Supplemental Digital Content is available in the text.
Background.
Use of higher-risk grafts in liver transplantation for patients with acute-on-chronic liver failure (ACLF) has been associated with poor outcomes. This study analyzes trends in liver transplantation outcomes for ACLF over time based on the donor risk index (DRI).
Methods.
Using the Organ Procurement and Transplantation Network and the United Network for Organ Sharing registry, 17 300 ACLF patients who underwent liver transplantation between 2002 and 2019 were evaluated. Based on DRI, adjusted hazard ratios for 1-y patient death were analyzed in 3 eras: Era 1 (2002–2007, n = 4032), Era 2 (2008–2013, n = 6130), and Era 3 (2014–2019, n = 7138). DRI groups were defined by DRI <1.2, 1.2–1.6, 1.6–2.0, and >2.0.
Results.
ACLF patients had significantly lower risks of patient death within 1 y in Era 2 (adjusted hazard ratio, 0.69; 95% confidence interval, 0.61-0.78; P < 0.001) and Era 3 (adjusted hazard ratio, 0.48; 95% confidence interval, 0.42-0.55; P < 0.001) than in Era 1. All DRI groups showed lower hazards in Era 3 than in Era 1. Improvement of posttransplant outcomes were found both in ACLF-1/2 and ACLF-3 patients. In ACLF-1/2, DRI 1.2 to 1.6 and >2.0 had lower adjusted risk in Era 3 than in Era 1. In ACLF-3, DRI 1.2 to 2.0 had lower risk in Era 3. In the overall ACLF cohort, the 2 categories with DRI >1.6 had significantly higher adjusted risks of 1-y patient death than DRI <1.2. When analyzing hazards in each era, DRI > 2.0 carried significantly higher adjusted risks in Eras 1 and 3‚ whereas DRI 1.2 to 2.0 had similar adjusted risks throughout eras. Similar tendency was found in ACLF-1/2. In the non-ACLF cohort, steady improvement of posttransplant outcomes was obtained in all DRI categories. Similar results were obtained when only hepatitis C virus-uninfected ACLF patients were evaluated.
Conclusions.
In ACLF patients, posttransplant outcomes have significantly improved, and outcomes with higher-risk organs have improved in all ACLF grades. These results might encourage the use of higher-risk donors in ACLF patients and provide improved access to transplant.
INTRODUCTION
Acute-on-chronic liver failure (ACLF) is a succinctly defined, systemic syndrome characterized by acute clinical deterioration in the setting of cirrhosis, development of organ failure (OF)‚ and high 28-d mortality.1,2 ACLF grade 3 (ACLF-3), defined by the presence of 3 or more OFs, has an especially pronounced short-term mortality that can approach 80% at 28 d.1,3 Liver transplantation (LT) represents the only opportunity for long-term survival in many of these patients.4-6
Timely LT is critical because of the high waitlist mortality, especially in ACLF-3 patients, and because ACLF-3 patients who undergo LT within 30 d have better outcomes than those who wait longer.7 The use of higher-risk donor grafts might be one option to facilitate expedient LT; however, marginal or high donor risk index (DRI) grafts, historically, have resulted in suboptimal posttransplant outcomes in ACLF patients.7,8 One study found that a DRI >1.7 was independently associated with decreased 1-y survival in patients with ACLF-3.7 The use of marginal grafts for ACLF patients remains of imprecise benefit in that the risks associated with these grafts might outweigh the benefit of LT, especially in ACLF patients with multiple OFs.7,9
The use of higher-risk donor grafts has increased in the general liver transplant population over time. The outcomes with these grafts have improved because of several factors, including increased center experience combined with advances in surgical and medical care10; however, no studies have assessed the effect of this trend on posttransplant outcomes in ACLF patients. It remains unclear whether thresholds for utilizing higher-risk donors have changed over time and whether sicker ACLF patients benefit from high-risk donor grafts. This study aims to investigate trends of posttransplant outcomes in ACLF patients.
MATERIALS AND METHODS
Patient Cohort
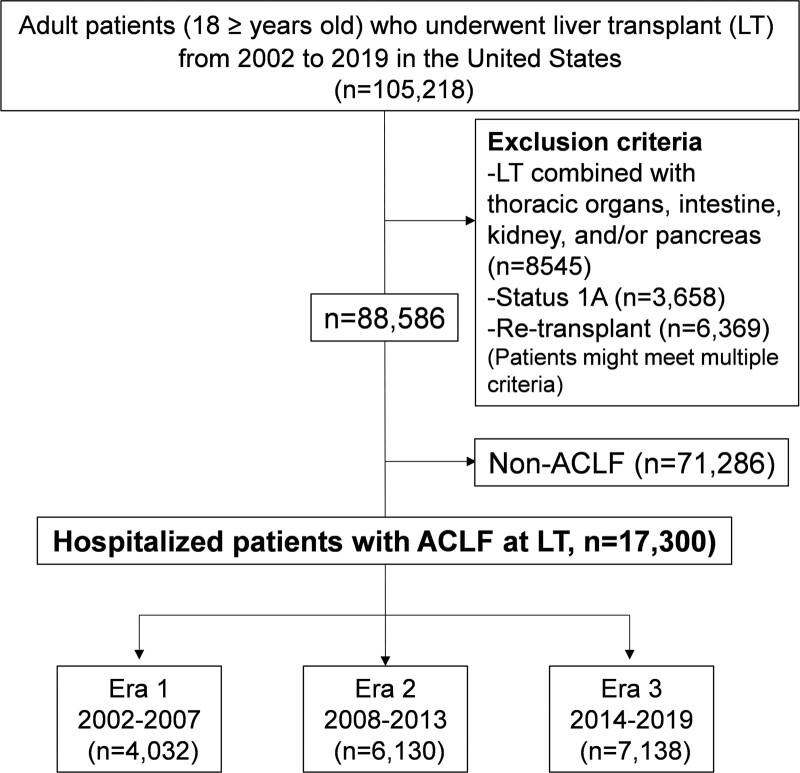
This study uses data from the Organ Procurement and Transplantation Network and United Network for Organ Sharing (OPTN/UNOS) in the Standard Transplant Analysis and Research file, which includes data on all patients who received an LT in the United States. Adult transplant patients (≥18 y old at LT) between January 1, 2002, and March 31, 2019, were evaluated. Patients with status 1A or retransplant or who underwent LT combined with thoracic organs, intestine, kidney, and pancreas were excluded (Figure 1).
FIGURE 1.
Flow chart of study population selection. ACLF, acute-on-chronic liver failure; LT, liver transplant.
Patients with ACLF were identified using the European Association for the Study of the Liver-Chronic Liver Failure criteria at the time of LT.1 In the OPTN/UNOS registry, specific OFs were assessed according to the presence of coagulopathy (international normalized ratio ≥2.5), liver failure (total bilirubin ≥12 mg/dL), renal failure (creatinine ≥2.0 mg/dL or dialysis), renal insufficiency (creatinine 1.5–1.9 mg/dL), grade 3 through 4 encephalopathy, circulatory failure (vasopressor requirement), and respiratory failure (mechanical ventilation requirement) in patients with a single manifestation of hepatic decompensation defined by either ascites or encephalopathy. In addition, ACLF population was limited to hospitalized patients based on the criteria reported in a previous study.1 We categorized all patients into 3 grades of ACLF based on the numbers of OFs at LT: ACLF-1 (single renal failure, renal insufficiency with nonrenal OF, or grade 1 through 2 encephalopathy with nonrenal OF), ACLF-2 (2 OFs), and ACLF-3 (3 or more OFs).7,8,11
This study was approved for an institutional review board waiver after an institutional review board review.
Trends in Posttransplant Outcomes Over Eras by DRI Category
The primary endpoint was 1-y posttransplant patient survival. To assess the trend of outcomes over time in ACLF patients, we categorized the study period into three 5-y eras: Era 1 (2002–2007), Era 2 (2008–2013), and Era 3 (2014–2019). Adjusted hazards of patient death within 1 y in Eras 2 and 3 were compared with Era 1 in the overall ACLF patient cohort and within each DRI category. In this analysis, hazards were adjusted for recipient variables and donor variables not included in the DRI formula. Recipient variables included age, gender, body mass index (BMI), diabetes, ethnicity, primary diagnosis (hepatitis C virus [HCV; LI_DGN 4104, 4106, 4204, 4206, 4593], nonalcoholic steatohepatitis [NASH, LI_DGN 4214], cholestatic liver disease [LI_DGN 4220-4265], alcohol-related liver disease [LI_DGN 4215-4217], metabolic liver disease [LI_DGN 4300-4315], and others), UNOS region, and ACLF grade 1 through 3. Donor variables included BMI and gender. As a subgroup analysis, posttransplant outcome trends were also assessed in ACLF-1 combined with ACLF-2 and ACLF-3.
Transplant outcomes over time have been affected by advances in HCV therapy. Era 3 coincides with the emergence of direct-acting antiviral agents and improved LT outcomes in HCV-infected patients. Therefore, a subgroup analysis was performed to assess the trends of posttransplant outcomes according to HCV status.
Analysis of Adjusted Hazards of 1-Y Patient Death Based on DRI in Each Era
Adjusted hazards of 1-y post-LT patient death in each DRI category (DRI 1.2–1.6, 1.6–2.0, >2.0 [ref: 0–1.2]) were analyzed using the Cox proportional hazards model. In this analysis, hazards were adjusted for the same recipient‚ and donor variables were used in the analysis over eras. As a subgroup analysis, adjusted hazards of patient death within 1 y in each DRI category were also analyzed by ACLF-1 combined with ACLF-2 and ACLF-3.
Analysis of Donor Risk Factors in Each Era
Donor risk factors for post-LT patient death within 1 y were analyzed separately in each era in ACLF patients. Donor risk factors were adjusted for recipient variables. Donor variables included age, gender, BMI, ethnicity, organ share (local, regional, or national), donor type (donation after brain death donor or donation after circulatory death [DCD] donor), cold ischemia time (CIT), cause of death (trauma, anoxia, or cerebrovascular accident), and graft type (whole or split liver graft). As the cutoffs of CIT, 6 h (median), 8 h (75 percentile in this cohort), and 12 h were used.10 Recipient variables included age, gender, BMI, ethnicity, diabetes, primary diagnosis, transplant type (liver alone or liver-kidney), UNOS region, and ACLF grade 1 through 3.
Posttransplant Outcome Analysis in Patients With Non-ACLF
One-year patient survival rates in each era and adjusted hazards of 1-y patient death based on DRI category were separately analyzed in the non-ACLF population. Patients with status 1A or retransplant or who underwent LT combined with thoracic organs, intestine, kidney, and pancreas were excluded (Figure 1). In this analysis, hazards were adjusted for recipient variables and donor variables not included in the DRI formula. Recipient variables included age, gender, BMI, diabetes, ethnicity, primary diagnosis, UNOS region, the Model for End-stage Liver Disease score, Karnofsky performance status score (10%–30%, 40%–60%, 70%–100%), encephalopathy (none/grades 1–2 or grades 3–4), ascites (absent/slight or moderate), life-support requirement, and status of dialysis (yes/no). Donor variables included BMI and gender.
Statistical Analysis
Data were summarized using the median with interquartile range for continuous variables and percentages for discrete variables. Comparisons of continuous variables and discrete variables were performed using the Kruskal-Wallis test and chi-square test, respectively. Patients were analyzed from time of LT using the Kaplan-Meier method‚ and groups were compared with log-rank tests. Risk factors for posttransplant patient death and the hazard risk were analyzed using Cox proportional hazards models. A P < 0.05 was considered statistically significant. All statistical analyses were completed using SPSS version 25 (IBM, Chicago, IL).
RESULTS
Patient Characteristics
A total of 17 300 patients with ACLF were evaluated. Of these, 4032 (23.3%), 6130 (35.4%), and 7138 (41.3%) were transplanted in Era 1, Era 2, and Era 3, respectively (Figure 1). The following recipient characteristics were greater in Era 3 than in Era 1 and Era 2 (Table 1): median age (Era 1: 52.0 versus Era 2: 55.0 versus Era 3: 55.0 y; P < 0.001), Model for End-stage Liver Disease score (33.0 versus 35.0 versus 36.0; P < 0.001), proportion of NASH (2.4% versus 9.3% versus 16.2%; P < 0.001) and alcohol-related liver disease (26.2% versus 25.3% versus 37.3%; P < 0.001), diabetes (20.6% versus 23.1% versus 23.6%; P = 0.001), liver failure (36.8% ver sus 38.6% versus 46.4%; P < 0.001), renal failure (32.1% versus 32.5% versus 38.6%; P < 0.001), coagulopathy (26.0% versus 27.6% versus 35.1%; P < 0.001), ACLF-2 (34.2% versus 37.0% versus 39.2%), and ACLF-3 (34.1% versus 34.1% versus 36.3%; P < 0.001). In ACLF patients undergoing transplant, duration on the waitlist was shorter in Era 3 than in Eras 1 and 2 (20 versus 21 versus 13 d; P < 0.001).
TABLE 1.
Characteristics at transplant in patients with ACLF
| Era 12002–2007(n = 4032) | Era 22008–2013(n = 6130) | Era 32014–2019(n = 7138) | P | ||
|---|---|---|---|---|---|
| Recipient characteristics | |||||
| Age (y), median [IQR] | 52.0 [46.0–58.0] | 55.0 [49.0–60.0] | 55.0 [47.0–61.0] | <0.001 | |
| Gender n, (%) | Male | 2674 (66.3) | 3904 (63.7) | 4291 (60.1) | <0.001 |
| Female | 1358 (33.7) | 2226 (36.3) | 2847 (39.9) | ||
| BMI (kg/m2), median [IQR] | 27.7 [24.4–32.1] | 28.7 [25.1–33.0] | 29.1 [25.3–33.7] | <0.001 | |
| Ethnicity n, (%) | White | 2761 (68.5) | 4153 (67.7) | 4864 (68.1) | 0.001 |
| Black | 343 (8.5) | 598 (9.8) | 557 (7.8) | ||
| Others | 928 (23.0) | 1379 (22.5) | 1717 (24.1) | ||
| Diagnosis n, (%) | HCV | 1247 (30.9) | 1845 (30.1) | 1005 (14.1) | <0.001 |
| NASH | 95 (2.4) | 570 (9.3) | 1156 (16.2) | ||
| CLD | 305 (7.6) | 489 (8.0) | 527 (7.4) | ||
| ALD | 1057 (26.2) | 1552 (25.3) | 2665 (37.3) | ||
| Metabolic | 80 (2.0) | 147 (2.4) | 160 (2.2) | ||
| Others | 1248 (31.0) | 1527 (24.9) | 1625 (22.8) | ||
| Diabetes n, (%) | 796 (20.6) | 1401 (23.1) | 1678 (23.6) | 0.001 | |
| Liver failure n, (%) | 1485 (36.8) | 2367 (38.6) | 3310 (46.4) | <0.001 | |
| Renal failure n, (%) | 1293 (32.1) | 1995 (32.5) | 2758 (38.6) | <0.001 | |
| Respiratory failure n, (%) | 302 (7.5) | 351 (5.7) | 433 (6.1) | 0.001 | |
| Coagulopathy n, (%) | 1047 (26.0) | 1693 (27.6) | 2506 (35.1) | <0.001 | |
| MELD score, median [IQR] | 33.0 [28.0–39.0] | 35.0 [30.0–40.0] | 36.0 [32.0–40.0] | <0.001 | |
| ACLF grades, n (%) | ACLF-1 | 1277 (31.7) | 1774 (28.9) | 1749 (24.5) | <0.001 |
| ACLF-2 | 1379 (34.2) | 2268 (37.0) | 2799 (39.2) | ||
| ACLF-3 | 1376 (34.1) | 2088 (34.1) | 2590 (36.3) | ||
| Days on waitlist (d), median [IQR] | 20 [6–152] | 21 [6–155] | 13 [4–83] | <0.001 | |
| Transplant centers, n | 108 | 117 | 118 | – | |
| Case numbers of ACLF at each center, median [IQR] | 64 [28–117] | 74 [36–140] | 91 [44–144] | 0.017 | |
| Donor characteristics | |||||
| Age (y), median [IQR] | 41.0 [24.0–54.0] | 40.0 [26.0–53.0] | 38.0 [27.0–52.0] | 0.080 | |
| Gender n, (%) | Male | 2440 (60.5) | 3613 (58.9) | 4349 (60.9) | 0.056 |
| Female | 1592 (39.5) | 2517 (41.1) | 2789 (39.1) | ||
| BMI (kg/m2), median [IQR] | 25.6 [22.6–29.2] | 26.2 [23.1–30.2] | 26.7 [23.4–30.8] | <0.001 | |
| Ethnicity n, (%) | White | 2754 (68.3) | 3894 (63.5) | 4437 (62.2) | <0.001 |
| Black | 480 (11.9) | 952 (15.5) | 1157 (16.2) | ||
| Others | 798 (19.8) | 1284 (20.9) | 1544 (21.6) | ||
| Cause of death n, (%) | Trauma | 1655 (41.0) | 2193 (35.8) | 2308 (32.3) | <0.001 |
| Anoxia | 535 (13.3) | 1484 (24.2) | 2521 (35.3) | ||
| Cerebrovascular | 1706 (42.3) | 2278 (37.2) | 2113 (29.6) | ||
| Others | 136 (3.4) | 175 (2.9) | 196 (2.7) | ||
| Donor type n, (%) | DBD | 3866 (95.9) | 5896 (96.2) | 6918 (96.9) | 0.001 |
| DCD | 127 (3.1) | 204 (3.3) | 189 (2.6) | ||
| LDLT | 39 (1.0) | 30 (0.5) | 31 (0.4) | ||
| Allocation type n, (%) | Local | 2904 (72.0) | 4250 (69.3) | 3288 (46.1) | <0.001 |
| Regional | 945 (23.4) | 1726 (28.2) | 3740 (52.4) | ||
| National | 183 (4.5) | 154 (2.5) | 110 (1.5) | ||
| Split graft n, (%) | 66 (1.6) | 50 (0.8) | 34 (0.5) | <0.001 | |
| CIT (h), median [IQR] | 7.0 [5.2–9.0] | 6.2 [5.0–8.0] | 6.0 [4.8–7.4] | <0.001 | |
| DRI, median [IQR] | 1.5 [1.3–1.8] | 1.5 [1.3–1.8] | 1.5 [1.3–1.7] | <0.001 |
Bold type indicates statistically significant differences.
ACLF, acute-on-chronic liver failure; ALD, alcohol-related liver disease; BMI, body mass index; CIT, cold ischemia time; CLD, cholestatic liver disease; DBD, donation after brain death; DCD, donation after circulatory death; DRI, donor risk index; HCV, hepatitis C virus; IQR, interquartile range; MELD, model for end-stage liver disease; NASH, nonalcoholic steatohepatitis.
In terms of donor characteristics, donors in Era 3, compared with Eras 1 and 2, had higher BMI (25.6 versus 26.2 versus 26.7 kg/m2; P < 0.001) and shorter CIT (7.0 versus 6.2 versus 6.0 h; P < 0.001). In Era 3, there was a higher proportion of donors with anoxia as the cause of death (13.3% versus 24.2% versus 35.3%; P < 0.001) and regional share donors (23.4% versus 28.2% versus 52.4%; P < 0.001), whereas the percent of split liver grafts (1.6% versus 0.8% versus 0.5%; P < 0.001) was lower. The median volume of LT performed for ACLF at each center increased significantly over time (64 versus 74 versus 91 cases per center per era; P = 0.017) (Table 1).
Trends in Posttransplant Outcomes Over Eras by DRI Category
In ACLF patients, 1-y patient survival rates in Era 1, Era 2, and Era 3 have improved steadily from 80.4% to 85.2% to 89.4%. All differences are statistically significant (P < 0.001; Table S1, SDC, http://links.lww.com/TXD/A402). For ACLF-1 combined with ACLF-2, 1-y patient survival improved from 82.4% to 87.3% to 90.1% (P < 0.001)‚ and for ACLF-3 patients, 1-y patient survival increased from 76.5% to 81.1% to 88.1% (P < 0.001). ACLF patients had significantly lower risks of patient death within 1 y in Era 2 (adjusted hazard ratio [aHR], 0.69; 95% confidence interval [CI], 0.61-0.78; P < 0.001) and Era 3 (aHR, 0.48; 95% CI, 0.42-0.55; P < 0.001) than in Era 1. When ACLF patients were stratified into the 4 DRI groups, all 4 groups had significantly lower adjusted risks in Era 3 than in Era 1 (Table 2). In the subgroup analysis of ACLF-1 and 2 patients combined, lower risks in Era 3 were found in the overall, DRI 1.2 to 1.6‚ and DRI >2 categories. ACLF-3 patients had significantly lower risks of patient death within 1 y in Era 2 (aHR, 0.74; 95% CI, 0.61-0.89; P = 0.001) and Era 3 (aHR, 0.43; 95% CI, 0.35-0.53; P < 0.001) than in Era 1. The 2 DRI categories between 1.2 and 2.0 had significantly lower adjusted risks in Era 3 than in Era 1, whereas the DRI >2.0 categories in Era 2 and Era 3 had risk comparable to Era 1 (Table 2). For ACLF-3 patients who received a donor graft with DRI between 1.6 and 2.0, 1-y patient survival went from 75.9% to 78.1% to 86.9% (P < 0.001). One-year patient survival rates in each Era based on the DRI group are shown in Table S1 (SDC, http://links.lww.com/TXD/A402).
TABLE 2.
Adjusted hazards of 1-y patient death in each DRI category according to eras (ref: Era 1 [2002–2007])
| Era 2 (2008–2013) | Era 3 (2014–2019) | |||
|---|---|---|---|---|
| aHR (95% CI) | P | aHR (95% CI) | P | |
| Overall ACLF | ||||
| Overall | 0.69 (0.61-0.78) | <0.001 | 0.48 (0.42-0.55) | <0.001 |
| 0 < DRI ≦ 1.2 | 0.90 (0.60-1.36) | 0.623 | 0.60 (0.39-0.94) | 0.025 |
| 1.2 < DRI ≦ 1.6 | 0.64 (0.53-0.76) | <0.001 | 0.39 (0.32-0.48) | <0.001 |
| 1.6 < DRI ≦ 2.0 | 0.92 (0.72-1.17) | 0.483 | 0.70 (0.54-0.90) | 0.005 |
| 2.0 < DRI | 0.68 (0.49-0.94) | 0.019 | 0.61 (0.43-0.87) | 0.006 |
| ACLF-1 and 2 | ||||
| Overall | 0.66 (0.57-0.77) | <0.001 | 0.52 (0.44-0.62) | <0.001 |
| 0 < DRI ≦ 1.2 | 0.73 (0.43-1.23) | 0.236 | 0.66 (0.39-1.14) | 0.137 |
| 1.2 < DRI ≦ 1.6 | 0.70 (0.55-0.90) | 0.005 | 0.47 (0.36-0.63) | <0.001 |
| 1.6 < DRI ≦ 2.0 | 0.91 (0.66-1.26) | 0.572 | 0.80 (0.58-1.12) | 0.197 |
| 2.0 < DRI | 0.62 (0.42-0.92) | 0.016 | 0.51 (0.33-0.78) | 0.002 |
| ACLF-3 | ||||
| Overall | 0.74 (0.61-0.89) | 0.001 | 0.43 (0.35-0.53) | <0.001 |
| 0 < DRI ≦ 1.2 | 1.52 (0.75-3.07) | 0.244 | 0.59 (0.27-1.31) | 0.194 |
| 1.2 < DRI ≦ 1.6 | 0.56 (0.42-0.73) | <0.001 | 0.31 (0.23-0.43) | <0.001 |
| 1.6 < DRI ≦ 2.0 | 0.95 (0.66-1.36) | 0.786 | 0.59 (0.40-0.87) | 0.008 |
| 2.0 < DRI | 0.98 (0.52-1.85) | 0.955 | 0.86 (0.45-1.66) | 0.648 |
| Non-ACLF | ||||
| Overall | 0.69 (0.64-0.75) | <0.001 | 0.47 (0.43-0.52) | <0.001 |
| 0 < DRI ≦ 1.2 | 0.90 (0.66-1.22) | 0.490 | 0.66 (0.48-0.92) | 0.013 |
| 1.2 < DRI ≦ 1.6 | 0.71 (0.62-0.81) | <0.001 | 0.47 (0.40-0.55) | <0.001 |
| 1.6 < DRI ≦ 2.0 | 0.71 (0.62-0.83) | <0.001 | 0.51 (0.44-0.61) | <0.001 |
| 2.0 < DRI | 0.68 (0.57-0.82) | <0.001 | 0.44 (0.36-0.54) | <0.001 |
Bold type indicates statistically significant differences.
ACLF, acute-on-chronic liver failure; aHR, adjusted hazard ratio; CI, confidence interval; DRI, donor risk index.
In the subgroup analysis of HCV patients, ACLF patients overall and those with DRI 1.2 to 1.6 had significantly lower adjusted risks of patient death within 1 y in Era 3 than in Era 1. A steady improvement was found in ACLF-1 and 2 with DRI 1.2 to 1.6. ACLF-3 patients overall and those with DRI 1.2 to 1.6 had lower risk in Era 3 than in Era 1 (Table S2, SDC, http://links.lww.com/TXD/A402). In non-HCV patients, overall ACLF patients and those with DRI >1.2 categories had significantly lower adjusted risks of patient death within 1 y in Era 3 than in Era 1. A steady improvement was reproduced for each ACLF grade (Table S3, SDC, http://links.lww.com/TXD/A402).
Adjusted Hazards of Patient Death Within 1 Y Based on DRI Category in Each Era
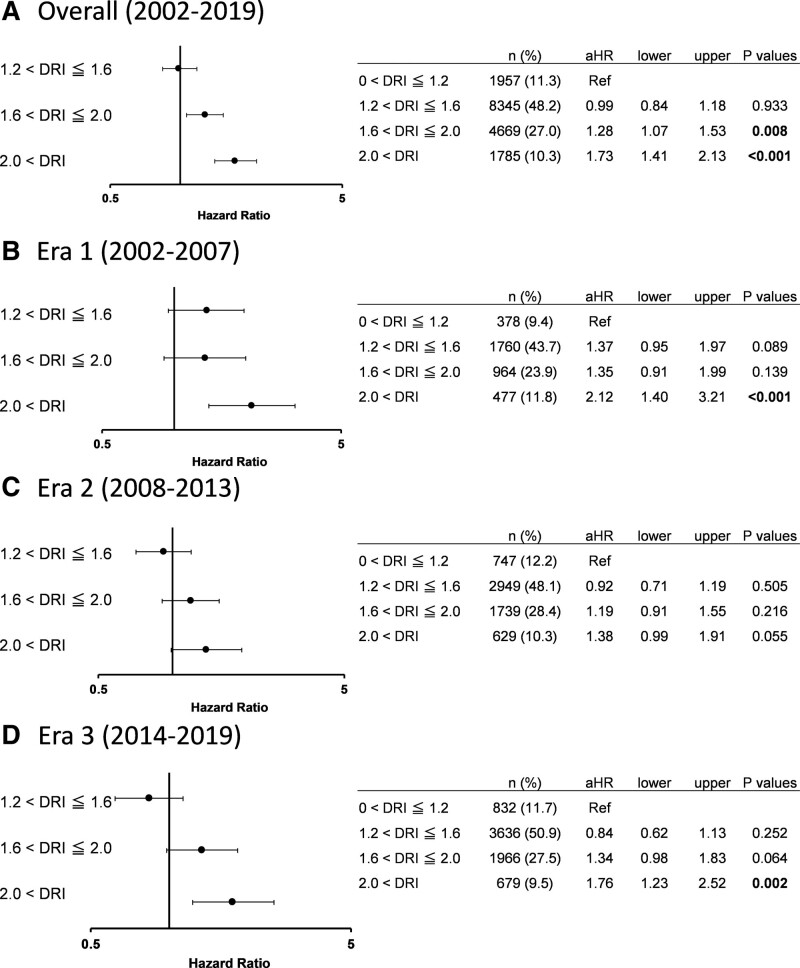
In the overall ACLF cohort, the 2 categories with DRI >1.6 had significantly higher adjusted risks of 1-y patient death. When analyzing hazards in each era, DRI >2.0 carried significantly higher adjusted risks in Era 1 and 3‚ whereas DRI 1.2 to 2.0 had similar adjusted risks compared with DRI 0 to 1.2 throughout eras (Figure 2).
FIGURE 2.
Adjusted hazards of 1-y patient death in each DRI category in ACLF (ref: 0 < DRI ≦ 1.2). A, Overall (2002–2019). B, Era 1 (2002–2007). C, Era 2 (2008–2013). D, Era 3 (2014–2019). ACLF, acute-on-chronic liver failure; aHR, adjusted hazard ratio; DRI, donor risk index.
In the subgroup analysis of ACLF-1 and 2, DRI >2.0 had higher adjusted risk of patient death in each era. Adjusted HRs have decreased in the DRI >2.0 category over time (Figure S1, SDC, http://links.lww.com/TXD/A402). In patients with ACLF-3, DRI 1.2 to 1.6 in Era 1 and DRI >2.0 in Era 3 were associated with an increased risk of 1-y patient death, whereas in Era 2, no DRI groups were associated with risk (Figure S2, SDC, http://links.lww.com/TXD/A402).
Subgroup Analysis of Posttransplant Outcomes Over Era by DCD Status
In 17 300 ACLF patients, 520 patients (3.0%) received a graft from a DCD donor. In ACLF patients who received a graft from a DCD donor, Era 2 (aHR, 0.85; 95% CI, 0.43-1.68; P = 0.64) and Era 3 (aHR, 0.60; 95% CI, 0.27-1.34; P = 0.21) had similar aHRs of patient death within 1 y compared with Era 1. In ACLF patients without a DCD donor, Era 2 (aHR, 0.74; 95% CI, 0.65-0.84; P < 0.001) and Era 3 (aHR, 0.53; 95% CI, 0.46-0.62; P < 0.001) had significantly lower risks than Era 1.
Donor Risk Factors for Patient Death Within 1 Y in Each Era
In the overall ACLF cohort, donor factors associated with 1-y patient death on multivariate analysis include older donor age (51–60, 61–70, >70 y), DCD donor, prolonged CIT (6–8, 8–12 h), and CVA as the cause of death. When analyzing donor risk factors in each era separately, older donor age (51–60, 61–70, >70 y), Black donor race, DCD donor, and CIT 8 to 12 h remained independent risk factors in Era 3. The risks of CIT 6 to 8 h and CVA as the cause of death have diminished over time, and these were no longer independent risk factors in Era 3 (Table 3).
TABLE 3.
Multivariable analysis of donor risk factors for 1-y patient death in ACLF patients
| Overall(2002–2019) | Era 1(2002–2007) | Era 2(2008–2013) | Era 3(2014–2019) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| aHR (95% CI) | P | aHR (95% CI) | P | aHR (95% CI) | P | aHR (95% CI) | P | ||
| Age (y) | ≤40 | Ref | Ref | Ref | Ref | ||||
| 41–50 | 1.15 (1.00-1.33) | 0.050 | 1.16 (0.88-1.52) | 0.304 | 1.09 (0.87-1.36) | 0.445 | 1.18 (0.92-1.51) | 0.191 | |
| 51–60 | 1.22 (1.06-1.41) | 0.007 | 1.07 (0.80-1.44) | 0.654 | 1.22 (0.97-1.53) | 0.086 | 1.30 (1.01-1.67) | 0.042 | |
| 61–70 | 1.57 (1.31-1.88) | <0.001 | 1.43 (1.02-2.02) | 0.041 | 1.45 (1.09-1.91) | 0.010 | 1.76 (1.28-2.44) | 0.001 | |
| 70< | 2.03 (1.51-2.75) | <0.001 | 1.50 (0.91-2.46) | 0.112 | 1.85 (1.12-3.06) | 0.017 | 2.70 (1.48-4.91) | 0.001 | |
| Gender | Male | Ref | Ref | Ref | Ref | ||||
| Female | 1.04 (0.93-1.15) | 0.502 | 0.83 (0.67-1.02) | 0.075 | 1.08 (0.92-1.27) | 0.360 | 1.15 (0.96-1.38) | 0.141 | |
| BMI (kg/m2) | <18.5 | 0.77 (0.53-1.10) | 0.149 | 1.07 (0.59-1.94) | 0.825 | 0.56 (0.30-1.06) | 0.076 | 0.77 (0.39-1.50) | 0.435 |
| 18.5–24.9 | Ref | Ref | Ref | Ref | |||||
| 25.0–29.0 | 0.90 (0.80-1.02) | 0.087 | 0.81 (0.64-1.02) | 0.070 | 0.92 (0.76-1.10) | 0.346 | 1.01 (0.82-1.25) | 0.919 | |
| ≥30 | 0.88 (0.77-0.99) | 0.042 | 0.97 (0.75-1.24) | 0.786 | 0.84 (0.69-1.03) | 0.099 | 0.92 (0.73-1.15) | 0.440 | |
| Ethnicity | White | Ref | Ref | Ref | Ref | ||||
| Black | 1.08 (0.94-1.25) | 0.285 | 1.19 (0.88-1.61) | 0.249 | 0.84 (0.66-1.06) | 0.140 | 1.45 (1.15-1.82) | 0.002 | |
| Others | 1.10 (0.96-1.25) | 0.165 | 1.18 (0.92-1.51) | 0.204 | 1.15 (0.94-1.41) | 0.171 | 1.05 (0.83-1.32) | 0.702 | |
| Organ share | Local | Ref | Ref | Ref | Ref | ||||
| Regional | 0.88 (0.79-0.99) | 0.026 | 0.98 (0.77-1.24) | 0.845 | 0.90 (0.75-1.08) | 0.250 | 1.11 (0.92-1.34) | 0.279 | |
| National | 1.10 (0.83-1.46) | 0.505 | 1.08 (0.68-1.72) | 0.754 | 1.14 (0.73-1.78) | 0.566 | 1.11 (0.60-2.06) | 0.735 | |
| Donor type | DBD | Ref | Ref | Ref | Ref | ||||
| DCD | 1.77 (1.37-2.29) | <0.001 | 1.51 (0.92-2.50) | 0.105 | 1.91 (1.32-2.78) | 0.001 | 1.67 (1.01-2.78) | 0.048 | |
| CIT (h) | ≤6 | Ref | Ref | Ref | Ref | ||||
| 6–8 | 1.14 (1.01-1.28) | 0.035 | 1.13 (0.88-1.44) | 0.353 | 1.14 (0.96-1.37) | 0.145 | 1.04 (0.85-1.28) | 0.681 | |
| 8–12 | 1.39 (1.22-1.59) | <0.001 | 1.37 (1.07-1.76) | 0.012 | 1.22 (0.99-1.51) | 0.064 | 1.29 (1.01-1.65) | 0.041 | |
| >12 | 1.36 (0.98-1.88) | 0.065 | 1.45 (0.91-2.32) | 0.118 | 0.83 (0.45-1.54) | 0.560 | 1.59 (0.74-3.38) | 0.233 | |
| Cause of death | Trauma | Ref | Ref | Ref | Ref | ||||
| Anoxia | 1.08 (0.94-1.25) | 0.253 | 1.14 (0.83-1.57) | 0.430 | 1.11 (0.89-1.38) | 0.376 | 1.22 (0.97-1.53) | 0.091 | |
| CVA | 1.23 (1.07-1.41) | 0.003 | 1.39 (1.08-1.79) | 0.010 | 1.30 (1.05-1.62) | 0.016 | 1.02 (0.79-1.31) | 0.904 | |
| Graft type | Whole | Ref | Ref | Ref | Ref | ||||
| Split | 1.24 (0.70-2.20) | 0.466 | 1.18 (0.48-2.90) | 0.718 | 0.94 (0.35-2.53) | 0.896 | 1.65 (0.52-5.21) | 0.392 |
Bold type indicates statistically significant differences.
ACLF, acute-on-chronic liver failure; aHR, adjusted hazard ratio; BMI, body mass index; CI, confidence interval; CIT, cold ischemia time; CVA, cerebrovascular accident; DBD, donation after brain death; DCD, donation after circulatory death.
Posttransplant Outcomes in Patients Without ACLF
In non-ACLF patients, 1-y patient survival rates in Era 1, Era 2, and Era 3 have improved steadily from 89.5% to 91.7% to 93.6%. Patient survival rates in DRI categories were summarized in Table S1 (SDC, http://links.lww.com/TXD/A402). Non-ACLF patients had significantly lower risks of patient death within 1 y in Era 2 (aHR, 0.69; 95% CI, 0.64-0.75; P < 0.001) and Era 3 (aHR, 0.47; 95% CI, 0.43-0.52; P < 0.001) than in Era 1. When non-ACLF patients were stratified into the 4 DRI categories, all 4 categories had significantly lower adjusted risks in Era 3 than in Era 1 (Table 2).
DISCUSSION
In this study, we demonstrate that posttransplant outcomes have significantly improved over time both in ACLF and non-ACLF patients. ACLF patients with DRI 1.2 to 1.6 had lower risk of patient death in Era 2 and 3 than in Era 1 regardless of ACLF grades. Those with DRI >2.0 had lower risk of patient death in Eras 2 and 3 than in Era 1 in ACLF-1 and 2‚ whereas this finding was not observed in ACLF-3. In the overall ACLF cohort, adjusted hazards of patients with DRI 1.2 to 1.6 grafts have decreased over time. In ACLF-1 and 2, the similar results were obtained‚ whereas in ACLF-3, no association between risks of patient death in each DRI category and eras was found. Our findings suggest that the improvement in posttransplant outcomes was observed in the ACLF cohort and the appropriate use of moderate-risk donors (DRI, 1.2–1.6) for ACLF patients may increase access to transplant without compromising posttransplant outcomes. Although special caution should be taken for the use of high-risk donors (DRI >2.0), the findings allow for a wider acceptance of marginal grafts in ACLF patients.
There are several reasons for the improvement in posttransplant outcomes. Performing LT in critically ill ACLF patients with higher-risk grafts represents a significant challenge to transplant centers. ACLF patients require specialized care that includes the identification and treatment of precipitating factors, stabilization of the patient, rapid and accurate risk assessment for LT, appropriate donor selection, and optimal perioperative management. The current data document a clear increase in ACLF volume per center over time‚ and centers have undoubtedly benefitted from this experience. Transplant center experience has been associated with improved outcomes in patients with acute liver failure.12 Specific and standardized improvements in the care of critically ill cirrhotic patients have also undoubtedly helped with improved posttransplant outcomes.13,14 Given the younger donor age, shorter CIT, and lower proportion of national donors seen in Era 3, we considered that the improvement might be attributable to careful donor selection at transplant centers. One factor that does not appear to be the primary reason for improved outcomes is the use of direct-acting antiviral therapy for HCV patients in Era 315,16 in that comparable improvement over time were seen in both HCV and non-HCV patients.
In addition, it was reported that post-LT outcomes with extended criteria donors‚ such as older donors or DCD donors‚ have been improving over time.10,17-19 This improvement has been driven by a better understanding of how to successfully utilize these organs through better donor and recipient matching and the refinement of the procurement operation.19 In this study, to address the effect of DCD grafts, we have analyzed trends of posttransplant outcomes in ACLF patients according to the presence of DCD donors. We showed that DCD donors were used only in 3.0% of ACLF patients, and posttransplant outcomes have improved over time regardless of DCD or donation after brain death donors. Based on this finding, we considered that the improvement of outcomes in ACLF patients might not be related to the improved outcomes of LT from DCD donors. In our cohort, improvement was also found in non-ACLF patients‚ which is consistent with previous reports.10,20 This improvement in the general population in the modern era could be one of the reasons for the improvement of outcomes in ACLF patients.
We did find a decrease in time on the waiting list for transplanted recipients especially in Era 3‚ and this may have contributed to the improved outcomes. ACLF patients experience organ damage that can worsen with time‚ and ACLF-3 patients have significant 30-d waitlist mortality. One study of improved transplant outcomes in ACLF-3 patients noted a median time from listing to transplant of 8 d.4 A previous analysis of ACLF patients in the UNOS registry noted that LT within 30 d of listing was associated with an improved outcome.7
Specific donor risk factors associated with patient death within 1 y were defined in each era. It is important to note that older donor grafts (>51 y), grafts from Black donors, DCD grafts‚ and grafts with prolonged CIT (8–12 h) remained independent risk factors in Era 3. Although an improvement in posttransplant outcomes in ACLF population was found over time, the use of grafts with higher DRI (>2.0) is still associated with an increased risk of patient death within 1 y in this era. It might be noted that ACLF-3 patients receiving grafts with DRI >2.0 in Era 3 still had 1-y patient survival of 78.8%‚ which markedly exceeds a reported 1-y survival of 7.9% without LT4; however, LT in this setting would come across issues of cost, organ utilization‚ and regulatory guardrails for expected outcomes. Centers will have to weigh the risks of higher-risk donors with the risks of longer waiting times on a case-by-case basis.
Since 2006, DRI has been used as a useful metric of donor quality in multiple studies and enhanced our understanding of donor factors and their impact on outcomes.21 This index has helped the decision-making process during an organ offer; however, DRI was derived from data before the Model for End-stage Liver Disease era, and it has been reported that there are some limitations of donor quality evaluation using DRI in the recent era because of the change of recipient and donor characteristics,21 policy changes over the decades,22,23 and the improvement of LT outcomes with the use of extended criteria donor grafts.10 In this study, CIT 6 to 8 and >12 h, organ share (local/regional/national), cause of donor death, and graft type (whole/split) did not relate to patient survival after LT in Era 3. This finding suggests that further research is encouraged to update DRI to reflect these changes in the current era.
There are limitations of this study that bear comment. First, both posttransplant and waitlist outcomes need to be taken into account to assess the prognostic impact of LT; however, intention-to-treat survival in ACLF patients is difficult to evaluate because ACLF is a dynamic syndrome and their ACLF status might be changed during the waiting time.11 Second, each OF, ascites, or encephalopathy might be misclassified or underestimated in the UNOS registry because this information is based on subjective evaluation. Third, some clinical information necessary for defining ACLF (such as the onset of ACLF) and selecting or grading ACLF patients is unavailable in the UNOS registry. Decompensating events such as variceal hemorrhage are not captured. We used mechanical ventilation as a surrogate marker of respiratory failure based on previous studies.7,8 Although macrosteatosis in the graft is reported to be associated with worse posttransplant outcomes,24 it is not included in the DRI and is not included in this study because of a large number of missing values in the UNOS registry. Fourth, although we created 5-y era groups to evaluated possible trends of posttransplant outcomes to equalize the number of patients in each era group, there have been several changes in the liver graft allocation system22,23 during this study period‚ which could have affected posttransplant outcomes. We selected the ACLF cohort based on their clinical status at the time of transplant but not at the time of listing. Therefore, the recent changes in the liver allocation would not necessarily affect their posttransplant outcomes. In addition, other important outcome metrics such as cost and complication rates are beyond the scope of this analysis. Despite these limitations, this is the first study investigating the trend of the outcomes with the use of higher-risk donors in ACLF patients by utilizing the UNOS registry.
In conclusion, our study demonstrated that posttransplant outcomes have significantly improved over time despite increased acuity of illness in ACLF patients. The use of marginal liver grafts might be considered rather than waiting for the use of ideal grafts for ACLF patients. Although the use of marginal grafts with multiple risk factors should be considered judiciously, our results suggest the potential to expand the donor pool and transplant access for ACLF patients while maintaining excellent transplant outcomes.
ACKNOWLEDGMENTS
The UNOS has supplied the data reported here as the contractor for the OPTN. The interpretation and reporting of these data are the responsibility of the authors and in no way should be seen as an official policy of or interpretation by the OPTN or the US government.
Supplementary Material
Footnotes
The authors declare no funding or conflicts of interest.
T.K., Y.K., T.I., and S.N. participated in research design, in the writing of the article, in the performance of the research, and in data analysis. S.S. and T.S. participated in research design, in the writing of the article, and in the performance of the research. M.L. participated in the writing of the article, in the performance of the research, and in data analysis. D.K. participated in the writing of the article and in the performance of the research. M.S.A. participated in research design and in the performance of the research.
Supplemental digital content (SDC) is available for this article. Direct URL citations appear in the printed text, and links to the digital files are provided in the HTML text of this article on the journal’s Web site (www.transplantationdirect.com).
REFERENCES
- 1.Moreau R, Jalan R, Gines P, et al. Acute-on-chronic liver failure is a distinct syndrome that develops in patients with acute decompensation of cirrhosis. Gastroenterology. 2013;144:1426–1437, 1437.e1421–1429. [DOI] [PubMed] [Google Scholar]
- 2.Shi Y, Yang Y, Hu Y, et al. Acute-on-chronic liver failure precipitated by hepatic injury is distinct from that precipitated by extrahepatic insults. Hepatology (Baltimore, Md). 2015;62:232–242. [DOI] [PubMed] [Google Scholar]
- 3.Arroyo V, Moreau R, Jalan R, et al. Acute-on-chronic liver failure: a new syndrome that will re-classify cirrhosis. J Hepatol. 2015;62(Suppl 1):S131–S143. [DOI] [PubMed] [Google Scholar]
- 4.Artru F, Louvet A, Ruiz I, et al. Liver transplantation in the most severely ill cirrhotic patients: a multicenter study in acute-on-chronic liver failure grade 3. J Hepatol. 2017;67:708–715. [DOI] [PubMed] [Google Scholar]
- 5.Sundaram V, Shah P, Wong RJ, et al. Patients with acute on chronic liver failure grade 3 have greater 14-day waitlist mortality than status-1a patients. Hepatology (Baltimore, Md). 2019;70:334–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thuluvath PJ, Thuluvath AJ, Hanish S, et al. Liver transplantation in patients with multiple organ failures: feasibility and outcomes. J Hepatol. 2018;69:1047–1056. [DOI] [PubMed] [Google Scholar]
- 7.Sundaram V, Jalan R, Wu T, et al. Factors associated with survival of patients with severe acute-on-chronic liver failure before and after liver transplantation. Gastroenterology. 2019;156:1381–1391.e1383. [DOI] [PubMed] [Google Scholar]
- 8.Sundaram V, Mahmud N, Perricone G, et al. Long-term outcomes of patients undergoing liver transplantation for acute-on-chronic liver failure. Liver Transpl. 2020;26:1594–1602. [DOI] [PubMed] [Google Scholar]
- 9.Karvellas CJ, Francoz C, Weiss E. Liver transplantation in acute-on-chronic liver failure. Transplantation. 2021;105:1471–1481. [DOI] [PubMed] [Google Scholar]
- 10.Zhang T, Dunson J, Kanwal F, et al. Trends in outcomes for marginal allografts in liver transplant. JAMA Surg. 2020;155:926–932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sundaram V, Kogachi S, Wong RJ, et al. Effect of the clinical course of acute-on-chronic liver failure prior to liver transplantation on post-transplant survival. J Hepatol. 2020;72:481–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wong NZ, Schaubel DE, Reddy KR, et al. Transplant center experience influences spontaneous survival and waitlist mortality in acute liver failure: an analysis of the UNOS database. Am J Transplant. 2021;21:1092–1099. [DOI] [PubMed] [Google Scholar]
- 13.McPhail MJ, Shawcross DL, Abeles RD, et al. Increased survival for patients with cirrhosis and organ failure in liver intensive care and validation of the chronic liver failure-sequential organ failure scoring system. Clin Gastroenterol Hepatol. 2015;13:1353–1360.e1358. [DOI] [PubMed] [Google Scholar]
- 14.Nadim MK, Durand F, Kellum JA, et al. Management of the critically ill patient with cirrhosis: a multidisciplinary perspective. J Hepatol. 2016;64:717–735. [DOI] [PubMed] [Google Scholar]
- 15.Flemming JA, Kim WR, Brosgart CL, et al. Reduction in liver transplant wait-listing in the era of direct-acting antiviral therapy. Hepatology (Baltimore, Md). 2017;65:804–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nagai S, Collins K, Chau LC, et al. Increased risk of death in first year after liver transplantation among patients with nonalcoholic steatohepatitis vs liver disease of other etiologies. Clin Gastroenterol Hepatol. 2019;17:2759–2768.e2755. [DOI] [PubMed] [Google Scholar]
- 17.Haugen CE, Holscher CM, Luo X, et al. Assessment of trends in transplantation of liver grafts from older donors and outcomes in recipients of liver grafts from older donors, 2003-2016. JAMA Surg. 2019;154:441–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Croome KP, Lee DD, Keaveny AP, et al. Improving national results in liver transplantation using grafts from donation after cardiac death donors. Transplantation. 2016;100:2640–2647. [DOI] [PubMed] [Google Scholar]
- 19.Croome KP, Taner CB. The changing landscapes in DCD liver transplantation. Curr Transplant Rep. 2020:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kwong AJ, Kim WR, Lake JR, et al. OPTN/SRTR 2019 annual data report: liver. Am J Transplant. 2021;21(Suppl 2):208–315. [DOI] [PubMed] [Google Scholar]
- 21.Flores A, Asrani SK. The donor risk index: a decade of experience. Liver Transpl. 2017;23:1216–1225. [DOI] [PubMed] [Google Scholar]
- 22.Kim WR, Biggins SW, Kremers WK, et al. Hyponatremia and mortality among patients on the liver-transplant waiting list. N Engl J Med. 2008;359:1018–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Feng S, O’Grady J. Share 35: a liver in time saves lives? Am J Transplant. 2015;15:581–582. [DOI] [PubMed] [Google Scholar]
- 24.Spitzer AL, Lao OB, Dick AA, et al. The biopsied donor liver: incorporating macrosteatosis into high-risk donor assessment. Liver Transpl. 2010;16:874–884. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.