Abstract
Background and aims
Published studies on coronavirus disease 19 (COVID-19) associated rhino-orbito-cerebral mucormycosis (CAROCM) were primarily descriptive. Therefore, we aimed to identify features of COVID-19 that could predispose to CAROCM and explore the pathogenic pathways.
Patients and methods
This retrospective hospital-based study was done during the first (March 2020 - January 2021) and the second (February 2021 - June 2021) waves of the COVID-19 pandemic. Subjects were grouped into four categories: first-wave CAROCM (n-4); second-wave CAROCM (n-27); first-wave non-mucor COVID (n-75), and second-wave non-mucor COVID (n-50). Data elements included age, gender, comorbidities, COVID-19 severity, steroid therapy, peak values of interleukin-6 (IL-6), serum ferritin and D-dimer, nadir values of absolute lymphocyte count (ALC), absolute neutrophil count (ANC) and platelet count (Pl. C).
Results
Thirty-one patients of CAROCM were included. The mean (SD) age was 51.26 (11.48) years. 27 (87.1%) were aged ≥ 40 years and males. Severe COVID-19 was seen more often in the second wave than the first wave (P-0.001). CAROCM group was significantly younger (P-0.008) and showed a higher incidence of uncontrolled diabetes (P-0.001) and renal dysfunction (P-0.004) than non-mucor COVID. While IL-6, ferritin and D-dimer were significantly elevated in CAROCM than non-mucor COVID, clinical severity, ANC, ALC and Pl. C showed no significant difference.
Conclusion
CAROCM is seen often in middle-aged diabetic males with uncontrolled hyperglycaemia, diabetic ketoacidosis, renal dysfunction and those infected by more transmissible delta variants and treated with steroids. IL-6, D-dimer, serum ferritin are more often elevated in CAROCM and might play a pathogenic role.
Keywords: Coronavirus disease-19, Rhino-orbito-cerebral mucormycosis, Delta variants, Diabetes, Hyperferritinemia, Cytokine storm
Introduction
Severe acute respiratory syndrome Coronavirus 2 (SARS CoV2) is a highly transmissible virus and causes Coronavirus disease 19 (COVID-19) [1]. From the time of its first report in December 2019 from Wuhan city, China, the disease has snowballed in no time to other parts of the world and was declared a pandemic by World Health Organisation (WHO) on March 11, 2020 [2]. India has witnessed the second wave of COVID-19 in the early and mid-2021, and during this phase, there had been an exponential increase in the incidence of mucormycosis, a deadly fungal infection. As of June 28 2021, the reported number of cases was 40,845 and 31,344 were rhino-cerebral [3]. Rhino-orbito-cerebral form of mucormycosis (ROCM) is potentially lethal, and the reported fatality rate ranged between 50% and 80% [4]. In India, this peril has become the double trouble, an epidemic of mucormycosis during the pandemic of COVID-19, leading to further strain on health resources [5]. The published reports on COVID-19 associated rhino-orbito-cerebral mucormycosis (CAROCM) from India were primarily descriptive and elaborated on the clinical features, radiological findings, treatment and outcomes [6], [7], [8], [9], [10], [11]. There were no systematic studies about the putative causative factors of this super-infection. This study attempted to identify the specific clinical and laboratory features in COVID-19 that are likely to predispose to ROCM and explore the possible pathogenic pathways.
Patients and methods
This retrospective cohort study was done in a tertiary care multi-speciality hospital, CARE Hospitals, Hyderabad, Telangana province, India. It was conducted as per the Ethical principles of the Declaration of Helsinki and Good Clinical Practices. The Institutional Ethics Committee of CARE hospital has approved the study (Ref. No. IEC/CARE/20,689/2021/OS; approved on June 17 2021). The ethics committee approved the consent waiver.
All admitted consecutive patients of CAROCM between March 2020 and June 2021 were enrolled in the study. Those between March 2020 and January 2021 were classified as first wave CAROCM cohort, and those between February 2021 and June 2021 were classified as second wave CAROCM cohort. Consecutive non-mucor COVID-19 patients during the first and second pandemic waves were also included for comparison. Seventy-five COVID-19 patients without mucormycosis admitted in April 2020 were classified as first wave non-mucor COVID-19 cohort and fifty COVID-19 patients without mucormycosis admitted in April 2021 as second wave non-mucor COVID-19 cohort. The patients with mucormycosis limited to the nasal cavity and paranasal sinuses (PNS) (without extension to orbital or cerebral areas) and non-COVID mucormycosis were excluded from the study.
Diagnosis of COVID-19 was based on a positive nasopharyngeal swab for reverse transcriptase-polymerase chain reaction (RT-PCR) SARS CoV2, by TaqPathTM COVID-19 Combo Kit (Applied Biosystems, Thermo Fisher Scientific, Massachusetts, USA). The clinical severity of COVID-19 was classified as non-severe and severe based on WHO classification. CAROCM patients underwent contrast magnetic resonance imaging (MRI) of the brain, orbits and PNS. Computed tomography (CT) of PNS was done in selected patients based on the treating physician's clinical judgment. Diagnosis of mucormycosis was based on clinical and radiological features along with microbiological or histopathological findings. CAROCM was designated as possible if the clinical features are associated with concurrent or recent COVID-19. It was designated probable if the clinical features are supported by characteristic nasal endoscopy or MRI or CT scan. It was considered proven if there was microbiological stains/culture or histopathological evidence.
Data collected from CAROCM patients included demographic data, the time interval between the onset of COVID-19 and mucormycosis, co-morbidities, use of steroids in the preceding three weeks (cumulative dose) and other immunosuppressant drugs, clinical severity, peak titres of interleukin-6 (IL-6), serum ferritin and D-dimer, nadir values of absolute lymphocyte count (ALC), absolute neutrophil count (ANC) and platelet count (Pl. C). The onset of ROCM was defined concurrent if contemporaneous with COVID-19 or as sequential if it developed following the onset of COVID-19. Diabetes was classified into longstanding diabetes mellitus (DM), denovo DM and diabetic ketoacidosis (DKA). The outcome was classified into eye rescued, vision rescued, and a life rescued. Data collected from non-mucor COVID-19 patients included demographic data, co-morbidities, steroids and other immunosuppressant drug usages, clinical severity of COVID-19, peak titres of IL-6, serum ferritin, D-dimer, nadir values of ALC, ANC and Pl.C.
Statistical analysis was performed using SPSS version 21.0 (Armonk, NY: IBM Corp.). All continuous variables were reported as mean with standard deviation (SD) or median with inter-quartile range (IQR). To compare differences in the continuous variables between non-mucor COVID-19 and CAROCM, an unpaired t-test or Mann-Whitney U test was used, and the Chi-square test was used for proportions. Probability (P) values less than 0.05 were considered significant.
Results
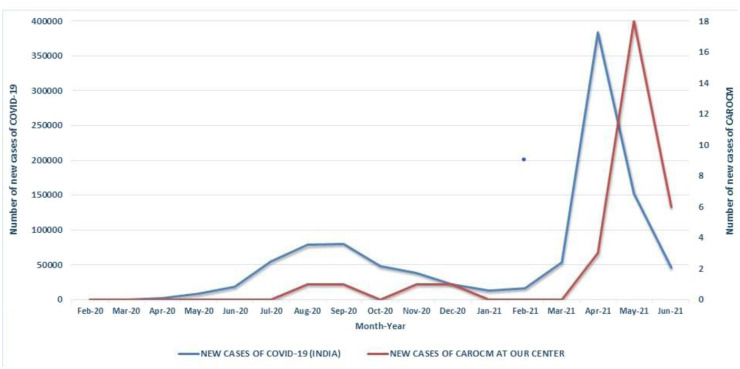
A total of thirty-one patients of CAROCM were included in this study during the specified period. The mean ± SD age was 51.26 ± 11.48 years. 27 (87.1%) patients were aged ≥ 40 years and males. Of them, 4 (12.9%) were from the first wave, and the remaining 27 (87.1%) were during the second pandemic wave (Fig. 1 ). Comparing non-mucor COVID-19 between the first and second waves showed a higher incidence of severe COVID-19 in the second wave (16% versus 49%; p <0.001).
Fig. 1.
Line diagram showing the trend of newly diagnosed cases of COVID-19 in India (blue) and rhino-orbito-cerebral mucormycosis cases of our study (red).
Clinical severity of COVID-19 was mild in 17 (54.84%), moderate in 4 (12.90%) and severe in 10 (32.26%) patients. Therapy for COVID-19 included corticosteroids in 25 (80.64%), Remdesivir in 14 (45.16%), Bariticinib and Bevacizumab in one each (3.22%). Prednisolone equivalent dose used was greater than 400 mg in 22 (70.96%). Co-morbid diseases included DM in 31 (100%), hypertension in 15 (48.4%), renal dysfunction in 8 (25.8%), coronary artery disease in 4 (12.9%), hypothyroidism in 2 (6.45%), bronchial asthma in 3 (9.68%), epilepsy and stroke in one each (3.22%). DM was de-novo detected in 7 (22.6%) and longstanding in the remaining 24 (77.4%) subjects. Uncontrolled DM and DKA were noted in 25 (80.65%) and 7 (22.6%), respectively. The median (IQR) of ANC and ALC (cells/mm3) were 6460 (4532–7905) and 900(532–1160), respectively. ALC was less than 1100 cells/mm3 in 19 (61.29%) patients. Values of inflammatory markers were not available in all subjects. Values of IL-6 (pg/ml) were available in 17, and it was elevated in all of them. The median (IQR) was 56.37 (30.6–203.89). Values of serum ferritin (ng/ml) were available in 20 patients and elevated in 13. The median (IQR) was 907.35 (676.7 - 1260). Values of D-dimer (ng/ml) were available in 21 patients and elevated in 11. The median (IQR) was 450 (222 - 824). The onset of ROCM was concurrent in 3 (9.67%) and sequential in 28 (90.32%). The mean ± duration between the onset of COVID-19 and CAROCM in the sequential group was 16.28±6.6 days. Features of COVID-19 and ROCM in the CAROCM cohort are shown in Tables 1 and 2 , respectively. Clinical, radiological and histopathological features are shown in Fig. 2, Fig. 3 .
Table 1.
. COVID-19 features in rhino-orbito-cerebral mucormycosis cases.
| Case no | Age/ Sex | Month & Year | Severity of COVID-19 † | Remdesivir or Biological therapies | Prednisolone Equivalent dose (mg) | Diabetes | DKA ‡ | Hypertension | Renal dysfunction | ANC § (cells/mm3) | ALC ¶ (cells/mm3) | IL-6 †† (pg/ml) | Serum Ferritin (ng/ml) | D-Dimer | Platelet count (x105 cells/mm3) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 57/M | November 2020 | Mild | – | 0 | + | – | + | + | 4592 | 2262 | 28.2 | ND ‡‡ | 180 | 3.79 |
| 2 | 67/M | December 2020 | Mild | – | 1000 | + | – | + | – | 3795 | 900 | 45.4 | 682 | 650 | 3.78 |
| 3 | 70/M | August 2020 | Mild | – | 500 | + | – | – | – | 4532 | 523 | ND‡‡ | ND‡‡ | ND‡‡ | 2.45 |
| 4 | 52/M | September 2020 | Mild | – | 937.5 | + | – | – | – | 5148 | 726 | ND‡‡ | ND‡‡ | ND‡‡ | 1.98 |
| 5 | 60/M | April 2021 | Mild | – | 533.5 | + | – | – | + | 8008 | 372 | 18.2 | 1500 | 4300 | 0.69 |
| 6 | 30/M | April 2021 | Moderate | Remdesivir | 1375 | + (Denovo) | + | – | + | 17,520 | 1314 | 141.78 | 1500 | 1493 | 2.77 |
| 7 | 58/F | April 2021 | Mild | – | 0 | + | – | + | – | 6500 | 1140 | ND‡‡ | 980 | ND‡‡ | 2.91 |
| 8 | 54/M | May 2021 | Severe | Remdesivir | 500 | + (Denovo) | – | – | – | 7600 | 1120 | 1172 | ND‡‡ | 2965 | 1.38 |
| 9 | 47/M | May 2021 | Mild | – | 266 | + (Denovo) | – | + | – | 6515 | 750 | 80.37 | 741 | 517 | 3.18 |
| 10 | 45/M | May 2021 | Mild | – | 550 | + | + | – | + | 5762 | 603 | ND‡‡ | ND‡‡ | ND‡‡ | 1.55 |
| 11 | 56/M | May 2021 | Moderate | Remdesivir | 400 | + | – | + | – | 6324 | 436 | 91.2 | 1080 | 780 | 1.45 |
| 12 | 61/F | May 2021 | Mild | – | 140 | + | – | + | – | 9452 | 3475 | ND‡‡ | 374.5 | 296 | 2.42 |
| 13 | 25/M | May 2021 | Mild | – | 293 | + (Denovo) | + | – | + | 1500 | 1136 | ND‡‡ | 1500 | 150 | 1.32 |
| 14 | 41/M | May 2021 | Mild | Remdesivir | 500 | + | – | + | – | 3186 | 2014 | ND‡‡ | 804.9 | 200 | 1.98 |
| 15 | 56/M | May 2021 | Severe | Remdesivir, Barcitinib | 0 | + | – | + | – | 3023 | 517 | ND‡‡ | 974 | ND‡‡ | 0.95 |
| 16 | 64/M | May 2021 | Moderate | Remdesivir | 550 | + | – | + | – | 7390 | 1124 | ND‡‡ | 1320 | ND‡‡ | 3.3 |
| 17 | 46/M | May 2021 | Mild | – | 0 | + | – | – | + | 6460 | 627 | ND‡‡ | ND‡‡ | ND‡‡ | 2.76 |
| 18 | 45/M | May 2021 | Severe | Remdesivir | 675 | + (Denovo) | – | + | – | 8032 | 1160 | 118.5 | 242 | 868 | 1.22 |
| 19 | 48/M | May 2021 | Mild | – | 400 | + | – | – | – | 7340 | 724 | 28.83 | 207.7 | 292 | 2.95 |
| 20 | 47/M | May 2021 | Moderate | Remdesivir | 625 | + | – | + | – | 6790 | 1260 | ND‡‡ | ND‡‡ | ND‡‡ | 1.78 |
| 21 | 48/M | May 2021 | Severe | – | 563 | + | + | – | – | 7905 | 425 | 266 | 1500 | 659 | 0.57 |
| 22 | 45/M | May 2021 | Severe | Remdesivir | 500 | + | – | – | + | 4800 | 920 | 42 | 450 | 480 | 1.72 |
| 23 | 42/M | May 2021 | Mild | – | 100 | + | + | + | – | 4000 | 900 | ND‡‡ | ND‡‡ | ND‡‡ | 1.5 |
| 24 | 56/M | June 2021 | Severe | Remdesvir Bevacizumab | 738 | + | + | + | + | 4284 | 612 | ND‡‡ | 759 | 140 | 1.2 |
| 25 | 60/F | June 2021 | Severe | Remdesivir | 542 | + | + | + | + | 5496 | 463 | 445.7 | 840.7 | 450 | 2.63 |
| 26 | 42/F | May 2021 | Mild | – | 480 | + | + | – | – | 6240 | 735 | 32.5 | 743.5 | 234 | 1.67 |
| 27 | 38/M | May 2021 | Severe | Remdesivir | 1055.5 | + | – | – | – | 2948 | 532 | 56.37 | 1056 | 260 | 1.33 |
| 28 | 33/M | June 2021 | Severe | Remdesivir | 627.5 | + | – | – | – | 10,080 | 440 | 7.8 | 984 | 288 | 4.67 |
| 29 | 53/M | June 2021 | Mild | – | 0 | + (Denovo) | – | – | – | 8946 | 975 | 54.7 | ND‡‡ | 210 | 2.45 |
| 30 | 69/M | June 2021 | Mild | – | 0 | + | – | – | – | 7528 | 1432 | ND‡‡ | ND‡‡ | ND‡‡ | 2.67 |
| 31 | 70/M | June-2021 | severe | Remdesivir | 527 | + | + | + | + | 13,072 | 1520 | 1004.88 | ND‡‡ | 889 | 1.20 |
† COVID-19: Corona virus disease 2019; ‡ DKA: Diabetic ketoacidosis; § ANC: Absolute neutrophil count; ¶ ALC: Absolute leukocyte count; †† IL-6: Interleukin 6; ‡‡ ND: Not done.
Table 2.
Clinical features, treatment and outcome of COVID-19 associated rhino-orbito-cerebral mucormycosis cases.
| Case no | Onset type (Concurrent or Sequential) | Time (in days) between COVID-19 † and Mucormycosis | Presenting Symptoms | Cranial nerves involved | Nasal cavity and paranasal sinus debridement (Yes/No) | Palatal Resection/ Maxillectomy (Yes/No) | Orbital Exentration (Yes/No) | Craniotomy (Yes/No) | Drugs | Classification | Vision Salvage (Yes/No) | Eye Salvage(Yes/No) | Life Salvage (Yes/No) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Concurrent | 0 | Headache; vision loss purulent discharge from nose | II | Y | Y/N | N | Craniotomy with abscess drainage | LAmB‡, ISZ§ | Confirmed | Y | Y | Y |
| 2 | Sequential | 30 | Nasal crusting and block | None | Y | N/N | N | N | LAmB‡, POS¶, ISZ§ | Confirmed | Y | Y | Y |
| 3 | Sequential | 14 | Nasal crusting and block | None | Y | N/N | N | N | LAmB‡, POS¶ | Confirmed | Y | Y | Y |
| 4 | Concurrent | 0 | Right-sided Headache; Nasal crusting, vision loss in right eye | II,III,IV,V,VI | Y | N/N | Y | N | LAmB‡, POS¶ | Confirmed | N | N | Y |
| 5 | Sequential | 14 | Headache; Left eye Swelling & vision loss; Ptosis; Altered sensorium | II,III,IV,V,VI | Y | Y/Y | Y | N | ISZ§ | Confirmed | N | N | N |
| 6 | Sequential | 14 | Left-sided vision loss and facial numbness; Altered sensorium | II,III,IV,V,VI | N | N/N | N | N | ISZ§ | Probable | N | N | N |
| 7 | Sequential | 3 | Right-sided facial and eye swelling and decreased vision; left hemiparesis | II,III,IV,V | Y | Y/Y | N | N | LAmB‡, POS¶ | Confirmed | N | Y | Y |
| 8 | Sequential | 20 | Left-sided ocular pain, swelling & vision loss | II,III,IV,V,VI | Y | Y/N | Y | N | LAmB‡ | Confirmed | N | N | N |
| 9 | Sequential | 13 | Headache, left-sided ocular pain, swelling and vision loss | II,III,IV,V,VI | Y | Y/N | Y | N | LAmB‡, ISZ§ | Confirmed | N | N | Y |
| 10 | Sequential | 16 | Right-sided ocular pain, swelling and vision loss; left Hemiparesis; altered sensorium | II,III,IV,V,VI | Y | Y/Y | Y | N | ISZ§, POS¶ | Confirmed | N | N | N |
| 11 | Sequential | 14 | Headache; bilateral nasal block | II,III,IV,V,VI | Y | N/N | N | Bilateral frontal craniotomy | LAmB‡, POS¶ | Confirmed | Y | Y | Y |
| 12 | Sequential | 13 | Headache; left-sided ocular pain, swelling and vision loss | II,III,IV,V,VI | Y | N/N | Y | N | POS¶ | Confirmed | N | N | N |
| 13 | Sequential | 14 | Headache; left-sided ocular pain swelling, vision loss and ptosis; right hemiparesis | II,III,IV,V,VI | Y | N/Y | Y | Left Decompressive craniotomy | LAmB‡ | Confirmed | N | N | Y |
| 14 | Sequential | 8 | Headache; left-sided facial pain | V | Y | N/N | N | N | LAmB‡ | Confirmed | Y | Y | Y |
| 15 | Sequential | 27 | Altered sensorium | CNBT†† | Y | N/N | N | N | LAmB‡ | Confirmed | N | N | N |
| 16 | Sequential | 15 | Headache; nasal crusting and diplopia | II,III,IV | Y | Y/Y | N | N | LAmB‡ | Confirmed | Y | Y | Y |
| 17 | Sequential | 7 | Headache; right-sided vision loss | II,III,IV,VI | Y | Y/N | Y | N | LAmB‡, ISZ§ | Confirmed | N | N | N |
| 18 | Sequential | 10 | Left-sided facial pain; nose block | CNBT†† | Y | N/N | N | N | LAmB‡, ISZ§ | Confirmed | N | N | N |
| 19 | Sequential | 14 | Left-sided periorbital pain and facial numbness | V | Y | N/N | N | N | ISZ§ | Confirmed | Y | Y | Y |
| 20 | Sequential | 16 | Left-sided periorbital pain and nose block | None | Y | N/N | N | N | POS¶ | Confirmed | Y | Y | Y |
| 21 | Sequential | 12 | Headache, bilateral eye swelling, ptosis and vision loss | II,III,IV,V,VI (Bilateral) | Y | N/N | N | N | LAmB‡, ISZ§ | Confirmed | N | N | N |
| 22 | Sequential | 27 | Headache; left-sided nose block and double vision | III,V | Y | N/N | N | N | LAmB‡, POS¶ | Confirmed | Y | Y | Y |
| 23 | Sequential | 14 | Headache, Left sided vision loss | II, III,IV,V,VI | Y | N/N | Y | N | LamB‡ | Confirmed | N | N | Y |
| 24 | Sequential | 22 | Headache, Right Frontal Nose block, Bilateral | None | Y | N/N | N | Y with abscess drainage | LamB‡ | Confirmed | Y | Y | Y |
| 25 | Sequential | 20 | Bilateral frontal headache | None | Y | N/N | N | Y(Bilateral frontal craniotomy) | LamB‡ | Confirmed | N | N | N |
| 26 | Sequential | 28 | Facial Deviation to the right, Difficulty in swallowing | VII.IX,X,XII | Y | N/N | N | N | LamB‡, POS¶ | Confirmed | Y | Y | Y |
| 27 | Sequential | 16 | Headache and left eye vision loss | II, III,IV,V,VI | Y | N/N | Y | N | LamB‡, POS¶ | Confirmed | N | N | Y |
| 28 | Sequential | 19 | Headache right frontal,right eye vision loss | II, III,IV,V,VI | Y | N/N | Y | N | LamB‡, | Confirmed | N | N | Y |
| 29 | Concurrent | 0 | Headache, Bilateral vision loss | II,III,IV,V,VI (Bilateral) | Y | N/N | N | N | LamB‡,POS¶ | Confirmed | N | Y | Y |
| 30 | Sequential | 13 | Headache, Left side vision loss | II, III,IV,V,VI | Y | N/N | N | N | LamB‡ | Confirmed | N | Y | Y |
| 31 | Sequential | 25 | Altered sensorium | CNBT†† | N | N/N | N | N | ISZ§, LamB‡ | Probable | N | N | N |
†COVID-19: Corona virus disease 2019; ‡ LAmB: Liposomal Amphotericin; § ISZ: Isuvaconazole; ¶ POS: Posaconazole; †† CNBT: Could not be tested.
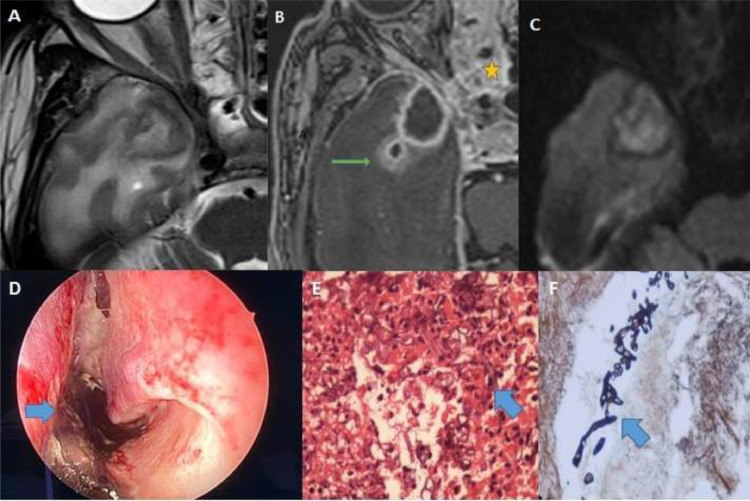
Fig. 2.
(A–F): MR axial images of case no. 1 showing a hypointense lesion on T2 with perilesional edema in the right temporal lobe (A); peripherally enhancing with daughter lesion posterolateral to larger lesion on contrast with ethmoidal sinusitis (yellow star) and sphenoid sinusitis(B); lesion showing central diffusion restriction (C); nasal endoscopy of the same patient showing black fungal material on the nasal septum (D); the histopathological picture of sinus mucosa showing broad aseptate hyphae suggestive of Mucor sp.(E); gomori methenamine silver stain showing fungal elements (F).
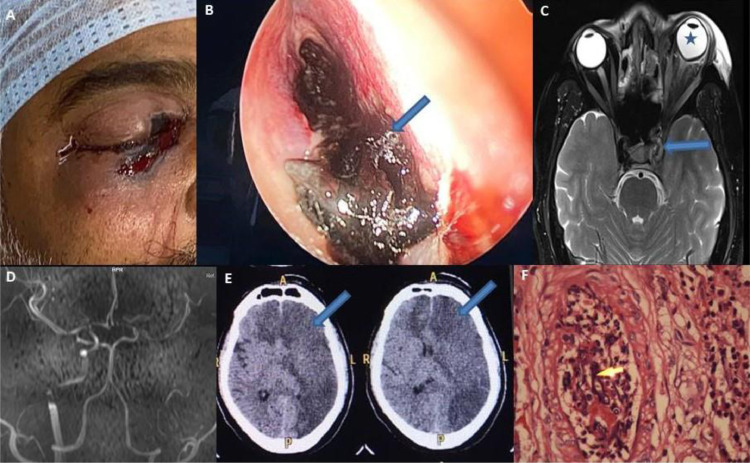
Fig. 3.
(A–F): Clinical photograph of case no. 6 showing blackish periorbital discolouration (A); nasal endoscopy of the same patient showing black fungal material in the nasal cavity (B); MR axial images of the brain and orbit of case no. 9 showing enlargement of the left cavernous sinus (arrow) and guitar pick sign (blue star) (C); 3D Time of flight images of case no. 9 showing non-visualization of the left intracranial portion of the internal carotid artery (D); CT brain of the same patient showing left-hemispheric infarction and right anterior cerebral artery infarction (E); the histopathological picture of sinus mucosa showing angioinvasion (shown in yellow arrow) (F).
We limited the comparative assessment of non-mucor COVID-19 and CAROCM to only the second wave as there were only four patients of CAROCM in the first wave. Second wave data analysis showed CAROCM patients to be younger (49.74±11.19 versus 58.92±15.29; p 0.008) with higher incidence of DM (100% versus 50%; p <0.001) and renal dysfunction (25.9% versus 4%; p 0.004) than non-mucor COVID-19. Serum IL-6 (80.6 versus 29.4; p 0.007), serum ferritin (974 versus 138.9; p <0.001) and D-dimer (450 versus 215; p 0.048) were higher in CAROCM than non-mucor COVID-19. There was no significant difference in the severity of COVID-19, ANC, ALC and Pl. C values between the two groups. Comparative assessment between all non-mucor COVID-19 and CAROCM patients (first and the second wave included) showed similar results (Table 3 ).
Table 3.
Comparison of non-mucor Covid and CAROCM cohorts.
| Parameter | Both waves Non-Mucor COVID-19† (N = 125) | Both waves Mucor COVID-19 † (N = 31) | Second wave Non-Mucor COVID-19† (N = 50) | Second wave Mucor COVID-19† (N = 27) | P value* | P value** |
|---|---|---|---|---|---|---|
| Age (Mean±SD)‡ | 60.71±4.7 | 51.26±11.477 | 68.92±7.17 | 49.74±11.19 | 0.053 | 0.008 |
| Male gender n(%) | 93(74.4) | 27 (87.1) | 36(72) | 23(85.2) | 0.133 | 0.192 |
| Severe COVID-19† n(%) | 36(28.8) | 10 (32.3) | 24(48) | 10(37) | 0.705 | 0.355 |
| Diabetes n(%) | 51(40.8) | 31 (100) | 25(50) | 27(100) | 0.000 | 0.000 |
| Hypertension n(%) | 54(43.2) | 15 (48.4) | 25(50) | 13 (48.1) | 0.603 | 0.877 |
| Renal Dysfunction n(%) | 4(3.2) | 8 (25.8) | 2(4) | 7(25.9) | 0.000 | 0.004 |
| IL-6§ Median (IQR)¶ | 29.4(15.2–54.7) | 56.37 (30.6–203.89) | 29.4 (19.63–50.76) | 80.37 (32.5–266) | 0.04 | 0.007 |
| Serum Ferritin Median (IQR)¶ | 154.2 (72.9–310.85) | 907.35 (696.7–1260) | 138.9 (70.8–269.95) | 974 (741–1320) | 0.000 | 0.000 |
| D-Dimer Median (IQR)¶ | 249 (132.5–660.75) | 450 (222–824) | 215 (114–650.25) | 450 (234–868) | 0.065 | 0.048 |
| ALC†† Median (IQR)¶ | 1035 (660–1512) | 900 (532–1160) | 1013 (660–1417) | 900 (532 - 1160) | 0.173 | 0.323 |
| ANC‡‡ Median (IQR)¶ | 3822 (2543–5784) | 6460 (4532–7905) | 3549 (2446.5–5892) | 6515 (4800–8008) | 0.000 | 0.000 |
| Platelet Count Median (IQR)¶ | 1.85 (1.4–2.51) | 1.98 (1.33–2.7) | 1.52 (1.15–2.08) | 1.72 (1.32–2.76) | 0.524 | 0.114 |
†COVID-19: Corona virus disease-2019; ‡ SD: Standard deviation; §IL-6: Interleukin-6; ¶ IQR: Interquartile range; †† ALC: Absolute leukocyte count; ‡‡ ANC: Absolute neutrophil count; * Non-mucor COVID and Mucor COVID of both waves;.** Non-mucor and Mucor of second wave.
Discussion
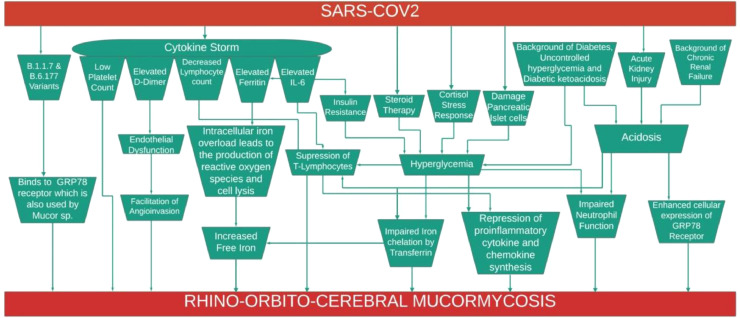
Analysis of patients’ data in the second wave showed that patients with CAROCM were younger (49.74±11.19 versus 58.92±15.29; p 0.008). The risk factors in developing CAROCM included male gender, diabetes, uncontrolled hyperglycaemia and DKA at presentation, renal impairment and corticosteroid therapy. These risk factors were noted irrespective of the clinical severity of COVID-19. The majority of patients who developed CAROCM had lymphopenia, elevated IL-6, ferritin and D-dimer. CAROCM developed more often after the onset of COVID-19, primarily within ten days. Based on our data, we have hypothesised the likely pathogenetic mechanisms causing ROCM in COVID-19 (Fig. 4 ). In a given patient, more than one mechanism may be responsible.
Fig. 4.
Flowchart showing the putative pathogenesis of COVID-19 associated Rhino-orbito-cerebral mucormycosis.
Our study showed that COVID-19 was more severe in the second than in the first wave (16% versus 49%; p <0.001). Moreover, the number of CAROCM cases was markedly higher in the second wave, similar to the observations in the published literature [9], [10], [11]. These observations could partly be related to the new variants of concern in the SARS COV2. Two variants, B.1.1.7 and B.6.177, were highly prevalent during the second COVID-19 wave in India [12]. It has been reported by Ibrahim et al. that apart from angiotensin converting enzyme (ACE) receptor-2, these variants could also use glucose-regulated protein (GRP) 78 receptor for endocytosis which also happens to be the one used by Mucorales for entry into the cell [13]. In a stressful condition like COVID-19, cells accumulate an excessively high number of unfolded viral structural proteins in the endoplasmic reticulum leading to overexpression of GRP78 at the cell surface, making them more susceptible to mucormycosis. This probably could explain both the higher transmissibility of SARS CoV2 and the surge in CAROCM in the second wave [3,14].
DM is a well-established predisposing factor for ROCM. Prevalence of DM and DKA was reported to be high in COVID-19 patients compared to the general population [15]. All the patients of CAROCM in our study had DM, and the incidence of DM between CAROCM patients and non-mucor patients in the second wave was statistically significant (100% versus 50%; p <0.001). In the majority, diabetes was uncontrolled. SARS-CoV2 can cause hyperglycemia due to one or more of the following mechanisms: damage to pancreatic islet cells bearing angiotensin convertase enzyme 2 receptors, insulin resistance due to cytokine storm, acute cortisol stress response and corticosteroid therapy [16].
RECOVERY trial showed that administration of dexamethasone (6 mg/day) for ten days (cumulative prednisolone equivalent dose: 400 mg) decreased the 28-day mortality in moderate to severe COVID-19 patients [17]. These results facilitated the rampant use of different types of corticosteroid at varying doses and durations, especially in rural and semi-urban India, even in asymptomatic and milder cases [18]. This was evidently clear in the CAROCM cohort of our study. On one hand, majority of the mild COVID-19 patients in the CAROCM cohort received steroid therapy and on the other hand, most of them received a cumulative steroid dose greater than 400 mg. Corticosteroid therapy may augment hyperglycaemia, impair neutrophil function, and repress many genes' transcription, which encode pro-inflammatory chemokines and cytokines. They also cause lymphopenia [19]. In the present study, the median (IQR) of ANC and ALC (cells/mm3) were 6460 (4532–7905) and 900 (532–1160), respectively; median ANC values between non-mucor and mucor ROCM in the second wave were statistically significantly different. Apart from steroids, the administration of biologicals targeting T or B cells and cytokines render them ineffective in clearing fungal elements.
About 20% of our patients with CAROCM had DKA and acute or chronic renal impairment. The incidence of renal impairment was significantly higher in patients with CAROCM (25.9% versus 4%; p = 0.004) than in non-mucor COVID-19 patients. Acidosis can predispose to mucormycosis by impairing iron chelation by transferrin and make free iron available for the growth of the fungus. Acidosis also enhancing cellular expression of the GRP78 receptor that binds to CotH on Mucorales hyphae that aid in fungal invasion and endothelial injury. Acidosis also suppresses T-lymphocytes, decreases gamma interferon production, and impairs phagocyte mediated killing [20].
Cytokine storm in COVID-19 poses a significant risk factor for developing ROCM. It is characterized by lymphocytopenia, thrombocytopenia, elevated IL-6 and other cytokines, D-dimer and ferritin [21]. Most of the patients in this study showed abnormalities in one or more of these inflammatory markers. Hypercytokinemia and significantly elevated IL-6 levels augment the apoptosis of lymphocytes by promoting Fas and Fas ligand interaction. SARS-CoV2 infection was found to interfere with T-cell expansion and also causes T-cell exhaustion [22]. Neutrophilia is a more common finding in COVID-19. Neutrophil dysfunction due to corticosteroid therapy and lymphopenia are other contributing factors [23].
COVID-19 is associated with deranged iron metabolism that leads to hyperferritinemia. Serum ferritin was markedly elevated (974 versus 138.9; p <0.001) in patients with CAROCM compared to non-mucor COVID-19 patients. Hyperglycaemia and acidosis result in glycosylation of iron-binding proteins and decreases their affinity for iron, thereby increasing serum free iron levels. Elevated IL-6 induces ferritin synthesis and hyperferritinemia, resulting in increased intracellular iron content. Reactive oxygen species produced by the intracellular iron stores leads to tissue damage and release of free iron into circulation. These processes provide a fertile milieu for the growth and invasion of Mucorales [24].
COVID-19 is a hypercoagulable state. D-dimer was significantly higher (450 vs. 215; p 0.048) in patients with CAROCM. D-dimer causes enhanced endothelial dysfunction, platelet aggregation and widespread micro-thrombi, and this provides fertile ground for propagating angioinvasive Mucorales [25].
To conclude, delta variants of SARS CoV2 are more transmissible and prone to ROCM. It is commonly seen in middle-aged diabetic males with uncontrolled sugars, diabetic ketoacidosis, renal dysfunction and those treated with any corticosteroid dose. Elevated IL-6, D-dimer, serum ferritin are more often seen in COVID-19 patients who developed CAROCM and might involve in the pathophysiology of ROCM in patients with COVID-19. The limitations of our study were: retrospective nature of the study and small sample size, and inflammatory markers were not measured at admission in the entire study cohort. Free iron levels were not done in any of the patients.
Declaration of Competing Interest
None of the authors have any conflict of interest.
Acknowledgements
Authors thank Deepika and Kiran ESS for technical assistance, Skanda mitra for developing figure 4.
References
- 1.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., Zhang L., et al. Clinical features of patients infected with 2019 novel Coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. Feb 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cucinotta D., Vanelli M. WHO declares COVID-19 a pandemic. Acta Biomed. 2020;91(1):157–160. doi: 10.23750/abm.v91i1.9397. Mar 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rahman F.I., Islam M.R., Bhuiyan M.A. Mucormycosis or black fungus infection is a new scare in South Asian countries during the COVID-19 pandemic: associated risk factors and preventive measures. J Med Virol. 2021;93(12):6447–6448. doi: 10.1002/jmv.27207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rocha I.C.N., Hasan M.M., Goyal S., Patel T., Jain S., Ghosh A., et al. COVID-19 and mucormycosis syndemic: double health threat to a collapsing healthcare system in India. Trop Med Int Health. 2021 doi: 10.1111/tmi.13641. Jun 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Verma D.K., Bali R.K. COVID-19 and mucormycosis of the craniofacial skeleton: causal, contributory or coincidental? J Maxillofac Oral Surg. 2021;20(2):1–2. doi: 10.1007/s12663-021-01547-8. Mar 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moorthy A., Gaikwad R., Krishna S., Hegde R., Tripathi K.K., Kale P.G., et al. SARS-CoV-2, uncontrolled diabetes and corticosteroids-an unholy trinity in invasive fungal infections of the maxillofacial region? A retrospective, multi-centric analysis. J Maxillofac Oral Surg. 2021:1–8. doi: 10.1007/s12663-021-01532-1. Mar 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.John T.M., Jacob C.N., Kontoyiannis D.P. When uncontrolled diabetes mellitus and severe COVID-19 converge: the perfect storm for mucormycosis. J Fungi (Basel). 2021 Apr 15;7(4):298. doi: 10.3390/jof7040298. [DOI] [PMC free article] [PubMed]
- 8.Garg D., Muthu V., Sehgal I.S., Ramachandran R., Kaur H., Bhalla A., et al. Coronavirus disease (Covid-19) associated mucormycosis (CAM): case report and systematic review of literature. Mycopathologia. 2021;186(2):289–298. doi: 10.1007/s11046-021-00528-2. May. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Patel A., Agarwal R., Rudramurthy S.M., Shevkani M., Xess I., Sharma R., et al. Multicenter epidemiologic study of Coronavirus disease-associated mucormycosis, India. Emerg Infect Dis. 2021;27(9) doi: 10.3201/eid2709.210934. Jun 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sen M., Honavar S.G., Bansal R., Sengupta S., Rao R., Kim U., et al. Epidemiology, clinical profile, management, and outcome of COVID-19-associated rhino-orbital-cerebral mucormycosis in 2826 patients in India - Collaborative OPAI-IJO study on mucormycosis in COVID-19 (COSMIC), report 1. Indian J Ophthalmol. 2021;69(7):1670–1692. doi: 10.4103/ijo.IJO_1565_21. Jul. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pal R., Singh B., Bhadada S.K., Banerjee M., Bhogal R.S., Hage N., et al. COVID-19-associated mucormycosis: an updated systematic review of literature. Mycoses. 2021 doi: 10.1111/myc.13338. Jun 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vaidyanathan G. Coronavirus variants are spreading in india-What scientists know so far (news in focus).Nature 2021; 593:321–2 . [DOI] [PubMed]
- 13.Ibrahim I.M., Abdelmalek D.H., Elshahat D.H. COVID-19 spike-host cell receptor GRP78 binding site prediction. J Infect. 2020;80(5):554–562. doi: 10.1016/j.jinf.2020.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chandra S., Rawal R. The surge in Covid related mucormycosis. J Infect. 2021 doi: 10.1016/j.jinf.2021.06.008. Jun 12S0163-4453(21)00288-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goldman N., Fink D., Cai J., Lee Y.N., Davies Z. High prevalence of COVID-19-associated diabetic ketoacidosis in UK secondary care. Diabetes Res Clin Pract. 2020;166 doi: 10.1016/j.diabres.2020.108291. Aug. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kothandaraman N., Rengaraj A., Xue B., Yew W.S., Velan S.S., Karnani N., et al. COVID-19 endocrinopathy with hindsight from SARS. Am J Physiol Endocrinol Metab. 2021;320(1):E139–E150. doi: 10.1152/ajpendo.00480.2020. Jan 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Horby P., Lim W.S., Emberson J.R., Mafham M., Bell J.L., Linsell L., et al. Dexamethasone in Hospitalized Patients with Covid-19. N Engl J Med. 2021;384(8):693–704. doi: 10.1056/NEJMoa2021436. Feb 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rodriguez-Morales A.J., Sah R., Millan-Oñate J., Gonzalez A., Montenegro-Idrogo J.J., Scherger S., et al. COVID-19 associated mucormycosis: the urgent need to reconsider the indiscriminate use of immunosuppressive drugs. Ther Adv Infect Dis. 2021;18:8. doi: 10.1177/20499361211027065. Jun20499361211027065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Coutinho A.E., Chapman K.E. The anti-inflammatory and immunosuppressive effects of glucocorticoids, recent developments and mechanistic insights. Mol Cell Endocrinol. 2011;335(1):2–13. doi: 10.1016/j.mce.2010.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ibrahim A.S., Spellberg B., Walsh T.J., Kontoyiannis D.P. Pathogenesis of mucormycosis. Clin Infect Dis. 2012;54(Suppl 1):S16–S22. doi: 10.1093/cid/cir865. FebSuppl 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fajgenbaum D.C., June C.H. Cytokine storm. N Engl J Med. 2020;3;383(23):2255–2273. doi: 10.1056/NEJMra2026131. Dec. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bhaskar S., Sinha A., Banach M., Mittoo S., Weissert R., Kass J.S., Rajagopal S., Pai A.R., Kutty S. Cytokine storm in COVID-19-immunopathological mechanisms, clinical considerations, and therapeutic approaches: the REPROGRAM consortium position paper. Front Immunol. 2020;10:11. doi: 10.3389/fimmu.2020.01648. jul1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tavakolpour S., Rakhshandehroo T., Wei E.X., Rashidian M. Lymphopenia during the COVID-19 infection: what it shows and what can be learned. Immunol Lett. 2020;225:31–32. doi: 10.1016/j.imlet.2020.06.013. Sep. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pilmis B., Alanio A., Lortholary O., Lanternier F. Recent advances in the understanding and management of mucormycosis. F1000Res. 2018 doi: 10.12688/f1000research.15081.1. Sep 7;7:F1000 Faculty Rev-1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kichloo A., Dettloff K., Aljadah M., Albosta M., Jamal S., Singh J., Wani F., Kumar A., Vallabhaneni S., Khan M.Z. COVID-19 and hypercoagulability: a review. Clin Appl Thromb Hemost. 2020;26 doi: 10.1177/1076029620962853. Jan-Dec. [DOI] [PMC free article] [PubMed] [Google Scholar]