ABSTRACT
The study aimed to evaluate the prognostic value of CD169 expression in tumor-infiltrating macrophages from regional lymph nodes (RLN) in various tumors. In order to identify eligible articles, PubMed, EMBASE, Web of Science, and Cochrane Library were used to conduct a systematic search. Pooled hazard ratios (HRs) or odds ratios (ORs) with corresponding 95% confidence intervals (CIs) were adopted to assess the relationship between CD169 expression and overall survival (OS) and clinicopathological characteristics. Ten studies, including eleven cohorts with 1699 patients, were enrolled. We found that high CD169+ expression in tumor-infiltrating macrophages from RLN was associated with a favorable OS (HR = 0.56, 95%CI: 0.39–0.79, P = 0.001). Subgroup analysis showed that high CD169+ expression had more predictive power in digestive system tumors (HR = 0.52, 95%CI: 0.42–0.67, <0.001). In addition, high CD169 expression was significantly linked with lymph node metastasis (OR = 0.66, 95%CI: 0.47–0.94, P = 0.020) and TNM stage (OR = 0.62, 95%CI: 0.48–0.80, P < 0.001). High CD169 expression in tumor-infiltrating macrophages from RLN was correlated with favorable survival outcome in patients with malignancies. CD169 may be a novel and effective prognostic marker, especially for digestive system tumors.
KEYWORDS: CD169, macrophages, prognosis, meta-analysis
GRAPHICAL ABSTRACT
Introduction
The incidence and mortality of cancer is rapidly growing because of the aging and growth of the population as well as socio-economic development [1,2]. According to the statistics, 18.1 million newly diagnosed cancer cases and 9.6 million cancer-related deaths occurred in 2018 [1]. Despite advanced drug treatments and surgical methods, the therapeutic response differs significantly in patients, and the overall prognosis of most cancers is still not very satisfactory [3]. The complex molecular mechanisms are the features of cancer cells. Therefore, it is imperative for us to explore applicable predictive biomarkers so that we enable to accurately evaluate accurately the therapeutic effect and prognosis of patients with malignancies.
Macrophages, an indispensable component of the innate immune system, are spread across the body [4]. Plenty of tumor-associated macrophages are detected in tumors, and them contribute to the major inflammatory infiltrate in various malignancies [5,6]. Macrophages have both pro- and anti-tumorigenic functions in different local tumor environmental conditions. The regional lymph nodes(RLN) is one of the first major components of the immune system that comes into contact with tumor cells or tumor cell products, and plays an important role in generating tumor-directed immune responses [7]. The macrophages located in RLN also play an important role in tumor immunology through endocytosing the fragmented dead tumor cells of the sinus area of the lymph nodes [7–10]. These findings suggest that regional lymph nodes or tumor-infiltrating macrophages represent a pivotal component in various tumor immunology.
CD169 (also known as Siglec-1) is a surface marker of macrophages and belongs to the sialic-acid-binding immunoglobulin-like lectin family, which can mediate cell-cell interactions via glycan recognition [11,12]. Nowadays, researchers recommended that CD169 expression in tumor-infiltrating macrophage from RLN served as a promising prognostic marker of multiple cancers [13–19]. However, their conclusions were contradictory. The prognostic value of CD169 expression in tumor-infiltrating macrophages from RLN was still unclear. Therefore, we conducted this meta-analysis to clarify the prognostic power of CD169 in cancers.
Materials and methods
Search strategy
A systematic search for eligible articles was conducted through PubMed, Web of Science, EMBASE, and Cochrane Library database. The search deadline was up to December 2020. The following keywords were used: ‘CD169’ OR ‘sialoadhesin’ OR ‘Siglec-1’ AND ‘cancer’ OR ‘carcinoma’ OR ‘neoplasm’ OR ‘tumor’ AND ‘prognosis’ OR ‘prognostic’ OR ‘survival’ OR ‘outcome’ AND ‘Macrophage’. Additionally, the reference lists of the included studies were explored for potentially relevant articles.
Study selection
The searching tasks were carried out independently by three authors (Kong WH, Wei and Liu RQ). Selection criteria employed in this meta-analysis to incorporate eligible studies were as follows: 1) Original English publication, 2) investigated the association between CD169 and single or multiple malignancies, 3) focusing on the CD169 and clinical outcomes.
Data extraction and quality assessment
Data extraction was performed by three reviewers (Kong WH, Wei M and Liu RQ) independently. The fourth reviewer (Zhang JL) would be involved in the discussion when any discrepancies were met. Extracted data and information included as follows: 1) The first author and the year of publication, 2) Article nationality, 3) Type of cancer, 4) The case number of included studies, 5) Median age of patients, 6) Test method of CD169 expression, 7) Definition of high or low CD169+ macrophages, 8) Location of the specimen, 9) Survival results, 10) Follow-up months. The quality of each included study was evaluated using the Newcastle-Ottawa scale (NOSs) [20,21]. NOS score from 0–4 was defined as low quality, 5–6 as medium quality, and 7–9 as high quality.
Statistical analysis
The pooled hazard ratios (HRs)or odds ratios (ORs) with the 95% confidence intervals (CIs) were calculated to illustrate the relationship between CD169 expression and survival outcome and clinicopathological characteristics. Cochran’s Q test and I2 statistics were performed to evaluate heterogeneity [22,23]. Heterogeneity was perceived as significant when I2 > 50% or p < 0.1, which determined the adoption of random effect models. Otherwise, fixed effect models were chosen. Moreover, the stability of the merged result was assessed by sensitivity analysis. Begg’s and Egger’s tests were utilized to detect the publication bias [24]. STATA 12.0 (StataCorp, College Station, TX, USA) was employed to conduct statistical analyses. P less than 0.05 was considered as statistical difference.
Results
Brief introduction
CD169 macrophages have been reported to play a pivotal role in anti-tumor immunity, but its prognostic value remains controversy. Therefore, a meta-analysis evaluating the prognostic value value of CD169 expression in tumor-infiltrating macrophages from regional lymph nodes was conducted. We retrieved articles about the relationship between CD169 expression and prognostic value in multiple tumors from the database. Through meta-analysis, we found that the high CD169 expression was significantly related to favorable prognosis, lymph node metastasis, and TNM stage.
Literature search and study characteristics
Literature selection flow was shown in Figure 1 in accordance with the PRISMA statement [25]. A total of 50 studies were initially identified through the search strategy described above. However, 15 studies were removed for duplication, and 25 studies were excluded based on the titles, abstracts, and full texts. Eventually, ten studies with 1699 patients were incorporated in the present meta-analysis. Table 1. summarizes the baselines characteristics of selected ten studies. The malignant neoplasm contained bladder cancer, esophageal cancer, hepatocellular carcinoma, gastric cancer, colorectal carcinoma, endometrial carcinoma, melanoma, breast cancer, prostate cancer, and bladder urothelial carcinoma. Surgically resected tissues were collected to detect the CD169 expression in all studies. As for survival results, overall survival (OS) was reported in nine studies; one research displayed the OS and relapse-free survival (RFS). All the included studies were retrospective. Follow-up time among studies varied from 82 months to 250 months in ten studies. The quality assessment of the included studies was presented in Table 2.
Figure 1.
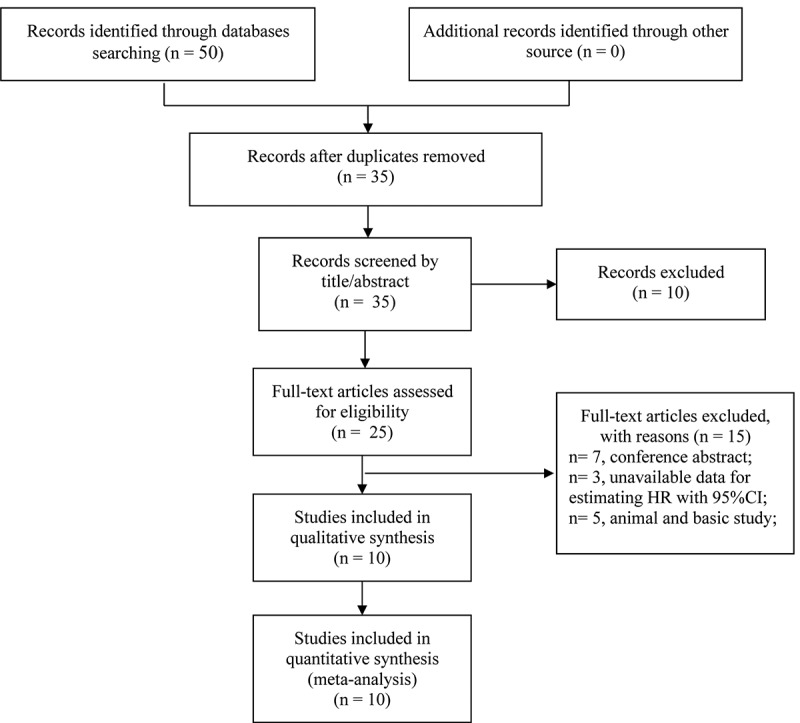
Flow diagram of the study selection process
Table 1.
Baseline characteristics of studies included in the meta-analysis
| Study | Origin | Cancer Type | Cases (low/high) | Gender (male/female) | Tumor stage | Median age (range) | Test method | Definition of high or low CD169+ macrophages | Location | Survival results | Maximum months of follow-up |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Asano 2018 | Japan | Bladder cancer | 44 (26/18) | 35/9 | TNM (I–IV) | 70 (49–85) | IHC | Mean number of cells and mean intensity | Regional lymph nodes | OSMA | 133 |
| Hiroto 2018 | Japan | Esophageal cancer | 182 (101/81) | 160/22 | TNM (I–IV) | NA | IHC | Mean densities | Regional lymph nodes | OSMA | 140 |
| Li 1# 2017 | China | Hepatocellular carcinoma | 188 (94/94) | 156/29 | TNM (I–III) | 50 (13–76) | IHC | Mean densities | Intra-tumor | OSMA | 120 |
| Li 2# 2017 | China | Gastric cancer | 132 (66/66) | 95/37 | TNM (I–IV) | 69 (28–78) | IHC | Mean densities | Intra-tumor | OSMA | 120 |
| Ohnishi 2013 | Japan | Colorectal carcinoma | 83 (45/38) | 48/35 | TNM (I–IV) | 64 (29–90) | IHC | Mean number of cells | Regional lymph nodes | OSUA | 100 |
| Ohnishi 2016 | Japan | Endometrial carcinoma | 79 (39/40) | NA | FIGO (I–IV) | 59 (30–74) | IHC | Mean number of cells | Regional lymph nodes | OSUA | 120 |
| Saito 2015 | Japan | Melanoma | 84 | 36/48 | Stage (0–4) | 69 (34–91) | IHC | Mean number of cells | Regional lymph nodes | OSMA | 100 |
| Shiota 2016 | Japan | Breast Cancer | 146 | 73/73 | Stage (1–3) | 56 (NA) | IHC | Mean number of cells | Regional lymph nodes | OSMA, RFSMA | 159 |
| Strömvall 2017 | Sweden | Prostate cancer | 109 (27/82) | NA | Gleason (6–9) | NA | IHC | Mean densities | Regional lymph nodes | OSMA | 250 |
| Wang 2015 | China | Bladder carcinoma | 302 (151/151) | 262/40 | TNM (0-IV) | 60 (15–90) | IHC | Mean number of cells and mean intensity | Intra-tumor | OSMA | 82 |
| Zhang 2016 | China | Hepatocellular carcinoma | 328 (164/164) | 292/36 | TNM (I–III) | 48 (20–78) | IHC | Mean densities | Intra-tumor | OSMA | 96 |
This means that these two different studies are from the same article.
Abbreviations: NA, not available; IHC, immunohistochemistery; OS, overall survival; RFS, recurrence-free survival; MA, multivariate analysis; UA, univariate analysis.
Table 2.
Newcastle -Ottawa Quality Assessment Scale
| Author | Year | Selection | Comparability | Outcome | Total |
|---|---|---|---|---|---|
| Asano et al. | 2018 | ☆☆ | ☆☆ | ☆☆☆ | 7 |
| Hiroto et al | 2018 | ☆☆ | ☆☆ | ☆☆ | 6 |
| Li et al. 1 | 2017 | ☆☆ | ☆☆ | ☆☆ | 6 |
| Li et al. 2 | 2017 | ☆☆ | ☆☆ | ☆☆ | 6 |
| Ohnishi et al. | 2013 | ☆ | ☆☆ | ☆☆☆ | 6 |
| Ohnishi et al. | 2016 | ☆☆ | ☆☆ | ☆☆☆ | 7 |
| Saito et al. | 2015 | ☆☆ | ☆☆ | ☆☆☆ | 7 |
| Shiota et al. | 2016 | ☆☆ | ☆☆ | ☆☆ | 6 |
| Stromvall et al. | 2017 | ☆☆ | ☆☆ | ☆☆☆ | 7 |
| Wang et al. | 2015 | ☆ | ☆☆ | ☆☆☆ | 6 |
| Zhang et al. | 2016 | ☆☆ | ☆☆ | ☆☆☆ | 7 |
Association between CD169 expression and OS
Ten studies reported the association between CD169 expression and OS. we found a significant relationship between high CD169 expression and longer OS in cancers (HR: 0.56, 95%CI: 0.39–0.79, P = 0.001) (Figure 2). Additionally, the heterogeneity among the studies (I2 = 56.2%, P = 0.011) was significant. To explore the source of heterogeneity, subgroup analysis for OS was performed (Table 3). The results were displayed in Table 3. We found that the sources of heterogeneity included country, tumor type, sample size, definition of high or low, macrophages location, analysis type and NOS score. In addition, we also observed that high CD169+ expression had more predictive power in digestive system tumors (HR = 0.52, 95%CI: 0.42–0.67, <0.001).
Figure 2.
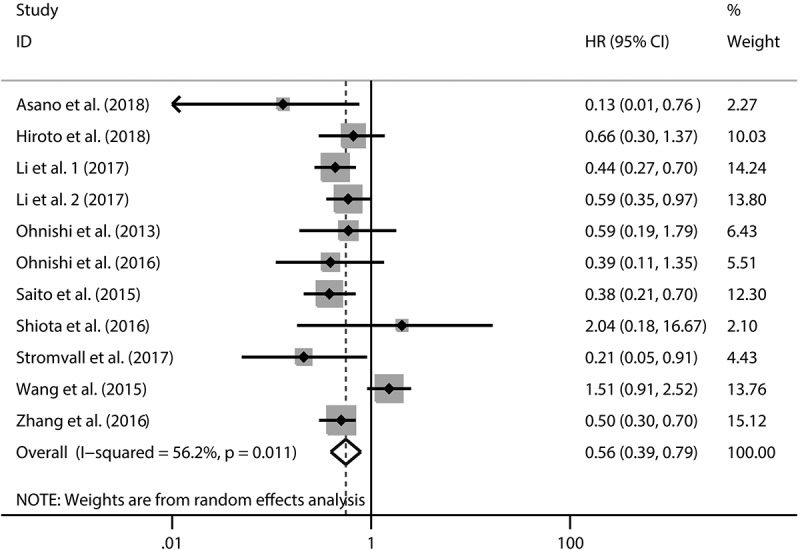
Forest plot of the overall survival analysis
Table 3.
Subgroup analysis for overall survival
| Stratified analysis | No. of cohorts | Pooled HR (95% CI) | P-value | Heterogeneity |
||
|---|---|---|---|---|---|---|
| I2 (%) | P-value | Model | ||||
| Country | ||||||
| Janpan | 6 | 0.48(0.32, 0.71) | <0.001 | 0 | 0.482 | Random |
| China | 4 | 0.66(0.39, 1.12) | 0.124 | 79.9 | 0.002 | Random |
| Sweden | 1 | 0.21(0.05, 0.90) | 0.035 | - | - | Random |
| Tumor type | ||||||
| Digestive system | 5 | 0.52(0.42, 0.67) | <0.001 | 0 | 0.877 | Random |
| Urinary system | 3 | 0.42(0.08, 2.34) | 0.324 | 80.5 | 0.006 | Random |
| Others | 3 | 0.42(0.25, 0.71) | 0.001 | 0 | 0.370 | Random |
| Sample size | ||||||
| ≤ 100 | 4 | 0.39(0.24, 0.63) | <0.001 | 0 | 0.678 | Random |
| >100 | 7 | 0.63(0.41, 0.98) | 0.039 | 67.0 | 0.006 | Random |
| Definition of high or low | ||||||
| Mean number of cells | 4 | 0.45(0.28, 0.72) | 0.001 | 0 | 0.515 | Random |
| Mean densities | 5 | 0.50(0.39, 0.65) | <0.001 | 0 | 0.629 | Random |
| Both | 2 | 0.56(0.05, 5.95) | 0.632 | 78.6 | 0.031 | Random |
| Macrophages location | ||||||
| Regional lymph nodes | 7 | 0.45(0.31, 0.66) | <0.001 | 0 | 0.471 | Random |
| Intra-tumor | 4 | 0.66(0.39, 1.12) | 0.124 | 79.9 | 0.002 | Random |
| Analysis type | ||||||
| Univariate | 2 | 0.49(0.21, 1.13) | 0.095 | 0 | 0.630 | Random |
| Multivariate | 9 | 0.56(0.38, 0.84) | 0.004 | 64.3 | 0.004 | Random |
| NOS score | ||||||
| ≤6 | 6 | 0.73(0.45, 1.18) | 0.198 | 64.4 | 0.015 | Random |
| >6 | 5 | 0.42(0.31, 0.58) | <0.001 | 0 | 0.597 | Random |
Association between CD169 expression and clinicalpathological parameters
We summarized the data to assess the relationship between CD169 expression and clinicopathological characteristics (Table 4). The combined results indicated that high CD169 expression was obviously related with TNM stage (III–IV vs I–II) (OR = 0.62, 95% CI: 0.48, 0.80, P < 0.001) and Lymph node metastasis (yes vs no)(OR = 0.66, 95% CI: 0.47–0.94; P = 0.020). However, the correlation was not observed in vascular invasion and histological grade. We believed that high CD169 expression may play an important role in preventing tumor invasion and lymph node metastasis
Table 4.
Association between CD169+ macrophages and clinicopathological features
| Clinicopathological parameter | No. of cohorts | Model | OR (95% CI) | P-value | Heterogeneity test |
|
|---|---|---|---|---|---|---|
| I2(%) | P-value | |||||
| Lymph node metastasis (yes vs no) | 6 | Fixed | 0.66(0.47,0.94) | 0.020 | 0.8 | 0.411 |
| TNM stage (III–IV vs I–II) | 7 | Fixed | 0.62(0.48,0.80) | <0.001 | 0 | 0.839 |
| Vascular invasion (yes vs no) | 4 | Fixed | 0.79(0.52,1.20) | 0.271 | 0 | 0.999 |
| Histological grade (III vs I–II) | 4 | Fixed | 1.26(0.90,1.76) | 0.177 | 41.0 | 0.165 |
Sensitivity analysis
Sensitivity analysis was employed to assess the influence of each individual study on the pooled HR. The results did not vary substantially with the exclusion of each research, which demonstrated stability and reliability of our results (Figure 3).
Figure 3.
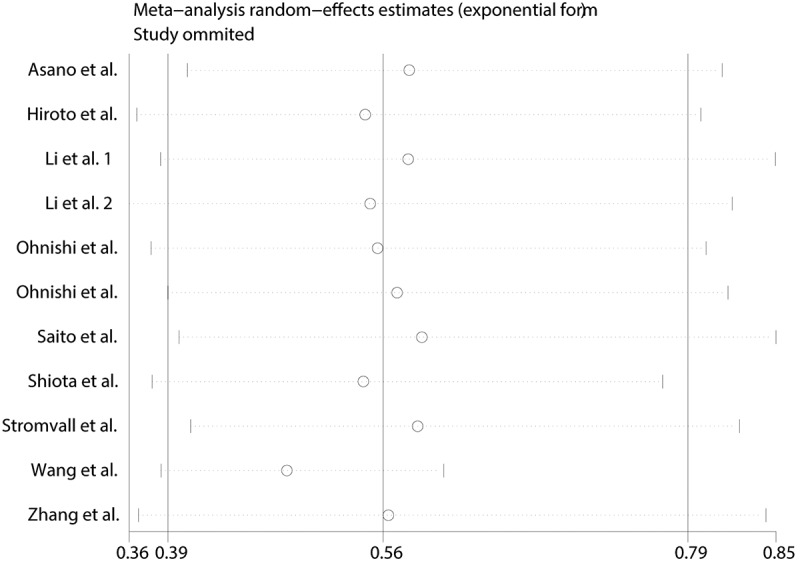
Sensitivity analysis of overall survival in this meta-analysis
Publication bias
The publication bias was detected by Begg’s tests and Egger’s tests. P-values for the Begg’s and Egger’s tests for OS were 0.876 and 0.587, respectively. P values were greater than 0.05, indicating no publication bias (Figure 4).
Figure 4.
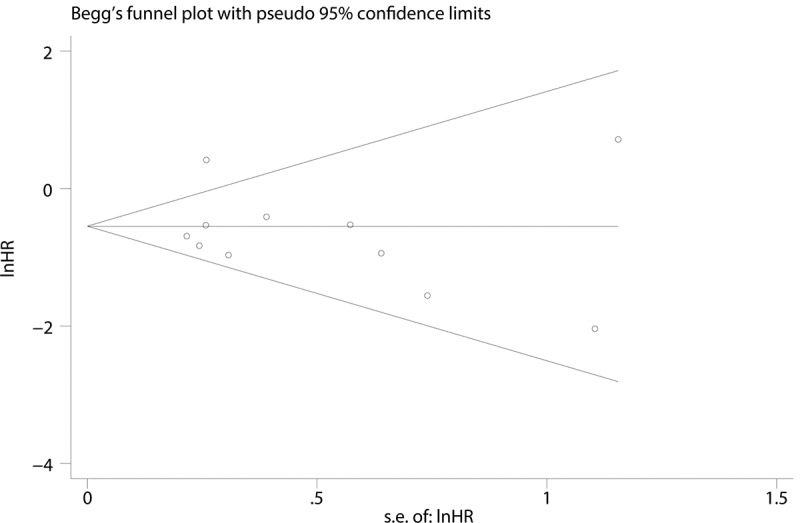
Begg’s funnel plot with pseudo 95% CI of the publication bias for overall survival
Discussion
Macrophages, which are consisted of diverse subpopulations based on specific markers, have diverse functions, including both pro-tumor and anti-tumor functions [26,27]. Macrophages in various cancers differ in distribution and composition patterns. Sinus macrophages play a pivotal role in anti-tumor immunity by endocytosing dead tumor cells, presenting antigens, and activating tumor antigen-specific lymphocytes [10,28]. CD169, which is a sialic acid receptor expressed on macrophages, is involved in the process of cell-cell interactions and cell-pathogen interactions. Increasing evidences have suggested that CD169+ macrophages play a crucial role in the anti-tumor immune response [28,29].
In the present study, a significant relationship was revealed between the high CD169 expression and OS (HR: 0.56, 95%CI: 0.39–0.79, P = 0.001). Subgroup analysis showed that the abundance of CD169 macrophages had a significant relationship with OS in Japan (HR: 0.48, 95%CI: 0.32–0.71, P < 0.001) and Sweden (HR: 0.21, 95%CI: 0.05–0.90, P = 0.035). We speculated that this may be caused by differences in genes, geography and climate. Moreover, high CD169+ expression had more predictive power in digestive system tumors(HR = 0.52, 95%CI: 0.42–0.67, <0.001). Additionally, CD169 macrophages from regional lymph nodes correlated with OS (HR: 0.45, 95% CI: 0.31–0.66, P < 0.001) rather than that intra-tumor macrophages (HR: 0.66, 95% CI: 0.39–1.12, P = 0.124). It suggested that macrophage’s location should be taken into consideration when using CD169 macrophages as prognostic value. In addition. It revealed that lymph node metastasis (OR: 0.66, 95% CI: 0.47–0.94, P = 0.020) and TNM stage (OR: 0.62, 95% CI: 0.48–0.80, P < 0.001) were associated prominently with high CD 169 macrophages.
Mechanisms underlying the prognostic value of CD169 expression in macrophages may be as follows. Touko Asano et al. revealed that the amount of CD169+ macrophages significantly correlated with the abundance of CD8+ cells and the favorable survival in bladder cancer, suggesting that CD169+ macrophages may play an anti-tumor role by boosting cytotoxic T-cell-mediated anti-tumor immunity [18]. Similarly, positive results between the density of CD169+ macrophages and CD8+ T cell infiltration were discovered in other cancers, including hepatocellular carcinoma, gastric cancer, and colorectal carcinoma [13,30]. Ohnishi and his colleagues identified that sinus macrophages and CD8+ T cells interaction were mediated by CD169–CD43 ligation in a regional lymph node, which may be one of the mechanisms for the proliferation of CD8+ T cells. Additionally, Hiroto Takeya et al. revealed a positive association between higher CD169 expression and density of tumor-infiltrating lymphocytes in esophageal cancer who underwent neoadjuvant chemotherapy, indicating that high CD169 expression plays a crucial role in inducing anti-cancer immune responses [19]. Moreover, activating NK cell-mediated anti-tumor immunity may be one of the mechanisms. Based on Garcia and Coombes’s experiment, Koji Ohnishi and his colleagues demonstrated that CD169+ macrophages activate infiltrating NK cells in the tumor by direct contact with CD57+ NK cells in RLN [15]. Besides, regulatory T cells (Treg) were suppressed by interaction with CD 169 and result in inflammation [31]. Taken together with our meta-analysis, we believed CD169+ macrophages may play a crucial role in anti-tumor immunity.
Several limitations should be taken into consideration. Firstly, all included studies were small retrospective studies. Secondly, the definition of the high CD169 expression varied among different researches. Thirdly, due to the limited included articles, we performed a subgroup analysis of the same systemic tumors. However, due to differ in their ontogeny, management and prognosis, the subgroup analysis may lead to erroneous results. Finally, significant heterogeneity is found in this meta-analysis, and the results should be treated with caution
Conclusion
High CD169 expression in macrophages from RLN predicted favorable survival outcome in patients with cancers, especially for digestive system tumors. CD169 could be an ideal prognositic marker in tumors. CD169 can be used to determine the prognosis of tumor patients and help clinicians to implement personalized treatment in advance. However, due to unavoidable restrictions, more large-scale, multi-center studies are needed to confirm our findings.
Funding Statement
This work was supported by the 2020 Anhui Medical University School Fund [2020xkj180]; the Youth Training Program of the First Affiliated Hospital of Anhui Medical University [2021kj24]; Jieping Medical Foundation [320.6750.2020-17-5].
Data Availability
The data used to support the findings of this research are available from the corresponding author upon request.
Disclosure statement
The authors declare that they have no conflicts of interest.
Author contributions
Data curation& Software: Weihao Kong, Meng Weiand Rongqiang Liu,
Supervision: Xingyu Wang
Writing–original draft: Weihao Kong, Meng Wei and Rongqiang Liu
Writing–review & editing: Jianlin Zhang and Xingyu Wang
All authors read and approved the final manuscript.
References
- [1].Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. [DOI] [PubMed] [Google Scholar]
- [2].Omran AR. The epidemiologic transition. A theory of the epidemiology of population change. Milbank Mem Fund Q. 1971;49(4):509–538. [PubMed] [Google Scholar]
- [3].Siegel RL, Miller KD, Jemal A.. Cancer statistics, 2019. CA Cancer J Clin. 2019;69(1):7–34. [DOI] [PubMed] [Google Scholar]
- [4].Wynn TA, Chawla A, Pollard JW. Macrophage biology in development, homeostasis and disease. Nature. 2013;496(7446):445–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Ruffell B, Coussens LM. Macrophages and therapeutic resistance in cancer. Cancer Cell. 2015;27(4):462–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Noy R, Pollard JW. Tumor-associated macrophages: from mechanisms to therapy. Immunity. 2014;41(1):49–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Lores B, Garcia-Estevez JM, Arias C. Lymph nodes and human tumors (review). Int J Mol Med. 1998;1(4):729–733. [DOI] [PubMed] [Google Scholar]
- [8].Swartz MA. Immunomodulatory roles of lymphatic vessels in cancer progression. Cancer Immunol Res. 2014;2(8):701–707. [DOI] [PubMed] [Google Scholar]
- [9].Hickok DF, Miller L, Harris L. Regional hyperplastic lymph nodes in breast cancer: the role of lymphocytes and nodal macrophages. An immunological study with a five-year follow-up. Surgery. 1977;82(5):710–715. [PubMed] [Google Scholar]
- [10].Gray EE, Cyster JG. Lymph node macrophages. J Innate Immun. 2012;4(5–6):424–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Crocker PR, Paulson JC, Varki A. Siglecs and their roles in the immune system. Nat Rev Immunol. 2007;7(4):255–266. [DOI] [PubMed] [Google Scholar]
- [12].Macauley MS, Crocker PR, Paulson JC. Siglec-mediated regulation of immune cell function in disease. Nat Rev Immunol. 2014;14(10):653–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Ohnishi K, Komohara Y, Saito Y, et al. CD169-positive macrophages in regional lymph nodes are associated with a favorable prognosis in patients with colorectal carcinoma. Cancer Sci. 2013;104(9):1237–1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Saito Y, Ohnishi K, Miyashita A, et al. Prognostic significance of CD169+ lymph node sinus macrophages in patients with malignant melanoma. Cancer Immunol Res. 2015;3(12):1356–1363. [DOI] [PubMed] [Google Scholar]
- [15].Ohnishi K, Yamaguchi M, Erdenebaatar C, et al. Prognostic significance of CD169-positive lymph node sinus macrophages in patients with endometrial carcinoma. Cancer Sci. 2016;107(6):846–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Shiota T, Miyasato Y, Ohnishi K, et al. The clinical significance of CD169-positive lymph node macrophage in patients with breast cancer. PloS One. 2016;11(11):e0166680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Stromvall K, Sundkvist K, Ljungberg B, et al. Reduced number of CD169(+) macrophages in pre-metastatic regional lymph nodes is associated with subsequent metastatic disease in an animal model and with poor outcome in prostate cancer patients. Prostate. 2017;77(15):1468–1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Asano T, Ohnishi K, Shiota T, et al. CD169-positive sinus macrophages in the lymph nodes determine bladder cancer prognosis. Cancer Sci. 2018;109(5):1723–1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Takeya H, Shiota T, Yagi T, et al. High CD169 expression in lymph node macrophages predicts a favorable clinical course in patients with esophageal cancer. Pathol Int. 2018;68(12):685–693. [DOI] [PubMed] [Google Scholar]
- [20].Zhang C, Ren X, Zhang W, et al. Prognostic and clinical significance of long non-coding RNA SNHG12 expression in various cancers. Bioengineered. 2020;11(1):1112–1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Liu R, Kong W, Zheng S, et al. Prognostic significance of microRNA miR-24 in cancers: a meta-analysis. Bioengineered. 2021;12(1):450–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Peters JL, Sutton AJ, Jones DR, et al. Comparison of two methods to detect publication bias in meta-analysis. Jama. 2006;295(6):676–680. [DOI] [PubMed] [Google Scholar]
- [23].Jackson D, White IR, Riley RD. Quantifying the impact of between-study heterogeneity in multivariate meta-analyses. Stat Med. 2012;31(29):3805–3820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ (Clin Res Ed). 1997;315(7109):629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol. 2009;62(10):1006–1012. [DOI] [PubMed] [Google Scholar]
- [26].Ostuni R, Kratochvill F, Murray PJ, et al. Macrophages and cancer: from mechanisms to therapeutic implications. Trends Immunol. 2015;36(4):229–239. [DOI] [PubMed] [Google Scholar]
- [27].Mills CD, Lenz LL, Harris RA. A breakthrough: macrophage-directed cancer immunotherapy. Cancer Res. 2016;76(3):513–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Asano K, Nabeyama A, Miyake Y, et al. CD169-positive macrophages dominate antitumor immunity by crosspresenting dead cell-associated antigens. Immunity. 2011;34(1):85–95. [DOI] [PubMed] [Google Scholar]
- [29].Zhang Y, Li JQ, Jiang ZZ, et al. CD169 identifies an anti-tumour macrophage subpopulation in human hepatocellular carcinoma. J Pathol. 2016;239(2):231–241. [DOI] [PubMed] [Google Scholar]
- [30].Li JQ, Yu XJ, Wang YC, et al. Distinct patterns and prognostic values of tumor-infiltrating macrophages in hepatocellular carcinoma and gastric cancer. Cancer Sci. 2017;15(1):37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Wu C, Rauch U, Korpos E, et al. Sialoadhesin-positive macrophages bind regulatory T cells, negatively controlling their expansion and autoimmune disease progression. J Iimmunol. 2009;182(10):6508–6516. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used to support the findings of this research are available from the corresponding author upon request.


