ABSTRACT
MicroRNA 200a (miR-200a) can inhibit the activation and proliferation of hepatic stellate cells (HSCs) through the transforming growth factor-β (TGF-β) signaling pathway, and improve fibrotic lesions. However, to date, there is no study exploring the role of miR-200a in schistosomiasis liver fibrosis (SLF). In this study, 64 healthy female Balb/c mice were selected and randomly divided into four groups: normal control group (non-infected schistosomiasis group), schistosomiasis model group, Lenti-NC group (lentivirus-negative control group), and Lenti-miR-200a group (lentivirus experimental group). Fluorescence quantitative PCR detection was used to measure the expression level of RNA. HE and Masson staining were used to observe the pathological changes of mouse liver tissue. Furthermore, ELISA was used to detect the serum concentrations of inflammation factors. We found that the expression level of miR-200a in liver tissues gradually decreased with the development of SLF. However, fibrosis factors (α-SMA and TGF-β2) and inflammatory cytokines (IL-4 and IFN-γ) in liver tissues and serum increased and the expression level of Colla I reached its peak in the 6th week of infection. Besides, compared with the schistosomiasis group and Lenti-NC group, the Lenti-NC group had lower levels of α-SMA, TGF-β2 and Colla I (P > 0.05). Furthermore, inflammatory cells and blue collagen fibers appeared and they increased with the development of infection in the schistosomiasis group and Lenti-NC group, but these changes reduced significantly in Lenti-miR-200a group. Our study demonstrated that upregulation of miR-200a might contribute to inhibiting schistosomiasis liver fibrosis.
KEYWORDS: Mir-200a, schistosoma japonicum, tgf-β, liver fibrosis
Graphical abstract
1. Introduction
Schistosomiasis japonica, a zoonotic disease, is endemic in the south of the Yangtze River of China and seriously endangers human health [1]. The main lesion of this disease is due to the deposition of schistosome eggs in the liver and intestine tissues. The soluble antigens of the eggs induce the infiltration of inflammatory cells and release of cytokine, forming a granulomatous inflammation reaction centered on the eggs and leading to abnormal proliferation of connective tissue and excessive deposition of extracellular matrix (ECM) which leads to fibrotic lesions. Schistosomiasis liver fibrosis (SLF) is the main cause of death of schistosomiasis. Exploring the mechanism of liver fibrosis and identifying targets to inhibit liver fibrosis have become the focuses of researches on schistosomiasis japonica [2,3].
Liver fibrosis caused by Schistosoma japonicum infection is mainly due to the continuous stimulation of soluble egg antigen (SEA) of schistosome eggs. Interleukin −6 (IL-6) and γ-interferon (IFN-γ) are secreted by Th1 cells, which makes the imbalance of immune response in the host. When a large number of eggs are deposited, SEA induces the rapid transformation of Th1 to Th2 immune response, producing a large amount of IL-4, IL-6 and other cytokines, and inducing macrophages, eosinophils, and fibroblasts to gather around the eggs and then form granulomas and fibrosis [4].
In addition, current studies have found that the main mechanism of schistosomiasis liver fibrosis is the activation of hepatic stellate cells (HSCs), which causes the abnormal expression of collagen and the excessive deposition of ECM. Under normal physiological conditions, HSCs are in a resting state, mainly distributed in the hepatic sinus gap and are used to store vitamin A [5]. When liver is injured chronically, the resting HSCs are activated and transformed into myofibroblasts, secreting a large amount of ECM and a variety of pro-fibrotic cytokines, such as α-smooth muscle agonist protein (α-SMA), transforming growth factor – β2 (TGF-β2), etc. Differentiated myofibroblasts synthesize and secrete a large amount of ECM containing collagen fibers, which leads to an imbalance in degradation and synthesis of ECM and contributes to the destruction of liver structure and ultimately the occurrence of liver fibrosis [6].
MicroRNAs (miRNA) are a type of single-stranded non-coding short-stranded RNA that regulate the post-transcriptional expression of messenger RNAs (mRNAs) mainly by targeting the 3ʹ untranslated region of messenger mRNAs, thereby regulating biological properties such as cell activation, proliferation, apoptosis, and migration [7]. In malignant tumors, heart, and kidney diseases, miRNAs have emerged as specific biomarkers and functioned as targets for disease therapy [8]. miR-200a, a member of the miR-200 family, is a miRNA formed by the primitive transcription code of a polycistron with a size of about 7.5 kb, encoding a gene on chromosome 1 and, like other miR-200 miRNAs, has biological characteristics such as gene timing, tissue specificity and high conservation. The target genes they act on are mainly related to protein phosphorylation, cell activation, and gene transcription [9]. Recently, increasing evidence has demonstrated that aberrantly expressed miR-200a is considered to be a regulator in some fibrosis diseases. It has been that miR-200a plays an important role in cardiac fibrosis, renal fibrosis, and liver fibrosis [10].
To date, several studies explored the effect and possible pathways of miR-200a on CCl4-induced liver fibrosis [9,11,12]. However, there is no report on the role of miR-200a in SLF. Therefore, in this study, we will explore the effect of miR-200a on Schistosoma japonicum-induced liver fibrosis by constructing miR-200a lentiviral vectors delivered to schistosomes infected mice. We hypothesize that miR-200a might play a role in SLF and hope that this study is able to provide a new potential target for the treatment of liver fibrosis in schistosomiasis.
2. Materials and methods
2.1. Experimental animals and grouping
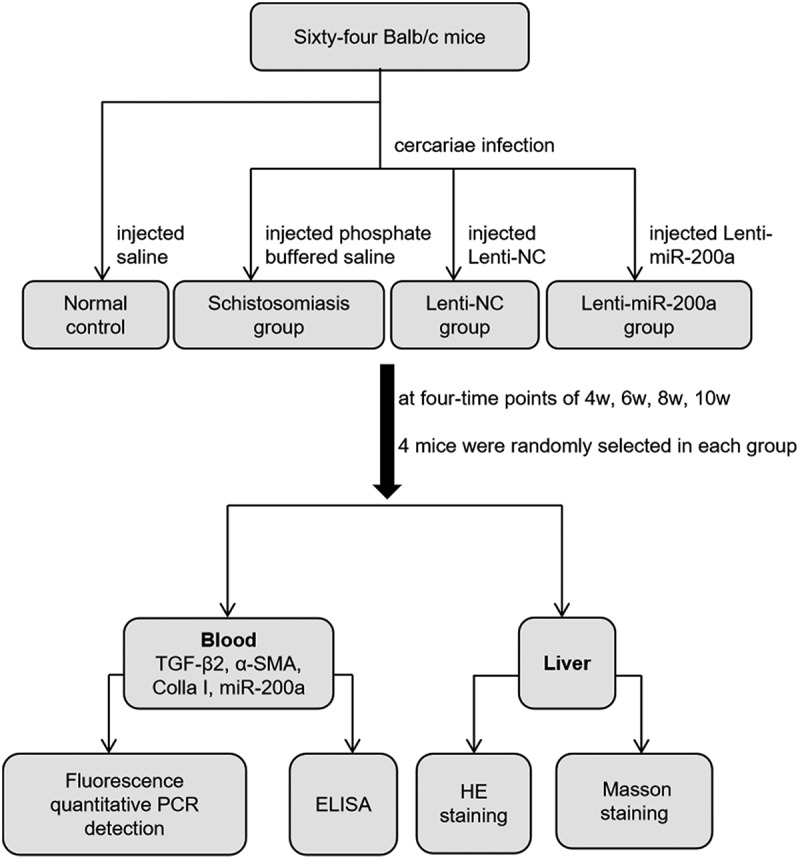
Sixty-four healthy female Balb/c mice (Changsha Slack Jingda Experimental Animal Co., Ltd., China) aged 6 to 8 weeks were selected and randomly divided into four groups, each with 16 mice, grouped as follows: normal control group (non-infected schistosomiasis group), schistosomiasis group (schistosomiasis model group), Lenti-NC group (the lentiviral empty vector injection group after schistosomiasis infection was the lentivirus negative control group), Lenti-miR-200a group (the lentivirus/miR-200a injection group after schistosomiasis infection was the lentivirus experimental group).
In a small conical flask with dechlorinated water at room temperature of about 28°C, positive Oncomelania infected with Schistosoma cercariae (Yueyang Institute of Parasitic Disease Control, Hunan Province, China) were filled, and the mouth of the conical flask was sealed with a filter to prevent the cercariae from crawling out. The conical flask was directly irradiated with light so as to induce the cercariae to release the larvae. Firstly, the microscope was used to count the caecilians, and the mice would be infected according to 16 ± 2 caecilians per mouse. Followed by removing the abdominal hair of the mice, the caecilians were picked on the coverslip using an inoculation loop, and the coverslip was applied to the abdomen of the mice via the abdominal skin dressing method, and then the patch was discarded after about 20 minutes of infection. Ten days after the mice were infected with cercariae, the mice were injected with an equal volume of 60 μL of saline, phosphate buffered saline (PBS),1 × 109 TU/mL Lenti-NC (Shanghai Jimma Gene Biotechnology Co., Ltd, China.) and 1 × 109 TU/mL Lenti-miR-200a (Shanghai Jimma Gene Biotechnology Co., Ltd, China.) through the tail vein, respectively. The mice were fed routinely and four-time points of 4 , 6 , 8 and 10 w after infection. Four mice in each group were randomly taken blood from the eyeballs of each mouse (about 0.8–1.2 mL), and finally executed and the liver tissues were collected for further experimental research.
2.2. Fluorescence quantitative PCR detection
The expression of miR-200 c, TGF-β2, α-SMA, and Colla I in mice liver were tested in all four groups at four-time points. Trizol reagent was used to extract total RNA from liver tissue. RNA was reverse transcribed into cDNA according to miRNA reverse transcription kit, placed on a PCR machine at 55°C for 8 minutes, 45°C for 1 hour, and 85°C for 4 minutes to obtain inactivated reverse transcriptase for PCR reaction. The detail of gene primer information is shown in Table 1. Each reverse transcription product was proportionally prepared in three tubes of reaction system: 7.5 μM gene primer (Shanghai Shenggong Technology Service Co., Ltd., China) 2.5 μL, 2× qPCR Mix 12.0 μL, reverse transcription product 3.0 μL, ddH2O 7.5 μL. PCR amplification: 45°C 30 minutes, 94°C 3 minutes, cycle (40 times), 94°C 10 s→55°C 20 s, dissolution curve 72°C→94°C, heating 1°C every 20s. The results were processed by the ΔΔCT method:
Table 1.
Primer information
| Primer name | Sequence |
|---|---|
| Hum-miR-200a Hum-TGF-β2 Mmu-α-SMA Mmu-Colla I Mmu-TGF-β2 Mmu-IFN-γ Mmu-IL-4 U6 |
Forward(5′–3′) CGTGGAGTCGGCAATTCAGTTGAGACATCGTT Reverse(5′–3′) ACACTCCAGCTGGGTAACACTGTCTGGTAA Forward(5′–3′) TTGTGCTCCAGACAGTCCCAG Reverse(5′–3′) CAATCCGTTGTTCAGGCACTCT Forward(5′–3′) TCCCTGGAGAAGAGCTACGAA Reverse(5′–3′) ATAGGTGGTTTCGTGGATGCC Forward(5′–3′) TGACTGGAAGAGCGGAGAGT Reverse(5′–3′) AGACGGCTGAGTAGGGAACA Forward(5′–3′) TGGTGTTGTACAGGCTGAGG Reverse(5′–3′) CCACAAAGACAGGAACCTGG Forward(5′–3′) GCTTTGCAGCTCTTCCTCAT Reverse(5′–3′) TCTTCCACATCTATGCCACTTG Forward(5′–3′) CGTCTGTAGGGCTTCCAAGG Reverse(5′–3′) AGGCTACGAAAAAGCCCGAA Forward(5′–3′) CTCGCTTCGGCAGCACA |
ΔCT = CT target gene-CT β-actin
ΔΔCT = ΔCT experiment-ΔCT control amplification factor = 2-ΔΔCT.
2.3. Mouse liver histopathology HE and Masson staining
100 mg of fresh mouse liver tissue was taken and put into 4% paraformaldehyde fixative. The fixed liver tissue was washed, dehydrated, transparent, embedded in wax, sliced (thickness of about 4 μm), and then stained by HE and Masson staining, respectively, [13,14]. HE staining was performed by dewaxing, ethanol elution, rinsing with running water, staining with hematoxylin staining solution for 6 min, 0.5% hydrochloric acid ethanol for 3 min, and 0.5% eosin staining solution for 1 min. 0.5% light ammonia returned to blue for 40 seconds, 0.5% eosin staining solution for 1 minute, dehydrated in 95% and 100% ethanol for 12 minutes, transparent with xylene four times for 10 minutes each, and sealed with neutral resin gel before microscopic observation of pathological changes in mouse liver tissue. Masson staining was performed according to the operating procedure after fixation of liver tissue by washing, dehydration, transparency, and immersion wax embedding, and then sliced. The slices were first stained with R1 nuclear staining solution for 1 minute, R2 plasma staining solution for 60 seconds, and R3 yellow solution for 8 minutes to pour off the separation solution, and directly stained with R4 blue re-staining solution, poured off the staining solution. rinsed with 95% ethanol. After drying, use neutral resin glue to seal the liver tissue and then observe the pathological changes of mouse liver tissue under a microscope.
2.4. ELISA to detect the expression levels of IL-4, IFN-γ and ALT in mouse serum
Use the kits (Nanjing Jiancheng Institute of Bioengineering, China) to detect the serum levels of IL-4, IFN-γ and ALT. All the procedures have strictly followed the instructions. The reagents and serum samples were first taken out of the refrigerator and left for 30 minutes at room temperature, and then the absorbance OD values of each well were measured sequentially on the enzyme standardizer with the wavelength set at 450 nm in accordance with the sample addition, warming, plate washing, enzyme addition, warming, plate washing, color development, termination, and colorimetric determination. Finally, we compared with the standard curve and calculated the corresponding measurement value. (OD value = determination well OD value – compare the OD value of the well). The colorimetric analysis should be completed within 20 minutes after the addition of the termination solution.
2.5. Statistical analysis
Experimental results were expressed as mean±standard deviation ( ±SD). The comparison between groups was performed by one-way ANOVA. The SPSS 20.0 software was used for statistical analysis. GraphPad Prism 5.0 was used for drawing processing of line graphs and histograms. P < 0.05 was considered statistically significant.
±SD). The comparison between groups was performed by one-way ANOVA. The SPSS 20.0 software was used for statistical analysis. GraphPad Prism 5.0 was used for drawing processing of line graphs and histograms. P < 0.05 was considered statistically significant.
3. Results
In this study, 64 healthy female Balb/c mice were selected and randomly divided into four groups: normal control group, schistosomiasis group, Lenti-NC group, and Lenti-miR-200a group. We explored the effect of miR-200a on Schistosoma japonicum-induced liver fibrosis by constructing miR-200a lentiviral vectors delivered to schistosomes infected mice. The expression level of miR-200a, fibrosis factors (α-SMA and TGF-β2) and inflammatory cytokines (IL-4 and IFN-γ) in liver tissues and serum were tested. We found a critical role of miR-200a in the development of SLF.
3.1. The expression of miR-200a in liver fibrosis in mice infected with Schistosoma
To explore whether miR-200a has a certain correlation with schistosomiasis liver fibrosis, we constructed a mouse model of schistosomiasis liver fibrosis. Using fluorescent quantitative PCR detection, it was found that the expression level of miR-200a in mouse liver tissues decreased with the prolongation of infection time (Figure 1 A). In contrast, the expression levels of α-SMA and TGF-β2 mRNA in the liver tissues of mice increased significantly with the prolongation of schistosomiasis infection time (Figure 1 B). Compared with the fourth week, the expression level of Colla I mRNA increased significantly in the sixth and eighth week and reached the peak in the sixth week (Figure 1 C).
Figure 1.
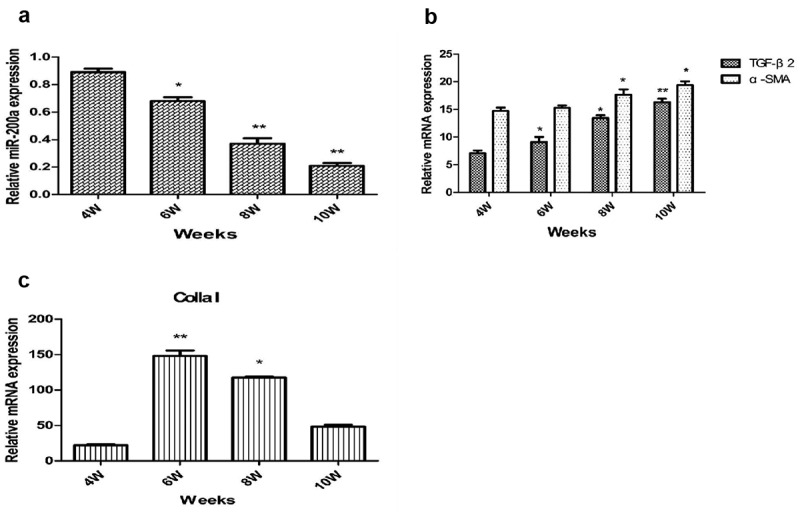
qRT-PCR identification of miR-200a (a), TGF-β2 and α-SMA (b), and Colla I (c) on expression in liver tissues of mice infected with S. japonicum **: P < 0.01 vs 4 w *: P < 0.05 vs 4 w.
3.2. Overexpression of lentivirus miR-200a can alleviate schistosomiasis liver fibrosis
In order to further study the effect of up-regulating the expression level of miR-200a in mice on schistosomiasis liver fibrosis, we delivered the lentivirus Lenti-miR-200a into mice infected with schistosome cercariae by tail vein injection, and detect the expression levels of miR-200a in the liver tissues of mice in each group at different time points by fluorescence quantitative PCR. The results showed that the expression level of miR-200a in the liver tissues of mice in the Lenti-miR-200a group increased significantly in the fourth and sixth weeks, and its expression gradually decreased with the extension of the infection time (Figure 2 A).
Figure 2.
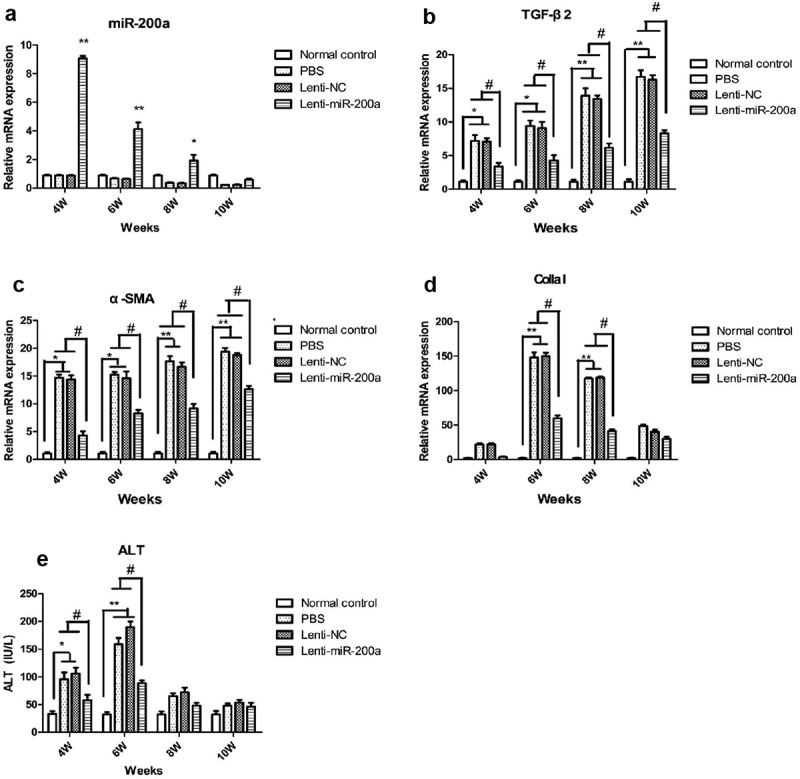
qRT-PCR identification of miR-200a (a), TGF-β2 (b), α-SMA (c), CollaI (d) and serum ALT (e) on expression in liver tissues of mice infected with S. japonicum. **: P < 0.01 vs normal control *: P < 0.05 vs normal control #: P < 0.05 vs schistosomiasis and Lenti-NC
Meanwhile, the expressions of TGF-β2 and α-SMA mRNA in the liver were detected, and the results showed that the expression levels of TGF-β2 and α-SMA mRNA in liver tissues of mice in the schistosomiasis group and Lenti-NC groups were found to be significantly increased with the extension of schistosome infection time, while the expression levels of TGF-β2 and α-SMA mRNA in the liver tissues of the Lenti-miR-200a group were significantly lower than that in the schistosomiasis group and the Lenti-NC group (P < 0.05) (Figure 2 B and C). The expression of Colla I mRNA in the liver was detected, and the results showed that with the extension time of schistosome infection, the expression of Colla I mRNA in the liver tissue of mice in the schistosomiasis group and Lenti-NC group started to rise in the fourth week, and peaked in the sixth week. The expression level of Colla I mRNA in the liver tissue of mice in the Lenti-miR-200a group was significantly lower than that in the schistosomiasis group and Lenti-NC group at each time point (Figure 2d). By detecting the ALT level, it was found that the ALT expression level in the serum of mice in the schistosomiasis group and Lenti-NC group began to increase significantly in the fourth week and reached the peak in the sixth week, while the ALT level in the serum of the Lenti-miR-200a group mice. The expression level after schistosomiasis infection was significantly lower than that of the schistosomiasis group and Lenti-NC group (P < 0.05) (Figure 2 E).
3.3. Overexpression of lentivirus miR-200a can alleviate the pathological condition of schistosomiasis liver fibrosis
HE staining revealed that the liver tissue of mice in the Lenti-miR-200a group showed significantly less egg deposition, hepatocyte necrosis, and inflammatory cell infiltration at all time points of infection compared with the schistosomiasis group and the Lenti-NC group (Figure 3). Masson staining indicated that the collagen fibers in the liver tissue of the Lenti-miR-200a group at each time point of infection were significantly reduced compared with the schistosomiasis group and the Lenti-NC group (Figure 4).
Figure 3.
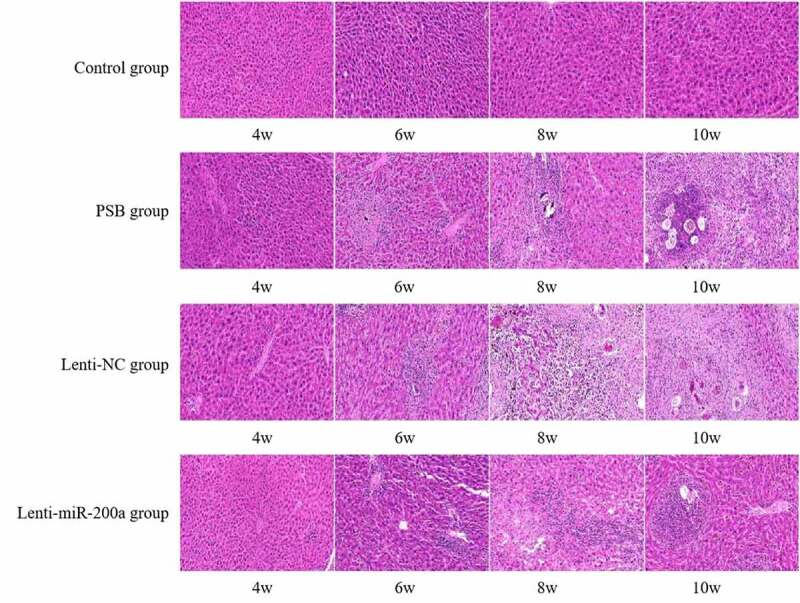
Liver tissue of the mice infected with S. japonicum by HE staining
Figure 4.
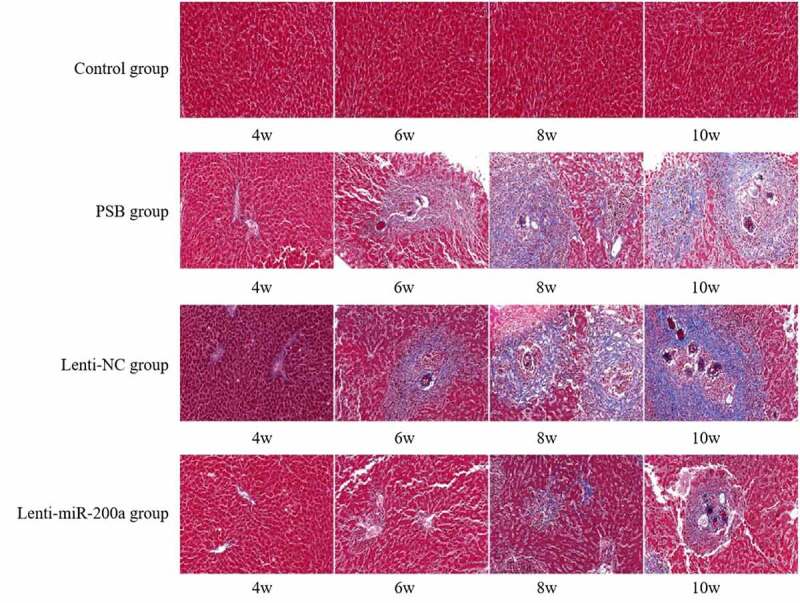
Liver tissue of the mice infected with S. japonicum by Masson staining
Normal control group (4th, 6th, 8th, 10th weeks) in Figure 3: The liver lobules of the liver tissues of mice had a complete structure. The hepatocytes were arranged radially around the central vein in a polygonal shape without deformation, necrosis, and inflammatory cell infiltration. A small number of inflammatory cells were infiltrated in the liver tissue of mice in the fourth week of the schistosomiasis group and Lenti-NC group. A small number of eggs were deposited in the liver tissue of the mice. A large number of inflammatory cells can be seen in the sixth week of the schistosomiasis group and Lenti-NC group. A large number of egg granulomas can be seen in the liver tissue of mice, and the liver cells had different degrees of deformation and necrosis in the eighth and 10th weeks of the schistosomiasis group and Lenti-NC group. In the Lenti-miR-200a group, the egg deposition, hepatocyte necrosis, and inflammatory cell infiltration in the liver tissues of the mice were significantly reduced compared with the schistosomiasis group and the Lenti-NC group at each time point after infection.
Normal control group (4th, 6th, 8th, 10th weeks) in Figure 4: There was no collagen fiber distribution in mouse hepatocytes and liver sinusoids. A small amount of blue collagen fibers can be seen in the liver tissue of mice in the 4th week of the schistosomiasis group and Lenti-NC group. Collagen in the liver tissue of mice increased, and a large amount of blue around the egg nodules can be seen. Collagen fibers, liver lobule structure disordered in the 6th week of the schistosomiasis group and Lenti-NC group. The blue collagen fibers around the egg granuloma were fused with each other in the liver tissue of mice, some of the blue collagen fibers were deformed and widened, and hepatocytes were visible in varying degrees of deformity and necrosis, and the lesions aggravated with the extension of the infection time in the 8th and 10th weeks of the schistosomiasis group and Lenti-NC group. The changes of collagen fibers in the liver tissue of mice in the Lenti-miR-200a group at each time point of infection were significantly less than those in the schistosomiasis group and the Lenti-NC group.
3.4. The effect of lentiviral miR-200a overexpression on cytokines
Fluorescence quantitative PCR was used to detect IL-4 and IFN-γ in the liver tissues of each group of mice at different time points. It was found that the IL-4 mRNA expression levels in the schistosomiasis group and Lenti-NC group increased from the fourth week after infection, peaked in the eighth week, and started to decrease in the 10th week. The IL-4 expression level in the miR-200a group was somewhat reduced compared with those in the schistosomiasis group and the Lenti-NC group (Figure 5 A). The level of IFN-γ mRNA in the schistosomiasis group and Lenti-NC group reached a peak in the fourth week after infection, and gradually decreased with the prolongation of the infection time. The expression level of IFN-γ in the miR-200a group was relatively lower than that in the schistosomiasis group and Lenti-NC group (Figure 5b).
Figure 5.
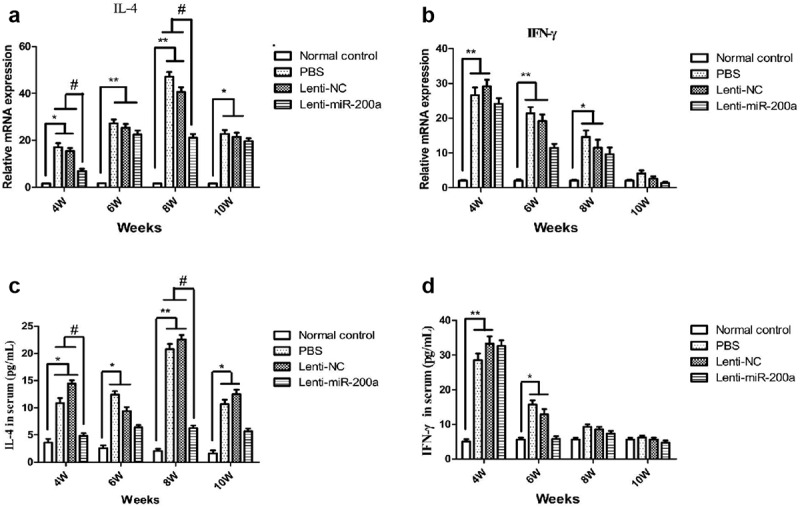
The effect of miR-200a on mRNA of IL-4 (a) and IFN-γ (b) by qRT-PCR, and serum level of IL-4 (c) and IFN-γ (d) by ELISA. **: P < 0.01 vs normal control *: P < 0.05 vs normal control #: P < 0.05 vs schistosomiasis and Lenti-NC
The results of ELISA showed that the IL-4 and IFN-γ levels in the serum of the mice at each time point were consistent with the mRNA expression levels in the liver tissues between the groups. The IL-4 expression level in the serum of mice in the miR-200a group was significantly reduced in the 8th week after infection compared with the schistosomiasis group and the Lenti-NC group (Figure 5 C). The expression level of serum IFN-γ of mice in the miR-200a group was relatively lower than that of the schistosomiasis group and Lenti-NC group in the sixth week after infection (Figure 5 D).
4. Discussion
At present, the mechanism of liver fibrosis and the treatment of liver fibrosis have become a hot spot in the research of schistosomiasis. If liver fibrosis can be effectively controlled, the disease can be delayed and improved [14]. In recent years, studies have shown that miR-200a can inhibit the activation and proliferation of HSCs through the transforming TGF-β signaling pathway and improve fibrotic lesions in animal models. This research provides a new target for the treatment of liver fibrosis [15]. In order to explore the role and mechanism of miR-200a in the formation of SLF, we constructed a miR-200a lentiviral vector to investigate the role and mechanism of miR-200a in SLF by up-regulating the expression of miR-200a in schistosomes infected mice.
In this study, the levels of inflammatory factors IL-4 and IFN-γ and the pro-fibrotic cytokines TGF-β and α-SMA were found to increase significantly with the progression of schistosomiasis. The results of this study were consistent with previous researches. It was indicated that when eggs of Schistosoma japonicum are deposited in the liver, causing persistent infection. That would lead to an imbalance between Th1 secretion of IFN-γ-mediated cellular immunity and Th2 secretion of IL-4-mediated humoral immunity [4], so the expression of IL-4 and IFN-γ in liver were increased. Besides, the continuous stimulation of SEA also induced the aggregation of inflammatory cells as well as fibroblasts, which was consistent with our HE and Masson staining results that a great number of inflammatory cell and collagen fibers can be found. Furthermore, studies have confirmed that the core mechanism of liver fibrosis is the activation of HSCs by TGF-β. The TGF-β signaling pathway can effectively prevent the activation of HSCs [16]. TGF-β2 is a multifunctional peptide, which plays a variety of biological roles in the process of cell growth and differentiation, and signals through receptors on the cell surface. The pathways regulate cell proliferation, secretion, and apoptosis [17]. It was reported that TGF-β2 expression is significantly upregulated in hepatic fibrosis animals, and in TGF-β1-activated HSCs, TGF-β2 expression increases with increasing TGF-β1 concentration. It was confirmed that TGF-β1 promotes TGF-β2 expression, and TGF-β2 promotes fibrosis in HSCs activated by the same TGF-β receptor [18].
The results showed that the expression level of miR-200a in liver tissues of the schistosomiasis model group gradually decreased with the prolongation of schistosomiasis infection time, indicating that miR-200a may be involved in the process of SLF. miR-200a plays an important role in liver fibrosis [9,11,12]. It has been reported that miR-200a is significantly downregulated in the lungs of rats with experimental lung fibrosis and patients with idiopathic pulmonary fibrosis [19]. And miR-200a has been also identified to be downregulated in TGF-β1-activated pancreatic stellate cells (PSCs) and forced miR-200a expression could attenuate TGF-β1-induced PSC activation and ECM formation by inhibiting the phosphatase and tensin homolog/AKT/mTOR [20]. Furthermore, similar to our result, studies reported that in the CCl4-induced liver fibrosis mouse model and also in vitro in TGF-β1-induced HSC activation, the expression level of miR-200a was down-regulated, and the expression was gradually down-regulated as the degree of liver fibrosis worsens [9,11,12].
In order to evaluate the role of miR-200a in the occurrence and development of SLF, we constructed a lentiviral vector miR-200a to study the effect and mechanism of miR-200a on liver fibrosis in a mouse model of schistosomiasis. In our results, the expression levels of α-SMA, Colla I and TGF-β2 decreased significantly compared with the schistosomiasis group and the Lenti-NC group, indicating that over-expression of miR-200a can inhibit liver fibrosis in mice with SLF. Through HE and Masson staining, we found that miR-200a could improve to a certain extent liver tissue worm egg granuloma and fibrotic lesions caused by schistosomiasis. In addition, the level of IL-4 in the Lenti-miR-200a group decreased significantly in the 4th and 8th weeks of infection, suggesting that miR-200a could inhibit the development of liver fibrosis to some extent. It has been reported that increasing expression of miR-200a attenuates HSC proliferation while knocking down miR-200a-promoted HSC proliferation [21]. And consistent with our result, it was reported that miR-200a upregulation contributed to the suppression of activated HSCs, leading to a reduction in cell proliferation, ECM production and α-SMA expression [22,23]. Yang et al. [9] demonstrated that miR-200a controlled hepatic stellate cell activation and fibrosis via SIRT1/Notch1 signal pathway. And Hu et al. [12] suggested that β-catenin was identified as a target gene of miR-200a and upregulation of miR-200a significantly attenuated the proliferation of HSC and reduced β-catenin expression. Furthermore, consistent with our findings, it has been demonstrated that the miR-200 family might regulate the occurrence and development of liver, kidney, and lung fibrosis by targeting TGF-β2 [24]. TGF-β2 is the target gene of miR-200a. A study has found that miR-200a can inhibit the activation of fibrosis by targeting TGF-β2, indicating that miR-200a can inhibit TGF-β through suppressing TGF-β2 and thus TGF-β [18]. Furthermore, Sun et al [11] reported that when activating HSCs through TGF-β1, the expression level of miR-200a showed a downward trend. When miR-200a mimics were transfected into HSCs, HSC activation and the production of fibrotic factors could be effectively inhibited.
5. Conclusion
In summary, our study is the first study to explore miR-200a on SLF combining serum and liver tissue of mice. This study found that the expression of miR-200a was down-regulated during the progression of schistosomiasis liver fibrosis. In addition, up-regulating the expression of miR-200a effectively inhibited the expression of α-SMA, Colla I and TGF-β2 mRNA, and significantly suppressed liver fibrosis and inflammatory response in mice with schistosomiasis. In a word, miR-200a plays an important regulatory role in schistosomiasis-induced liver fibrosis, and its mechanism may be related to the negative regulation of TGF-β2 expression by miR-200a. This research provides a new target for the treatment of schistosomiasis liver fibrosis.
Acknowledgements
None.
Funding Statement
The authors have no funding to report.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Authors contribution
Xu A. and Zhong G. designed the study. Wang J. did the literature research. Liu C. and Liu Y. conducted the experiments and acquired the data. Xu A. performed the statistical analyses. Xu A. drafted the manuscript. Wang W. reviewed the paper. All authors read and approved the final version.
Highlights
1. The expression of miR-200a was down-regulated during the progression of schistosomiasis liver fibrosis;
2. Up-regulating the expression of miR-200a effectively inhibited the expression of α-SMA, Colla I and TGF-β2;
3. miR-200a can improve to a certain extent liver tissue worm egg granuloma and fibrotic lesions caused by schistosomiasis.
Consent for publication
Not applicable.
Ethic approval and informed consent
The study was approved by the ethics committee of Hunan Aerospace Hospital.
References
- [1].Sotillo J, Doolan D, Loukas A.. Recent advances in proteomic applications for schistosomiasis research: potential clinical impact. Expert Rev Proteomics. 2017;14(2):171–183. [DOI] [PubMed] [Google Scholar]
- [2].Li JZ, Zhi MX, Si MD, et al. Endemic status of schistosomiasis in People’s Republic of China in 2017. Zhongguo Xue Xi Chong Bing Fang Zhi Za Zhi. 2018;30(5):481–488. [DOI] [PubMed] [Google Scholar]
- [3].Song L, Wu X, Sacko M, et al. History of schistosomiasis epidemiology, current status, and challenges in China: on the road to schistosomiasis elimination. Parasitol Res. 2016;115(11):4071–4081. [DOI] [PubMed] [Google Scholar]
- [4].Brandon-Warner E, Feilen NA, Culberson CR, et al. Processing of miR17-92 cluster inhepatic stellate cells promotes hepatic fibrogenesis during alcoholinduced injury. Alcohol Clin Exp Res. 2016;40(7):1430–1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Lee YS, Kim SY, Ko E, et al. Exosomes derived from palmitic acid treated hepatocytes induce fibrotic activation of hepatic stellatecells. Sci Rep. 2017;7(1):3710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Gressner AM, Werskirchen R.. Modern pathogenetic concepts of liver fibrosis suggest stellate cells and TGF-beta as major players and therapeutic targets. J Cell Mol Med. 2006;10(1):76–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Su J, Zhang A, Shi Z, et al. Micro RNA-200a suppresses the Wnt/β-catenin signaling pathway by interacting with β-catenin. Int J Oncol. 2012;40(40):1162–1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].He X, Tang R, Sun Y, et al. MicroR-146 blocks the activation of M1 macrophage by targeting signal transducer and activator of transcription 1 in hepatic schistosomiasis. Ebiomedicine. 2016;13:339–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Yang JJ, Tao H, Liu LP, et al. MiR-200a controls hepatic stellate cell activation and fibrosis via sirt1/notch1 signal pathway. Inflamm Res. 2017;66(4):341–352. [DOI] [PubMed] [Google Scholar]
- [10].Yang S, Banerjee S, de Freitas A, et al. Participation of miR-200 in pulmonary fibrosis. Am J Pathol. 2012;180(2):484–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Sun X, He Y, Ma TT, et al. Participation of miR-200a in TGF-β1-mediated hepatic stellate cell activation. Mol Cell Biochem. 2014. Mar;388(1–2):11–23. [DOI] [PubMed] [Google Scholar]
- [12].Hu BL, Shi C, Lei RE, et al. Interleukin-22 ameliorates liver fibrosis through miR-200a/beta-catenin. Sci Rep. 2016. Nov 7;6(1):36436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Arjmand A, Tsipouras MG, Tzallas AT, et al. Quantification of liver fibrosis—A comparative study. Appl Sci. 2020;10(2):447. [Google Scholar]
- [14].Ge WS, Wang YJ, Wu JX, et al. Beta-catenin is overexpressed in hepatic fibrosis and blockage of wnt/beta-catenin signaling inhibits hepatic stellate cell activation. Mol Med Rep. 2014;9(6):2145–2151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Ang S, Banerjee S, de Freitas A, et al. Participation of miR-200 in pulmonary fibrosis. Am J Pathol. 2012;180(2):484–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Herbert DR, Orekov T, Perkins C, et al. IL-10 and TGF-beta redundant lyprotect against severe liver in jury and mortality during acute schistosomiasis. J Immunol. 2008;181(10):7214–7220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Chen X, Shi C, Meng X, et al. Inhibition of Wnt/β-caten in signaling suppresses bleomycin-inducedpulmonary fibrosisby attenuating the expression of TGF-β1 and FGF-2. Exp MolPathol. 2016;101:22–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Wang B. miR-200a Prevents renal fibrogenesis through repression of TGF-β2 expression. Diabetes. 2011;60(1):280–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Yang S, Banerjee S, de Freitas A, et al. Participation of miR-200 in pulmonary fibrosis. Am J Pathol. 2012. Feb;180(2):484–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Xu M, Wang G, Zhou H, et al. TGF-β1-miR-200a-PTEN induces epithelial-mesenchymal transition and fibrosis of pancreatic stellate cells. Mol Cell Biochem. 2017. Jul;431(1–2):161–168. [DOI] [PubMed] [Google Scholar]
- [21].Li L, Ran J, Li L, et al. Gli3 is a novel downstream target of miR-200a with an anti-fibrotic role for progression of liver fibrosis in vivo and in vitro. Mol Med Rep. 2020. Apr;21(4):1861–1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Xiong M, Jiang L, Zhou Y, et al. The miR-200 family regulates TGF-β1-induced renal tubular epithelial to mesenchymal transition through Smad pathway by targeting ZEB1 and ZEB2 expression. Am J Physiol Renal Physiol. 2012. Feb 1;302(3):F369–79. [DOI] [PubMed] [Google Scholar]
- [23].Yu F, Zheng Y, Hong W, et al. Micro RNA-200a suppresses epithelial-to-mesenchymal transition in rat hepatic stellate cells via GLI family zinc finger 2. Mol Med Rep. 2015. Dec;12(6):8121–8128. [DOI] [PubMed] [Google Scholar]
- [24].Humphries B, Yang C. The microRNA-200 family:Small molecules with novel roles in cancer development, progression and therapy. Oncotarget. 2015;6(9):6472–6498. [DOI] [PMC free article] [PubMed] [Google Scholar]


