ABSTRACT
Enhanced serum secreted clusterin (sCLU) protein was associated with progression, poor prognosis and chemotherapy sensitivity evaluation in malignant patients. However, the clinical significance of serum sCLU protein levels in patients with invasive breast cancer (IBC) is unknown. In this study, the serum sCLU protein in 2648 patients with IBC was detected. The diagnostic value and treatment responses of serum sCLU protein in patients with IBC were also performed. The results showed that the serum sCLU protein level was significantly higher in IBC patients compared to the healthy controls (P < 0.0001), and strongly correlated with higher clinical tumor stage (P < 0.001), lymph node metastasis (P < 0.001), shorter overall survival (OS) (P = 0.032) and disease-free survival (DFS) (P = 0.029), respectively. Using the cutoff value of 18.46 μg/mL, the sensitivity and specificity were 86.26% and 73.46% to separate IBC patients from noncancerous and healthy controls. The postoperative patients showed lower serum sCLU levels compared to the preoperative patients (P = 0.003). The chemoresistant patients showed higher serum sCLU levels compared to the chemosensitive patients (P < 0.001). These data indicated that serum sCLU levels are effective indicators for diagnosis and chemotherapy sensitivity evaluation in patients with IBC.
KEYWORDS: Invasive breast cancer, diagnosis, prognosis, chemotherapy, clusterin
Graphical abstract
Introduction
Breast cancer is the most common cancer in Chinese women, with 12.2% of all newly diagnosed breast cancers and 9.6% of all deaths from breast cancer worldwide [1]. With the improvement of early diagnosis technology of breast cancer, 5–10% of patients still have distant metastases at the time of diagnosis [2]. Standard chemotherapy and hormonal therapy options can not significantly reduce recurrence and metastasis, which results in 90% of the deaths in breast cancer patients [2–4]. Numerous prognostic factors are frequently used for making clinical decisions in breast cancer [5,6], but they are not routinely used in clinical practice because of their certain limitations and defect [7,8]. Therefore, it is urgent to find highly sensitive and specific molecular markers for diagnosis, prognostic judgment and evaluation of treatment response in patients with breast cancer.
Epidermal growth factor receptor (EGFR) serves as a co-target for dual/pan-EGFR-inhibitors in breast cancer [9]. Current evidence indicates associations between low baseline serum-EGFR and shorter survival or reduced response to treatment in patients with advanced breast cancer, especially in patients with estrogen and/or progesterone receptor-positive tumors. However, the prognostic and predictive value of EGFR and EGFR-ligands in blood has only been investigated in highly selected subsets of breast cancer patients and most studies were small [9–11]. The non-coding RNA sequences have been shown to be dysregulated in breast cancer. Despite showing immense promise, miRNAs have not been successfully implemented in the clinical setting due to a lack of a standardized approach which has resulted in conflicting results [12]. However, further studies are required to specifically isolate cancer exosomes from patient plasma and establish exosome microRNA signatures indicative of breast cancer. Following a diagnosis of breast cancer, measurement of serum CA 15–3 or CEA or uPA/PAI-1 and Oncotype DX may be used for determining prognosis and identifying lymph node-negative patients who may be spared from having to receive adjuvant chemotherapy [13]. However, the sensitivity and specificity of these biomarkers are relatively low for breast cancer.
Clusterin (CLU), a highly conserved 78–80 kDa heterodimeric glycoprotein, widely exists in a variety of tissues, cells and body fluids. CLU exists in at least two sets of isoforms: The secreted forms (sCLU) (∼75-80 kDa) and non-secreted nuclear forms (nCLU) (∼45-60 kDa). sCLU exhibits pro-survival functions [14–17] and nCLU appears to be pro-apoptotic functions [18,19]. sCLU is overexpressed in many types of malignant tumors, and inforced sCLU expression could promote carcinogenesis and tumor progression [20–23]. In addition, sCLU was also used as a marker to evaluate diagnosis and metastasis potential in many malignant tumors [24–27]. Previous study showed that sCLU was overexpressed in osteosarcoma (OS), and higher sCLU expression was related to metastasis and chemoresistance [28]. Higher preoperative sCLU expression in breast cancer was associated with CAF resistance and TNF-alpha-induced apoptosis in breast cancer cells in vitro [29,30]. Furthermore, targeting sCLU inhibited tumor growth and metastasis in breast cancer cells in vivo [31]. In this study, we evaluated whether serum sCLU levels could be used as a biomarker for the diagnosis and evaluating treatment responses in breast cancer patients with higher sensitivity and specificity.
Materials and Methods
Patients and serum samples
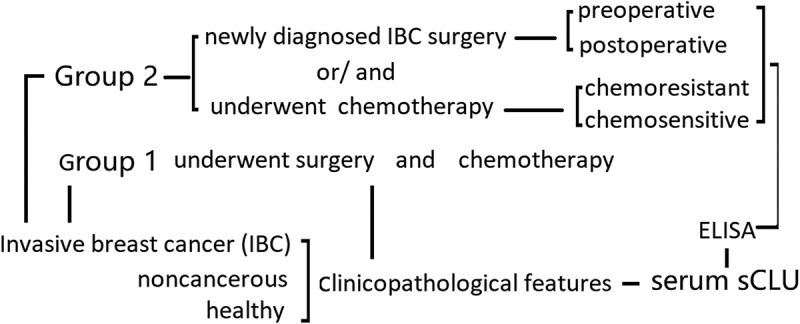
The patients with invasive breast cancer (IBC), noncancerous patients and healthy controls were collected between Jan 2012 and March 2014. The patients were divided into two groups: the first group was the control group and the IBC patients that underwent surgery or chemotherapy. Among them,1998 patients with pathologically confirmed IBC, 180 patients with pathologically confirmed noncancerous breast lesions and 170 healthy controls. The clinicopathological parameters were evaluated. The patients without pathologic diagnosis or with multiple cancers, or with adjuvant radiotherapy, or lost after operation were not included in the study. Plasma samples were collected and stored at −20°C from all participants. The clinicopathologic characteristics of the IBC patients were summarized in (Table 1).
Table 1.
Clinicopathologic characteristics of patients with invasive breast cancer
| Characteristic | Static group (n = 1998) | Dynamic group (n = 650) |
|
|---|---|---|---|
| Surgery (n = 160) | Chemotherapy (n = 490) | ||
| Age (years) | |||
| <50 | 923 | 73 | 178 |
| ≥50 | 1075 | 87 | 312 |
| Tumor size (Diameter) | |||
| ≤ 2 cm | 288 | 18 | 36 |
| >2 cm≤5 cm | 1350 | 106 | 370 |
| >5 cm | 360 | 36 | 84 |
| Histopathological Grades | |||
| G1 | 230 | 19 | 61 |
| G2 | 1478 | 121 | 348 |
| G3 | 290 | 20 | 81 |
| Clinical Stages | |||
| I | 208 | 14 | 41 |
| II | 970 | 80 | 231 |
| III | 820 | 66 | 218 |
| Lymph metastasis | |||
| Yes | 1180 | 94 | 316 |
| No | 818 | 66 | 174 |
| Distant metastasis | |||
| Yes | 115 | 19 | 64 |
| No | 1883 | 141 | 426 |
| Subtype | |||
| Luminal A | 320 | 21 | 59 |
| Luminal B | 1111 | 92 | 293 |
| HER2 (+) | 211 | 16 | 48 |
| Triple negative | 356 | 31 | 90 |
| Menopause | |||
| Yes | 798 | 70 | 216 |
| No | 1200 | 90 | 274 |
| ER | |||
| Positive(+) | 1313 | 102 | 322 |
| Negative(-) | 685 | 58 | 168 |
| PR | |||
| Positive(+) | 1017 | 91 | 286 |
| Negative(-) | 981 | 69 | 204 |
sThe second groups were the newly diagnosed IBC patients (n = 650), of which 160 patients received surgery and 490 patients underwent chemotherapy. For surgical patients, serum samples were collected 24 h before surgery, 48 h-72 h days after surgery, respectively. For chemotherapy patients, the baseline serum samples were collected before the first chemotherapy cycle and subsequent serum samples in any treatment cycle. All patients follow the standard chemotherapy regimen. Two milliliters of whole blood was collected and centrifuged and then stored at −20°C. This study was approved by the affiliated Hospital of Qingdao University. Written informed consent was obtained from each individual or patient.
ELISA detection of serum sCLU
Serum sCLU protein was detected and calculated using an ELISA kit (Boster Biotechnology Company, Wuhan, China). The procedures were carried out according to the introduction of the manufacturer. All serum were stored at −80°C before measurements were taken, and both the standards and samples were run in triplicate. The OD450 was calculated by subtracting the background value, and standard curves were plotted using the CurveExpert 1.3 software program. Each sample was repeated at least 3 times.
Statistical analysis
Statistical analyses were performed using SPSS.22 software. The t-test or the rank-sum test was used to evaluate the statistical differences. The Youden index, calculated as (sensitivity + specificity-1) was estimated to determine optimal cutoff values. The sensitivity and specificity were compared using receiver operating characteristic (ROC) curves. The associations between disease-free survival and overall survival and sCLU levels were estimated by the Kaplan–Meier, log-rank test, and Cox proportional hazard regression models. P < 0.05 was considered significant.
Results
Plasma levels of sCLU in IBC patients
The serum sCLU was assessed using ELISA in 1998 IBC patients,180 noncancerous patients and 170 healthy controls. The data showed that the serum sCLU protein levels were significantly higher in patients with IBC (79.6 ± 11.5) μg/mL than those of the healthy controls (10.4 ± 4.8) μg/mL and noncancerous controls (19.5 ± 5.6) μg/mL (P < 0.0001, respectively), indicating that serum sCLU protein levels could be used as a biomarker for the IBC diagnosis.
Serum sCLU levels and clinicopathological features in IBC patients
In the first cohort of 1998 participants, sCLU level was higher in IBC patients with higher clinical stages (III)(92.6 μg/mL ± 13.8 μg/mL) and lymph metastasis (89.7 μg/mL ± 12.6 μg/mL) compared to the lower clinical stages (I+ II) (56.4 μg/mL ± 12.8 μg/mL) and without lymph metastasis (60.4 μg/mL ± 11.8 μg/mL) (Table 2, P < 0.0001, respectively). No relationship was found between levels of serum sCLU and age, tumor size, grade, distant metastasis, subtype, menopause, etc. (Table 2). These observations indicated that the progression of IBC is associated with increased serum sCLU levels.
Table 2.
Relationship between sCLU levels and clinicopathological factors in 1998 IBC patients
| Group | sCLU level (μg/mL) | P-value |
|---|---|---|
| Age (years) | 0.089 | |
| <50 | 78.9 ± 11.6 | |
| ≥50 | 81.3 ± 11.7 | |
| Tumor size | 0.163 | |
| ≤ 2 cm | 77.7 ± 10.3 | |
| >2 cm | 80.4 ± 11.5 | |
| Grade | 0.251 | |
| G1-G1 | 78.3 ± 12.4 | |
| G3 | 80.6 ± 11.8 | |
| Clinical Stages | <0.0001 | |
| I–II | 56.4 ± 12.8 | |
| III | 92.6 ± 13.8 | |
| Lymph metastasis | <0.0001 | |
| Yes | 89.7 ± 12.6 | |
| No | 60.4 ± 11.8 | |
| Distant metastasis | 0.173 | |
| Yes | 82.6 ± 13.5 | |
| No | 78.4 ± 12.7 | |
| Subtype | 0.494 | |
| Luminal A | 78.5 ± 12.7 | |
| Luminal B | 77.6 ± 13.1 | |
| HER2 (+) | 81.6 ± 13.6 | |
| Triple negative | 82.6 ± 12.7 | |
| Menopause | 0.560 | |
| Yes | 79.0 ± 12.5 | |
| No | 79.8 ± 12.2 | |
| ER | 0.137 | |
| Positive(+) | 81.6 ± 12.5 | |
| Negative(-) | 78.4 ± 12.5 | |
| PR | 0.382 | |
| Positive(+) | 78.4 ± 11.8 | |
| Negative(-) | 80.9 ± 12.6 |
We also found that serum sCLU protein levels >18.46 μg/mL would predict IBC patients with 86.26% sensitivity and 73.46% specificity. The AUC was 0.87 (Figure 1). Log-rank test of OS curves revealed that high serum sCLU levels were associated with the shorter OS (P = 0.032, Figure 2a) and DFS in IBC patients (P = 0.029, Figure 2b).
Figure 1.
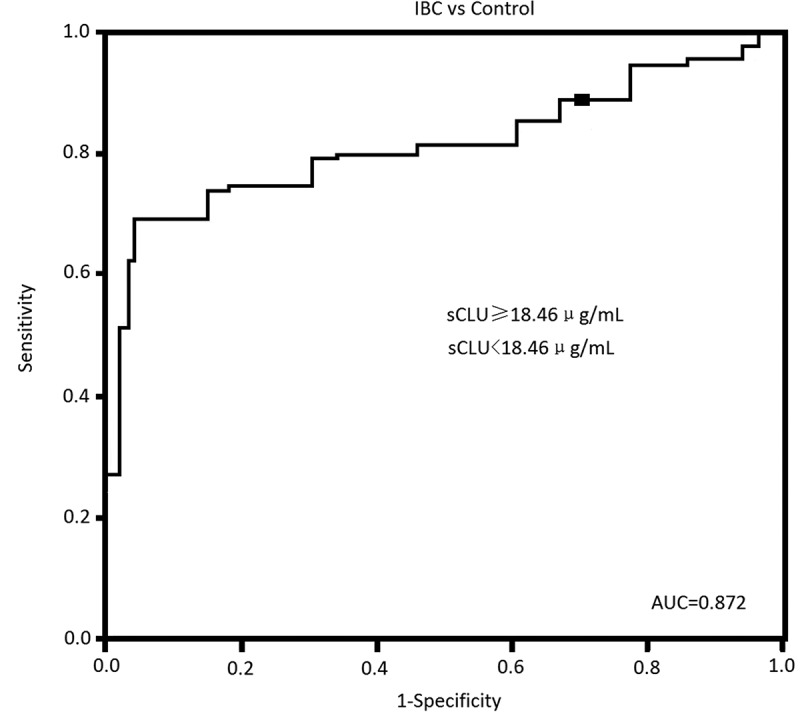
Serum sCLU levels in IBC and controls. Serum sCLU yielded an AUC value of 0.87 in distinguishing IBC from controls
Figure 2.
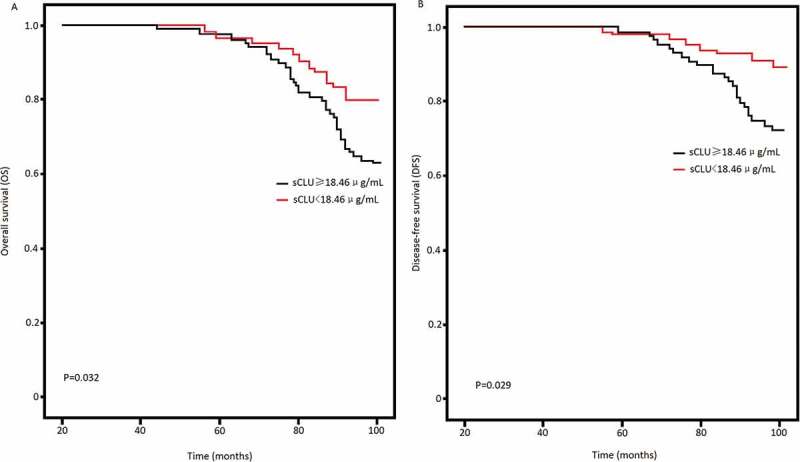
Kaplan-Meier survival curves. Percent survival rate was stratified by serum sCLU level. A, overall survival of patients;B, progression-free survival
Detection of serum sCLU levels in newly diagnosed IBC patients
Using cutoff value 18.46 μg/mL as the positive value for surgical patients, a statistically significant association was observed between preoperative and postoperative serum sCLU levels (63.65 μg/mL vs. 35.14 μg/mL, P = 0.003; Figure 3a). In addition, serum sCLU levels were significantly higher in the chemoresistant patients than the chemosensitive patients (673.4 μg/mL vs. 292.7 μg/mL, p < 0.001, Figure 3 B).
Figure 3.
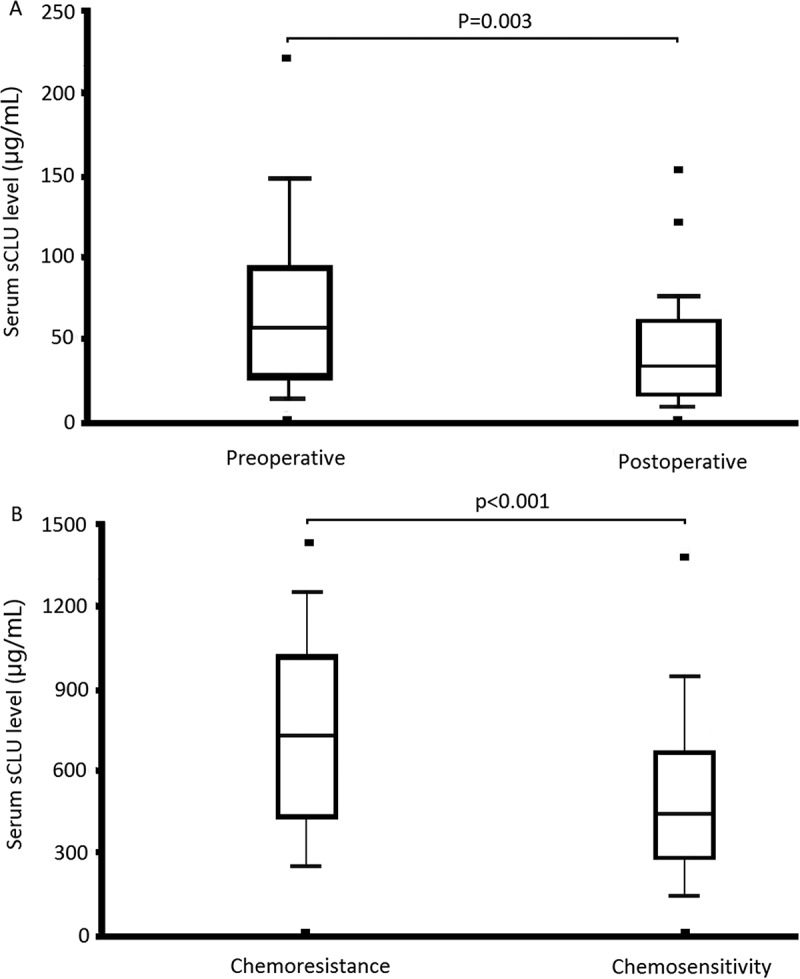
ELISA detection of serum sCLU levels. A, Surgical patients; B, Chemotherapy patients
Discussion
In this study, we first detected the serum sCLU protein levels in IBC patients, noncancerous patients, and healthy controls and assess its diagnostic value and treatment responses in patients with IBC. The results showed that enhanced sCLU protein levels were found in patients with IBC than that in controls. Using 18.46 μg/mL as the cutoff value, the sCLU had a higher sensitivity and specificity in diagnosis of IBC, indicating that serum sCLU protein levels could be as a biomarker for the diagnosis of IBC.
Clusterin encodes a 449 amino acid precursor polypeptide which is cleaved to form the mature secreted clusterin protein which functions to scavenge and chaperone damaged proteins in the extracellular compartment of tissues [18,19]. Other functional variants of clusterin are generated by alternative splicing of the mRNA, producing a cytoplasmic variant, which can translocate to the nucleus and influence cell survival [19]. Clusterin is not detected in normal tissue, but overexpressed in many cancer tissues or cells [20–23]; therefore, the apparent ubiquitous presence of clusterin has the potential for it to be used as a biomarker for health and in disease. Previous reports have correlated clusterin expression with cell responses to stress [32], cell damage recovery [33], senescence [34], tumorigenesis and apoptosis [35]. Up-regulation of clusterin in breast cancers has been previously reported, but whether serum sCLU is enhanced still unknown. A previous study has reported that reduced sCLU might reflect poor outcomes of biliary atresia patients and have potential as a novel biomarker for the disease severity following Kasai-operation [36]. In human colon cancer, significant increase of sCLU in the serum of CRC patients as compared with controls; in addition, sCLU in blood showed a 55.6% sensitivity and 100% specificity [37]. In HCC and prostate Cancer patients, serum clusterin was significantly elevated, suggesting that serum sCLU is a potential novel serum marker for HCC and prostate Cancer [38,39]. In this report, we have demonstrated that serum sCLU levels were higher in IBC patients, especially in advanced IBC (stage III) and with lymph metastasis compared to the early-stage (stage I–II) and without lymph metastasis. Furthermore, the patients with higher sCLU levels have short survival than the patients with lower sCLU levels, suggesting that serum sCLU could be a useful prognostic marker in IBC. Expression patterns of clusterin at different stages indicate that this protein functions in the maintenance and/or progression of tumors, but it is not clear whether it plays a role in tumor establishment.
Elevated production of clusterin antigen, if secreted from tumor cells, may be detected in body fluids, such as serum. Therefore, serum sCLU could decrease after resection of tumors containing high expression of clusterin antigen. In our study, serum levels of sCLU protein showed a statistically significant difference between preoperative and postoperative patients, and lower serum levels of sCLU protein were shown in the postoperative patients than the preoperative patients. These data indicated that serum levels of sCLU protein could predict the surgery responses of patients with IBC.
sCLU expression is documented to lead to broad-based resistance to anticancer agents and other unrelated chemotherapeutic agents, and targeted death-inducing molecules, tumor necrosis factor, Fas and TRAIL, or histone deacetylase inhibitors can also be mediated by sCLU [40]. Mechanistically sCLU could mediate multidrug resistance to a broad range of unrelated chemotherapeutic agents. sCLU is also readily induced by therapeutic agents and confers survival advantage to cancer cells [41]. Therefore, sCLU ablation via specific antisense oligonucleotides as a means to chemosensitization toward clinically established drugs could be a potent strategy to overcome drug resistance. Serum sCLU levels could be effectively reduced with effective chemotherapeutic agents [42]. In the study, we found that serum sCLU protein was higher in the chemoresistant patients compared to the chemosensitive patients. These data demonstrated that serum sCLU levels could predict the chemotherapy responses in patients with IBC. However, some limitations exist in the study that some clinicopathologic data were not enough.
Conclusion
The study aimed to investigate the relationship between serum secreted clusterin (sCLU) protein and clinicopathological characteristics and chemotherapy sensitivity evaluation in IBC. It was demonstrated that the serum sCLU protein level was significantly higher in IBC patients, and strongly correlated with higher clinical tumor stage, lymph node metastasis, shorter overall survival and disease-free survival (DFS). Serum sCLU protein was also higher in the chemoresistant IBC patients. Therefore, detection of serum of sCLU could help to predict prognosis and monitor the treatment responses. However, there are a number of challenges to overcome in order to successfully translate these promising laboratory results into efficacious therapies in clinical practice.
Supplementary Material
Disclosure statement
No potential conflict of interest was reported by the authors.
Highlights
Serum sCLU level was higher in patients with invasive breast cancer
Higher serum sCLU level was related to high tumor stage and lymph metastasis
Lower serum sCLU level was observed in the postoperative patients
Higher serum sCLU level was observed in the chemoresistant patients
Supplementary material
Supplemental data for this article can be accessed here.
References
- [1].Fan L, Strasser-Weippl K, Li JJ, et al. Breast cancer in China. Lancet Oncol. 2014;15(7):e279–289. [DOI] [PubMed] [Google Scholar]
- [2].Itani N, Grogan N, Mott S, et al. Metastatic presentations of previously treated early-stage breast cancer patients and association with survival. Clin Breast Cancer. 2019;3:S1526-8209:30735–30739. DOI: 10.1016/j.clbc.2019.11.004 [DOI] [PubMed] [Google Scholar]
- [3].Skuli SJ, Sheng JY, Bantug ET, et al. Survivorship care visits in a high-risk population of breast cancer survivors. Breast Cancer Res Treat. 2019;173(3):701–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Early Breast Cancer Trialists’ Collaborative G . Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-y survival: an overview of the randomised trials. Lancet. 2005;365(9472):1687–1717. . [DOI] [PubMed] [Google Scholar]
- [5].Cardoso F, Kyriakides S, Ohno S, et al. ESMO guidelines committee. early breast cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2019;30:1674. [DOI] [PubMed] [Google Scholar]
- [6].Ikubo A, Matsufuji S, Morifuji Y, et al. clinical features, prognosis, diagnostic approaches and treatment of multiple primary malignancies in the digestive system. Anticancer Res. 2019;39(12):6863–6870. [DOI] [PubMed] [Google Scholar]
- [7].Burton C, Ma Y.. Current trends in cancer biomarker discovery using urinary metabolomics: achievements and new challenges. Curr Med Chem. 2019;26(1):5–28. [DOI] [PubMed] [Google Scholar]
- [8].Ryška A. Molecular pathology in real time. Cancer Metastasis Rev. 2016;35(1):129–140. [DOI] [PubMed] [Google Scholar]
- [9].Lipton A, Leitzel K, Ali SM, et al. Human epidermal growth factor receptor 2 (HER2) extracellular domain levels are associated with progression-free survival in patients with HER2-positive metastatic breast cancer receiving lapatinib monotherapy. Cancer. 2011;117(21):5013–5020. [DOI] [PubMed] [Google Scholar]
- [10].Kjaer IM, Bechmann T, Brandslund I, et al. Prognostic and predictive value of EGFR and EGFR-ligands in blood of breast cancer patients: a systematic review. Clin Chem Lab Med. 2018;56(5):688–701. [DOI] [PubMed] [Google Scholar]
- [11].Finn RS, Gagnon R, Di Leo A, et al. Prognostic and predictive value of HER2 extracellular domain in metastatic breast cancer treated with lapatinib and paclitaxel in a randomized phase III study. J Clin Oncol. 2009;27(33):5552–5558. [DOI] [PubMed] [Google Scholar]
- [12].Joyce DP, Kerin MJ, Dwyer RM. Exosome-encapsulated microRNAs as circulating biomarkers for breast cancer.Int. J Cancer. 2016;139(7):1443–1448. [DOI] [PubMed] [Google Scholar]
- [13].Duffy MJ, Walsh S, McDermott EW, et al. Biomarkers in breast cancer: where are we and where are we going? Adv Clin Chem. 2015;71:1–23. [DOI] [PubMed] [Google Scholar]
- [14].Rizzi F, Coletta M, Bettuzzi S. Chapter 2: clusterin (CLU): from one gene and two transcripts to many proteins. Adv Cancer Res. 2009;104:9–23. [DOI] [PubMed] [Google Scholar]
- [15].Moretti RM, Montagnani Marelli M, Mai S, et al. Clusterin isoforms differentially affect growth and motility of prostate cells: possible implications in prostate tumorigenesis. Cancer Res. 2007;67(21):10325–10333. [DOI] [PubMed] [Google Scholar]
- [16].Xiu P, Xu Z, Liu F, et al. Downregulating sCLU enhances the sensitivity of hepatocellular carcinoma cells to gemcitabine by activating the intrinsic apoptosis pathway. Dig Dis Sci. 2014;59(8):1798–1809. [DOI] [PubMed] [Google Scholar]
- [17].Shepherd CE, Affleck AJ, Bahar AY, et al. Intracellular and secreted forms of clusterin are elevated early in Alzheimer’s disease and associate with both Aβ and tau pathology. Neurobiol Aging. 2019;19:30389–30386. [DOI] [PubMed] [Google Scholar]
- [18].Kim N, Choi WS. Proapoptotic role of nuclear clusterin in brain. Anat Cell Biol. 2011;44(3):169–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Pucci S, Polidoro C, Joubert A, et al. Ku70, ku80, and sclusterin: a cluster of predicting factors for response to neoadjuvant chemoradiation therapy in patients with locally advanced rectal cancer. Int J Radiat Oncol Biol Phys. 2017;97(2):381–388. [DOI] [PubMed] [Google Scholar]
- [20].Peng M, Deng J, Zhou S, et al. The role of clusterin in cancer metastasis. Cancer Manag Res. 2019;11:2405–2414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Henderson-Jackson EB, Nasir A, Chen DT, et al. cytoplasmic clusterin expression correlates with pancreatic neuroendocrine tumor size and pathological stage. Pancreas. 2013;42(6):967–970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Zoubeidi A, Chi K, Gleave M. Targeting the cytoprotective chaperone, clusterin, for treatment of advanced cancer. Clin Cancer Res. 2010;16(4):1088–1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Yang GF, Li XM, Xie D. Overexpression of clusterin in ovarian cancer is correlated with impaired survival. Int J Gynecol Cancer. 2009;19(8):1342–1346. [DOI] [PubMed] [Google Scholar]
- [24].Pins MR, Fiadjoe JE, Korley F, et al. Clusterin as a possible predictor for biochemical recurrence of prostate cancer following radical prostatectomy with intermediate Gleason scores: a preliminary report. Prostate Cancer Prostatic Dis. 2004;7(3):243–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Pucci S, Bonanno E, Sesti F, et al. Clusterin in stool: a new biomarker for colon cancer screening? Am J Gastroenterol. 2009;104(11):2807–2815. [DOI] [PubMed] [Google Scholar]
- [26].Hazzaa SM, Elashry OM, Afifi IK. Clusterin as a diagnostic and prognostic marker for transitional cell carcinoma of the bladder. Pathol Oncol Res. 2010;16(1):101–109. [DOI] [PubMed] [Google Scholar]
- [27].Rodríguez-Piñeiro AM, García-Lorenzo A, Blanco-Prieto S, et al. Secreted clusterin in colon tumor cell models and its potential as diagnostic marker for colorectal cancer. Cancer Invest. 2012;30(1):72–78. [DOI] [PubMed] [Google Scholar]
- [28].Ma J, Gao W, Gao J. sCLU as prognostic biomarker and therapeutic target in osteosarcoma. Bioengineered. 2019;10(1):229–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Niu ZH, Wang Y, Chun B, et al. Secretory clusterin (sCLU) overexpression is associated with resistance to preoperative neoadjuvant chemotherapy in primary breast cancer. Eur Rev Med Pharmacol Sci. 2013;17(10):1337–1344. [PubMed] [Google Scholar]
- [30].Wang Y, Wang X, Zhao H, et al. Clusterin confers resistance to TNF-alpha-induced apoptosis in breast cancer cells through NF-kappaB activation and Bcl-2 overexpression. J Chemother. 2012;24(6):348–357. [DOI] [PubMed] [Google Scholar]
- [31].Niu Z, Li X, Hu B, et al. Small interfering RNA targeted to secretory clusterin blocks tumor growth, motility, and invasion in breast cancer. Acta Biochim Biophys Sin. 2012;44(12):991–998. [DOI] [PubMed] [Google Scholar]
- [32].Janiszewska E, EM K. Could the glycosylation analysis of seminal plasma clusterin become a novel male infertility biomarker? Mol Reprod Dev. 2020;87(5):515–524. [DOI] [PubMed] [Google Scholar]
- [33].Guo J, Guan Q, Liu X, et al. Relationship of clusterin with renal inflammation and fibrosis after the recovery phase of ischemia-reperfusion injury. BMC Nephrol. 2016;17(1):133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Zhang Y, Zhang Y, Xiao Y, et al. Expression of Clusterin suppresses Cr(VI)-induced premature senescence through activation of PI3K/AKT pathway. Ecotoxicol Environ Saf. 2019;183:109465. [DOI] [PubMed] [Google Scholar]
- [35].Tellez T, Garcia-Aranda M, Redondo M. The Role of Clusterin in Carcinogenesis and its Potential Utility as Therapeutic Target. Curr Med Chem. 2016;23(38):4297–4308. [DOI] [PubMed] [Google Scholar]
- [36].Udomsinprasert W, Poovorawan Y, Chongsrisawat V, et al. Decreased circulating clusterin reflects severe liver complications after hepatoportoenterostomy of biliary atresia. Sci Rep. 2020;10(1):19736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Pucci S, Bonanno E, Sesti F, et al. Clusterin in stool: a new biomarker for colon cancer screening? Am J Gastroenterol. 2009;104(11):2807–2815. [DOI] [PubMed] [Google Scholar]
- [38].Kimura A, Sogawa K, Satoh M, et al. The application of a three-step serum proteome analysis for the discovery and identification of novel biomarkers of hepatocellular carcinoma. Int J Proteomics. 2012;2012:623190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Čapoun O, Soukup V, Kalousová M, et al. Diagnostic importance of selected protein serum markers in the primary diagnostics of prostate cancer. Urol Int. 2015;95(4):429–435. [DOI] [PubMed] [Google Scholar]
- [40].Djeu JY, Wei S. Clusterin and chemoresistance. Adv Cancer Res. 2009;105:77–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Hoeller C, Pratscher B, Thallinger C, et al. Clusterin regulates drug-resistance in melanoma cells. J Invest Dermatol. 2005;124(6):1300–1307. [DOI] [PubMed] [Google Scholar]
- [42].Chi KN, Chin JL, Winquist E, et al. Multicenter phase II study of combined neoadjuvant docetaxel and hormone therapy before radical prostatectomy for patients with high risk localized prostate cancer.J. Urol. 2008;180(2):565–570. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


