Abstract
Children with obesity are at risk for numerous health problems, including nonalcoholic fatty liver disease (NAFLD). This review focuses on progress made in the epidemiology of NAFLD in children for the years 2015–2020. The estimated prevalence of NAFLD in children with obesity is 26%. The incidence of NAFLD in children has risen rapidly over the past decade. An understanding of the reasons for this rise is incomplete, but over the past 5 years, many studies have provided additional insight into the complexity of risk factors, diagnostic approaches, and associated comorbidities. Risk factors for NAFLD are wide ranging, including perinatal factors involving both the mother and newborn, as well as environmental toxin exposure. Progress made in noninvasive assessment will be critical to improving issues related to variability in approach to screening and diagnosis of NAFLD in children. The list of serious comorbidities observed in children with NAFLD continues to grow. Notably, for many of these conditions, such as diabetes and depression, the rates observed have exceeded the rates reported in children with obesity without NAFLD. Recent advancements reviewed show an increased awareness of this problem, while also calling attention to the need for additional research to guide successful efforts at prevention and treatment.
Keywords: Nonalcoholic Steatohepatitis, Steatosis, Maternal-Fetal Medicine, Diabetes Mellitus, Dyslipidemias, Depression
1. INTRODUCTION
Obesity has become one of the most important public health issues in the United States and many other countries.1 Children with obesity are at risk for numerous health problems, including nonalcoholic fatty liver disease (NAFLD). NAFLD is characterized by accumulation of macrovesicular fat droplets in hepatocytes in patients whom other causes of liver disease (including chronic alcohol use) are excluded.2 In 2006, the Study of Child and Adolescent Epidemiology (SCALE) first showed that NAFLD was a common problem in children with an estimated prevalence of 9.6%.3 Subsequently, NAFLD became one of the fastest growing areas for clinical and translational research in pediatric gastroenterology.4 This includes studies on the incidence of NAFLD in children, the risk factors for NAFLD, as well as associated co-morbidities in children with NAFLD. This review will focus on the progress made in the epidemiology of NAFLD in children from 2015 through 2020. A summary of key findings is shown in Figure 1.
Figure 1. Key Findings in Epidemiology of NAFLD in Children 2015–2020.
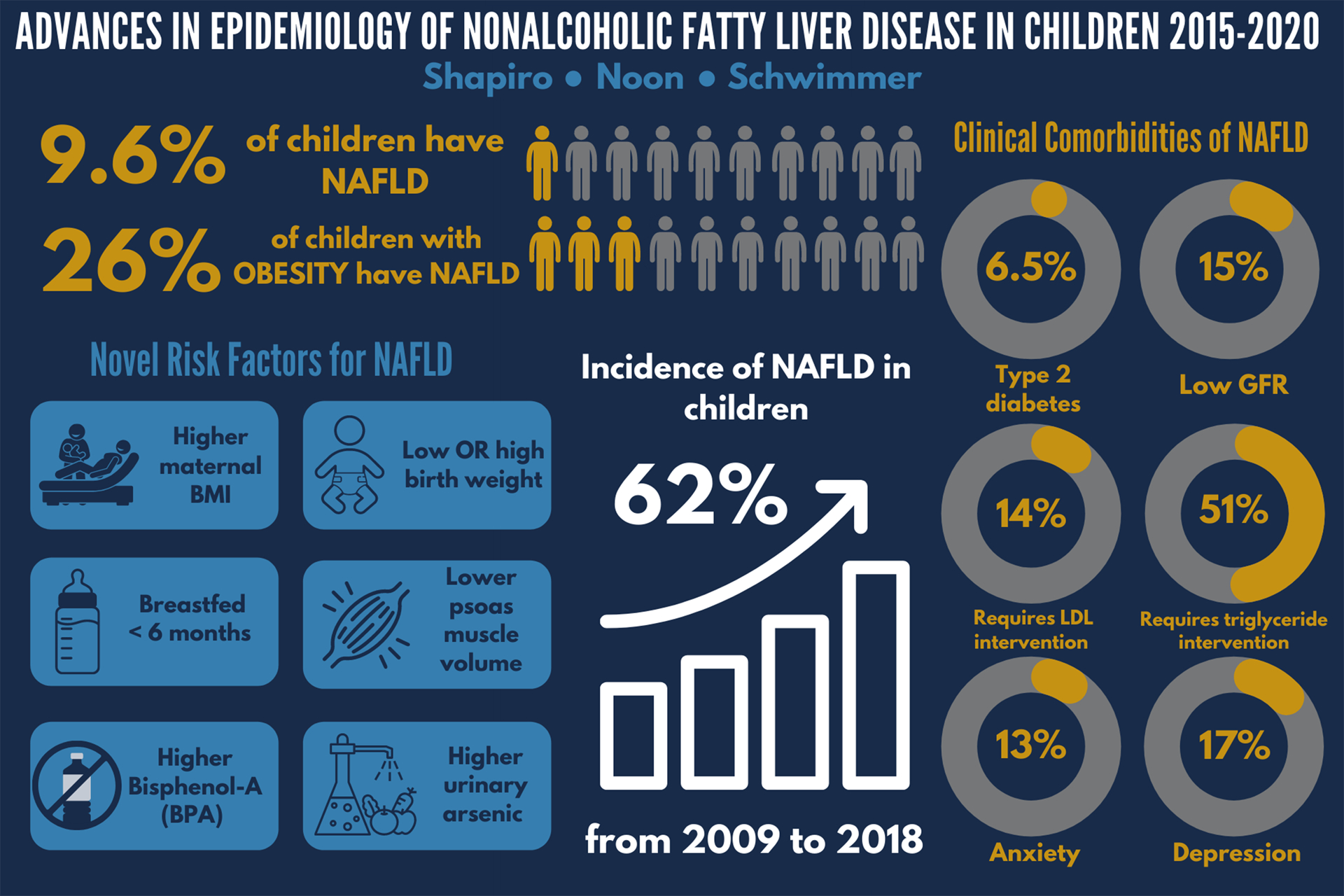
This figure captures the key results from many of the papers discussed in this review. The details and references for each are included within the body of the manuscript.
2. DIAGNOSIS OF NAFLD
In the early definition for nonalcoholic steatohepatitis (NASH) in children, Schwimmer et al identified important differences in liver histology between children and adults.5 A key observation was that there were two different forms of steatohepatitis; one characterized by ballooning degeneration and perisinusoidal fibrosis, and one characterized by portal inflammation and portal fibrosis. As a follow-up to those early studies, Africa et al evaluated children with NAFLD in which the steatosis was exclusively portal (zone 1) and those in which the steatosis was exclusively perisinusoidal (zone 3).6 Children with zone 1 steatosis were significantly younger than children with zone 3 steatosis. In addition, children with zone 1 steatosis were more likely to have advanced fibrosis, and children with zone 3 steatosis were more likely to have steatohepatitis. These data suggest that for a comprehensive understanding of pediatric NAFLD, one should account for the zonality of steatosis in studies of the pathophysiology, natural history, and response to treatment.
2.1. Progress in Non-invasive Assessment of NAFLD
The diagnosis of NAFLD is an ongoing challenge because a complete clinical diagnosis requires both liver histology and the exclusion of alternate causes of liver steatosis and inflammation. Therefore, there have been extensive efforts to develop noninvasive imaging tools for diagnosis and determination of disease severity. Increasingly, epidemiology studies are reliant on these imaging tools.
Liver magnetic resonance imaging (MRI) has evolved from measurement of hepatic fat fraction or signal fat fraction to the ability to measure proton density fat fraction (PDFF). Liver MRI PDFF removes the major confounding effects present in hepatic fat fraction in order to provide a more accurate and precise measure of liver fat content. The MRI Rosetta Stone Project showed that advanced magnitude based Liver MRI PDFF correlates well with hepatic steatosis assessed by liver histology.7 The choice of any single cutoff for normal liver and fatty liver is a tradeoff between sensitivity and specificity. Therefore, we generated a nomogram to predict the probability of the histologic steatosis stage from any given liver MRI PDFF value.7 With the advances in liver MRI, pediatric clinical trials are increasingly using this imaging tool to assess the primary outcome. Therefore, Goyal et al performed the STEATOSIS study in order to assess the normal fluctuation in liver MRI PDFF over 12 weeks in adolescents with NASH.8 The mean absolute change in liver MRI-PDFF was −0.8% and the mean relative change was −3.8%. For 95 percent of adolescents, that change in liver MRI-PDFF ranged from −5.8% to +4.2%. Thus, an intervention group would need to demonstrate a greater change than these values to be considered clinically relevant.
Controlled attenuation parameter (CAP) is a technique that uses the information collected during the measurement of liver stiffness by vibration controlled elastography (FibroScan) to quantify the attenuation of the ultrasound wave. The CAP measurement has been proposed as a tool to evaluate steatosis. Shin et al evaluated 76 children with NAFLD by both liver MRI-PDFF and CAP.9 The mean CAP values were significantly higher in children with NAFLD (309 dB/m) compared to 10 children with normal BMI and normal liver chemistry (225 dB/m), and they proposed an optimal cutoff of 241 dB/m. CAP values were only moderately correlated with liver MRI-PDFF values for the children overall, and CAP values were not correlated with liver MRI-PDFF in children with BMI ≥ 30. Runge et al evaluated 60 children with severe obesity using both liver MR spectroscopy PDFF and CAP.10 In children without steatosis, the median CAP value was 253 dB/m, and in children with fatty liver, it was 327 dB/m. They proposed that the optimal CAP cutoff value for detecting fatty liver was 277 dB/m, which had an overall diagnostic accuracy of 73%. To date, CAP remains an experimental technique in children awaiting further validation and standardization.
In addition to the correct diagnosis of NAFLD, the severity of liver disease is an important clinical parameter that has traditionally been assessed as the stage of fibrosis present in liver histology. MRI has been used to estimate the severity of liver fibrosis in the form of magnetic resonance elastography (MRE) to measure liver stiffness noninvasively by analyzing the propagation of shear waves transmitted into the abdomen. The MRI Assessment Guiding NAFLD Evaluation and Treatment (MAGNET) was a multi-center study in children with NAFLD that compared MRE-measured hepatic stiffness to liver histology fibrosis stage.11 The overall diagnostic accuracy to detect liver fibrosis was 72%. In order to develop normative data for MRE in children, Sawh et al evaluated 81 children free from liver disease by history, exam, laboratory analyses, and liver MRI-PDFF.12 Depending upon the MRE threshold value used, 74 to 85% of healthy children free from liver disease were correctly identified as not having any liver fibrosis. In the STEATOSIS study, Goyal et al also evaluated the stability of MRE derived shear stiffness over 12 weeks.8 MRE shear stiffness demonstrated greater variability than was observed for liver MRI-PDFF. There was a moderate correlation between individual measures of liver stiffness at baseline and week 12. Collectively, these studies showed that liver MRE holds promise as a noninvasive tool to evaluate liver fibrosis. However, further technological refinements are needed to advance its use in the clinical management of children with NAFLD.
3. PREVALENCE AND INCIDENCE OF NAFLD
3.1. Prevalence of NAFLD in Children with Obesity
Although obesity has been well-established as the largest risk factor for NAFLD, there was a lack of uniform agreement on the estimated prevalence of NAFLD in children with obesity. The estimated prevalence of NAFLD in children with obesity ranged from 1.7% all the way to 85%, thus having enormous repercussions on the screening and management of this patient population. Therefore, Yu et al evaluated 408 obese children from the community ages 9 to 17 for NAFLD.13 Patients underwent MRI studies to measure proton density fat fractions in the liver, a biomarker for hepatic steatosis. The overall estimated prevalence of NAFLD in children with obesity was 26%, with a prevalence of 29.4% in males and 22.6% in females. Alanine Aminotransferase (ALT) was also evaluated with respect to diagnostic accuracy for NAFLD. In males with obesity, a cutoff point of 42 U/L provided a diagnostic accuracy of 80%, and for females with obesity, a cutoff point of 30 U/L provided a diagnostic accuracy of 80%.. By combining with fasting insulin levels, individuals were able to be sorted with more granular probabilities with a clinical decision tree. The prevalence of NAFLD is higher in patients with severe obesity. Xanthakos and her collaborators from the Teen-LABS study investigated 242 adolescents undergoing bariatric surgery.14 An intraoperative liver biopsy was performed in 68% of patients at the time of weight loss surgery. Adolescents that had a liver biopsy had significantly higher BMIs and greater rates of dyslipidemia than those who did not have a liver biopsy. In those patients that had a liver biopsy, the mean age was 16.9 years and the mean BMI was 51.6. In this group, 59% had NAFLD confirmed histologically.
3.2. Clinical Incidence of NAFLD
In 2007, the American Academy of Pediatrics recommended that children with obesity should be screened for NAFLD. In 2017, The North American Society for Pediatric Gastroenterology Hepatology and Nutrition supplemented this recommendation with the use of ALT measurement to screen for NAFLD in children with obesity or overweight beginning at age 9 years. Guidelines leave it up to clinical judgement on how to proceed once there is a positive screening test. Despite these recommendations, little was known about rates of clinical diagnosis or screening practices. Many investigators had speculated that the incidence of NAFLD in children was increasing, but this had not been formally studied. Sahota et al looked at the screening practices and annual incidence of NAFLD in children within a large health maintenance organization.15 Using electronic health records, 7,884,884 patient years were evaluated. Screening was performed in 54% of children with obesity and was positive in 8.9% when using an ALT level greater than 54 U/L and 28.4% when using an ALT level of greater than 30 U/L. Notably, only 12.3% of children with elevated ALT received further workup for NAFLD. The substantial gap between the number of positive screening test results and the number of children who received a diagnosis of NAFLD may be explained by a number of factors. Specifically, guidelines are unclear as to how physicians should proceed when an elevated ALT is found. Furthermore, confusion exists in ALT interpretation based on differences between ALT normal reference ranges and biologically-based normal values for ALT. In addition, children with older age, higher BMI, and higher degree of ALT elevation were more likely to have a comprehensive evaluation. In this study, the incidence rate of NAFLD in children increased by 62% from 36.0 per 100,000 in 2009 to 58.2 per 100,000 in 2018. The number of children diagnosed with NAFLD increased each year over the course of a decade, and the annual rate rose faster in more recent years. This increase in incidence was due to a combination of factors, including an increase in the absolute number of children with NAFLD as well as improvements in screening for NAFLD. However, many children with NAFLD are likely to go undiagnosed due to both the underutilization of screening and the complexity of clinical diagnosis.
4. RISK FACTORS FOR NAFLD
4.1. Perinatal Factors
In addition to obesity, risk factors for NAFLD, including age, ethnicity, birth weight and muscle mass, have been identified. Increasing evidence shows that factors during gestation may have a long-lasting influence on metabolic health. In a multicenter, cross-sectional study of 538 children with biopsy-proven NAFLD, Newton et al investigated the relationship between birth weight and severity of NAFLD. Among children with NAFLD, both low birth weight and high birth weight were overrepresented, suggesting that these may be risk factors for developing NAFLD.16 In addition, birthweight was associated with the severity of NAFLD. Specifically, those with low birthweight had a higher risk for more severe fibrosis, and those with high birth weight had a higher risk for more severe steatosis. Maternal obesity at the time of delivery has also been identified as a risk factor for NAFLD. Cantoral et al studied 631 mothers at the time of delivery and their offspring were followed over time.17 They found that mothers with higher body mass index at delivery had children that were more likely to have NAFLD in early adulthood as assessed by MRI PDFF.17 Whether high BMI at delivery reflected obesity prior to conception or excessive weight gain during pregnancy was not delineated. However, both of these factors may be relevant. In the Raine cohort, mothers were enrolled during pregnancy and their offspring were followed through 17 years of age.18 At age 17, those children who had been exclusively breastfed for greater than 6 months had reduced odds of having NAFLD compared to those children who had been exclusively breastfed for less than 6 months. The raw prevalence rates were 11.9% vs 17.9%, and this was associated with a 36% reduction in the odds for NAFLD after adjustment for maternal pre-pregnancy obesity and obesity in the adolescent.
4.2. Race and Ethnicity
Studies have shown that the risk for NAFLD differs by race and ethnicity. Through the Child and Adolescent Trial for Cardiovascular Health (CATCH) in California, Louisiana, Minnesota, and Texas, we first demonstrated that Hispanic adolescents with obesity were more likely to have elevated ALT than White adolescents with obesity, and Black adolescents with obesity were less likely to have elevated ALT than White adolescents with obesity.19 In SCALE, it was shown that fatty liver was most prevalent in children and adolescents of Hispanic ethnicity (11.8%) and least prevalent among children and adolescents of Black race (1.5%).3 The prevalence of fatty liver was estimated to be 10.2% in children and adolescents of Asian race and 8.6% in children and adolescents of White race.3 Sahota et al showed that the highest rates of NAFLD were in children of Hispanic ethnicity followed by children of Pacific Islander race.15 The higher prevalence of NAFLD in children of Hispanic ethnicity may be related to factors including genetics, access to health care, and/or the prevalence of other conditions such as hypertriglyceridemia and type 2 diabetes.20 With regard to genetic factors, the rs738409 single-nucleotide polymorphism (SNP) in the patatin-like phospholipase 3 gene (PNPLA3) has been previously associated with hepatic steatosis. Allele frequency was consistent with observed epidemiology, with children of Hispanic ethnicity having the highest allele frequency (0.49) and children of Black race having the lowest allele frequency (0.17).21 Recently in children, Tang et al showed that the G allele was associated with 3 times the odds of having NAFLD compared to the C allele.22
4.3. Environmental Toxins
Increasingly, environmental toxins have been implicated as potential causes or contributors to NAFLD. Verstraete et al studied the relationship between urinary Bisphenol-A (BPA) levels and suspected NAFLD, defined as ALT ≥30 IU/L in combination with overweight or obesity, in 944 adolescents.23 BPA is a known endocrine disruptor synthetically used in the manufacture of polycarbonate plastics and resins lining food and beverage containers. Higher urinary BPA levels were associated with higher rates of suspected NAFLD, although this finding was not consistent across the full range of BPA levels observed.23 In another study of 8,516 adolescents and adults, Frediani et al looked at the association between urinary arsenic levels and elevated ALT (ALT >25 IU/L in boys, >22 IU/L in girls, >30 IU/L in adult males, and >19 IU/L in adult females).24 Arsenic is a chemical present in soil and groundwater, through which it contaminates drinking water and food products such as rice, cereals, seafood, and poultry. The odds of elevated ALT were 20% higher for those in the second quartile of urinary arsenic levels and 100% higher for those in the fourth quartile compared to the first quartile of arsenic levels.24 The study did not separate adolescents from adults; therefore, whether this risk in adolescents is higher, lower, or the same as adults remains to be determined.
4.4. Body Composition
In addition to excess body fat, the specific location of fat and/or the amount or quality of lean body mass influences the risk for NAFLD. Using data from NHANES for the years 2005–2014, Selvakumar et al evaluated 1,482 adolescents with body mass index <85th percentile and found that 8% had unexplained ALT elevation.25 The authors considered this to represent suspected NAFLD and thus speculated that lean NAFLD may be common in adolescents within the US. Reduced muscle mass contributes to reduced whole body insulin sensitivity, a condition in adults known as sarcopenia. Yodoshi et al performed a retrospective review of 336 patients with NAFLD (100 diagnosed by biopsy and 236 diagnosed by MRI) and assessed the psoas muscle from clinical MRI images.26 Lower total psoas muscle surface area adjusted for height was associated with both greater histopathological steatosis severity and liver PDFF in children.
5. COMORBID CONDITIONS ASSOCIATED WITH NAFLD
NAFLD is associated with several comorbidities, and more recently, the clinical relevance of these associations has been better characterized, including relationships with diabetes, dyslipidemia, mental health, kidney disease, and obstructive sleep apnea. Although screening is recommended for hypertension, dyslipidemia, and type 2 diabetes in children with NAFLD, Shapiro et al found that only half (47.5%) of pediatric gastroenterologists screened for these comorbidities.27 Notably, time constraints were associated with poor screening. Providers who spent more than 25 minutes at the initial visit were more likely to screen, illustrating the need for sufficient time to address comorbidities in children with NAFLD.
5.1. Type 2 Diabetes
While the relationship between NAFLD and type 2 diabetes has been well-defined in the adult population, Newton et al demonstrated its prevalence in the pediatric population.28 This multi-center, cross-sectional study included 675 children with biopsy-proven NAFLD. Among children with NAFLD, nearly 30% had abnormal glucose metabolism, 6.5% had type 2 diabetes, and 23% had prediabetes. Children with type 2 diabetes or prediabetes were at greater risk for NASH.
5.2. Kidney Disease
In adults, the presence and severity of NAFLD are associated with higher rates and greater severity of chronic kidney disease. In children with NAFLD, one factor that may place them at a greater risk for chronic kidney disease is hypertension. One potential risk factor for chronic kidney disease in children with NAFLD is the high rate of hypertension.29 Pacifico et al measured estimated glomerular filtration rate (eGFR) and urinary albumin excretion in 596 children with overweight or obesity and 130 controls with healthy weight.30 The group with overweight or obesity was separated into 268 with NAFLD and 328 without NAFLD based upon MRI hepatic fat fraction of ≥ 5%. Children with NAFLD had a higher rate of having an abnormal eGFR, 35%, compared to 24% of children with overweight or obesity without NAFLD and 5% of children with healthy weight. For children with NAFLD, the abnormality in eGFR was equally split between having low eGFR and hyperfiltration. The prevalence of microalbuminuria was also significantly greater in children with NAFLD (9.3%) compared to children with overweight or obesity without NAFLD (4.0%) and healthy controls (0%). More recently, Yodoshi et al confirmed the high prevalence of renal impairment in children with NAFLD.31 They observed very similar rates in a retrospective study of 179 children with biopsy-confirmed NAFLD -- 15% had low eGFR and 20% had glomerular hyperfiltration.
5.3. Dyslipidemia
Pediatric NAFLD has been associated with dyslipidemia, yet it was unknown how frequently this dyslipidemia required clinical action. Recently, Harlow et al followed 585 children with biopsy-proven NAFLD in a multicenter, longitudinal cohort study.32 Overall, 14% of children with NAFLD met clinical intervention thresholds for LDL cholesterol, and 51% of children with NAFLD met clinical intervention thresholds for triglycerides. With one year of standard-of-care lifestyle intervention, half of children met their LDL cholesterol goal. However, only 25% of children met their triglyceride target goal after one year of standard-of-care lifestyle intervention.
5.4. Depression and Anxiety
While the association between NAFLD and the aforementioned comorbidities has been well-established, its interconnection with mental health conditions has been understudied. In a longitudinal cohort study of 160 adolescents with biopsy-proven NAFLD, Noon et al demonstrated that the mental health burden adolescents with NAFLD face is substantial.33 Over a four year average following diagnosis with NAFLD, 1 in 6 adolescents with NAFLD developed clinically relevant depression. Notably, adolescents with NAFLD whose liver chemistry worsened over time were more likely to develop depression. In this cohort, the cumulative prevalence of anxiety was 12.5%. The average time from diagnosis of NAFLD to a clinical diagnosis of an anxiety disorder was 3 years. Notably, neither BMI nor BMI z-score were risk factors for incident depression or anxiety.
5.5. Obstructive Sleep Apnea
In 2014, initial studies by Sundaram et al and Nobili et al reported that approximately 60% of children with NAFLD had an apnea-hypopnea index >1.0.34,35 In an expansion of one of these studies, Sundaram et al performed sleep studies in 36 adolescents with biopsy-proven NAFLD and found that 24 (67%) had OSA as defined by an apnea-hypopnea index >2.0.36 Higher NAFLD Activity Score (NAS) was associated with higher apnea-hypopnea index.
6. CONCLUSION
NAFLD is a common disease among children with obesity with an estimated prevalence of 26%. The incidence of NAFLD has risen rapidly in children over the past decade. An understanding of the reasons for this rise are incomplete, but over the past 5 years, many studies have provided additional insight into the complexity of risk factors, diagnostic approaches, and associated comorbidities. Risk factors for NAFLD are wide ranging, including perinatal factors involving both the mother and newborn, as well as environmental toxin exposure. The extent to which any specific risk factor underlies the increased incidence in NAFLD merits further evaluation. The variability in the approach to screening and diagnosis is a limitation to both advancements in understanding the disease as well as to its management. The progress made in noninvasive assessment will be critical to improving upon these issues. With respect to MRI, progress in the measurement of steatosis is closest to use in clinical settings, whereas progress in the measurement of fibrosis is still largely a research tool in children. In addition, the list of serious comorbidities observed in children with NAFLD continues to grow. Notably, for many of these conditions, such as diabetes and depression, the rates observed have exceeded the rates reported in children with obesity without NAFLD. These comorbidities should be considered when caring for patients with NAFLD. The recent advancements in epidemiology reviewed herein show that there has been an increased awareness of this problem, while also calling attention to the need for additional research to guide successful efforts at prevention and treatment.
Conflict of Interest:
The current study was. supported by the National Institutes of Health Grants UL1TR001442. The funders did not participate in the collection, management, analysis, and interpretation of the data; or preparation of the manuscript. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. Dr. Schwimmer also reports grants to UC San Diego from Intercept, Genfit, and Seraphina; and consulting for Merck. Ms Noon and Dr. Shapiro report no conflicts.
References
- 1.Health Effects of Overweight and Obesity in 195 Countries over 25 Years. N Engl J Med. 2017;377(1):13–27. doi: 10.1056/NEJMoa1614362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schwimmer JB, Newton KP, Awai HI, et al. Paediatric gastroenterology evaluation of overweight and obese children referred from primary care for suspected non-alcoholic fatty liver disease. Aliment Pharmacol Ther. 2013;38(10):1267–1277. doi: 10.1111/apt.12518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schwimmer JB, Deutsch R, Kahen T, Lavine JE, Stanley C, Behling C. Prevalence of fatty liver in children and adolescents. Pediatrics. 2006;118(4):1388–1393. doi: 10.1542/peds.2006-1212 [DOI] [PubMed] [Google Scholar]
- 4.Schwimmer MH, Sawh MC, Heskett KM, Goyal NP, Newton KP, Schwimmer JB. A Bibliometric Analysis of Clinical and Translational Research in Pediatric Gastroenterology from 1970 to 2017. J Pediatr Gastroenterol Nutr. 2018;67(5):564–569. doi: 10.1097/MPG.0000000000002056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schwimmer JB, Behling C, Newbury R, et al. Histopathology of pediatric nonalcoholic fatty liver disease. Hepatol Baltim Md. 2005;42(3):641–649. doi: 10.1002/hep.20842 [DOI] [PubMed] [Google Scholar]
- 6.Africa JA, Behling CA, Brunt EM, et al. In Children with Nonalcoholic Fatty Liver Disease, Zone 1 Steatosis is Associated with Advanced Fibrosis. Clin Gastroenterol Hepatol Off Clin Pract J Am Gastroenterol Assoc. 2018;16(3):438–446.e1. doi: 10.1016/j.cgh.2017.02.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schwimmer JB, Middleton MS, Behling C, et al. Magnetic resonance imaging and liver histology as biomarkers of hepatic steatosis in children with nonalcoholic fatty liver disease. Hepatol Baltim Md. 2015;61(6):1887–1895. doi: 10.1002/hep.27666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goyal NP, Sawh MC, Ugalde-Nicalo P, et al. Evaluation of Quantitative Imaging Biomarkers for Early-phase Clinical Trials of Steatohepatitis in Adolescents. J Pediatr Gastroenterol Nutr. 2020;70(1):99–105. doi: 10.1097/MPG.0000000000002535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shin J, Kim M-J, Shin HJ, et al. Quick assessment with controlled attenuation parameter for hepatic steatosis in children based on MRI-PDFF as the gold standard. BMC Pediatr. 2019;19(1):112. doi: 10.1186/s12887-019-1485-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Runge JH, van Giessen J, Draijer LG, et al. Accuracy of controlled attenuation parameter compared with ultrasound for detecting hepatic steatosis in children with severe obesity. Eur Radiol. 2021;31(3):1588–1596. doi: 10.1007/s00330-020-07245-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schwimmer JB, Behling C, Angeles JE, et al. Magnetic resonance elastography measured shear stiffness as a biomarker of fibrosis in pediatric nonalcoholic fatty liver disease. Hepatology. 2017;66(5):1474–1485. doi: 10.1002/hep.29241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sawh MC, Newton KP, Goyal NP, et al. Normal range for MR elastography measured liver stiffness in children without liver disease. J Magn Reson Imaging. 2020;51(3):919–927. doi: 10.1002/jmri.26905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yu EL, Golshan S, Harlow KE, et al. Prevalence of Nonalcoholic Fatty Liver Disease in Children with Obesity. J Pediatr. 2019;207:64–70. doi: 10.1016/j.jpeds.2018.11.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xanthakos SA, Jenkins TM, Kleiner DE, et al. High Prevalence of Nonalcoholic Fatty Liver Disease in Adolescents Undergoing Bariatric Surgery. Gastroenterology. 2015;149(3):623–634.e8. doi: 10.1053/j.gastro.2015.05.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sahota AK, Shapiro WL, Newton KP, Kim ST, Chung J, Schwimmer JB. Incidence of Nonalcoholic Fatty Liver Disease in Children: 2009–2018. Pediatrics. 2020;146(6). doi: 10.1542/peds.2020-0771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Newton KP, Feldman HS, Chambers CD, et al. Low and High Birth Weights Are Risk Factors for Nonalcoholic Fatty Liver Disease in Children. J Pediatr. 2017;187:141–146.e1. doi: 10.1016/j.jpeds.2017.03.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cantoral A, Montoya A, Luna‐Villa L, et al. Overweight and obesity status from the prenatal period to adolescence and its association with non-alcoholic fatty liver disease in young adults: cohort study. BJOG Int J Obstet Gynaecol. 2020;127(10):1200–1209. doi: 10.1111/1471-0528.16199 [DOI] [PubMed] [Google Scholar]
- 18.Ayonrinde OT, Oddy WH, Adams LA, et al. Infant nutrition and maternal obesity influence the risk of non-alcoholic fatty liver disease in adolescents. J Hepatol. 2017;67(3):568–576. doi: 10.1016/j.jhep.2017.03.029 [DOI] [PubMed] [Google Scholar]
- 19.Schwimmer JB, McGreal N, Deutsch R, Finegold MJ, Lavine JE. Influence of gender, race, and ethnicity on suspected fatty liver in obese adolescents. Pediatrics. 2005;115(5):e561–565. doi: 10.1542/peds.2004-1832 [DOI] [PubMed] [Google Scholar]
- 20.Saab S, Manne V, Nieto J, Schwimmer JB, Chalasani NP. Nonalcoholic Fatty Liver Disease in Latinos. Clin Gastroenterol Hepatol Off Clin Pract J Am Gastroenterol Assoc. 2016;14(1):5–12; quiz e9–10. doi: 10.1016/j.cgh.2015.05.001 [DOI] [PubMed] [Google Scholar]
- 21.Romeo S, Kozlitina J, Xing C, et al. Genetic variation in PNPLA3 confers susceptibility to nonalcoholic fatty liver disease. Nat Genet. 2008;40(12):1461–1465. doi: 10.1038/ng.257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tang S, Zhang J, Mei TT, et al. Association of PNPLA3 rs738409 G/C gene polymorphism with nonalcoholic fatty liver disease in children: a meta-analysis. BMC Med Genet. 2020;21(1):163. doi: 10.1186/s12881-020-01098-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Verstraete SG, Wojcicki JM, Perito ER, Rosenthal P. Bisphenol a increases risk for presumed non-alcoholic fatty liver disease in Hispanic adolescents in NHANES 2003–2010. Environ Health. 2018;17. doi: 10.1186/s12940-018-0356-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Frediani JK, Naioti EA, Vos MB, Figueroa J, Marsit CJ, Welsh JA. Arsenic exposure and risk of nonalcoholic fatty liver disease (NAFLD) among U.S. adolescents and adults: an association modified by race/ethnicity, NHANES 2005–2014. Environ Health Glob Access Sci Source. 2018;17(1):6. doi: 10.1186/s12940-017-0350-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Conjeevaram Selvakumar PK, Kabbany MN, Lopez R, Rayas MS, Lynch JL, Alkhouri N. Prevalence of Suspected Nonalcoholic Fatty Liver Disease in Lean Adolescents in the United States: J Pediatr Gastroenterol Nutr. 2018;67(1):75–79. doi: 10.1097/MPG.0000000000001974 [DOI] [PubMed] [Google Scholar]
- 26.Yodoshi T, Orkin S, Clachar A-CA, et al. Muscle Mass Is Linked to Liver Disease Severity in Pediatric Nonalcoholic Fatty Liver Disease. J Pediatr. 2020;223:93–99.e2. doi: 10.1016/j.jpeds.2020.04.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shapiro WL, Yu EL, Arin JC, et al. Clinical Practice Approach to Nonalcoholic Fatty Liver Disease by Pediatric Gastroenterologists in the United States. J Pediatr Gastroenterol Nutr. 2019;68(2):182–189. doi: 10.1097/MPG.0000000000002194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Newton KP, Hou J, Crimmins NA, et al. Prevalence of Prediabetes and Type 2 Diabetes in Children With Nonalcoholic Fatty Liver Disease. JAMA Pediatr. 2016;170(10):e161971. doi: 10.1001/jamapediatrics.2016.1971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schwimmer JB, Zepeda A, Newton KP, et al. Longitudinal assessment of high blood pressure in children with nonalcoholic fatty liver disease. PloS One. 2014;9(11):e112569. doi: 10.1371/journal.pone.0112569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pacifico L, Bonci E, Andreoli GM, et al. The Impact of Nonalcoholic Fatty Liver Disease on Renal Function in Children with Overweight/Obesity. Int J Mol Sci. 2016;17(8):1218. doi: 10.3390/ijms17081218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yodoshi T, Arce-Clachar AC, Sun Q, et al. Glomerular Hyperfiltration Is Associated with Liver Disease Severity in Children with Nonalcoholic Fatty Liver Disease. J Pediatr. 2020;222:127–133. doi: 10.1016/j.jpeds.2020.03.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Harlow KE, Africa JA, Wells A, et al. Clinically Actionable Hypercholesterolemia and Hypertriglyceridemia in Children with Nonalcoholic Fatty Liver Disease. J Pediatr. 2018;198:76–83.e2. doi: 10.1016/j.jpeds.2018.02.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Noon S, D’Annibale D, Schwimmer M, et al. Incidence of Depression and Anxiety in a Cohort of Adolescents with Nonalcoholic Fatty Liver Disease. J Pediatr Gastroenterol Nutr. 2020;Publish Ahead of Print. doi: 10.1097/MPG.0000000000003024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sundaram SS, Sokol RJ, Capocelli KE, et al. Obstructive Sleep Apnea and Hypoxemia Are Associated with Advanced Liver Histology in Pediatric Nonalcoholic Fatty Liver Disease. J Pediatr. 2014;164(4):699–706.e1. doi: 10.1016/j.jpeds.2013.10.072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nobili V, Cutrera R, Liccardo D, et al. Obstructive sleep apnea syndrome affects liver histology and inflammatory cell activation in pediatric nonalcoholic fatty liver disease, regardless of obesity/insulin resistance. Am J Respir Crit Care Med. 2014;189(1):66–76. doi: 10.1164/rccm.201307-1339OC [DOI] [PubMed] [Google Scholar]
- 36.Sundaram SS, Halbower A, Pan Z, et al. Nocturnal hypoxia-induced oxidative stress promotes progression of pediatric non-alcoholic fatty liver disease. J Hepatol. 2016;65(3):560–569. doi: 10.1016/j.jhep.2016.04.010 [DOI] [PMC free article] [PubMed] [Google Scholar]


