Abstract
Objectives
This study aimed to explore the regularity of S-RBD domain antibody reactivity after immunization with inactivated SARS-CoV-2 vaccine and evaluate the effect of this vaccine on the immune response.
Design or methods
Venous blood samples were collected from 1156 healthcare workers who participated in the phase III clinical trial of the SARS-CoV-2 inactivated vaccine. The S-RBD domain antibody levels in the serum were detected by ELISA 14 days after the first and second active immunization, respectively.
Results
The positive rates after inoculation of the first and second vaccination of S-RBD domain antibody against SARS-CoV-2 were 28.03% and 86.76%, respectively. The mean inhibition rate of S-RBD domain antibody against positive samples was 57.18 ± 18.87% after the second vaccination at 14 days. Sex and age had no effects on the positive rate. The positive rate was decreased in the high BMI group. Single-factor logistic analysis showed that there was no significant correlation between age and positive rate. BMI was negatively correlated with the positive rate.
Conclusions
After 2 immunizations, the positive rate of SARS-CoV-2 S-RBD domain antibody was high, and the vaccine had good immunogenicity. The improvement of the immune strategy should focus on the effects of BMI and other factors.
Keywords: COVID-19, Inactivated SARS-CoV-2 vaccine, S-RBD domain antibody, Immunogenicity, BMI
Introduction
The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) lead to the outbreak of coronavirus disease 2019 (COVID-19) pandemic (Tsang et al., 2021). This infection outbreak has posed a serious threat to global trade and public health (Cutler, 2020; Antia and Halloran, 2021). By November 9, 2021, the number of confirmed cases of COVID-19 has exceeded 250 million, and the number of deaths has exceeded 5 million according to the WHO. No biomarkers have been proved to be effective in the treatment of COVID-19 due to various restraints (Bivona et al., 2021). At present, vaccination is considered to be the most effective option to prevent the spread of viral infection (Dagotto et al. 2020; Krammer 2020). Neutralizing antibodies block viral infection and reflect the intensity of the body's humoral immune response (Häfner, 2019). It has been discovered in the study that SARS-CoV-2 mainly activates intracellular endocytosis through the combination of and human angiotensin converting enzyme 2 (hACE2), and thus enters into the human spike protein (S protein) receptor-binding region (S-RBD) cells and initiates the related signals of virus replication (Amanat and Krammer, 2020). Thus, neutralizing antibodies against the RBD domain of the S protein can block viral infection.
Protection evaluation of vaccines often requires tracking the health of the vaccinated population for several years to obtain convincing data. However, the ongoing development of the pandemic worldwide has not allowed sufficient time for adequate vaccine protection analysis Therefore, it is necessary to establish a method that can indirectly evaluate the protective effect of vaccines (Hodgson et al., 2021). Several detecting kits were applied in this field. Gambino et al. certifies that the immunochromatographic rapid test and the chemiluminescence immunoassay have a good concordance (Gambino et al., 2020). According to the existing research reports, detecting the level of neutralizing antibodies against the RBD domain can theoretically become indirect evidence to evaluate the effectiveness of the vaccine (Jin et al., 2021).
At present, there are few reports on the production of neutralizing antibodies in people immunized with SARS-CoV-2 inactivated vaccine. This study intended to detect and analyze the level of S-RBD antibodies in people who were voluntarily vaccinated with inactivated SARS-CoV-2 vaccine (Vero) in hospitals, to find out the influencing factors and rules of S-RBD antibody production, to provide a reference basis for improving the immunization strategy, and to provide data support for the newly established experimental method.
Materials and methods
Patients and sample collection
This study was a prospective observational cohort study. A total of 1156 healthcare workers who completed 2 immunizations with inactivated SARS-Cov-2 vaccine (Vero Cell) at Taian City Central Hospital between December 2020 and January 2021 were enrolled in this investigation. The mean age of the included subjects was 37.63 ± 10.34 years (18–60 years), including 868 females and 288 males (Table 1 ). The inactivated vaccine was from the Beijing Institute of Biological Products (Beijing, China). The vaccination was carried out in full accordance with the technical guidelines for vaccination against Covid-19 (中华人民共和国国家卫生健康委员会, 2021) as a 2-dose regimen with an interval of 3 weeks. All these personnel were not affected by SARS-CoV-2. The following populations were excluded: history of contact with a confirmed case of COVID-19, severe systemic immune disease, and suspected symptoms of COVID-19. The venous blood was collected on the 14th day of the first and second needle inoculation. The serum specimens were collected after centrifugation for the following detection. Sex refers to biological differences between males and females and it is assigned at birth as male or female based on visible anatomy. This study was performed in accordance with the Declaration of Helsinki; the design and procedure of this study were approved by Taian City Central Hospital. All participants signed the written informed consent.
Table 1.
Clinical data of the study population
| Variables | N (%) | Mean ± SD |
|---|---|---|
| Sex | ||
| male | 288 (24.91) | - |
| female | 868 (75.09) | - |
| Age (years) | ||
| ≤ 30 | 439 (37.98) | 24.20 ± 3.21 |
| 30-40 | 465 (40.22) | 35.17 ± 2.87 |
| 40-50 | 149 (12.89) | 44.62 ± 2.62 |
| >50 | 103 (8.91) | 53.88 ± 3.14 |
| BMI (kg/m2) | ||
| ≤ 21.00 | 281 (24.31) | 19.25 ± 1.10 |
| 21.00-25.00 | 519 (44.90) | 22.96 ± 1.14 |
| >25.00 | 9356 (30.79) | 27.01 ± 0.81 |
| Total number of individuals | 1156 | - |
Noted: BMI, body mass index.
Enzyme-linked immunosorbent assay (ELISA)
The anti-SARS-Cov-2 neutralizing antibody ELISA kits from Nanjing Vazyme Biotech Co., Ltd. (Nanjing, China) were used to detect the neutralizing antibodies. The microplate was coated with recombinant human ACE2 receptor protein (hACE2) and horseradish peroxidase-labeled RBD protein (HRP-RBD) was used to prepare the enzyme conjugate. In the first step of the reaction, the sample was preincubated with HRP-RBD outside the ELISA plate at 37°C for 30 minutes. And then, the previous mixture was transferred into the hACE2-coated plate and incubated for 20 minutes, where HRP-RBD without binding to the neutralizing antibody binds to hACE2. After washing, the TMB substrate was added, and the substrate produced a colored product under the action of enzyme. After termination, the absorbance value was immediately read at the wavelength of 450 nm. The absorbance is inversely proportional to the effective activity of the anti-RBD neutralizing antibody in the sample. The signal inhibition rate (SIR) was calculated with the equation SIR = (1 – OD of sample/mean OD of negative control) × 100%; where SIR <20% indicated that neutralizing antibody was absent and the SIR ≥20% indicated the presence of SARS-CoV-2 neutralizing antibody.
Statistical analysis
SPSS21.0 statistical software and GraphPad were used for data processing and statistical analysis. The counting data were expressed in the number of cases or percentages. The student t test, ANOVA, and χ 2 test were used for comparison among groups. The single factor logistic regression was used to analyze the correlations between SIR and clinical characteristics. The difference was statistically significant (p <0.05).
Results
Total SIR from all subjects
SARS-CoV-2 S-RBD domain antibody was positive in 324 cases, with a positive rate of 28.03% after 14 days from the first injection. A total of 1003 SARS-CoV-2 S-RBD domain antibodies were positive, with a positive rate of 86.76% after 14 days of the second injection. The average inhibition rate of neutralization antibody in positive samples (57.18 ± 18.87%)was significantly increased in the second vaccination group than that in individuals after the first vaccination (29.64 ± 8.66%) (Figure 1 , p <0.001).
Figure 1.
The SIR of neutralizing antibodies was elevated after the second vaccination compared with the first vaccination. ***P < 0.001
Difference in SIR according to sex, age, and BMI
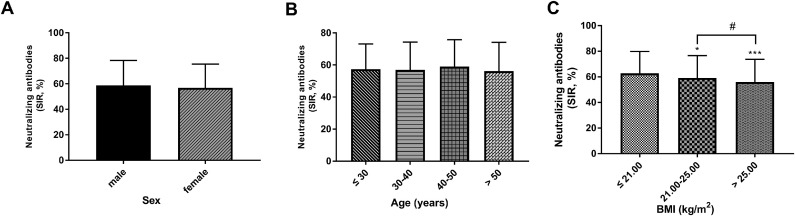
Fourteen days after the second injection, there were 250 males and 753 females in the 1003 positive samples. The results showed that there was no significant difference in the positive rate between males and females (Table 2 , p >0.05). In positive samples, there was no significant difference in the average value of neutralizing antibody among different sexes (male: 58.67 ± 19.62% vs female: 56.79±18.66%; Figure 2 A, p = 0.172).
Table 2.
Difference in positive rate according to parameters
| Variables | Positive | P value |
|---|---|---|
| N (%) | ||
| Sex | 0.172 | |
| male | 250 (86.81) | |
| female | 753 (86.75) | |
| Age (years) | 0.212 | |
| ≤ 30 | 374 (85.19) | |
| 30-40 | 402 (86.45) | |
| 40-50 | 137 (91.94) | |
| >50 | 90 (87.37) | |
| BMI (kg/m2) | 0.045 | |
| ≤ 21.00 | 251 (89.32) | |
| 21.00-25.00 | 456 (87.86) | |
| >25.00 | 296 (83.15) | |
| Number of individuals | 1003 |
Noted: BMI, body mass index.
Figure 2.
The difference in SIR according to sex, age, and BMI. A The SIR results of sex. B The comparison of SIR in different age groups. C Different SIRs in the different BMI groups. *P < 0.05, ***P < 0.001, compared with the ≤ 21.00 group; # P < 0.05, compared with the 21.00-25.00 group.
The patients were divided into four groups based on age: ≤30 years, 30–40 years, 40–50 years, and >50 years. The positive rate was 85.19% in the ≤30 years group, 86.45% in the 30–40 years group, 91.95% in the 40–50 years group, and 87.37% in the >50 years group (Table 2, p >0.05). No significance was found in the positive rate of different age groups (Table 2, p >0.05). As shown in Figure 2B, age could not influence the levels of SIR in samples of positive individuals (p >0.05).
According to the quartile of BMI, the patients were divided into 4 groups: ≤21.00 kg/m2, 21.00–25.00 kg/m2, and >25.00 kg/m2. The results showed that there was a significant difference in the positive rate among different groups (Table 2, p <0.05). In addition, in Figure 2C, the SIR values in the 21.00–25.00 kg/m2 and >25.00 kg/m2 group were less than the ≤21.00 kg/m2 group (p <0.05). The average SIR value was less in the >25.00 kg/m2 group than the 21.00–25.00 kg/m2 group (Figure 2C, p <0.05).
Correlations of SIR with characteristics
Univariate logistic analysis used the positive SARS-CoV-2 S-RBD domain antibody as the dependent variable and characteristics as the independent variable. As shown in Table 3 , there was no significant correlation between positive rate and sex (OR = 1.005; 95% CI = 0.678–1.489; p = 0.981) and age (OR = 1.012; 95% CI = 0.995–1.029, p = 0.170). In addition, there was a significant negative correlation between BMI and positive rate (Table 3; OR = 0.930; 95% CI = 0.902-0.959; p <0.01).
Table 3.
Association of different variables with the SIR
| Variables | OR | 95% CI | P value |
|---|---|---|---|
| Sex | 1.005 | 0.678-1.489 | 0.981 |
| Age (years) | 1.012 | 0.995-1.029 | 0.170 |
| BMI (kg/m2) | 0.930 | 0.902-0.959 | < 0.01 |
Noted: SIR, signal inhibition rate; BMI, body mass index.
Discussion
Since the outbreak of the COVID-19 pandemic, it has posed a serious threat to the safety and health of the general public and has brought a great impact on the development of the world economy (Niu et al., 2020; Li XZ et al., 2021). At present, the COVID-19 pandemic situation still continues to spread at a high rate, and China's epidemic prevention and control have achieved significant results; however, the import of overseas epidemic diseases poses a greater risk of local spread (Li Z et al., 2021). Recently, imported epidemic diseases causing local epidemic spread have been reported in some parts of China, resulting in the serious situation of normalized epidemic prevention and control in winter and spring. Vaccination is a powerful measure to address and control further transmission and outbreaks of COVID-19 (Monrad, 2020).
On the basis of ensuring safety, effectiveness is a key indicator in evaluating whether a vaccine can be marketed, and the intensity and durability of the immune response are also key indicators in evaluating the effectiveness of a vaccine (Mao et al., 2013). The results of this study showed that 14 days after the first dose of inoculation, the positive rate of SARS-CoV-2 S-RBD domain antibody was 28.03%, and the average inhibition rate of S-RBD domain antibody of positive samples was 29.64 ± 8.66 %. After 14 days of the second needle inoculation, the positive rate of SARS-CoV-2 S-RBD domain antibody was 86.76%, and the average inhibition rate of positive sample S-RBD domain antibody was 57.18 ± 18.87%. As a result, the positive rate of S-RBD domain antibody was high after 14 days of 2 active immunizations, which was consistent with the seroconversion rate of neutralizing antibody to 85.70% after 14 days of vaccine injection reported in the second clinical phase of relevant SARS-CoV-2 inactivated vaccine (Xia et al., 2020). In addition, due to different experimental methods, the antibody titer of the S-RBD domain could not be compared with other literature reports, and the results of the significantly increased inhibition rate of neutralizing antibodies after the second immunization also showed that the immune population achieved a good humoral immune response. A large number of research data have shown that the antibody levels of people with SARS-CoV-2 will decline over time, especially in asymptomatic people in whom the decline is more significant (Rapid Decay of Anti-SARS-CoV-2 Antibodies in Persons with Mild Covid-19, 2020; Long et al., 2020). Currently, it is not clear that the durability of vaccine-induced S-RBD domain antibody levels requires 6 months or more of follow-up testing to determine the status of S-RBD domain antibody in the immune population.
It has been reported that there is no difference in IgG antibody concentration between mild, normal, and recovered male and female patients (Huang et al., 2020; Jiang et al., 2020; Zeng et al., 2020). The incidence and mortality of severe cases in male patients are higher than those in female patients, and the possible underlying reasons are not yet clear (Sohn et al., 2021). However, the dynamics of the SARS-CoV-2 IgG antibody were different between male and female patients (Zeng et al., 2020). Therefore, the dynamic change of antibody level may be the reason for the incidence and mortality of severe diseases. Comparing the positive rate and inhibition rate of S-RBD domain antibodies between men and women in the immunized population, it was found that there was no statistical difference. The subsequent dynamic changes of the S-RBD domain antibody still needed to be tracked and detected. In addition, this study was divided into 4 groups according to age quartile. The results showed that there was no significant difference in the positive rate between groups. Univariate logistic analysis showed that there was no significant correlation between age and positive rate, indicating that the SARS-CoV-2 inactivated vaccine could get an ideal humoral immune effect in all age groups of 20–60 years.
Studies have found that obese people have an increased risk of severe COVID-19 (Caci et al., 2020). Body mass index is commonly used internationally to measure the degree of obesity and health (Zhu et al., 2021). A large meta-analysis of 75 studies shows that obesity may be an important risk factor affecting the clinical outcomes of SARS-CoV-2 pneumonia (Aminian and Tu, 2021). In this study, positive rate showed a significant downward trend with the increase of BMI. In addition, BMI had a significant negative correlation with the positive rate. It could be deduced that BMI might be an important factor in regulating the levels of S-RBD domain antibody in the immune population. According to the grouping comparison results, BMI should be considered as an important factor in planning immune strategy to achieve better humoral immunity. However, the mRNA BNT162b2 vaccines from Pfizer-BioNTech show age-, time-, and sex-related differences after administration (Lo Sasso et al., 2021). This conclusion was inconsistent with our findings, which might be because we used inactivated vaccine, whereas the mRNA vaccine was used in the previously mentioned article.
The production of an antibody response is generally one of the manifestations of vaccine protection efficacy. Based on the results of existing research, the ability of human body to produce Covid-19-related antibody after inoculation with this vaccine is ideal. Of course, this is not entirely equivalent to vaccine effectiveness being desirable. Whether the S-RBD domain antibody captured by the experimental method is a valid neutralizing antibody, especially for the validity of recently reported SARS-CoV-2 variants in various countries; other aspects need to be verified by further pseudovirus and true virus experiments. Therefore, ongoing monitoring and further analysis of S-RBD domain antibody levels in the vaccinated population are still needed.
Conclusion
In conclusion, we analyzed the reaction law of the population producing antibodies against the RBD domain after immunization with SARS-CoV-2 inactivated vaccine. These results indicated that the inactivated SARS-CoV-2 vaccine could induce a high level of S-RBD domain antibody response, independent of sex and age. In addition, BMI might influence the level of S-RBD domain antibody produced by SARS-CoV-2 inactivated vaccine.
Acknowledgments
Conflicts of Interest
The authors declare no conflict of interest.
Ethical Approval statement
This study was performed in accordance with the Declaration of Helsinki; the design and procedure of this study were approved by Taian City Central Hospital. All participants signed the written informed consent.
Acknowledgments
The research was supported by Dr. Jinpeng Bi from Nanjing Vazyme Biotechnology Co., Ltd.
References
- Amanat F, Krammer F. SARS-CoV-2 Vaccines: Status Report. Immunity. 2020;52:583–589. doi: 10.1016/j.immuni.2020.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aminian A, Tu C. Association of Bariatric Surgery with Clinical Outcomes of SARS-CoV-2 Infection: a Systematic Review and Meta-analysis in the Initial Phase of COVID-19 Pandemic. Obesity surgery. 2021;31:2419–2425. doi: 10.1007/s11695-020-05213-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antia R, Halloran ME. Transition to endemicity: Understanding COVID-19. Immunity. 2021;54:2172–2176. doi: 10.1016/j.immuni.2021.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bivona G, Agnello L, Ciaccio M. Biomarkers for Prognosis and Treatment Response in COVID-19 Patients. Annals of laboratory medicine. 2021;41:540-8. doi: 10.3343/alm.2021.41.6.540. [DOI] [PMC free article] [PubMed]
- Caci G, Albini A, Malerba M, Noonan DM, Pochetti P, Polosa R. COVID-19 and Obesity: Dangerous Liaisons. Journal of clinical medicine. 2020;9 doi: 10.3390/jcm9082511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cutler D. How Will COVID-19 Affect the Health Care Economy? Jama. 2020;323:2237–2238. doi: 10.1001/jama.2020.7308. [DOI] [PubMed] [Google Scholar]
- Dagotto G, Yu J, Barouch DH. Approaches and Challenges in SARS-CoV-2 Vaccine Development. Cell host & microbe. 2020;28:364–370. doi: 10.1016/j.chom.2020.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gambino CM, Lo Sasso B, Colomba C, Giglio RV, Agnello L, Bivona G, et al. Comparison of a rapid immunochromatographic test with a chemiluminescence immunoassay for detection of anti-SARS-CoV-2 IgM and IgG. Biochemia medica. 2020;30 doi: 10.11613/bm.2020.030901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Häfner S. Guns N' viruses. Microbes and infection. 2019;21:125-8. doi: 10.1016/j.micinf.2019.01.001. [DOI] [PMC free article] [PubMed]
- Hodgson SH, Mansatta K, Mallett G, Harris V, Emary KRW, Pollard AJ. What defines an efficacious COVID-19 vaccine? A review of the challenges assessing the clinical efficacy of vaccines against SARS-CoV-2. The Lancet Infectious diseases. 2021;21:e26-e35. doi: 10.1016/s1473-3099(20)30773-8. [DOI] [PMC free article] [PubMed]
- Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet (London, England) 2020;395:497–506. doi: 10.1016/s0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang F, Deng L, Zhang L, Cai Y, Cheung CW, Xia Z. Review of the Clinical Characteristics of Coronavirus Disease 2019 (COVID-19) Journal of general internal medicine. 2020;35:1545–1549. doi: 10.1007/s11606-020-05762-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin P, Li J, Pan H, Wu Y, Zhu F. Immunological surrogate endpoints of COVID-2019 vaccines: the evidence we have versus the evidence we need. Signal transduction and targeted therapy. 2021;6:48. doi: 10.1038/s41392-021-00481-y. [DOI] [PMC free article] [PubMed]
- Krammer F. SARS-CoV-2 vaccines in development. Nature. 2020;586:516–527. doi: 10.1038/s41586-020-2798-3. [DOI] [PubMed] [Google Scholar]
- Li XZ, Gao S, Fu YK, Martcheva M. Modeling and Research on an Immuno-Epidemiological Coupled System with Coinfection. Bulletin of mathematical biology. 2021;83:116. doi: 10.1007/s11538-021-00946-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Zhang B, Wu X, Yang M, Zhang Q, Xiang G, et al. What is the status of nucleic acid contamination in 2019-nCOV vaccination sites? Can it be avoided? Epidemiology and infection. 2021;149:1–23. doi: 10.1017/s0950268821001394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo Sasso B, Giglio RV, Vidali M, Scazzone C, Bivona G, Gambino CM, et al. Evaluation of Anti-SARS-Cov-2 S-RBD IgG Antibodies after COVID-19 mRNA BNT162b2 Vaccine. Diagnostics (Basel, Switzerland) 2021:11. doi: 10.3390/diagnostics11071135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long QX, Tang XJ, Shi QL, Li Q, Deng HJ, Yuan J, et al. Clinical and immunological assessment of asymptomatic SARS-CoV-2 infections. Nature medicine. 2020;26:1200–1204. doi: 10.1038/s41591-020-0965-6. [DOI] [PubMed] [Google Scholar]
- Mao Q, Cheng T, Zhu F, Li J, Wang Y, Li Y, et al. The cross-neutralizing activity of enterovirus 71 subgenotype c4 vaccines in healthy chinese infants and children. PloS one. 2013;8:e79599. doi: 10.1371/journal.pone.0079599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monrad JT. Ethical considerations for epidemic vaccine trials. Journal of medical ethics. 2020;46:465–469. doi: 10.1136/medethics-2020-106235. [DOI] [PubMed] [Google Scholar]
- Niu S, Tian S, Lou J, Kang X, Zhang L, Lian H et al. Clinical characteristics of older patients infected with COVID-19: A descriptive study. Archives of gerontology and geriatrics. 2020;89:104058. doi: 10.1016/j.archger.2020.104058. [DOI] [PMC free article] [PubMed]
- Rapid Decay of Anti-SARS-CoV-2 Antibodies in Persons with Mild Covid-19. The New England journal of medicine. 2020;383:e74. doi: 10.1056/NEJMx200017. [DOI] [PMC free article] [PubMed]
- Sohn SY, Hearing J, Mugavero J, Kirillov V, Gorbunova E, Helminiak L, et al. Interferon-Lambda Intranasal Protection and Differential Sex Pathology in a Murine Model of SARS-CoV-2 Infection. mBio. 2021;12 doi: 10.1128/mBio.02756-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsang HF, Chan LWC, Cho WCS, Yu ACS, Yim AKY, Chan AKC, et al. An update on COVID-19 pandemic: the epidemiology, pathogenesis, prevention and treatment strategies. Expert review of anti-infective therapy. 2021;19:877–888. doi: 10.1080/14787210.2021.1863146. [DOI] [PubMed] [Google Scholar]
- Xia S, Duan K, Zhang Y, Zhao D, Zhang H, Xie Z, et al. Effect of an Inactivated Vaccine Against SARS-CoV-2 on Safety and Immunogenicity Outcomes: Interim Analysis of 2 Randomized Clinical Trials. Jama. 2020;324:951–960. doi: 10.1001/jama.2020.15543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng F, Dai C, Cai P, Wang J, Xu L, Li J, et al. A comparison study of SARS-CoV-2 IgG antibody between male and female COVID-19 patients: A possible reason underlying different outcome between sex. Journal of medical virology. 2020;92:2050–2054. doi: 10.1002/jmv.25989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu C, Fu Z, Liu L, Shi X, Li Y. Health risk assessment of PM(2.5) on walking trips. Scientific reports. 2021;11:19249. doi: 10.1038/s41598-021-98844-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 中华人民共和国国家卫生健康委员会 新冠病毒疫苗接种技术指南(第一版) 中华临床感染病杂志. 2021;14:89–90. [Google Scholar]