ABSTRACT
Previous studies have demonstrated that protein tyrosine phosphatase 1B (PTP1B) can promote tumor progression in breast cancer, colon cancer and prostate cancer. Additionally, PTP1B also acts as a tumor suppressor in esophageal cancer and lymphoma. These findings suggest that PTP1B functions as a double-faceted molecule in tumors. However, the role of PTP1B in malignant melanoma (MM) is still unknown. PTP1B expression in normal and melanoma tissues was evaluated by GEO analysis and immunohistochemistry. The effects of PTP1B on cell migration and invasion were evaluated in melanoma cells with up – and downregulated PTP1B expression. In this study, we initially demonstrated that the expression of PTP1B in malignant melanoma tissue is significantly higher than its expression in benign nevus tissue and indicated poor survival of malignant melanoma patients. In vitro studies have demonstrated that inhibition of PTP1B suppresses and overexpression of PTP1B promotes migration and invasion of melanoma cells. Moreover, we found that PTP1B could interact with Src via coimmunoprecipitation and dephosphorylation of the Src at Tyr530 site. Collectively, our study revealed that PTP1B can promote melanoma cell metastasis by interacting with Src and provides a theoretical basis for future applications of PTP1B inhibitors in the treatment of malignant melanoma.
KEYWORDS: Melanoma, ptp1b, src, metastasis
1. Introduction
Cutaneous malignant melanoma is one of the most lethal cancers in the world, and its incidence has increased in recent years [1,2]. Localized melanoma can be treated quite successfully with surgery but will metastasize rapidly if not caught early [3]. Although targeted therapy agents such as BRAF and MEK kinase inhibitors, as well as immune checkpoint inhibitors, have been widely used in the treatment of malignant melanoma, many patients with metastatic melanoma cannot achieve tumor remission [4,5]. Thus, it is urgent to explore potential therapeutic targets, elucidate the biological mechanism underlying tumorigenesis and cancer progression, and develop novel therapeutic strategies against malignant melanoma.
Protein tyrosine phosphatase 1B (encoded by PTPN1) is recognized as a potential therapeutic target for the treatment of diabetes, obesity and cancer [6,7]. Numerous studies have reported that PTP1B-deficient mice are hypersensitive to insulin and resistant to obesity induced by a high-fat diet [8]. In addition to its role in regulating insulin, PTP1B has a dual role in cancer development. PTP1B expression was increased in prostate [9], breast [10], ovarian [11], lung [12], colon [13], gastric [14] and pancreatic [15] cancers. In colon cancer, PTP1B has been shown to activate Src by dephosphorylating its negative regulatory residue [13]. PTP1B has also been shown to be a positive mediator of ErbB2-induced signals that trigger breast tumorigenesis and to be required for ErbB2 transformation in breast epithelial cells [16]. In contrast, PTP1B acts as a tumor suppressor in hematopoietic malignancies, such as classical Hodgkin’s lymphoma and B-cell–like diffuse large-cell lymphomas [17,18]. However, the association between PTP1B and MM has never been described.
Src is one of the members of the Src kinase family and plays an important role in cell proliferation, differentiation, movement and localization [19]. Its three main domains are the Src homology domains SH2 and SH3 and catalytic domain, which contains an active kinase. Both SH2 and SH3 are required for protein-protein interactions. A large number of studies have found that the human Src gene product c-Src is overexpressed and highly activated in a variety of human tumor cells, and Src activation promotes tumor progression [20]. Src is activated by protein–protein interactions or phosphorylation events. Tyr419 and Tyr530 are the two main phosphorylation sites in Src [21]. Tyr419 can be internally phosphorylated in the presence of a mitotic signal that removes the activation ring from the catalytic pocket. This allows phosphorylated Src to activate downstream targets that promote cell proliferation [22]. Phosphorylation of Tyr530 by other proteins negatively regulates Src and inactivates its kinase activity by the blocking catalytic pocket [23]. However, the biological functions of PTP1B and Src in MM are unknown.
In this study, we identified PTP1B as a tumor promoter in which PTP1B expression increases with tumor progression. We detected the expression of PTP1B in human melanoma tissues and evaluated its function in melanoma metastasis in vivo and in vitro. Moreover, human phospho-kinase array and co-IP analysis revealed a critical role for PTP1B in melanoma metastasis through dephosphorylating Src.
In the present study, we hypothesized that PTP1B is involved in the metastasis of MM by activating Src. To prove this hypothesis, we evaluated the expression of PTP1B in human melanoma tissues and detected its effects on melanoma cell migration and invasion in vivo and in vitro. Additionally, the relationship between PTP1B and Src in MM was investigated.
2. Methods
2.1. Cell culture and transfection
The human melanoma cell lines MV3 and A2058 used in this study were obtained from ATCC. MV3 cells were cultured in RPMI 1640, and A2058 cells were cultured in DMEM; both media were supplemented with 10% FBS, 100 IU/mL penicillin, and 100 mg/mL streptomycin. Cells were maintained at 37°C with 5% CO2. PTP1B-targeted shRNA (shPTP1B, 5′ – GGAAGAGACCCAGGAGGATAA-3′) was synthesized to knock down endogenous PTP1B expression in melanoma cells, and a nonhuman homologous shRNA sequence was used as a negative control (shCon, 5′-TTCTCCGAACGTGTCACGT-3′). For the generation of lentivirus, 293 T cells were transfected with optimized packaging plasmid (pAXA2, pVSV-G) along with shPTP1B or shCon vector by Lipofectamine (Invitrogen). After 72 h, virus-containing medium was collected and used for infection of target cells in the presence of polybrene. Then, the stably infected cells were selected by puromycin (5 μg/mL).
2.2. Patient samples
The tissue microarray was obtained from Xi’an Alenabio.com (China), and detailed information on this microarray has been previously described [24].
2.3. Immunohistochemistry
The paraffin-embedded sections were deparaffinized, rehydrated and then exposed to the primary antibody (PTP1B,1:100 dilution) at 4°C overnight. After they were washed three times with phosphate-buffered saline (PBS), the sections were incubated with the secondary antibody at room temperature for 30 min, followed by treatment with a 3-amino-9-ethylcarbozole (AEC) solution for 1 min. Then, the sections were stained with hematoxylin to identify morphological changes. The PTP1B staining intensity was independently evaluated and scored by two pathologists. The IHC score was calculated as previously described [25].
2.4. Cell proliferation assay
Cell proliferation was detected with a cell counting kit-8 assay (CCK-8, Dojindo). The cells were seeded in a 96-well plate in RPMI 1640 supplemented with 10% FBS. The absorbance was detected at 450 nm on a TECAN microplate reader (Mechelen, Belgium).
2.5. Migration and invasion assay
Cell migration was detected with the wound healing assay or Transwell assay as previously described [26]. Cell invasion was detected using FalconTM Cell Culture Inserts (BD353097, BD company, USA, New Jersey) according to the manufacturer’s instructions. First, 100 μl of Matrigel (BD Biosciences, USA) (5 mg/ml) diluted with 500 μl of DMEM was added to the upper chamber of the insert and incubated for 60 min at room temperature. Cells suspended in DMEM at a density of 1 × 106 cells/ml were then added to the upper chamber (100 μl/each), while 500 μl of medium was added to the lower chamber. After incubation at 37°C for 24 h, the cells on the membrane surface of the upper chamber were carefully removed, and cells on the bottom surface were fixed in 10% formaldehyde for 15 min, stained with 5% crystal violet for 30 min and observed under a microscope. The numbers of infiltrating cells were counted and photographed from five randomly selected fields. All experiments were performed in duplicate and repeated three times.
2.6. Co-immunoprecipitation
Whole-cell lysates were precleared and incubated with mouse anti-HA antibody (51,064, 1:100, Proteintech). The IP targets were disassociated from the immobilized antibodies on AminoLink Plus Resin. Then, the eluted proteins were resolved using SDS–PAGE through 10% gels and subjected to by Western blot using mouse anti-PTP1B monoclonal antibody (201,974, 1:1000, Abcam) or rabbit anti-Src antibody (1:2000, Proteintech).
2.7. Western blotting analysis
Cultured cells and tumor tissues from nude mice were lysed with ice-cold RIPA buffer containing freshly added PMSF. Total protein was quantified using a BCA Protein Assay Kit (Beyotime, Guangzhou, China). Cell lysates (40ug) were separated by SDS–PAGE and transferred onto a PVDF membrane (Millipore, Billerica, MA, USA). Membranes were blocked in 5% bovine serum albumin (BSA) in 1× Tris-buffered saline containing 0.05% Tween 20 (TBST) for 1 h at room temperature and then incubated with primary antibodies overnight at 4°C, followed by incubation with HRP-conjugated secondary antibodies for 1.5 h at room temperature. The primary antibodies used were as follows: anti-PTPN1 antibody (201,974, 1:1000, Abcam), anti-HA antibody (51,064, 1:2000, Proteintech), anti-Src antibody (19,096, 1:2000, Proteintech), and anti-β-actin antibody (60,008–1, Proteintech). The secondary antibodies used were as follows: anti-rabbit (7404, CST) and anti-mouse (7076, CST). The bands were visualized using enhanced chemiluminescence (ECL) reagent (WBULS0500, Millipore) with the Tanon 5200 system (Tanon, Shanghai, China). The Human Phospho-Kinase Array (ARY003C, R&D Systems) was used according to the array procedure, and the intensities were analyzed by Image J.
2.8. In vivo tumor xenograft study
Five-to seven-week-old female BALB/c nude mice were randomized and assigned to the control or experimental groups. A lung metastasis model was established by tail vein injections of 1 × 105 cells and was analyzed at 6 weeks after injection. Before the mice were sacrificed, we used PET-CT to evaluate lung metastasis in each group. 18-FDG was injected into the tail vein, and mice were scanned after 30 minutes. The SUV value was analyzed in each group, and then the mice were sacrificed. The lung metastases were detected by HE staining.
2.9. Statistical analyses
Statistical analyses were performed using GraphPad Prism software with unpaired t-test with Welch’s correction for comparison of two groups or one-way ANOVA with Tukey’s test for comparing more than two groups. In figures, unless otherwise noted, data are presented as the means ± SEM, where P ≤ 0.05 (*), P ≤ 0.01 (**), P ≤ 0.001 (***), and P ≤ 0.0001 (****) were considered statistically significant, and ‘NS’ indicates not statistically significant.
All experiments were performed at least three repetitions to ensure the accuracy of data.
Results
PTP1B can promote tumor progression in breast cancer, colon cancer and prostate cancer. However, the clinical and biological functions of PTP1B in MM are unknown. In this study, we hypothesized that PTP1B is involved in the progression of MM. We detected the expression of PTP1B in human melanoma tissues and evaluated its function in melanoma metastasis in vivo and in vitro. Moreover, human phospho-kinase array and co-IP analysis revealed a critical role for PTP1B in melanoma metastasis through dephosphorylating Src.
PTP1B expression is highly elevated in malignant melanoma and correlates with poor survival rates
PTP1B expression in melanoma and noncancerous tissues was analyzed in samples from GEO databases. As shown in Figure 1a, PTP1B mRNA was overexpressed in melanoma compared with normal tissues. Moreover, PTP1B expression was upregulated in metastatic melanoma compared with primary melanoma (Figure 1b&c). Survival analysis showed that PTP1B overexpression was related to poor survival rates in patients (Figure 1d). All these data indicate that PTP1B may promote melanoma progression. Therefore, we used AEC (Aminoethyl carbazole) IHC to detect the protein expression of PTP1B in melanoma and nontumor sample tissues. As shown in Figure 1e, PTP1B expression was detected in the cytoplasm (red color) and was upregulated in melanoma samples compared with nevi (Figure 1f). Collectively, these results indicate that PTP1B is a tumor promoter for MM.
Figure 1.
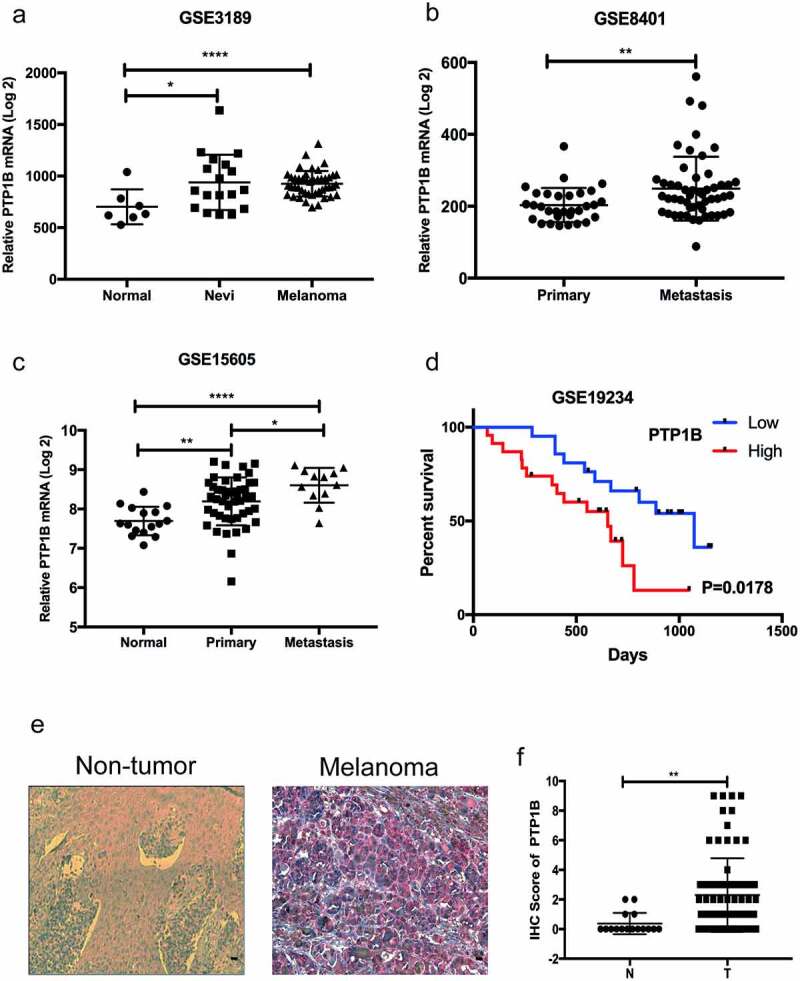
PTP1B expression is highly elevated in malignant melanoma and correlates with poor survival
A. PTP1B mRNA expression analysis in MM, nevi and normal tissues (GSE3189; Normal, n = 7; Nevi, n = 18; Melanoma, n = 45).B. PTP1B mRNA expression analysis in primary and metastatic tissues (GSE8401; Primary, n = 31; Metastasis, n = 52).C. PTP1B mRNA expression analysis in normal, primary and metastatic tissues (GSE15605; Normal, n = 16; Primary, n = 46; Metastasis, n = 12)D. Overall survival of MM patients based on PTP1B mRNA expression (GSE19234; High, n = 23; Low, n = 21).E. PTP1B protein expression in nontumor and melanoma tissues by immunohistochemical AEC staining. Original magnification 400 × .F. PTP1B expression was analyzed in nontumor and melanoma tissues (Normal, n = 18; Melanoma, n = 82).
PTP1B deficiency inhibits the horizontal migration of melanoma cells
We used lentivirus to establish PTP1B-upregulated and PTP1B-downregulated A2058 and MV3 cells and detected proliferation in the experimental and control groups by performing a CCK-8 assay. As shown in Figure 2a&b, knockdown or overexpression of PTP1B had no effect on the growth of melanoma cells. Therefore, we used a wound healing assay to further analyze the biological role of PTP1B in horizontal cell migration. As shown in Figure 2c, downregulation of PTP1B significantly inhibited horizontal migration. In contrast, upregulation of PTP1B promoted horizontal migration of melanoma cells.
Figure 2.
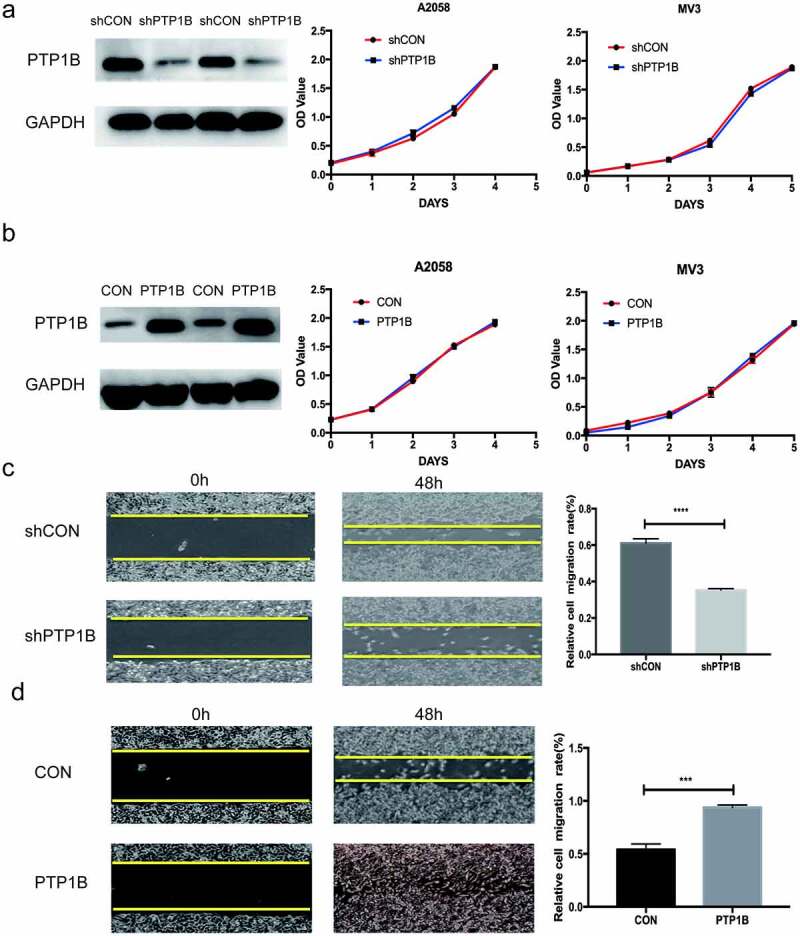
PTP1B deficiency inhibits the horizontal migration of melanoma cells without affecting cell proliferation
A The proliferation ability of A2058 and MV3 cells with knockdown of PTP1B was assessed by the CCK-8 assay (OD value 450 nm).B The proliferation ability of A2058 and MV3 cells with overexpression of PTP1B was assessed by the CCK-8 assay (OD value 450 nm).C The horizontal migration ability of A2058 cells with knockdown of PTP1B was assessed by the wound healing assay at 48 h.D The horizontal migration ability of A2058cells with overexpression of PTP1B was assessed by the wound healing assay at 48 h.
PTP1B-overexpressing melanoma cells promote vertical migration and invasion
Similar to the wound healing assay, the vertical migration assay (Figure 3a&b) showed that downregulation of PTP1B significantly inhibited vertical cell migration and that upregulation of PTP1B promoted melanoma vertical cell migration. Then, melanoma cell invasion was evaluated (Figure 3c&d). Knockdown of PTP1B significantly inhibited cell invasion. In contrast, overexpression of PTP1B promoted melanoma cell invasion. Therefore, inhibition of PTP1B suppressed MM cell migration and invasion.
Figure 3.
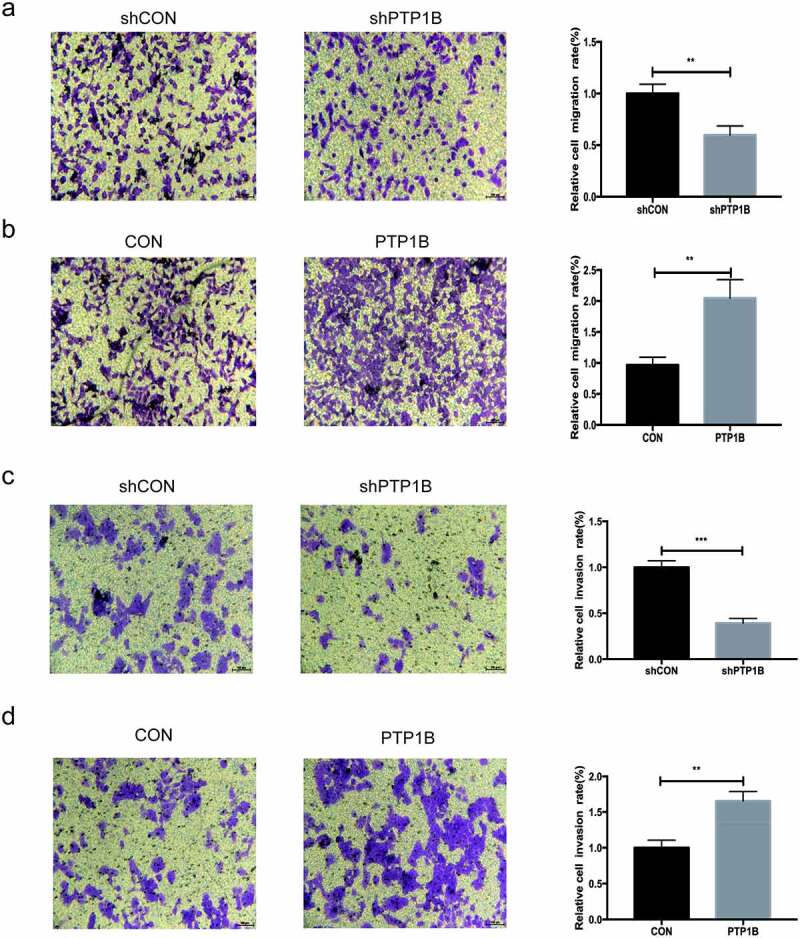
PTP1B-overexpressing melanoma cells show increased vertical migration and invasion
A&B The vertical migration ability of MV3 cells with knockdown or overexpression of PTP1B was assessed by the Transwell migration assay.C&D The invasion ability of A2058 cells with knockdown or overexpression of PTP1B was assessed by the Transwell invasion assay.
PTP1B promotes tumor metastasis in vivo
Lung is the commonest site for the occurrence of melanoma distant metastasis, and the prognosis of patients with lung metastasis is poor, median survival with lung metastasis was only 7 months. As PTP1B influence the metastasis of melanoma in vitro, we further confirm this function in vivo. A lung metastasis model was used to evaluate the effects of PTP1B in vivo. PET-CT images (Figure 4a) showed that the lung area (red arrow) was obviously more metabolically active in the OE group than in the control group. Moreover, the SUV value in the lung area was four times higher in the PTP1B OE group than in the control group (Figure 4b), which means that the OE group had more lung tumors than did the control group. HE staining was used to detect the number of metastases in the lung area. As shown in Figure 4c&d, the number and area of metastases in the OE group were ten times greater than those in the control group. These results again show that PTP1B could promote the metastasis of MM.
Figure 4.
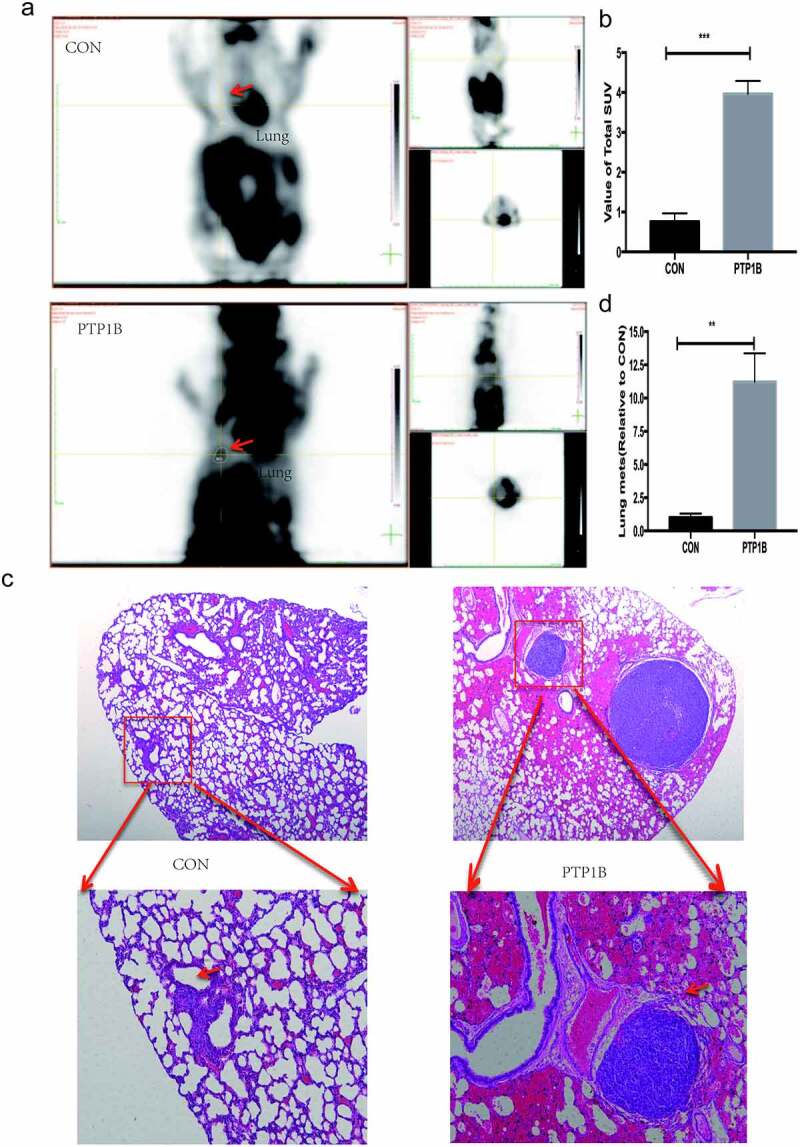
PTP1B promotes tumor metastasis in vivo
A. PET-CT images showing the lung metastasis area (red arrow).B. Analysis of the SUV value in the lung metastases.C. Images of lung metastases subjected to HE staining. Original magnification, 40× and 200 × .D. Analysis of the relative lung metastasis based on the number and area of metastases.
PTP1B regulates melanoma cell migration by interacting with Src
As PTP1B is a tyrosine phosphatase, the Human Phospho-Kinase Array was used to analyze the potential protein(s) affected by PTP1B. Figure 5a shows that the phosphorylation level of many proteins was inhibited in PTP1B-knockdown cells. As shown in Figure 5b, the phosphorylation of PLC, SRC, STAT5, WNK1 and P53 was decreased. PTP1B is reported to interact with Src and STAT5, so the phosphorylation of Src and STAT5 was detected by Western blots, and Src Tyr530 levels were measured in cells with up – and downregulated PTP1B expression. The phosphorylation level of Src at Tyr530 was increased in PTP1B-downregulated cells and decreased in PTP1B-overexpressing cells (Figure 5c). Co-IP assays showed that PTP1B could interact with Src (Figure 5d), which was previously reported in colon cancer. Together, these results show that PTP1B promotes MM metastasis and predicts poor survival rates.
Figure 5.
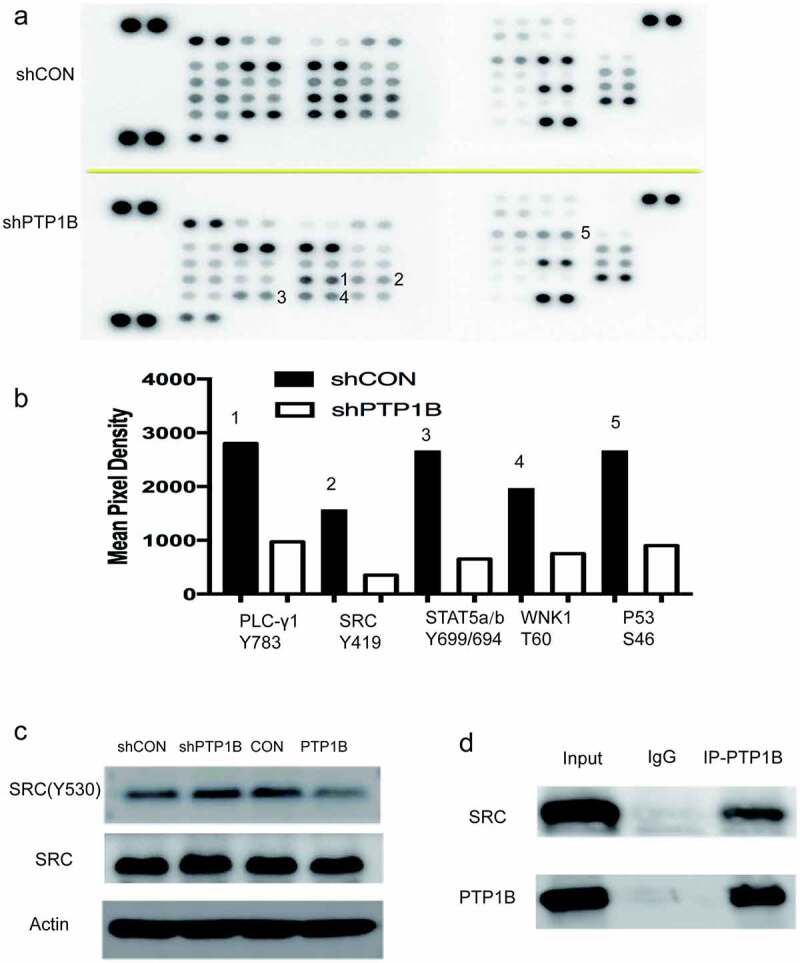
PTP1B regulates melanoma cell migration by interacting with Src
A. Phosphorylation levels of proteins in A2058 cells with knockdown of PTP1B were detected in the Human Phospho-Kinase Array.B. Mean pixel density shows changes in the phosphorylation status of proteins.C. The phosphorylation level of Src Tyr530 was detected in cells with knockdown or overexpression of PTP1B.D. Proteins pulled down with PTP1B-HA antibody were evaluated by western blotting for the presence of PTP1B and Src.
Discussion
In this study, we report that PTP1B is overexpressed in MM tissues and that downregulation of PTP1B suppresses the migration and invasion of melanoma cells. Moreover, we discovered an association between PTP1B and Src in the progression of MM. As a tyrosine phosphatase, PTP1B was reported to be involved in the progression of many cancers by dephosphorylating key proteins. In esophageal squamous cell carcinoma, PTP1B promotes cell invasion and migration by dephosphorylating MYH9 at Y1408, which results in increased EGFR expression [27]. In colorectal carcinoma, PTP1B directly dephosphorylates PITX1 at Y160, Y175 and Y179, which destabilizes PITX1 and consequently results in downregulation of the PITX1/p120RasGAP axis [28]. However, the biological function of PTP1B in MM is unknown.
The carboxyl terminal residue Tyr530 in human c-Src is an important site for the regulation of c-Src tyrosine kinase activity [29,30]. When Tyr530 is phosphorylated, it binds to the SH2 domain of Src. The combination of the two makes the molecule curl, and then the catalytic pocket is covered, which will inhibit the tyrosine kinase activity of c-Src. Protein tyrosine phosphatase family members were found to be physiological regulators of Src [31–33]. Experiments show that PKC can act on PTPα and that the catalytic activity of PKC increases and enhances PTPα activity, which results in the automatic phosphorylation of Tyr419 to further activate Src [34]. Another protein tyrosine phosphatase, PTPλ, can dephosphorylate c-Src. This dephosphorylation can occur at the Tyr419 site but mainly occurs at the Tyr530 site [35]. Moreover, PTP1C can dephosphorylate the Tyr419 and Tyr530 sites but has a stronger effect on the Tyr530 site [36]. Using the Human Phospho-Kinase Array, we found that the phosphorylation level of Tyr419 was inhibited when PTP1B was downregulated. Because Src activity is mainly reduced after phosphorylation of the Tyr530 site, we detected the phosphorylation level at Tyr530. As shown in Figure 5c, the phosphorylation level at Tyr530 was inhibited in the PTP1B-overexpressing group, which indicated that PTP1B might dephosphorylate Src at Tyr530. Moreover, a co-IP assay showed that PTP1B could interact with Src. All these findings indicate that PTP1B might promote the metastasis of MM by interacting with Src and dephosphorylating the Tyr530 site. However, further studies are required to further elucidate the detailed mechanism between PTP1B and Src.
In summary, PTP1B is overexpressed in MM tissues and promotes metastasis by binding Src and dephosphorylating the Tyr530 site. Therefore, elucidating the function of PTP1B could pave the way for improved therapy for MM.
Acknowledgements
Support for the present study was funded by National Natural Science Foundation of China (No. 81802724)
Funding Statement
This work was supported by the National Natural Science Foundation of China [81802724]; National Natural Science Foundation of China [81802724].
Conclusion
The present study provides new insights into PTP1B function in human melanoma tissues. Our data showed that downregulation of PTP1B expression suppresses and overexpression of PTP1B promotes cell migration and invasion. Moreover, overexpression of PTP1B causes significant progression of established lung metastasis in nude mice by targeting Src, suggesting that PTP1B is as a novel therapeutic target for the management of MM.
Abbreviation
MM (Malignant Melanoma), PTP1B (Protein tyrosine phosphatase 1B), PTPN1 (Protein Tyrosine Phosphatase Non-Receptor Type 1), PET-CT (positron emission tomography / computedtomography), SUV (standard uptake value), AEC (Aminoethyl carbazole), IHC Immunohistochemistry, GEO (Gene Expression Omnibus).
Disclosure statement
No potential conflict of interest was reported by the author(s).
Author contributions
Qiang Wang and Yuyan Pan performed all the experiments and prepared the manuscript. Liping Zhao and Fazhi Qi helped analyze the data. Jiaqi Liu conceptualized, planned this study.
References
- [1].Schadendorf D, van Akkooi ACJ, Berking C, et al. Melanoma. Lancet. 2018;392(10151):971–984. [DOI] [PubMed] [Google Scholar]
- [2].Siegel RL, Miller KD, Jemal A.. Cancer statistics, 2020. CA Cancer J Clin. 2020;70(1):7–30. [DOI] [PubMed] [Google Scholar]
- [3].Kudchadkar RR, Lowe MC, Khan MK, et al. Metastatic melanoma. CA Cancer J Clin. 2020;70(2):78–85. [DOI] [PubMed] [Google Scholar]
- [4].Sun J, Carr MJ, Khushalani NI.. Principles of targeted therapy for melanoma. Surg Clin North Am. 2020;100(1):175–188. [DOI] [PubMed] [Google Scholar]
- [5].Albittar AA, Alhalabi O, Glitza Oliva IC. Immunotherapy for melanoma. Adv Exp Med Biol. 2020;1244:51–68. [DOI] [PubMed] [Google Scholar]
- [6].Cho H. Protein tyrosine phosphatase 1B (PTP1B) and obesity. Vitam Horm. 2013;91:405–424. [DOI] [PubMed] [Google Scholar]
- [7].Hussain H, Green IR, Abbas G, et al. Protein tyrosine phosphatase 1B (PTP1B) inhibitors as potential anti-diabetes agents: patent review (2015-2018). Expert Opin Ther Pat. 2019;29(9):689–702. [DOI] [PubMed] [Google Scholar]
- [8].Dodd GT, Xirouchaki CE, Eramo M, et al. Intranasal targeting of hypothalamic PTP1B and TCPTP reinstates leptin and insulin sensitivity and promotes weight loss in obesity. Cell Rep. 2019;28(11):2905–2922.e2905. [DOI] [PubMed] [Google Scholar]
- [9].Labbé DP, Uetani N, Vinette V, et al. PTP1B deficiency enables the ability of a high-fat diet to drive the invasive character of PTEN-deficient prostate cancers. Cancer Res. 2016;76(11):3130–3135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Yu M, Liu Z, Liu Y, et al. PTP 1B markedly promotes breast cancer progression and is regulated by miR-193a-3p. FEBS J. 2019;286(6):1136–1153. [DOI] [PubMed] [Google Scholar]
- [11].Wang W, Cao Y, Zhou X, et al. PTP1B promotes the malignancy of ovarian cancer cells in a JNK-dependent mechanism. Biochem Biophys Res Commun. 2018;503(2):903–909. [DOI] [PubMed] [Google Scholar]
- [12].Liu H, Wu Y, Zhu S, et al. PTP1B promotes cell proliferation and metastasis through activating src and ERK1/2 in non-small cell lung cancer. Cancer Lett. 2015;359(2):218–225. [DOI] [PubMed] [Google Scholar]
- [13].Zhu S, Bjorge JD, Fujita DJ. PTP1B contributes to the oncogenic properties of colon cancer cells through Src activation. Cancer Res. 2007;67(21):10129–10137. [DOI] [PubMed] [Google Scholar]
- [14].Wang N, She J, Liu W, et al. Frequent amplification of PTP1B is associated with poor survival of gastric cancer patients. Cell Cycle. 2015;14(5):732–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Xu Q, Wu N, Li X, et al. Inhibition of PTP1B blocks pancreatic cancer progression by targeting the PKM2/AMPK/mTOC1 pathway. Cell Death Dis. 2019;10(12):874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Arias-Romero LE, Saha S, Villamar-Cruz O, et al. Activation of Src by protein tyrosine phosphatase 1B is required for ErbB2 transformation of human breast epithelial cells. Cancer Res. 2009;69(11):4582–4588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Zahn M, Marienfeld R, Melzner I, et al. A novel PTPN1 splice variant upregulates JAK/STAT activity in classical Hodgkin lymphoma cells. Blood. 2017;129(11):1480–1490. [DOI] [PubMed] [Google Scholar]
- [18].Lu X, Malumbres R, Shields B, et al. PTP1B is a negative regulator of interleukin 4-induced STAT6 signaling. Blood. 2008;112(10):4098–4108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Roskoski R Jr. Src protein-tyrosine kinase structure, mechanism, and small molecule inhibitors. Pharmacol Res. 2015;94:9–25. [DOI] [PubMed] [Google Scholar]
- [20].Patel A, Sabbineni H, Clarke A, et al. Novel roles of Src in cancer cell epithelial-to-mesenchymal transition, vascular permeability, microinvasion and metastasis. Life Sci. 2016;157:52–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Amata I, Maffei M, Pons M. Phosphorylation of unique domains of Src family kinases. Front Genet. 2014;5:181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Zhang H, Forman HJ. TGFβ1 rapidly activates Src through a non-canonical redox mechanism. Free Radic Biol Med. 2014;75(Suppl 1):S4. [DOI] [PubMed] [Google Scholar]
- [23].Wang J, Yu L, Zheng X. PTPα-mediated Src activation by EGF in human breast cancer cells. Acta Biochim Biophys Sin (Shanghai). 2013;45(4):320–329. [DOI] [PubMed] [Google Scholar]
- [24].Wang Q, Qian L, Tao M, et al. Knockdown of DEAD-box RNA helicase 52 (DDX52) suppresses the proliferation of melanoma cells in vitro and of nude mouse xenografts by targeting c-Myc. Bioengineered. 2021;12(1):3539–3549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Wang Q, Peng R, Wang B, et al. Transcription factor KLF13 inhibits AKT activation and suppresses the growth of prostate carcinoma cells. Cancer Biomarkers. 2018;22(3):533–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Ying J, Wang Q, Lu L, et al. Fermitin family homolog 2 (Kindlin-2) affects vascularization during the wound healing process by regulating the Wnt/β-catenin signaling pathway in vascular endothelial cells. Bioengineered. 2021;12(1):4654–4665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Pan BQ, Xie ZH, Hao JJ, et al. PTP1B up-regulates EGFR expression by dephosphorylating MYH9 at Y1408 to promote cell migration and invasion in esophageal squamous cell carcinoma. Biochem Biophys Res Commun. 2020;522(1):53–60. [DOI] [PubMed] [Google Scholar]
- [28].Teng HW, Hung MH, Chen LJ, et al. Corrigendum: Protein tyrosine phosphatase 1B targets PITX1/p120RasGAP thus showing therapeutic potential in colorectal carcinoma. Sci Rep. 2017;7(1):45612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Egan C, Pang A, Durda D, et al. Activation of Src in human breast tumor cell lines: elevated levels of phosphotyrosine phosphatase activity that preferentially recognizes the Src carboxy terminal negative regulatory tyrosine 530. Oncogene. 1999;18(5):1227–1237. [DOI] [PubMed] [Google Scholar]
- [30].Zhu S, Bjorge JD, Fujita DJ. SRC is dephosphorylated at tyrosine 530 in human colon carcinomas. Chin J Cancer Res. 2011;23(3):229–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Pallen CJ. Protein tyrosine phosphatase α (PTPα): a Src family kinase activator and mediator of multiple biological effects. Curr Top Med Chem. 2003;3(7):821–835. [DOI] [PubMed] [Google Scholar]
- [32].Cardone L, Carlucci A, Affaitati A, et al. Mitochondrial AKAP121 binds and targets protein tyrosine phosphatase D1, a novel positive regulator of src signaling. Mol Cell Biol. 2004;24(11):4613–4626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Zhang Z, Lin SY, Neel BG, et al. Phosphorylated alpha-actinin and protein-tyrosine phosphatase 1B coregulate the disassembly of the focal adhesion kinase x Src complex and promote cell migration. J Biol Chem. 2006;281(3):1746–1754. [DOI] [PubMed] [Google Scholar]
- [34].Brandt DT, Goerke A, Heuer M, et al. Protein kinase C delta induces Src kinase activity via activation of the protein tyrosine phosphatase PTP alpha. J Biol Chem. 2003;278(36):34073–34078. [DOI] [PubMed] [Google Scholar]
- [35].Fang KS, Sabe H, Saito H, et al. Comparative study of three protein-tyrosine phosphatases. Chicken protein-tyrosine phosphatase lambda dephosphorylates c-Src tyrosine 527. J Biol Chem. 1994;269(31):20194–20200. [PubMed] [Google Scholar]
- [36].Somani AK, Bignon JS, Mills GB, et al. Src kinase activity is regulated by the SHP-1 protein-tyrosine phosphatase. J Biol Chem. 1997;272(34):21113–21119. [DOI] [PubMed] [Google Scholar]


