunisia, Holstein cattle were less infected with A. marginale than other breeds (Schwyz and crossbreeds) (Belkahia et al., 2015 ▶; M’ghirbi et al., 2016 ▶). The lower prevalence of anaplasmosis in native breeds compared to pure breeds, that are mainly imported from Europe, might be due to greater resistance to anaplasmosis or the circulation of host-adapted strains in the north Africa (M’ghirbi et al., 2016 ▶; Ben Said et al., 2018b ▶).
Larger herds had lower rates of anaplasmosis possibly due to stricter hygienic measures and more effective management and control strategies. Consistent with our findings, the incidence of anaplasmosis within herds was negatively related to herd size in the United States (Alderink and Dietrich, 1983 ▶). Previous studies have shown that owners of larger flocks and herds have a significantly better understanding of parasites and diseases (Sazmand et al., 2020 ▶). This factor is crucial in the monitoring and treatment of sick animals.
We found a statistically higher incidence of anaplasmosis in cattle with risk factors associated with ticks and tick bites. In particular, 73.08% of seropositive animals were infested with ticks, 83.8% did not receive regular acaricides treatment, and 88.5% went out to graze. Furthermore, a significantly higher number of seropositive cattle was recorded in the summer season. These factors are recognized risks in the epidemiology of bovine anaplasmosis (Aubry and Geale, 2011 ▶; Ben Said et al., 2018b ▶). Anaplasma marginale is transmitted essentially by ticks, however, mechanical transmission through bites of flies and by instruments frequently used in veterinary practice may also occur (Kocan et al., 2015 ▶). It has also been suggested that transplacental transmission may contribute to the epidemiology of bovine anaplasmosis in some regions (Aubry and Geale, 2011 ▶). However, the burden of alternative routes of Anaplasma transmission in Egypt requires further investigations.
Species-specific PCRs for the detection of active infection with A. marginale revealed that the pathogen was actively circulating in 20% of examined population. In molecular epidemiology studies in Egypt, 10.6–92.7% of tested cattle were PCR positive (Table 3). In other north African countries, infection rates in cattle based on molecular studies were 4.7–25.4% in Tunisia, 21.9% in Morocco, 6.1% in Sudan, and 11.1% in Algeria (Ben Said et al., 2018b ▶). However, the different prevalence rates reported for these countries could be explained in part by the difference in sampling seasons, i.e. late spring and early summer, which is favorable to the spread of ticks compared to the winter season with minimal tick activity (Aubry and Geale, 2011 ▶).
Understanding the phylogenetic relationships between A. marginale isolates is important for performing an informative intraspecific diversity analysis contributing to better prevention and control of this bacterium. Thus, the sequencing of the partial msp4 gene was used for an analysis of the diversity of our Egyptian A. marginale isolates. The detection of two different A. marginale isolates in this study, in the second cluster, suggests that phylo-geographical resolution may be obtained at the regional level (de la Fuente et al., 2003 ▶; de la Fuente et al., 2004 ▶), but not when the analysis is conducted worldwide. This heterogeneity could be explained, in part, by the importation of live cattle and/or the dissemination of Anaplasma spp. infected ticks with migratory birds which are proven by several studies from different countries (Alekseev et al., 2001 ▶; Ogden et al., 2008 ▶; Hildebrandt et al., 2010 ▶; Kang et al., 2013 ▶).
When examining 130 cattle for A. centrale and A. bovis, no cases of infection were detected. In accordance with our result, these Anaplasma species were not diagnosed in previous studies in Egypt (El-Ashker et al., 2015 ▶; AL-Hosary et al., 2020 ▶; Nasreldin et al., 2020 ▶). Anaplasma centrale, which is less pathogenic than A. marginale, causes mild signs in cattle and is considered a naturally attenuated subspecies. It has therefore been widely used as a live vaccine against A. marginale (Kocan et al., 2010 ▶). In North Africa, the molecular prevalence of up to 15.1% and 39.4% have been recorded in Tunisia and Algeria (Belkahia et al., 2015 ▶; Rjeibi et al., 2017 ▶). Anaplasma bovis that infects circulating monocytes and tissue macrophages of domestic and wild ruminants, is also usually asymptomatic however, can cause a variety of clinical signs, including a reduction in body weight, fever, anemia, depression, lymphadenopathy, rarely abortion, and death in some cases (Noaman and Shayan, 2010 ▶). Although it has not been detected in Egypt, the DNA of A. bovis was reported in all domestic ruminant species i.e. cattle, sheep, and goats in Tunisia and Algeria (Ben Said et al., 2018b ▶). Given the low prevalence of A. bovis in cattle populations in North Africa and West Asia (Noaman et al., 2016 ▶), a higher number of tested samples from more regions are required in Egypt.
Although cattle have been traditionally studied for A. marginale, buffaloes and camels can also be infected with hemoparasites from cattle and transmit them to cattle in mixed grazing areas (Sazmand et al., 2016 ▶; Elhariri et al., 2017 ▶; Sazmand et al., 2019 ▶). Moreover, wild species such as hedgehogs and their ticks and fleas could act as reservoirs for anaplasmosis (Khodadadi et al., 2021 ▶; Bezerra-Santos et al., 2021 ▶). Hence, for a sustainable control of anaplasmosis in Egypt, different domestic and wild mammals should be involved.
As a limitation of this study, only seropositive cattle were selected for PCR. However, it was shown that PCR is capable to detect more infected cattle (AL-Hosary et al., 2020 ▶). Hence, the burden of bovine anaplasmosis in the investigated areas is possibly higher. Furthermore, infection of Egyptian cattle with A. platys, and A. platys-like bacteria (AL-Hosary et al., 2020 ▶; Tumwebaze et al., 2020 ▶) and exposure of dogs to A. phagocytophilum and A. platys (Selim et al., 2021 ▶) suggest that various Anaplasma species are present in the country; so investigation of both classified and unclassified Anaplasma spp. in future studies in Egypt would shed light on the true incidence of bovine anaplasmosis.
This serological and molecular surveillance study showed the presence of A. marginale in cattle of Kafr El-Sheikh, Al-Gharbia, and Menofia. However, the connection between Anaplasma species and their tick vectors remains largely unknown and merits further investigation. Considering that the infection spreads mainly through bites of ixodid ticks, effective ectoparasite control strategies, regular examination of cattle, and successful chemoprophylaxis are advocated.
Conflicts of interest
The authors declare no conflict of interest.
Acknowledgements
The authors thank the veterinarians for their support and help in providing data and sample collection throughout the study.
References
- Abdel-Shafy S, Allam N, Mahmoud M. Molecular description of Anaplasma biodiversity regarding 16SrDNA, msp4, hsp60, and rpoB profiles in ixodid ticks infesting animals from some Egyptian Provinces. Bull. Natl. Res. Cent. 2016;41:121–136. [Google Scholar]
- Ait Hamou S, Rahali T, Sahibi H, Belghyti D, Losson B, Goff W, Rhalem A. Molecular and serological prevalence of Anaplasma marginale in cattle of North Central Morocco. Res. Vet. Sci. 2012;93:1318–1323. doi: 10.1016/j.rvsc.2012.02.016. [DOI] [PubMed] [Google Scholar]
- Alanazi AD, Nguyen VL, Alyousif MS, Manoj RR, Alouffi AS, Ridolfi D, Sazmand A, Mendoza-Roldan JA, Dantas-Torres F, Otranto D. Ticks and associated pathogens in camels (Camelus dromedarius) from Riyadh Province, Saudi Arabia. Parasit. Vectors. 2020;13 doi: 10.1186/s13071-020-3973-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alderink FJ, Dietrich R. Economic and epidemiological implications of anaplasmosis in Texas beef cattle herds. Bulletin of Texas Agricultural Experiment Station, Number 1426 . 1983:66–75. [Google Scholar]
- Alekseev AN, Dubinina HV, Semenov AV, Bolshakov CV. Evidence of ehrlichiosis agents found in ticks (Acari: Ixodidae) collected from migratory birds. J. Med. Entomol. 2001;38:471–474. doi: 10.1603/0022-2585-38.4.471. [DOI] [PubMed] [Google Scholar]
- AL-Hosary A, Răileanu C, Tauchmann O, Fischer S, Nijhof AM, Silaghi C. Epidemiology and genotyping of Anaplasma marginale and co-infection with piroplasms and other Anaplasmataceae in cattle and buffaloes from Egypt. Parasit. Vectors. 2020;13:495. doi: 10.1186/s13071-020-04372-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- AL-Hosary A, Răileanu C, Tauchmann O, Fischer S, Nijhof AM, Silaghi C. Tick species identification and molecular detection of tick-borne pathogens in blood and ticks collected from cattle in Egypt. Ticks Tick Borne Dis. 2021;12:101676. doi: 10.1016/j.ttbdis.2021.101676. [DOI] [PubMed] [Google Scholar]
- Altschul SF, Madden TL, Schäffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aubry P, Geale DW. A review of bovine anaplasmosis. Transbound. Emerg. Dis. 2011;58:1–30. doi: 10.1111/j.1865-1682.2010.01173.x. [DOI] [PubMed] [Google Scholar]
- Battilani M, De Arcangeli S, Balboni A, Dondi F. Genetic diversity and molecular epidemiology of Anaplasma. Infect. Genet. Evol. 2017;49:195–211. doi: 10.1016/j.meegid.2017.01.021. [DOI] [PubMed] [Google Scholar]
- Belkahia H, Said MB, Alberti A, Abd K, Issaoui Z, Hattab D, Gharbi M, Messadi L. First molecular survey and novel genetic variants’ identification of Anaplasma marginale A centrale and A bovis in cattle from Tunisia. Infect. Genet. Evol. 2015;34:361–371. doi: 10.1016/j.meegid.2015.06.017. [DOI] [PubMed] [Google Scholar]
- Ben Said M, Asker AB, Belkahia H, Ghribi R, Selmi R, Messadi L. Genetic characterization of Anaplasma marginale strains from Tunisia using single and multiple gene typing reveals novel variants with an extensive genetic diversity. Ticks Tick Borne Dis. 2018a;9:1275–1285. doi: 10.1016/j.ttbdis.2018.05.008. [DOI] [PubMed] [Google Scholar]
- Ben Said M, Belkahia H, Alberti A, Zobba R, Bousrih M, Yahiaoui M, Daaloul-Jedidi M, Mamlouk A, Gharbi M, Messadi L. Molecular survey of Anaplasma species in small ruminants reveals the presence of novel strains closely related to A phagocytophilum in Tunisia. Vector Borne Zoonotic Dis. 2015;15:580–590. doi: 10.1089/vbz.2015.1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben Said M, Belkahia H, El Mabrouk N, Saidani M, Alberti A, Zobba R, Cherif A, Mahjoub T, Bouattour A, Messadi L. Anaplasma platys-like strains in ruminants from Tunisia. Infect. Genet. Evol. 2017a;49:226–233. doi: 10.1016/j.meegid.2017.01.023. [DOI] [PubMed] [Google Scholar]
- Ben Said M, Belkahia H, El Mabrouk N, Saidani M, Hassen MB, Alberti A, Zobba R, Bouattour S, Bouattour A , Messadi L. Molecular typing and diagnosis of Anaplasma spp closely related to Anaplasma phagocytophilum in ruminants from Tunisia. Ticks Tick borne Dis. 2017b;8:412–422. doi: 10.1016/j.ttbdis.2017.01.005. [DOI] [PubMed] [Google Scholar]
- Ben Said M, Belkahia H, Messadi L. Anaplasma spp in North Africa: a review on molecular epidemiology, associated risk factors and genetic characteristics. Ticks Tick Borne Dis. 2018b;9:543–555. doi: 10.1016/j.ttbdis.2018.01.003. [DOI] [PubMed] [Google Scholar]
- Ben Said M, Belkahia H, Selmi R, Messadi L. Computational selection of minimum length groESL operon required for Anaplasma species attribution and strain diversity analysis. Mol. Cell. Probes. 2019;48:101467. doi: 10.1016/j.mcp.2019.101467. [DOI] [PubMed] [Google Scholar]
- Bezerra-Santos MA, Sgroi G, Mendoza-Roldan JA, Khedri J, Camarda A, Iatta R, Sazmand A, Otranto D. Ectoparasites of hedgehogs: From flea mite phoresy to their role as vectors of pathogens. Int. J. Parasitol. Parasites Wildl. 2021;15:95–104. doi: 10.1016/j.ijppaw.2021.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung C, Wilson C, Bandaranayaka-Mudiyanselage CB, Kang E, Adams DS, Kappmeyer LS, Knowles DP, McElwain TF, Evermann JF, Ueti MW. Improved diagnostic performance of a commercial Anaplasma antibody competitive enzyme-linked immunosorbent assay using recombinant major surface protein 5–glutathione S-transferase fusion protein as antigen. J. Vet. Diagn. Investig. 2014;26:61–71. doi: 10.1177/1040638713511813. [DOI] [PubMed] [Google Scholar]
- Dantas-Torres, F, Otranto, D. Anaplasmosis. In: Marcondes, CB, editor. Arthropod borne diseases. Cham, Switzerland: Springer; 2017. pp. 2015– 222. [Google Scholar]
- de la Fuente J, Thomas EJG, Van Den Bussche RA, Hamilton RG, Tanaka EE, Druhan SE, Kocan KM. Characterization of Anaplasma marginale isolated from North American bison. Appl. Environ. Microbiol. 2003;69:5001–5005. doi: 10.1128/AEM.69.8.5001-5005.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Fuente J, Vicente JN, Höfle U, Ruiz-Fons F, De Mera IGF, Van Den Bussche RA, Kocan KM, Gortazar C. Anaplasma infection in free-ranging Iberian red deer in the region of Castilla-La Mancha, Spain. Vet. Microbiol. 2004;100:163–173. doi: 10.1016/j.vetmic.2004.02.007. [DOI] [PubMed] [Google Scholar]
- Dreher U, de la Fuente J, Hofmann-Lehmann R, Meli ML, Pusterla N, Kocan K, Woldehiwet Z, Braun U, Regula G, Staerk K. Serologic cross-reactivity between Anaplasma marginale and Anaplasma phagocytophilum. Clin. Diagn. Lab. Immunol. 2005;12:1177–1183. doi: 10.1128/CDLI.12.10.1177-1183.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Ashker M, Hotzel H, Gwida M, El-Beskawy M, Silaghi C, Tomaso H. Molecular biological identification of Babesia, Theileria, and Anaplasma species in cattle in Egypt using PCR assays, gene sequence analysis and a novel DNA microarray. Vet. Parasitol. 2015;207:329–334. doi: 10.1016/j.vetpar.2014.12.025. [DOI] [PubMed] [Google Scholar]
- El-Dakhly KM, Arafa WM, Soliman S, Abdel-Fatah OR, Wahba AA, Esteve-Gasent MD, Holman PJ. Molecular detection, phylogenetic analysis, and genetic diversity of Theileria annulata, Babesia bigemina, and Anaplasma marginale in cattle in three districts of Egypt. Acta Parasitol. 2020;65:620–627. doi: 10.2478/s11686-020-00189-z. [DOI] [PubMed] [Google Scholar]
- Elhariri MD, Elhelw RA, Hamza DA, Soliman DE. Molecular detection of Anaplasma marginale in the Egyptian water buffaloes (Bubalus bubalis) based on major surface protein 1α. J. Egypt Soc. Parasitol. 2017;47:247–252. [Google Scholar]
- El-Naga T, Barghash S. Blood parasites in camels (Camelus dromedarius) in Northern West Coast of Egypt. J. Bacteriol. Parasitol. 2016;7:1000258. [Google Scholar]
- Felsenstein J. Confidence limits on phylogenies: an approach using the bootstrap. Evolution. 1985;39:783–791. doi: 10.1111/j.1558-5646.1985.tb00420.x. [DOI] [PubMed] [Google Scholar]
- Fereig RM, Mohamed SG, Mahmoud HY, AbouLaila MR, Guswanto A, Nguyen TT, Mohamed AEA, Inoue N, Igarashi I, Nishikawa Y. Seroprevalence of Babesia bovis B bigemina, Trypanosoma evansi, and Anaplasma marginale antibodies in cattle in southern Egypt. Ticks Tick Borne Dis. 2017;8:125–131. doi: 10.1016/j.ttbdis.2016.10.008. [DOI] [PubMed] [Google Scholar]
- Hall TA. 1999) BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Sym. Ser. 41:95–98. [Google Scholar]
- Hildebrandt A, Franke J, Meier F, Sachse S, Dorn W, Straube E. The potential role of migratory birds in transmission cycles of Babesia spp Anaplasma phagocytophilum, and Rickettsia spp. Ticks Tick Borne Dis. 2010;1:105–107. doi: 10.1016/j.ttbdis.2009.12.003. [DOI] [PubMed] [Google Scholar]
- Jongejan F, Uilenberg G. The global importance of ticks. Parasitology. 2004;129:S3–S14. doi: 10.1017/s0031182004005967. [DOI] [PubMed] [Google Scholar]
- Kang JG, Kim HC, Choi CY, Nam HY, Chae HY, Chong ST, Klein TA, Ko S, Chae JS. Molecular detection of Anaplasma, Bartonella, and Borrelia species in ticks collected from migratory birds from Hong-do Island, Republic of Korea. Vector Borne Zoonotic Dis. 2013;13:215–225. doi: 10.1089/vbz.2012.1149. [DOI] [PubMed] [Google Scholar]
- Kawahara M, Rikihisa Y, Lin Q, Isogai E, Tahara K, Itagaki A, Hiramitsu Y, Tajima T. Novel genetic variants of Anaplasma phagocytophilum, Anaplasma bovis, Anaplasma centrale, and a novel Ehrlichia sp in wild deer and ticks on two major islands in Japan. Appl. Environm. Microbiol. 2006;72:1102–1109. doi: 10.1128/AEM.72.2.1102-1109.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khodadadi N, Nabavi R, Sarani A, Saadati D, Ganjali M, Mihalca AD, Otranto D, Sazmand A. Identification of Anaplasma marginale in long-eared hedgehogs (Hemiechinus auritus) and their Rhipicephalus turanicus ticks in Iran. Ticks Tick Borne Dis. 2021;12:101641. doi: 10.1016/j.ttbdis.2020.101641. [DOI] [PubMed] [Google Scholar]
- Kocan KM, de la Fuente J, Blouin EF, Coetzee JF, Ewing S. The natural history of Anaplasma marginale. Vet. Parasitol. 2010;167:95–107. doi: 10.1016/j.vetpar.2009.09.012. [DOI] [PubMed] [Google Scholar]
- Kocan KM, de la Fuente J, Cabezas-Cruz A. The genus Anaplasma: new challenges after reclassifica-tion. Rev. Sci. Tech. 2015;34:577–586. doi: 10.20506/rst.34.2.2381. [DOI] [PubMed] [Google Scholar]
- Lew-Tabor A, Valle MR. A review of reverse vaccinology approaches for the development of vaccines against ticks and tick borne diseases. Ticks Tick Borne Dis. 2016;7:573–585. doi: 10.1016/j.ttbdis.2015.12.012. [DOI] [PubMed] [Google Scholar]
- Loftis AD, Reeves WK, Szumlas DE, Abbassy MM, Helmy IM, Moriarity JR, Dasch GA. Rickettsial agents in Egyptian ticks collected from domestic animals. Exp. Appl. Acarol. 2006;40:67–81. doi: 10.1007/s10493-006-9025-2. [DOI] [PubMed] [Google Scholar]
- M’ghirbi Y, Bèji M, Oporto B, Khrouf F, Hurtado A, Bouattour A Anaplasma marginale and A phagocytophilum in cattle in Tunisia. Parasit. Vectors. 2016;9:556. doi: 10.1186/s13071-016-1840-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasreldin N, Ewida RM, Hamdon H, Elnaker YF. Molecular diagnosis and biochemical studies of tick-borne diseases (anaplasmosis and babesiosis) in Aberdeen Angus Cattle in New Valley, Egypt. Vet. World. 2020;13:1884. doi: 10.14202/vetworld.2020.1884-1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noaman V, Nabinejad A, Shahmoradi A, Esmaeilkhanian S. Molecular detection of bovine leukocytic Anaplasma species in Isfahan, Iran. Res. Mol. Med. 2016;4:47–51. [Google Scholar]
- Noaman V, Shayan P. Molecular detection of Anaplasma bovis in cattle from central part of Iran. Vet. Res. Forum. 2010;1:117–122. [Google Scholar]
- Ogden NH, Lindsay LR, Hanincová K, Barker IK, Bigras-Poulin M, Charron DF, Heagy A, Francis CM, O’Callaghan CJ, Schwartz I. Role of migratory birds in introduction and range expansion of Ixodes scapularis ticks and of Borrelia burgdorferi and Anaplasma phagocytophilum in Canada. Appl. Environ. Microbiol. 2008;74:1780–1790. doi: 10.1128/AEM.01982-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OIE, World Organization for Animal Health. Bovine anaplasmosis manual of diagnostic tests and vaccines for terrestrial animals. Paris, France: 2018. [ (accessed 28 December 2021)]. https://www.oie.int/fileadmin/Home/eng/Health_standards/tahm/3.04.01_BOVINE_ANAPLASMOSIS.pdf . [Google Scholar]
- Parvizi O, El-Adawy H, Melzer F, Roesler U, Neubauer H, Mertens-Scholz K. Seroprevalence and molecular detection of bovine anaplasmosis in Egypt. Pathogens. 2020;9:64. doi: 10.3390/pathogens9010064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radwan M, Abdel Fatah A, El Hamied O. Epidemiological studies and molecular diagnosis of Anaplasma marginale in cattle and biochemical changes associated with it in Kaliobia Governorate. Am. J. Infec. Dis. Microbiol. 2013;1:46–49. [Google Scholar]
- Rjeibi MR, Ayadi O, Rekik M, Gharbi M. Molecular survey and genetic characterization of Anaplasma centrale, A marginale and A bovis in cattle from Algeria. Transbound. Emerg. Dis. 2018;65:456–464. doi: 10.1111/tbed.12725. [DOI] [PubMed] [Google Scholar]
- Saitou N, Nei M. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 1987;4:406–425. doi: 10.1093/oxfordjournals.molbev.a040454. [DOI] [PubMed] [Google Scholar]
- Salih DA, Abdel Rahman MB, Mohammed AS, Ahmed R, Kamal S, El Hussein AM. Seroprevalence of tick-borne diseases among cattle in the Sudan. Parasitol. Res. 2009;104:845–850. doi: 10.1007/s00436-008-1265-0. [DOI] [PubMed] [Google Scholar]
- Salih DA, Hassan S, Julla I, Kyule M, Zessin KH, El Hussein A. Distribution and application of ELISA for the seroprevalence of tick-borne diseases in Central Equatoria State, Sudan. Transbound. Emerg Dis. 2008;55:257–262. doi: 10.1111/j.1865-1682.2008.01032.x. [DOI] [PubMed] [Google Scholar]
- Salm F, Younis E, Hegazy N, El-Sawalhy A. Epidemiological studies on bovine anaplasmosis. Bull. Anim. Health Prod. Afr. 2011;59:179–189. [Google Scholar]
- Sazmand A, Alipoor G, Zafari S, Zolhavarieh SM, Alanazi AD, Sargison ND. Assessment of knowledge, attitudes and practices relating to parasitic diseases and anthelmintic resistance among livestock farmers in Hamedan, Iran. Front. Vet. Sci. 2020;7:584323. doi: 10.3389/fvets.2020.584323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sazmand A, Eigner B, Mirzaei M, Hekmatimoghaddam S, Harl J, Duscher GG, Fuehrer HP, Joachim A. Molecular identification of hemoprotozoan parasites in camels (Camelus dromedarius) of Iran. Iran J. Parasitol. 2016;11:568–573. [PMC free article] [PubMed] [Google Scholar]
- Sazmand A, Harl J, Eigner B, Hodžić A, Beck R, Hekmatimoghaddam S, Mirzaei M, Fuehrer HP, Joachim A. Vector-borne bacteria in blood of camels in Iran: New data and literature review. Comp. Immunol. Microbiol. Infect. Dis. 2019;65:48–53. doi: 10.1016/j.cimid.2019.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selim A, Alanazi AD, Sazmand A, Otranto D. Seroprevalence and associated risk factors for vector-borne pathogens in dogs from Egypt. Parasit. Vectors. 2021;14:175. doi: 10.1186/s13071-021-04670-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selmi R, Ben Said M, Dhibi M, Ben Yahia H, Abdelaali H, Messadi L. Genetic diversity of groEL and msp4 sequences of Anaplasma ovis infecting camels from Tunisia. Parasitol. Int. 2020;74:101980. doi: 10.1016/j.parint.2019.101980. [DOI] [PubMed] [Google Scholar]
- Selmi R, Said MB, Dhibi M, Yahia HB, Messadi L. Improving specific detection and updating phylogenetic data related to Anaplasma platys-like strains infecting camels (Camelus dromedarius) and their ticks. Ticks Tick Borne Dis. 2019;10:101260. doi: 10.1016/j.ttbdis.2019.07.004. [DOI] [PubMed] [Google Scholar]
- Sharifiyazdi H, Jafari S, Ghane M, Nazifi S, Sanati A. Molecular investigation of Anaplasma and Ehrlichia natural infections in the dromedary camel (Camelus dromedarius) in Iran. Comp. Clin. Pathol. 2017;26:99–103. [Google Scholar]
- Tamura K, Nei M. Estimation of the number of nucleotide substitutions in the control region of mitochondrial DNA in humans and chimpanzees. Mol. Biol. Evol. 1993;10:512–526. doi: 10.1093/oxfordjournals.molbev.a040023. [DOI] [PubMed] [Google Scholar]
- Thrusfield M, Christley R, Brown H, Diggle PJ, French N, Howe K, et al . Surveys. In: hrusfield M, Christley R, editors. Veterinary epidemiology. 4th Edn. West Sussex: John Wiley & Sons Ltd; 2018. pp. 270–295. [Google Scholar]
- Torina A, Agnone A, Blanda V, Alongi A, D’Agostino R, Caracappa S, Marino AM, Di Marco V, de laFuente J. Development and validation of two PCR tests for the detection of and differentiation between Anaplasma ovis and Anaplasma marginale. Ticks Tick Borne Dis. 2012;3:283–287. doi: 10.1016/j.ttbdis.2012.10.033. [DOI] [PubMed] [Google Scholar]
- Tumwebaze MA, Lee SH, Moumouni PFA, Mohammed-Geba K, Sheir SK, Galal-Khallaf A, Abd El Latif HM, Morsi DS, Bishr NM, Galon EM. First detection of Anaplasma ovis in sheep and Anaplasma platys-like variants from cattle in Menoufia governorate, Egypt. Parasitol. Int. 2020;78:102150. doi: 10.1016/j.parint.2020.102150. [DOI] [PubMed] [Google Scholar]
- Uilenberg G. International collaborative research: significance of tick-borne hemoparasitic diseases to world animal health. Vet. Parasitol. 1995;57:19–41. doi: 10.1016/0304-4017(94)03107-8. [DOI] [PubMed] [Google Scholar]
- Younis E, Hegazy N, El-Deeb W, El-Khatib R. Epidemiological and biochemical studies on bovine anaplasmosis in Dakahlia and Demiatta governorates in Egypt. Bull. Anim. Health Prod. Afri. 2009 doi: 10.4314/bahpa.v57i4.51668. [Google Scholar]
- Ziam H, Benaouf H. Prevalence of blood parasites in cattle from wilayates of Annaba and El Tarf east Algeria. Arch. Inst. Pasteur Tunis. 2004;81:27–30. [PubMed] [Google Scholar]
- Zobba R, Anfossi AG, Pinna Parpaglia ML, Dore GM, Chessa B, Spezzigu A, Rocca S, Visco S, Pittau M, Alberti A. Molecular investigation and phylogeny of Anaplasma spp in Mediterranean ruminants reveal the presence of neutrophil-tropic strains closely related to A. platys. Appl. Environ. Microbiol. 2014;80:271–280. doi: 10.1128/AEM.03129-13. [DOI] [PMC free article] [PubMed] [Google Scholar]