Abstract
INTRODUCTION:
The association between nonalcoholic fatty liver disease (NAFLD) and colorectal cancer (CRC) has been controversial. Using the new consensus-driven definition, we evaluated the association of metabolic dysfunction–associated fatty liver disease (MAFLD) with the risk of developing CRC.
METHODS:
From a nationwide health screening database, we included 8,933,017 participants (48.6% male) aged 40–64 years between 2009 and 2010. Participants were categorized by the presence of fatty liver disease (FLD)—NAFLD and MAFLD, separately—and by the combination of the 2 definitions: neither FLD, NAFLD only, MAFLD only, or both FLD. The primary outcome was the development of CRC.
RESULTS:
Among the participants, 2,517,330 (28.2%) had NAFLD, and 3,337,122 (37.4%) had MAFLD, whereas 2,465,151 (27.6%) met both NAFLD and MAFLD definitions. Over a median follow-up period of 10.1 years, 60,888 new CRC cases developed. NAFLD and MAFLD were each associated with a significantly higher risk of developing CRC. When the neither FLD group was the reference, multivariable-adjusted hazard ratios (95% confidence interval) for CRC were 1.16 (1.06–1.28) in the NAFLD only group, 1.18 (1.16–1.20) in the both FLD group, and 1.32 (1.28–1.35) in the MAFLD only group. The presence of advanced liver fibrosis further increased CRC risk in each FLD group.
DISCUSSION:
FLD was associated with a higher risk of CRC development. CRC risk was higher in the presence of MAFLD, especially when accompanied by liver fibrosis.
INTRODUCTION
Nonalcoholic fatty liver disease (NAFLD) often carries metabolic dysfunction such as type 2 diabetes mellitus (T2DM) and obesity, which are significantly associated with increased risks of cardiovascular events and cancers (1). Extrahepatic malignancy is the second leading cause of death, after cardiovascular disease, among patients with NAFLD (2). In a recent T2DM cohort study, NAFLD was an independent risk factor for mortality from any type of malignancy (3).
Colorectal cancer (CRC) is among the most common malignancies globally (4). CRC and NAFLD share common risk factors, such as obesity, metabolic syndrome, and T2DM (5). However, the association between NAFLD and CRC remains controversial. In a Chinese cross-sectional study, NAFLD was associated with 1.87 times higher odds of having CRC (6). A Korean cohort study also confirmed that women with NAFLD were at 3.08 times higher risk for developing CRC (7). In contrast, other studies reported null or inverse associations between NAFLD and CRC (8,9), wherein 1 study even suggested that NAFLD prolonged overall survival among patients with CRC (9). NAFLD exhibits a wide disease spectrum ranging from simple steatosis to nonalcoholic steatohepatitis (NASH) or cirrhosis. CRC risk may be particularly higher in NAFLD with advanced fibrosis than without (10). Accordingly, the association between NAFLD and CRC risk may differ according to proportions of simple steatosis, with a favorable clinical course (11), and NASH or cirrhosis, being the most progressive and severe conditions (12). This heterogeneity may invoke controversy regarding the NAFLD-CRC association.
Recently, a new definition was proposed for metabolic dysfunction-associated fatty liver disease (MAFLD) (13,14). In contrast to the NAFLD criteria, the diagnosis of MAFLD is based on the presence of metabolic dysfunction among people with fatty liver. Although further validation is desirable, the transition from NAFLD to MAFLD may facilitate identification of metabolically complicated fatty liver superimposed on other causes of chronic liver diseases such as viral hepatitis or alcoholism. Therefore, one may hypothesize that MAFLD has a stronger association with CRC than conventional NAFLD. Using a nationwide health screening database, we investigated whether fatty liver disease (FLD)―NAFLD or MAFLD―was associated with CRC risk and how the change from NAFLD to MAFLD would impact CRC risk stratification.
METHODS
Data source
We used a nationwide health information database provided by the National Health Insurance Service (NHIS), which includes deidentified medical claim records for the entire South Korean population. The NHIS is the single provider of universal health care coverage in Korea. The NHIS database contains sociodemographic details, reimbursement claims with International Classification of Diseases, 10th revision (ICD-10) coding, health checkup results, and death information (15). This data source was described in previous studies (16,17). The current study protocol was approved by the Institutional Review Board of Yonsei University Health System, Seoul, Korea (#Y-2020-0133). Informed consent was waived, as this was a retrospective study of deidentified, routinely collected data.
Study population
We identified 10,186,076 adults aged 40–64 years who underwent routine NHIS health examinations between 2009 and 2010. If a participant had multiple examinations during this period, the first record was used as the baseline. After excluding participants with incomplete information (n = 601,677), those with previous cancer or inflammatory bowel disease before the baseline (n = 645,643), and those who developed CRC or died within 60 days of follow-up (n = 5,739), a final analytical sample of 8,933,017 participants was achieved.
Key variables and outcome
Clinical and biochemical measurements and responses to lifestyle questionnaires were collected during routine biennial health examinations provided to all Korean adults. Health examination centers were designated and quality controlled according to relevant national laws and regulations. The details of health examinations have been described elsewhere (15). The collected variables included body mass index, waist circumference, blood pressure, blood glucose, lipids, liver enzymes, tobacco use, alcohol consumption, and exercise frequency. T2DM, hypertension, and dyslipidemia (see Table S1, Supplementary Digital Content 1, http://links.lww.com/CTG/A735) were defined according to Korean guidelines (18–20). Medication use, concomitant liver disease, and Charlson Comorbidity Index (21) were determined from insurance claims data during a 2-year look-back period from the baseline.
Hepatic steatosis was assessed using the fatty liver index (FLI) detailed in (see Table S2, Supplementary Digital Content 1, http://links.lww.com/CTG/A735). The use of FLI for large epidemiologic studies was supported by the European Clinical Practice Guidelines. FLI was validated in the Korean population with an area under the receiver operating characteristic curve of 0.87, although the cutoff should be lower than that used in Western populations (22). The lower cutoff of FLI ≥30 was used in this study. Other validated steatosis models (22)—namely, hepatic steatosis index (HSI ≥36) and simple NAFLD score (SNS ≥8)—were used in sensitivity analyses.
NAFLD was defined as the presence of hepatic steatosis without excessive alcohol consumption (≥210 g/wk in men; ≥140 g/wk in women) or concomitant liver disease (see Table S1, Supplementary Digital Content 1, http://links.lww.com/CTG/A735). MAFLD was defined as the presence of hepatic steatosis with 1 or more of the following: (1) overweight or obese (BMI ≥23 kg/m2; by the Asia-Pacific criteria); (2) T2DM; or (3) at least 2 metabolic abnormalities described in (see Supplementary Method, Table S1, http://links.lww.com/CTG/A735) (14). Participants were initially classified by the presence of FLD according to each definition. Subsequently, for comparison between NAFLD and MAFLD, participants were further categorized into 4 (2 × 2 combination) mutually exclusive groups: neither FLD, NAFLD only, MAFLD only, or both FLD. Among participants with FLD, the presence of advanced liver fibrosis was determined by a BARD score ≥2, as described in (see Supplementary Method, Table S1, http://links.lww.com/CTG/A735).
The primary outcome was CRC development, defined as the first hospitalization with ICD-10 codes C18-C21 (23), recorded on or before December 31, 2019. Participants who did not have any event were censored at the date of death, last follow-up, or December 31, 2019, whichever came first. Death was ascertained by linkage to the national registry via resident registration numbers.
Statistical analysis
Baseline characteristics were reported as frequency and percentage or median and interquartile range. Incidence rates were calculated as the number of events per 100,000 person-years of follow-up. The cumulative incidence of CRC was estimated using the Kaplan-Meier method. Hazard ratios (HRs) and 95% confidence intervals (CIs) were calculated using Cox proportional hazards models. The proportionality of hazards was confirmed via graphical inspection of log-minus-log plots and Schoenfeld residuals. HRs were adjusted for age, sex, household income quartile, residential area, Charlson Comorbidity Index, aspirin use, nonsteroidal anti-inflammatory drug use, tobacco smoking, and exercise frequency. Covariables were selected a priori on the basis of possible associations with FLD and CRC (24,25). The presence of overweight/obesity, diabetes, hypertension, and dyslipidemia, as well as alcohol intake and other liver diseases, represents an intrinsic difference between the definitions of NAFLD and MAFLD. As such, these factors were not adjusted for in the comparison between NAFLD and MAFLD.
The following sensitivity analyses were conducted. First, we tested whether the association between MAFLD and the CRC risk differed when patients were stratified by age (in 5-year intervals), sex, and other risk factors. Second, different biochemical models for the assessment of hepatic steatosis (i.e., hepatic steatosis index ≥36 and simple NAFLD score ≥8) were used to repeat our main analyses. Third, a lower threshold of ≥1 instead of ≥2 metabolic abnormalities for lean/nondiabetic MAFLD (see Supplementary Method, Supplementary Table S1, http://links.lww.com/CTG/A735) was used to relieve stringency, considering the lack of data for fasting insulin and C-reactive protein levels in our cohort (26). Fourth, we tested the association between MAFLD and CRC after additionally adjusting for alcohol intake, concomitant liver diseases, and metabolic risk factors. The association persisted when propensity score weighting was used to balance all covariates between the MAFLD and non-MAFLD groups (27). Analyses were conducted using SAS version 9.4 (SAS Institute Cary, NC) and R version 3.5.3 (R Foundation for Statistical Computing, Vienna, Austria).
RESULTS
Baseline characteristics
The study included 8,933,017 participants (median age, 50 years; 48.6% male), among whom 2,517,330 (28.2%) had NAFLD, and 3,337,122 (37.4%) had MAFLD. By either definition, participants with FLD were more likely to be male and wealthy, live in a rural area, and have other metabolic risk factors (Table 1). By combination of the 2 definitions, 2,465,151 (27.6%) participants met both NAFLD and MAFLD criteria (both FLD), whereas 52,179 (0.6%) and 871,971 (9.8%) participants met only NAFLD or MAFLD definition (i.e. NAFLD only and MAFLD only), respectively (see Supplementary Method, Supplementary Table S2, http://links.lww.com/CTG/A735, Supplementary Material). Participants in the NAFLD only group were lean, nondiabetic, and less likely to have other comorbidities or use aspirin/NSAIDs regularly than those with MAFLD. Participants in the MAFLD only group had concomitant liver disease or consumed alcohol excessively but otherwise had similar characteristics compared with those in the both FLD group.
Table 1.
Baseline characteristics by the presence of fatty liver disease (FLD)
| Variable | NAFLD | MAFLD | ||
| No | Yes | No | Yes | |
| N = 6,415,687 | N = 2,517,330 | N = 5,595,895 | N = 3,337,122 | |
| Age, yr | 50 [44–56] | 51 [46–57] | 50 [44–56] | 51 [45–57] |
| Sex | ||||
| Female | 3,754,082 (58.5) | 834,565 (33.2) | 3,652,353 (65.3) | 936,294 (28.1) |
| Male | 2,661,605 (41.5) | 1,682,765 (66.8) | 1,943,542 (34.7) | 2,400,828 (71.9) |
| Household incomea | ||||
| Q4, highest | 2,298,689 (35.8) | 954,600 (37.9) | 1,992,955 (35.6) | 1,260,334 (37.8) |
| Q3 | 1,574,034 (24.5) | 648,905 (25.8) | 1,355,583 (24.2) | 867,356 (26.0) |
| Q2 | 1,247,004 (19.4) | 462,451 (18.4) | 1,090,192 (19.5) | 619,263 (18.6) |
| Q1, lowest | 1,295,960 (20.2) | 451,374 (17.9) | 1,157,165 (20.7) | 590,169 (17.7) |
| Residential area | ||||
| Metropolitan | 2,979,725 (46.4) | 1,118,594 (44.4) | 2,626,049 (46.9) | 1,472,270 (44.1) |
| Urban | 2,273,931 (35.4) | 888,115 (35.3) | 1,987,777 (35.5) | 1,174,269 (35.2) |
| Rural | 1,162,031 (18.1) | 510,621 (20.3) | 982,069 (17.5) | 690,583 (20.7) |
| Charlson Comorbidity Index | ||||
| 0 | 4,622,176 (72.0) | 1,803,511 (71.6) | 4,031,969 (72.1) | 2,393,718 (71.7) |
| 1 | 1,594,330 (24.9) | 616,531 (24.5) | 1,399,832 (25.0) | 811,029 (24.3) |
| ≥2 | 199,181 (3.1) | 97,288 (3.9) | 164,094 (2.9) | 132,375 (4.0) |
| Overweight/obese | 3,166,860 (49.4) | 2,319,290 (92.1) | 2,384,773 (42.6) | 3,101,377 (92.9) |
| Diabetes mellitus | 481,376 (7.5) | 388,616 (15.4) | 310,075 (5.5) | 559,917 (16.8) |
| Hypertension | 1,586,277 (24.7) | 1,053,871 (41.9) | 1,169,407 (20.9) | 1,470,741 (44.1) |
| Dyslipidemia | 1,959,970 (30.5) | 1,578,997 (62.7) | 1,440,589 (25.7) | 2,098,378 (62.9) |
| Viral hepatitis | 332,744 (5.2) | 0 | 198,503 (3.5) | 134,241 (4.0) |
| Alcoholic liver diseaseb | 845,461 (13.2) | 0 | 101,676 (1.8) | 743,785 (22.3) |
| Other liver diseasec | 99,698 (1.6) | 0 | 52,426 (0.9) | 47,272 (1.4) |
| Regular aspirin use | 367,040 (5.7) | 270,687 (10.8) | 266,478 (4.8) | 371,249 (11.1) |
| Regular NSAID use | 152,650 (2.4) | 82,196 (3.3) | 132,126 (2.4) | 102,720 (3.1) |
| Alcohol consumption | ||||
| None | 3,677,021 (57.3) | 1,324,109 (52.6) | 3,579,631 (64.0) | 1,421,499 (42.6) |
| Moderate | 1,698,417 (26.5) | 1,193,221 (47.4) | 1,624,713 (29.0) | 1,266,925 (38.0) |
| Excessive | 1,040,249 (16.2) | 0 | 391,551 (7.0) | 648,698 (19.4) |
| Tobacco use | ||||
| Never | 4,322,103 (67.4) | 1,296,374 (51.5) | 4,102,537 (73.3) | 1,515,940 (45.4) |
| Past | 828,374 (12.9) | 520,405 (20.7) | 599,747 (10.7) | 749,032 (22.4) |
| Current | 1,265,210 (19.7) | 700,551 (27.8) | 893,611 (16.0) | 1,072,150 (32.1) |
| Exercise frequency | ||||
| ≥3/wk | 1,658,320 (25.8) | 600,477 (23.9) | 1,440,139 (25.7) | 818,658 (24.5) |
| 1–2/wk | 1,637,481 (25.5) | 724,153 (28.8) | 1,402,965 (25.1) | 958,669 (28.7) |
| None | 3,119,886 (48.6) | 1,192,700 (47.4) | 2,752,791 (49.2) | 1,559,795 (46.7) |
| BARD score ≥2 | NA | 1,712,545 (68.0) | NA | 2,310,900 (69.2) |
| Follow-up, yr | 10.1 [9.4–10.4] | 10.1 [9.4–10.4] | 10.1 [9.4–10.4] | 10.1 [9.4–10.4] |
Values as frequency (%), median [interquartile range], or mean ± standard deviation.
Household income categorized based on quartiles among the entire Korean population.
Diagnosed as alcoholic liver disease or fatty liver with excessive alcohol consumption.
Other liver disease including toxic liver disease, autoimmune hepatitis, biliary cholangitis, Wilson disease, or hemochromatosis.
MAFLD, metabolic dysfunction–associated fatty liver disease; NAFLD, nonalcoholic fatty liver disease; NSAID, nonsteroidal anti-inflammatory drug.
CRC risk according to FLD groups
During a median follow-up period of 10.1 years, 60,888 participants developed CRC. The CRC incidence rate was 85.7 per 100,000 person-years in participants with NAFLD and 63.2 per 100,000 person-years in participants without NAFLD. The incidence rate was 89.9 per 100,000 person-years in those with MAFLD and 57.5 per 100,000 person-years, without MAFLD. The cumulative CRC incidence was higher in participants with FLD by either definition than in those without FLD. When participants were categorized by a combination of the 2 criteria, the cumulative CRC incidence was highest in the MAFLD only group, followed by the both FLD and NAFLD only groups and then by the neither FLD group (Figure 1). After multivariable adjustment, NAFLD and MAFLD were associated with a 1.10 (95% CI, 1.09–1.12) and 1.21 (95% CI, 1.19–1.23) times higher CRC risk, respectively, compared with the absence of each condition (Table 2, Model 3). When the neither FLD group was the reference, HRs (95% CI) for incident CRC were 1.16 (1.06–1.28) in the NAFLD only group, 1.18 (1.16–1.20) in the both FLD group, and 1.32 (1.28–1.35) in the MAFLD only group (Table 2, Model 3).
Figure 1.
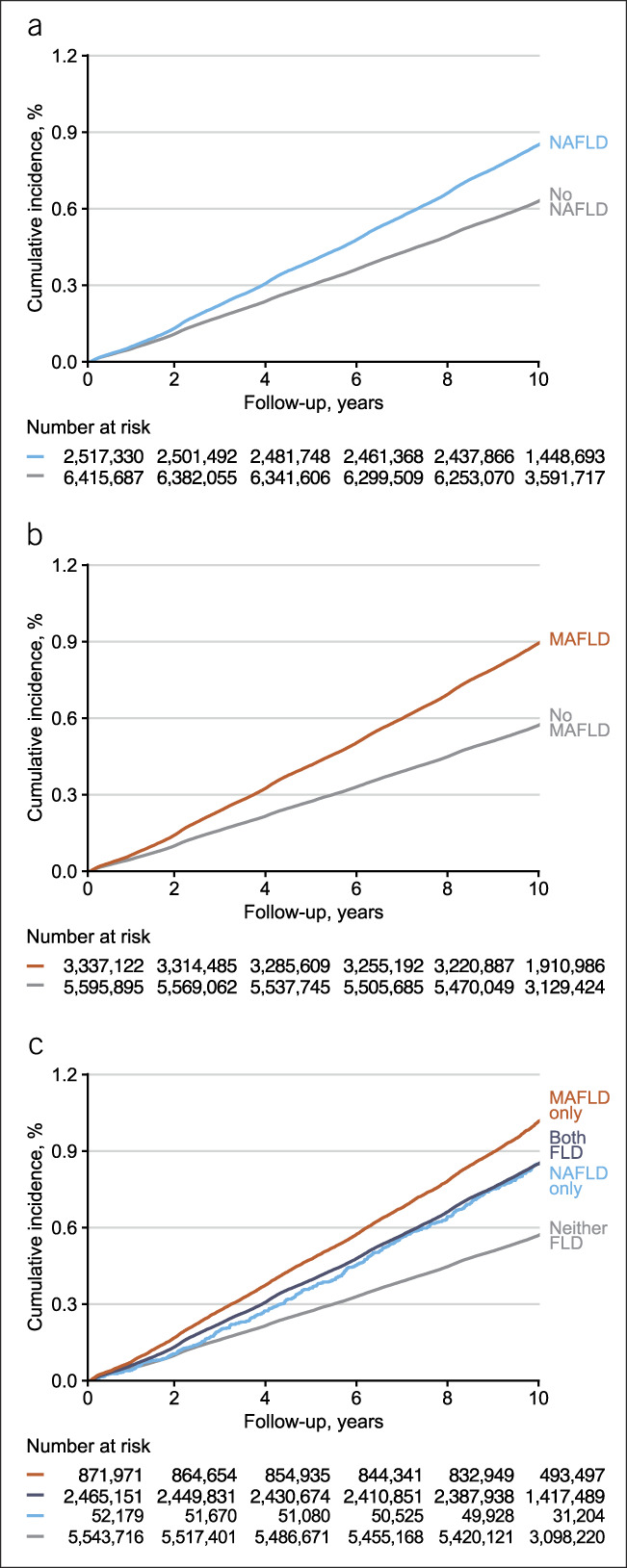
Cumulative colorectal cancer incidence by Kaplan-Meier methods according to the presence and combination of NAFLD and/or MAFLD. (a) Cumulative incidence by the presence of NAFLD. (b) Cumulative incidence by the presence of MAFLD. (c) Cumulative incidence by combination of NAFLD and MAFLD—neither FLD, NAFLD only, MAFLD only, or both FLD. FLD, fatty liver disease; MAFLD, metabolic dysfunction–associated fatty liver disease; NAFLD, nonalcoholic fatty liver disease.
Table 2.
CRC risk according to the presence and combination of NAFLD and/or MAFLD
| Presence of FLD | Events | Person-years | Ratea | Hazard ratio (95% confidence interval) | |||
| Model 1 | Model 2 | Model 3 | Model 4 | ||||
| NAFLD | |||||||
| No | 39,780 | 62,898,545 | 63.2 | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| Yes | 21,108 | 24,624,987 | 85.7 | 1.36 (1.33–1.38) | 1.09 (1.08–1.11) | 1.10 (1.09–1.12) | 1.06 (1.04–1.08) |
| MAFLD | |||||||
| No | 31,603 | 54,940,578 | 57.5 | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| Yes | 29,285 | 32,582,954 | 89.9 | 1.56 (1.54–1.59) | 1.21 (1.19–1.23) | 1.21 (1.19–1.23) | 1.19 (1.17–1.21) |
| Combination | |||||||
| Neither FLD | 31,177 | 54,433,056 | 57.3 | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | — |
| NAFLD only | 426 | 507,522 | 83.9 | 1.47 (1.33–1.61) | 1.20 (1.09–1.32) | 1.16 (1.06–1.28) | — |
| Both FLD | 20,682 | 24,117,464 | 85.8 | 1.50 (1.47–1.52) | 1.17 (1.15–1.19) | 1.18 (1.16–1.20) | — |
| MAFLD only | 8,603 | 8,465,489 | 101.6 | 1.77 (1.73–1.82) | 1.34 (1.31–1.37) | 1.32 (1.28–1.35) | — |
Model 1 was unadjusted.
Model 2 was adjusted for age and sex.
Model 3 was further adjusted for household income quartile, residential area, Charlson Comorbidity Index, aspirin use, nonsteroidal anti-inflammatory drug use, tobacco smoking, and exercise frequency.
For the NAFLD analyses, Model 4 was adjusted for overweight/obesity, diabetes, hypertension, and dyslipidemia in addition to Model 3.
For the MAFLD analyses, Model 4 was adjusted for alcohol intake and concomitant liver diseases in addition to Model 3.
Rate per 100,000 person-years.
CRC, colorectal cancer; FLD, fatty liver disease; MAFLD, metabolic dysfunction–associated fatty liver disease; NAFLD, nonalcoholic fatty liver disease.
CRC risk according to FLD and advanced liver fibrosis
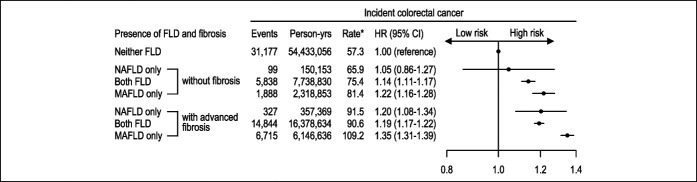
FLD groups were further subcategorized by the presence of advanced liver fibrosis. Participants with FLD and advanced liver fibrosis were at higher CRC risk than those with simple steatosis but without advanced liver fibrosis by either NAFLD or MAFLD definition (see Supplementary Method, Supplementary Table S3, http://links.lww.com/CTG/A735, Supplementary Material). With or without fibrosis, the both FLD and MAFLD only groups were associated with a significantly higher CRC risk compared with the neither FLD group (Figure 2). The presence of advanced fibrosis in these FLD groups was associated with a greater CRC risk. The NAFLD only group without advanced liver fibrosis was not associated with elevated CRC risk (HR, 1.05; 95% CI, 0.86–1.27).
Figure 2.
Colorectal cancer risk according to combination of NAFLD/MAFLD with or without advanced fibrosis. *Rate per 100,000 person-years. All models were adjusted for age, sex, household income quartile, residential area, Charlson Comorbidity Index, aspirin use, nonsteroidal anti-inflammatory drug use, tobacco smoking, and exercise frequency. MAFLD, metabolic dysfunction–associated fatty liver disease; NAFLD, nonalcoholic fatty liver disease.
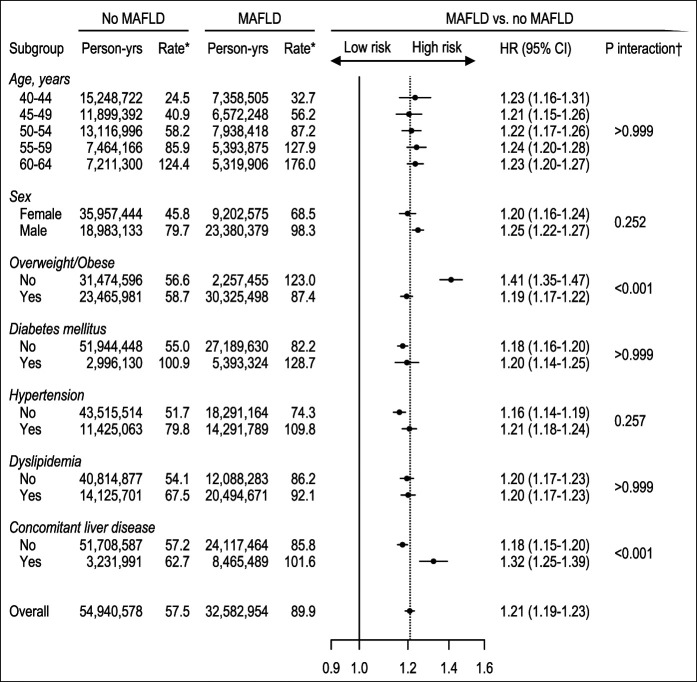
Sensitivity analyses
We conducted the following sensitivity analyses. First, when stratified by age, sex, overweight/obesity, diabetes, hypertension, dyslipidemia, and concomitant liver disease, the association between MAFLD and CRC risk was significant across all subgroups (Figure 3). MAFLD was significantly associated with the CRC risk in patients of all age groups, including those as young as 40–44 years (HR, 1.23; 95% CI, 1.16–1.31). The HRs for incident CRC were particularly higher in subgroups with lean MAFLD (1.41; 95% CI, 1.35–1.47; P < 0.001 for interaction) and concomitant liver disease (1.32; 95% CI, 1.25–1.39; P < 0.001 for interaction). Second, using different biochemical models for the assessment of hepatic steatosis, the both FLD and MAFLD only groups were repeatedly associated with higher CRC risk, whereas the association in the NAFLD only group was inconsistent (see Supplementary Method, Supplementary Table S4, http://links.lww.com/CTG/A735). Third, using the lower threshold of ≥1 instead of ≥2 metabolic abnormalities for lean/nondiabetic MAFLD, the HR for incident CRC was similar in terms of point estimates with that of the original analysis (see Supplementary Method, Supplementary Table S5, http://links.lww.com/CTG/A735, Supplementary Material). Finally, after adjusting for alcohol intake, concomitant liver diseases, and metabolic risk factors in the multivariate model, the association between MAFLD and CRC remained significant (HR, 1.16; 95% CI, 1.13–1.18; see Supplementary Method, Supplementary Table S6, http://links.lww.com/CTG/A735, Supplementary Material). The association between MAFLD and CRC persisted when propensity score weighting was used to balance all covariates (HR 1.14; 95% CI, 1.12-1.17; see Table S6, supplementary Digital Content 1, http://links.lww.com/CTG/A735 and Figure S2, Supplementary Digital Content 1, http://links.lww.com/CTG/A735, Supplementary Material).
Figure 3.
Subgroup analysis of the association of MAFLD with colorectal cancer risk. *Rate per 100,000 person-years. †P for interaction adjusted for multiple comparison (Bonferroni corrected). All models were adjusted for age, sex, household income quartile, residential area, Charlson Comorbidity Index, aspirin use, nonsteroidal anti-inflammatory drug use, tobacco smoking, and exercise frequency. MAFLD, metabolic dysfunction–associated fatty liver disease.
DISCUSSION
In this large population-based cohort study, we found that the risk of developing CRC was significantly higher in participants with FLD than in those without; CRC risk was further elevated in the presence of MAFLD. The MAFLD only group, representing MAFLD with concomitant causes of liver diseases such as viral hepatitis or alcoholism, was associated with the highest CRC risk. When stratified according to fibrotic burden, participants with advanced liver fibrosis were at higher risks of CRC development than those without in all FLD groups: the NAFLD only, both FLD, and MAFLD only groups.
Biological pathways associated with CRC development in people with FLD are not yet fully understood. Elevated levels of insulin and insulin-like growth factor 1 associated with insulin resistance may promote growth and antiapoptosis of CRC (28). Proinflammatory cytokines and adipokines, including tumor necrosis factor alpha, interleukin 6, adiponectin, and leptin, may also affect development of CRC (29). Mechanisms underlying FLD and CRC should be further studied.
Our study has several clinical implications. First, our results confirm that FLD is independently associated with CRC risk. Although several studies had reported similar associations (6,7,30), the notion was disputed by other studies (8,9). This inconsistency may stem from the wide spectrum of NAFLD, ranging from simple steatosis with favorable clinical courses (11) to NASH or cirrhosis with unfavorable outcomes (12). In our study, FLD was consistently associated with CRC risk in the presence or absence of liver fibrosis, suggesting that the risk of CRC development may be elevated even in a mild form of FLD without progression to advanced fibrosis. The significant association between FLD and incident CRC has important implications for CRC screening strategies, as demonstrated in a study by Zhang et al.(31), where patients with NAFLD who had not undergone colonoscopy exhibited a higher CRC risk than the general population, whereas those who had undergone colonoscopy evidenced a lower risk. We also found that MAFLD was associated with a higher risk of CRC even in the subgroup of youngest age (40–44 years), which is below the recommended CRC screening age (32). Further studies should explore whether CRC surveillance should commence at a younger age in patients with FLD.
Second, this is the first study to elucidate the association between MAFLD, by the new definition, and the risk of CRC development. As CRC is associated with metabolic abnormalities (e.g., central obesity, metabolic syndrome, and diabetes), which are diagnostic features of MAFLD (5), it is reasonable that the new MAFLD criteria identify more accurately fatty liver associated with a high CRC risk. Our findings extend the growing body of literature, suggesting that MAFLD, unlike NAFLD, identifies more patients with comorbidities, advanced fibrosis, poorer prognosis, and a higher risk of extrahepatic complications (26,33–35). Notably, the CRC risk was higher in the nonoverweight than the overweight MAFLD subgroup, possibly because patients with nonoverweight MAFLD would, by definition, meet other metabolic criteria such as diabetes, hypertension, or dyslipidemia (14). However, it is also possible that a reduced skeletal muscle mass may contribute to fibrotic progression and poor outcomes in patients with FLD (36). Further studies should explore the clinical characteristics and mechanisms underlying lean MAFLD and the CRC risk.
Third, we demonstrated that the risk of CRC development among participants with MAFLD (or with NAFLD, likewise) differed according to presence of advanced liver fibrosis. Liver fibrosis is a well-known determinant of unfavorable outcomes in patients with FLD or chronic liver disease (37). Therefore, the severity of liver fibrosis may be a useful predictor of future CRC risk in MAFLD. Although multiple noninvasive surrogates for liver fibrosis were not tested, this is the first study to signify the potential importance of liver fibrosis evaluation for the assessment of CRC risk. Further study is needed to determine which noninvasive surrogates should be used and how CRC risk can be stratified according to fibrotic burden.
Fourth, the risk of CRC development was higher in the MAFLD only group, such as patients with FLD and coexisting viral hepatitis or alcoholism, compared to the other FLD groups. Similarly, the relative CRC risk associated with MAFLD was greater among patients with concomitant liver disease. These findings are well supported by several recent studies. In a Taiwanese population-based case-control study (n > 69,000) (38), HBV infection was associated with a 1.27-fold higher odds of CRC. Likewise, in a meta-analysis of 12 studies, chronic viral hepatitis was associated with a 1.32-fold higher odds of CRC (39). In a Korean study (n = 1,448) (40), significant alcohol consumption (defined as alcohol consumption of >30 g/d in men and >20 g/d in women) was associated with a 1.86 times higher risk of overall colorectal neoplasia at surveillance colonoscopy. Therefore, we speculate that FLD with metabolic and other combined etiologies for liver disease may confer an elevated risk of developing CRC. Future studies should address how to establish colonoscopic screening strategies in people with dual-etiology MAFLD.
We acknowledge several limitations of our study. First, the study used a routinely collected health screening database, in which no pathology or imaging data and only a limited number of clinical/biochemical variables were available. In addition, measurements may not have been as rigorously standardized as those in prospective studies. Second, as an observational study, residual confounding, particularly by diet, medications, genetic predisposition, or psychosocial factors, cannot be excluded. Third, detection bias may have been in play because patients with FLD may exhibit comorbid conditions and may thus require more frequent screening than others, increasing the likelihood of CRC detection. However, the association between MAFLD and CRC remained significant after fully adjusting for (or balancing) all covariates, including comorbidities. Finally, we studied only middle-aged Korean adults; this may limit the generalizability of our work to other demographic or age groups. However, cancer screening strategies generally target middle-aged populations, for whom cancer prevention and early detection exert the largest economic and public health impacts. Despite the limitations, our study of a large population-based cohort revealed an association between MAFLD and CRC, thus adding important evidence to the literature on the CRC risk of patients with FLD. Furthermore, we are the first to show that the fibrotic burden may be associated with a higher risk of CRC in patients with FLD.
In conclusion, FLD was associated with a higher risk of CRC development. The presence of MAFLD components may further increase CRC risk. Assessment of liver fibrosis may help identify a high-risk subgroup for CRC among people with FLD.
CONFLICTS OF INTEREST
Guarantor of the article: Seung Up Kim, MD, PhD, and Hyeon Chang Kim, MD, PhD, FAHA.
Specific author contributions: All authors conceived and designed the study. H.L. conducted statistical analyses, and all authors interpreted the findings. H.L. and H.W.L. drafted the manuscript. S.U.K and H.C.K critically reviewed the manuscript for key intellectual content. All authors approved the final manuscript. S.U.K. and H.C.K. are the guarantors and, as such, had full access to the data and take responsibility for its integrity and accuracy.
Financial support: This study was supported by the Korea Health Technology R&D Project through the Korea Health Industry Development Institute funded by the Ministry of Health and Welfare (HI13C0715) and the Basic Science Research Program through the National Research Foundation of Korea funded by the Ministry of Science, ICT and Future Planning (2019R1A2C4070136), Republic of Korea.
Potential competing interests: None to report.
Study Highlights.
WHAT IS KNOWN
✓ Colorectal cancer (CRC) and fatty liver disease share common risk factors including obesity, metabolic syndrome, and type 2 diabetes mellitus.
✓ In contrast to nonalcoholic fatty liver disease, the diagnosis of metabolic dysfunction–associated fatty liver disease (MAFLD) is based on presence of metabolic dysfunction among people with fatty liver.
WHAT IS NEW HERE
✓ CRC risk is higher in the presence of MAFLD, especially when accompanied by liver fibrosis.
Acknowledgement
This study used the National Health Insurance Service database (NHIS-2021-1-333).
Footnotes
SUPPLEMENTARY MATERIAL accompanies this paper at http://links.lww.com/CTG/A735
Hokyou Lee, MD and Hye Won Lee, MD contributed equally to this work.
Contributor Information
Hokyou Lee, Email: hokyou.lee@yuhs.ac.
Hye Won Lee, Email: lorry-lee@yuhs.ac.
Seung Up Kim, Email: ksukorea@yuhs.ac.
Hyeon Chang Kim, Email: hckim@yuhs.ac.
References
- 1.Adams LA, Anstee QM, Tilg H, et al. Non-alcoholic fatty liver disease and its relationship with cardiovascular disease and other extrahepatic diseases. Gut 2017;66:1138–53. [DOI] [PubMed] [Google Scholar]
- 2.Ekstedt M, Hagström H, Nasr P, et al. Fibrosis stage is the strongest predictor for disease-specific mortality in NAFLD after up to 33 years of follow-up. Hepatology 2015;61:1547–54. [DOI] [PubMed] [Google Scholar]
- 3.Adams LA, Harmsen S, St Sauver JL, et al. Nonalcoholic fatty liver disease increases risk of death among patients with diabetes: A community-based cohort study. Am J Gastroenterol 2010;105:1567–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 2015;136:E359–86. [DOI] [PubMed] [Google Scholar]
- 5.Chiu HM, Lin JT, Shun CT, et al. Association of metabolic syndrome with proximal and synchronous colorectal neoplasm. Clin Gastroenterol Hepatol 2007;5:221–9. quiz 141. [DOI] [PubMed] [Google Scholar]
- 6.Lin XF, Shi KQ, You J, et al. Increased risk of colorectal malignant neoplasm in patients with nonalcoholic fatty liver disease: A large study. Mol Biol Rep 2014;41:2989–97. [DOI] [PubMed] [Google Scholar]
- 7.Lee YI, Lim YS, Park HS. Colorectal neoplasms in relation to non-alcoholic fatty liver disease in Korean women: A retrospective cohort study. J Gastroenterol Hepatol 2012;27:91–5. [DOI] [PubMed] [Google Scholar]
- 8.Basyigit S, Uzman M, Kefeli A, et al. Absence of non-alcoholic fatty liver disease in the presence of insulin resistance is a strong predictor for colorectal carcinoma. Int J Clin Exp Med 2015;8:18601–10. [PMC free article] [PubMed] [Google Scholar]
- 9.You J, Huang S, Huang GQ, et al. Nonalcoholic fatty liver disease: A negative risk factor for colorectal cancer prognosis. Medicine (Baltimore) 2015;94:e479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ahn JS, Sinn DH, Min YW, et al. Non-alcoholic fatty liver diseases and risk of colorectal neoplasia. Aliment Pharmacol Ther 2017;45:345–53. [DOI] [PubMed] [Google Scholar]
- 11.Cotter TG, Rinella M. NAFLD 2020: The state of the disease. Gastroenterology 2020;158:1851-1864. [DOI] [PubMed] [Google Scholar]
- 12.Taylor RS, Taylor RJ, Bayliss S, et al. Association between fibrosis stage and outcomes of patients with non-alcoholic fatty liver disease: A systematic review and meta-analysis. Gastroenterology 2020;158:1611–25.e12. [DOI] [PubMed] [Google Scholar]
- 13.Eslam M, Sanyal AJ, George J. MAFLD: A consensus-driven proposed nomenclature for metabolic associated fatty liver disease. Gastroenterology 2020;158:1999–2014.e1. [DOI] [PubMed] [Google Scholar]
- 14.Eslam M, Newsome PN, Sarin SK, et al. A new definition for metabolic dysfunction-associated fatty liver disease: An international expert consensus statement. J Hepatol 2020;73:202–9. [DOI] [PubMed] [Google Scholar]
- 15.Seong SC, Kim YY, Park SK, et al. Cohort profile: The national health insurance service-national health screening cohort (NHIS-HEALS) in Korea. BMJ Open 2017;7:e016640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee H, Yano Y, Cho SMJ, et al. Cardiovascular risk of isolated systolic or diastolic hypertension in young adults. Circulation 2020;141:1778–86. [DOI] [PubMed] [Google Scholar]
- 17.Lee H, Park S, Kim HC. Temporal and geospatial trends of hypertension management in Korea: A nationwide study 2002-2016. Korean Circ J 2019;49:514–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim HC, Cho SMJ, Lee H, et al. Korea hypertension fact sheet 2020: Analysis of nationwide population-based data. Clin Hypertens 2021;27:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cho SMJ, Lee H, Lee H-H, et al. Dyslipidemia fact sheets in Korea 2020: An analysis of nationwide population-based data. J Lipid Atheroscler 2021;10:202–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee H-H, Cho SMJ, Lee H, et al. Korea heart disease fact sheet 2020: Analysis of nationwide data. Korean Circ J 2021;51:495–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Quan H, Li B, Couris CM, et al. Updating and validating the Charlson comorbidity index and score for risk adjustment in hospital discharge abstracts using data from 6 countries. Am J Epidemiol 2011;173:676–82. [DOI] [PubMed] [Google Scholar]
- 22.Lee YH, Bang H, Park YM, et al. Non-laboratory-based self-assessment screening score for non-alcoholic fatty liver disease: Development, validation and comparison with other scores. PLoS One 2014;9:e107584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Seo HJ, Oh IH, Yoon SJ. A comparison of the cancer incidence rates between the national cancer registry and insurance claims data in Korea. Asian Pac J Cancer Prev 2012;13:6163–8. [DOI] [PubMed] [Google Scholar]
- 24.Younossi Z, Anstee QM, Marietti M, et al. Global burden of NAFLD and NASH: Trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol 2018;15:11–20. [DOI] [PubMed] [Google Scholar]
- 25.Keum N, Giovannucci E. Global burden of colorectal cancer: Emerging trends, risk factors and prevention strategies. Nat Rev Gastroenterol Hepatol 2019;16:713–32. [DOI] [PubMed] [Google Scholar]
- 26.Lee H, Lee YH, Kim SU, et al. Metabolic dysfunction-associated fatty liver disease and incident cardiovascular disease risk: A nationwide cohort study. Clin Gastroenterol Hepatol 2021;19:2138–47.e10. doi: 10.1016/j.cgh.2020.12.022. [DOI] [PubMed] [Google Scholar]
- 27.Li F, Thomas LE, Li F. Addressing extreme propensity scores via the overlap weights. Am J Epidemiol 2019;188:250–7. [DOI] [PubMed] [Google Scholar]
- 28.Ze EY, Kim BJ, Jun DH, et al. The fatty liver index: A simple and accurate predictor of colorectal adenoma in an average-risk population. Dis Colon Rectum 2018;61:36–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wong VW, Hui AY, Tsang SW, et al. Metabolic and adipokine profile of Chinese patients with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol 2006;4:1154–61. [DOI] [PubMed] [Google Scholar]
- 30.Lee JM, Park YM, Yun JS, et al. The association between nonalcoholic fatty liver disease and esophageal, stomach, or colorectal cancer: National population-based cohort study. PLoS One 2020;15:e0226351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang X, Wong VW, Yip TC, et al. Colonoscopy and risk of colorectal cancer in patients with nonalcoholic fatty liver disease: A retrospective territory-wide cohort study. Hepatol Commun 2021;5:1212–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hong SN, Kim JH, Choe WH, et al. Prevalence and risk of colorectal neoplasms in asymptomatic, average-risk screenees 40 to 49 years of age. Gastrointest Endosc 2010;72:480–9. [DOI] [PubMed] [Google Scholar]
- 33.Wai-Sun Wong V, Lai-Hung Wong G, Woo J, et al. Impact of the new definition of metabolic associated fatty liver disease on the epidemiology of the disease. Clin Gastroenterol Hepatol 2021;19:2161–71.e5. doi: 10.1016/j.cgh.2020.10.046. [DOI] [PubMed] [Google Scholar]
- 34.Yamamura S, Eslam M, Kawaguchi T, et al. MAFLD identifies patients with significant hepatic fibrosis better than NAFLD. Liver Int 2020;40:3018–30. doi: 10.1111/liv.14675.liv.14675-41. [DOI] [PubMed] [Google Scholar]
- 35.Nguyen VH, Le MH, Cheung RC, et al. Differential clinical characteristics and mortality outcomes in persons with NAFLD and/or MAFLD. Clin Gastroenterol Hepatol 2021;19:2172–81.e6. doi: 10.1016/j.cgh.2021.05.029. [DOI] [PubMed] [Google Scholar]
- 36.Lee YH, Kim SU, Song K, et al. Sarcopenia is associated with significant liver fibrosis independently of obesity and insulin resistance in nonalcoholic fatty liver disease: Nationwide surveys (KNHANES 2008-2011). Hepatology 2016;63:776–86. [DOI] [PubMed] [Google Scholar]
- 37.Vilar-Gomez E, Calzadilla-Bertot L, Wai-Sun Wong V, et al. Fibrosis severity as a determinant of cause-specific mortality in patients with advanced nonalcoholic fatty liver disease: A multi-national cohort study. Gastroenterology 2018;155:443–57.e17. [DOI] [PubMed] [Google Scholar]
- 38.Su FH, Le TN, Muo CH, et al. Chronic hepatitis B virus infection associated with increased colorectal cancer risk in Taiwanese population. Viruses 2020;12:97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hong SW, Choi WM, Hwang HW, et al. Chronic viral hepatitis is associated with colorectal neoplasia: A systematic review and meta-analysis. Dig Dis Sci 2021;66:3715–24. doi: 10.1007/s10620-020-06745-x. [DOI] [PubMed] [Google Scholar]
- 40.Yang YJ, Bang CS, Choi JH, et al. Alcohol consumption is associated with the risk of developing colorectal neoplasia: Propensity score matching analysis. Sci Rep 2019;9:8253–10. [DOI] [PMC free article] [PubMed] [Google Scholar]