Abstract
INTRODUCTION:
Antibiotic use has emerged as a risk factor for colorectal neoplasia and is hypothesized as a contributor to the rising incidence of colorectal cancer under age 50 years or early-onset colorectal cancer (EOCRC). However, the impact of antibiotic use and risk of EOCRC is unknown.
METHODS:
We conducted a population-based case-control study of CRC among individuals aged ≥18 years in the Epidemiology Strengthened by histoPathology Reports in Sweden (ESPRESSO) cohort (2006–2016). The primary outcome was EOCRC. A secondary outcome was CRC at any age. Incident CRC was pathologically confirmed, and for each, up to 5 population-based controls were matched on age, sex, county of residence, and calendar year. We assessed prescriptions until 6 months before CRC diagnosis. Conditional logistic regression was used to estimate adjusted odds ratios (aORs) and 95% confidence intervals (CIs).
RESULTS:
We identified 54,804 cases of CRC (2,557 EOCRCs) and 261,089 controls. Compared with none, previous antibiotic use was not associated with EOCRC risk after adjustment for potential confounders (aOR 1.06, 95% CI: 0.96, 1.17) with similarly null findings when stratified by anatomic tumor site. In contrast, previous antibiotic use was weakly associated with elevated risk for CRC at any age (aOR 1.05, 95% CI: 1.02, 1.07). A potential but modest link between broad-spectrum antibiotic use and EOCRC was observed (aOR 1.13, 95% CI: 1.02, 1.26).
DISCUSSION:
We found no conclusive evidence that antibiotics are associated with EOCRC risk. Although antibiotic use was weakly associated with risk of CRC at any age, the magnitude of association was modest, and the study period was relatively short.
INTRODUCTION
Contrasting with overall declines in colorectal cancer (CRC) incidence, rates have increased dramatically among those aged 20–49 years (1), particularly in Sweden with an annual percent change of 2.8% from 1991 to 2015 for ages 20–39 years and 1.2% for ages 40–49 years, respectively (2). Early-onset CRC (EOCRC) or CRC before age 50 years is detected at more advanced stages than later-onset CRC (CRC after age 50 years) with more aggressive tumors and greater years of life lost (3–6). This concerning trend has led to updated guidelines from both the American Cancer Society and draft recommendations from the United States Preventative Services Task Force advising average-risk screening begin at age 45 years rather than 50 years (7,8). Although these changes could lead to the earlier detection of colorectal neoplasia, primary disease prevention—the cornerstone of most public health efforts—is relatively underexplored in EOCRC. Despite previous investigations implicating obesity (9,10), a sedentary lifestyle (11), and metabolic syndrome (12) as potential targets for intervention, the identification of other modifiable risk factors and how they might differ from later-onset CRC remain a high unmet need.
Because EOCRC has been rising predominantly in Western populations and developing regions in Asia (13), increased sanitation and the widespread use of broad-spectrum antibiotics (14–16) have been proposed as reasons for why this emerging disparity in disease burden is becoming more apparent. Previous studies have demonstrated that up to ⅓ of all antibiotic dispensations may be inappropriate (17,18). Although campaigns to encourage antibiotic stewardship have seen recent success, relatively modest decreases in prescribing patterns have only been apparent in the last decade, likely too recent to have influenced the alarming upward trends in EOCRC (19–22), even in countries like Sweden where a strategic program to decrease antibiotic usage has been in place since the early 1990s (23–26). More dramatic decreases in antibiotic usage may be possible if informed by compelling data on how it might influence risk of gastrointestinal (GI) diseases, including CRC.
With greater appreciation for the critical role of the gut microbiome in GI disease development, so too has concern that antibiotics that perturb these microbial communities generate the dysregulated host/microbial interactions that promote early colorectal carcinogenesis. Despite this compelling rationale, the relationship between antibiotic usage and subsequent risk of EOCRC remains to be elucidated. Thus, to our knowledge, we conducted the largest investigation exploring the potential association between antibiotic use and risk of EOCRC in a large nationally representative case-control study in Sweden.
METHODS
Study population
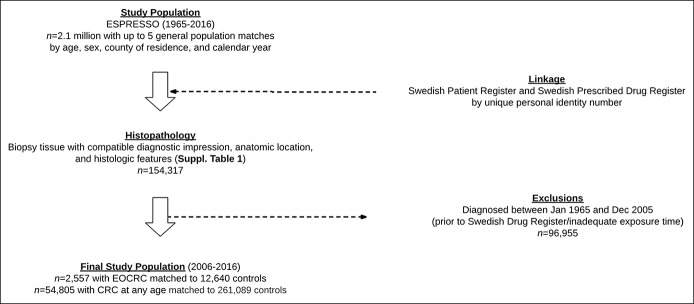
In Sweden, access to health care is universal and tax funded. This includes prescription drug coverage (27). The Swedish National Board of Health and Welfare has collected patient-level information on hospital discharges nationwide since 1987 in the Swedish Patient Register (28). Each record includes sex, date of birth, dates of hospital admission, and procedural and discharge diagnoses by International Classification of Diseases (ICD) code (Figure 1). In 2001, this registry was expanded to include outpatient specialty care, including visits to gastroenterology or oncology providers. The positive predictive value for most diagnoses in this register ranges between 85% and 95% (28).
Figure 1.
Flowchart of participant selection. EOCRC, early-onset colorectal cancer; ESPRESSO, Epidemiology Strengthened by histoPathology Reports in Sweden.
We integrated these national registry data with the Epidemiology Strengthened by histoPathology Reports in Sweden (ESPRESSO) study (29). Briefly, the ESPRESSO study is a comprehensive data harmonizing effort involving all 28 pathology laboratories in Sweden and any GI pathology reports generated for clinical care or research purposes between 1965 and 2017. This consortium has enrolled more than 2.1 million unique individuals with detailed information on GI topography (i.e., the anatomic location of the obtained tissue), morphological appearance, and pathologist's diagnostic impression. As previously described (29), at the time of ESPRESSO inclusion, individuals were paired with up to 5 controls from the general population, matched on the basis of age, sex, calendar year, and county. Finally, since July 2005, the Swedish Prescribed Drug Register has collected information on all medications, including antibiotics, prescribed to the entire Swedish population, including date of redemption, amount dispensed, and dose allotted (29,30).
Patient-level information from ESPRESSO and both national registries (Patient Register and the Prescribed Drug Register) was linked by a unique personal identity number assigned at birth or at the time when permanent residence was established. To ensure adequate time at risk (e.g., to allow for accrual of antibiotic dispensations), we defined the study baseline as January 1, 2006. Thus, our study encompasses all consecutive eligible patients for the period of overlap during which the National Patient Register, the ESPRESSO study, and the Prescribed Drug Register were each actively enrolling plus a 6-month lead-in period (January 1, 2006, to December 31, 2016). This investigation was approved by the Stockholm Ethics Review Board (Protocol 2014/1287-31/4). Because of the strict registry-based nature of the study, informed consent was waived.
Ascertainment of outcomes
Our primary outcome was incident CRC diagnosed under age 50 years. An a priori secondary outcome was CRC at any age. Using predefined anatomic and histologic criteria, as well as the attending pathologist's diagnostic impression (see Supplementary Table 1, Supplementary Digital Content 1, http://links.lww.com/CTG/A737), we identified individuals in the ESPRESSO study with GI tract histopathology compatible with the diagnosis of incident CRC from January 2006 to December 2016. We then cross-referenced potential cases and the entirety of their inpatient and outpatient records in search of at least 1 ICD code consistent with CRC, thus requiring both compatible histopathology and registry-level case confirmation. We abstracted information on the date of diagnosis and tumor location with CRCs of the cecum, ascending colon, hepatic flexure, transverse colon, and splenic flexure defined as proximal, descending or sigmoid colon defined as distal, and rectum or rectosigmoid junction defined as rectal, respectively. We excluded those with CRC-compatible pathology or ICD diagnostic coding before our study baseline (January 2006). The date of CRC diagnosis was defined as the earliest among the dates of relevant pathology findings and the first appearance of a CRC-related diagnosis code (Figure 1).
Ascertainment of primary exposure and other covariates
Cumulative antibiotic usage before CRC diagnosis or matching (primary exposure) was defined as the cumulative sum of antibiotic dispensations. To account for the possibility of reverse causation or antibiotic therapy prescribed for symptoms related to undiagnosed CRC, we did not count dispensations in the 6 months before CRC diagnosis/matching. Antibiotic use was assessed in the Swedish Prescribed Drug Register and categorized using established World Health Organization Anatomical Therapeutic Chemical (ATC) codes for the therapeutic subgroup of antibacterials approved for systemic usage (see Supplementary Table 2, Supplementary Digital Content 1, http://links.lww.com/CTG/A737). We collected information on the number of dispensations, the cumulative number of days prescribed, as well as the cumulative defined daily dose, ATC class of antibiotics (penicillins, cephalosporins, macrolides, quinolones, tetracyclines, sulfonamides, and other), bacterial target (aerobic vs anaerobic microbes), and spectrum of coverage (broad vs narrow).
We obtained data on level of education (≤9 years, 10–12 years, ≥13 years, and unknown) from Statistics Sweden and the longitudinal integrated database for health insurance and labor market studies (28). This information is available for more than 98% of all working-age individuals. We also calculated the number of inpatient and outpatient encounters (continuous) for each participant during the study period up until the censoring date (6 months before the time of matching). We used validated procedure codes to identify the number of previous lower endoscopies, including colonoscopy (9011, 9023, 4688, 4689, 4674, 4684, UJF32, and UJF35) and sigmoidoscopy (9012, 4685, UJF42, and UJF45), also accrued until 6 months before match. Consistent with previous validated methods using ICD codes (31–33), we calculated Charlson Comorbidity Index (CCI) scores, an established composite measure of overall health and predictor of 10-year mortality (33).
Statistical analysis
To evaluate the association between antibiotic therapy and risk of EOCRC, we performed conditional logistic regression to estimate crude and multivariable-adjusted odds ratios (aORs) and 95% confidence intervals (CIs) conditioned on matching criteria (age, sex, calendar year at the time of match, and county of residence) and further adjusted for potential confounding factors (number of previous lower endoscopies, educational attainment, and health care utilization). Tests for linear trend were calculated using the midpoint of each frequency category as a continuous variable. Two-sided P values of <0.05 were considered statistically significant.
As a secondary analysis, we assessed antibiotics and risk of CRC regardless of age. We also performed subgroup analyses according to the spectrum of antibiotic coverage (broad vs narrow), whether they targeted aerobic or anaerobic bacteria, class of antibiotic therapy prescribed (penicillins, tetracyclines, quinolones, macrolides, sulfonamides and trimethoprim, cephalosporins, other nonpenicillin beta-lactams, and other), and anatomic location of EOCRC. Statistical analyses were performed using R 4.0.1 (Vienna, Austria).
Role of funding sources
Sponsors had no role in study design, the collection, analysis, and interpretation of data, report writing, or the decision to submit for publication. All authors had access to the study data and reviewed and approved the final manuscript.
RESULTS
We identified 154,317 individuals aged ≥18 years in the ESPRESSO study with GI tract biopsies or surgical specimens consistent with the diagnosis of EOCRC. After excluding patients with CRC-compatible tissue or a relevant CRC ICD diagnosis code before study baseline, as well as individuals without adequate exposure time using our prespecified lead-in period, we enrolled 54,804 cases of CRC (of whom, 2,557 were incident EOCRC) and 261,089 matched controls between January 1, 2006, and December 31, 2016.
As expected, EOCRC cases were comparable to their matched controls on the basis of mean age (42.9 years) and sex (46% female) (Table 1). Cases had higher levels of health care engagement in both the inpatient and outpatient settings, although notably, CCI scores were grossly comparable. CRC cases diagnosed at any age (and their matched controls), as expected, were older than those in the EOCRC analysis, and trends in health care utilization and CCI were comparable (see Supplementary Table 3, http://links.lww.com/CTG/A737).
Table 1.
Characteristics of colorectal cancer cases before age 50 years and controls at the index dates, 2006–2016
| Cases (n = 2,557) | Controls (n = 12,640) | |
| Age, yr | 42.9 (6.0) | 42.9 (6.1) |
| Female, % | 1,182 (46.2) | 5,866 (46.3) |
| Year of diagnosis, % | ||
| 2006–2007 | 438 (17.1) | 2,173 (17.1) |
| 2008–2010 | 722 (28.2) | 3,577 (28.2) |
| 2011–2013 | 782 (30.6) | 3,877 (30.6) |
| ≥2014 | 615 (24.1) | 3,047 (24.0) |
| Educational attainment | ||
| ≤9 yr | 284 (11.0) | 1,423 (11.3) |
| 10–12 yr | 1,280 (50.1) | 6,216 (49.0) |
| ≥13 yr | 968 (37.9) | 4,835 (38.1) |
| Unknown | 25 (1.0) | 200 (1.6) |
| Number of previous clinic visits | 9.7 (16.2) | 7.0 (12.4) |
| Number of previous hospitalizations | 3.6 (5.5) | 2.8 (4.8) |
| Number of previous lower endoscopies | 0.2 (1.3) | 0.02 (0.17) |
| Charlson Comorbidity Index | 0.2 (0.9) | 0.1 (0.5) |
Mean (SD) is shown for continuous variables, and percentage is shown for categorical variables.
Compared with no previous use, any antibiotic use was associated with an 18% increased risk of EOCRC (OR 1.18, 95% CI: 1.07, 1.29; Table 2). However, this finding was significantly attenuated after further adjustment for CCI, number of previous lower endoscopies, health care engagement, and educational attainment (aOR 1.06, 95% CI: 0.96, 1.17). The modest increase in risk was found to be statistically significant among previous users of broad-spectrum antibiotics (aOR 1.13, 95% CI: 1.02, 1.26). No clear association according to aerobic or antiaerobic coverage or anatomic therapeutic chemical (ATC) subclass was identified.
Table 2.
Association between use of oral antibiotics and risk of early-onset colorectal cancer (age < 50 years)
| Nonusers | Users | OR (95% CI) | ||||
| Cases (n) | Controls (n) | Cases (n) | Controls (n) | Model 1a | Model 2b | |
| All antibiotics | 1,141 | 6,091 | 1,416 | 6,583 | 1.18 (1.07, 1.29) | 1.06 (0.96, 1.17) |
| Spectrum of coverage | ||||||
| Broad spectrum | 1768 | 9,330 | 789 | 3,344 | 1.27 (1.15, 1.40) | 1.13 (1.02, 1.26) |
| Narrow spectrum | 1,395 | 7,186 | 1,162 | 5,488 | 1.10 (1.01, 1.21) | 1.01 (0.92, 1.11) |
| Bacterial target | ||||||
| Antiaerobic | 1,171 | 6,244 | 1,386 | 6,430 | 1.18 (1.07, 1.29) | 1.07 (0.96, 1.17) |
| Antianaerobic | 2,258 | 11,414 | 299 | 1,260 | 1.21 (1.05, 1.38) | 1.01 (0.87, 1.17) |
| ATC class | ||||||
| Penicillin | 1,481 | 7,550 | 1,076 | 5,124 | 1.08 (0.98, 1.18) | 1.00 (0.91, 1.10) |
| Tetracyclines | 2,118 | 10,722 | 439 | 1952 | 1.15 (1.02, 1.29 | 1.04 (0.92, 1.18) |
| Quinolones | 2,343 | 11,952 | 214 | 722 | 1.52 (1.29, 1.78) | 1.17 (0.98, 1.40) |
| Macrolides | 2,338 | 11,636 | 219 | 1,038 | 1.05 (0.90, 1.22) | 0.88 (0.75, 1.04) |
| Sulfonamides and trimethoprim | 2,453 | 12,213 | 104 | 461 | 1.13 (0.91, 1.41) | 0.95 (0.75, 1.21) |
| Cephalosporins and other nonpenicillin Beta-lactams |
2,412 | 12,066 | 145 | 608 | 1.20 (0.99, 1.44) | 1.08 (0.88, 1.32) |
| Other | 2,462 | 12,230 | 95 | 444 | 1.08 (0.85, 1.36) | 0.92 (0.72, 1.18) |
Bold text indicates P value <0.05.
ATC, Anatomic Therapeutic Chemical CI, confidence interval; OR, odds ratio.
Conditional logistic regression was used with matching for age (continuous), sex (binary), calendar year at the time of match, and county of residence.
Multivariable conditional logistic regression models were further adjusted for education (9 years or less, 10–12 years, ≥13 years, and missing), number of clinic visits (continuous), number of hospitalizations (continuous), Charlson Comorbidity Score (continuous), and number of previous lower endoscopies (continuous).
We observed no clear association between the frequency of antibiotic dispensation and risk of EOCRC (Table 3). A sensitivity analysis using cumulative number of days prescribed and the cumulative defined daily dose did not materially alter our findings (data not shown). No significant heterogeneity was observed in risk for EOCRC with previous use of any antibiotics by coverage or target when we conducted stratified analyses by tumor location (Table 4).
Table 3.
Association between cumulative dispensations of antibiotics and risk of early-onset colorectal cancer (<age 50 years)
| No previous use | 1 previous dispensation | 2 previous dispensations | 3-5 previous dispensations | ≥6 previous dispensations | P for trend | |
| All antibiotics | ||||||
| No. of cases | 1,141 | 518 | 342 | 363 | 193 | |
| No. of controls | 6,091 | 2,606 | 1,444 | 1716 | 817 | |
| OR (95% CI)a | 1 (ref) | 1.05 (0.93, 1.18) | 1.19 (1.03, 1.37) | 1.04 (0.90, 1.21) | 0.93 (0.76, 1.14) | 0.68 |
| Spectrum of coverage | ||||||
| Broad spectrum | ||||||
| No. of cases | 1768 | 417 | 181 | 138 | 53 | |
| No. of controls | 9,330 | 1817 | 766 | 578 | 183 | |
| OR (95% CI)a | 1 (ref) | 1.19 (1.05, 1.35) | 1.14 (0.95, 1.37) | 1.06 (0.86, 1.31) | 0.76 (0.52, 1.11) | 0.42 |
| Narrow spectrum | ||||||
| No. of cases | 1,395 | 554 | 283 | 253 | 72 | |
| No. of controls | 7,186 | 2,713 | 1,232 | 1,198 | 345 | |
| OR (95% CI)a | 1 (ref) | 1.01 (0.90, 1.13) | 1.11 (0.95, 1.29) | 0.97 (0.82, 1.15) | 0.82 (0.61, 1.10) | 0.72 |
| Bacterial target | ||||||
| Antiaerobic | ||||||
| No. of cases | 1,171 | 561 | 309 | 361 | 155 | |
| No. of controls | 6,244 | 2,725 | 1,424 | 1,604 | 677 | |
| OR (95% CI)a | 1 (ref) | 1.07 (0.95, 1.20) | 1.07 (0.92, 1.25) | 1.11 (0.96, 1.28) | 0.89 (0.71, 1.11) | 0.66 |
| Antianaerobic | ||||||
| No. of cases | 2,258 | 221 | 43 | 24 | 11 | |
| No. of controls | 11,414 | 940 | 211 | 96 | 13 | |
| OR (95% CI)a | 1 (ref) | 1.06 (0.90, 1.25) | 0.84 (0.59, 1.19) | 0.86 (0.51, 1.44) | 0.63 (0.20, 2.00) | 0.49 |
Bold text indicates P value <0.05.
CI, confidence interval; OR, odds ratio.
Multivariable conditional logistic regression was used with matching for age (continuous), sex (binary), calendar year at the time of match, and county of residence and adjusted for education (9 years or less, 10–12 years, ≥13 years, and missing), number of clinic visits (continuous), number of hospitalizations (continuous), Charlson Comorbidity Score (continuous), and number of previous lower endoscopies (continuous).
Table 4.
Association between use of oral antibiotics and risk of early-onset colorectal cancer (age < 50 years) by anatomic location
| Nonusers | Users | OR (95% CI) | ||||
| Cases (n) | Controls (n) | Cases (n) | Controls (n) | Model 1a | Model 2b | |
| Proximal colon | ||||||
| All antibiotics | 92 | 481 | 126 | 587 | 1.14 (0.83, 1.57) | 0.99 (0.71, 1.39) |
| Spectrum of coverage | ||||||
| Broad spectrum | 152 | 778 | 66 | 290 | 1.18 (0.84, 1.64) | 0.94 (0.65, 1.35) |
| Narrow spectrum | 113 | 578 | 105 | 490 | 1.11 (0.82, 1.52) | 1.04 (0.75, 1.45) |
| Bacterial target | ||||||
| Antiaerobic | 94 | 496 | 124 | 572 | 1.17 (0.85, 1.61) | 1.01 (0.72, 1.42) |
| Antianaerobic | 194 | 968 | 24 | 100 | 1.21 (0.74, 1.96) | 0.79 (0.45, 1.41) |
| Distal colon | ||||||
| All antibiotics | 131 | 685 | 178 | 852 | 1.11 (0.85, 1.45) | 1.05 (0.79, 1.39) |
| Spectrum of coverage | ||||||
| Broad spectrum | 210 | 1,088 | 99 | 449 | 1.15 (0.88, 1.53) | 1.12 (0.84, 1.50) |
| Narrow spectrum | 157 | 831 | 152 | 706 | 1.15 (0.88, 1.50) | 1.08 (0.81, 1.43) |
| Bacterial target | ||||||
| Antiaerobic | 134 | 698 | 175 | 839 | 1.11 (0.85, 1.44) | 1.04 (0.79, 1.38) |
| Antianaerobic | 264 | 1,353 | 45 | 184 | 1.26 (0.87, 1.80) | 1.13 (0.77, 1.66) |
| Rectal | ||||||
| All antibiotics | 424 | 2,198 | 506 | 2,380 | 1.12 (0.96, 1.31) | 1.04 (0.88, 1.22) |
| Spectrum of coverage | ||||||
| Broad spectrum | 659 | 3,386 | 271 | 1,192 | 1.18 (1.00, 1.38) | 1.06 (0.90, 1.26) |
| Narrow spectrum | 524 | 2,589 | 406 | 1989 | 1.01 (0.87, 1.18) | 0.93 (0.79, 1.09) |
| Bacterial target | ||||||
| Antiaerobic | 437 | 2,247 | 493 | 2,331 | 1.10 (0.94, 1.28) | 1.01 (0.86, 1.19) |
| Antianaerobic | 824 | 4,133 | 106 | 445 | 1.20 (0.95, 1.50) | 0.98 (0.77, 1.26) |
CI, confidence interval.
Conditional logistic regression was used with matching for age (continuous), sex (binary), calendar year at the time of match, and county of residence.
Multivariable conditional logistic regression models were further adjusted for education (9 years or less, 10–12 years, ≥13 years, and missing), number of clinic visits (continuous), number of hospitalizations (continuous), Charlson Comorbidity Score (continuous), and number of previous lower endoscopies (continuous).
When we assessed the link between antibiotics and CRC diagnosed at any age, previous antibiotic therapy was associated with risk of CRC, demonstrating modestly elevated risk for any vs no previous use of antibiotics, particularly for broad spectrum (any vs no previous use: aOR 1.05, 95% CI: 1.03, 1.07). Risk did not seem to be significantly elevated for narrow-spectrum antibiotics and antianaerobic coverage, although users of several common classes of antibiotics, including penicillins and tetracyclines, had slight increases in risk compared with nonusers (see Supplementary Table 4, Supplementary Digital Content 1, http://links.lww.com/CTG/A737).
DISCUSSION
In a population-based case-control study of over 50,000 cases of CRC, among whom more than 2,500 were diagnosed before age 50 years, antibiotic usage was not convincingly associated with risk of EOCRC with the possible exception of broad-spectrum antibiotic usage. However, we did not observe a clear dose-dependent relationship between broad-spectrum antimicrobials and EOCRC, and this result should be interpreted with caution. In addition, we reaffirmed previous evidence demonstrating an association between antibiotic use and CRC diagnosed at any age (34).
Our results warrant further discussion in the context of previous work in this area. A recent investigation linked antibiotic usage and colorectal carcinogenesis using a similar nationally representative matched case-control design but was unable to fully interrogate the relationship between antibiotics and EOCRC, as Zhang et al. (34) specifically excluded individuals aged <40 years. In addition, previous studies have not only demonstrated that life-course antibiotics may be associated with the development of colorectal adenomas (35)—the most common precursor lesions to both EOCRC (36) and CRC at any age (37)—but some antibiotic subclasses may be linked to colorectal precursor lesions in individuals aged <50 years (38). Furthermore, our findings of a modest association between antibiotic usage and later-onset CRC with null results for EOCRC may reflect an inflection point for what has been a relatively recent and successful Swedish public health campaign encouraging antibiotic stewardship and more restrained prescribing practices (23–25). Whether this public health effort can reverse alarming trends in EOCRC risk remains to be seen.
The lack of a clear relationship between antibiotics and early tumorigenesis could suggest that certain antibiotics may be associated with the development of precursor lesions, but not their acceleration through the adenoma-carcinoma sequence through which most EOCRCs arise. Conversely, a longer induction/latency period or time at risk and more granular information related to the timing of antibiotic exposure (e.g., during childhood) may be required to capture the potentially elevated EOCRC risk conferred on individuals using antibiotics, a potential explanation for our null findings.
This investigation was strengthened by the inclusion of all consecutive eligible patients with new-onset EOCRC from a population-based register over a 10-year study period. In Sweden, medication coverage is universal with virtually complete information on dispensations, including antibiotics, minimizing ascertainment bias (<0.3% of all prescriptions lack identifying information) (39). We used stringent outcome ascertainment, requiring both compatible histopathologic findings and confirmatory ICD coding for cases adjudication. We considered the possibility of reverse causation (therapy initiated for undiagnosed CRC) or confounding by indication (therapy initiated for GI infections/bacterial translocation related to new CRC) and attempted to mitigate this possibility by accounting for prescriptions at least 6 months before diagnosis. Thus, we can be more confident that antibiotic usage was not likely due to undiagnosed CRC. Finally, our conservative coding of the date of diagnosis (at the time of either the earliest EOCRC-compatible histopathology or ICD coding) further minimizes the time period for which antibiotic dispensations could be attributable to symptoms of CRC.
We acknowledge limitations. As with all large-scale pharmacoepidemiologic studies, medication dispensation through the Swedish Prescribed Drug Register may not capture actual usage, although because of the short-term nature of most antibiotics and the presumption that dispensations were most frequently attributable to positive symptoms suggestive of an infection, adherence was not likely a major issue or subject to differential misclassification by case/control status. We did not capture route of antibiotic administration (intravenous, oral, or other), which several prehuman investigations have suggested could differentially influence gut microbial communities (40,41), although most exposed patients would have received oral antibiotics in this prescription database. In addition, the Prescribed Drug Register was not established until 2005, limiting the window for exposure assessment, including the use of antibiotics during childhood—especially early life—a time of critical importance in the development of gut microbial plasticity and resilience (42–44). Finally, given the observational nature and epidemiologic scale of this registry-level investigation, the possibility of unmeasured confounding remains, including more detailed information on the presence of familial CRC syndromes and more established lifestyle risk factors for CRC such as diet (45), alcohol, sedentary behavior and physical activity (11), and obesity (10), although none are clearly linked to established differences in antibiotic dispensation patterns.
In this prospective population-scale case-control study, previous antibiotic usage was not associated with an increase in the risk of EOCRC, despite other investigations previously linking their use to CRC diagnosed at older ages. Subgroup analyses demonstrated a potential link between broad-spectrum antibiotics and only a small but statistically significant elevation of risk for CRC at any age, the latter being consistent with previous investigations. These findings, as well as heterogeneous risk estimates based on antibiotic class and coverage, should be contextualized by our observation that even statistically significant differences were modest in magnitude and, thus, of unclear clinical importance. Our results will need to be validated in other populations given Sweden's lower antibiotic dispensation patterns compared with other nations (46), and although these results may be reassuring on some level, antibiotic stewardship and prescriber restraint should be practiced regardless, particularly for broad-spectrum antibiotics.
CONFLICTS OF INTEREST
Guarantor of the article: Jonas Ludvigsson, MD, PhD.
Specific author contributions: L.H.N., Y.C., and J.F.L: study concept and design. J.F.L: acquisition of data. All coauthors: analysis and interpretation of data. L.H.N. and Y.C.: drafting of the manuscript. All authors: critical revision of the manuscript for important intellectual content. N.B., L.H.N., and Y.C: statistical analysis. J.F.L: study supervision.
Financial support: J.F.L. coordinates a study on behalf of the Swedish IBD Quality Register (SWIBREG). This study has received funding from Janssen Corporation. This work was supported by the National Institutes of Health and the National Institute of Diabetes and Digestive and Kidney Disease (K23DK125838 to L.H.N.); the American Gastroenterological Association (Research Scholars Award to L.H.N.); the Crohn's and Colitis Foundation (Research Fellowship Award and Career Development Award to L.H.N. and Senior Investigator Award to A.T.C. and H.K.); the National Cancer Institute (R01CA202704 and R35 CA253185 to A.T.C.; R00CA215314 to MS); the American Cancer Society (MRSG-17-220-01—NEC to M.S.); and the Massachusetts General Hospital (Stuart and Suzanne Steel Research Scholars Award to A.T.C.).
Potential competing interests: None to report.
Study Highlights.
WHAT IS KNOWN
✓ Early-onset colorectal cancer (CRC) before age 50 years is rising.
✓ Antibiotic use has been linked to CRC after age 50 years, but no study has focused on early-onset CRC.
WHAT IS NEW HERE
✓ Overall antibiotic use was not associated with early-onset CRC risk.
✓ A possible link between broad-spectrum antibiotic use and early-onset CRC was observed.
Footnotes
SUPPLEMENTARY MATERIAL accompanies this paper at http://links.lww.com/CTG/A737
Long H. Nguyen and Yin Cao contributed equally to this work.
Contributor Information
Yin Cao, Email: yin.cao@wustl.edu.
Nurgul Batyrbekova, Email: nurgul.batyrbekova@scanddev.se.
Bjorn Roelstraete, Email: bjorn.roelstraete@ki.se.
Wenjie Ma, Email: wma6@mgh.harvard.edu.
Hamed Khalili, Email: hkhalili@mgh.harvard.edu.
Mingyang Song, Email: mis911@mail.harvard.edu.
Andrew T. Chan, Email: achan@mgh.harvard.edu.
Jonas F. Ludvigsson, Email: jonasludvigsson@yahoo.com.
REFERENCES
- 1.Hofseth LJ, Hebert JR, Chanda A, et al. Early-onset colorectal cancer: Initial clues and current views. Nat Rev Gastroenterol Hepatol 2020;17:352–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vuik FE, Nieuwenburg SA, Bardou M, et al. Increasing incidence of colorectal cancer in young adults in Europe over the last 25 years. Gut 2019;68:1820–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pearlman R, Frankel WL, Swanson B, et al. Prevalence and spectrum of germline cancer susceptibility gene mutations among patients with early-onset colorectal cancer. JAMA Oncol 2017;3:464–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lortet-Tieulent J, Soerjomataram I, Lin CC, et al. U.S. Burden of cancer by race and ethnicity according to disability-adjusted life years. Am J Prev Med 2016;51:673–81. [DOI] [PubMed] [Google Scholar]
- 5.Abualkhair WH, Zhou M, Ahnen D, et al. Trends in incidence of early-onset colorectal cancer in the United States among those approaching screening age. JAMA Netw Open 2020;3:e1920407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chang DT, Pai RK, Rybicki LA, et al. Clinicopathologic and molecular features of sporadic early-onset colorectal adenocarcinoma: An adenocarcinoma with frequent signet ring cell differentiation, rectal and sigmoid involvement, and adverse morphologic features. Mod Pathol 2012;25:1128–39. [DOI] [PubMed] [Google Scholar]
- 7.Wolf AMD, Fontham ETH, Church TR, et al. Colorectal cancer screening for average-risk adults: 2018 guideline update from the American Cancer Society. CA Cancer J Clin 2018;68:250–81. [DOI] [PubMed] [Google Scholar]
- 8.Recommendation draft: Colorectal cancer: screening. (https://www.uspreventiveservicestaskforce.org/uspstf/recommendation/colorectal-cancer-screening). Accessed December 3, 2021, USPSTF Program Office, Rockville, MD. Published 2021. [Google Scholar]
- 9.Low EE, Demb J, Liu L, et al. Risk factors for early-onset colorectal cancer. Gastroenterology 2020;159:492–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu PH, Wu K, Ng K, et al. Association of obesity with risk of early-onset colorectal cancer among women. JAMA Oncol 2019;5:37–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nguyen LH, Liu PH, Zheng X, et al. Sedentary behaviors, TV viewing time, and risk of young-onset colorectal cancer. JNCI Cancer Spectr 2018;2:pky073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen H, Zheng X, Zong X, et al. Metabolic syndrome, metabolic comorbid conditions and risk of early-onset colorectal cancer. Gut October 2021;70:1147–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Siegel RL, Torre LA, Soerjomataram I, et al. Global patterns and trends in colorectal cancer incidence in young adults. Gut 2019;68:2179–85. [DOI] [PubMed] [Google Scholar]
- 14.Baggs J, Fridkin SK, Pollack LA, et al. Estimating national trends in inpatient antibiotic use among US hospitals from 2006 to 2012. JAMA Intern Med 2016;176:1639–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Roumie CL, Halasa NB, Grijalva CG, et al. Trends in antibiotic prescribing for adults in the United States—1995 to 2002. J Gen Intern Med 2005;20:697–702. ( 10.1111/j.1525-1497.2005.0148.x). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Allwell-Brown G, Hussain-Alkhateeb L, Kitutu FE, et al. Trends in reported antibiotic use among children under 5 years of age with fever, diarrhoea, or cough with fast or difficult breathing across low-income and middle-income countries in 2005-17: A systematic analysis of 132 national surveys from 73 countries. Lancet Glob Health 2020;8:e799–e807. [DOI] [PubMed] [Google Scholar]
- 17.Antibiotic Use in Outpatient Settings, 2017. (https://www.cdc.gov/antibiotic-use/stewardship-report/outpatient.html). Published August 8, 2019. Accessed October 4, 2020. Center for Disease Control and Prevention. Atlanta, GA. [Google Scholar]
- 18.Fridkin S, Baggs J, Fagan R, et al. Vital signs: Improving antibiotic use among hospitalized patients. MMWR Morb Mortal Wkly Rep 2014;63:194–200. [PMC free article] [PubMed] [Google Scholar]
- 19.Vaz LE, Kleinman KP, Raebel MA, et al. Recent trends in outpatient antibiotic use in children. Pediatrics 2014;133:375–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kinlaw AC, Stürmer T, Lund JL, et al. Trends in antibiotic use by birth season and birth year. Pediatrics 2017;140:e20170441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Finkelstein JA, Raebel MA, Nordin JD, et al. Trends in outpatient antibiotic use in 3 health plans. Pediatrics 2019;143:e20181259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nguyen LH, Ma W, Wang DD, et al. Association between sulfur-metabolizing bacterial communities in stool and risk of distal colorectal cancer in men. Gastroenterology 2020;158:1313–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mölstad S, Löfmark S, Carlin K, et al. Lessons learnt during 20 years of the Swedish strategic programme against antibiotic resistance. Bull World Health Organ 2017;95:764–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tyrstrup M, Beckman A, Mölstad S, et al. Reduction in antibiotic prescribing for respiratory tract infections in Swedish primary care- a retrospective study of electronic patient records. BMC Infect Dis 2016;16:709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hanberger H, Skoog G, Ternhag A, et al. Antibiotic consumption and antibiotic stewardship in Swedish hospitals. Ups J Med Sci 2014;119:154–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nguyen LH, Örtqvist AK, Cao Y, et al. Antibiotic use and the development of inflammatory bowel disease: A national case-control study in Sweden. Lancet Gastroenterol Hepatol 2020;5:986–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wettergren B, Blennow M, Hjern A, et al. Child health systems in Sweden. J Pediatr 2016;177:S187–S202. ( 10.1016/j.jpeds.2016.04.055). [DOI] [PubMed] [Google Scholar]
- 28.Ludvigsson JF, Andersson E, Ekbom A, et al. External review and validation of the Swedish national inpatient register. BMC Public Health 2011;11:450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ludvigsson JF, Lashkariani M. Cohort profile: ESPRESSO (Epidemiology Strengthened by histoPathology Reports in Sweden). Clin Epidemiol 2019;11:101–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wallerstedt SM, Wettermark B, Hoffmann M. The first decade with the Swedish prescribed drug register - a systematic review of the output in the scientific literature. Basic Clin Pharmacol Toxicol 2016;119:464–9. [DOI] [PubMed] [Google Scholar]
- 31.Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J Chronic Dis 1987;40:373–83. [DOI] [PubMed] [Google Scholar]
- 32.Ludvigsson JF, Appelros P, Askling J, et al. Adaptation of the Charlson comorbidity index for register-based research in Sweden. Clin Epidemiol 2021;13:21–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sundararajan V, Henderson T, Perry C, et al. New ICD-10 version of the Charlson comorbidity index predicted in-hospital mortality. J Clin Epidemiol 2004;57:1288–94. [DOI] [PubMed] [Google Scholar]
- 34.Zhang J, Haines C, Watson AJM, et al. Oral antibiotic use and risk of colorectal cancer in the United Kingdom, 1989-2012: A matched case-control study. Gut 2019;68:1971–8. [DOI] [PubMed] [Google Scholar]
- 35.Cao Y, Wu K, Mehta R, et al. Long-term use of antibiotics and risk of colorectal adenoma. Gut 2018;67(4):672–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cao Y, Harrison T, Liu J, et al. 1015 integrative molecular marker analyses OF early-onset colorectal cancer support the importance OF the traditional adenoma-carcinoma sequence. Gastroenterology 2020;158:202. ( 10.1016/s0016-5085(20)31191-4) (rg/issue/S0016-5085(20)×6001-×?pageStart=10). [DOI] [Google Scholar]
- 37.Nguyen LH, Goel A, Chung DC. Pathways of colorectal carcinogenesis. Gastroenterology 2020;158:291–302. ( 10.1053/j.gastro.2019.08.059). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Song M, Nguyen LH, Emilsson L, et al. Antibiotic use associated with risk of colorectal polyps in a nationwide study. Clin Gastroenterol Hepatol 2021;19:1426–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wettermark B, Hammar N, Fored CM, et al. The new Swedish Prescribed Drug Register--opportunities for pharmacoepidemiological research and experience from the first six months. Pharmacoepidemiol Drug Saf 2007;16:726–35. [DOI] [PubMed] [Google Scholar]
- 40.Zhang L, Huang Y, Zhou Y, et al. Antibiotic administration routes significantly influence the levels of antibiotic resistance in gut microbiota. Antimicrob Agents Chemother 2013;57:3659–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kelly SA, Nzakizwanayo J, Rodgers AM, et al. Antibiotic therapy and the gut microbiome: Investigating the effect of delivery route on gut pathogens. ACS Infect Dis 2021;7:1283–96. [DOI] [PubMed] [Google Scholar]
- 42.Robertson RC, Manges AR, Finlay BB, et al. The human microbiome and child growth - first 1000 days and beyond. Trends Microbiol 2019;27:131–47. [DOI] [PubMed] [Google Scholar]
- 43.Stewart CJ, Ajami NJ, O'Brien JL, et al. Temporal development of the gut microbiome in early childhood from the TEDDY study. Nature 2018;562:583–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Örtqvist AK, Lundholm C, Halfvarson J, et al. Fetal and early life antibiotics exposure and very early onset inflammatory bowel disease: A population-based study. Gut 2019;68:218–25. [DOI] [PubMed] [Google Scholar]
- 45.J Natl Cancer Inst. 2021. May 4;113(5):543–552. [Google Scholar]
- 46.Summary of the Latest Data on Antibiotic Consumption in EU. 2016. (https://www.ecdc.europa.eu/en/publications-data/summary-latest-data-antibiotic-consumption-eu-2016). Published November 18, 2016. Accessed November 10, 2020. European Centre for Disease Prevention and Control. Stockholm, Sweden. [Google Scholar]