Abstract
The present study investigated the feasibility of automating the specimen-pipetting component of sample preparation in the LCx Chlamydia assay (LCx-CT assay; Abbott Laboratories, Chicago, Ill.) by using a commercially available liquid-handling system (Tecan Genesis RSP100; Tecan Inc., Research Triangle Park, N.C.). The Tecan instrument proved to be comparable in both precision and accuracy to a manual multipipettor (Eppendorf model 4850; Eppendorf Scientific, Westbury, N.Y.). The Tecan instrument was extensively checked for evidence of specimen-to-specimen transfer, and no level of contamination sufficient to generate a signal above the background in the LCx-CT assay was detected. Finally, pipetting speed was significantly improved by using the Tecan instrument. A mean time of 2.5 min was required to pipette a complete LCx-CT assay carousel (20 samples and 4 controls) with the Tecan instrument, whereas 8.4 min was required to pipette a comparable number of samples manually (P < 0.001).
Detection of Chlamydia trachomatis in urogenital specimens by nucleic acid amplification (NAA) assays, including the LCx Chlamydia assay (LCx-CT assay; Abbott Laboratories, Chicago, Ill.), has become firmly established as the standard of care for the diagnosis of infection with this organism (3, 6, 9). Although the LCx-CT assay has excellent performance characteristics (4, 7, 8), a significant drawback of this and many other first-generation NAA tests for C. trachomatis is their relative lack of automation, making them significantly more labor intensive than alternative methodologies for detection of this organism. The specimen preparation component of the LCx-CT assay includes pipetting of fixed volumes of processed samples into amplification vials containing prealiquoted amplification reagents. This process is typically performed with a manually operated single-channel pipettor, a laborious and ergonomically problematic operation for moderate- to high-volume laboratories. Automated liquid-handling devices, such as the Tecan Genesis RSP100 (Tecan Inc., Research Triangle Park, N.C.) automated pipettor, have been shown to be highly effective at increasing throughput and decreasing labor expenditure in a number of high-volume applications (1, 5). There have, however, been no published evaluations describing the use of this type of instrument in NAA applications, with their more stringent requirements for pipetting accuracy and precision and concern about cross-specimen transfer leading to false-positive results (2).
Study institution.
The clinical microbiology laboratory at Hennepin County Medical Center (HCMC) has been performing the LCx NAA assays for C. trachomatis (LCx-CT) and Neisseria gonorrhoeae (LCx-GC) since January 1997. The annual volume of tests was 38,034 (22,485 for the LCx-CT assay and 15,549 for the LCx-GC assay) in 1997 and has risen to a projected (on the basis of data collected from January through September 2000) 53,877 tests (30,897 for the LCx-CT assay and 22,980 for the LCx-GC assay) in 2000. The typical daily work flow is illustrated in Fig. 1 and is performed by a single technologist with four thermocyclers and four LCx analyzers. The mean positivity rates for the populations served by HCMC since the introduction of the LCx assays are 8.07 and 3.26% for C. trachomatis and N. gonorrhoeae, respectively.
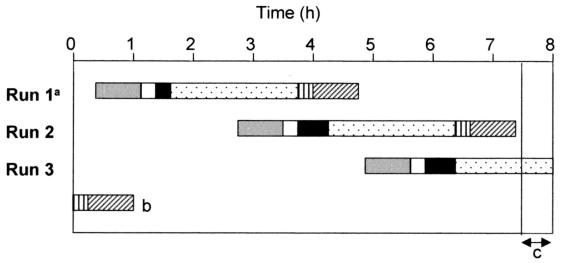
FIG. 1.
Typical daily LCx assay workflow at HCMC enables analysis of a maximum of 120 samples tested for both C. trachomatis and N. gonorrhoeae in an 8-h shift by one technologist using four thermocyclers and four LCx analyzers. a, each run consists of 40 samples (80 tests) plus controls; b, completion of run 3 from the previous day; c, results reporting and instrument cleanup. The following assay procedures are represented by the indicated symbols: sample preparation, solid gray bars; sample transfer to amplification vials, open bars; thermocycler loading, solid black bars; amplification, dotted bars; LCx analyzer loading, vertically hatched bars; product detection, diagonally hatched bars.
All clinical samples used for determination of the performance characteristics of the Tecan automated pipettor in the Abbott LCx-CT assay were endocervical swab specimens submitted to the HCMC clinical microbiology laboratory for testing for C. trachomatis. After routine testing was completed, discarded materials were retained (frozen at −70°C) for use in the study.
The precisions of the Tecan automated pipettor and the Eppendorf 4850 manual pipettor were determined gravimetrically. Multiple (n = 24) 100-μl aliquots of water were pipetted, using 1,000-μl maximum-volume pipette tips, into preweighed LCx amplification vials, and the volume dispensed was determined with an analytical balance (AE-163; Mettler Instrument Corp., Highstown, N.J.). Coefficients of variation (CV) are shown in Table 1 and were comparable for both instruments.
TABLE 1.
Performance characteristics of the Tecan automated pipettor in the LCx-CT assay
| Parameter (no. of replicates) and sample | Median (range) LCx signal (cps) | % CV |
|---|---|---|
| Precision (24)a | NDb | 2.2c |
| Reproducibility | ||
| Positive sample pools (10) | 1,437.6 (1,380.5–1,492.7) | 2.4 (1.6–3.0) |
| Positive specimens (15, in triplicate) | 1,418.9 (504.0–1,857.3) | 4.4 (0.5–20.3) |
| Negative specimens (54, in triplicate) | 15.3 (7.2–31.3) | 5.1 (0.0–28.5) |
Precision was assessed by replicate pipetting 100-μl aliquots of water.
ND, not done.
A CV of 1.0% was obtained with a manual pipettor.
The reproducibility of the Tecan automated pipettor in the LCx-CT assay was assessed in three independent studies. Serial dilutions of known C. trachomatis-positive specimens were prepared by using pooled negative specimens as a diluent. A total of 10 such positive pools were then multiply pipetted (10 replicates) into LCx-CT assay amplification vials by using the Tecan automated pipettor, and the mean signal and percent CV were calculated for each pool (Table 1).
A total of 69 previously analyzed specimens (15 specimens positive for C. trachomatis and 54 specimens negative for C. trachomatis) were repeatedly pipetted (three replicates) with the Tecan automated pipettor and then retested by the LCx-CT assay. The mean signal and CV for each group of replicates were determined (Table 1).
One hundred previously analyzed clinical samples (24 samples positive for C. trachomatis and 76 samples negative for C. trachomatis) were simultaneously pipetted into amplification vials with either the Tecan automated pipettor or the Eppendorf 4850 manual pipettor and then analyzed by the LCx-CT assay. Entirely concordant results were obtained, with no statistically significant differences in qualitative results obtained with the two pipettors. The mean ± standard deviation (SD) signals generated from positive specimens were 1,708.3 ± 184.4 cps for manually pipetted samples and 1,694.6 ± 164.4 cps for samples pipetted with the Tecan automated pipettor. The corresponding values for negative specimens were 19.2 ± 4.7 and 16.6 ± 4.0 cps for the Eppendorf and Tecan pipettors, respectively.
Two studies were conducted to evaluate the risk of cross-sample contamination when the Tecan automated pipettor was used. In the first study, a total of 16 high-level-positive samples (mean signal, 1871.8 ± 132.8 cps; mean signal-to-cutoff ratio, 3.24 ± 0.28) were evenly interspersed among 64 uninoculated sample collection vials. All 80 samples were then pipetted into LCx-CT assay amplification vials with the Tecan automated pipettor and analyzed. The mean ± SD signal generated from the negative samples was 15.7 ± 1.9 cps (range, 12.3 to 22.7 cps). The second study was performed immediately after the completion of the first cross-sample contamination study. A total of 80 uninoculated sample collection vials were placed on the Tecan instrument, and an aliquot (100 μl) of sample diluent was transferred from each collection vial into an LCx-CT amplification vial. The activated vials were then run through the standard LCx amplification and product detection protocol. These vials yielded a mean signal of 14.6 ± 1.4 cps (range, 12.0 to 17.8 cps). In addition, upon completion of the evaluation of the Tecan automated pipettor, multiple areas of the instrument including the base plate, permanent pipettors, specimen vial racks, amplification vial racks, pipette tip discard tray, and wash station were sampled by using LCx specimen collection swabs. Swabs were then expressed into sample collection vials and analyzed for the presence of both C. trachomatis and N. gonorrhoeae DNA by standard LCx protocols. All swab samples taken from the Tecan instrument were negative for C. trachomatis and N. gonorrhoeae when subjected to testing by the LCx assays.
An initial comparison of the time required to pipette samples manually and with the Tecan automated pipettor was performed by pipetting the contents of four complete LCx carousels (a total of 80 samples) by both methods and recording the time required to complete the process. A mean time of 2.5 min was required to pipette the contents of a complete LCx-CT carousel (20 samples and 4 controls) with the Tecan automated pipettor, whereas 8.4 min was required to pipette a comparable number of samples manually (P < 0.001). Upon completion of a probationary training and evaluation period, a real-time analysis of the workload requirement for routine testing by the LCx assay in our laboratory (a combination of the LCx-CT and the LCx-GC assays) was performed with the Tecan automated pipettor. Technologists performing LCx assays recorded the total number of billable analyses performed (excluding all required controls and repeat tests) and the time taken to complete all daily tasks, including accessioning of specimens, reporting of results, and instrument maintenance, for 30 consecutive workdays. The data generated during that study were compared with those obtained during a similar study conducted in October 1997 when all specimens were manually pipetted (Table 2).
TABLE 2.
Results of independent real-time evaluations of the workload requirement for the Abbott LCx assays when addition of samples to amplification vials was performed with a manual pipettor (Eppendorf 4850) or an automated pipettor (Tecan Genesis RSP100 automated pipettor)
| Pipetting method | Test period (days) | Total no. of LCx assays (no. of LCx-CT assays; no. of LCx-GC assays) | Total labor (min) | Workload per LCx test (min) |
|---|---|---|---|---|
| Manual | 30 | 4,020 (2,370; 1,650) | 14,807 | 3.68 |
| Automated | 29 | 5,018 (2,844; 2,174) | 13,185 | 2.60a |
P < 0.001.
The results of the present study demonstrate that the use of an automated pipetting instrument, in this instance, the Tecan Genesis RSP100 instrument, can improve the efficiency of the Abbott LCx NAA assays for C. trachomatis and N. gonorrhoeae without compromising the integrity of the results obtained. Use of the Tecan automated pipettor in our laboratory has enabled us to accommodate increases in test volumes for the LCx-CT and the LCx-GC assays without concomitant increases in technologist time. Indeed, routine use of the Tecan automated pipettor has resulted in a decrease in the typical daily workload from 8.22 h in 1997 (in which pipetting was exclusively manual) to 6.92 h in 2000 (Table 2) over a time period in which the annual volume of LCx tests has increased by almost 20,000. The performance of the Tecan automated pipettor in the LCx-CT assay was, as far as we were able to ascertain, entirely comparable to that achieved with a manual pipettor (the Eppendorf 4850 pipettor). There was no evidence that the use of an automated pipettor adversely affected any of the performance characteristics of the assay. Although the level of additional automation afforded by use of the Tecan automated pipettor is relatively small, the labor savings realized by using the automated pipettor in this high-volume molecular biology-based application were still considerable. In addition, although difficult to measure quantitatively, the ergonomic benefits of using an automated pipettor for high-volume molecular biology-based assays are significant and further increase the benefit-to-cost ratio of acquiring such a device. The present study illustrates that NAA assays are amenable to automation and that the increased instrumentation cost can often be more than offset by a concomitant decrease in labor cost.
Acknowledgments
This study was supported in part by a grant from Abbott Laboratories.
REFERENCES
- 1.Ertingshausen G, Shapiro S I, Green G, Zborowski G. Adaptation of a T3-uptake test and of radioimmunoassays for serum digoxin, thyroxine, and triiodothyronine to an automated radioimmunoassay system—“Centria”. Clin Chem. 1975;21:1305–1313. [PubMed] [Google Scholar]
- 2.Gronowski A M, Copper S, Baorto D, Murray P R. Reproducibility problems with the Abbott Laboratories LCx assay for Chlamydia trachomatis and Neisseria gonorrhoeae. J Clin Microbiol. 2000;38:2416–2418. doi: 10.1128/jcm.38.6.2416-2418.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guaschino S, De Seta F. Update on Chlamydia trachomatis. Ann N Y Acad Sci. 2000;900:293–300. doi: 10.1111/j.1749-6632.2000.tb06241.x. [DOI] [PubMed] [Google Scholar]
- 4.Lee H H, Chernesky M A, Schachter J, Burczak J D, Andrews W W, Muldoon S, Leckie G, Stamm W E. Diagnosis of Chlamydia trachomatis genitourinary infection in women by ligase chain reaction assay of urine. Lancet. 1995;345:213–216. doi: 10.1016/s0140-6736(95)90221-x. [DOI] [PubMed] [Google Scholar]
- 5.Liebl B, Anhaupl T, Haen E, Gunster B, Georgieff M. A partially automated radioligand binding assay system for use in clinical and pharmaceutical research. J Recept Res. 1993;13:369–378. doi: 10.3109/10799899309073667. [DOI] [PubMed] [Google Scholar]
- 6.Quinn T C. DNA amplification assays: a new standard for diagnosis of Chlamydia trachomatis infections. Ann Acad Med Singapore. 1995;24:627–633. [PubMed] [Google Scholar]
- 7.Schachter J, Stamm W E, Quinn T C, Andrews W W, Burczak J D, Lee H H. Ligase chain reaction to detect Chlamydia trachomatis infection of the cervix. J Clin Microbiol. 1994;32:2540–2543. doi: 10.1128/jcm.32.10.2540-2543.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stary A, Schuh E, Kerschbaumer M, Gotz B, Lee H. Performance of transcription-mediated amplification and ligase chain reaction assays for detection of chlamydial infection in urogenital samples obtained by invasive and noninvasive methods. J Clin Microbiol. 1998;36:2666–2670. doi: 10.1128/jcm.36.9.2666-2670.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Taylor-Robinson D. Evaluation and comparison of tests to diagnose Chlamydia trachomatis genital infections. Hum Reprod. 1997;12(11 Suppl.):113–120. [PubMed] [Google Scholar]