Abstract
The halogens chlorine (Cl2) and bromine (Br2) are highly reactive oxidizing elements with widespread industrial applications and a history of development and use as chemical weapons. When inhaled, depending on the dose and duration of exposure, they cause acute and chronic injury to both the lungs and systemic organs that may result in the development of chronic changes (such as fibrosis) and death from cardiopulmonary failure. A number of conditions, such as viral infections, coexposure to other toxic gases, and pregnancy increase susceptibility to halogens significantly. Herein we review their danger to public health, their mechanisms of action, and the development of pharmacological agents that when administered post-exposure decrease morbidity and mortality.
Keywords: antioxidants, bromine, ARDS, chlorine, heme
Introduction
The halogens chlorine (Cl2) and bromine (Br2) are highly reactive, oxidizing elements with widespread industrial applications and a history of development and use as chemical weapons. Both are encountered in gaseous form at room temperature and both are only slightly soluble in water with solubilities of 7.25 and 42 g/L at 20°C, respectively. Despite their low physical solubility, both Cl2 and Br2 produce high concentrations of their hydrolysis products due to their high reactivity with water. In the reactions
and
they readily produce hypohalous (HOCl and HOBr) and hydrohalic acids (HCl and HBr), also known as hydrogen halydes. While hypohalous acids dissociate relatively poorly and do not reduce pH substantially, they are highly reactive. Hydrohalic acids are not reactive but dissociate readily to protons and halide ions, producing acidic pH. The high reactivity of hypohalous acids with organic molecules is considered to be the main cause of halogen toxicity to all living things.
There are numerous instances of both intentional and unintentional human exposure to halogen gasses resulting in morbidity and in some cases mortality. Depending on the concentration and duration of exposure, symptoms range from mild mucosal membrane irritation to severe lung and cardiac injury, in some cases culminating in death (1–3). Survivors may develop chronic ailments including pulmonary and cardiac fibrosis (3, 4). Exposure of pregnant animals to halogens results in significant mortality and cardiovascular injury to both the dams and their fetuses (5).
There has been significant interest in developing suitable animal and cell models to investigate the mechanisms by which Cl2 and Br2 damage cardiomyocytes as well as lung epithelial, smooth muscle, and endothelial cells along with developing countermeasures which may prevent and reverse this injury. Herein, we briefly review the epidemiology of halogen gas exposure, summarize what is known concerning the generation of secondary reactive intermediates and how these species contribute to pulmonary and systemic injury, and review some of the most promising countermeasures.
Halogens and Public Health
Cl2 and Br2 have widespread industrial applications and are used to sterilize drinking water, to treat swimming pools and in numerous industrial applications including the production of paper, plastic, dyes, textiles, and medicines (2, 6, 7). Most human exposures occur accidentally during manufacturing, transportation, or storage of halogens for industrial purposes. Exposures to Cl2 and Br2 at water treatment facilities and swimming pools are well documented. The toxic properties of halogens and their derivatives were inadvertently exhibited near the start of the 1900s during administration of chloroform as an anesthetic where the open flames used to illuminate the operating rooms of the time caused its decomposition into Cl2 and phosgene (COCl2) leading to respiratory tract symptoms and in some cases the death of patients and physicians (8). Simultaneously, efforts to harness this toxic potential for use on the battlefields of World War I were ongoing. Br2-based tear gas agents, along with Cl2, COCl2, diphosgene, C4H8Cl2S (mustard gas), and various combinations of each, were all refined and deployed at various conflicts (6). Unfortunately, the use of halogen-based chemical weapons has continued into the modern era with Cl2 attacks documented in Iraq and during the ongoing Syrian civil war (1, 9) and with Br2 storage tanks in Israel serving as the apparent target of a failed terrorist attack in 2004 (10). The US Department of Homeland Security identified industrial sites dedicated to Cl2 as possible targets of terrorist activity, with some projections estimating that a successful attack could result in thousands of fatalities and 100,000 hospitalization (The Homeland Security Council. Planning Scenarios: Executive Summaries. 004; 8-1).
Lessons Learned from Accidental Halogen Exposures
As mentioned, industrial and manufacturing mishaps represent the most likely interface between humans and halogen gas. Through exploring the results of these accidents, one can begin to piece together a picture of both the acute and long-term sequelae resulting from various exposure intensities. Thirty large-scale Cl2-related accidents have occurred in urban centers over the past two decades with hundreds of documented smaller scale incidents. In one prominent example, a train was accidentally diverted causing a collision with a parked train car carrying Cl2. The tanker ruptured, spilling 54,422 kg (120,000 lb) of Cl2 and engulfing the city of Graniteville in a toxic cloud of gas. Eight victims died before arrival to a hospital. Seventy-one patients were hospitalized due to acute Cl2 exposure. Of these, there was 1 in-hospital death and 25 (35%) individuals who required intensive care (11). Patients who survive acute lung injury frequently develop chronic lung disease, with airflow obstruction, fibrosis, airway hyperreactivity, and impaired gas exchange (3). These patients are more likely to require hospital admission (1, 12) and more susceptible to bacterial pneumonia (13). People that smoke and those with respiratory infections and asthma are much more sensitive to the toxic effects of Cl2 and may experience airway hyperresponsiveness at concentrations that cause no symptoms in people with no respiratory disease (7, 14–17).
Airway hyperresponsiveness, termed reactive airways dysfunction syndrome, is also reported in individuals exposed to Cl2, and asthma-like symptoms can persist for long periods (11). In the year following the Graniteville train accident, a 4% decrease in mean forced expiratory volume at 1 s (FEV1) was observed in patients when compared with test results the year before the incident. During the subsequent year, a partial recovery in the forced vital capacity was noted, but the predicted average FEV1 continued to decrease over time. Severe annual FEV1 decline was most prevalent in the year of the accident and independent of smoking status (1, 3, 4, 18). Intriguingly, hospitalizations for hypertension doubled from 110 per 10,000 residents in 2005 to 220 hospitalizations per 10,000 residents in 2012 in the larger Graniteville population. This increase was found in all age groups, races, and both sexes. Cardiomegaly was observed during autopsy in eight of the nine immediate victims (11).
In other accidents, exposure to high levels of Cl2 gas led to vascular injury and depressed of cardiac function (19). Risk of stroke over time may also be impacted due to oxidation of low-density lipoproteins (LDL) by hypochlorous acid and the generated oxidized LDL contributing to the vessel wall inflammation and thus promoting atherosclerosis (20, 21).
Chemical accidents due to inadvertent release of Br2 have occurred in Netherlands, Germany, Belgium, Israel, United States, Russia, and many other countries (22). A major train accident involving Br2 occurred in Chelyabinsk, Russia (population 1.1 million) leading to 42 hospitalizations and 200 patients requiring medical attention. In another train accident, a freight train collided with three rail cars in Dimona Israel, one of which was carrying liquid bromine. There are numerous reports of incidents worldwide, where trucks carrying Br2 overturned, spilling their contents in the atmosphere.
Presently, treatments following halogen gas exposure are mainly supportive, including humidified oxygen administration for correction of hypoxemia, β2-agonists for reversal of for bronchospasm, and antibiotics for potential infections (23). In severe cases of acute respiratory distress syndrome (ARDS), positive pressure ventilation with or without intubation may be necessary. Corticosteroids and sodium bicarbonate can alleviate ARDS following Cl2 exposure in animal studies, but similar efficacy in humans has yet to be demonstrated (24, 25).
Animal Models of Cl2 Toxicity
Mice, rats, rabbits, pigs, sheep, dogs, and monkeys have been used to study the cardiopulmonary sequelae of exposure to Cl2 and the response to various treatments (reviewed in Refs. 1, 2). Rodents are either placed in environmental chambers (whole body exposures) or breathe Cl2 via a head-only exposure system. The advantages and disadvantages of each system have been previously described in detail (26). One important consideration is that Cl2 injury to the skin induces the endoplasmic reticulum stress and unfolded protein response which may contribute to lung injury (27). Notably, animals exposed through nose-only exposure require restraint which may impose an additional stress. Rodents can be exposed to halogens either while sedated or when fully conscious (and treated with an analgesic rather than anxiolytic). On the other hand, large animals need to be anesthetized, intubated, and ventilated. Thus halogens are delivered through the endotracheal tube.
Experiments in rodents show that the degree of lung damage after Cl2 exposure depends on total dose (i.e., concentration × time of exposure) as well as the specifics of exposure concentration and time (28). Acute exposure to doses of 1 ppm may cause irritation (29), and chronic exposure to even lower doses (0.1–0.4 ppm) may lead to ocular irritation and degeneration of the airway epithelium (30, 31). Irritant effects are primarily mediated through transient receptor potential ankyrin 1 (TRPA1) ion channels and their interplay with neurons within the airways that mediates the noxious reflex to inhaled irritant exposure, including the cough reflex (32, 33). These channels, which are found in trigeminal nerve fibers (upper airway innervation) as well as in sensory fibers of the vagus nerve (larynx and lower airways), mediate protective mechanisms including the cough reflex, sneezing, decreased respiratory rate, sensations of irritation and pain, and glandular secretions (33, 34). TRPA1 channels act through increasing intracellular calcium and other cations, thus promoting neuronal excitation (35). Pharmacologic inhibition of TRPA1 channels before exposure to Cl2 prevents the decrease in respiratory rate. The consequent increase in respiratory rate improves ventilation but, as an undesirable consequence, may result in higher levels of lung injury because of the higher levels of inhaled oxidant gases due to the increase in minute ventilation (32, 36). On the other hand, inhibition of TRPA1 channels decreases lung injury and inflammation post-acrolein exposure, an unsaturated aldehyde generated during incomplete combustion as in tobacco smoke and indoor fire (37, 38). Mice exposed to Cl2 develop decreased locomotion, which was alleviated by the administration of buprenorphine, a common analgesic agent, indicating that this effect is probably due Cl2-induced pain, because of activation of TRPA1 channels (39). The effects of TRPA1 inhibitors in halogen-induced toxicity are currently under investigation.
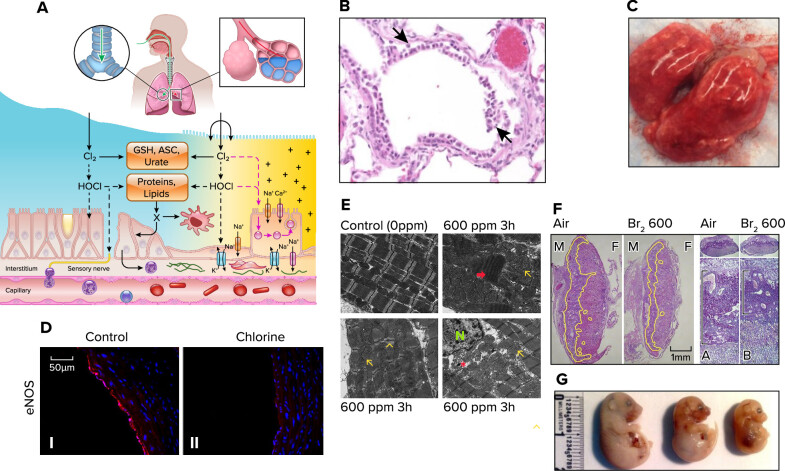
To draw valid conclusions from animal studies that will prove relevant to humans, it is important to use exposure models that approximate conditions found during release of Cl2 in the atmosphere. With the use of validated plume models (40) in the Graniteville accident, it was determined that Cl2 levels during a 30-min exposure period were 4,428, 550, and 161 ppm at 0.2, 0.5 and 1 km downwind from the epicenter of the accident. Persons exposed to 550 ppm Cl2 for 30 min required hospitalization, and a number of them develop ARDS. Similarly, the large majority of rodents exposed to 500–600 ppm Cl2 for 30 min survive the exposure although they develop bradypnea and signs of respiratory distress (41, 42). At various times in the first 48 h post-exposure, rodents develop airway epithelial sloughing, interstitial and alveolar edema secondary to injury of the microvascular and alveolar epithelial cells, massive infiltration of inflammatory cells (mainly neutrophils), hypoxemia and respiratory acidosis, impaired surfactant function, decreased active Na+ transport across alveolar epithelial cells, airway hyperreactivity, alveolar hypercoagulation, and systemic hypocoagulation (2, 41, 43–53). In addition, significant cardiac injury as well as injury to placentas and developing fetuses of pregnant mice were present as well (FIGURE 1).
FIGURE 1.
Halogen (Cl2 and Br2)-induced acute lung and systemic injury A: schema showing interaction of halogens with various components of the epithelial lining fluid and lung epithelial cells. At higher concentrations (>50 ppm), inhaled halogens reach the distal lung regions (from Ref. 48, with permission from the publisher). ASC, ascorbate; GSH, reduced glutathione. B: extensive denudation of airway epithelium in a rat exposed to Cl2 (400 ppm for 30 min) and returned to room air for 24 h. Lung sections were stained with hematoxylin-eosin. C: lungs of a mouse exposed to Cl2 (600 ppm and 45 min) and returned to room air for 24 h. There was significant hemorrhage and alveolar spaces were filled with protein-rich edema. D: Cl2 decreases endothelial nitric oxide synthase (eNOS) protein expression. I and II: representative immunofluorescence images for eNOS staining from aorta collected 24 h after exposure to air (I) or Cl2 (400 ppm, 30 min) (II). Red: eNOS; blue: Hoechst staining for nuclei (modified from Ref. 46, with permission from the publisher). E: bromine (Br2) inhalation causes disruption of the cardiac cytoskeleton and loss of the normal highly organized linear mitochondrial sarcomere integrity. Representative transmission electron microscopy (×3,200) images demonstrating a normal control heart (top left). The remaining 3 images demonstrate myofibrillar loss, contraction band necrosis (red arrow), loss of I bands, and disruption of z-disks (yellow arrowheads) in the left ventricle 3 h after Br2 exposure in addition to mitochondrial swelling (yellow arrows) and cristae lysis (red asterisk) [nucleus (N)] (modified from Ref. 54, with permission from the publisher). F: pregnant [embryonic day 14.5 (E14.5)] mice were exposed to air or 600 ppm Br2 for 30 min, returned to room. Representative hematoxylin-eosin-stained placenta sections at E18.5 with the junctional zone demarcated with yellow highlighting (left) as well as periodic acid-Schiff staining (right) of Br2-exposed pregnant mice revealed a reduced junctional zone (black bars) at E18.5 (modified from Ref. 55, with permission from the publisher). M, male; F, female; A, air; B, bromide. G: pregnant mice were exposed to air or Br2 (400 or 600 ppm for 30 min) at E14.5 and were returned to room air. Fetuses were delivered by C-section on E18.5. Notice marked fetal growth restriction that was dependent on Br2 concentration (modified from Ref. 55).
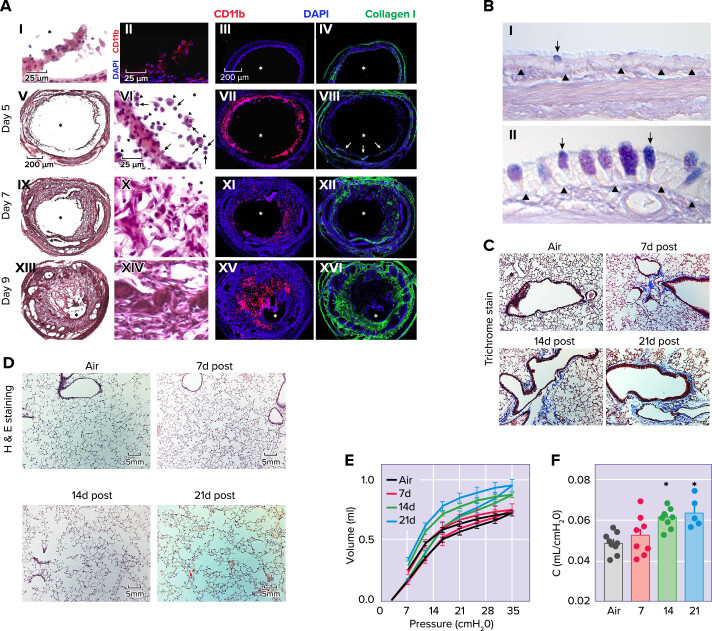
More than 50% of mice or rats exposed to 600 ppm Cl2 for 30–45 min die from respiratory failure within 5 days post-exposure. Animals that survive the initial Cl2 insult exhibit resolution of many of the initial symptoms observed in the acute phase of injury post-exposure but often develop recurring or progressively worsening elevated airway resistance in response to methacholine challenge, mucous cell metaplasia, abnormal epithelial repair, airway fibrosis, alveolar enlargement, increased respiratory system compliance, bronchiolitis obliterans, and demonstrate changes in gene expression (3, 7, 49, 56–68) (FIGURE 2). Furthermore, Cl2 and HOCl react with amino acids in carbohydrate recognition domains of surfactant protein (SP)-A, hindering the capability to bind and eliminate pathogens (69). HOCl disrupts disulfide crosslinking of SP-D and inhibits pathogen aggregating activity after an infectious challenge (122, 123). Exposure of mice to Cl2 increases their susceptibility infection by the mold Aspergillus fumigatus by 500-fold (70). The diminished antifungal defense is potentially explained by the inhibitory effect of Cl2 exposure on superoxide generation and production of IL-17A and IL-22 by myeloid cells in the lung (70).
FIGURE 2.
Halogen (Cl2 and Br2) induced chronic lung injury A: loss of epithelial integrity results in inflammatory cell infiltration, mesenchyme infiltration, and collagen deposition. I: hematoxylin-eosin staining of tracheal epithelium, 2 days after exposure to Cl2 (350 ppm for 30 min). II: immunofluorescent staining for CD11b (red) in a similar day 2 tissue section. In I and II, scale bars = 25 μm. Immunofluorescent staining of either CD11b (red) or collagen I (green) in a control tracheal section and sections harvested on days 5, 7, and 9 after high-dose Cl2 and CD11b (III, VII, XI, and XV, respectively) and collagen I (IV, VIII, XII, and XVI, respectively; scale bar, = 200 μm). In VII, arrows denote collagen 1+ cells infiltrating the lumen. Hematoxylin-and-eosin staining of tracheal sections harvested on days 5, 7, and 9 after high-dose Cl2 (V, IX, and XII, respectively; scale bar, = 200 μm); magnified images are presented VI, X, and XIV, respectively (scale bar, = 25 μm) In VI, arrowheads denote macrophages, whereas arrows denote neutrophils. All tissue sections are representative of 5–6 tracheas at each time. Asterisks denote the lumen (from Ref. 66, with permission from the publisher). B: histopathological effects of Cl2 inhalation in proximal airways, 7 days after exposure. Rats were exposed to air or 400 ppm Cl2 for 30 min. Tissue was collected 7 days after exposure, embedded in paraffin, and stained with Alcian blue and period acid-Schiff stain. I and II: representative light micrographs are of proximal airways from air-exposed (I) or 400 ppm Cl2 for 30 min (II). Arrows, mucous cells; arrowheads, basal lamina. Magnification bar = 50 μm (modified from Ref. 59, with permission from the publisher). C and D: male C57BL/6 mice were exposed to air or Br2 gas (400 ppm, 30 min) and returned to room air: peripheral lung tissue with Masson’s trichrome (C) or hematoxylin-eosin stain (H&E; D); notice increased accumulation collagen deposition (blue stain) primarily around airways on days 14 and 21 after Br2 (C) and marked enlargement of alveolar spaces (modified from Ref. 68, with permission from the publisher). E and F: quasistatic inflation and deflation pressure volume lung relationships (measured by flexiVent) (E) and lung compliance (F), measured as the slope of the deflation pressure-volume curve of mice exposed to Br2 gas (400 ppm, 30 min) and returned to room air at the indicated time points (modified from Ref. 68).
Hemodynamic responses of pigs to lung injury resemble those of humans (71). Thus experiments in larger animals allow a more complete assessment of cardiopulmonary and systemic changes. When anesthetized and ventilated pigs were exposed to 400 ppm Cl2 for 15 min and then returned to room air, they developed arterial hypoxemia and pulmonary arterial hypertension within 30 min post-exposure. Although they demonstrated improvement over the next 5–7 h, both variables remained abnormal up to 23 h post-exposure (72). In another study, Gunnarsson et al. (73) reported that exposure of young pigs to 140 ppm Cl2 for 10 min led to severe hypoxemia, increased pulmonary pressure, and decreased lung compliance starting at 1 h post-exposure. Microscopic examination of lung tissue showed sloughing of the bronchial epithelium and early infiltration with leukocytes, but the alveolar structure remained generally intact. Interstitial edema and increased number of inflammatory cells in the lung spaces appeared at the later stages of exposure.
It is important to note that these exposures were conducted in the context of general anesthesia, endotracheal intubation, and mechanical positive pressure ventilation. Thus these findings may not be generalizable to an awake, spontaneously ventilating human exposed to Cl2 in the industrial or chemical warfare scenario. For instance, Cl2 delivery to the distal airways is likely greater when introduced via an endotracheal tube. In addition, airway reflexes are inherently ablated in the process of induction of general anesthesia to facilitate endotracheal intubation further compounding the limitations of extrapolating these data to human exposures. Despite these limitations, taken as a whole, these studies indicate that exposures of both small and large animals to Cl2 in concentrations likely to be encountered in the vicinity of industrial accidents compromise gas exchange through dose-dependent damage to the respiratory and alveolar epithelia. Exposures cause significant atelectasis and ventilation-perfusion (VA/Q) mismatch leading to severe hypoxemia and decreased lung compliance most likely due to injury to pulmonary surfactant (48, 49). Injury to the alveolar and pulmonary vasculature results over time in the accumulation of protein-rich (noncardiogenenic) edema fluid in both the interstitial and eventually in the alveolar space. Alveolar edema is exacerbated by injury to epithelial Na+ channels during and following exposure of rodents to Cl2 (47, 74), decreasing the ability of alveolar cells to clear edema fluid. Most importantly, these effects persist long after the exposure and result in either death from respiratory failure or the development of chronic lung injury such as fibrosis and bronchiolitis obliterans.
Animal Models of Br2 Toxicity
Humans exposed to 0.9 ppm Br2 for 5 min developed cough, eye, and airwar irritation leading bronchospasm (22, 75). At higher doses, Br2 exposure leads to acute lung injury and adult respiratory distress syndrome. Survivors may develop reactive airway disease and pulmonary fibrosis (76). Additional information of the toxic effects of Br2 inhalation comes from animal studies. Bitron and Aharonson (77) found that the median lethal time (Lt50) at a concentration of 750 ppm was 9 min, and the Lt50 at a concentration of 240 ppm was 100 min. No deaths occurred during a 5-min exposure at 750 ppm or during a 20-min exposure at 240 ppm. Schlagbauer and Henschler (78) reported a 30-min lethal concentration (LC50) for mice exposed to Br2 was 174 ppm. In other studies, mice exposed to 600 ppm of Br2 gas for 30 min manifest significant lung injury within 24 h post-exposure, as demonstrated by increased protein extravasation, total cell infiltration into the bronchoalveolar lavage fluid, disruption of the airway parenchyma, and increased lung cellularity (22, 79). In these mice, Br2 gas inhalation resulted in lung protein oxidation, increased airway resistance following methacholine challenge, elevated lung wet-to-dry weight ratio, and correlates with the observed hypoxemia and uncompensated respiratory acidosis. There is also significant inhibition of lung alveolar Na+ transport which worsens pulmonary edema (74). In mice exposed to Br2 at 600 ppm for 30 min, there was a gradual increase in mortality, reaching 50% by 5 days post-exposure.
Recently Aggarwal et al. (68) reported that mice that survived exposure to Br2 at 400 ppm for 30 min developed significant emphysematous-type changes 14–21 days later characterized by significant alveolar enlargement, increased pulmonary compliance, and increased total lung capacity along with increased levels of lung elastase. Peribronchial fibrosis was also noted at that time but the overall phenotype was predominantly consistent with changes seen in pulmonary emphysema (FIGURE 2). The mechanisms involved are discussed in detail in the following section.
Cardiovascular Effects of Halogen Toxicity
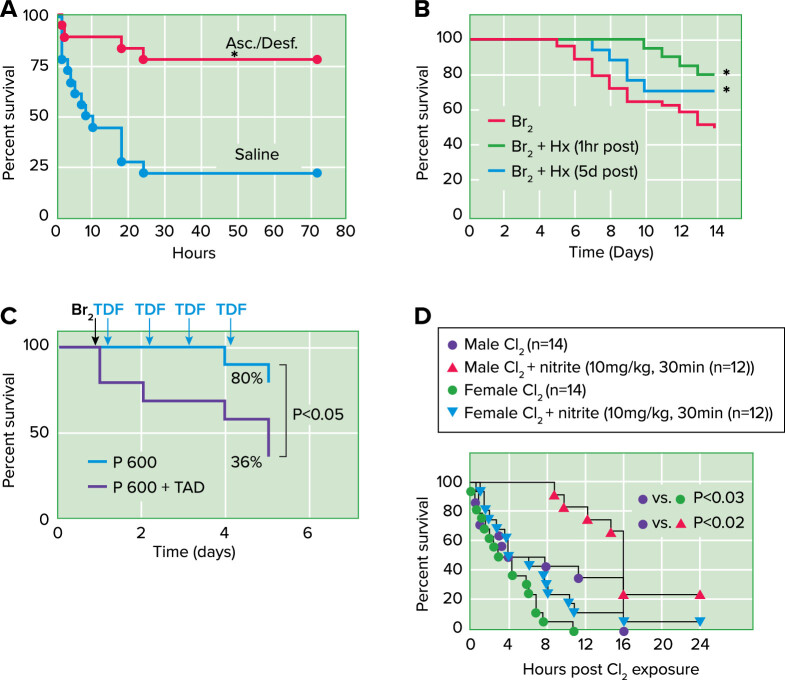
Cl2 exposure has also been shown to injure systemic organs and impair vascular function (FIGURE 1). Rats exposed to Cl2 (250–400 ppm) for 30 min had significantly decreased endothelial nitric oxide synthase (eNOS) protein expression and displayed marked attenuation in acetylcholine-induced eNOS-dependent vasodilation at 24 to 48 h post-exposure, suggesting that nitric oxide (NO) dysregulation may underlie the pathophysiology of Cl2-inhalation induced systemic endothelial dysfunction (46). However, during the initial stages of Cl2 induced injury inflammatory cell derived inducible NO synthase (iNOS) may compensate for the loss of eNOS and prevent the increase in the blood pressure. However, when iNOS was inhibited by administration of 1,400 W, significantly higher systemic blood pressures were recorded (46). This observation led some investigators to consider administration of nitrite, which is converted to NO in hypoxic and acidotic tissue, as a therapeutic agent. Post-Cl2 administration of nitrite in rats and mice improved hypoxemia, injury to the alveolar and airway epithelia, lung inflammation pulmonary edema, and survival within 24 h post-exposure (42, 45, 80, 81) (FIGURE 3).
FIGURE 3.
Countermeasures against halogen toxicity. Post-Cl2 administration of antioxidants increase survival Mice were exposed to 600 ppm of Cl2 for 45 min and returned to room air. A: they received intramuscular injections of ascorbate (Asc; 2 mg) and deferoxamine (Desf; 0.3 mg) in saline starting at 1 h after exposure and every 12 h thereafter up to 60 h after exposure. They also received aerosols of ascorbate (150 mg/mL) and deferoxamine (0.3577 mg/mL) at 1.5, 24, and 48 h after exposure in sterile water. Control mice received vehicle (saline for intramuscular injections; sterile water for aerosols) instead of antioxidants using identical protocols. Data points were fitted with Kaplan-Meier survival curves and compared with the log-rank test (P = 0.0007) (from Ref. 41). B: post-Br2 exposure administration of hemopexin increases survival. C57BL/6 mice were exposed to Br2 (400 ppm for 30 min) and returned to room air. At either 1 h or 5 days post-exposure they were given a single intraperitoneal injection of purified human hemopexin (Hx) (4 µg/g body wt) or vehicle. Survival was assessed in the next 14 days. The Kaplan-Meier curve demonstrated that Hx reduced mortality after Br2 exposure, even when was given 5 days later. *P < 0.05 vs. Br2 + saline by one-way ANOVA followed by Tukey’s post hoc test (68). C: post-Br2 administration of tadalafil (Cialis; TDF or TAD) decreases mortality of pregnant (P) mice. Nonpregnant (NP) and P gestational day 14.5 (E14.5) mice were exposed to air or to Br2 at 600 ppm for 30 min and returned to room air; they received tadalafil (TAD; 2 mg/kg body weight in 0.1 mL of sterile saline) or vehicle via oral gavage at 1 h post-exposure and every 24 h thereafter. Data show Kaplan-Meyer curves of P and NP mice with tadalafil or vehicle, post-Br2 exposure. NP mice exposed to Br2 and returned to room air lived longer than similarly exposed P mice (*P < 0.05) (from Ref. 55). D: post-Cl2 administration of nitrite improves survival. Male or female mice were exposed to Cl2 at 600 ppm, 45 min and then brought back to room air, and nitrite was administered by intramuscular injection 30 min post-exposure. Data show Kaplan-Meier survival curves. P < 0.03 between male and female Cl2-alone groups; P < 0.02 for nitrite therapy in males and P = 0.09 for nitrite therapy in females.
Cardiotoxicity leads to severe short-term sequelae after Cl2-exposure. In rats, exposure to 500 ppm Cl2 for 30 min increased the concentration of lactate in the coronary sinus, indicative of a myocardial oxygen supply/demand mismatch and consequent anaerobic metabolism. In these experiments, Cl2 also attenuated myocardial contractile force, decreased systemic blood pressure, and led to biventricular failure and substantial mortality (19, 82). An increased left ventricular ejection fraction was also observed. The authors surmised that this effect was related to a decrease in systemic afterload in concert with a hyperadrenergic state and a consequent increase in inotropy (82).
Similarly, significant cardiac damage was found after exposure of rats to Br2, characterized by increased troponin I, heart-type fatty acid-binding protein, and NH2-terminal pro-brain natriuretic peptide. In these experiments, left ventricular (LV) systolic and diastolic dysfunction was observed at 7 days post-exposure (54). Lambert et al. (55) also reported that exposure of pregnant mice to Br2 led to significant cardiac injury (to be reviewed in more detail in a subsequent section). Cumulatively these studies indicate that cardiovascular derangements caused by acute exposure to either Cl2 or Br2 may lead to cardiac dysfunction, hypotension, and even biventricular failure.
Halogens and the Proteome
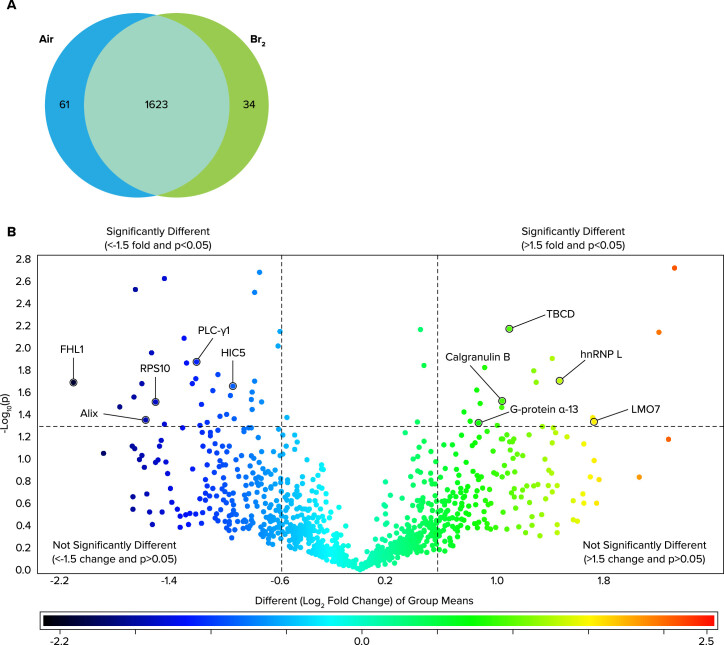
Addis et al. (84) used a combination of proteomics analysis of lung tissues along with a systems biology analysis to identify the lung proteome of mice exposed to Br2 and returned to room for 24 h. They reported alterations in proteins coinciding with regulation of three processes: 1) exosome secretion, 2) inflammation, and 3) vascular permeability (FIGURE 4). Similarly, confluent monolayers of lung cells in primary culture exhibited zona occludens-1 dissociated from cell wall localization, increased phosphorylation, internalization of E-cadherin, and increased actin stress fiber formation 24 h post-exposure to Br2 exposure, consistent with increased permeability.
FIGURE 4.
Global lung protein changes 24 h post-Br2 exposure. Adult male C57BL/6 mice, 8–10 wk old, were exposed to Br2 gas (600 ppm for 30 min) or air in environmental chambers and returned to room air Twenty-four hours later, their lungs were removed and proteins were processed for global proteomics analysis. A: Venn diagram demonstrating the total number of proteins identified across both groups in addition to those proteins found to be significantly changed following exposure to Br2 (increased vs. the other group). B: volcano plot of the log10(P) value vs. log2 fold change (Br2/air) demonstrating the distribution of the entire data set of proteins with upper limits (above the line) indicating statistically significant changes and outer limits (to the right and left of each line) indicating significant fold changes as outlined in materials and methods under statistics. Note that although fold change is visualized as log2, the cutoff value of ±1.5 was applied to the fold change before logging, thereby yielding the indicated ±0.6 limits. Various proteins that play a role in vascular permeability are identified by the arrows (from Ref. 84).
Mechanisms of Halogen Toxicity
Inhaled halogens exert their toxicity by 1) direct interaction of Cl2 and Br2 with important cellular targets in the epithelial lining fluid or on the surface of lung airway and epithelial cells; 2) by generating long-acting toxic intermediates capable of reaching sites (such as the heart and placenta) which are beyond their mean free paths; and 3) by increasing levels of reactive oxygen and nitrogen species which promote an exuberant inflammatory response.
Direct Interactions of Halogens with Biological Targets
The toxicities of halogens have been mainly attributed to the reactivities of HOCl and HOBr, generated during the reaction of Cl2 and Br2 with water as detailed in the introduction. HOCl and HOBr, like Cl2 and Br2, are strong oxidants and electrophiles. HOCl is a stronger oxidant and a weaker electrophile, while Br2 is a stronger electrophile and a weaker oxidant of the two, which is possibly behind differences in their toxic effects (85). HCl and HBr are neutralized by bicarbonate in the epithelial lining fluid (ELF) (86, 87) and probably do not play a major role in halogen toxicity. Prior studies illustrate that Cl2 gas is more toxic to the lung than aerosolized HCl (65). However, aspiration of the stomach contents or instillation of large quantities of HCl at pH <2 are known to damage the blood gas barrier and result in acute and chronic lung injury via neutrophil dependent mechanisms (24, 88, 89).
Based on the reaction rate constants and the large concentrations of ascorbate and reduced glutathione in the ELF, Cl2 and Br2 will react with ascorbate and reduced glutathione, which exist in mM concentrations in the ELF, before being converted to HOCl (48, 90). Exposure of rats to Cl2 decreases ascorbate in bronchoalveolar lavage fluid and lung tissues (49, 91); furthermore, aerosolized and intramuscular injections of ascorbate and deferoxamine, an iron chelator, in rats and mice post-Cl2 exposure replenished lung ascorbate stores and decreased lipid peroxidation, airway hyperresponsiveness inflammation, the accumulation of plasma proteins in the alveolar space, and airway thickening and hyperplasia, and mortality (41, 49, 59) (FIGURE 3). Other antioxidants such as dimethylthiourea (92) and N-acetylcysteine (NAC) (93) have also shown some effectiveness in reducing various indices of lung injury (but not mortality) when administered in mice either pre or post-Cl2 exposure. NAC also potentiated the effects of corticosteroids (94) as well as ascorbate and deferoxamine (49) in decreasing Cl2 toxicity. In addition, AEOL 10150, a compound that scavenges peroxynitrite, inhibits lipid peroxidation, and has superoxide dismutase and catalase-like activities, diminished airway hyperresponsiveness and lung injury in Cl2-exposed mice (63). The results of various countermeasures in mitigating the extent of halogen injury to adult and newborn animals are shown in Table 1 and FIGURE 3.
Table 1.
Mitigation of Cl2 and Br2 injury in animals by various classes of compounds
| Target Pathway(s)/Agents | Reference No. | Species | Halogen | Variables Affected |
|---|---|---|---|---|
| Antioxidants | ||||
| N-acetylcysteine | (93) | Isolated perfused lungs; rats | Cl2 | Cytokines, airway contraction. permeability |
| Dimethylthiourea | (92) | Mice | Cl2 | Permeability. lipid peroxidation |
| AEOL 10150 | (63) | Mice | Cl2 | Airway hyperreactivity. Permeability. inflammatory cells |
| Ascorbic acid plus deferoxamine | (41, 59) | Mice | Cl2 | Survival, permeability. airway hyperplasia. inflammatory cells |
| Anti-inflammatory | ||||
| Corticosteroids | (95) | Pigs | Cl2 | Airway pressures. arterial blood gases. lung wet/dry weights |
| cAMP modulation | ||||
| Forskolin | (96) | Mice | Cl2 | Na+ channel activation |
| Rolipram | (50) | Mice | Cl2 | Pulmonary edema, airway hyperreactivity |
| β2-Agonists | (57) | Mice | Cl2 | Airway hyperreactivity, inflammation, cytokines |
| NO modulation | ||||
| Nitrite | (42, 80, 97) | Rats, mice, rabbits | Cl2 | Permeability, inflammation, survival, inflammatory cell accumulation, airway reactivity, vessel dilation |
| Heme and coagulation | ||||
| Aerosolized heparin | (44) | Mice | Cl2 | Coagulation, permeability, inflammatory cells |
| Hemopexin | (68, 74) | Mice | Cl2 and Br2 | Permeability, inflammatory cells, airway hyperreactivity, survival, Na+ channels, wet/dry weights, pulmonary emphysema, fibrosis |
| TRP ion channels | ||||
| TRPV4 inhibitors | Mice | Cl2, HCl | Pulmonary edema, oxygen saturation, inflammatory cells, cytokines, permeability | |
| SERCA activators | ||||
| Ranolazine | (98) | Rats, cardiomyocytes | Cl2 | Prevented cardiomyocyte death in rats, prevented mitochondrial injury in cells |
| Hyaluronan | ||||
| Yabro | (58, 99) | Mice, human cells | Cl2 and Br2 | Airway hyperreactivity, permeability, inflammatory cells, membrane potential, RhoA, calcium sensor |
| Type 5-phosphodiesterase inhibitors | ||||
| Tadalafil | (5, 55) | Pregnant mice | Br2 | Increased survival, fetal growth, systemic blood pressure, inflammation, decreased placental injury |
| VEGF | ||||
| VEGF-121 | (83) | Pregnant mice | Br2 | Lung permeability, lung wet/dry weights, survival, decreased placental injury, fetal growth development |
NO, nitric oxide; TRP, transient receptor potential; SERCA, sarcoendoplasmic Ca2+ ATPase.
Important targets of Cl2, HOCl, and OCl− include proteins and amino acids like cysteine (100, 101), methionine, side-chain amino groups in amino acids, terminal amino groups (creating corresponding chloramines) (102), and aromatic amino acids like tyrosine (creating 3-chlorotyrosine) (103–105). Chloramines are intrinsically toxic and cause injury directly as well as synergizing to exacerbate the Cl2/HOCl/OCl− damage (47, 98, 106–109). HOCl activates the mitogen-activated protein kinase pathway, and the cell-permeable glycine and taurine chloramines regulate NF-κB activity through oxidation of IκBa (110, 111). The consequent release of chemokines and cytokines by inflammatory cells stimulates the secondary release of millimolar concentrations of HOCl, generated through catalytic activity of eosinophil and neutrophil-derived peroxidases with Cl− and H2O2 functioning as substrates (112). HOCl also damages DNA and impairs poly-ADP-ribose polymerase, a DNA-repair enzyme (113, 114), thus impairing the repair of damaged DNA.
Contributing to the overall pro-oxidant environment, exposure of mice to Cl2 gas upregulates the inducible form of nitric oxide synthase (iNOS) in alveolar epithelial cells and macrophages (65). However, as mentioned previously, NO derived from iNOS may be essential in maintaining blood pressure following Cl2 induced injury to eNOS. In addition, one study reported that exposure of iNOS(−/−) mice to Cl2 does not blunt the hyperresponsiveness of airway resistance to methacholine as compared with wild type mice (57).
Pulmonary surfactant, consisting of lipids and four specific protein (SP-A, B, C, and D), is a critical Cl2 target. Higher minimum surface tensions of concentrated bronchoalveolar lavage fluid were measured post-Cl2 exposure in rats (49), although levels of surfactant lipids were normal. These findings are consistent with injury to the lipid soluble surfactant apoproteins SP-B and SP-C, which are responsible for interacting with surfactant lipids to generate a minimum surface tension while surface area decreases, thus preventing alveolar collapse. Damage to SP-B and SP-C has been reported following exposure of rabbits to hyperoxia (115, 116) and other oxidants (116) as well as exposure of surfactant mixtures containing surfactant lipids and SP-B and SP-C to peroxynitrite (117), HOCl, and Fenton reagents (118). Increased levels of plasma proteins in the ELF due to increased permeability of the blood gas barrier following exposure to halogens (2, 48, 49, 68, 79) may further interfere with the ability of pulmonary surfactant to reach a minimum surface tension (53, 119). Damage to the pulmonary surfactant system will result in hypoxemia and may contribute to the development of ARDS (116). Exposure of surfactant protein A (SP-A) to HOCl or peroxynitrite led to oxidation, nitration, and chlorination of amino acids found within the carbohydrate recognition domain, impairing mannose residue binding, a step required for sequestration and elimination of pathogens (69, 120, 121). Surfactant protein D (SP-D), like SP-A, is a lectin with a variety of important roles in the innate immune response and maintenance of physiology respiratory homeostasis. Exposing SP-D to HOCl prevented pathogen aggregation and led to generation of abnormal disulfide cross-linked oligomers (122, 123). Therefore, it is reasonable to conclude that after Cl2 exposure, mammals are at increased risk of bacterial pneumonia due to a compromised innate immune response.
Active transport of Na+ ions down the electrochemical gradient generated by the Na/K/ATPase from the epithelial lining fluid to the interstitium is an important function of a normal functioning alveolar epithelium (124–126). Sodium (Na+) ions enter epithelial cells through amiloride-sensitive (ENaC) or cation channels located in their apical membranes and are extruded into the interstitium by the basolateral Na/K/ATPase. To maintain electroneutrality, Cl− ions passively move across paracellular junctions of alveolar epithelial cells or transcellularly. Vectorial ionic movement of Na+ and Cl− ions is followed passively by fluid from the airspace to the interstitium [the physiological concept of airspace fluid clearance (AFC)]. This is necessary to allow the reabsorption of pulmonary edema fluid (reviewed in Refs. 126, 127). Patients with acute lung injury with intact AFC have lower mortality and lower hospital stays as compared with those with compromised AFC (128, 129).
A variety of studies have shown that halogens damage the activity of amiloride-sensitive Na+ channels either directly or by upregulation of signal transduction pathways. Direct recordings of single Na+ channels activity in alveolar type I and type II cells in the lungs of mice at 1 and 24 h post-exposure to either Cl2 or Br2 in their native environment (lung slices) showed significant injury to ENaC (74, 96), as evidenced by decreased single channel activity. Exposure of alveolar type II (ATII) cell monolayers to Cl2 increased levels of reactive intermediates, leading to ERK1/2 phosphorylation and decreased apical ENaC and transepithelial Na+ transport; these changes were prevented and reversed by inhibitors of ERK1/2 and of the proteasomes and lysosome systems (96). Chloramines formed by the reactions of HOCl with taurine inhibited ENaC activity across Xenopus oocytes injected with the three ENaC subunits (47). These data indicate that halogens inhibit ENaC and may contribute to the development of pulmonary edema seen posthalogen exposure. Agents that upregulate cAMP levels, such as rolipram (50), β-agonists (57), forskolin, and cAMP (47, 96), increase Na+ transport, decreased pulmonary edema in Cl2-exposed mice by increasing ENaC levels and activity. Agents that increase cAMP also improve airway hyperresponsiveness by relaxing airway smooth muscles. These finding explain the beneficial role of β-agonists in persons exposed to halogens.
Reactive Species: Mitochondrial Bioenergetic Dysfunction
Mitochondria are key regulators of cell survival in response to stress, and this has stimulated the development of mitochondrially targeted therapies (130–132). The exposure of cells to reactive oxygen species (ROS) generated during inflammation decrease mitochondrial quality, as evidenced by damage to the respiratory chain, damage to mitochondrial DNA, and increased generation of ROS (133–138). The continual generation of reactive oxygen species by the respiratory chain may promote proinflammatory signaling and thus prevent cellular recovery (134, 139, 140) along with activating a number of danger signals, which play a critical pathogenic role by activating downstream inflammatory cascades. Progressive injury to mitochondria results in the opening of the mitochondrial permeability transition pore, release of cytochrome c, and necrotic or apoptotic cell death (141–143). Maintaining mitochondrial quality control through selective removal of damaged, ROS-generating mitochondria, is an important property of macroautophagy known as mitophagy (130, 144). Proteins or organelles modified by reactive species are targeted for removal by the lysosomal-autophagy system (130, 145, 146).
In vitro studies showed that exposure of human club cell-like lung epithelial cells (H441) to Cl2 gas decreased their maximal mitochondrial oxygen consumption rate (OCR) (43) and their bioenergetic reserve capacity, an index of cellular energy for repair and resistance to oxidative damage (138). Cl2 also increased nonmitochondrial OCR, which represents an increased capacity to generate reactive oxygen species (43). Along these lines, Cl2 increased superoxide production in the mitochondria of H441 cells (43) (FIGURE 5) and in primary cultures of alveolar type II cells (96) for up to 12 h post-exposure. Treatment of Cl2-exposed H441 cells with MitoQ, an antioxidant targeted to the mitochondria), can attenuate the Cl2-dependent reduction in maximal OCR (43). Cl2 also reduced the mitochondrial membrane potential, and this effect was similarly attenuated following MitoQ pretreatment (43). Cl2 significantly decreased the maximum extracellular acidification rate, which suggests a Cl2-dependant impairment in glycolysis (44). Cl2 specifically inhibits complex I and II of the mitochondrial electron transport chain (43). Increased reactive oxygen species were detected in lungs of Cl2- and Br2-exposed mice as shown by the presence of products of malondialdehyde adducts and free heme (22, 68, 74). In addition, F2a-isoprostanes (prostaglandin F2-like compounds derived from the nonenzymatic oxidation of arachidonic acid by chloramines or HOCl) were detected in lung tissue of rats exposed to Cl2 (48).
FIGURE 5.
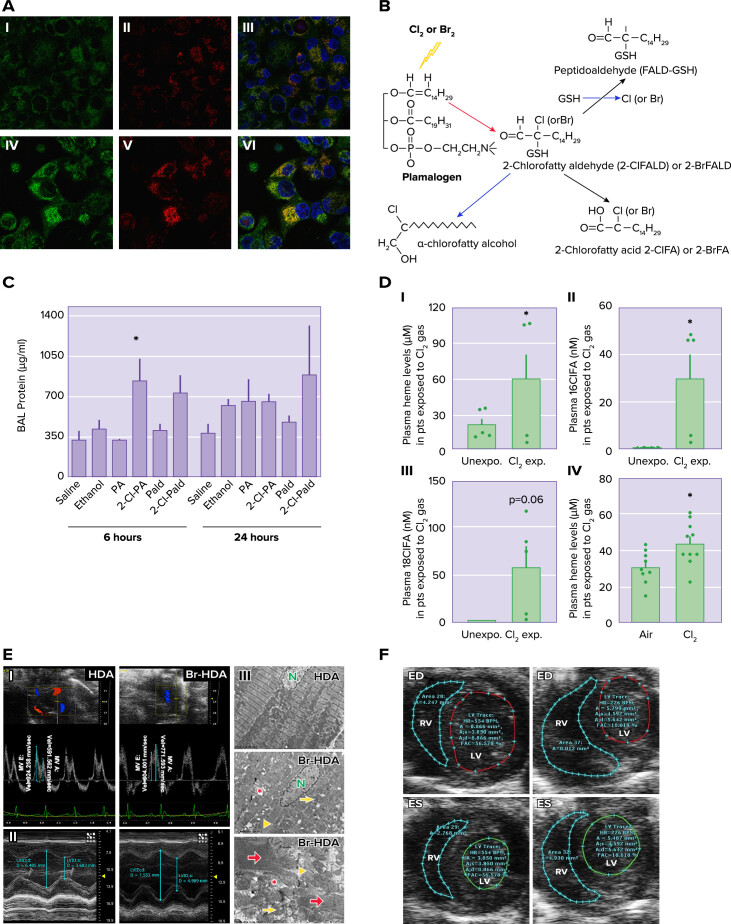
Production of secondary mediators A: exposure to Cl2 increases mitochondrial superoxide/hydrogen peroxide. I–VI: H441 cells immersed in 50 µl of artificial epithelial lining fluid were exposed to 95% air-5% CO2 as control (I–III) or Cl2 (100 ppm for 15 min) (IV–VI) and returned to 95% air-5% CO2 for 1 h. Before imaging with confocal laser microscopy, the cells were incubated with MitoSOX (red) for 15 min and MitoTracker (green). II and V: most of the Cl2-exposed H441 cells showed higher levels of red fluorescence compared with controls. VI: MitoSOX fluorescence localized with MitoTracker (yellow), consistent with the presence of reactive species in mitochondria. Nuclei were counterstained with DAPI (blue). Quantitative analysis showed increased production of reactive intermediates at 6 h post-exposure (modified from Ref. 43, with permission from the publisher). B: plasmalogen-derived Cl2 and Br2 oxidation products. The vinyl ether bond of plasmalogens is targeted by Cl2, Br2, HOCl, and HOBr resulting in 2-chlorofatty(bromo)aldehyde production including 2-Cl(Br)-Pald and 2-Cl(Br)-Sald. The 2-chloro(bromo)fatty aldehydes are either oxidized to the 2-chloro(bromo)fatty acids and 2-Cl(Br)-PA and 2-Cl(Br)l-SA or reduced to the 2-chloro(bromo)fatty alcohols, 2-chloro(bromo)palmitoyl alcohol, and 2-chloro(bromo)stearoyl alcohol. Alternatively, nucleophilic attack of 2-chloro(bromo)fatty aldehydes by GSH results in either palmitaldehyde or stearaldehyde GSH adduct formation. R1 = C14H29 or C16H33 (modified from Ref. 147). C: effects of Cl-lipids on lung permeability and inflammation. C57bl/6 male mice were exposed to saline, ethanol, palmitate (PA), palmitic aldehyde (Pald), 2-Cl-PA, or 2-Cl-Pald by intranasal administration and lung injury was assessed by measuring total protein in the bronchoalveolar lavage levels of protein. Data are means ± SE (n = 4–6). *P < 0.05 relative to corresponding native fatty acid (from Ref. 147). BAL, bronchoalveolar lavage. D: plasma cell-free heme (CFH) and chlorinated lipids are elevated in humans and mice exposed to Cl2 gas. Blood was collected from patients (pts) exposed to Cl2 gas in the emergency room of the University of Alabama at Birmingham, 3–4 h post-exposure, stored for 72 h at 4°C at which time it was analyzed. Blood from human volunteers as controls was treated in the same fashion. I: plasma CFH levels in persons exposed to Cl2 were higher than the age- and sex-matched human controls. II and III: Cl2-exposed individuals also had elevated levels of 16ClFA (n = 4–5) (II) and 18ClFA (III). Similarly, adult male C57BL/6 mice exposed to Cl2 gas (400 ppm, 30 min) had increased levels of heme in plasma 24 h post-exposure. Individual values and means ± SE. *P < 0.05 vs. unexposed humans or air exposed mice; by unpaired t test (from Ref. 74). E: cardiac dysfunction and cardiomyocyte death with 2-bromohexadecanal (Br-HDA). I: left ventricular (LV) diastolic dysfunction was reflected by the decrease in mitral valve (MV) early and late wave velocities 4 h after injection of Br-HDA into the LV cavity. II: Br-HDA caused an increase in LV end-diastolic dimension and end-systolic dimension, resulting in a decrease in fractional shortening. Image demonstrates M-mode echocardiography of the LV before and 4 h after intracardiac injection of Br-HDA. III: Br-HDA caused extensive disruption of the cardiac cytoskeleton and loss of the normal highly organized linear mitochondrial sarcomere integrity. Transmission electron microscopy (×3,200 at top and middle and ×8,000 at bottom) demonstrated contraction band necrosis (red arrows), loss of I bands, and disruption of z-disk (yellow arrowheads) in the LV of rats administered Br-HDA as well as mitochondrial swelling (yellow arrows) and cristae lysis (red asterisk) (from Ref. 54). F: Br2-exposed pregnant mice exhibit diminished cardiac function at gestational day 18.5 (E18.5). Nonpregnant and pregnant (E14.5) mice were exposed to air or 600 ppm Br2 for 30 mi and returned to room air. Representative echosonography left ventricular (LV) and right ventricular (RV) traces at E18.5 of pregnant mice exposed to air (left) or Br2 with demarcation of ventricular sizes (ED, end diastole; ES, end systole) (from Ref. 55).
Providing further evidence for mitochondrial injury of H441 cells by exposure to Cl2, upregulation of autophagy by pretreatment with trehalose (which activates autophagy) improved bioenergetics function. Conversely, the administration of the autophagy inhibitor 3-methyladenine exacerbated the deterioration of bioenergetics after Cl2 exposure. Critically, treatment of mice with trehalose decreased some manifestations of Cl2-induced lung injury (43).
Injured epithelial cells produce mediators including reactive oxygen species, cytokines, and platelet-activating factor leading to recruitment of inflammatory cells (mainly neutrophils). Significant increases in a number of cytokines (such as TNFa, IL6, and KC) were observed in the plasma and lung tissues post-Cl2 and -Br2 exposures (57, 79). These cytokines stimulate release of arachidonic acid and result in eicosanoid production, further stimulating the secretion of mucus and exacerbating inflammation.
Mitochondrial injury may also mediate Cl2-induced cardiotoxicity. As briefly mentioned previously, rats exposed to Cl2 showed a decrease in ATP content in primary cardiomyocytes along with an increase in lactate within the coronary sinus indicative of increased myocardial anaerobic metabolism (98). Sedlic et al. (148) attributed this impaired mitochondrial function to Cl2 reactants in the circulation as well as chlorination causing inactivation of cardiac sarcoendoplasmic Ca2+ ATPase (SERCA) with a corresponding cytosolic Ca2 + overload. Increased cytosolic Ca2+ leads to a concurrent increase in the mitochondrial production of reactive oxygen species. Additionally, reactive oxygen species in turn cause disturbances in cytosolic Ca2+ homeostasis and may contribute to myocardial dysfunction by bromine (149). SERCA regulates Ca2+ homeostasis in the heart through transport of cytosolic Ca2+ into the sarcoendoplasmic reticulum (lowering intracellular Ca2+). SERCA activity is impaired by HOCL (98, 150). Notably, ranolazine (a SERCA stabilizer) and istaroxime (a SERCA activator) preserve ATP levels, preserve the membrane potential across the mitochondria, and, importantly, can prevent Cl2-induced cardiomyocyte death after Cl2 exposure (98). Similar results were reported following exposure of rats to Br2 (FIGURE 1) (54).
Formation of Secondary Mediators: Halogenated Lipids
Plasmalogens are important components of plasma membrane phospholipids in mammals (151). They facilitate solvation of transmembrane ion channels and transport proteins and storage of arachidonic acid (152). A number of studies have shown that Cl2 and Br2 (along with the hydration products HOCl and HOBr) react with lung plasmalogens to form halogenated lipid aldehydes (147, 153). Major molecular species of the reactions include 2-(chloro)bromopalmitaldehyde (2-XPALD) and 2-(chloro)bromostearaldehyde (2-XrSALD), which are either reduced to alcohol or oxidized to 2-(chloro)bromomopalmitic acid (2-XPA) and 2-chloro(bromo)stearic acid (2-XSA), respectively (FIGURE 5). The fatty acids exist either in the esterified or free forms (154). 2-BrPA is a potent inhibitor of mitochondrial fatty acid oxidation (155) and of protein palmitoylation (156). Endogenously generated, myeloperoxidase-derived α-ClFALD has also been implicated in eNOS inhibition (157), neutrophil chemotaxis (158), and myocardial contractile dysfunction (159). Thus α-ClFALD may be responsible for the vascular eNOS inhibition reported in mice post-Cl2 (45, 46) exposure. Decreased NO bioavailability was also reported in pregnant mice exposed to Br2, which contributed to the subsequent development of a preeclamptic-like syndrome and higher incidence of fetal demise (5, 55, 83). Furthermore, incubation of lung cells with brominated lipids (fatty acids and aldehydes) resulted in activation of RhoA and its downstream kinase Rho-associated kinase 2 (ROCK-2), coinciding with significant increases in permeability as detected by measuring significant decreases of electrical resistance. The decreased barrier function was accompanied by formation of actin stress fibers, phosphorylation and internalization of VE-cadherin as well as disruption of ZO-1 localization (84). Hartman et al. (160) also reported that a synthetic analogue of chlorofatty acids localized in the Weibel-Palade bodies of human endothelial cells resulting in the release of P-selectin, von Willebrand factor, and angiopoietin-2 from endothelial cells . Chlorinated fatty acids also lead to platelet aggregation due to neutrophil adherence and contribute to an increase in endothelial permeability, a phenomenon related to the release of angiopoietin-2 (160).
Chlorinated and brominated fatty acids and aldehydes were detected in large quantities in the plasma, lung tissues, and bronchoalveolar lavage of mice and rats up to 24–48 h post-Cl2 and -Br2 exposure (79, 161) in the left ventricular tissues of rats exposed to Br2 (54), as well as in the plasma of humans at 6 h post-exposure to Cl2 (74) (FIGURE 5). Intranasal administration of 2-Cl-PA or 2-Cl-PALD at doses like those formed in the lung after Cl2 gas exposure led to increased permeability of lung epithelial cells to plasma proteins, neutrophil influx, and systemic endothelial dysfunction characterized by loss of eNOS-dependent vasodilation (FIGURE 5) (147). Furthermore, injection of brominated aldehydes into the left ventricular cavity of an air breathing rats caused acute left ventricular enlargement with extensive disruption of the sarcomeric architecture and mitochondrial damage (54). These findings suggest that the halogen-induced mitochondrial injury may be mediated at least in part by halogenated lipids (FIGURE 5).
Chlorinated lipids have also been detected in the lung of patients with ARDS secondary to sepsis (162) and their levels correlated with the severity of clinical symptoms. In this case, HOCl originated from the action of neutrophil myeloperoxidase on H2O2 and chloride ions. However, the concentrations measured in persons exposed to Cl2 (74) were a least 100-fold higher than what has been reported in patients with sepsis (162). Overall, these findings indicate that halogenated fatty acids may be both biomarkers of and contributing to the pathogenesis of halogen cardiopulmonary injury.
In addition to lung and cardiac cells, halogenated fatty acids are known to damage red blood cells, causing the release of free heme in the plasma. Heme is an essential functional group of many intracellular proteins. However, nonencapsulated cell-free heme (CFH), a breakdown component of proteins such as hemoglobin, myoglobin, cytochromes, is a significant source of reactive species, impairs cellular integrity (163), and is implicated in the pathogenesis of several disorders (164–170). CFH is an abundant source of redox-active iron capable of damaging lipids, proteins, and DNA (171) and causing cell necrosis (172). Under normal conditions, circulating CFH is maintained at low levels by serum albumin, haptoglobin, and most importantly hemopexin (173–175). Significant concentrations of nonencapsulated free heme are present in the plasma, lung tissue, and bronchoalveolar lavage of mice post-Cl2 and -Br2 exposure (68, 74, 79) (FIGURE 5). In addition, free heme was detected in the plasma of humans exposed to Cl2 gas in a recent incident in Birmingham, AL at 6 h post-exposure (74) (FIGURE 5).
As mentioned above, it is highly unlikely that inhaled Cl2 or Br2 will be present in the plasma. This has led investigators to question whether chlorinated lipids are responsible for red blood cell (RBC) injury. Ex vivo incubation of RBC with chlorinated and brominated lipids followed by mechanical agitation resulted in the release of free heme (74). There was significant carbonylation of RBC membranes following incubation with halogenated lipids, as shown by oxyblot analysis (68, 74, 84). Studies of RBCs isolated from mice 24 h postbromine exposure identified six putative carbonylation sites in lysines within the spectrin α-chain and one site in the β-spectrin, while Cl2 caused carbonylation only in the α-spectrin chain (74). Carbonylation of spectrin may form aggregates, which may destabilize the RBC membrane rendering RBCs more susceptible to mechanical stress as they cross capillaries (176). In a positive feedback loop, free heme can destabilize the RBC membrane by altering the conformation of cytoskeletal proteins, such as spectrin, protein 4.1 (177), and other cytoskeletal proteins (178, 179).
Two lines of evidence point to the potential importance of heme in the pathophysiology of halogen toxicity. First, mice overexpressing heme oxygenase-1 (HO-1), which catalyzes the first and rate-limiting step in heme degradation into equimolar amounts of iron, carbon monoxide (CO), and biliverdin, exhibit decreased mortality and lung injury when exposed to Br2, while mice lacking HO-1 are highly susceptible to exposures to both Cl2 and Br2 (79). Second, injection of hemopexin, an endogenous plasma protein with a high affinity for heme, decreased lung heme levels and lung oxidative stress, ameliorating the associated tissue damage and respiratory dysfunction after both Br2 and Cl2 inhalation as well as the development of pulmonary fibrosis and emphysema, and prolonged survival posthalogen exposure (68, 74) (FIGURE 3).
Low-Molecular Weight Hyaluronan
Increased airway resistance and airway hyperresponsiveness (AHR) are important pathological events following exposure to Cl2 and Br2, and may result in persistent asthma-like symptoms and exacerbation of allergic airway inflammation (56, 57, 65), which may progress to lung fibrosis (60, 61, 68). Below we discuss some findings indicating that low-molecular-weight hyaluronan (LMW-HA), generated by the action of halogens, on high-molecular-weight hyaluronan (HMW-HA) may be an important mediator of halogen toxicity.
HMW-HA, a major structural component of the extracellular matrix, promotes cell survival and has antiangiogenic properties and anti-inflammatory effects on immune cells mediated by binding to its membrane receptors, CD44 TLR2, and TLR4 (180–183). On the contrary, the binding of LMW-HA (L-HA ∼300 kDa) fragments to the same receptors act as endogenous innate immune ligands and promote inflammatory responses, angiogenesis, and epithelial to mesenchymal transition (180, 181, 184, 185). LMW-HA fragments stimulate cytokine production and activate the innate immune response via binding to CD44 and Toll-like receptor (TLR) signaling in an MyD-88- and NF-κB-dependent fashion, whereas HMW-HA inhibits TLR-2 signaling in vitro and in vivo (185). Binding of either HMW-HA or LMW-HA to CD44 is enhanced by the inter-α-inhibitor (IαI), a serum protease inhibitor consisting of three polypeptides (180, 186–188). LMW-HA is both necessary and sufficient for the development of AHR after exposure to ozone (180, 189) and ischemia-reperfusion (190) among other injury patterns. LMW-HA leads to an increase in permeability through activation of RhoA and ROCK, triggering cytoskeletal reorganization, and through inhibition of cell-cell junctional integrity (191). Conversely, the administration of HMW-HA is protective in injury models including ozone exposure (189), bleomycin administration (192), smoke inhalation, and sepsis (193, 194).
In a series of experiments, Lazrak et al. (99) showed the presence of LMW-HA in the bronchoalveolar lavage fluid and the peribronchial spaces of C57BL/6 mice exposed to Cl2 for up to 24 h post-exposure. The increase in LMW-HA was accompanied by an increase in hyalruonan synthases expression and presence of hyaluronidases. LMW-HA was also increased in bronchoalveolar lavage fluid of mice exposed to Br2 (58). Instilling HMW-HA in the nares posthalogen-exposure decreased AHR (58, 99). Cl2-induced AHR was mitigated by instillation of an antibody against the inter-α-trypsin inhibitor (IαI), which inhibited HA signaling (99). Mice infected with respiratory syncytial virus (RSV) develop an increased sensitivity to Cl2, and this sensitivity was attenuated by high molecular weight hyaluronan (15).
In vitro studies demonstrated that exposure of human airway smooth muscle to Cl2, Br2, or to LMW-HA increased intracellular Ca2+ levels and RhoA activity. This resulted in activation of the RhoA downstream kinase ROCK2 and activation of TMEM16, a Ca2+-activated Cl− channel, resulting in membrane depolarization (58). Post-exposure administration of HMW-HA reversed these sequelae. Activation of RhoA leads to AHR, alters airway smooth muscle contractility, and leads to an increase in pulmonary vascular permeability (195–197). In subsequent experiments, it was shown that LMW-HA increased the expression of calcium-sensing receptor (Ca-SR) 24 h post-Cl2 or -Br2 (58). The effect of LMW-HA on Ca-SR was reversed when cells were treated with HMW-HA, added after the incubation with LMW-HA. Instillation of Ca-SR inhibitor (calcilytic) NPS2143, in the external nares of mice at 1 h and 23 h post-Br2 exposure, resulted in normal airway responsiveness to methacholine (58). HMW-HA reversed the LMW-HA Ca-SR overexpression by either inhibiting downstream signaling cascades or by displacing LMW-HA from its receptors. The mechanisms involved are currently under investigation.
HMW-HA may be fragmented to LMW-HA by a variety of mechanisms. Reactive oxygen species (ROS), including superoxide, hydrogen peroxide, nitric oxide and peroxynitrite, hypochlorous, and hypobromous acids, are known to degrade HMW-HA (88, 99, 198–200). Inhaled ozone and Cl2 can fragment HMW-HA, while neutralization of ROS, through superoxide dismutase or its mimetics, decreases HA degradation and resulting inflammation (201, 202). Whether halogenated lipids or nonencapsulated heme contributes to the fragmentation of hyaluronan has not been determined.
While generally the impact of the halogens is remarkably similar, it is interesting to note that although Cl2 and Br2 are both halogens, there are important differences in their physical and chemical properties and reactivity with biomolecules. To name just a few: 1) Br2 is almost 10 times more soluble than Cl2 and thus likely to react more readily on biological targets in upper airways; 2) chlorination of the plasmalogen vinyl ether bond occurs at acidic pH (203), while bromination occurs at neutral pH (204); 3) 2-bromofatty acids have a reduced charge in their carboxyl group compared with 2-chlorofatty acid due to the disparate electron withdrawing properties of these two halogens; and 4) while Br2 exposure increases LMW-HA along with AHR through similar mechanisms as Cl2 (119), the AHR observed after Cl2 exposure is due to an increase in Newtonian resistance (large airway resistance) whereas the AHR seen after Br2 exposure is related to tissue viscoelasticity and resistance (possibly related to small airway resistance) (58).
Toxic Effects on the Parturient
A detailed review of the impact of halogen gas exposure on the parturient was recently published (5). Vulnerable populations may demonstrate unique and specific sequelae after exposure to halogen gas. Up to 5% of women of reproductive age in the United States are pregnant or within 6 wk postpartum (205). Physiologic maternal adaptations begin early in pregnancy to prime the cardiopulmonary system for the demands of carrying a fetus to term, laboring, and the immediate postpartum period. These changes may create a milieu more susceptible to certain cardiopulmonary toxins. Fortunately, halogen exposure during pregnancy is rare; thus animal models are relied on to explore and understand these differential risks and sequelae unique to the parturient. Exposure of pregnant mice to Br2 leads to a preeclamptic-like syndrome characterized by increased systemic and pulmonary pressures, endothelial dysfunction, a decrease in cardiac output, placental disruption, an increase in inflammatory cytokines, and fetal growth restriction (5, 55, 83) (FIGURES 1 AND 5). Exposed pregnant mice demonstrate an increase in the level of soluble fms-like tyrosine kinase 1 (sFlt-1) over time that corresponds to worsening pulmonary edema. Notably, pregnant halogen-exposed mice respond favorably to treatment with exogenous vascular endothelial growth factor (VEGF) and tadalafil (a phosphodiesterase-5 specific inhibitor) with increased maternal survival (FIGURE 3) and attenuation of pulmonary and cardiac injury (5, 55, 83). Neither exogenous VEGF nor tadalafil is efficacious in reducing injury in nonpregnant mice indicating that this is a unique phenomenon to pregnancy. The precise mechanisms by which halogen inhalation leads to placental hypoperfusion and injury, causes alterations in the ratio of sFlt-1:VEGFR1, and culminates in fetal demise have not been identified. Halogenated lipids, nonencapsulated heme, as well as the inflammatory response likely all contribute to this injury.
Halogens Impair Newborn Lung Development
Damage to the developing lung can lead to impaired development and permanent adverse pulmonary remodeling. In some cases, this could lead to early mortality or a lifelong increased risk of respiratory disease(s). In the single study in the literature, Jilling et al. (206) reported that exposure of newborn mice to bromine for 30 min at postnatal day 3 had impaired alveolar development characterized by alveolar simplification, decreased lung compliance, hypoxemia, and increased mRNAs of inflammatory cytokines in lung tissues. In addition, gene expression analysis of lung tissues revealed persistent abnormalities in gene expression profiles in genes involved in pulmonary development. Clearly, additional studies are needed to understand the mechanisms involved.
Conclusions
Exposure of humans and animals to the halogens Cl2 and Br2 damages the cardiopulmonary system and may, in severe cases, cause death from cardiopulmonary failure. Survivors may develop significant sequalae including pulmonary and cardiac fibrosis, increased compliance and alveolar simplification, and airway hyperresponsiveness. Pregnant animals are particularly vulnerable to halogens and develop preeclampsia even after a short exposure to halogens. Fetuses are born stillborn or with severe growth restrictions while newborns exposed to halogens develop alveolar simplification and bronchopulmonary dysplasia type symptoms. The toxic effects of halogens are exerted by oxidizing and carbonylating targets in the epithelial lining fluid and lung epithelial cells or by generating reactive intermediates and reactive species capable of reaching distant targets (such as the vascular system, heart, and placenta). Although several countermeasures demonstrate significant promise in reversing cardiopulmonary injury in animals, none of them have been approved for human treatment. Since clinical trials cannot be conducted, additional work is needed to satisfy Food and Drug Administration requirements under the Animal Rule.
Acknowledgments
This work was supported by the CounterACT Program; National Institutes of Health Office of the Director; National Institute of Neurological Disorders and Stroke; National Institute of Environmental Health Sciences Grants 5UO1-ES-026458, 3UO1-ES-026458 03S1, and 5UO1-ES-027697) (to S. Matalon and T. Jilling); and National Heart, Lung, and Blood Institute Grant K12-HL-143958 (to S. Aggarwal). D. R. Addis is supported by a National Institutes of Health Ruth L. Kirschstein National Research Service Award T32-HL-129948.
No conflicts of interest, financial or otherwise, are declared by the authors.
D.R.A., S.A., A.L., and T.J. prepared figures; D.R.A., S.A., A.L., T.J., and S.M. drafted manuscript; D.R.A., S.A., A.L., and T.J. edited and revised manuscript; D.R.A., S.A., A.L., T.J., and S.M. approved final version of manuscript.
References
- 1.Summerhill EM, Hoyle GW, Jordt SE, Jugg BJ, Martin JG, Matalon S, Patterson SE, Prezant DJ, Sciuto AM, Svendsen ER, White CW, Veress LA, ATS Terrorism and Inhalational Disasters Section of the Environmental, Occupational, and Population Health Assembly. An Official American Thoracic Society Workshop Report: Chemical Inhalational Disasters. Biology of Lung Injury, Development of Novel Therapeutics, and Medical Preparedness. Ann Am Thorac Soc 14: 1060–1072, 2017. doi: 10.1513/AnnalsATS.201704-297WS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Carlisle M, Lam A, Svendsen ER, Aggarwal S, Matalon S. Chlorine-induced cardiopulmonary injury. Ann N Y Acad Sci 1374: 159–167, 2016. doi: 10.1111/nyas.13091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hoyle GW, Svendsen ER. Persistent effects of chlorine inhalation on respiratory health. Ann N Y Acad Sci 1378: 33–40, 2016. doi: 10.1111/nyas.13139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Clark KA, Karmaus WJ, Mohr LC, Cai B, Balte P, Gibson JJ, Ownby D, Lawson AB, Vena JE, Svendsen ER. Lung function before and after a large chlorine gas release in Graniteville, South Carolina. Ann Am Thorac Soc 13: 356–363, 2016. doi: 10.1513/AnnalsATS.201508-525OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Addis DR, Lambert JA, Ford DA, Jilling T, Matalon S. Halogen gas exposure: toxic effects on the parturient. Toxicol Mech Methods 31: 272–287, 2020. doi: 10.1080/15376516.2020.1736702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Evans RB. Chlorine: state of the art. Lung 183: 151–167, 2005. doi: 10.1007/s00408-004-2530-3. [DOI] [PubMed] [Google Scholar]
- 7.White CW, Martin JG. Chlorine gas inhalation: human clinical evidence of toxicity and experience in animal models. Proc Am Thorac Soc 7: 257–263, 2010. doi: 10.1513/pats.201001-008SM. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Firth PG. Of penguins, pinnipeds, and poisons: anesthesia on Elephant Island. Anesthesiology 125: 25–33, 2016. doi: 10.1097/ALN.0000000000001151. [DOI] [PubMed] [Google Scholar]
- 9.Cave D, Fadam A. Iraq insurgents employ chlorine in bomb attacks (Online). New York Times. 2007. https://www.nytimes.com/2007/02/22/world/middleeast/22iraq.html
- 10.Makarovsky I, Markel G, Hoffman A, Schein O, Brosh-Nissimov TM, Finkelstien A, Tashma Z, Dushnitsky T, Eisenkraft A. Bromine–the red cloud approaching. Isr Med Assoc J 9: 677–679, 2007. [PubMed] [Google Scholar]
- 11.Van Sickle D, Wenck MA, Belflower A, Drociuk D, Ferdinands J, Holguin F, Svendsen E, Bretous L, Jankelevich S, Gibson JJ, Garbe P, Moolenaar RL. Acute health effects after exposure to chlorine gas released after a train derailment. Am J Emerg Med 27: 1–7, 2009. doi: 10.1016/j.ajem.2007.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mackie E, Svendsen E, Grant S, Michels JE, Richardson WH. Management of chlorine gas-related injuries from the Graniteville, South Carolina, train derailment. Disaster Med Public Health Prep 8: 411–416, 2014. doi: 10.1017/dmp.2014.81. [DOI] [PubMed] [Google Scholar]
- 13.Cucinell SA. Review of the toxicity of long-term phosgene exposure. Arch Environ Health 28: 272–275, 1974. doi: 10.1080/00039896.1974.10666485. [DOI] [PubMed] [Google Scholar]
- 14.D'Alessandro A, Kuschner W, Wong H, Boushey HA, Blanc PD. Exaggerated responses to chlorine inhalation among persons with nonspecific airway hyperreactivity. Chest 109: 331–337, 1996. doi: 10.1378/chest.109.2.331. [DOI] [PubMed] [Google Scholar]
- 15.Song W, Yu Z, Doran SF, Ambalavanan N, Steele C, Garantziotis S, Matalon S. Respiratory syncytial virus infection increases chlorine-induced airway hyperresponsiveness. Am J Physiol Lung Cell Mol Physiol 309: L205–L210, 2015. doi: 10.1152/ajplung.00159.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Deschamps D, Soler P, Rosenberg N, Baud F, Gervais P. Persistent asthma after inhalation of a mixture of sodium hypochlorite and hydrochloric acid. Chest 105: 1895–1896, 1994. doi: 10.1378/chest.105.6.1895. [DOI] [PubMed] [Google Scholar]
- 17.Demeter SL, Cordasco EW. Reactive airway disease after chlorine gas exposure. Chest 102: 984, 1992. doi: 10.1378/chest.102.3.984b. [DOI] [PubMed] [Google Scholar]
- 18.Ginsberg JP, Holbrook JR, Chanda D, Bao H, Svendsen ER. Posttraumatic stress and tendency to panic in the aftermath of the chlorine gas disaster in Graniteville, South Carolina. Soc Psychiatry Psychiatr Epidemiol 47: 1441–1448, 2012. doi: 10.1007/s00127-011-0449-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zaky A, Ahmad A, Dell'Italia LJ, Jahromi L, Reisenberg LA, Matalon S, Ahmad S. Inhaled matters of the heart.Cardiovasc Regen Med 2: e997, 2015. doi: 10.14800/crm.997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Daugherty A, Dunn JL, Rateri DL, Heinecke JW. Myeloperoxidase, a catalyst for lipoprotein oxidation, is expressed in human atherosclerotic lesions. J Clin Invest 94: 437–444, 1994. doi: 10.1172/JCI117342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Podrez EA, Abu-Soud HM, Hazen SL. Myeloperoxidase-generated oxidants and atherosclerosis. Free Radic Biol Med 28: 1717–1725, 2000. doi: 10.1016/S0891-5849(00)00229-X. [DOI] [PubMed] [Google Scholar]
- 22.Lam A, Vetal N, Matalon S, Aggarwal S. Role of heme in bromine-induced lung injury. Ann N Y Acad Sci 1374: 105–110, 2016. doi: 10.1111/nyas.13086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sexton JD, Pronchik DJ. Chlorine inhalation: the big picture. J Toxicol Clin Toxicol 36: 87–93, 1998. doi: 10.3109/15563659809162593. [DOI] [PubMed] [Google Scholar]
- 24.Zhou T, Song WF, Shang Y, Yao SL, Matalon S. Halogen inhalation-induced lung injury and acute respiratory distress syndrome. Chin Med J (Engl) 131: 1214–1219, 2018. doi: 10.4103/0366-6999.231515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pascuzzi TA, Storrow AB. Mass casualties from acute inhalation of chloramine gas. Mil Med 163: 102–104, 1998. doi: 10.1093/milmed/163.2.102. [DOI] [PubMed] [Google Scholar]
- 26.Cheng YS, Bowen L, Rando RJ, Postlethwait EM, Squadrito GL, Matalon S. Exposing animals to oxidant gases: nose only vs. whole body. Proc Am Thorac Soc 7: 264–268, 2010. doi: 10.1513/pats.201001-001SM. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li C, Weng Z, Doran SF, Srivastava RK, Afaq F, Matalon S, Athar M. Chlorine induces the unfolded protein response in murine lungs and skin. Am J Respir Cell Mol Biol 49: 197–203, 2013. doi: 10.1165/rcmb.2012-0488RC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hoyle GW, Chang W, Chen J, Schlueter CF, Rando RJ. Deviations from Haber's Law for multiple measures of acute lung injury in chlorine-exposed mice. Toxicol Sci 118: 696–703, 2010. doi: 10.1093/toxsci/kfq264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Barrow CS, Alarie Y, Warrick JC, Stock MF. Comparison of the sensory irritation response in mice to chlorine and hydrogen chloride. Arch Environ Health 32: 68–76, 1977. doi: 10.1080/00039896.1977.10667258. [DOI] [PubMed] [Google Scholar]
- 30.Wolf DC, Morgan KT, Gross EA, Barrow C, Moss OR, James RA, Popp JA. Two-year inhalation exposure of female and male B6C3F1 mice and F344 rats to chlorine gas induces lesions confined to the nose. Fundam Appl Toxicol 24: 111–131, 1995. doi: 10.1093/toxsci/24.1.111. [DOI] [PubMed] [Google Scholar]
- 31.Klonne DR, Ulrich CE, Riley MG, Hamm TE Jr, Morgan KT, Barrow CS. One-year inhalation toxicity study of chlorine in rhesus monkeys (Macaca mulatta). Fundam Appl Toxicol 9: 557–572, 1987. doi: 10.1093/toxsci/9.3.557. [DOI] [PubMed] [Google Scholar]
- 32.Bessac BF, Jordt SE. Sensory irritation by toxic gases-mechanisms, health effects and countermeasures. Proc Am Thorac Soc 7: 269–277, 2010. doi: 10.1513/pats.201001-004SM. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bessac BF, Sivula M, von Hehn CA, Escalera J, Cohn L, Jordt SE. TRPA1 is a major oxidant sensor in murine airway sensory neurons. J Clin Invest 118: 1899–1910, 2008. doi: 10.1172/JCI34192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Escalera J, von Hehn CA, Bessac BF, Sivula M, Jordt SE. TRPA1 mediates the noxious effects of natural sesquiterpene deterrents. J Biol Chem 283: 24136–24144, 2008. doi: 10.1074/jbc.M710280200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Talavera K, Startek JB, Alvarez-Collazo J, Boonen B, Alpizar YA, Sanchez A, Naert R, Nilius B. Mammalian transient receptor potential TRPA1 channels: from structure to disease. Physiol Rev 100: 725–803, 2020. doi: 10.1152/physrev.00005.2019. [DOI] [PubMed] [Google Scholar]
- 36.Bessac BF, Sivula M, von Hehn CA, Caceres AI, Escalera J, Jordt SE. Transient receptor potential ankyrin 1 antagonists block the noxious effects of toxic industrial isocyanates and tear gases. FASEB J 23: 1102–1114, 2009. doi: 10.1096/fasebj.23.1_supplement.580.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bautista DM, Jordt SE, Nikai T, Tsuruda PR, Read AJ, Poblete J, Yamoah EN, Basbaum AI, Julius D. TRPA1 mediates the inflammatory actions of environmental irritants and proalgesic agents. Cell 124: 1269–1282, 2006. doi: 10.1016/j.cell.2006.02.023. [DOI] [PubMed] [Google Scholar]
- 38.Conklin DJ, Haberzettl P, Jagatheesan G, Kong M, Hoyle GW. Role of TRPA1 in acute cardiopulmonary toxicity of inhaled acrolein. Toxicol Appl Pharmacol 324: 61–72, 2017. doi: 10.1016/j.taap.2016.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Filippidis AS, Zarogiannis SG, Randich A, Ness TJ, Matalon S. Assessment of locomotion in chlorine exposed mice by computer vision and neural networks. J Appl Physiol (1985) 112: 1064–1072, 2012. doi: 10.1152/japplphysiol.01023.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jani DD, Reed D, Feigley CE, Svendsen ER. Modeling an irritant gas plume for epidemiologic study. Int J Environ Health Res 26: 58–74, 2016. doi: 10.1080/09603123.2015.1020414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zarogiannis SG, Jurkuvenaite A, Fernandez S, Doran SF, Yadav AK, Squadrito GL, Postlethwait EM, Bowen L, Matalon S. Ascorbate and deferoxamine administration after chlorine exposure decrease mortality and lung injury in mice. Am J Respir Cell Mol Biol 45: 386–392, 2011. doi: 10.1165/rcmb.2010-0432OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Honavar J, Doran S, Oh JY, Steele C, Matalon S, Patel RP. Nitrite therapy improves survival postexposure to chlorine gas. Am J Physiol Lung Cell Mol Physiol 307: L888–L894, 2014. doi: 10.1152/ajplung.00079.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jurkuvenaite A, Benavides GA, Komarova S, Doran SF, Johnson M, Aggarwal S, Zhang J, Darley-Usmar VM, Matalon S. Upregulation of autophagy decreases chlorine-induced mitochondrial injury and lung inflammation. Free Radic Biol Med 85: 83–94, 2015. doi: 10.1016/j.freeradbiomed.2015.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zarogiannis SG, Wagener BM, Basappa S, Doran S, Rodriguez CA, Jurkuvenaite A, Pittet JF, Matalon S. Postexposure aerosolized heparin reduces lung injury in chlorine-exposed mice. Am J Physiol Lung Cell Mol Physiol 307: L347–L354, 2014. doi: 10.1152/ajplung.00152.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Honavar J, Bradley E, Bradley K, Oh JY, Vallejo MO, Kelley EE, Cantu-Medellin N, Doran S, Dell’Italia LJ, Matalon S, Patel RP. Chlorine gas exposure disrupts nitric oxide homeostasis in the pulmonary vasculature. Toxicology 321: 96–102, 2014. doi: 10.1016/j.tox.2014.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Honavar J, Samal AA, Bradley KM, Brandon A, Balanay J, Squadrito GL, MohanKumar K, Maheshwari A, Postlethwait EM, Matalon S, Patel RP. Chlorine gas exposure causes systemic endothelial dysfunction by inhibiting endothelial nitric oxide synthase-dependent signaling. Am J Respir Cell Mol Biol 45: 419–425, 2011. doi: 10.1165/rcmb.2010-0151OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Song W, Wei S, Zhou Y, Lazrak A, Liu G, Londino JD, Squadrito GL, Matalon S. Inhibition of lung fluid clearance and epithelial Na+ channels by chlorine, hypochlorous acid, and chloramines. J Biol Chem 285: 9716–9728, 2010. doi: 10.1074/jbc.M109.073981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yadav AK, Bracher A, Doran SF, Leustik M, Squadrito GL, Postlethwait EM, Matalon S. Mechanisms and modification of chlorine-induced lung injury in animals. Proc Am Thorac Soc 7: 278–283, 2010. doi: 10.1513/pats.201001-009SM. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Leustik M, Doran S, Bracher A, Williams S, Squadrito GL, Schoeb TR, Postlethwait E, Matalon S. Mitigation of chlorine-induced lung injury by low-molecular-weight antioxidants. Am J Physiol Lung Cell Mol Physiol 295: L733–L743, 2008. doi: 10.1152/ajplung.90240.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chang W, Chen J, Schlueter CF, Rando RJ, Pathak YV, Hoyle GW. Inhibition of chlorine-induced lung injury by the type 4 phosphodiesterase inhibitor rolipram. Toxicol Appl Pharmacol 263: 251–258, 2012. doi: 10.1016/j.taap.2012.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hoyle GW. Mitigation of chlorine lung injury by increasing cyclic AMP levels. Proc Am Thorac Soc 7: 284–289, 2010. doi: 10.1513/pats.201001-002SM. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tian X, Tao H, Brisolara J, Chen J, Rando RJ, Hoyle GW. Acute lung injury induced by chlorine inhalation in C57BL/6 and FVB/N mice. Inhal Toxicol 20: 783–793, 2008. doi: 10.1080/08958370802007841. [DOI] [PubMed] [Google Scholar]
- 53.Massa CB, Scott P, Abramova E, Gardner C, Laskin DL, Gow AJ. Acute chlorine gas exposure produces transient inflammation and a progressive alteration in surfactant composition with accompanying mechanical dysfunction. Toxicol Appl Pharmacol 278: 53–64, 2014. doi: 10.1016/j.taap.2014.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ahmad S, Masjoan Juncos JX, Ahmad A, Zaky A, Wei CC, Bradley WE, Zafar I, Powell P, Mariappan N, Vetal N, Louch WE, Ford DA, Doran SF, Matalon S, Dell’Italia LJ. Bromine inhalation mimics ischemia-reperfusion cardiomyocyte injury and calpain activation in rats. Am J Physiol Heart Circ Physiol 316: H212–H223, 2019. doi: 10.1152/ajpheart.00652.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lambert JA, Carlisle MA, Lam A, Aggarwal S, Doran S, Ren C, Bradley WE, Dell'italia L, Ambalavanan N, Ford DA, Patel RP, Jilling T, Matalon S. Mechanisms and treatment of halogen inhalation-induced pulmonary and systemic injuries in pregnant mice. Hypertension 70: 390–400, 2017. doi: 10.1161/HYPERTENSIONAHA.117.09466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Balakrishna S, Song W, Achanta S, Doran SF, Liu B, Kaelberer MM, Yu Z, Sui A, Cheung M, Leishman E, Patel RP, Jilling T, Matalon S. TRPV4 inhibition counteracts edema and inflammation and improves pulmonary function and oxygen saturation in chemically induced acute lung injury. Am J Physiol Lung Cell Mol Physiol 307: L158–L172, 2014. doi: 10.1152/ajplung.00065.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Song W, Wei S, Liu G, Yu Z, Estell K, Yadav AK, Schwiebert LM, Matalon S. Postexposure administration of a beta2-agonist decreases chlorine-induced airway hyperreactivity in mice. Am J Respir Cell Mol Biol 45: 88–94, 2011. doi: 10.1165/rcmb.2010-0226OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lazrak A, Yu Z, Doran S, Jian MY, Creighton J, Laube M, Garantziotis S, Prakash YS, Matalon S. Upregulation of airway smooth muscle calcium-sensing receptor by low-molecular-weight hyaluronan. Am J Physiol Lung Cell Mol. Physiol 318: L459–L471, 2020. doi: 10.1152/ajplung.00429.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fanucchi MV, Bracher A, Doran SF, Squadrito GL, Fernandez S, Postlethwait EM, Bowen L, Matalon S. Post-exposure antioxidant treatment in rats decreases airway hyperplasia and hyperreactivity due to chlorine inhalation. Am J Respir Cell Mol Biol 46: 599–606, 2012. doi: 10.1165/rcmb.2011-0196OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mo Y, Chen J, Humphrey DM Jr, Fodah RA, Warawa JM, Hoyle GW. Abnormal epithelial structure and chronic lung inflammation after repair of chlorine-induced airway injury. Am J Physiol Lung Cell Mol Physiol 308: L168–L178, 2014. doi: 10.1152/ajplung.00226.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mo Y, Chen J, Schlueter CF, Hoyle GW. Differential susceptibility of inbred mouse strains to chlorine-induced airway fibrosis. Am J Physiol Lung Cell Mol Physiol 304: L92–L102, 2013.doi: 10.1152/ajplung.00272.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.McGovern TK, Goldberger M, Allard B, Farahnak S, Hamamoto Y, O'Sullivan M, Hirota N, Martel G, Rousseau S, Martin JG. Neutrophils mediate airway hyperresponsiveness following chlorine-induced airway injury in the mouse. Am. J Respir Cell Mol Biol 52: 513–522, 2015. doi: 10.1165/rcmb.2013-0430OC. [DOI] [PubMed] [Google Scholar]
- 63.McGovern T, Day BJ, White CW, Powell WS, Martin JG. AEOL10150: a novel therapeutic for rescue treatment after toxic gas lung injury. Free Radic Biol Med 50: 602–608, 2011. doi: 10.1016/j.freeradbiomed.2010.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tuck SA, Ramos-Barbon D, Campbell H, McGovern T, Karmouty-Quintana H, Martin JG. Time course of airway remodelling after an acute chlorine gas exposure in mice. Respir Res 9: 61, 2008. doi: 10.1186/1465-9921-9-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Martin JG, Campbell HR, Iijima H, Gautrin D, Malo JL, Eidelman DH, Hamid Q, Maghni K. Chlorine-induced injury to the airways in mice. Am J Respir Crit Care Med 168: 568–574, 2003. doi: 10.1164/rccm.200201-021OC. [DOI] [PubMed] [Google Scholar]
- 66.O'Koren EG, Hogan BL, Gunn MD. Loss of basal cells precedes bronchiolitis obliterans-like pathological changes in a murine model of chlorine gas inhalation. Am. J Respir Cell Mol Biol 49: 788–797, 2013. doi: 10.1165/rcmb.2012-0369OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Leikauf GD, Pope-Varsalona H, Concel VJ, Liu P, Bein K, Brant KA, Dopico RA, Di YP, Jang AS, Dietsch M, Medvedovic M, Li Q, Vuga LJ, Kaminski N, You M, Prows DR. Functional genomics of chlorine-induced acute lung injury in mice. Proc Am Thorac Soc 7: 294–296, 2010. doi: 10.1513/pats.201001-005SM. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Aggarwal S, Ahmad I, Lam A, Carlisle MA, Li C, Wells JM, Raju SV, Athar M, Rowe SM, Dransfield MT, Matalon S. Heme scavenging reduces pulmonary endoplasmic reticulum stress, fibrosis, and emphysema. JCI Insight 3: e120694, 2018. doi: 10.1172/jci.insight.120694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Davis IC, Zhu S, Sampson JB, Crow JP, Matalon S. Inhibition of human surfactant protein A function by oxidation intermediates of nitrite. Free Radic Biol Med 33: 1703–1713, 2002. doi: 10.1016/S0891-5849(02)01170-X. [DOI] [PubMed] [Google Scholar]
- 70.Gessner MA, Doran SF, Yu Z, Dunaway CW, Matalon S, Steele C. Chlorine gas exposure increases susceptibility to invasive lung fungal infection. Am J Physiol Lung Cell Mol Physiol 304: L765–L773, 2013. doi: 10.1152/ajplung.00030.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Borg T, Alvfors A, Gerdin B, Modig J. A porcine model of early adult respiratory distress syndrome induced by endotoxaemia. Acta Anaesthesiol Scand 29: 814–830, 1985. doi: 10.1111/j.1399-6576.1985.tb02306.x. [DOI] [PubMed] [Google Scholar]
- 72.Wang J, Winskog C, Edston E, Walther SM. Inhaled and intravenous corticosteroids both attenuate chlorine gas-induced lung injury in pigs. Acta Anaesthesiol Scand 49: 183–190, 2005. doi: 10.1111/j.1399-6576.2004.00563.x. [DOI] [PubMed] [Google Scholar]
- 73.Gunnarsson M, Walther SM, Seidal T, Bloom GD, Lennquist S. Exposure to chlorine gas: effects on pulmonary function and morphology in anaesthetised and mechanically ventilated pigs. J Appl Toxicol 18: 249–255, 1998. doi:. [DOI] [PubMed] [Google Scholar]
- 74.Aggarwal S, Lazrak A, Ahmad I, Yu Z, Bryant A, Mobley JA, Ford DA, Matalon S. Reactive species generated by heme impair alveolar epithelial sodium channel function in acute respiratory distress syndrome. Redox Biol 36: 101592, 2020. doi: 10.1016/j.redox.2020.101592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Rupp H, Henschler D. [Effect of low chlorine and bromine concentrations on man] Int Arch Arbeitsmed 23: 79–90, 1967. [PubMed] [Google Scholar]
- 76.Woolf A, Shannon M. Reactive airways dysfunction and systemic complaints after mass exposure to bromine. Environ Health Perspect 107: 507–509, 1999. doi: 10.1289/ehp.99107507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bitron MD, Aharonson EF. Delayed mortality of mice following inhalation of acute doses of CH2O, SO2Cl2, and Br2. Am Ind Hyg Assoc J 39: 129–138, 1978. doi: 10.1080/0002889778507726. [DOI] [PubMed] [Google Scholar]
- 78.Schlagbauer M, Henschler D. [Toxicity of chlorine and bromine in single and repeated inhalation]. Int Arch Arbeitsmed 23: 91–98, 1967. doi: 10.1007/BF00376171. [DOI] [PubMed] [Google Scholar]
- 79.Aggarwal S, Lam A, Bolisetty S, Carlisle MA, Traylor A, Agarwal A, Matalon S. Heme attenuation ameliorates irritant gas inhalation-induced acute lung injury. Antioxid Redox Signal 24: 99–112, 2016. doi: 10.1089/ars.2015.6347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yadav AK, Doran SF, Samal AA, Sharma R, Vedagiri K, Postlethwait EM, Squadrito GL, Fanucchi MV, Roberts LJ, Patel RP, Matalon S. Mitigation of chlorine gas lung injury in rats by postexposure administration of sodium nitrite. Am J Physiol Lung Cell Mol Physiol 300: L362–L369, 2011. doi: 10.1152/ajplung.00278.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Samal AA, Honavar J, Brandon A, Bradley KM, Doran S, Liu Y, Dunaway C, Steele C, Postlethwait EM, Squadrito GL, Fanucchi MV, Matalon S, Patel RP. Administration of nitrite after chlorine gas exposure prevents lung injury: effect of administration modality. Free Radic Biol Med . 53: 1431–1439, 2012. doi: 10.1016/j.freeradbiomed.2012.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zaky A, Bradley WE, Lazrak A, Zafar I, Doran S, Ahmad A, White CW, Dell'Italia LJ, Matalon S, Ahmad S. Chlorine inhalation-induced myocardial depression and failure. Physiol Rep 3: e12439, 2015. doi: 10.14814/phy2.12439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Addis DR, Lambert JA, Ren C, Doran S, Aggarwal S, Jilling T, Matalon S. Vascular Endothelial growth factor-121 administration mitigates halogen inhalation-induced pulmonary injury and fetal growth restriction in pregnant mice. J Am Heart Assoc 9: e013238, 2020. doi: 10.1161/jaha.119.013238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Addis DR, Aggarwal S, Doran SF, Jian MY, Ahmad I, Kojima K, Ford DA, Matalon S, Mobley JA. Vascular permeability disruption explored in the proteomes of mouse lungs and human microvascular cells following acute bromine exposure. Am J Physiol Lung Cell Mol Physiol 319: L337–L359, 2020. doi: 10.1152/ajplung.00196.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ximenes VF, Morgon NH, de Souza AR. Hypobromous acid, a powerful endogenous electrophile: Experimental and theoretical studies. J Inorg Biochem 146: 61–68, 2015. doi: 10.1016/j.jinorgbio.2015.02.014. [DOI] [PubMed] [Google Scholar]
- 86.Nielson DW, Goerke J, Clements JA. Alveolar subphase pH in the lungs of anesthetized rabbits. Proc Natl Acad Sci U S A 78: 7119–7123, 1981. doi: 10.1073/pnas.78.11.7119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Nielson DW. Electrolyte composition of pulmonary alveolar subphase in anesthetized rabbits. J Appl Physiol (1985). 60: 972–979, 1986. doi: 10.1152/jappl.1986.60.3.972. [DOI] [PubMed] [Google Scholar]
- 88.Zhou T, Yu Z, Jian MY, Ahmad I, Trempus C, Wagener BM, Pittet JF, Aggarwal S, Garantziotis S, Song W, Matalon S. Instillation of hyaluronan reverses acid instillation injury to the mammalian blood gas barrier. Am J Physiol Lung Cell Mol Physiol 314: L808–L821, 2018. doi: 10.1152/ajplung.00510.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Folkesson HG, Matthay MA, Hebert CA, Broaddus VC. Acid aspiration-induced lung injury in rabbits is mediated by interleukin-8-dependent mechanisms. J Clin Invest 96: 107–116, 1995. doi: 10.1172/JCI118009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Squadrito GL, Postlethwait EM, Matalon S. Elucidating mechanisms of chlorine toxicity: reaction kinetics, thermodynamics, and physiological implications. Am J Physiol Lung Cell Mol Physiol 299: L289–L300, 2010. doi: 10.1152/ajplung.00077.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Bracher A, Doran SF, Squadrito GL, Postlethwait EM, Bowen L, Matalon S. Targeted aerosolized delivery of ascorbate in the lungs of chlorine-exposed rats. J. Aerosol Med. Pulm. Drug Deliv 25: 333–341, 2012. doi: 10.1089/jamp.2011.0963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.McGovern TK, Powell WS, Day BJ, White CW, Govindaraju K, Karmouty-Quintana H, Lavoie N, Tan JJ, Martin JG. Dimethylthiourea protects against chlorine induced changes in airway function in a murine model of irritant induced asthma. Respir Res 11: 138, 2010. doi: 10.1186/1465-9921-11-138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Agren L, Elfsmark L, Akfur C, Hagglund L, Ekstrand-Hammarstrom B, Jonasson S. N-acetyl cysteine protects against chlorine-induced tissue damage in an ex vivo model. Toxicol Lett 322: 58–65, 2020. doi: 10.1016/j.toxlet.2020.01.006. [DOI] [PubMed] [Google Scholar]
- 94.Wigenstam E, Koch B, Bucht A, Jonasson S. N-acetyl cysteine improves the effects of corticosteroids in a mouse model of chlorine-induced acute lung injury. Toxicology 328: 40–47, 2015. doi: 10.1016/j.tox.2014.12.008. [DOI] [PubMed] [Google Scholar]
- 95.Wang J, Zhang L, Walther SM. Administration of aerosolized terbutaline and budesonide reduces chlorine gas-induced acute lung injury. J Trauma 56: 850–862, 2004. doi: 10.1097/01.TA.0000078689.45384.8B. [DOI] [PubMed] [Google Scholar]
- 96.Lazrak A, Chen L, Jurkuvenaite A, Doran SF, Liu G, Li Q, Lancaster JR Jr, Matalon S. Regulation of alveolar epithelial Na+ channels by ERK1/2 in chlorine-breathing mice. Am J Respir Cell Mol Biol 46: 342–354, 2012. doi: 10.1165/rcmb.2011-0309OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Honavar J, Doran S, Ricart K, Matalon S, Patel RP. Nitrite therapy prevents chlorine gas toxicity in rabbits. Toxicol Lett 271: 20–25, 2017. doi: 10.1016/j.toxlet.2017.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ahmad S, Ahmad A, Hendry-Hofer TB, Loader JE, Claycomb WC, Mozziconacci O, Schoneich C, Reisdorph N, Powell RL, Chandler JD, Day BJ, Veress LA, White CW. Sarcoendoplasmic reticulum Ca(2+) ATPase. A critical target in chlorine inhalation-induced cardiotoxicity. Am J Respir Cell Mol Biol 52: 492–502, 2015. doi: 10.1165/rcmb.2014-0005OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lazrak A, Creighton J, Yu Z, Komarova S, Doran SF, Aggarwal S, Emala CW Sr, Stober VP, Trempus CS, Garantziotis S, Matalon S. Hyaluronan mediates airway hyperresponsiveness in oxidative lung injury. Am J Physiol Lung Cell Mol.Physiol 308: L891–L903, 2015. doi: 10.1152/ajplung.00377.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.den Hartog GJ, Haenen GR, Vegt E, van der Vijgh WJ, Bast A. Efficacy of HOCl scavenging by sulfur-containing compounds: antioxidant activity of glutathione disulfide? Biol Chem 383: 709–713, 2002. doi: 10.1515/bc.2002.073. [DOI] [PubMed] [Google Scholar]
- 101.Hawkins CL, Pattison DI, Davies MJ. Hypochlorite-induced oxidation of amino acids, peptides and proteins. Amino Acids 25: 259–274, 2003. doi: 10.1007/s00726-003-0016-x. [DOI] [PubMed] [Google Scholar]
- 102.Weiss SJ, Klein R, Slivka A, Wei M. Chlorination of taurine by human neutrophils. Evidence for hypochlorous acid generation. J Clin Invest 70: 598–607, 1982. doi: 10.1172/jci110652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Crow JP. Measurement and significance of free and protein-bound 3-nitrotyrosine, 3-chlorotyrosine, and free 3-nitro-4-hydroxyphenylacetic acid in biologic samples: a high-performance liquid chromatography method using electrochemical detection. Methods Enzymol. 301: 151–160, 1999. doi: 10.1016/S0076-6879(99)01078-2. [DOI] [PubMed] [Google Scholar]
- 104.Hazen SL, Hsu FF, Mueller DM, Crowley JR, Heinecke JW. Human neutrophils employ chlorine gas as an oxidant during phagocytosis. J Clin Invest 98: 1283–1289, 1996. doi: 10.1172/JCI118914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Hazen SL, Heinecke JW. 3-Chlorotyrosine, a specific marker of myeloperoxidase-catalyzed oxidation, is markedly elevated in low density lipoprotein isolated from human atherosclerotic intima. J Clin Invest 99: 2075–2081, 1997. doi: 10.1172/JCI119379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Foote CS, Goyne TE, Lehrer RI. Assessment of chlorination by human neutrophils. Nature 301: 715–716, 1983. doi: 10.1038/301715a0. [DOI] [PubMed] [Google Scholar]
- 107.Pattison DI, Hawkins CL, Davies MJ. Hypochlorous acid-mediated protein oxidation: how important are chloramine transfer reactions and protein tertiary structure? Biochemistry 46: 9853–9864, 2007. doi: 10.1021/bi7008294. [DOI] [PubMed] [Google Scholar]
- 108.Robaszkiewicz A, Bartosz G, Soszyński M. N-chloroamino acids cause oxidative protein modifications in the erythrocyte membrane. Mech Ageing Dev 129: 572–579, 2008. doi: 10.1016/j.mad.2008.05.007. [DOI] [PubMed] [Google Scholar]
- 109.Song W, Matalon S. Modulation of alveolar fluid clearance by reactive oxygen-nitrogen intermediates. Am J Physiol Lung Cell Mol Physiol 293: L855–L858, 2007. doi: 10.1152/ajplung.00305.2007. [DOI] [PubMed] [Google Scholar]
- 110.Midwinter RG, Vissers MC, Winterbourn CC. Hypochlorous acid stimulation of the mitogen-activated protein kinase pathway enhances cell survival. Arch Biochem Biophys 394: 13–20, 2001. doi: 10.1006/abbi.2001.2530. [DOI] [PubMed] [Google Scholar]
- 111.Midwinter RG, Cheah FC, Moskovitz J, Vissers MC, Winterbourn CC. IkappaB is a sensitive target for oxidation by cell-permeable chloramines: inhibition of NF-kappaB activity by glycine chloramine through methionine oxidation. Biochem J 396: 71–78, 2006. doi: 10.1042/BJ20052026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Weiss SJ. Tissue destruction by neutrophils. N Engl J Med 320: 365–376, 1989. doi: 10.1056/NEJM198902093200606. [DOI] [PubMed] [Google Scholar]
- 113.Whiteman M, Jenner A, Halliwell B. Hypochlorous acid-induced base modifications in isolated calf thymus DNA. Chem Res Toxicol 10: 1240–1246, 1997. doi: 10.1021/tx970086i. [DOI] [PubMed] [Google Scholar]
- 114.Van Rensburg CE, Van Staden AM, Anderson R. Inactivation of poly (ADP-ribose) polymerase by hypochlorous acid. Free Radic Biol Med 11: 285–291, 1991. doi: 10.1016/0891-5849(91)90125-M. [DOI] [PubMed] [Google Scholar]
- 115.Holm BA, Notter RH, Siegle J, Matalon S. Pulmonary physiological and surfactant changes during injury and recovery from hyperoxia. J Appl Physiol (1985) 59: 1402–1409, 1985. doi: 10.1152/jappl.1985.59.5.1402. [DOI] [PubMed] [Google Scholar]
- 116.Holm BA, Matalon S. Role of pulmonary surfactant in the development and treatment of adult respiratory distress syndrome. Anesth Analg 69: 805–818, 1989. [PubMed] [Google Scholar]
- 117.Haddad IY, Ischiropoulos H, Holm BA, Beckman JS, Baker JR, Matalon S. Mechanisms of peroxynitrite-induced injury to pulmonary surfactants. Am J Physiol Lung Cell Mol Physiol 265: L555–L564 1993. doi: 10.1152/ajplung.1993.265.6.L555. [DOI] [PubMed] [Google Scholar]
- 118.Rodriguez-Capote K, Manzanares D, Haines T, Possmayer F. Reactive oxygen species inactivation of surfactant involves structural and functional alterations to surfactant proteins SP-B and SP-C. Biophys J 90: 2808–2821, 2006. doi: 10.1529/biophysj.105.073106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Holm BA, Notter RH, Finkelstein JN. Surface property changes from interactions of albumin with natural lung surfactant and extracted lung lipids. Chem Phys Lipids 38: 287–298, 1985. doi: 10.1016/0009-3084(85)90022-2. [DOI] [PubMed] [Google Scholar]
- 120.Haddad IY, Crow JP, Hu P, Ye Y, Beckman J, Matalon S. Concurrent generation of nitric oxide and superoxide damages surfactant protein A. Am J Physiol Lung Cell Mol Physiol 267: L242–L249, 1994. doi: 10.1152/ajplung.1994.267.3.L242. [DOI] [PubMed] [Google Scholar]
- 121.Haddad IY, Beckman JS, Garver RI, Matalon S. Structural and functional alterations of surfactant protein A by peroxynitrite. Chest 105: 84S, 1994. doi: 10.1378/chest.105.3_supplement.84s. [DOI] [PubMed] [Google Scholar]
- 122.Crouch EC, Hirche TO, Shao B, Boxio R, Wartelle J, Benabid R, McDonald B, Heinecke J, Matalon S, Belaaouaj A. Myeloperoxidase-dependent inactivation of surfactant protein D in vitro and in vivo. J Biol Chem 285: 16757–16770, 2010. doi: 10.1074/jbc.M109.097048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Matalon S, Shrestha K, Kirk M, Waldheuser S, McDonald B, Smith K, Gao Z, Belaaouaj A, Crouch EC. Modification of surfactant protein D by reactive oxygen-nitrogen intermediates is accompanied by loss of aggregating activity, in vitro and in vivo. FASEB J. 23: 1415–1430, 2009. doi: 10.1096/fj.08-120568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Matalon S, Bartoszewski R, Collawn JF. Role of epithelial sodium channels in the regulation of lung fluid homeostasis. Am J Physiol Lung Cell Mol Physiol 309: L1229–L1238, 2015. doi: 10.1152/ajplung.00319.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Matalon S, O’Brodovich H. Sodium channels in alveolar epithelial cells: molecular characterization, biophysical properties, and physiological significance. Annu Rev Physiol 61: 627–661, 1999. doi: 10.1146/annurev.physiol.61.1.627. [DOI] [PubMed] [Google Scholar]
- 126.Matalon S, Lazrak A, Jain L, Eaton DC. Invited review: biophysical properties of sodium channels in lung alveolar epithelial cells. J Appl Physiol (1985) 93: 1852–1859, 2002. doi: 10.1152/japplphysiol.01241.2001. [DOI] [PubMed] [Google Scholar]
- 127.Matthay MA, Folkesson HG, Clerici C. Lung epithelial fluid transport and the resolution of pulmonary edema. Physiol Rev. 82: 569–600, 2002. doi: 10.1152/physrev.00003.2002. [DOI] [PubMed] [Google Scholar]
- 128.Matthay MA, Wiener-Kronish JP. Intact epithelial barrier function is critical for the resolution of alveolar edema in humans. Am Rev Respir Dis 142: 1250–1257, 1990. doi: 10.1164/ajrccm/142.6_Pt_1.1250. [DOI] [PubMed] [Google Scholar]
- 129.Zhu S, Ware LB, Geiser T, Matthay MA, Matalon S. Increased levels of nitrate and surfactant protein a nitration in the pulmonary edema fluid of patients with acute lung injury. Am J Respir Crit Care Med 163: 166–172, 2001. doi: 10.1164/ajrccm.163.1.2005068. [DOI] [PubMed] [Google Scholar]
- 130.Lee J, Giordano S, Zhang J. Autophagy, mitochondria and oxidative stress: cross-talk and redox signalling. Biochem J 441: 523–540, 2012. doi: 10.1042/BJ20111451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Lemasters JJ, Theruvath TP, Zhong Z, Nieminen AL. Mitochondrial calcium and the permeability transition in cell death. Biochim Biophys Acta 1787: 1395–1401, 2009. doi: 10.1016/j.bbabio.2009.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Murphy MP. Targeting lipophilic cations to mitochondria. Biochim Biophys Acta 1777: 1028–1031, 2008. doi: 10.1016/j.bbabio.2008.03.029. [DOI] [PubMed] [Google Scholar]
- 133.Whiteman M, Spencer JP, Szeto HH, Armstrong JS. Do mitochondriotropic antioxidants prevent chlorinative stress-induced mitochondrial and cellular injury? Antioxid Redox Signal 10: 641–650, 2008. doi: 10.1089/ars.2007.1879. [DOI] [PubMed] [Google Scholar]
- 134.Klamt F, Shacter E. Taurine chloramine, an oxidant derived from neutrophils, induces apoptosis in human B lymphoma cells through mitochondrial damage. J Biol Chem 280: 21346–21352, 2005. doi: 10.1074/jbc.M501170200. [DOI] [PubMed] [Google Scholar]
- 135.Whiteman M, Rose P, Siau JL, Cheung NS, Tan GS, Halliwell B, Armstrong JS. Hypochlorous acid-mediated mitochondrial dysfunction and apoptosis in human hepatoma HepG2 and human fetal liver cells: role of mitochondrial permeability transition. Free Radic Biol Med 38: 1571–1584, 2005. doi: 10.1016/j.freeradbiomed.2005.02.030. [DOI] [PubMed] [Google Scholar]
- 136.Jaeschke H, Gores GJ, Cederbaum AI, Hinson JA, Pessayre D, Lemasters JJ. Mechanisms of hepatotoxicity. Toxicol Sci 65: 166–176, 2002. doi: 10.1093/toxsci/65.2.166. [DOI] [PubMed] [Google Scholar]
- 137.Kon K, Kim JS, Jaeschke H, Lemasters JJ. Mitochondrial permeability transition in acetaminophen-induced necrosis and apoptosis of cultured mouse hepatocytes. Hepatology 40: 1170–1179, 2004. doi: 10.1002/hep.20437. [DOI] [PubMed] [Google Scholar]
- 138.Hill BG, Benavides GA, Lancaster JR, Ballinger S, Dell’italia L, Zhang J, Darley-Usmar VM. Integration of cellular bioenergetics with mitochondrial quality control and autophagy. Biol Chem 393: 1485–1512, 2012. doi: 10.1515/hsz-2012-0198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Gutierrez J, Ballinger SW, Darley-Usmar VM, Landar A. Free radicals, mitochondria, and oxidized lipids: the emerging role in signal transduction in vascular cells. Circ Res 99: 924–932, 2006. doi: 10.1161/01.RES.0000248212.86638.e9. [DOI] [PubMed] [Google Scholar]
- 140.Koenitzer JR, Freeman BA. Redox signaling in inflammation: interactions of endogenous electrophiles and mitochondria in cardiovascular disease. Ann N Y Acad Sci 1203: 45–52, 2010. doi: 10.1111/j.1749-6632.2010.05559.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Lemasters JJ. V. Necrapoptosis and the mitochondrial permeability transition: shared pathways to necrosis and apoptosis. Am J Physiol Gasteroenterol Physiol 276: G1–G6, 1999. doi: 10.1152/ajpgi.1999.276.1.G1. [DOI] [PubMed] [Google Scholar]
- 142.Jaeschke H, Lemasters JJ. Apoptosis versus oncotic necrosis in hepatic ischemia/reperfusion injury. Gastroenterology 125: 1246–1257, 2003. doi: 10.1016/S0016-5085(03)01209-5. [DOI] [PubMed] [Google Scholar]
- 143.Kim JS, Qian T, Lemasters JJ. Mitochondrial permeability transition in the switch from necrotic to apoptotic cell death in ischemic rat hepatocytes. Gastroenterology 124: 494–503, 2003. doi: 10.1053/gast.2003.50059. [DOI] [PubMed] [Google Scholar]
- 144.Kim JS, Nitta T, Mohuczy D, O'Malley KA, Moldawer LL, Dunn WA Jr, Behrns KE. Impaired autophagy: a mechanism of mitochondrial dysfunction in anoxic rat hepatocytes. Hepatology 47: 1725–1736, 2008. doi: 10.1002/hep.22187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Ryter SW, Nakahira K, Haspel JA, Choi AM. Autophagy in pulmonary diseases. Annu Rev Physiol 74: 377–401, 2012. doi: 10.1146/annurev-physiol-020911-153348. [DOI] [PubMed] [Google Scholar]
- 146.Goldman SJ, Taylor R, Zhang Y, Jin S. Autophagy and the degradation of mitochondria. Mitochondrion 10: 309–315, 2010. doi: 10.1016/j.mito.2010.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Ford DA, Honavar J, Albert CJ, Duerr MA, Oh JY, Doran S, Matalon S, Patel RP. Formation of chlorinated lipids post-chlorine gas exposure. J Lipid Res 57: 1529–1540, 2016. doi: 10.1194/jlr.M069005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Sedlic F, Sepac A, Pravdic D, Camara AK, Bienengraeber M, Brzezinska AK, Wakatsuki T, Bosnjak ZJ. Mitochondrial depolarization underlies delay in permeability transition by preconditioning with isoflurane: roles of ROS and Ca2+. Am J Physiol Cell Physiol 299: C506–C515, 2010. doi: 10.1152/ajpcell.00006.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Yancey DM, Guichard JL, Ahmed MI, Zhou L, Murphy MP, Johnson MS, Benavides GA, Collawn J, Darley-Usmar V, Dell’Italia LJ. Cardiomyocyte mitochondrial oxidative stress and cytoskeletal breakdown in the heart with a primary volume overload. Am J Physiol Heart Circ Physiol 308: H651–H663, 2015. doi: 10.1152/ajpheart.00638.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Cook NL, Viola HM, Sharov VS, Hool LC, Schoneich C, Davies MJ. Myeloperoxidase-derived oxidants inhibit sarco/endoplasmic reticulum Ca2+-ATPase activity and perturb Ca2+ homeostasis in human coronary artery endothelial cells. Free Radic Biol Med 52: 951–961, 2012. doi: 10.1016/j.freeradbiomed.2011.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Rana FR, Harwood JS, Mautone AJ, Dluhy RA. Identification of phosphocholine plasmalogen as a lipid component in mammalian pulmonary surfactant using high-resolution 31P NMR spectroscopy. Biochemistry 32: 27–31, 1993. doi: 10.1021/bi00052a005. [DOI] [PubMed] [Google Scholar]
- 152.Ford DA, Gross RW. Plasmenylethanolamine is the major storage depot for arachidonic acid in rabbit vascular smooth muscle and is rapidly hydrolyzed after angiotensin II stimulation. Proc Natl Acad Sci U S A 86: 3479–3483, 1989. doi: 10.1073/pnas.86.10.3479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Duerr MA, Palladino END, Hartman CL, Lambert JA, Franke JD, Albert CJ, Matalon S, Patel RP, Slungaard A, Ford DA. Bromofatty aldehyde derived from bromine exposure and myeloperoxidase and eosinophil peroxidase modify GSH and protein. J Lipid Res 59: 696–705, 2018. doi: 10.1194/jlr.M083279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Albert CJ, Thukkani AK, Heuertz RM, Slungaard A, Hazen SL, Ford DA. Eosinophil peroxidase-derived reactive brominating species target the vinyl ether bond of plasmalogens generating a novel chemoattractant, alpha-bromo fatty aldehyde. J Biol Chem 278: 8942–8950, 2003. doi: 10.1074/jbc.m211634200. [DOI] [PubMed] [Google Scholar]
- 155.Chase JF, Tubbs PK. Specific inhibition of mitochondrial fatty acid oxidation by 2-bromopalmitate and its coenzyme A and carnitine esters. Biochem J 129: 55–65, 1972. doi: 10.1042/bj1290055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Draper JM, Smith CD. Palmitoyl acyltransferase assays and inhibitors (Review). Mol Membr Biol 26: 5–13, 2009. doi: 10.1080/09687680802683839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Marsche G, Heller R, Fauler G, Kovacevic A, Nuszkowski A, Graier W, Sattler W, Malle E. 2-chlorohexadecanal derived from hypochlorite-modified high-density lipoprotein-associated plasmalogen is a natural inhibitor of endothelial nitric oxide biosynthesis. Arterioscler Thromb Vasc Biol 24: 2302–2306, 2004. doi: 10.1161/01.ATV.0000148703.43429.25. [DOI] [PubMed] [Google Scholar]
- 158.Thukkani AK, Hsu FF, Crowley JR, Wysolmerski RB, Albert CJ, Ford DA. Reactive chlorinating species produced during neutrophil activation target tissue plasmalogens: production of the chemoattractant, 2-chlorohexadecanal. J Biol Chem 277: 3842–3849, 2002. doi: 10.1074/jbc.M109489200. [DOI] [PubMed] [Google Scholar]
- 159.Thukkani AK, Martinson BD, Albert CJ, Vogler GA, Ford DA. Neutrophil-mediated accumulation of 2-ClHDA during myocardial infarction: 2-ClHDA-mediated myocardial injury. Am J Physiol Heart Circ Physiol 288: H2955–H2964, 2005. doi: 10.1152/ajpheart.00834.2004. [DOI] [PubMed] [Google Scholar]
- 160.Hartman CL, Duerr MA, Albert CJ, Neumann WL, McHowat J, Ford DA. 2-Chlorofatty acids induce Weibel-Palade body mobilization. J Lipid Res 59: 113–122, 2018.doi: 10.1194/jlr.M080200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Oh MW, Honavar J, Doran S, Benavides G, Darley-Usmar V, Matalon S, Ford DA, Patel RP. Chlorinated fatty acids are biomarkers and potential mediators of chlorine gas toxicity. Free Radic Biol Med 76: S165–S166 2014. doi: 10.1016/j.freeradbiomed.2014.10.053. [DOI] [Google Scholar]
- 162.Meyer NJ, Reilly JP, Feng R, Christie JD, Hazen SL, Albert CJ, Franke JD, Hartman CL, McHowat J, Ford DA. Myeloperoxidase-derived 2-chlorofatty acids contribute to human sepsis mortality via acute respiratory distress syndrome. JCI Insight 2: e96432, 2017. doi: 10.1172/jci.insight.96432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Ryter SW, Tyrrell RM. The heme synthesis and degradation pathways: role in oxidant sensitivity. Heme oxygenase has both pro- and antioxidant properties. Free Radic Biol Med 28: 289–309, 2000. doi: 10.1016/S0891-5849(99)00223-3. [DOI] [PubMed] [Google Scholar]
- 164.Balla J, Vercellotti GM, Jeney V, Yachie A, Varga Z, Jacob HS, Eaton JW, Balla G. Heme, heme oxygenase, and ferritin: how the vascular endothelium survives (and dies) in an iron-rich environment. Antioxid Redox Signal 9: 2119–2137, 2007. doi: 10.1089/ars.2007.1787. [DOI] [PubMed] [Google Scholar]
- 165.Gaggar A, Patel RP. There is blood in the water: hemolysis, hemoglobin, and heme in acute lung injury. Am J Physiol Lung Cell Mol Physiol 311: L714–L718, 2016.doi: 10.1152/ajplung.00312.2016. [DOI] [PubMed] [Google Scholar]
- 166.Kato GJ, Steinberg MH, Gladwin MT. Intravascular hemolysis and the pathophysiology of sickle cell disease. J Clin Invest 127: 750–760, 2017. doi: 10.1172/JCI89741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Stapley R, Rodriguez C, Oh JY, Honavar J, Brandon A, Wagener BM, Marques MB, Weinberg JA, Kerby JD, Pittet JF, Patel RP. Red blood cell washing, nitrite therapy, and antiheme therapies prevent stored red blood cell toxicity after trauma-hemorrhage. Free Radic Biol Med 85: 207–218, 2015. doi: 10.1016/j.freeradbiomed.2015.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Lin T, Maita D, Thundivalappil SR, Riley FE, Hambsch J, Van Marter LJ, Christou HA, Berra L, Fagan S, Christiani DC, Warren HS. Hemopexin in severe inflammation and infection: mouse models and human diseases. Crit Care 19: 166, 2015. doi: 10.1186/s13054-015-0885-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Wagener BM, Hu PJ, Oh JY, Evans CA, Richter JR, Honavar J, Brandon AP, Creighton J, Stephens SW, Morgan C, Dull RO, Marques MB, Kerby JD, Pittet JF, Patel RP. Role of heme in lung bacterial infection after trauma hemorrhage and stored red blood cell transfusion: a preclinical experimental study. PLoS Med 15: e1002522, 2018. doi: 10.1371/journal.pmed.1002522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Larsen R, Gozzelino R, Jeney V, Tokaji L, Bozza FA, Japiassu AM, Bonaparte D, Cavalcante MM, Chora A, Ferreira A, Marguti I, Cardoso S, Sepúlveda N, Smith A, Soares MP. A central role for free heme in the pathogenesis of severe sepsis. Sci Transl Med 2: 51ra71, 2010. doi: 10.1126/scitranslmed.3001118. [DOI] [PubMed] [Google Scholar]
- 171.Higdon AN, Benavides GA, Chacko BK, Ouyang X, Johnson MS, Landar A, Zhang J, Darley-Usmar VM. Hemin causes mitochondrial dysfunction in endothelial cells through promoting lipid peroxidation: the protective role of autophagy. Am J Physiol Heart Circ Physiol 302: H1394–H1409, 2012. doi: 10.1152/ajpheart.00584.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Fortes GB, Alves LS, de OR, Dutra FF, Rodrigues D, Fernandez PL, Souto-Padron T, De Rosa MJ, Kelliher M, Golenbock D, Chan FK, Bozza MT. Heme induces programmed necrosis on macrophages through autocrine TNF and ROS production. Blood 119: 2368–2375, 2012. doi: 10.1182/blood-2011-08-375303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Janz DR, Bastarache JA, Sills G, Wickersham N, May AK, Bernard GR, Ware LB. Association between haptoglobin, hemopexin and mortality in adults with sepsis. Crit Care 17: R272, 2013. doi: 10.1186/cc13108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Heide K, Haupt H, Stoeriko K, Schultze HE. On the heme-binding capacity of hemopexin. Clin Chim Acta 10: 460–469, 1964. doi: 10.1016/0009-8981(64)90176-7. [DOI] [PubMed] [Google Scholar]
- 175.Graw JA, Mayeur C, Rosales I, Liu Y, Sabbisetti VS, Riley FE, Rechester O, Malhotra R, Warren HS, Colvin RB, Bonventre JV, Bloch DB, Zapol WM. Haptoglobin or hemopexin therapy prevents acute adverse effects of resuscitation after prolonged storage of red cells. Circulation 134: 945–960, 2016. doi: 10.1161/CIRCULATIONAHA.115.019955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Szweda-Lewandowska Z, Krokosz A, Gonciarz M, Zajeczkowska W, Puchala M. Damage to human erythrocytes by radiation-generated HO* radicals: molecular changes in erythrocyte membranes. Free Radic Res 37: 1137–1143, 2003. doi: 10.1080/10715760310001604152. [DOI] [PubMed] [Google Scholar]
- 177.Liu SC, Zhai S, Lawler J, Palek J. Hemin-mediated dissociation of erythrocyte membrane skeletal proteins. J Biol Chem 260: 12234–12239, 1985. doi: 10.1016/S0021-9258(17)39015-4. [DOI] [PubMed] [Google Scholar]
- 178.Solar I, Dulitzky J, Shaklai N. Hemin-promoted peroxidation of red cell cytoskeletal proteins. Arch Biochem Biophys 283: 81–89, 1990. doi: 10.1016/0003-9861(90)90615-6. [DOI] [PubMed] [Google Scholar]
- 179.Shaklai N, Solar I. Residual hemin in the membrane is a cause of red cell distortion. Prog Clin Biol Res 319: 575–585, 1989. [PubMed] [Google Scholar]
- 180.Garantziotis S, Li Z, Potts EN, Lindsey JY, Stober VP, Polosukhin VV, Blackwell TS, Schwartz DA, Foster WM, Hollingsworth JW. TLR4 is necessary for hyaluronan-mediated airway hyperresponsiveness after ozone inhalation. Am J Respir Crit Care Med 181: 666–675, 2010.doi: 10.1164/rccm.200903-0381OC. [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- 181.Jiang D, Liang J, Noble PW. Regulation of non-infectious lung injury, inflammation, and repair by the extracellular matrix glycosaminoglycan hyaluronan. Anat Rec (Hoboken) 293: 982–985, 2010. doi: 10.1002/ar.21102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Scheibner KA, Lutz MA, Boodoo S, Fenton MJ, Powell JD, Horton MR. Hyaluronan fragments act as an endogenous danger signal by engaging TLR2. J Immunol 177: 1272–1281, 2006. doi: 10.4049/jimmunol.177.2.1272. [DOI] [PubMed] [Google Scholar]
- 183.Forteza R, Lieb T, Aoki T, Savani RC, Conner GE, Salathe M. Hyaluronan serves a novel role in airway mucosal host defense. FASEB J 15: 2179–2186, 2001. doi: 10.1096/fj.01-0036com. [DOI] [PubMed] [Google Scholar]
- 184.Li Y, Jiang D, Liang J, Meltzer EB, Gray A, Miura R, Wogensen L, Yamaguchi Y, Noble PW. Severe lung fibrosis requires an invasive fibroblast phenotype regulated by hyaluronan and CD44. J Exp Med 208: 1459–1471, 2011. doi: 10.1084/jem.20102510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Jiang D, Liang J, Noble PW. Hyaluronan as an immune regulator in human diseases. Physiol Rev. 91: 221–264, 2011. doi: 10.1152/physrev.00052.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Bost F, Diarra-Mehrpour M, Martin JP. Inter-alpha-trypsin inhibitor proteoglycan family–a group of proteins binding and stabilizing the extracellular matrix. Eur J Biochem 252: 339–346, 1998. doi: 10.1046/j.1432-1327.1998.2520339.x. [DOI] [PubMed] [Google Scholar]
- 187.Malki N, Balduyck M, Maes P, Capon C, Mizon C, Han KK, Tartar A, Fournet B, Mizon J. The heavy chains of human plasma inter-alpha-trypsin inhibitor: their isolation, their identification by electrophoresis and partial sequencing. Differential reactivity with concanavalin A. Biol Chem Hoppe Seyler 373: 1009–1018, 1992. doi: 10.1515/bchm3.1992.373.2.1009. [DOI] [PubMed] [Google Scholar]
- 188.Lazrak A, Jurkuvenaite A, Ness EC, Zhang S, Woodworth BA, Muhlebach MS, Stober VP, Lim YP, Garantziotis S, Matalon S. Inter-alpha-inhibitor blocks epithelial sodium channel activation and decreases nasal potential differences in DeltaF508 mice. Am J Respir Cell Mol Biol 50: 953–962, 2014. doi: 10.1165/rcmb.2013-0215OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Garantziotis S, Li Z, Potts EN, Kimata K, Zhuo L, Morgan DL, Savani RC, Noble PW, Foster WM, Schwartz DA, Hollingsworth JW. Hyaluronan mediates ozone-induced airway hyperresponsiveness in mice. J Biol Chem 284: 11309–11317, 2009. doi: 10.1074/jbc.M802400200. [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- 190.Eldridge L, Moldobaeva A, Wagner EM. Increased hyaluronan fragmentation during pulmonary ischemia. Am J Physiol Lung Cell Mol Physiol 301: L782–L788, 2011. doi: 10.1152/ajplung.00079.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Lennon FE, Singleton PA. Hyaluronan regulation of vascular integrity. Am J Cardiovasc Dis 1: 200–213, 2011. [PMC free article] [PubMed] [Google Scholar]
- 192.Jiang D, Liang J, Fan J, Yu S, Chen S, Luo Y, Prestwich GD, Mascarenhas MM, Garg HG, Quinn DA, Homer RJ, Goldstein DR, Bucala R, Lee PJ, Medzhitov R, Noble PW. Regulation of lung injury and repair by Toll-like receptors and hyaluronan. Nat Med 11: 1173–1179, 2005.doi: 10.1038/nm1315. [DOI] [PubMed] [Google Scholar]
- 193.Huang PM, Syrkina O, Yu L, Dedaj R, Zhao H, Shiedlin A, Liu YY, Garg H, Quinn DA, Hales CA. High MW hyaluronan inhibits smoke inhalation-induced lung injury and improves survival. Respirology 15: 1131–1139, 2010. doi: 10.1111/j.1440-1843.2010.01829.x. [DOI] [PubMed] [Google Scholar]
- 194.Liu YY, Lee CH, Dedaj R, Zhao H, Mrabat H, Sheidlin A, Syrkina O, Huang PM, Garg HG, Hales CA, Quinn DA. High-molecular-weight hyaluronan–a possible new treatment for sepsis-induced lung injury: a preclinical study in mechanically ventilated rats. Crit Care 12: R102, 2008. doi: 10.1186/cc6982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Chiba Y, Matsusue K, Misawa M. RhoA, a possible target for treatment of airway hyperresponsiveness in bronchial asthma. J Pharmacol Sci 114: 239–247, 2010.doi: 10.1254/jphs.10r03cr. [DOI] [PubMed] [Google Scholar]
- 196.Goto K, Chiba Y, Matsusue K, Hattori Y, Maitani Y, Sakai H, Kimura S, Misawa M. The proximal STAT6 and NF-kappaB sites are responsible for IL-13- and TNF-alpha-induced RhoA transcriptions in human bronchial smooth muscle cells. Pharmacol Res 61: 466–472, 2010. doi: 10.1016/j.phrs.2009.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Kudo M, Melton AC, Chen C, Engler MB, Huang KE, Ren X, Wang Y, Bernstein X, Li JT, Atabai K, Huang X, Sheppard D. IL-17A produced by alphabeta T cells drives airway hyper-responsiveness in mice and enhances mouse and human airway smooth muscle contraction. Nat Med 18: 547–554, 2012. doi: 10.1038/nm.2684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Rees MD, McNiven TN, Davies MJ. Degradation of extracellular matrix and its components by hypobromous acid. Biochem J 401: 587–596, 2007. doi: 10.1042/BJ20061236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Stankovska M, Hrabarova E, Valachova K, Molnarova M, Gemeiner P, Soltes L. The degradative action of peroxynitrite on high-molecular-weight hyaluronan. Neuro Endocrinol Lett 27: 31–34, 2006. [PubMed] [Google Scholar]
- 200.Soltes L, Mendichi R, Kogan G, Schiller J, Stankovska M, Arnhold J. Degradative action of reactive oxygen species on hyaluronan. Biomacromolecules. 7: 659–668, 2006. doi: 10.1021/bm050867v. [DOI] [PubMed] [Google Scholar]
- 201.Gao F, Koenitzer JR, Tobolewski JM, Jiang D, Liang J, Noble PW, Oury TD. Extracellular superoxide dismutase inhibits inflammation by preventing oxidative fragmentation of hyaluronan. J Biol Chem 283: 6058–6066, 2008. doi: 10.1074/jbc.M709273200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Campo GM, Avenoso A, D'Ascola A, Scuruchi M, Nastasi G, Micali A, Puzzolo D, Pisani A, Calatroni A, Campo S. The SOD mimic MnTM-2-PyP(5+) reduces hyaluronan degradation-induced inflammation in mouse articular chondrocytes stimulated with Fe (II) plus ascorbate. Int J Biochem Cell Biol 45: 1610–1619, 2013. doi: 10.1016/j.biocel.2013.05.007. [DOI] [PubMed] [Google Scholar]
- 203.Albert CJ, Crowley JR, Hsu FF, Thukkani AK, Ford DA. Reactive chlorinating species produced by myeloperoxidase target the vinyl ether bond of plasmalogens: identification of 2-chlorohexadecanal. J Biol Chem 276: 23733–23741, 2001. doi: 10.1074/jbc.M101447200. [DOI] [PubMed] [Google Scholar]
- 204.Albert CJ, Crowley JR, Hsu FF, Thukkani AK, Ford DA. Reactive brominating species produced by myeloperoxidase target the vinyl ether bond of plasmalogens: disparate utilization of sodium halides in the production of alpha-halo fatty aldehydes. J Biol Chem 277: 4694–4703, 2002. doi: 10.1074/jbc.M110875200. [DOI] [PubMed] [Google Scholar]
- 205.Mosher WD, Martinez GM, Chandra A, Abma JC, Willson SJ. Use of contraception and use of family planning services in the United States: 1982-2002. Adv Data 350: 1–36, 2004. [PubMed] [Google Scholar]
- 206.Jilling T, Ren C, Yee A, Aggarwal S, Halloran B, Ambalavanan N, Matalon S. Exposure of neonatal mice to bromine impairs their alveolar development and lung function. Am J Physiol Lung Cell Mol Physiol 314: L137–L143, 2018. doi: 10.1152/ajplung.00315.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]