Abstract
The incidence of depression has increased significantly during the COVID-19 pandemic. This disease is closely associated with serotonin 1A (5-HT1A) receptor and often treated by complex prescription containing Curcuma wenyujin Y. H. Chen et C. Ling. Therefore, we hypothesized that this herb contains bioactive compounds specially binding to the receptor. However, the rapid discovery of new ligands of 5-HT1A receptor is still challenging due to the lack of efficient screening methods. To address this problem, we developed and characterized a novel approach for the rapid screening of ligands by using immobilized 5-HT1A receptor as the chromatographic stationary phase. Briefly, haloalkane dehalogenase was fused at the C-terminal of 5-HT1A receptor, and the modified 5-HT1A receptor was immobilized on amino-microspheres by the reaction between haloalkane dehalogenase and 6-chlorohexanoic acid linker. Scanning electron microscope and X-ray photo-electron were used to characterize the morphology and element of the immobilized receptor. The binding of three specific ligands to 5-HT1A receptor was investigated by two different methods. Moreover, we examined the feasibility of 5-HT1A receptor colume in high throughput screening of new ligands from complex systems as exemplified by Curcuma wenyujin Y. H. Chen et C. Ling. Gweicurculactone, 2-hydroxy-1-(3,4-dihydroxybenzene)-7-(4′-hydroxybezene)-heptane and curcuminol F were identified as the ligands of 5-HT1A receptor with the binding energies of −7.06 kcal/mol, −7.77 kcal/mol and −5.26 kcal/mol, respectively. Collectively, these results indicated that the immobilized 5-HT1A receptor was capable of screening bioactive compound from complex system, providing an effective methodology for high throughput screening.
Keywords: G protein-coupled receptor, 5-HT1A receptor, Halo-tag, Affinity chromatography, One-step immobilization, High-throughput screening
Abbreviations: 5-HT, Serotonin; 5-HT1A, Serotonin 1A; BCA, Bicinchoninic acid; Compound 2, 2-Hydroxy-1-(3,4-dihydroxy benzene)-7-(4′- hydroxybezene)-heptane; DIEA, N,N-diisopropylethylamine; Halo, Haloalkane dehalogenase; HATU, O-(7-azabenzotriazol-1-yl)-N,N,N′,N′-tetramethyluronium hexafluoro phosphate; LB, Luria-Bertani; SDS-PAGE, Sodium dodecyl sulfate-polyacrylamide gel electrophoresis
Graphical Abstract
1. Introduction
Depression is a severe mental illness that affects more than 300 million people worldwide with high morbidity and mortality, the prevalence of which has been increased markedly in the past decades [1]. Indeed, depression was recognized as one of the leading causes of global burden in 2018 [2]. Despite this impending crisis, the persistent lack of progress in pharmacotherapy stands in sharp contrast to the rapid pandemic magnitude of the disease, suggesting that novel drug candidates are urgently needed. Curcuma wenyujin Y. H. Chen et C. Ling is a valuable traditional medicine that possesses multiple pharmacological properties, such as anti-oxidant, anti-proliferative, anti-microbial, anti-fungal and anti-inflammatory effects [3]. Many complex prescriptions containing Curcuma wenyujin Y. H. Chen et C. Ling have been utilized to alleviate depression for years [4]. Serotonin (5-HT) is a fundamental neurotransmitter that mediates important brain functions. Modern pharmacological research shows that 5-HT1A receptor contributes much to the pathophysiology and successful treatment of many psychiatric disorders, especially for depression [5]. Therefore, we hypothesize that Curcuma wenyujin Y. H. Chen et C. Ling may ameliorate depression by modulating 5-HT1A receptor. However, the studies of Curcuma wenyujin Y. H. Chen et C. Ling were primarily concentrated in β-elemene and its effect on tumors, while the active pharmaceutical ingredient and possible mechanism of this herb against mental diseases remain elusive.
The traditional strategy for bioactive compound identification, which involves the extraction, isolation, chemical manipulation and spectroscopic analysis, is time-consuming and laborious. In addition, the minor and unstable components may be lost during the separation process. Although some active compounds have been identified based on this strategy, the specific protein targets of these compounds are largely unknown, which severely impedes their further research. Therefore, it is necessary to develop new methods to identify bioactive compounds from natural products. Up to now, a series of technologies has been developed to advance the identification of bioactive compounds from natural products, among which high-performance affinity chromatography is the most notable one [6]. In this method, the target protein is immobilized on solid matrix to synthesize affinity stationary phase, and the conservation of biological activity in the process of protein immobilization is of particular importance to detection performance. Over the last decade, Wainer et al. constructed some G-protein-coupled receptor stationary phases and applied them in drug-receptor interaction analysis, such as β2-adrenergic receptor [7], G protein-coupled receptor 17 [8] and cannabinoid receptor [9]. However, these stationary phases were prepared through physical adsorption and the long-term performance stability needed to be improved.
To address this problem, our group has focused on the preparation of more stable stationary phase for years. We found the specificity and stability of stationary phase can be markedly improved by high specific covalent reaction between the immobilized tag and its substrate [6]. Haloalkane dehalogenase (Halo) is a commercially available engineered tag that specifically interacts with its substrate like 6-chlorohexanoic acid [10]. Briefly, halides were removed from 6-chlorohexanoic acid by halo through a mechanism of nucleophilic displacement. A covalent alkyl-enzyme intermediate was formed during catalysis between the terminal chloride in chloroalkane linker with Asp106 in halo tag. This study was designed to examine whether halo-tag can be used to immobilize serotonin 1A (5-HT1A) receptor, and the feasibility of immobilized 5-HT1A receptor in high throughput screening of the receptor-binding ligands from complex systems as exemplified by Curcuma wenyujin Y. H. Chen et C. Ling.
2. Theory
2.1. Langmuir isotherm
The Langmuir isotherm is the most extensively used, semi-empirical model to describe the homogeneous adsorption process of liquid-solid chromatographic equilibria [11]:
| (1) |
where q and [C] represent the solute concentrations in the stationary phase and mobile phase, respectively; q s is the saturation capacity of the colume and b is the equilibrium constant.
2.2. The bi-Langmuir isotherm
The bi-Langmuir isotherm is usually used to describe the adsorption behavior of the nonhomogeneous surface that possesses two types of independent adsorption sites [12]:
| (2) |
qs,1 and q s,2 represent the saturation capacities of the two types of the ligand binding sites, respectively. Moreover, they follow the relationship of q s = q s,1 + q s,2 .
2.3. Frontal analysis
Frontal analysis is a widespread method for the determination of solutions with known concentrations of solutes, involving a stepwise increase in ligand concentrations that pass through 5-HT1A receptor colume to generate various breakthrough curves. If there is only one type of immobilized ligand site, the double-reciprocal form of Langmuir isotherm equation can be used to describe the interaction between the receptor and the ligand. If there are two kinds of immobilized ligand sites and both sites have different stabilities for the adsorbed complexes, the double-reciprocal form of Bi-Langmuir isotherm equation can be applied to describe the interaction between the receptor and the ligand.
2.4. Injection amount-dependent analysis
Injection amount-dependent analysis is a novel methodology for drug-receptor interaction analysis that firstly proposed by our group in 2014 [13]. The binding of a ligand to the immobilized protein is widely considered as an equilibrium between the process of adsorption and desorption in affinity chromatography (L + A ⇌ LA, where A, L and LA represent the ligand, protein and ligand-protein complex, respectively). Under the assumption that i) the adsorption sites are uniformly distributed on the stationary phase; ii) the longitudinal diffusion is negligible; iii) the equilibrium of adsorption and desorption can be rapidly achieved, the interaction between the receptor and the ligand can be described as follows:
| (3) |
k is the capacity factor of the ligand, which can be calculated by (tR-t0)/t0. n I is the molar amount of injected solute, n t the number of binding sites on the 5-HT1A receptor colume, K a is the equilibrium association constant, and V m represents the void time of the 5-HT1A receptor colume.
3. Experimental section
3.1. Materials and instruments
Reference standards of zolmitriptan (CAS 139264-17-8) and 5-HT (CAS 153-98-0) were obtained from Macklin Biochemical Co., Ltd. (Shanghai, China). YL-0919 (CAS 1339339-18-2) was from Tianjin Kailiqi Biopharma Technology Co., Ltd. (Tianjin, China). Ampicillin was obtained from Shanghai regal Biology Technology Co., Ltd. (Shanghai, China). Yeast extract and peptone were supplied by Amresco (Houston, Texas, USA). N,N-diisopropylethylamine (DIEA) and O-(7-azabenzotriazol-1-yl)-N,N,N′,N′-tetramethyluronium hexafluoro phosphate (HATU) were bought from Sigma-Aldrich (St. Louis, MO, USA) and Macklin Biochemical Co., Ltd. (Shanghai, China), respectively. Amino polystyrene microspheres (particle size 7 µm, pore size 300 Å) were purchased from Knowledge & Benefit Sphere Tech. Co., Ltd. (Suzhou, China). Other materials were analytically pure reagents unless stated otherwise.
3.2. The preparation of halo-tagged 5-HT1A receptor stationary phase
Double stranded DNA fragment of Homo sapiens 5-HT1A receptor (Genbank accession: NC_000005.10) plus the HaloTag (Genbank accession: ADN27525.1) was synthesized by Sangon Biotech and inserted into the vector pET15b through the restriction site of Nco I and Xho I. The resulting protein of interest therefore contained a halo-tag on its C-terminal. After transforming the recombinant plasmid into Escherichia coli BL21 (DE3) cells, the modified Escherichia coli BL21 (DE3) cells were incubated in Luria-Bertani (LB) medium for 12.0 h at 37 ℃. Moreover, they were propagated in auto-induction medium for another 12.0 h at the same temperature. The cells were collected by centrifugation at 8000 rpm for 15.0 min, and the 14.0 g cell pellet was suspended in 140 mL phosphate buffer (20 mM, pH 7.4) to obtain the supernatant for immobilization.
The halo-tagged 5-HT1A receptor was immobilized on the surface of amino polystyrene microspheres as follows. Briefly, 1.0 g amino polystyrene microspheres (amino content: 450 μmol/g microsphere, 1.0 eq) were immersed in 8.0 mL dimethylformamide containing 83.0 mg 6-chlorocaproic acid (1.2 eq) and 237.5 μL DIEA (3.0 eq). The reaction was initiated by adding 209.5 mg HATU (1.2 eq) to the mixture at the room temperature for 4.0 h. In addition, the microspheres were washed by DMF, methanol and phosphate buffer (20 mM, pH 7.4), respectively. Subsequently, the rinsed microspheres were reacted with above-mentioned cell lysates for 3.0 h to prepared halo-tagged 5-HT1A receptor stationary phase, and the stainless-steel columes (4.6 mm * 3.0 cm) were filled with immobilized receptor using phosphate buffer (20 mM, pH 7.4) under a pressure of 200 bar.
3.3. The characterization of 5-HT1A receptor colume
Sodium nitrite was used to determine the void time of chromatographic system as it had no specific interaction with 5-HT1A receptor. YL-0919, zolmitriptan, 5-HT (the agonists of 5-HT1A receptor) and bambuterol (an agonist of β2-adrenergic receptor) were utilized to evaluate the specificity of 5-HT1A receptor colume. Phosphate buffer (50 mM, pH 7.0) +5% isopropanol (V/V) was used as the mobile phase and the flow rate was 0.3 mL/min. Moreover, the stability of the colume was examined by 5-HT with continuous use over 30 days and the mobile phase was phosphate buffer (20 mM, pH 7.4) at 0.2 mL/min.
3.4. The chromatographic condition
All experiments were carried out on Agilent 1100 series apparatus equipped with high-performance liquid chromatographic system. YL-0919 and zolmitriptan were dissolved in methanol, and the solution of 5-HT was prepared by water. The detection wavelengths for sodium nitrate and bambuterol were 220 nm; 217 nm for YL-0919, 5-HT; and 227 nm for zolmitriptan. All chromatographic studies were performed at 30 ℃ with an injection volume of 10 μL for each drug.
3.5. The chromatographic procedure
3.5.1. Frontal analysis
The mobile phases for YL-0919, zolmitriptan, and 5-HT were phosphate buffer (50 mM, pH 7.0) +5% isopropanol (V/V), phosphate buffer (20 mM, pH 7.4) +5% isopropanol (V/V), phosphate buffer (20 mM, pH 7.4), respectively. The colume was equilibrated with the mobile phase for 30 min before analysis. In this section, the concentrations were 62.5 nM-80.0 μM (62.5 nM, 125.0 nM, 0.5 μM, 1.0 μM, 2.0 μM, 4.0 μM, 8.0 μM, 20.0 μM, 40.0 μM, 80.0 μM) for YL-0919 at the flow rate of 0.3 mL/min; 15.6 nM-128.0 μM (15.6 nM, 31.2 nM, 62.5 nM, 125.0 nM, 1.0 μM, 2.0 μM, 4.0 μM, 8.0 μM, 16.0 μM, 32.0 μM, 64.0 μM and 128.0 μM) for zolmitriptan at the flow rate of 0.2 mL/min; 62.5 nM-160.0 μM (62.5 nM, 125.0 nM, 250.0 nM, 2.0 μM, 4.0 μM, 8.0 μM, 20.0 μM, 40.0 μM, 80.0 μM, 160.0 μM) for 5-HT at the flow rate of 0.2 mL/min.
3.5.2. Injection amount-dependent analysis
The mobile phases for YL-0919, zolmitriptan and 5-HT were the same as those of frontal analysis. In this section, the concentrations were 3–3072 μM (3 μM, 6 μM, 12 μM, 24 μM, 48 μM, 192 μM, 384 μM, 768 μM, 1536 μM and 3072 μM) for YL-0919 at the flow rate of 0.3 mL/min; 1–2048 μM (1 μM, 2 μM, 4 μM, 8 μM, 128 μM, 256 μM, 512 μM, 1024 μM and 2048 μM) for zolmitriptan at the flow rate of 0.2 mL/min; 0.75–6144 μM (0.75 μM, 1.5 μM, 3 μM, 6 μM, 192 μM, 384 μM, 768 μM, 1536 μM, 3072 μM and 6144 μM) for 5-HT at the flow rate of 0.2 mL/min.
3.6. Molecular docking
The structure of 5-HT1A receptor (PDB: 7e2z) was used to construct the molecular docking model. All water molecules and ligands were removed before generating the model. The three-dimensional structure of YL-0919, zolmitriptan, 5-HT, gweicurculactone, 2-hydroxy-1-(3,4-dihydroxy benzene)-7-(4′- hydroxybezene)-heptane (compound 2) and curcuminol F were built by ChemDraw Ultra 19.0. Automated docking was performed by using the Lamarckian Genetic Algorithm in the software of Autodock 4.2.5, which was downloaded from the website of https://www.scripps.edu. Both the structure of 5-HT1A receptor and YL-0919/zolmitriptan/5-HT/gweicurculactone /compound 2/curcuminol F were converted to pdbqt format before initiating the docking process. Docking poses with 10 optimized conformations were selected for further analysis and the software of Discovery studio 4.5 was used to visualize the docking conformations.
3.7. Screening of bioactive compounds from Curcuma wenyujin Y. H. Chen et C. Ling
The halo-tagged 5-HT1A receptor colume was utilized to screen the bioactive compounds in Curcuma wenyujin Y. H. Chen et C. Ling. Ammonium acetate buffer (10 mM, pH 7.4) was used as the mobile phase and the flow rate was 0.2 mL/min under the detection wavelength of 217 nm. The chromatographic peak that had a retention time longer than void time was collected and recognized as the bioactive compounds specifically targeting 5-HT1A receptor. The retained fraction was further analyzed by high-performance liquid chromatography-mass spectrometry through an Inertsil ODS-3-C18 colume (5 µm, 4.6 mm × 150 mm). Mass spectrometry detection was performed using the positive mode with the scan range of 100–2000 amu and the mobile phase was 10% methanol solution (0–5 min), 60% methanol solution (5–15 min) at 0.3 mL/min ( Fig. 1).
Fig. 1.
The fabrication of halo-tagged 5-HT1A receptor colume and bioactive compound screening from Curcuma wenyujin Y. H. Chen et C. Ling.
4. Results and discussion
4.1. The expression of halo-tagged 5-HT1A receptor in Escherichia coli cells and protein immobilization
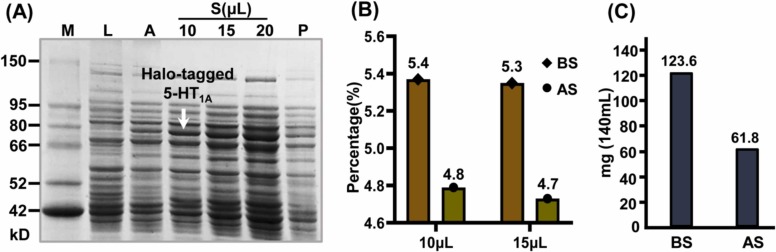
In this study, we expressed halo-tagged 5-HT1A receptor in Escherichia coli cells by using auto-induction medium. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) was employed to evaluate protein expression. By analyzing the marker bands, we found there was a linear relationship between band mobility and the logarithm of molecular weight, which can be utilized to calculate molecular weights of desired bands. A sharp new band of 80.0 kDa was appeared in the auto-induction medium compared with LB medium, indicating that 5-HT1A protein was successfully expressed ( Fig. 2A). The band was visible in the supernatant of cell lysates as well, while it was rarely appeared in the precipitation, suggesting that halo-tagged 5-HT1A protein were primarily distributed in the supernatant of Escherichia coli cell lysates and the supernatant was chosen for further analysis (Fig. 2A). Considering the size of halo-tag (33.0 kDa), we calculated that the molecular weight of 5-HT1A receptor was 47.0 kDa, which is consistent with previous studies [14].
Fig. 2.
The expression of halo-tagged 5-HT1A receptor by SDS-PAGE, and the amount of 5-HT1A receptor immobilized on amino polystyrene microspheres. (A) SDS-PAGE analysis of 5-HT1A receptor. Lane M, Lane L and Lane A were the protein marker, LB medium and auto-induction medium, respectively. Lane S: the supernatant of cell lysates; Lane P: the precipitation of cell lystates. (B) The relative contents of 5-HT1A receptor in the supernatant before immobilization and the supernatant after immobilization. (C) The amount of 5-HT1A receptor in the 140 mL supernatant of cell lysates before immobilization and after immobilization. BS: before immobilization, AS: after immobilization.
To further investigate how much 5-HT1A protein was immobilized on the surface of amino polystyrene microspheres, we conducted grayscale analysis in SDS-PAGE. As shown in Figs. S1 and Fig. 2B, the average relative contents of 5-HT1A protein in the supernatant of cell lysates before immobilization and after immobilization were 5.35% and 4.75%, respectively. Moreover, bicinchoninic acid (BCA) assay is widely applied in total protein quantitative analysis. We also determined the total protein concentrations of the supernatant of cell lysates before and after immobilization by BCA method, and the concentrations were 16.5 mg/mL and 9.3 mg/mL, respectively. Therefore, there was 123.6 mg 5-HT1A protein in 140 mL supernatant of cell lysates before immobilization and 61.8 mg 5-HT1A protein in 140 mL supernatant of cell lysates after immobilization (Fig. 2C). According to these data, it can be calculated that about 61.8 mg/g (7.72 × 10−7 mol) 5-HT1A protein was immobilized on the surface of microspheres. In brief, these results showed that 5-HT1A receptor was successfully expressed in Escherichia coli cells and about 7.72 × 10−7 mol 5-HT1A protein was immobilized on microsphere surface.
4.2. The surface characterization of stationary phase
To observe the morphological change in the surface of microspheres, scanning electron microscopy was firstly employed to characterize the features of linker- and receptor-conjugated microspheres. Owing to small size of 6-chlorocaproic acid, there was no significant difference between the bared amino polystyrene microspheres and 6-chlorocaproic acid coated gels, while the surface of microspheres became rougher when 5-HT1A receptor was modified, suggesting the successful immobilization of the protein ( Fig. 3A–F).
Fig. 3.
The surface characterization of stationary phase. The morphology characterization of amino-microspheres by scanning electron microscopy, bared amino-microspheres (A,D), 6-chlorohexanoic acid coated amino-microspheres (B, E), halo-tagged 5-HT1A receptor coated amino-microspheres (C, F); the element characterization of amino-microspheres by X-ray photoelectron spectroscopy, bared amino-microspheres (G), 6-chlorohexanoic acid coated amino-microspheres (H), halo-tagged 5-HT1A receptor coated amino-microspheres (I), the relative content of C, N, O, Cl element in amino-microspheres, 6-chlorohexanoic acid coated amino-microspheres and halo-tagged 5-HT1A receptor coated amino-microspheres (J).
To further confirm whether halo-tagged 5-HT1A receptor was successfully immobilized on the surface of microspheres, we also analyzed the surface element composition of synthesized stationary phase by using X-ray photoelectron spectroscopy. The results can be seen from Fig. 3G–J and Table 1. In 6-chlorocaproic acid coated gels, the relative content of C element was decreased compared with bared amino polystyrene microspheres, and the characteristic peak of Cl2p appeared at 198 eV. Furthermore, the relative content of N element was almost same, while the N 1 s peak shifted, which is due to the successful bonding of 6-chlorocaproic acid. After bonding of 5-HT1A receptor, the relative contents of N and O elements were dramatically increased owing to the abundance of amino and carboxylic groups in 5-HT1A receptor, while C content was further decreased, indicating the successful bonding of 5-HT1A receptor. Overall, C1 s peaks were mainly centered at ~284 eV, and the peak was probably associated with C C bond [15]; N1s peaks were mainly centered at ~400 eV, and the peak was probably associated with —NH— bond [16]; O1s peaks were mainly centered at ~531 eV, and peak was probably associated with C O bond [17]. Such phenomenon was consistent with our results. Taken together, these results demonstrated that 5-HT1A receptor was successfully immobilized on the surface of amino polystyrene microspheres.
Table 1.
The binding energy and relative content of C, N, O, Cl in amino-microsphere, 6-chlorohexanoic acid coated gel and halo-tagged 5-HT1A receptor coated gel.
|
Position (eV) |
Atomic/% |
|||||
|---|---|---|---|---|---|---|
| Amino- microsphere | 6-Chlorohexanoic acid coated gel | Halo-tagged 5-HT1A | Amino- microsphere | 6-Chlorohexanoic acid coated gel | Halo-tagged 5-HT1A | |
| C1 s | 283 | 283 | 284 | 86.46 | 85.39 | 77.77 |
| N1 s | 397 | 398 | 399 | 1.68 | 1.90 | 5.90 |
| O1 s | 531 | 531 | 531 | 11.87 | 11.71 | 15.39 |
| Cl2p | — | 198 | 199 | 0 | 1.00 | 0.94 |
4.3. The specificity and stability characterization of stationary phase
In this study, YL-0919, zolmitriptan, 5-HT (the agonists of 5-HT1A receptor), bambuterol (an agonist of β2-adrenergic receptor) and sodium nitrite were utilized to evaluate the specificity of 5-HT1A receptor colume. As shown in Fig. 4A, the retention time of YL-0919, zolmitriptan and 5-HT was 14.8 min, 6.6 min and 4.2 min, respectively, which is substantially longer than the void time measured by sodium nitrite (1.6 min). Moreover, bambuterol (an agonist of β2-adrenergic receptor) displayed a retention time of 1.5 min under the same condition, showing little difference from the system void time. Therefore, halo-tagged 5-HT1A receptor colume exhibited high specificity in recognizing ligands that had specific affinity to the receptor.
Fig. 4.
The specificity and stability of 5-HT1A receptor colume. (A) The chromatogram of sodium nitrite, 5-HT, zolmitriptan and YL-0919 on halo-tagged 5-HT1A receptor colume. (B) The chromatograms of 5-HT on halo-tagged 5-HT1A receptor colume over thirty days.
Here, we immobilized halo-tagged 5-HT1A receptor on the surface of amino polystyrene microspheres by one-step strategy, and halo-tagged 5-HT1A receptor colume was promised to be more active and stable than stationary phase synthesized by other immobilization methods. Taking 5-HT as a probe, we evaluated the stability of halo-tagged 5-HT1A receptor colume by determining the peak profile and the retention time of the ligand. During a testing period of 30 days, we found neither the peak profile nor the retention time exhibited significant changes, suggesting that 5-HT1A receptor colume showed good stability in at least 30 days (Fig. 4B). Through extensive literature on access, we discovered that the stability of 5-HT1A receptor colume in our study was superior to that of other GPCR receptor colume [7]. Such improvement may be attributed to the use of one-step immobilized methodology in the preparation of stationary phase since the biological orthogonal interaction between halo-tag and 5-HT1A receptor was more robust and specific than weak forces.
4.4. 5-HT1A receptor-ligand interaction analysis by frontal analysis
Frontal analysis is a classic chromatographic method where ligands are continuously infused over the stationary phase. It has been widely used to explore the interaction between the stationary phase and its ligand with high precision and accuracy. As shown in Fig. 5, typical breakthrough curves were obtained by frontal analysis. The breakthrough time was negatively correlated with ligand concentrations, and there was one type of immobilized ligand sites for all three drugs. The association constants of YL-0919, zolmitriptan and 5-HT determined by frontal analysis were 1.44 × 105 M−1, 0.62 × 105 M−1 and 0.40 × 105 M−1, respectively ( Table 2). Moreover, Langmuir and bi-Langmuir isotherm models were utilized to validate the number of binding sites of YL-0919, zolmitriptan and 5-HT. After fitting the raw data of three drugs to isotherm models, we found all three drugs agreed well with the simulation of Langmuir model, and the results of bi-Langmuir model were consistent with those of Langmuir model as well.
Fig. 5.
The interactions of YL-0919, zolmitriptan and 5-HT with 5-HT1A receptor by frontal affinity chromatography. The breakthrough curves of YL-0919 (A), zolmitriptan (D) and 5-HT (G) by 5-HT1A receptor colume. The regression curves of YL-0919 (B), zolmitriptan (E) and 5-HT (H) obtained by plotting 1/q vs 1/[C]. Figs. C, F and I represents the Langmuir model and BiLangmuir model of YL-0919, zolmitriptan and 5-HT, respectively.
Table 2.
The Ka values of YL-0919, zolmitriptan and 5-HT by frontal analysis, injection amount-dependent method and radio-ligand binding assay.
| Ligands | Ka by frontal analysis (M−1) | Ka by injection amount- dependent method (M−1) | Ka by radio-ligand binding assay (M−1) |
|---|---|---|---|
| YL-0919 | (1.44 ± 0.02) × 105 | (2.39 ± 0.09) × 106 | 5.26 × 109 |
| Zolmitriptan | (0.62 ± 0.01) × 105 | (1.85 ± 0.12) × 106 | 5.56 × 108 |
| 5-HT | (0.40 ± 0.01) × 105 | (0.94 ± 0.13) × 106 | 3.45 × 108 |
In brief, these results indicated that there was one type of binding sites for YL-0919, zolmitriptan and 5-HT at concentrations less than 80 μM, 128 μM and 160 μM, respectively. Furthermore, our model was a powerful way to elucidate the interaction between 5-HT1A receptor and its ligand.
4.5. 5-HT1A receptor-ligand interaction analysis by injection amount-dependent method
Injection amount-dependent analysis is a novel methodology that proposed by our group. In previous studies, we successfully carried out myriad drug-receptor interaction analysis by injection amount-dependent method, such as β2-adrenergic receptor [18], angiotensin II type I receptor [19] and green fluorescence protein [20]. In this study, we investigated the binding of zolmitriptan, YL-0919 and 5-HT to 5-HT1A receptor through injection amount-dependent analysis by using 5-HT1A receptor as a probe. As shown in Fig. 6A, the retention time of YL-0919 was obviously decreased with the growing injection concentrations. Therefore, it is rational to explain the retention behavior of YL-0919 by injection amount-dependent method.
Fig. 6.
The interactions of YL-0919, zolmitriptan and 5-HT with 5-HT1A receptor by injection amount-dependent method. The chromatograms of YL-0919 (A), zolmitriptan (D) and 5-HT (G) achieved by using 5-HT1A receptor colume. The regression curves of YL-0919 (B), zolmitriptan (E) and 5-HT (H) obtained by plotting knI/(1 + k) vs kVm. Figs. C, F and I represents the structure of YL-0919, zolmitriptan and 5-HT, respectively.
Plotting the curve of knI/(1 + k) to kVm using Eq.(3), two linear relationships for zolmitriptan in the desired concentrations were observed, and the highest association constant of 2.39 × 106 M−1 was achieved at concentrations less than 192 μM with correlation coefficients (r2) of 0.9988 (Fig. 6B). Above 192 μM, YL-0919 presented much lower affinity to the receptor, and the number of weaker binding sites was ~100 fold larger than the high affinity sites. Collectively, these results indicated that the binding sites at low concentration showed higher affinity for the ligand, and contributed dominantly to the binding of 5-HT1A receptor, which is consistent with our previous studies [19], [21]. Similarly, zolmitriptan showed the highest association constant of 1.85 × 106 M−1 at concentrations less than 128 μM with correlation coefficients (r2) of 0.9590, and 5-HT showed the highest association constant of 0.94 × 106 M−1 at concentrations less than 192 μM with correlation coefficients (r2) of 0.9978 (Fig. 6D,E, G,H). Moreover, considering the structural differences in three ligands (Fig. 6C, F, I), it is essential to carry out molecular docking to investigate the main amino acid residues of three drugs in drug-receptor interaction.
4.6. The comparison of frontal analysis and injection amount-dependent method
Frontal analysis is one of the most common approaches in the analysis of drug-receptor interaction with the advantage of high accuracy, which was developed by Kasai. et al. in 1975 [22]. However, considering the disadvantages of time-consuming and ligand-consuming of frontal analysis in the investigation of drug-receptor interaction, injection amount-dependent method was employed to explore whether it could address these deficiencies. In this study, frontal analysis and injection amount-dependent method showed quite different results in the number of binding sites involved in the interaction of 5-HT1A receptor and YL-0919, zolmitriptan and 5-HT. There was only one type of immobilized ligand site by frontal analysis, while injection amount-dependent method presented a result of at least two immobilized ligand sites. Such result is reasonable since the ligand concentrations used in two methods were different, and there was both one type of binding sites for two methods at the same concentration range.
Moreover, the association constants of three ligands obtained from injection amount-dependent method were one order of magnitude larger than the results of frontal analysis (Table 2). In spite of the differences of association constant between two sets of data, same affinity rank order was generated by two methods (YL-0919 > zolmitriptan > 5-HT). By BCA assay, we discovered that about 7.72 × 10−7 mol 5-HT1A protein was immobilized on microspheres. Such result was in good agreement with the maximum binding sites of YL-0919 (7.53 × 10−7 mol), zolmitriptan (1.79 × 10−7 mol), and 5-HT (2.54 × 10−7 mol) as well.
Radioligand binding techniques are reliable tools that provide new insights into the nature of the receptors and the affinity values of ligands. To further check the reliability of our affinity stationary phase, we also compared the association constants of YL-0919, zolmitriptan and 5-HT with those of literature. By literature profiling, we found the association constants of YL-0919 and zolmitriptan to 5-HT1A receptor by radioligand binding assays was 5.26 × 109 M−1 [23] and 5.56 × 108 M−1 [24], respectively. Segu et al. found there was a series of high-affinity 5-HT1 binding sites in rat superior colliculus and the mean association constant of [3H]5-HT to 5-HT1 receptor was 3.45 × 108 M−1 [25]. The affinity rank order of three ligands was YL-0919 > zolmitriptan > 5-HT, which agreed well with our results. Considering the potential damage of radioligands to humans, it is rather practicable to perform drug-receptor interaction analysis by frontal analysis and injection amount-dependent method.
Taken together, injection amount-dependent method is a valuable methodology for ligands that are difficult or expensive to obtain. Moreover, according to the results from frontal analysis and injection amount-dependent method, we found halo-tag can be used to immobilized 5-HT1A receptor and halo-tagged 5-HT1A receptor colume can recognize its ligands with high specificity.
4.7. The mechanism of drug-receptor interaction in molecular docking
5-HT1A receptor is a prototypical 5-HT receptor that has been widely used to treat numerous diseases of central nervous system after binding to ligands. However, no structure of 5-HT1A receptor was reported in the past few decades until Xu. et al. firstly reported 5-HT1A receptor structure in 2021 [26]. In this section, we analyzed the drug-receptor interaction of 5-HT1A receptor by molecular docking, and the docking interaction diagrams of YL-0919, zolmitriptan and 5-HT to 5-HT1A receptor were illustrated in Fig. 7.
Fig. 7.
The molecular docking model of the interaction between 5-HT1A receptor and its ligand. The 5-HT1A receptor is shown as the red ribbon, and other colors represent different amino acid residues. 3D overview of the interaction between zolmitriptan (A), YL-0919 (B), 5-HT (C) and 5-HT1A receptor; 2D overview of the interaction between zolmitriptan (D), YL-0919 (E), 5-HT (F) and 5-HT1A receptor.
As shown in Fig. 7 and Table 3, van der Waals (Asn R:386, Gly R:389, Phe R:362, Thr R:196, Trp R:358, Val R:117), hydrogen bonds (Asp R:116, Ser 199, Tyr R:195), and hydrophobic interactions (Ala R:365, Cys R:120, Ile R:189, Phe R:361, Tyr R:390) were the main forces of 5-HT1A receptor -YL-0919 interaction; van der Waals (Ala R:203, Ala R:365, Ile R:113, Ile R:189, Phe R:112, Phe R:362, Ser R:199, Thr R:196, Thr R:200, Trp R:358, Tyr R:390, Val R:117), hydrogen bonds (Cys R:120) and hydrophobic interactions (Asp R:116, Phe R:361) were the main driving forces of 5-HT1A receptor-zolmitriptan interaction; van der Waals (Ala R:383, Asn R:386, Ile R:189, Phe R:112, Phe R:361, Tyr R:96, Tyr R:390), hydrogen bonds (Asp 116, Gln R:97), and hydrophobic interactions (Ala R:93, Trp R:387) were the main forces of 5-HT1A receptor-5-HT interaction. The amino acid residues that involved in 5-HT1A receptor-ligand interaction in our study were consistent with those reported in the literature [26]. Moreover, the rank order of 5-HT1A receptor-ligand binding energy was YL-0919 (−7.30 kcal/mol) < zolmitriptan (−6.20 kcal/mol) < 5-HT (−6.18 kcal/mol), which agrees well with the association constants determined by frontal analysis and injection amount-dependent method.
Table 3.
The interacting residues of YL-0919, zolmitriptan and 5-HT with 5-HT1A receptor by molecular docking and their binding forces.
| Residues | YL-0919 | Zolmitriptan | 5-HT |
|---|---|---|---|
| Ala R:93 | — | — | Pi-Alkyl |
| Ala R:203 | — | Van der Waals | — |
| Ala R:365 | Pi-Alkyl | Van der Waals | — |
| Ala R:383 | — | — | Van der Waals |
| Asn R:386 | Van der Waals | — | Van der Waals |
| Asp R:116 | Carbon hydrogen bond | Pi-Anion | Conventional hydrogen bond |
| Cys R:120 | Pi-Alkyl | Conventional hydrogen bond | — |
| Gln R:97 | — | — | Conventional hydrogen bond |
| Gly R:389 | Van der Waals | — | — |
| Ile R:113 | — | Van der Waals | — |
| Ile R:189 | Pi-Sigma | Van der Waals | Van der Waals |
| Phe R:112 | — | Van der Waals | Van der Waals |
| Phe R:361 | Pi-Pi stacked | Pi-pi T-shaped | Van der Waals |
| Phe R:362 | Van der Waals | Van der Waals | — |
| Ser R:199 | Conventional hydrogen bond | Van der Waals | — |
| Thr R:196 | Van der Waals | Van der Waals | — |
| Thr R:200 | — | Van der Waals | — |
| Trp R:358 | Van der Waals | Van der Waals | — |
| Trp R:387 | — | — | Pi-pi T-shaped |
| Tyr R:96 | — | — | Van der Waals |
| Tyr R:195 | Conventional hydrogen bond | — | — |
| Tyr R:390 | Pi-Pi stacked | Van der Waals | Van der Waals |
| Val R:117 | Van der Waals | Van der Waals | — |
“—”means that the residue is not existing in the interaction between zolmitriptan/YL-0919/5-HT with 5-HT1A receptor.
4.8. The identification of bioactive compounds in Curcuma wenyujin Y. H. Chen et C. Ling
In this section, we performed bioactive compound identification in Curcuma wenyujin Y. H. Chen et C. Ling by using 5-HT1A receptor colume. As shown in Fig. 8A, the peak I at 5.41 min represented the retained fraction that specifically targeted 5-HT1A receptor, while other peaks were recognized as non-binding compounds since their retention time was near to the void time of chromatographic system. According to high-performance liquid chromatography-mass spectrometry analysis, we discovered that there were mainly three compounds in peak I. They were identified as gweicurculactone (m/z 229.1 [M+H]+) [27], compound 2 (m/z 679.5 [2 M+NH4]+, m/z 701.5 [2 M+NH3+Na]+) and curcuminol F (m/z 814.6 [2 M+H]+) [28] with the retention time of 1.258 min, 6.269 min and 6.591 min, respectively (Fig. 8B–D).
Fig. 8.
Screening potential bioactive compounds in Curcuma wenyujin Y. H. Chen et C. Ling. The crude extracts of this herb on 5-HT1A receptor colume and total ion current of peak I (A); the compositions of peak I by HPLC-MS analysis (B,D); the interaction between gweicurculactone (E,F), compound 2 (G,H), curcuminol F (I,J) and 5-HT1A receptor by molecular docking.
In addition, we analyzed the interaction of these three compounds with 5-HT1A receptor by molecular docking. As shown in Fig. 8E–J and Table 4, van der Waals (Asn R:386, Asp R:116, Gln R:97, Thr R:188, Trp R:387), hydrogen bonds (Tyr R:390) and hydrophobic interactions (Ala R:93, Cys R:109, Cys R:187, Ile R:113, Ile R:189, Phe R:112, Trp R:102, Tyr R:96) were the main driving forces of 5-HT1A receptor-gweicurculactone interaction; van der Waals (Asp R:192, Ile R:124, Ile R:167, Leu R:366, Leu R:368, Phe R:361, Pro R 369, Ser R:199, Thr R:196, Val R:364), hydrogen bonds (Ala R:365, Ser R:190, Thr R:121) and hydrophobic interactions (Ala R:203, Cys R:120, Ile R:189, Lys R:191, Phe R:362, Tyr R:195, Val R:117) were the main driving forces of 5-HT1A receptor-compound 2 interaction; van der Waals (Ala R:365, Asn R:386, Gly R:382, Ile R:113, Ile R:385, Leu R:368, Lys R:191, Met R:377, Phe R:112, Phe R:361, Pro R:369, Thr R:188, Val R:364), hydrogen bonds (Ile R:189) and hydrophobic interactions (Cys R:109, Cys R:187, Tyr R:96) were the main driving forces of 5-HT1A receptor-curcuminol F interaction. The binding energy of gweicurculactone, compound 2 and curcuminol F was −7.06 kcal/mol, −7.77 kcal/mol and −5.26 kcal/mol, respectively. Collectively, gweicurculactone, compound 2 and curcuminol F were identified as potential ligands of 5-HT1A receptor in Curcuma wenyujin Y. H. Chen et C. Ling with relatively low binding energies. Van der Waals, hydrogen bonds and hydrophobic interactions were the main driving forces of 5-HT1A receptor–ligand interaction.
Table 4.
The interacting residues of gweicurculactone, compound 2, curcuminol F with 5-HT1A receptor by molecular docking and their binding forces.
| Residues | Gweicurculactone | Compound 2 | Curcuminol F |
|---|---|---|---|
| Ala R:93 | Pi-Sigma | — | — |
| Ala R:203 | — | Alkyl/Pi-Alkyl | — |
| Ala R:365 | — | Conventional hydrogen bond | Van der Waals |
| Asn R:386 | Van der Waals | — | Van der Waals |
| Asp R:116 | Van der Waals | — | — |
| Asp R:192 | — | Van der Waals | — |
| Cys R:109 | Alkyl/Pi-Alkyl | — | Pi-Alkyl |
| Cys R:120 | — | Alkyl/Pi-Alkyl | — |
| Cys R:187 | Alkyl/Pi-Alkyl | — | Pi-Lone Pair |
| Gln R:97 | Van der Waals | — | — |
| Gly R:382 | — | — | Van der Waals |
| Ile R:113 | Alkyl/Pi-Alkyl | — | Van der Waals |
| Ile R:124 | — | Van der Waals | — |
| Ile R:167 | — | Van der Waals | — |
| Ile R:189 | Alkyl/Pi-Alkyl | Alkyl/Pi-Alkyl | Conventional hydrogen bond |
| Ile R:385 | — | — | Van der Waals |
| Leu R:366 | — | Van der Waals | — |
| Leu R:368 | — | Van der Waals | Van der Waals |
| Lys R:191 | — | Pi-Cation | Van der Waals |
| Met R:377 | — | — | Van der Waals |
| Phe R:112 | Pi-pi T-shaped | — | Van der Waals |
| Phe R:361 | — | Van der Waals | Van der Waals |
| Phe R:362 | — | Pi-pi T-shaped | — |
| Pro R 369 | — | Van der Waals | Van der Waals |
| Ser R:190 | — | Conventional hydrogen bond | — |
| Ser R:199 | — | Van der Waals | — |
| Thr R:121 | — | Conventional hydrogen bond | — |
| Thr R:188 | Van der Waals | — | Van der Waals |
| Thr R:196 | — | Van der Waals | — |
| Trp R:102 | Alkyl/Pi-Alkyl | — | — |
| Trp R:387 | Van der Waals | — | — |
| Tyr R:96 | Pi-Sigma | — | Pi-pi T-shaped |
| Tyr R:195 | — | Alkyl/Pi-Alkyl | — |
| Tyr R:390 | Conventional hydrogen bond | — | — |
| Val R:117 | — | Alkyl/Pi-Alkyl | — |
| Val R:364 | — | Van der Waals | Van der Waals |
“—”means that the residue is not existing in the interaction between zolmitriptan/YL-0919/5-HT with 5-HT1A receptor.
In addition to gweicurculactone, compound 2 and curcuminol F, curcumin has also been reported to alleviate depression by increasing 5-HT levels via inhibiting monoamine oxidases [29], and modulating 5-HT1A/1B/5-HT2C receptors [30]. However, curcumin was not identified by 5-HT1A receptor colume in our study. Such phenomenon may be caused by the low affinity of curcumin to 5-HT1A receptor or its poor stability, and affinity chromatography colume that was suitable to perform drug-receptor interaction analysis for low-affinity ligands were needed in the future.
5. Conclusion
In this study, we synthesized a new affinity stationary phase by attaching 5-HT1A receptor on microspheres through the specific covalent reaction of halo-tag fused at the C-terminal of the receptor and 6-chlorohexanoic acid on the microsphere surface. The immobilized 5-HT1A receptor could recognize and separate new ligands from complex systems with high specificity. Given the fact that 5-HT1A receptor was immobilized through a one-step strategy, intensive purification procedures and loss of activity were largely avoided. Gweicurculactone, compound 2 and curcuminol F were bioactive compounds of Curcuma wenyujin Y. H. Chen et C. Ling that specifically bound to 5-HT1A receptor. Moreover, this method is applicable to the bioactive compound identification of other natural products that binds to 5-HT1A receptor as well. Altogether, we developed a potentially universal chromatographic based method for bioactive compound identification of natural products by using the immobilized 5-HT1A receptor as stationary phase. We believe that this study is of interest to a broad range of scientists who are interested in protein-ligand interaction analysis, high throughput screening, and drug discovery.
CRediT authorship contribution statement
Yuan-Yuan Chen: Investigation, Formal analysis, Data curation, Writing – original draft. Ya-Hui Jin: helped to carry out the experiments, Writing – review & editing. Aerduosi Shayiranbieke: helped to carry out the experiments, Writing – review & editing. Xue Zhao: helped to carry out the experiments, Writing – review & editing. Hu-Shuai Fan: helped to carry out the experiments, Writing – review & editing. Qian Li: Writing – review & editing, Funding acquisition. Xin-Feng Zhao: Conceptualization, Supervision, Funding acquisition. All authors have approved the final manuscript.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgment
We thank the National Natural Science Foundation of China (22074118 and 21974107) and the Key Research and Development Program of Shaanxi Province (2020ZDLSF05-07) for the financial support of this work.
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.jpba.2022.114632.
Appendix A. Supplementary material
Supplementary material
.
References
- 1.Gururajan A., Reif A., Cryan J.F., Slattery D.A. The future of rodent models in depression research. Nat. Rev. Neurosci. 2019;20(11):686–701. doi: 10.1038/s41583-019-0221-6. [DOI] [PubMed] [Google Scholar]
- 2.G.B.D. Disease, I. Injury, C. Prevalence Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392(10159):1789–1858. doi: 10.1016/S0140-6736(18)32279-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu R., Pei Q., Shou T., Zhang W., Hu J., Li W. Apoptotic effect of green synthesized gold nanoparticles from Curcuma wenyujin extract against human renal cell carcinoma A498 cells. Int. J. Nanomed. 2019;14:4091–4103. doi: 10.2147/IJN.S203222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xia X., Cheng G., Pan Y., Xia Z.H., Kong L.D. Behavioral, neurochemical and neuroendocrine effects of the ethanolic extract from Curcuma longa L. in the mouse forced swimming test. J. Ethnopharmacol. 2007;110(2):356–363. doi: 10.1016/j.jep.2006.09.042. [DOI] [PubMed] [Google Scholar]
- 5.Sharp T., Boothman L., Raley J., Queree P. Important messages in the ‘post’: recent discoveries in 5-HT neurone feedback control. Trends Pharmacol. Sci. 2007;28(12):629–636. doi: 10.1016/j.tips.2007.10.009. [DOI] [PubMed] [Google Scholar]
- 6.Zeng K., Li Q., Wang J., Yin G., Zhang Y., Xiao C., Fan T., Zhao X., Zheng X. One-step methodology for the direct covalent capture of GPCRs from complex matrices onto solid surfaces based on the bioorthogonal reaction between haloalkane dehalogenase and chloroalkanes. Chem. Sci. 2017;9(2):446–456. doi: 10.1039/c7sc03887a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beigi F., Chakir K., Xiao R.P., Wainer I.W. G-protein-coupled receptor chromatographic stationary phases. 2. Ligand-induced conformational mobility in an immobilized β2-adrenergic receptor. Anal. Chem. 2004;76(24):7187–7193. doi: 10.1021/ac048910c. [DOI] [PubMed] [Google Scholar]
- 8.Calleri E., Ceruti S., Cristalli G., Martini C., Temporini C., Parravicini C., Volpini R., Daniele S., Caccialanza G., Lecca D., Lambertucci C., Trincavelli M.L., Marucci G., Wainer I.W., Ranghino G., Fantucci P., Abbracchio M.P., Massolini G. Frontal affinity chromatography-mass spectrometry useful for characterization of new ligands for GPR17 receptor. J. Med. Chem. 2010;53(9):3489–3501. doi: 10.1021/jm901691y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moaddel R., Rosenberg A., Spelman K., Frazier J., Frazier C., Nocerino S., Brizzi A., Mugnaini C., Wainer I.W. Development and characterization of immobilized cannabinoid receptor (CB1/CB2) open tubular column for on-line screening. Anal. Biochem. 2011;412(1):85–91. doi: 10.1016/j.ab.2010.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Los G.V., Encell L.P., McDougall M.G., Hartzell D.D., Karassina N., Zimprich C., Wood M.G., Learish R., Ohana R.F., Urh M., Simpson D., Mendez J., Zimmerman K., Otto P., Vidugiris G., Zhu J., Darzins A., Klaubert D.H., Bulleit R.F., Wood K.V. HaloTag: a novel protein labeling technology for cell imaging and protein analysis. ACS Chem. Biol. 2008;3(6):373–382. doi: 10.1021/cb800025k. [DOI] [PubMed] [Google Scholar]
- 11.Miyabe K., Guiochon G. A kinetic study of mass transfer in reversed-phase liquid chromatography on a C18-silica gel. Anal. Chem. 2000;72(21):5162–5171. doi: 10.1021/ac0002801. [DOI] [PubMed] [Google Scholar]
- 12.Repo E., Kurniawan T.A., Warchol J.K., Sillanpaa M.E. Removal of Co(II) and Ni(II) ions from contaminated water using silica gel functionalized with EDTA and/or DTPA as chelating agents. J. Hazard. Mater. 2009;171(1–3):1071–1080. doi: 10.1016/j.jhazmat.2009.06.111. [DOI] [PubMed] [Google Scholar]
- 13.Zhao X., Li Q., Xiao C., Zhang Y., Bian L., Zheng J., Zheng X., Li Z., Zhang Y., Fan T. Oriented immobilisation of histidine-tagged protein and its application in exploring interactions between ligands and proteins. Anal. Bioanal. Chem. 2014;406(12):2975–2985. doi: 10.1007/s00216-014-7723-x. [DOI] [PubMed] [Google Scholar]
- 14.Heo S., Patil S.S., Jung G., Hoger H., Lubec G. A serotonin receptor 1A containing complex in hippocampus of PWD/PhJ mice is linked to training effects in the Barnes maze. Behav. Brain Res. 2011;216(1):389–395. doi: 10.1016/j.bbr.2010.08.018. [DOI] [PubMed] [Google Scholar]
- 15.Kamau G.N., Willis W.S., Rusling J.F. Electrochemical and electron spectroscopic studies of highly polished glassy carbon electrodes. Anal. Chem. 1985;57(2):545–551. doi: 10.1021/ac50001a049. [DOI] [PubMed] [Google Scholar]
- 16.Silva-Moraes M.O., Garcia-Basabe Y., de Souza R.F.B., Mota A.J., Passos R.R., Galante D., Fonseca Filho H.D., Romaguera-Barcelay Y., Rocco M.L.M., Brito W.R. Geometry-dependent DNA-TiO2 immobilization mechanism: a spectroscopic approach. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2018;199:349–355. doi: 10.1016/j.saa.2018.03.081. [DOI] [PubMed] [Google Scholar]
- 17.Yi S., Sun Y., Hu X., Xu H., Gao B., Wu J. Porous nano-cerium oxide wood chip biochar composites for aqueous levofloxacin removal and sorption mechanism insights. Environ. Sci. Pollut. Res. Int. 2018;25(26):25629–25637. doi: 10.1007/s11356-016-8342-1. [DOI] [PubMed] [Google Scholar]
- 18.Gao J., Chang Z., Tian R., Li P., Ahmad F., Jia X., Liang Q., Zhao X. Reversible and site-specific immobilization of β(2)-adrenergic receptor by aptamer-directed method for receptor-drug interaction analysis. J. Chromatogr. A. 2020;1622 doi: 10.1016/j.chroma.2020.461091. [DOI] [PubMed] [Google Scholar]
- 19.Liang Q., Fu X., Zhang J., Hao J., Feng G., Wang J., Li Q., Ahmad F., Zhao X. Immobilized angiotensin II type I receptor: a powerful method of high throughput screening for antihypertensive compound identification through binding interaction analysis. J. Chromatogr. A. 2020;1620 doi: 10.1016/j.chroma.2020.461003. [DOI] [PubMed] [Google Scholar]
- 20.Li Q., Wang J., Yang L., Gao X., Chen H., Zhao X., Bian L., Zheng X. Estimation of interaction between oriented immobilized green fluorescent protein and its antibody by high performance affinity chromatography and molecular docking. J. Mol. Recognit. 2015;28(7):438–446. doi: 10.1002/jmr.2460. [DOI] [PubMed] [Google Scholar]
- 21.Zhao X., Jin Y., Yuan X., Hou Z., Chen Z., Fu X., Li Q., Wang J., Zhang Y. Covalent inhibitor-based one-step method for endothelin receptor A immobilization: from ligand recognition to lead identification. Anal. Chem. 2020;92(20):13750–13758. doi: 10.1021/acs.analchem.0c01807. [DOI] [PubMed] [Google Scholar]
- 22.Kasai K., Ishii S. Quantitative analysis of affinity chromatography of trypsin. A new technique for investigation of protein-ligand interaction. J. Biochem. 1975;77(1):261–264. [PubMed] [Google Scholar]
- 23.Chen H.X., Jin Z.L., Zhang L.M., Xue R., Xu X.D., Zhao N., Qiu Z.K., Wang X.W., Zhang Y.Z., Yang R.F., Li Y.F. Antidepressant-like activity of YL-0919: a novel combined selective serotonin reuptake inhibitor and 5-HT1A receptor agonist. PLoS One. 2013;8(12) doi: 10.1371/journal.pone.0083271. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 24.Martin G.R., Robertson A.D., MacLennan S.J., Prentice D.J., Barrett V.J., Buckingham J., Honey A.C., Giles H., Moncada S. Receptor specificity and trigemino-vascular inhibitory actions of a novel 5-HT1B/1D receptor partial agonist, 311C90 (zolmitriptan) Br. J. Pharmacol. 1997;121(2):157–164. doi: 10.1038/sj.bjp.0701041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Segu L., Abdelkefi J., Dusticier G., Lanoir J. High-affinity serotonin binding sites: autoradiographic evidence for their location on retinal afferents in the rat superior colliculus. Brain Res. 1986;384(2):205–217. doi: 10.1016/0006-8993(86)91156-x. [DOI] [PubMed] [Google Scholar]
- 26.Xu P., Huang S., Zhang H., Mao C., Zhou X.E., Cheng X., Simon I.A., Shen D.D., Yen H.Y., Robinson C.V., Harpsoe K., Svensson B., Guo J., Jiang H., Gloriam D.E., Melcher K., Jiang Y., Zhang Y., Xu H.E. Structural insights into the lipid and ligand regulation of serotonin receptors. Nature. 2021;592(7854):469–473. doi: 10.1038/s41586-021-03376-8. [DOI] [PubMed] [Google Scholar]
- 27.Tungcharoen P., Wattanapiromsakul C., Tansakul P., Nakamura S., Matsuda H., Tewtrakul S. Antiinflammation constituents from Curcuma zedoaroides. Phytother. Res. 2018;32(11):2312–2320. doi: 10.1002/ptr.6173. [DOI] [PubMed] [Google Scholar]
- 28.Ma Z.J., Meng Z.K., Zhang P. Chemical constituents from the radix of Curcuma wenyujin. Fitoterapia. 2009;80(6):374–376. doi: 10.1016/j.fitote.2009.05.004. [DOI] [PubMed] [Google Scholar]
- 29.Zhang Y., Li L., Zhang J. Curcumin in antidepressant treatments: an overview of potential mechanisms, pre-clinical/clinical trials and ongoing challenges. Basic Clin. Pharmacol. Toxicol. 2020;127(4):243–253. doi: 10.1111/bcpt.13455. [DOI] [PubMed] [Google Scholar]
- 30.Wang R., Xu Y., Wu H.L., Li Y.B., Li Y.H., Guo J.B., Li X.J. The antidepressant effects of curcumin in the forced swimming test involve 5-HT1 and 5-HT2 receptors. Eur. J. Pharmacol. 2008;578(1):43–50. doi: 10.1016/j.ejphar.2007.08.045. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material